Abstract
Purpose
Adjuvant therapy using anti-GD2 monoclonal antibody and granulocyte-macrophage colony-stimulating factor (GM-CSF) has shown treatment success for patients with high-risk neuroblastoma (NB). Although there is ample evidence on how the antibody targets NB, in vivo contribution by GM-CSF remains unclear. This report investigates granulocyte activation and its correlation with treatment outcome.
Patients and Methods
Patients enrolled onto NCT00072358 received multiple treatment cycles, each consisting of anti-GD2 antibody 3F8 plus subcutaneous (SC) GM-CSF. Peripheral-blood (PB) samples from 151 patients were collected on day 0 and day 4 of cycle 1. PB from a subgroup of 35 patients had intravenous (IV) instead of SC GM-CSF during cycle 4. Samples were analyzed by flow cytometry for CD11a, CD63, CD87, and CD11b and its activation epitope CBRM1/5.
Results
Comparing cycle 1 day 4 PB samples with day 0 PB samples, five of five activation marker–positive granulocytes were significantly higher. The change in frequency and mean fluorescence intensity of CBRM1/5-positive granulocytes correlated with progression-free survival (PFS; P = .024 and P = .008, respectively). A multivariable analysis identified increasing CBRM1/5-positive granulocytes and missing killer immunoglobulin-like receptor ligand as positive independent prognostic factors for PFS, whereas second-line cyclophosphamide-based therapy before protocol entry negatively influenced outcome. Thirty-five patients who received SC GM-CSF at cycle 1 and IV GM-CSF at cycle 4 had significantly less CBRM1/5 activation after IV GM-CSF. In contrast, 63 patients who received SC GM-CSF at both cycles had comparable CBRM1/5 activation.
Conclusion
GM-CSF–induced granulocyte activation in vivo is associated with improved patient outcome. This activation was more apparent when GM-CSF was given by the SC route instead of IV route.
INTRODUCTION
Monoclonal antibody (MoAb) therapy is an accepted treatment modality for cancers in adult solid tumors, including colorectal and breast cancer, non–small-cell lung cancer, squamous cell carcinoma, and melanoma.1,2 However, this modality has remained inadequately exploited for the treatment of pediatric cancers. Unlike chemotherapy or radiation, MoAb is neither myelosuppressive nor genotoxic and generally has little long-term toxicity. These are critical considerations for young children. More importantly, MoAb is effective against malignant cells in blood, bone marrow, and bone, typical metastases found in high-risk neuroblastoma (NB).
GD2 is an adhesion molecule abundant on NB. It is also widely expressed in melanoma, small-cell lung cancer, bone or soft tissue sarcoma, retinoblastoma, and brain tumors.3 GD2 is rarely expressed in normal tissues, except neurons, skin cells, and pain fibers. Scintigraphy studies using radiolabeled MoAb confirm excellent tumor targeting.3 At least two antibody families against GD2 have been tested clinically (ie, murine 3F84 and ch14.185,6). They both mediate antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-mediated cytotoxicity of NB and melanoma cells in vitro.7–10 Used alone, ch14.18 improved overall survival and reduced the rate of bone marrow relapse.11 When combined with granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-2 after autologous stem-cell transplantation,12 statistically significant improvements in progression-free survival (PFS) and overall survival were documented at 2 years in a phase III randomized trial.13
Anti-GD2 MoAb mediates highly efficient ADCC of NB in the presence of human natural killer (NK) cells and granulocytes in vitro.14–16 Moreover, it induces complement-mediated cytotoxicity because NB cells lack decay-accelerating factor CD5517 and homologous restriction factor CD59.18 Complement deposition on NB cells can also enhance ADCC by activating the iC3b receptor on granulocytes.16,19 Neutrophil function depends on an array of adhesion molecules, including β2 integrin LFA-1 (CD11a/CD18) and membrane-activated complex 1 (Mac-1), also called CD11b/CD18 or CR3. CD11b/CD18 has been implicated in tumor ADCC mediated by anti-GD2 antibodies.16 CD11b is most efficient when activated (ie, when a conformational change within the N-terminal ligand-binding I domain creates a neoepitope known as CBRM1/5.20 Although upregulation of CD11b expression typically accompanies CD11b conformational activation in vitro,21 the role of CD11b conformational activation in vivo and its prognostic importance in patient outcome are not known.
Although chemotherapy leads to prolonged T-cell lymphopenia and immunosuppression,22 myeloid cells predictably recover, provided colony-stimulating factors are given. In two consecutive clinical trials of high-risk NB conducted at Memorial Sloan-Kettering Cancer Center, a combination of anti-GD2 MoAb 3F8 and GM-CSF was tested for its efficacy in prolonging survival among patients with minimal residual disease. Intravenous (IV) GM-CSF (ClinicalTrials.gov identifier: NCT00002560) was used in the first study,23 and subcutaneous (SC) GM-CSF was used in a subsequent trial (NCT00072358). In this report, granulocyte activation in patients treated on NCT00072358 was analyzed and correlated with treatment outcome. Activation markers including CD11b (and its activation epitope CBRM1/5), CD11a, CD63, and CD87 were examined. CD63, a member of the tetraspan membrane glycoprotein family,24 is an activation marker of neutrophils and basophils, and it is responsible for the retention and sorting of pro-neutrophil elastase in the primary granules of neutrophils.25 CD87 is a cellular urokinase-type plasminogen activation receptor found on granulocytes, monocytes, T cells, and NK cells. In neutrophils, activation triggers exocytosis of preformed CD87 on cytoplasmic granular membranes to the cell surface. Complex formation with urokinase-type plasminogen activation receptor facilitates the adhesive functions of CD11b. Given the prognostic importance of FcGR2A26 and inhibitory killer immunoglobulin-like receptor (KIR) mismatch,27 a multivariable analysis of known prognostic variables was also carried out.
PATIENTS AND METHODS
Treatment Protocol
The first 151 evaluable patients who received anti-GD2 MoAb 3F8 and yeast-derived human recombinant GM-CSF (Sargramostim; Immunex, Seattle, WA) on protocol IRB 03-077 (NCT00072358) were included in this analysis. Informed written consents for treatment and tests were obtained in accordance to the guidelines of the institutional review board. Patients received multiple cycles of SC GM-CSF plus IV 3F8 (Table 1). The dose of GM-CSF was 250 μg/m2 on days −5 to 1; the dose was escalated to 500 μg/m2 on days 2 to 4. Treatment cycles were repeated every 1 to 3 months for up to 2 years from study entry; all patients also received six cycles of oral isotretinoin after cycle 4 at the dosage previously described.28 Immunotherapy would be terminated if there was progressive disease. For patients with elevated human antimouse antibody titer (> 1,000 U/mL),29 treatment would be delayed until their titer turned negative.
Table 1.
Treatment Intervention and PB Collection
| Treatment Day | Day of the Week | Regular Cycle GM-CSF (μg/m2) | Cycle 4 GM-CSF (μg/m2)* | Antibody 3F8 | PB Collection† |
|---|---|---|---|---|---|
| −5 | Wednesday | 250 SC | 250 SC | ||
| −4 | Thursday | 250 SC | 250 SC | ||
| −3 | Friday | 250 SC | 250 SC | ||
| −2 | Saturday | 250 SC | 250 SC | ||
| −1 | Sunday | 250 SC | 250 SC | ||
| 0 | Monday | 250 SC | 250 IV | IV | First (day 0) collection‡ |
| 1 | Tuesday | 250 SC | 250 IV | IV | |
| 2 | Wednesday | 500 SC | 500 IV | IV | |
| 3 | Thursday | 500 SC | 500 IV | IV | |
| 4 | Friday | 500 SC | 500 IV | IV | Second (day 4) collection |
Abbreviations: GM-CSF, granulocyte-macrophage colony-stimulating factor; IV, intravenous; PB, peripheral blood; SC, subcutaneous.
The first 56 patients on protocol received IV GM-CSF on days 0 through 4 of treatment cycle 4; 35 of these patients had adequate blood from both cycle 1 and cycle 4 for activation marker analysis.
Both PB samples were collected on site for immediate marker analyses.
This sample was not collected on day −5, when GM-CSF was started usually at home or at home institutions because activation markers of neutrophils are labile and would likely be affected by storage and long-distance transport.
Patient Characteristics
Patients were required to have high-risk NB according to the International Neuroblastoma Staging System30 (ie, stage 4 or stage 3 with MYCN amplification). Of the 151 patients in this analysis, 88 were male, and 63 were female; all patients were enrolled before December 1, 2008. All seven stage 3 patients had MYCN-amplified (≥ 10 copies per genome) tumors. One hundred forty-three patients had stage 4 NB (40% with MYCN-amplified tumors) diagnosed at a median age of 3.3 years; 132 of 143 patients were diagnosed at ≥ 18 months of age, and all nine patients diagnosed before age 18 months had MYCN amplification. One patient diagnosed at age 1 year had progressive disease at 3 years before reinduction and immunotherapy. One patient with stage 4S disease experienced relapse with widespread bone and marrow disease at 30 months of age before reinduction and immunotherapy. Disease status at protocol entry included 48 patients with primary refractory NB, four patients with secondary refractory NB, and 99 patients in complete remission (CR)/very good partial remission (VGPR; 74 patients in first CR/VGPR and 25 patients in ≥ second CR/VGPR) in accordance with International Neuroblastoma Response Criteria.30 Sixty-eight of 151 patients (first CR/VGPR, n = 21; ≥ second CR/VGPR, n = 20; primary refractory, n = 25; and secondary refractory, n = 2) also required a second-line cyclophosphamide-based regimen before protocol entry to consolidate remission.31,32 Among the 151 patients, 35 patients received IV GM-CSF on days 0 through 4 of cycle 4 and had adequate blood samples from both cycle 1 (SC GM-CSF) and cycle 4 (IV GM-CSF) for activation marker comparisons. Another 63 patients received SC GM-CSF during cycle 4 and had adequate blood samples from both cycle 1 (SC GM-CSF) and cycle 4 (SC GM-CSF) for activation marker comparisons. Median follow-up among survivors was 50 months (range, 12 to 87 months).
Immunostaining of Peripheral-Blood Leukocytes With Activation Markers
In the 151 patients, peripheral blood (PB) from treatment cycle 1 (plus cycle 4 for 98 patients) was collected on day 0 and day 4 of 3F8 immunotherapy (Table 1). PB diluted in phosphate-buffered saline was centrifuged at 1,500 rpm for 5 minutes at 4°C. Cell pellets were reacted with a panel of fluorescent antibodies (CD3, CD14, and class-matched control immunoglobulin G) to gate on lymphocytes, monocytes, and granulocytes and to set cut points for positivity. Cells with geometric mean fluorescence intensity (MFI) greater than 95% of control antibody were called positive. The activation marker panel included CD11a (Serotec, Oxford, United Kingdom), CD11b (D12 clone from BD Biosciences, San Jose, CA; and VIM12 clone from Invitrogen, Carlsbad, CA), CD11b activation epitope CBRM1/5 (eBioscience, San Diego, CA), and CD63 and CD87 (both from BD Biosciences). Cell pellets were incubated with these antibodies on ice for 30 minutes and then washed with phosphate-buffered saline and centrifuged at 1,500 rpm for 5 minutes at 4°C. After incubation with red cell lysis buffer (Becton Dickinson, Franklin Lakes, NJ) for 15 minutes at room temperature, cells were washed and centrifuged. Then, 200 μL of 1% formalin was added, and each sample was analyzed by flow cytometry using FACSCalibur (Becton Dickinson).
FCGR2A polymorphism genotyping has been described previously.27 Allelic discrimination of FCGR2A was identified as R/R versus H/H versus H/R. HLA and KIR genotyping and analysis have also been previously detailed.27 Patients were scored as NK sensitive if any of the HLA class I ligands for his or her inhibitory KIR was absent.
Statistical Analysis
The t test was used to determine the statistical difference between day 0 and day 4. PFS was estimated using the Kaplan-Meier method, and Cox proportional hazards regression was used to determine the statistical importance of prognostic factors on PFS.
RESULTS
Comparing Leukocyte Populations in Day 0 and Day 4 PB Samples From 151 Patients Given SC GM-CSF
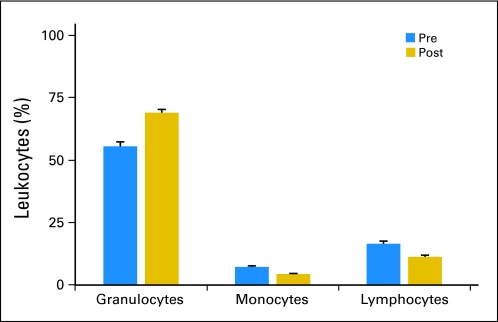
PB samples collected from treatment cycle 1 from 151 patients who were treated with SC GM-CSF plus antibody 3F8 were analyzed. Day 0 samples were obtained after 5 days of GM-CSF at 250 μg/m2. Day 4 samples were obtained after 4 more days of GM-CSF (2 days at 250 μg/m2/d, followed by 2 additional days at 500 μg/m2/d; Table 1). The day 0 sample WBC and absolute neutrophil count values were 11,400/μL ± 6,300/μL and 8,400/μL ± 5,400/μL, respectively, and the day 4 sample WBC and absolute neutrophil count values were 11,800/μL ± 8,100/μL and 8,300/μL ± 5,900/μL, respectively. By flow cytometry, granulocytes were the predominant population, with a 13.5% increase comparing day 4 with day 0 (Fig 1). In contrast, both monocytes and lymphocytes decreased by 2.9% and 5.3%, respectively. The change in all three populations was statistically significant (P < .001).
Fig 1.
Distribution of leukocyte population during cycle 1 of anti-GD2 antibody 3F8 plus subcutaneous granulocyte-macrophage colony-stimulating factor (GM-CSF). Day 0 (pre) samples were obtained after 5 days of GM-CSF at 250 μg/m2, and day 4 (post) samples were obtained after 4 more days of GM-CSF (2 days at 250 μg/m2/d, followed by 2 additional days at 500 μg/m2/d; mean + SEM; P < .001).
Change in Activation Marker Expression in Patients Given SC GM-CSF
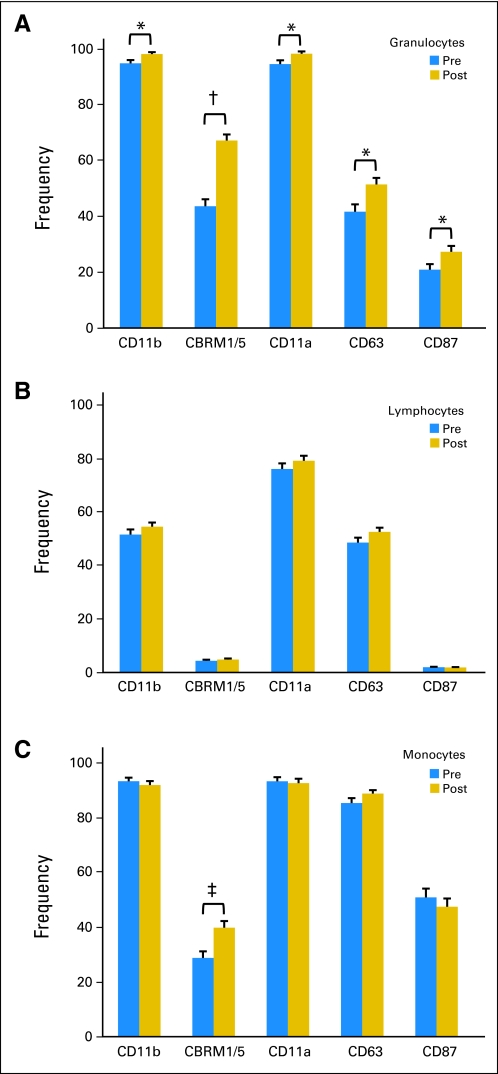
For each leukocyte population, frequency was defined as the percentage of cells positive for the specific activation marker in the respective leukocyte population. In the granulocyte population (Fig 2A), the frequency of all five activation marker–positive granulocytes in day 4 PB increased significantly compared with day 0 PB. This increase was particularly apparent for CBRM1/5, the activation epitope of CD11b, which increased from 43.6% to 67.2% (P < .0001). In contrast, none of the markers showed any statistical change in the lymphocyte population (Fig 2B). In the monocyte population, only CBRM1/5-positive cells increased from 28.9% to 39.9% (P < .001; Fig 2C).
Fig 2.
Frequency of activation marker in leukocyte population in day 0 (pre) and day 4 (post) peripheral blood of patients during cycle 1 of anti-GD2 antibody 3F8 plus subcutaneous granulocyte-macrophage colony-stimulating factor: (A) granulocytes, (B) lymphocytes, and (C) monocytes. Frequency was defined as the percentage of cells positive for the specific activation marker in the respective leukocyte population. (*) P < .03. (†) P < .0001. (‡) P < .001.
Correlation Between CD11b Activation and PFS
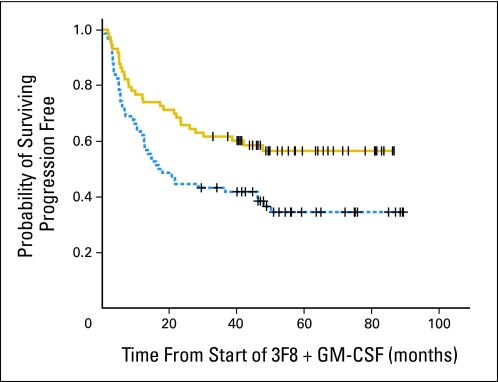
Increasing day 4 CBRM1/5-positive granulocytes were associated with the likelihood of better PFS by Kaplan-Meier analysis when the median was used as the cut point (P = .024, data not shown). Similar results were found using the ratio of day 4 MFI compared with day 0 MFI, with the median ratio as the cut point (Fig 3, P = .008). When patients were stratified according to their disease status at protocol entry, both CR/VGPR patients (n = 99) and primary refractory patients (n = 48) showed PFS advantage among patients with a greater day 4 MFI ratio (P = .009) or increased day 4 CBRM1/5-positive granulocytes (P = .025). When the absolute number of CBRM1/5-positive granulocytes in the day 0 sample was analyzed, there was no correlation with outcome. Similarly, the absolute number of CBRM1/5-positive granulocytes in the day 4 sample had no impact on PFS. In contrast, there was significant correlation when frequency change of CBRM 1/5-positive granulocytes from day 0 to day 4 samples was used as a prognostic variable. Other prognostic variables, including MYCN amplification, prior marrow or stem-cell transplantation, history of prior relapse, second-line cyclophosphamide-based therapy, disease status at enrollment (primary refractory v first CR/VGPR v second CR/VGPR), missing KIR ligand, and FcGR2A polymorphism, were also tested in univariate analyses (Table 2 and Appendix Table A1, online only). Variables with P < .1 were used to build the multivariable model. Only an increase in day 4 CBRM1/5-positive granulocytes and missing KIR ligand were found to have positive independent prognostic importance on PFS, whereas the need for an additional high-dose cyclophosphamide-based regimen before protocol entry had a negative independent impact on PFS (Table 2). Similar conclusions were drawn when MFI ratio was used instead of frequency change in CBRM1/5-positive granulocytes. The distribution of patients according to disease status at enrollment for these three independent risk factors is provided in Appendix Table A2 (online only).
Fig 3.
Correlation between mean fluorescence intensity (MFI) of CBRM1/5-positive granulocytes and progression-free survival among 147 patients treated with anti-GD2 antibody 3F8 plus subcutaneous granulocyte-macrophage colony-stimulating factor (GM-CSF). Median ratio of day 4 MFI over day 0 MFI was used as the cut point (solid line, > median, n = 73; dotted line, < median, n = 74; P = .008).
Table 2.
Univariate and Multivariate Analyses of Variables on Progression-Free Survival in 147 Patients Treated With Anti-GD2 Antibody 3F8 Plus Subcutaneous GM-CSF
| Variable | No. of Patients* |
P |
Hazard Ratio | 95% CI | |
|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | ||||
| Frequency change in CBRM1/5-positive granulocytes† | 147 | .024 | .005 | 0.51 | 0.32 to 0.81 |
| MYCN amplification | 140 | .32 | — | — | — |
| Prior bone marrow or stem-cell transplantation | 147 | .12 | — | — | — |
| History of prior relapse‡ | 147 | .025 | .092 | 1.81 | 0.91 to 3.59 |
| Second-line cyclophosphamide-based regimen | 147 | .001 | .001 | 2.24 | 1.39 to 3.63 |
| Disease status at enrollment§ | 147 | .008 | .67 | 0.95 | 0.75 to 1.21 |
| Missing KIR ligand | 147 | .073 | .034 | 0.60 | 0.38 to 0.96 |
| FcGR2A polymorphism‖ | 147 | .40 | — | — | — |
Abbreviations: GM-CSF, granulocyte-macrophage colony-stimulating factor; KIR, killer immunoglobulin-like receptor.
Four secondary refractory patients were excluded from this analysis.
Frequency was defined as the percentage of granulocyte cells positive for CBRM1/5 activation marker; change was measured from day 0 to day 4 using median cut point.
History of prior relapse included all patients in second complete remission/very good partial remission.
First complete remission/very good partial remission v primary refractory v second complete remission/very good partial remission.
R/R or R/H v H/H.
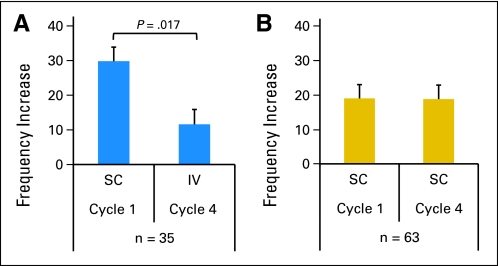
Assessing the Impact of SC Versus IV Route of GM-CSF Administration by Granulocyte CBRM1/5 Expression.
Given the patient-to-patient variability of granulocyte activation or excitability, each individual patient served as his or her own control. A total of 98 patients had adequate PB samples collected in both cycle 1 and cycle 4 for activation analysis; 35 patients received SC GM-CSF in cycle 1 and IV GM-CSF in cycle 4, whereas 63 patients received SC GM-CSF in both cycle 1 and cycle 4. CBRM1/5 activation (frequency increase from day 0 to day 4) during cycle 4 was compared with that during cycle 1 in these two patient subgroups. Patients (n = 35) given IV GM-CSF (cycle 4) had significantly less activation than when they were given SC GM-CSF (cycle 1; P = .017, Fig 4A). In contrast, among the 63 patients who were given SC GM-CSF during cycle 4, CBRM1/5 activation was comparable with activation in cycle 1, when they were also given SC GM-CSF (P = .97, Fig 4B).
Fig 4.
Comparing frequency change of CBRM1/5-positive granulocytes from day 0 to day 4 peripheral blood when patients were treated with (A) subcutaneous (sc) granulocyte-macrophage colony-stimulating factor (GM-CSF; cycle 1) and intravenous (iv) GM-CSF (cycle 4; n = 35) or (B) SC GM-CSF (cycles 1 and 4; n = 63).
DISCUSSION
Early in vitro studies showed that granulocytes mediate efficient ADCC of NB. Cytotoxicity was dependent on FcγRIIA (CD32) and CD11b/CD18 integrins but was not dependent on oxidative intermediates.14–16 Subsequent studies using Lym-1 antibody in B-cell lymphoma showed similar findings.33 In the presence of GM-CSF, anti-GD2 MoAb was effective against NB.13,23 Combining GM-CSF with anti-CD20 antibody was also found to have benefit in patients with advanced non-Hodgkin's lymphoma and Hodgkin's disease.34 Despite compelling in vitro evidence, the in vivo role of neutrophils in these antitumor responses was uncertain. This report provides the first evidence, to our knowledge, that granulocyte activation by GM-CSF in patients, as measured by the CD11b activation epitope CBRM1/5, may be an independent prognostic factor on outcome. This phenomenon held true for patients who were in first and second CR/VGPR, as well as those who had primary refractory NB before immunotherapy. Moreover, PB from patients with GM-CSF administered by SC route elicited greater granulocyte activation when compared with the IV route. This finding may account, in part, for the superior survival outcome of patients receiving SC GM-CSF (NCT00072358) when compared with patients receiving IV GM-CSF (NCT00002560).35 These data, together with the prognostic importance of FCGR2A (CD32) polymorphism among patients with primary refractory NB,26 gave credence that myeloid cells, specifically granulocytes, contributed to the antitumor response after combination therapy using anti-GD2 antibody and GM-CSF.
This study demonstrated that an increase in frequency or MFI of CBRM1/5-positive granulocytes during the first treatment cycle was a key favorable determinant of patient outcome. This correlation was somewhat unexpected, because multiple cycles were given to patients for up to 2 years on this protocol. These data suggest that the level of granulocyte excitability in patients receiving SC GM-CSF was similar throughout the repeated cycles and that the excitability determined as early as the first cycle was prognostic of treatment outcome. In addition, because the size of the circulating granulocyte population, relative to the marginating pool or to the marrow reserve, was variable between patients, the absolute granulocyte count or CBRM1/5-positive granulocyte count per microliter of blood might not be representative of the patient's total-body granulocyte pool.
The biologic effects of GM-CSF can be multifold.36 It stimulates production of myeloid cells, including granulocytes and monocytes/macrophages, accelerating hematopoietic recovery after dose-intensive chemotherapy.36 It also stimulates the maturation of monocytes into antigen-presenting cells. When locally combined with cancer vaccines,37 GM-CSF has shown clinical promise.36 However, GM-CSF is also a potent stimulator of myeloid-derived suppressor cells (MDSCs), which can interfere with both the innate and adaptive immune response.38,39 In mouse models, MDSCs are Gr-1+ and CD11b+ myeloid precursor cells that can differentiate into dendritic cells, macrophages, and neutrophils. MDSCs accumulate in the blood, lymph nodes, and bone marrow and at tumor sites to inhibit both adaptive and innate immunity.40–42 Tumor-infiltrating MDSCs have also been associated with adverse patient outcome.43 Furthermore, the stimulation of MDSCs by high GM-CSF levels could reduce vaccine response and possibly decrease antitumor efficacy in patients.38,39 It was reassuring that CD11b activation in our patients had positive instead of negative impact on patient outcome.
In addition to inducing MDSCs,44 high doses of GM-CSF (> 250 μg/m2/d) have also been implicated to have a negative effect on monocyte and lymphocyte ADCC.45 Hence, optimal doses of GM-CSF of 40 to 80 μg/d were suggested for vaccine development,46 and doses of 80 to 150 μg/d (< 150 μg/m2/d) were suggested for antibody therapy.45 However, in the randomized studies of ch14.18, GM-CSF at 250 μg/m2/d seemed beneficial for patient outcome.13 In our study, at an even higher dose (500 μg/m2/d for 3 days; Table 1), there was further granulocyte activation, which correlated with superior PFS.
Previous studies have also shown that after repeated daily injections of GM-CSF 250 μg/m2/d, peak serum concentration of GM-CSF gradually decreased on days 5 and 10 when compared with day 1. Such a decline in serum level was less pronounced in cycle 4 compared with cycle 1.45 Thus, serum GM-CSF levels could not have accounted for the smaller increase in CBRM1/5-positive granulocytes in PB in cycle 4 when GM-CSF was administered IV (Fig 4A). These results suggest that the SC route of GM-CSF administration was more effective than the IV route in granulocyte activation.
In addition to CBRM1/5 activation, two other independent prognostic factors were identified in the multivariable analysis, namely the favorable impact of missing KIR ligand and the negative impact when additional cyclophosphamide-based therapy was required to consolidate remission (Table 2). Inadequate response to induction therapy is well known to be an adverse prognostic factor in high-risk NB. In the randomized study carried out by the Children's Oncology Group, achieving CR/VGPR was a highly favorable predictor of outcome.47 In our study, patients with refractory disease or relapsed disease were treated with high-dose cyclophosphamide-based regimens containing vincristine with either topotecan or irinotecan.31 Once they achieved CR/VGPR or stable disease, they were enrolled onto the 3F8/GM-CSF protocol. Thus, it is not surprising that in the multivariable model, the necessity of a cyclophosphamide-based second-line regimen was a poor prognostic marker associated with refractory or high-risk disease.
Both in the context of autologous stem-cell transplantation27 and high-dose cyclophosphamide,48 missing KIR ligand seemed to be highly prognostic for patient outcome after treatment with antibody 3F8 plus GM-CSF. Although NK cells alone were suboptimal in killing NB,49 killing by both licensed and unlicensed NK cells was much more efficient in the presence of antibody 3F8.50 Previous studies in patients undergoing autotransplantation51 and more recently patients receiving hu14.18–interleukin-2 fusion protein52 have also implicated missing KIR ligand as a prognostic factor. In our analysis, lymphocytes had low CBRM1/5 expression, and as expected, it did not increase with GM-CSF. CBRM1/5 among the NK subset was not tested, but this may be an important marker to monitor in future studies. Although supporting the role played by NK cells in mediating antitumor response,27 this study strongly suggests that granulocyte and myeloid cell activation may be a crucial component in the overall strategy of antibody therapy in NB.53
Acknowledgment
We thank Brian Kushner, MD, Kim Kramer, MD, and Shakeel Modak, MD, and the entire neuroblastoma nursing staff for their dedicated care of our patients; our data management team; and in particular, Xiaodong Huang, BS, for her technical assistance.
Appendix
Table A1.
Relationship Between PFS and Frequency Change in CBRM1/5-Positive Granulocytes Stratified According to Patient and Tumor Characteristics
| Patient/Tumor Characteristic | No. of Patients | Frequency Change in CBRM1/5-Positive Granulocytes |
||||||
|---|---|---|---|---|---|---|---|---|
| > Median |
< Median |
P | ||||||
| No. of Patients | PFS (%) |
No. of Patients | PFS (%) |
|||||
| Mean | SD | Mean | SD | |||||
| Disease status at enrollment | ||||||||
| First CR/VGPR | 74 | 37 | 55 | 10 | 37 | 45 | 9 | .34 |
| ≥ Second CR/VGPR | 25 | 12 | 50 | 14 | 13 | 10 | 9 | .14 |
| Primary refractory | 48 | 24 | 52 | 11 | 24 | 25 | 10 | .16 |
| MYCN status | ||||||||
| Amplified | 58 | 30 | 66 | 9 | 28 | 31 | 10 | .051 |
| Nonamplified | 82 | 39 | 44 | 9 | 43 | 34 | 8 | .23 |
| Prior BM/SC transplantation | ||||||||
| Yes | 74 | 40 | 56 | 9 | 34 | 44 | 9 | .33 |
| No | 73 | 33 | 53 | 9 | 40 | 24 | 8 | .035 |
| Second-line cyclophosphamide-based regimen | ||||||||
| Yes | 66 | 35 | 46 | 9 | 31 | 14 | 8 | .008 |
| No | 81 | 38 | 62 | 9 | 42 | 46 | 8 | .26 |
| Missing KIR ligand | ||||||||
| Yes | 98 | 50 | 56 | 8 | 48 | 39 | 8 | .15 |
| No | 49 | 23 | 50 | 11 | 26 | 25 | 9 | .08 |
| FcGR2A polymorphism | ||||||||
| R/R or R/H | 110 | 57 | 54 | 7 | 53 | 36 | 7 | .13 |
| H/H | 37 | 16 | 56 | 12 | 21 | 27 | 10 | .07 |
| History of prior relapse | ||||||||
| Yes | 26 | 12 | 50 | 14 | 14 | 18 | 11 | .23 |
| No | 121 | 61 | 54 | 7 | 60 | 37 | 7 | .07 |
| Frequency change in CBRM1/5-positive granulocytes | 147 | 73 | 54 | 6 | 74 | 34 | 6 | .024 |
Abbreviations: BM, bone marrow; CR/VGPR, complete remission/very good partial remission; GM-CSF, granulocyte-macrophage colony-stimulating factor; KIR, killer immunoglobulin-like receptor; PFS, progression-free survival; SC, stem cell; SD, standard deviation.
Table A2.
Distribution of Patients According to Disease Status at Enrollment for Three Independent Risk Factors
| Disease Status | Total No. of Patients |
No. of Patients With Second-Line Cyclophosphamide-Based Therapy |
No. of Patients With KIR Ligand Missing |
|||
|---|---|---|---|---|---|---|
| Frequency Change > Median* | Frequency Change < Median* | Frequency Change > Median* | Frequency Change < Median* | Frequency Change > Median* | Frequency Change < Median* | |
| First CR/VGPR | 37 | 37 | 12 | 9 | 26 | 25 |
| ≥ Second CR/VGPR | 12 | 13 | 9 | 11 | 10 | 8 |
| Primary refractory | 24 | 24 | 14 | 11 | 14 | 15 |
Abbreviations: CR/VGPR, complete remission/very good partial remission; KIR, killer immunoglobulin-like receptor.
Frequency change in CBRM1/5-positive granulocytes.
Footnotes
Supported in part by Grant No. CA106450 from the National Institutes of Health, the Robert Steel Foundation, and Hope Street Kids.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Nai-Kong V. Cheung
Collection and assembly of data: Irene Y. Cheung, Katharine Hsu
Data analysis and interpretation: Irene Y. Cheung, Nai-Kong V. Cheung
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Boyiadzis M, Foon KA. Approved monoclonal antibodies for cancer therapy. Expert Opin Biol Ther. 2008;8:1151–1158. doi: 10.1517/14712598.8.8.1151. [DOI] [PubMed] [Google Scholar]
- 2.Yan L, Hsu K, Beckman RA. Antibody-based therapy for solid tumors. Cancer J. 2008;14:178–183. doi: 10.1097/PPO.0b013e318172d71a. [DOI] [PubMed] [Google Scholar]
- 3.Modak S, Cheung NK. Disialoganglioside directed immunotherapy of neuroblastoma. Cancer Invest. 2007;25:67–77. doi: 10.1080/07357900601130763. [DOI] [PubMed] [Google Scholar]
- 4.Cheung NK, Saarinen UM, Neely JE, et al. Monoclonal antibodies to a glycolipid antigen on human neuroblastoma cells. Cancer Res. 1985;45:2642–2649. [PubMed] [Google Scholar]
- 5.Mujoo K, Kipps TJ, Yang HM, et al. Functional properties and effect on growth suppression of human neuroblastoma tumors by isotype switch variants of monoclonal antiganglioside GD2 antibody 14.18. Cancer Res. 1989;49:2857–2861. [PubMed] [Google Scholar]
- 6.Gillies SD, Lo KM, Wesolowski J. High-level expression of chimeric antibodies using adapted cDNA variable region cassettes. J Immunol Methods. 1989;125:191–202. doi: 10.1016/0022-1759(89)90093-8. [DOI] [PubMed] [Google Scholar]
- 7.Barker E, Mueller BM, Handgretinger R, et al. Effect of a chimeric anti-ganglioside GD2 antibody on cell-mediated lysis of human neuroblastoma cells. Cancer Res. 1991;51:144–149. [PubMed] [Google Scholar]
- 8.Barker E, Reisfeld RA. A mechanism for neutrophil-mediated lysis of human neuroblastoma cells. Cancer Res. 1993;53:362–367. [PubMed] [Google Scholar]
- 9.Mueller BM, Reisfeld RA, Gillies SD. Serum half-life and tumor localization of a chimeric antibody deleted of the CH2 domain and directed against the disialoganglioside GD2. Proc Natl Acad Sci U S A. 1990;87:5702–5705. doi: 10.1073/pnas.87.15.5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mueller BM, Romerdahl CA, Gillies SD, et al. Enhancement of antibody-dependent cytotoxicity with a chimeric anti-GD2 antibody. J Immunol. 1990;144:1382–1386. [PubMed] [Google Scholar]
- 11.Simon T, Hero B, Faldum A, et al. Consolidation treatment with chimeric anti-GD2-antibody ch14.18 in children older than 1 year with metastatic neuroblastoma. J Clin Oncol. 2004;22:3549–3557. doi: 10.1200/JCO.2004.08.143. [DOI] [PubMed] [Google Scholar]
- 12.Gilman AL, Ozkaynak MF, Matthay KK, et al. Phase I study of ch14.18 with granulocyte-macrophage colony-stimulating factor and interleukin-2 in children with neuroblastoma after autologous bone marrow transplantation or stem-cell rescue: A report from the Children's Oncology Group. J Clin Oncol. 2009;27:85–91. doi: 10.1200/JCO.2006.10.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu AL, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kushner BH, Cheung NK. GM-CSF enhances 3F8 monoclonal antibody-dependent cellular cytotoxicity against human melanoma and neuroblastoma. Blood. 1989;73:1936–1941. [PubMed] [Google Scholar]
- 15.Kushner BH, Cheung NK. Clinically effective monoclonal antibody 3F8 mediates nonoxidative lysis of human neuroectodermal tumor cells by polymorphonuclear leukocytes. Cancer Res. 1991;51:4865–4870. [PubMed] [Google Scholar]
- 16.Kushner BH, Cheung NK. Absolute requirement of CD11/CD18 adhesion molecules, FcRII and the phosphatidylinositol-linked FcRIII for monoclonal antibody-mediated neutrophil antihuman tumor cytotoxicity. Blood. 1992;79:1484–1490. [PubMed] [Google Scholar]
- 17.Cheung NK, Walter EI, Smith-Mensah WH, et al. Decay-accelerating factor protects human tumor cells from complement-mediated cytotoxicity in vitro. J Clin Invest. 1988;81:1122–1128. doi: 10.1172/JCI113426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen S, Caragine T, Cheung NK, et al. CD59 expressed on a tumor cell surface modulates decay-accelerating factor expression and enhances tumor growth in a rat model of human neuroblastoma. Cancer Res. 2000;60:3013–3018. [PubMed] [Google Scholar]
- 19.Metelitsa LS, Gillies SD, Super M, et al. Antidisialoganglioside/granulocyte macrophage-colony-stimulating factor fusion protein facilitates neutrophil antibody-dependent cellular cytotoxicity and depends on FcgammaRII (CD32) and Mac-1 (CD11b/CD18) for enhanced effector cell adhesion and azurophil granule exocytosis. Blood. 2002;99:4166–4173. doi: 10.1182/blood.v99.11.4166. [DOI] [PubMed] [Google Scholar]
- 20.Diamond MS, Springer TA. A subpopulation of Mac-1 (CD11b/CD18) molecules mediates neutrophil adhesion to ICAM-1 and fibrinogen. J Cell Biol. 1993;120:545–556. doi: 10.1083/jcb.120.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vetvicka V, Thornton BP, Ross GD. Soluble beta-glucan polysaccharide binding to the lectin site of neutrophil or natural killer cell complement receptor type 3 (CD11b/CD18) generates a primed state of the receptor capable of mediating cytotoxicity of iC3b-opsonized target cells. J Clin Invest. 1996;98:50–61. doi: 10.1172/JCI118777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mackall CL. T-cell immunodeficiency following cytotoxic antineoplastic therapy: A review. Stem Cells. 2000;18:10–18. doi: 10.1634/stemcells.18-1-10. [DOI] [PubMed] [Google Scholar]
- 23.Kushner BH, Kramer K, Cheung NK. Phase II trial of the anti-G(D2) monoclonal antibody 3F8 and granulocyte-macrophage colony-stimulating factor for neuroblastoma. J Clin Oncol. 2001;19:4189–4194. doi: 10.1200/JCO.2001.19.22.4189. [DOI] [PubMed] [Google Scholar]
- 24.Levy S, Shoham T. The tetraspanin web modulates immune-signalling complexes. Nat Rev Immunol. 2005;5:136–148. doi: 10.1038/nri1548. [DOI] [PubMed] [Google Scholar]
- 25.Källquist L, Hansson M, Persson AM, et al. The tetraspanin CD63 is involved in granule targeting of neutrophil elastase. Blood. 2008;112:3444–3454. doi: 10.1182/blood-2007-10-116285. [DOI] [PubMed] [Google Scholar]
- 26.Cheung NK, Sowers R, Vickers AJ, et al. FCGR2A polymorphism is correlated with clinical outcome after immunotherapy of neuroblastoma with anti-GD2 antibody and granulocyte macrophage colony-stimulating factor. J Clin Oncol. 2006;24:2885–2890. doi: 10.1200/JCO.2005.04.6011. [DOI] [PubMed] [Google Scholar]
- 27.Venstrom JM, Zheng J, Noor N, et al. KIR and HLA genotypes are associated with disease progression and survival following autologous hematopoietic stem cell transplantation for high-risk neuroblastoma. Clin Cancer Res. 2009;15:7330–7334. doi: 10.1158/1078-0432.CCR-09-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid: Children's Cancer Group. N Engl J Med. 1999;341:1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 29.Cheung NK, Cheung IY, Canete A, et al. Antibody response to murine anti-GD2 monoclonal antibodies: Correlation with patient survival. Cancer Res. 1994;54:2228–2233. [PubMed] [Google Scholar]
- 30.Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11:1466–1477. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 31.Kushner BH, Kramer K, Modak S, et al. Camptothecin analogs (irinotecan or topotecan) plus high-dose cyclophosphamide as preparative regimens for antibody-based immunotherapy in resistant neuroblastoma. Clin Cancer Res. 2004;10:84–87. doi: 10.1158/1078-0432.ccr-1147-3. [DOI] [PubMed] [Google Scholar]
- 32.Kushner BH, Cheung IY, Kramer K, et al. High-dose cyclophosphamide inhibition of humoral immune response to murine monoclonal antibody 3F8 in neuroblastoma patients: Broad implications for immunotherapy. Pediatr Blood Cancer. 2007;48:430–434. doi: 10.1002/pbc.20765. [DOI] [PubMed] [Google Scholar]
- 33.Ottonello L, Epstein AL, Mancini M, et al. Monoclonal Lym-1 antibody-targeted lysis of B lymphoma cells by neutrophils: Evidence for two mechanisms of FcgammaRII-dependent cytolysis. J Leukoc Biol. 2000;68:662–668. [PubMed] [Google Scholar]
- 34.Rapoport AP, Meisenberg B, Sarkodee-Adoo C, et al. Autotransplantation for advanced lymphoma and Hodgkin's disease followed by post-transplant rituxan/GM-CSF or radiotherapy and consolidation chemotherapy. Bone Marrow Transplant. 2002;29:303–312. doi: 10.1038/sj.bmt.1703363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheung NK, Kushner BH, Kramer K, et al. Anti-GD2 murine monoclonal antibody (MoAb) 3F8 for consolidation of first complete/very good partial remission of high risk stage 4 neuroblastoma (NB). Presented at the Advances in Neuroblastoma Research 2010 Meeting; June 20-23, 2010; Stockholm, Sweden. [Google Scholar]
- 36.Arellano M, Lonial S. Clinical uses of GM-CSF, a critical appraisal and update. Biologics. 2008;2:13–27. doi: 10.2147/btt.s1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dranoff G, Jaffee E, Lazenby A, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young MR, Lathers DM. Myeloid progenitor cells mediate immune suppression in patients with head and neck cancers. Int J Immunopharmacol. 1999;21:241–252. doi: 10.1016/s0192-0561(99)00008-9. [DOI] [PubMed] [Google Scholar]
- 39.Filipazzi P, Valenti R, Huber V, et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25:2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 40.Rodríguez PC, Ochoa AC. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: Mechanisms and therapeutic perspectives. Immunol Rev. 2008;222:180–191. doi: 10.1111/j.1600-065X.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: Linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peranzoni E, Zilio S, Marigo I, et al. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol. 2010;22:238–244. doi: 10.1016/j.coi.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 43.Talmadge JE. Immune cell infiltration of primary and metastatic lesions: Mechanisms and clinical impact. Semin Cancer Biol. 2011;21:131–138. doi: 10.1016/j.semcancer.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 44.Serafini P, Carbley R, Noonan KA, et al. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. 2004;64:6337–6343. doi: 10.1158/0008-5472.CAN-04-0757. [DOI] [PubMed] [Google Scholar]
- 45.Liljefors M, Nilsson B, Mellstedt H, et al. Influence of varying doses of granulocyte-macrophage colony-stimulating factor on pharmacokinetics and antibody-dependent cellular cytotoxicity. Cancer Immunol Immunother. 2008;57:379–388. doi: 10.1007/s00262-007-0377-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parmiani G, Castelli C, Pilla L, et al. Opposite immune functions of GM-CSF administered as vaccine adjuvant in cancer patients. Ann Oncol. 2007;18:226–232. doi: 10.1093/annonc/mdl158. [DOI] [PubMed] [Google Scholar]
- 47.Matthay KK, Reynolds CP, Seeger RC, et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: A Children's Oncology Group study. J Clin Oncol. 2009;27:1007–1013. doi: 10.1200/JCO.2007.13.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Venstrom JM, Zheng J, Kushner B, et al. NK cell killer Ig-like receptor (KIR) genotype as a novel biomarker for neuroblastoma patients receiving anti-GD2 monoclonal antibody 3F8. Proceedings of the 101st Annual Meeting of the American Association for Cancer Research; April 17-21, 2010; Washington, DC. (abstr 5586) [Google Scholar]
- 49.Cho D, Shook DR, Shimasaki N, et al. Cytotoxicity of activated natural killer cells against pediatric solid tumors. Clin Cancer Res. 2010;16:3901–3909. doi: 10.1158/1078-0432.CCR-10-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nidale T, Galagher MM, Cheung NK, et al. Normally hyporesponsive unlicensed natural killer cells are strongly activated by neuroblastoma (NB) in the presence of anti-GD2 monoclonal antibody (MoAb). Presented at the 24th Annual Meeting of the American Society of Pediatric Hematology Oncology; April 13-16, 2011; Baltimore, MD. [Google Scholar]
- 51.Leung W, Handgretinger R, Iyengar R, et al. Inhibitory KIR-HLA receptor-ligand mismatch in autologous haematopoietic stem cell transplantation for solid tumour and lymphoma. Br J Cancer. 2007;97:539–542. doi: 10.1038/sj.bjc.6603913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Delgado DC, Hank JA, Kolesar J, et al. Genotypes of NK cell KIR receptors, their ligands, and Fcgamma receptors in the response of neuroblastoma patients to Hu14.18-IL2 immunotherapy. Cancer Res. 2010;70:9554–9561. doi: 10.1158/0008-5472.CAN-10-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schneider-Merck T, Lammerts van Bueren JJ, Berger S, et al. Human IgG2 antibodies against epidermal growth factor receptor effectively trigger antibody-dependent cellular cytotoxicity but, in contrast to IgG1, only by cells of myeloid lineage. J Immunol. 2010;184:512–520. doi: 10.4049/jimmunol.0900847. [DOI] [PubMed] [Google Scholar]