Abstract
Purpose
Our group has previously published the Graded Prognostic Assessment (GPA), a prognostic index for patients with brain metastases. Updates have been published with refinements to create diagnosis-specific Graded Prognostic Assessment indices. The purpose of this report is to present the updated diagnosis-specific GPA indices in a single, unified, user-friendly report to allow ease of access and use by treating physicians.
Methods
A multi-institutional retrospective (1985 to 2007) database of 3,940 patients with newly diagnosed brain metastases underwent univariate and multivariate analyses of prognostic factors associated with outcomes by primary site and treatment. Significant prognostic factors were used to define the diagnosis-specific GPA prognostic indices. A GPA of 4.0 correlates with the best prognosis, whereas a GPA of 0.0 corresponds with the worst prognosis.
Results
Significant prognostic factors varied by diagnosis. For lung cancer, prognostic factors were Karnofsky performance score, age, presence of extracranial metastases, and number of brain metastases, confirming the original Lung-GPA. For melanoma and renal cell cancer, prognostic factors were Karnofsky performance score and the number of brain metastases. For breast cancer, prognostic factors were tumor subtype, Karnofsky performance score, and age. For GI cancer, the only prognostic factor was the Karnofsky performance score. The median survival times by GPA score and diagnosis were determined.
Conclusion
Prognostic factors for patients with brain metastases vary by diagnosis, and for each diagnosis, a robust separation into different GPA scores was discerned, implying considerable heterogeneity in outcome, even within a single tumor type. In summary, these indices and related worksheet provide an accurate and facile diagnosis-specific tool to estimate survival, potentially select appropriate treatment, and stratify clinical trials for patients with brain metastases.
INTRODUCTION
Brain metastases are a common problem, with incidence estimates ranging from 100,000 to 300,000 patients per year.1,2 In the past, survival was uniformly poor, and a fatalistic futileness dominated management recommendations.2,3 With advances in systemic therapy and technology, including stereotactic radiosurgery, this nihilism has been replaced with the need to tailor therapy to appropriate subgroups, based on expected survival. In this context, it is well recognized that the prognosis for patients with brain metastases varies widely and a one-size-fits-all treatment paradigm is no longer appropriate; management decisions require a thorough understanding of projected survival.
The original work for this dates back to 1997, when Gaspar et al4 published a seminal report on a prognostic index for patients with brain metastases, the Radiation Therapy Oncology Group's (RTOG) Recursive Partitioning Analysis. The index was validated5 and quickly adopted for purposes of stratification in clinical trials. Weaknesses of the RTOG Recursive Partitioning Analysis are that it is not diagnosis specific and the data are considered legacy in nature, because of their vintage, and do not reflect current advances in systemic therapy.
The Graded Prognostic Assessment (GPA) is a newer prognostic index for patients with brain metastases.6 This prognostic index was originally developed from a database of 1,960 patients accrued to four Radiation Therapy Oncology Group (RTOG) protocols for patients with brain metastases.7–10 The original GPA was validated11 and refined with diagnosis-specific prognostic indices based on a second, independent, multi-institutional retrospective analysis of 4,259 other patients with brain metastases from breast carcinoma, small-cell and non–small-cell lung carcinoma, GI cancers, melanoma, and renal cell carcinoma.12,13 The breast cancer–specific GPA index was then further refined using additional variables, including human epidermal growth factor receptor 2 (HER2) and estrogen receptor (ER)/progesterone receptor (PR) status.14 In that study, two statistical methodologies, multivariate Cox regression (MCR) and recursive partitioning analysis (RPA), were used to identify and weight the prognostic factors that were significant for each diagnosis. The Lung-GPA and the Breast-GPA are being used to stratify patients in RTOG 1118, a randomized phase I/II trial of whole-brain radiation therapy (WBRT) with and without drug therapy, and the Breast-GPA has been adopted in RTOG 1119, a randomized phase II trial of WBRT versus WBRT plus lapatinib in patients with HER2-positive breast cancer and brain metastases.
Because the diagnosis-specific GPA indices have evolved since the initial reports, it was felt that it would be useful to collect and publish the updated indices in one concise and facile reference to facilitate their clinical use in a practical manner, especially in the context of a radiation oncology service or clinic.
METHODS
Patient Population
An institutional review board–approved retrospective database of patients treated for brain metastases between June 1993 and January 2010 was generated from the radiation oncology departments at 11 institutions. The number of patients included in this composite report is 3,940 (from the 4,259 patients entered into the database), which excluded patients with missing data for one or more prognostic factors, mostly patients with breast cancer with incomplete ER/PR/HER2 data. All of the 3,940 patients were treated for newly diagnosed brain metastases. Patients in this database completed treatment within 2 months of the diagnosis of brain metastases. The 2-month cutoff was made to exclude patients with recurrent brain metastases and was an approximation of when a patient who underwent surgery would complete WBRT. In other words, this entire database represents patients with newly diagnosed brain metastases. Patients with recurrent brain metastases were excluded.
Prognostic Factors for Survival
Survival time was measured from the time of first treatment for brain metastases to the date of death or last follow-up. Prognostic factors for survival were analyzed by MCR in the diagnosis-specific GPA report12 and by both MCR and RPA in the Breast-GPA publication.14 This dual MCR-RPA methodology has been previously shown to be an effective tool in the design of prognostic indices.15 Prognostic factors found to be significant by either method were weighted relative to the magnitude of their regression coefficients to define the GPA index.
MCR
Multivariate survival analysis was performed using the Cox proportional hazards model. The Cox model was stratified by institution to allow for potentially different shapes of the baseline hazard function. Factors initially considered were age, Karnofsky performance score, number of brain metastases, whether extracranial metastases were present, HER2/ER/PR status, and all possible two-way interactions. A forward selection procedure with a cutoff of P = .10 was used to establish the initial model. Analysis was performed using SAS version 9.2 (SAS Institute, Cary, NC).
RPA
In the Breast-GPA report,14 RPA was used to supplement MCR in the construction of the index. RPA splits the sample into two subgroups, or nodes, choosing a splitting rule from among all possible splits over all prognostic factors. The split that maximizes the homogeneity of each subgroup with respect to survival is chosen. This procedure is performed recursively to generate a tree, which is then pruned to an optimal size.16,17 This analysis was performed using R version 2.10.1 (R Development Core Team, 2009, http://www.r-project.org/).
Derivation of the GPA Indices
Prognostic factors found to be significant by either MCR12 or MCR and/or RPA14 were retained in the final MCR model to improve its prognostic ability. The relative magnitudes of the regression coefficients (ie, log hazard ratios) from the final model were used to design and weight the GPA, an additive point-based prognostic index. A score of 4.0 correlates with the best prognosis, and a score of 0.0 correlates with the worst prognosis. The GPA was refined by choosing prognostic factor splits and point groupings that maximized differentiation with respect to survival curves among classes, while keeping the total number of classes and factor levels to a manageable number for ease of use and to ensure that differences among classes were large enough to be clinically meaningful and statistically significant. For the Breast-GPA, RPA was also used to select splits of continuous factors. The Kaplan-Meier method was used to estimate the survival curve for each prognostic group. The log-rank test was used to test whether significant survival differences were present between adjacent classes and among all classes. A smartphone application for the GPA is in development.
RESULTS
Table 1 lists the median survival times (MSTs) for patients with brain metastases, overall, by diagnosis, and by GPA score. The overall MST for all patients was 7.16 months, but the MST varied from 2.79 to 25.30 months depending on diagnosis and GPA. The overall MSTs by histology for the various GPA classes were as follows: non–small-cell lung cancer, 7.00 months (range, 3.02 to 14.78 months); small-cell lung cancer, 4.90 months (range, 2.79 to 17.05 months); melanoma, 6.74 months (range, 3.38 to 13.32 months); renal cell carcinoma, 9.63 months (range, 3.27 to 14.77 months); breast cancer, 13.80 months (range, 3.35 to 25.30 months); and GI cancers, 5.36 months (range, 3.13 to 13.54 months).
Table 1.
Median Survival Time for Patients With Brain Metastases by DS-GPA Score
| Diagnosis | Overall |
DS-GPA Score |
P(log-rank) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0-1.0 |
1.5-2.0 |
2.5-3.0 |
3.5-4.0 |
|||||||||||||||||
| Survival Time (months) |
No. of Patients | Survival Time (months) |
Patients |
Survival Time (months) |
Patients |
Survival Time (months) |
Patients |
Survival Time (months) |
Patients |
|||||||||||
| Median | 95% CI | Median | 95% CI | No. | % | Median | 95% CI | No. | % | Median | 95% CI | No. | % | Median | 95% CI | No. | % | |||
| NSCLC | 7.00 | 6.53 to 7.50 | 1,833 | 3.02 | 2.63 to 3.84 | 254 | 14 | 5.49 | 4.83 to 6.40 | 705 | 38 | 9.43 | 8.38 to 10.80 | 713 | 40 | 14.78 | 11.80 to 18.80 | 161 | 9 | < .001 |
| SCLC | 4.90 | 4.30 to 6.20 | 281 | 2.79 | 1.83 to 3.12 | 65 | 23 | 4.90 | 4.04 to 6.51 | 119 | 42 | 7.67 | 6.27 to 9.13 | 84 | 30 | 17.05 | 4.70 to 27.43 | 13 | 5 | < .001 |
| Melanoma | 6.74 | 5.90 to 7.56 | 481 | 3.38 | 2.53 to 4.27 | 84 | 17 | 4.70 | 4.07 to 5.39 | 150 | 31 | 8.77 | 6.74 to 10.77 | 135 | 28 | 13.23 | 9.13 to 15.64 | 112 | 23 | < .001 |
| RCC | 9.63 | 7.66 to 10.91 | 286 | 3.27 | 2.04 to 5.10 | 43 | 15 | 7.29 | 3.73 to 10.91 | 76 | 27 | 11.27 | 8.80 to 14.80 | 104 | 36 | 14.77 | 9.73 to 19.79 | 63 | 22 | < .001 |
| Breast cancer | 13.80 | 11.53 to 15.87 | 400 | 3.35 | 3.13 to 3.78 | 23 | 6 | 7.70 | 5.62 to 8.74 | 104 | 26 | 15.07 | 12.94 to 15.87 | 140 | 35 | 25.30 | 23.10 to 26.51 | 133 | 33 | < .001 |
| GI cancer | 5.36 | 4.30 to 6.30 | 209 | 3.13 | 2.37 to 4.57 | 76 | 36 | 4.40 | 3.37 to 6.53 | 65 | 31 | 6.87 | 4.86 to 11.63 | 50 | 24 | 13.54 | 9.76 to 27.12 | 18 | 9 | < .001 |
| Other | 6.37 | 5.22 to 7.49 | 450 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Total | 7.16 | 6.83 to 7.52 | 3,940 | 3.10 | 2.83 to 3.45 | 545 | 16 | 5.40 | 4.90 to 5.89 | 1,219 | 35 | 9.63 | 8.74 to 10.58 | 1,226 | 35 | 16.73 | 14.65 to 18.80 | 500 | 14 | < .001 |
Abbreviations: DS-GPA, diagnosis-specific Graded Prognostic Assessment; NSCLC, non–small-cell lung cancer; RCC, renal cell carcinoma; SCLC, small-cell lung cancer.
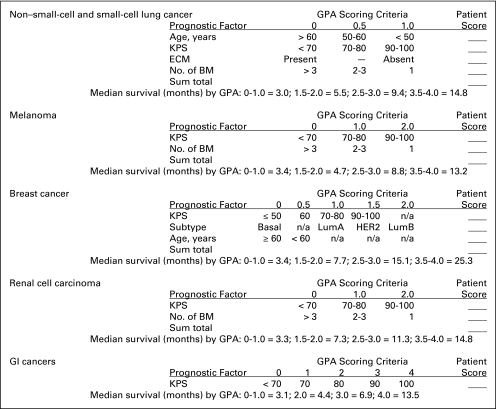
Figure 1 shows a practical and useful worksheet updating the diagnosis-specific GPA indices, based on significant prognostic factors and the scoring criteria. The sum of the points for each prognostic factor is the GPA for an individual patient. The estimated MST for each diagnosis and GPA is included in the worksheet for easy reference.
Fig 1.
Graded Prognostic Assessment (GPA) worksheet to estimate survival from brain metastases (BM) by diagnosis. Subtype: Basal: triple negative; LumA: ER/PR positive, HER2 negative; LumB: triple positive; HER2: ER/PR negative, HER2 positive. ECM, extracranial metastases; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; KPS, Karnofsky performance score; LumA, luminal A; LumB, luminal B; PR, progesterone receptor.
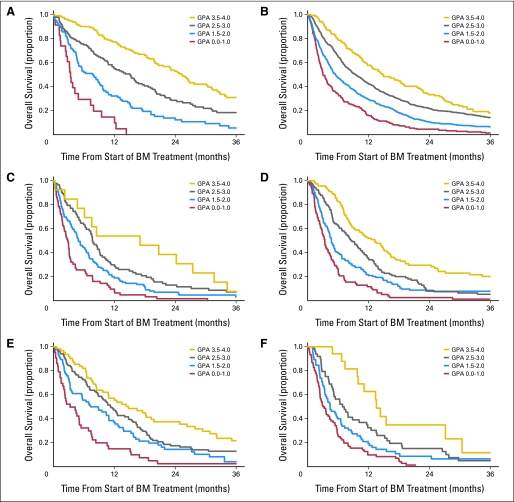
Figure 2 shows the Kaplan-Meier curve for survival by GPA group, demonstrating excellent separation between groups (P < .001). The MSTs for all patients with GPA scores of 0 to 1.0, 1.5 to 2.0, 2.5 to 3.0, and 3.5 to 4.0 were 3.10, 5.40, 9.63, and 16.73 months, respectively.
Fig 2.
Kaplan-Meier curves for survival for six diagnoses by Graded Prognostic Assessment (GPA) group, demonstrating excellent separation between groups (P < .001) for each diagnosis: (A) breast cancer; (B) non–small-cell lung cancer; (C) small-cell lung cancer; (D) melanoma; (E) renal cell carcinoma; and (F) GI cancer. BM, brain metastases.
Table 1 shows that in the database overall, the percentages of patients with a GPA of 0 to 1.0, 1.5 to 2.0, 2.5 to 3.0, and 3.5 to 4.0 were 16%, 35%, 35%, and 14%. The only diagnoses that vary from this pattern are breast cancer, in which only 6% of patients have a GPA of 0 to 1.0, and GI cancers, in which 36% of patients have a GPA of 0 to 1.0.
Table 2 demonstrates a multivariate analysis of risk of death and median survival by treatment and diagnosis. The percentages of patients with each diagnosis who received each treatment are also listed.
Table 2.
Multivariate Analysis of Risk of Death and Median Survival* by Treatment and Diagnosis
| Diagnosis | Total No. of Patients | Treatment |
|||||
|---|---|---|---|---|---|---|---|
| WBRT | SRS | WBRT + SRS | S + SRS | S + WBRT | S + WBRT + SRS | ||
| NSCLC | 1,833 | ||||||
| Risk of death† | |||||||
| HR | 1.0 | 0.62‡ | 0.54‡ | 0.48‡ | 0.48‡ | 0.39‡ | |
| 95% CI | 0.51 to 0.74 | 0.46 to 0.64 | 0.34 to 0.68 | 0.40 to 0.57 | 0.27 to 0.55 | ||
| P | < .001 | < .001 | < .001 | < .001 | < .001 | ||
| Median survival (months) | 3.53 | 9.86 | 12.72 | 11.86 | 11.66 | 12.06 | |
| Patients | |||||||
| No. | 768 | 395 | 339 | 58 | 212 | 61 | |
| % | 42 | 22 | 18 | 3 | 12 | 3 | |
| SCLC | 281 | ||||||
| Risk of death† | |||||||
| HR | 1.0 | 0.97 | 0.24‡ | 0.00 | 0.42‡ | 0.00 | |
| 95% CI | 0.41 to 2.26 | 0.10 to 0.59 | NA | 0.25 to 0.73 | NA | ||
| P | .94 | .002 | .99 | .002 | .98 | ||
| Median survival (months) | 4.24 | 6.90 | 15.23 | 12.02 | 14.66 | 14.95 | |
| Patients | |||||||
| No. | 229 | 13 | 21 | 1 | 16 | 1 | |
| % | 81 | 5 | 7 | 0.4 | 6 | 0.4 | |
| Melanoma | 481 | ||||||
| Risk of death† | |||||||
| HR | 1.0 | 0.73 | 0.79 | 0.64 | 0.61‡ | 0.51‡ | |
| 95% CI | 0.50 to 1.06 | 0.54 to 1.16 | 0.37 to 1.11 | 0.37 to 0.99 | 0.29 to 0.89 | ||
| P | .10 | .24 | .11 | .04 | .02 | ||
| Median survival (months) | 2.87 | 7.26 | 6.67 | 12.78 | 11.10 | 13.11 | |
| Patients | |||||||
| No. | 86 | 221 | 89 | 30 | 29 | 26 | |
| % | 18 | 46 | 19 | 6 | 6 | 5 | |
| Renal cell carcinoma | 286 | ||||||
| Risk of death† | |||||||
| HR | 1.0 | 0.83 | 0.70 | 0.87 | 0.66 | 0.68 | |
| 95% CI | 0.56 to 1.21 | 0.43 to 1.14 | 0.42 to 1.83 | 0.37 to 1.17 | 0.09 to 5.01 | ||
| P | .33 | .15 | .71 | .16 | .70 | ||
| Median survival (months) | 5.08 | 10.78 | 12.12 | 12.91 | 15.52 | 8.80 | |
| Patients | |||||||
| No. | 78 | 131 | 46 | 11 | 18 | 2 | |
| % | 27 | 46 | 16 | 4 | 6 | 1 | |
| Breast cancer | 400 | ||||||
| Risk of death† | |||||||
| HR | 1.0 | 1.07 | 0.74 | 0.59 | 0.72 | 0.47‡ | |
| 95% CI | 0.66 to 1.73 | 0.47 to 1.16 | 0.28 to 1.23 | 0.43 to 1.21 | 0.23 to 0.96 | ||
| P | .80 | .18 | .16 | .72 | .04 | ||
| Median survival (months) | 7.39 | 12.85 | 15.47 | 23.98 | 18.30 | 29.53 | |
| Patients | |||||||
| No. | 131 | 115 | 86 | 19 | 28 | 20 | |
| % | 33 | 29 | 22 | 5 | 7 | 5 | |
| GI cancer | 209 | ||||||
| Risk of death† | |||||||
| HR | 1.0 | 0.72 | 0.69 | 2.30 | 0.33‡ | 0.39‡ | |
| 95% CI | 0.40 to 1.28 | 0.39 to 1.22 | 0.43 to 12.4 | 0.19 to 0.56 | 0.17 to 0.90 | ||
| P | .26 | .21 | .33 | < .001 | .03 | ||
| Median survival (months) | 2.92 | 7.33 | 7.13 | 9.30 | 10.37 | 7.92 | |
| Patients | |||||||
| No. | 95 | 35 | 35 | 2 | 34 | 8 | |
| % | 45 | 17 | 17 | 1 | 16 | 4 | |
Abbreviations: HR, hazard ratio; NA, not applicable; NSCLC, non–small-cell lung cancer; S, surgery; SCLC, small-cell lung cancer; SRS, stereotactic radiosurgery; WBRT, whole-brain radiation therapy.
Median survival is based on one-sample Kaplan-Meier method.
Risk of death HR was normalized to patients treated with WBRT alone (HR, 1.0), calculated using multivariate Cox regression, adjusted for diagnosis-specific Graded Prognostic Assessment, and stratified by institution.
Statistically significantly better than WBRT alone.
DISCUSSION
The primary strengths of this study include the large sample size and the multi-institutional, multinational nature of the database. The weaknesses of this study include the following: the retrospective design and inherent selection bias; the long period of time (1985 to 2007) represented in this database, during which many changes in the management of patients with brain metastases occurred, including the advent of stereotactic radiosurgery, the widespread use of magnetic resonance imaging to monitor these patients, and improvements in chemotherapy to control systemic disease; patients with brain metastases who were not treated for their brain metastases are not included in this database, and thus, this database may overestimate survival of the overall population of patients with brain metastases; and the database did not have complete data on the size of brain metastases.
The use of the term “in selected patients” has become so ubiquitous in guidelines and review articles that it renders them edentulous. This creates a heuristic imbroglio of clinical science, defeating the purpose of the guidelines and suggesting almost any treatment option is acceptable. The diagnosis-specific GPA indices presented here hold several implications for clinical management and research involving patients with brain metastases.
These clinical implications and nuances for management include the following. First, there is marked heterogeneity in outcomes for patients with brain metastases, and these outcomes vary not only by diagnosis, but also by diagnosis-specific prognostic factors, as detailed herein. Because of this heterogeneity, we should not treat all patients with brain metastases the same way; treatment should be individualized, and the past philosophy of fatalistic futileness should be abandoned. In this respect, we are reminded of Sir William Osler's famous adage, “the greater the dogma, the greater the ignorance.”18
Second, as shown in Table 1, if a patient has a GPA of 0 to 1.0, regardless of diagnosis, their expected survival is approximately 3 months. For these patients, a more conservative treatment approach may be the best option.
Third, for patients with GPA scores greater than 1.0, the MST (Table 1) varies more by diagnosis, and more aggressive treatment strategies may be appropriate, but these retrospective data do not provide a basis for assuming that longer survival is a consequence of more aggressive treatment. Indeed, the survival by treatment data shown in Table 2 is certainly fraught with selection bias and should not be blindly applied or expected. Nonetheless, these data reflect patterns of care for patients with brain metastases over the last quarter century.
Fourth, performance status is prognostic in every diagnosis. Clinicians should take the time to accurately assess and document their patients' performance status.
Fifth, Figure 1 shows that the number of brain metastases is a significant prognostic factor for lung cancer, melanoma, and renal cell carcinoma, but not for breast or GI cancers. Patients should not be denied treatment because of the number of brain metastases.
Sixth, extracranial metastases are only prognostic in lung cancer, and not in melanoma, breast cancer, renal cell carcinoma, or GI cancers. The implication here is that patients with nonlung malignancies should not be denied aggressive treatment for their brain metastases because they have extracranial metastases.
Seventh, age is strongly prognostic in lung cancer, weakly prognostic in breast cancer, and not prognostic in melanoma, renal cell carcinoma, or GI cancers. Thus, age should not be used as a rationale to withhold aggressive treatment for nonlung malignancies.
Eighth, because lung cancer and brain metastases from lung cancer are so common, those patients have masked our understanding of the distinct course for patients with nonlung malignancies and brain metastases, as demonstrated earlier by the fifth, sixth, and seventh points.
Ninth, tumor subtype in breast cancer is of paramount importance and prognostic significance, but it is not as prognostic as the Breast-GPA index.14
Tenth, a disproportionate number of patients with GI cancers present with a GPA of 0 to 1.0. Whether this is a result of lack of screening magnetic resonance imaging in these patients versus other biologic reasons remains unclear, but the finding should serve as a reminder that brain metastases are not uncommon in patients with GI cancer.
Eleventh, clinicians may use the worksheet in Table 2 to calculate their patient's GPA score and estimate survival.
Twelfth, the GPA may be used for purposes of stratification in clinical trials dealing with patients with brain metastases. As noted, the GPA has already been adopted for stratification of two RTOG trials (1118 and 1119). In summary, these indices and worksheet provide a quick and user-friendly diagnosis-specific tool to estimate survival for patients with brain metastases.
Footnotes
Supported in part by Grant No. W81XWH-062-0033 from the US Department of Defense Breast Cancer Research Program (R.J.W.) and by National Institutes of Health Grant No. P30-CA77598 using the services of the Biostatistics and Bioinformatics Core, Masonic Cancer Center, University of Minnesota shared resource.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Minesh Mehta, Pharmacyclics (C), Apogenix (C) Consultant or Advisory Role: John Suh, Abbott (C); Nancy Lin, GlaxoSmithKline (C), Novartis (C), Geron (U); Minesh Mehta, Adnexus (C), Bayer Pharmaceuticals (C), Genentech (C), Schering-Plough (C), TomoTherapy (C) Stock Ownership: Minesh Mehta, Pharmacyclics, TomoTherapy Honoraria: None Research Funding: John Kirkpatrick, Varian Medical Systems; Nancy Lin, Genentech, GlaxoSmithKline, Boehringer Ingelheim, Geron Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Paul W. Sperduto, Ryan Shanley, Xianghua Luo, Minesh Mehta
Provision of study materials or patients: Paul W. Sperduto,David Roberge
Collection and assembly of data: Paul W. Sperduto, Norbert Kased, David Roberge, Zhiyuan Xu, Ryan Shanley, Xianghua Luo, Penny K. Sneed, Samuel T. Chao, John Suh, Amit Bhatt, Ashley W. Jensen,Paul D. Brown, Helen A. Shih, John Kirkpatrick, Laurie E. Gaspar, John B. Fiveash, Veronica Chiang, Jonathan P.S. Knisely,Christina Maria Sperduto
Data analysis and interpretation: Paul W. Sperduto, David Roberge, Ryan Shanley, Xianghua Luo, Penny K. Sneed, Robert J. Weil, Helen A. Shih, Nancy Lin, Minesh Mehta
Manuscript writing: All authors
Final approval of manuscript: All authors
Affiliations
Paul W. Sperduto, University of Minnesota Gamma Knife, Minneapolis Radiation Oncology; Ryan Shanley and Xianghua Luo, University of Minnesota, Minneapolis; Ashley W. Jensen and Paul D. Brown, Mayo Clinic, Rochester, MN; Norbert Kased and Penny K. Sneed, University of California, San Francisco, San Francisco, CA; Zhiyuan Xu, Samuel T. Chao, Robert J. Weil, and John Suh, Cleveland Clinic, Cleveland, OH; Amit Bhatt, University of Wisconsin, Madison, WI; Helen A. Shih, Massachusetts General Hospital, Harvard Medical School; Nancy Lin, Dana-Farber Cancer Institute, Boston, MA; John Kirkpatrick, Duke University Medical Center, Durham, NC; Laurie E. Gaspar, University of Colorado School of Medicine, Aurora, CO; John B. Fiveash, University of Alabama Medical Center at Birmingham, Birmingham, AL; Veronica Chiang, Yale University School of Medicine and Yale Cancer Center, New Haven, CT; Jonathan P.S. Knisely, Hofstra University School of Medicine and North Shore-Long Island Jewish Health System, Manhasset, NY; Christina Maria Sperduto, Dartmouth College, Hanover, NH; Minesh Mehta, Northwestern University, Chicago, IL; and David Roberge, McGill University Health Center, Montreal, Quebec, Canada.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Zimm S, Wampler GL, Stablein D, et al. Intracerebral metastases in solid-tumor patients: Natural history and results of treatment. Cancer. 1981;48:384–394. doi: 10.1002/1097-0142(19810715)48:2<384::aid-cncr2820480227>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 3.Posner JB. Neurologic Complications of Cancer. Philadelphia, PA: FA Davies; 1995. [Google Scholar]
- 4.Gaspar LE, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–751. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 5.Gaspar LE, Scott C, Murray K, et al. Validation of the RTOG recursive partitioning analysis (RPA) classification for brain metastases. Int J Radiat Oncol Biol Phys. 2000;47:1001–1006. doi: 10.1016/s0360-3016(00)00547-2. [DOI] [PubMed] [Google Scholar]
- 6.Sperduto PW, Berkey B, Gaspar LE, et al. A new prognostic index and comparison to three other indices for patients with brain metastases: An analysis of 1960 patients in the RTOG database. Int J Radiat Oncol Biol Phys. 2008;70:510–514. doi: 10.1016/j.ijrobp.2007.06.074. [DOI] [PubMed] [Google Scholar]
- 7.Komarnicky LT, Phillips TL, Martz K, et al. A randomized phase III protocol for the evaluation of misonidazole combined with radiation in the treatment of patients with brain metastases (RTOG79-16) Int J Radiat Oncol Biol Phys. 1991;20:53–58. doi: 10.1016/0360-3016(91)90137-s. [DOI] [PubMed] [Google Scholar]
- 8.Sause WT, Scott C, Kirsch R, et al. Phase I/II trial of accelerated fractionation in brain metastases, RTOG 85-28. Int J Radiat Oncol Biol Phys. 1993;26:653–657. doi: 10.1016/0360-3016(93)90284-3. [DOI] [PubMed] [Google Scholar]
- 9.Phillips TL, Scott CB, Leibel S, et al. Results of a randomized comparison of radiotherapy and bromodeoxyuridine to radiotherapy alone for brain metastases: Report of RTOG trial 89-05. Int J Radiat Oncol Biol Phys. 1995;33:339–348. doi: 10.1016/0360-3016(95)00168-X. [DOI] [PubMed] [Google Scholar]
- 10.Murray KJ, Scott C, Greenberg HM, et al. A randomized phase III study of accelerated hyperfractionation versus standard in patients with unresected brain metastases: A report of RTOG 9104. Int J Radiat Oncol Biol Phys. 1997;39:571–574. doi: 10.1016/s0360-3016(97)00341-6. [DOI] [PubMed] [Google Scholar]
- 11.Sperduto CM, Watanabe Y, Mullan J, et al. A validation study of a new prognostic index for patients with brain metastases: The graded prognostic assessment. J Neurosurg. 2008;109:87–89. doi: 10.3171/JNS/2008/109/12/S14. [DOI] [PubMed] [Google Scholar]
- 12.Sperduto PW, Chao ST, Sneed PK, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: A multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77:655–661. doi: 10.1016/j.ijrobp.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 13.Sperduto PW. What is your patient's GPA and why does it matter? Managing brain metastases and the cost of hope. Int J Radiat Oncol Biol Phys. 2010;77:643–644. doi: 10.1016/j.ijrobp.2010.02.038. [DOI] [PubMed] [Google Scholar]
- 14.Sperduto PW, Kased N, Roberge D, et al. The effect of tumor subtype on survival and the Graded Prognostic Assessment (GPA) for patients with breast cancer and brain metastases. Int J Radiat Oncol Biol Phys. 10.1016/j.ijrobp.2011.02.027 [epub ahead of print on April 14, 2011] [DOI] [PMC free article] [PubMed]
- 15.van Dijk MR, Steyerberg EW, Stenning SP, et al. Survival of patients with nonseminomatous germ cell cancer: A review of the IGCC classification by Cox regression and recursive partitioning. Br J Cancer. 2004;90:1176–1183. doi: 10.1038/sj.bjc.6601665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breiman L, Friedman JH, Olshen RA, et al. Classification and Regression Trees. Monterey, CA: Wadsworth; 1984. [Google Scholar]
- 17.Therneau TM, Atkinson EJ. Technical Report 61. Rochester, MN: Section of Biostatistics, Mayo Clinic; 1997. An Introduction to Recursive Partitioning Using the rpart Routine. [Google Scholar]
- 18.et al. Chauvinism in medicine, in Aequanimitas and Other Addresses. Philadelphia, PA: P. Blakiston's Son & Co; 1928. p. 300. [Google Scholar]