Abstract
Although intermediate filaments are one of three major cytoskeletal systems of vertebrate cells, they remain the least understood with respect to their structure and function. This is due in part to the fact that they are encoded by a large gene family which is developmentally regulated in a cell and tissue type specific fashion. This article is in honor of Ueli Aebi. It highlights the studies on IF that have been carried out by our laboratory for more than 40 years. Many of our advances in understanding IF are based on conversations with Ueli which have taken place during adventurous and sometimes dangerous hiking and biking trips throughout the world.
Keywords: intermediate filaments, vimentin, keratin, cell motility, phosphorylation
Introduction
This paper is written as a tribute to Ueli Aebi’s remarkable career in the fields of structural and cell biology. In honor of his formal retirement from the University of Basel we have chosen to relate our own history of discovery in the area of intermediate filament structure and function, a field of research in which Ueli has made major contributions.
Historical overview
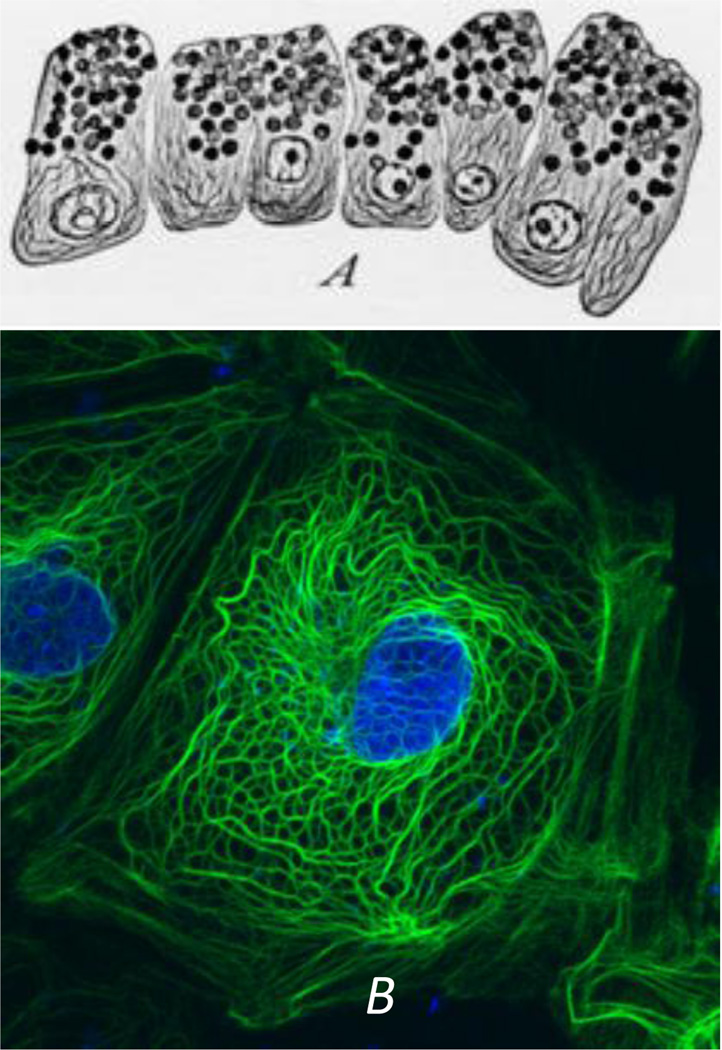
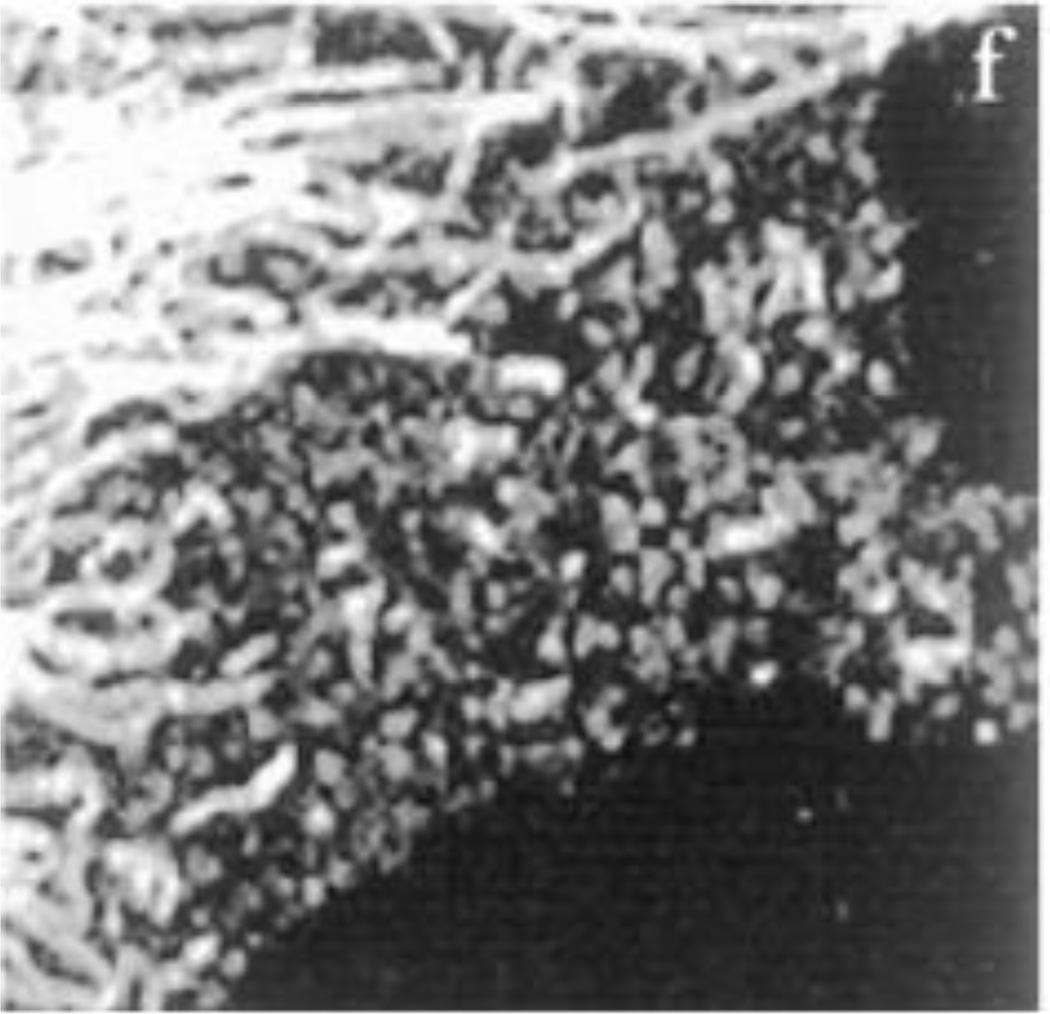
Intermediate filaments (IF) remain the least studied and understood of the three major cytoskeletal systems that are expressed in virtually all vertebrate and many invertebrate cell types. From an historical perspective, this is probably related to the fact that even though their constituent subunits typically form 10 nm filaments, the protein composition of these subunits is not conserved as they are encoded by ~75 different genes. Contributing to the complexity is the fact that the expression of these genes is developmentally regulated in a cell and tissue type specific fashion. As a result of the enormous diversity in their subunit composition, it took many years for cell biologists and biochemists to realize that all of the 10nm IF seen in different types of cells were derived from the same large protein family. It is likely that the first descriptions of IF were made over a hundred years ago by the pioneering studies of cytologists and histologists who developed various methods to generate contrast in the bright field microscope (for review see (Wilson, 1928)). These methods employed various fixatives and stains which revealed extensive arrays of filamentous structures in nerve cells and in epithelial cells (Wilson, 1928). In the former, the fibrillar patterns are highly reminiscent of the organization and distribution of neurofilaments composed of the type IV IF proteins (e.g., neurofilament triplet proteins) and in epithelial cells, the “fibrillae” or “tonofibrils”, are now known to be bundles of closely packed Types I and II keratin IF (Fig. 1A).
Fig. 1.
(A) Early observations of secretory epithelial cells in the mudpuppy pancreas. Note the “fibrillae” which most likely represent keratin IF bundles or tonofibrils. Work of Mathews in the laboratory of E.B. Wilson. From reference: (Wilson, 1928)). (B) A PtK2 epithelial cell fixed and processed for immunostaining with anti-keratin. Note the similarities in the tonofibrils seen in the preparations spanning 80 years of research.
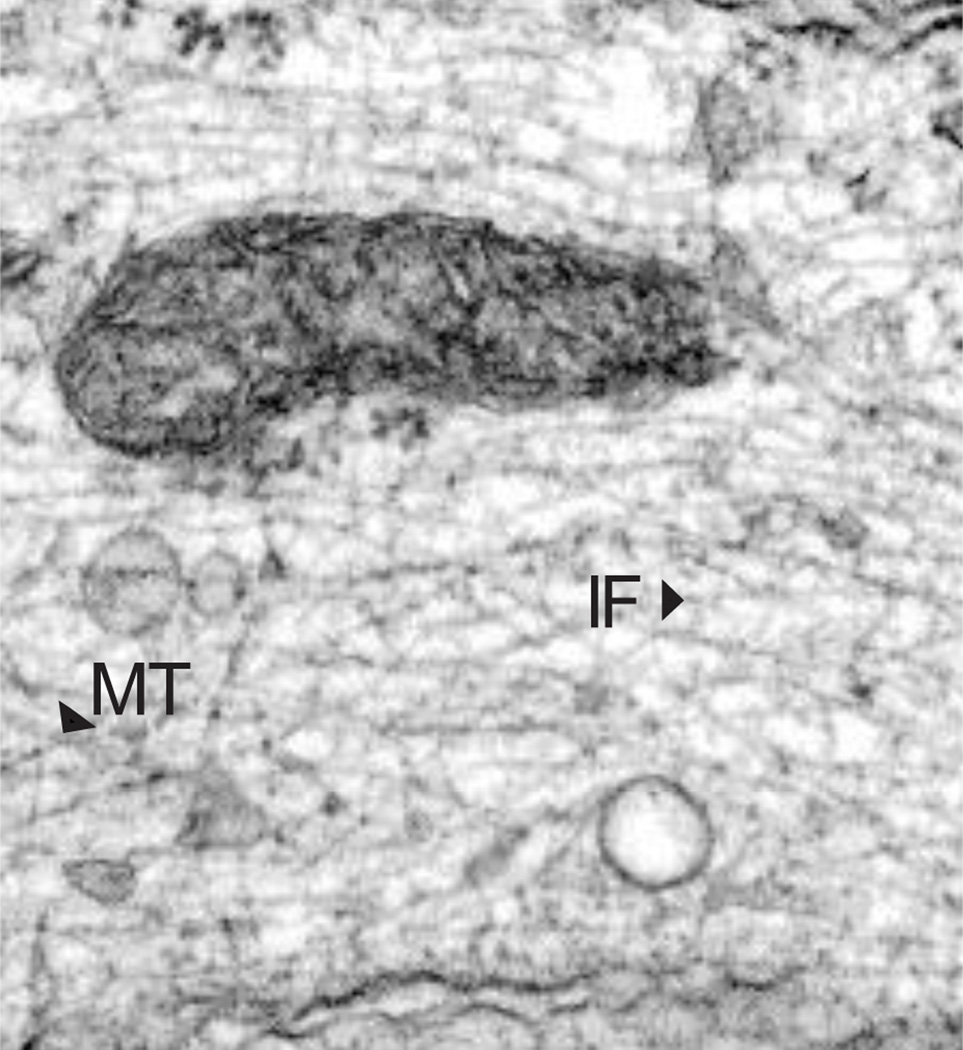
Following the introduction of techniques developed for studying cell fine structure using electron microscopy, 10 nm IF were described in numerous cell types (Goldman and Follett, 1969) (see Fig 2). However, the nomenclature used in the 1960s and 1970s to describe them caused significant confusion. During this period IF were called 10nm and 100 Angstrom filaments, decafilaments, 9 nm filaments, 110 nm filaments, 80–100 A filaments, ‘intermediate filaments’ in muscle cells (the latter name is based on the cross sectional diameter of IF which lies between actin and myosin filaments), neurofilaments in neurons, tonofilaments for the keratin IF in epithelial cells, glial fibrillary acidic filaments in glial cells and sarcoplasmic filaments for skeletal muscle cell IF. In addition, the names of their purported protein subunits varied widely and included muscle skeletin or desmin; glial fibrillary acidic protein (GFAP); fibroblast 10nm filament protein, fibroblast intermediate filament protein, vimentin or decamin; peripheral and brain neurofilament protein; keratin and tonofilament protein, etc. (for review see (Eriksson and Thornell, 1979)). Eventually, the name of intermediate filaments became widely accepted, based mainly on the fact that the diameter of IF was between that of actin/microfilaments and microtubules.
Fig. 2.
Electron micrograph of a human fibroblast showing the cytoplasm containing numerous 10 nm vimentin IF (IF) and a few microtubules (MT).
It was not until the late 1970s and early to mid-1980s that cell biologists began to realize that, although there were differences, there were also very significant similarities in the proteins comprising the different IF systems. These similarities began emerging when comparative structural and biochemical methodologies, along with the introduction of cloning and sequencing of cDNAs, made it possible to determine the specific properties of IF derived from a wide range of cell types. Now we know that IF assemble from a coiled-coiled dimer. The region responsible for dimer formation is the highly conserved, α-helix rich, central rod domain which is a common feature of all cytoskeletal IF proteins. These dimers associate in a hierarchical fashion to form IF which differ from each other primarily in the size and the amino acid composition of their N- and C- terminal non-ααhelical domains. These discoveries are based primarily on work from the laboratories of Peter Steinert, David Parry, Ueli Aebi and Harald Herrmann (Dowling et al., 1983; Herrmann et al., 1999; Herrmann et al., 1996; Parry et al., 1985; Parry et al., 1986; Parry et al., 2007; Steinert et al., 1976). During this same period, the development of antibodies directed against IF proteins and advancements in fluorescence microscopy revealed the presence of extensive arrays of complex cytoskeletal networks of IF, typically extending from the perinuclear region to the cell surface (Starger et al., 1978; Sun and Green, 1978) (Fig.1B). We now know that these complex networks engage in many functions ranging from cell shape determination and mechanics to signal transduction and cell motility.
Early observations leading to the isolation and initial biochemical characterizations of IF
Our first IF isolation procedures were based upon observations of live cells using polarized light microscopy. We discovered that during the early stages of the spreading of fibroblasts such as BHK-21 cells, there was a prominent juxtanuclear “birefringent sphere” (or juxtanuclear cap) which became elongated into birefringent fibers as cells flattened and took on the shape of fibroblasts. The middle of this spherical region was isotropic while the surrounding anisoptropic region exhibited positive birefringence with respect to its circumferential axis (Fig. 3). This latter finding indicated that filamentous components were oriented with their long axes wound around the center of the isotropic core. Electron microscopy revealed that the birefringent sphere consisted exclusively of closely associated masses of IF organized in the fashion predicted by the polarized light findings. No microtubules or microfilaments were detected within these juxtanuclear arrays of IF in the early stages of cell spreading. An association with microtubules only became apparent as the cells flattened into the typical shapes of fibroblasts. We also speculated that the organizational changes in IF were in some fashion responsible for aspects of the motile properties of cells (Goldman and Follett, 1970). Other factors involved in the formation of the juxtanuclear caps of IF were revealed in experiments in which we used colchicine to disassemble microtubules in BHK-21 cells. The results demonstrated that IF retracted in a retrograde fashion and became concentrated next to the nucleus to form a juxtanuclear cap, as the microtubules disassembled. Support for interactions between IFs and microtubules also came from our earlier electron microscope studies showing a close relationship between these two cytoskeletal elements (Goldman, 1971; Goldman and Follett, 1970) (see Fig 2). These results showed that the normal organization of IF in fibroblasts depends on microtubules (Goldman, 1971).
Fig. 3.
A living BHK-21 cell observed during the early stages of cell spreading by polarized light microscopy. The birefringent sphere comprised of dark and bright sectors surrounds an isotropic region (I). This structure is closely associated with the nucleus (N). From reference: (Goldman and Follett, 1970)
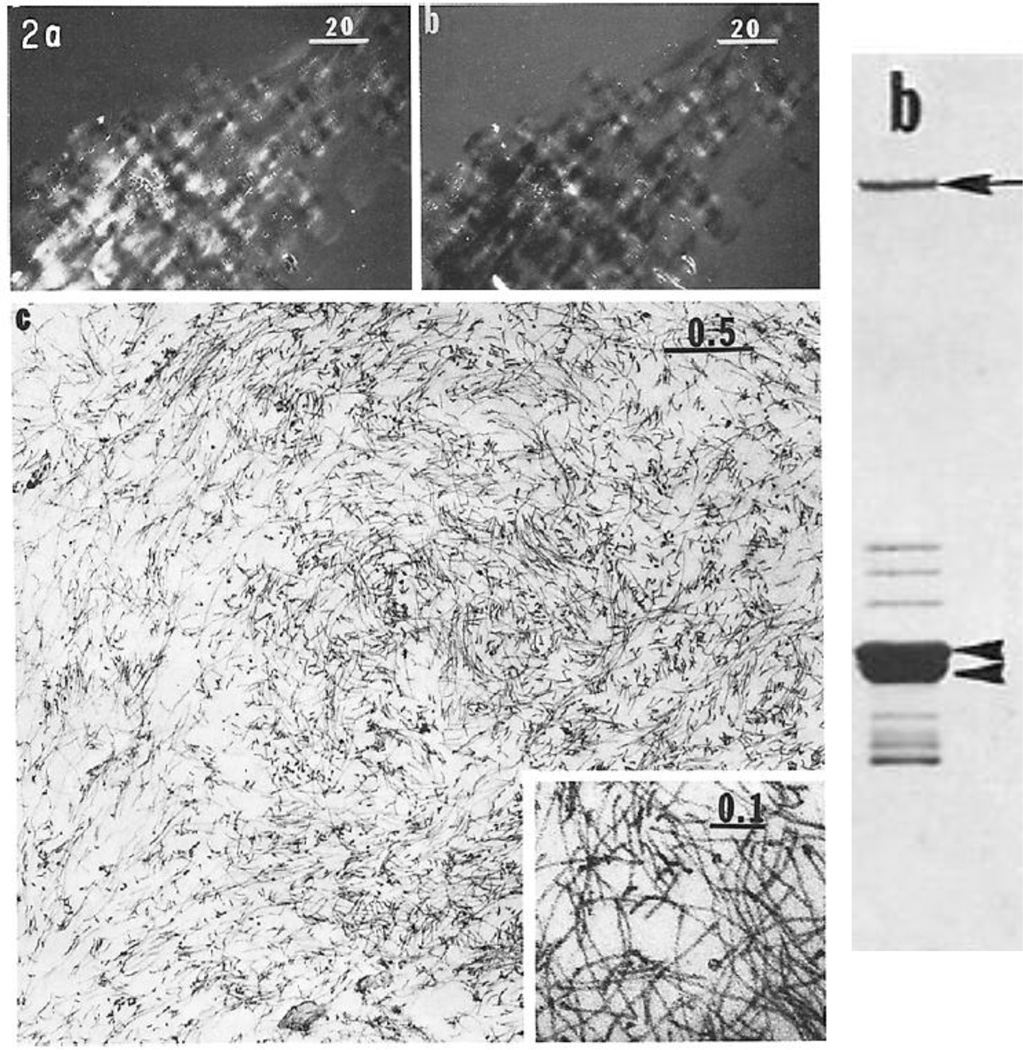
Based upon the observations of the formation of juxtanuclear IF caps, we developed a procedure for their preparation exploiting their close association with nuclei. Briefly, we lysed BHK-21cells either during the early stages of cell spreading or following colchicine treatment and subsequently fractionated the nuclei in these lysates with their IF caps attached. The nuclei were then lysed in high salt and subsequently digested with DNAase I to remove DNA/chromatin. This latter step also removed actin from these preparations (Hitchcock et al., 1976). This method rapidly produced relatively pure fractions of intact IF caps which retained their birefringent properties. Electron microscopy revealed that pellets of these IF caps contained large numbers of intact IF (Fig.4). When intact IF obtained from these preparations were analyzed by SDS-PAGE, the major proteins migrated as bands of ~54 and 55kD (arrowheads Fig.4) which later we realized consisted of two Type III IF protein subunits, vimentin and desmin (Starger and Goldman, 1977; Starger et al., 1978). We also observed a high molecular weight protein (arrow in figure) which was subsequently identified as the IF associated protein, plectin (Clubb et al., 2000). It is also interesting to note that the ~60–70 kD bands migrating above the cytoskeletal IF proteins were later identified as the Type V nuclear lamins (Aebi et al., 1986; Goldman et al., 1986; Zackroff et al., 1984) (Fig. 4).
Fig. 4.
Left panel; Panels a and b show pellets of isolated birefringent juxtanuclear caps at opposite compensator settings; panel c is an electron micrograph of a thin section of a similar pellet showing large numbers of IF (from reference: (Starger and Goldman, 1977)). The right panel shows a Coomassie stained SDS-gel of intact IF obtained from the caps (from reference: (Starger et al., 1978)).
Early insights into the common structural features of IF
We first began to realize that there may be similarities in IF from different cell types when we met Peter Steinert at the 1976 Cell Motility Meeting held at the Cold Spring Harbor laboratory (1976). Peter was one of the world’s leading authorities on the keratins. This stemmed from his Australian wool chemistry roots and his training under a distinguished hair and wool keratin protein chemist, George Rogers in the Department of Biochemistry at the University of Adelaide. It was at this meeting that Peter and I realized that the 10nm filaments my lab was studying and isolating from mesenchymal cells (e.g., vimentin IF) might be similar to the keratins that he was isolating from epithelial cells. Following this meeting Peter and I immediately initiated a collaboration which resulted in numerous publications until his untimely and tragic death in 2003. Our first paper demonstrated that the 10 nm filaments isolated from BHK-21 cells and keratin IF had similar structures as determined using optical rotary dispersion (ORD) and wide angle X-ray diffraction analyses. Specifically the results showed that both IF types contained ~42 % α-helix arranged in a coiled-coil configuration, features now known to typify all cytoskeletal IF (Steinert et al., 1978).
In vitro assembly of IF
The isolation of the juxtanuclear IF caps to obtain enriched preparations of their constituent proteins was soon replaced with a method for preparing “IF-enriched cytoskeletons”. This method was rapid and simple. It involved extracting BHK-21 cells in high salt/detergent containing solutions, followed by digestion with DNAase I to remove chromatin and also to depolymerize filamentous actin (see above). Under these conditions greatly enriched preparations of polymerized IF were obtained in milligram quantities in the absence of tubulin and actin. Further purification was achieved by the disassembly of these IF-enriched cytoskeletal preparations in low ionic strength buffers followed by reassembly under physiological conditions. After several cycles of disassembly and reassembly in vitro, minor “contaminating” proteins disappeared from the IF preparations. We developed a combined turbidimetric/negative staining assay by which we determined that the critical concentration required for the in vitro polymerization of the IF proteins (i.e., vimentin and desmin) was in the range of 0.05–0.15 mg/ml. The overall results of these studies showed that IF assembly in vitro involved a two-step nucleation-condensation reaction in which short IF form first, followed by elongation (Zackroff and Goldman, 1979).
Based upon our results with isolating and reassembling BHK-21 IF, we went on to develop a common method for preparing IF-enriched cytoskeletons from a wide variety of cell types (e.g., HeLa and PC12 cells). Incorporating the use of 8 molar urea as a universal solvent, a technique developed by Peter Steinert for epidermal keratins (Steinert et al., 1976), we were able to disassemble and reassemble different members of the IF family (e.g. see (Aynardi et al., 1984; Parysek and Goldman, 1987)). We also developed methods for the isolation and in vitro reconstitution of neuronal IF obtained from squid giant axons and also bovine spinal cords. In all cases the overall assembly properties were similar to those found for BHK-21 IF, except that neurofilaments typically contained 3–4 different IF proteins which formed complex heteropolymers. In addition we found that the exact conditions for solubilization/disassembly and reassembly of neurofilaments differed for each system (Zackroff and Goldman, 1980; Zackroff et al., 1982). These early studies on the identification of IF protein subunits and their in vitro assembly properties were followed by much more sophisticated structural analyses carried out in the laboratories of Ueli Aebi and Harald Herrmann. In a series of elegant studies, Ueli and Harald defined the steps involved in the polymerization of vimentin and desmin. The long term collaboration between these two groups led to the discovery of the unit length filament (ULF) which is an essential building block of cytoskeletal IF. Their work represents the cornerstone of our knowledge of the roles of the different subdomains of the protein chains in IF assembly mechanisms (Herrmann and Aebi, 1998; Herrmann and Aebi, 2004; Herrmann et al., 2007; Herrmann et al., 1996; Meier et al., 2009; Nicolet et al., 2010; Plodinec et al., 2011; Portet et al., 2009; Strelkov et al., 2003; Strelkov et al., 2002; Strelkov et al., 2001).
Early Insights into the Dynamic Properties of Intermediate Filaments
For many years cytoskeletal IF were considered to be static, “space filling” elements of the cytoplasm of non-muscle vertebrate cells. Historically this was primarily based upon the biochemical findings that they could be isolated intact as 10 nm filaments from cells (Starger et al., 1978) and there was little evidence for soluble pools of IF subunits (Soellner et al., 1985). These biochemical properties suggested that once IF proteins were synthesized and polymerized in cells, there was little subunit exchange. Indeed, this view is consistent with in vitro studies which demonstrate that there is hardly any exchange of subunits among filaments, even after two days of incubation (Winheim et al., 2011). These early studies suggested that the steady state for IF was regulated mainly by protein synthesis and degradation or post-translational modifications. These properties contrasted with those known for the other cytoskeletal systems, microtubules and actin/microfilaments, which depended on large pools of soluble subunits for regulating subunit exchange and their disassembly/reassembly. For these reasons the roles of IF in various cellular functions, such as motility, were largely ignored.
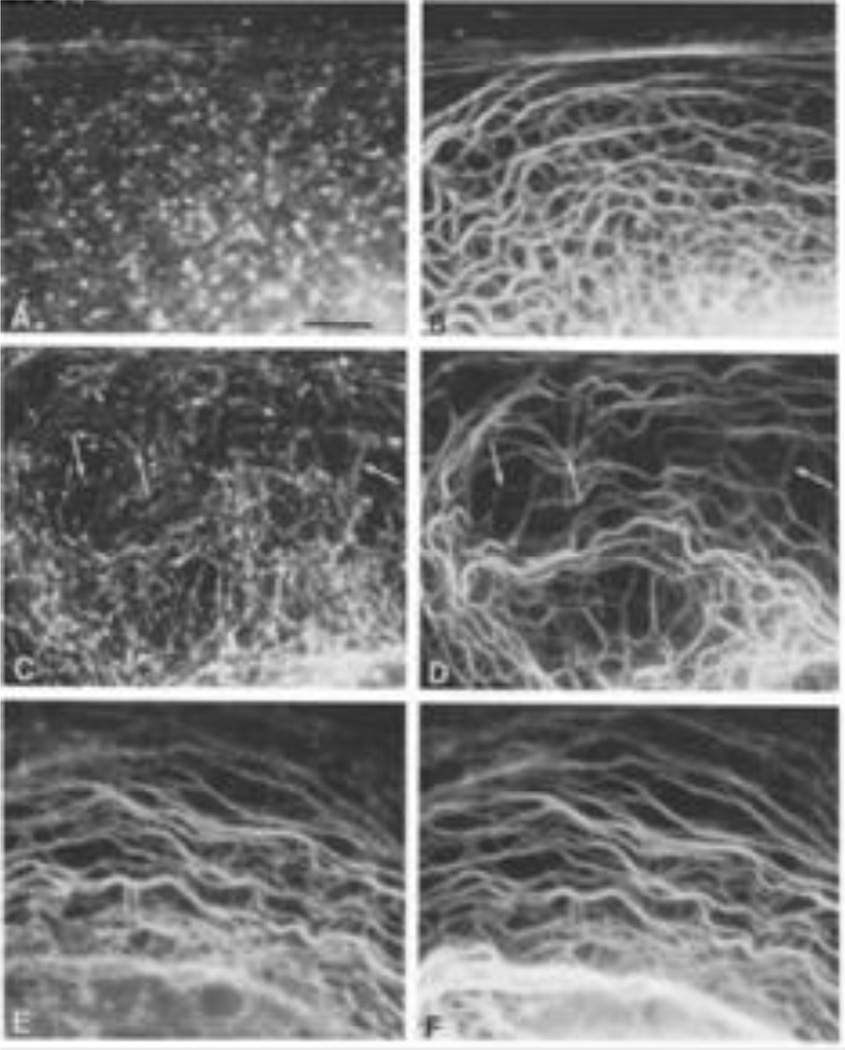
However, our early studies showing that IF were reorganized during spreading and shape formation in cultured cells led us to believe that they were, indeed, dynamic in living cells. Therefore we developed methods to determine the subunit exchange properties of IF in live cells. Initially this involved the use of soluble, biotinylated vimentin which was microinjected into BHK-21 cells. In order to obtain sufficient quantities of purified vimentin for tagging with biotin (this was the period prior to the introduction of bacterial expression systems), we used fresh cow lenses (Lieska et al., 1980). Importantly, the in vitro assembly properties of vimentin were preserved if the biotin was added during the polymerization reaction. The biotinylated vimentin was then solubilzed and microinjected into live BHK-21 cells which were fixed at different time intervals following injection. The results showed, by double label immunofluorescence, that the exogenous cow lens protein was incorporated into endogenous IF within 30 minutes. This demonstrated that IF were not static structures and provided early clues that they had dynamic properties in living cells (Vikstrom et al., 1989). Similar experiments were carried out on the keratin IF networks in epithelial cells. These studies involved a different part of the bovine anatomy, the cow’s tongue (also obtained at the slaughter house), which provided a source of keratin types I and II. The major keratins were separated by ion-exchange chromatography and then biotinylated as they formed the obligate hetero-polymers required for 10nm filaments to assemble in vitro. Following assembly, the two types of keratin were separated and only the biotinylated type I (which was soluble) was injected into live epithelial cells. Following fixation and processing for double label immunofluorescence at time intervals post injection, we found that within minutes small aggregates or particles of the injected keratin formed in close association with the endogenous bundles of keratin IF (tonofibrils). After 4hr, the exogenous keratin was fully integrated into the endogenous network (Miller et al., 1991) (Fig. 5). In a subsequent study we showed that the non-filamentous aggregates of biotinylated Type I keratin, which formed immediately following microinjection, also contained endogenous Type II keratin IF. Importantly, injections of significantly higher concentrations of the Type I protein resulted in the disassembly of the endogenous IF network. These results suggested that keratin IF were also regulated by an equilibrium between polymerized and non-polymerized subunits and suggested that heteropolymer keratin IF are dynamic cytoskeletal components (Miller et al., 1993). Other experiments shedding light on the dynamic properties of keratin IF involved expressing mutant forms of epidermal keratin 14 (K14) in simple epithelial cells (Albers and Fuchs, 1987; Albers and Fuchs, 1989).
Fig. 5.
Purified biotinylated Type I keratin was microinjected into PtK2 cells and cells were fixed and processed for immunofluorescence with anti-biotin (Fig 5, A, C, E) and anti-keratin (Fig. 5 B, D, Figure legends F) at different time intervals (20 min [A,B], 1hr [C,D] and 4hr [E,F] post-microinjection). Taken from reference: (Miller et al., 1991).
Further insights into the dynamic properties of vimentin IF were derived from fluorescence recovery after photobleaching (FRAP) analyses. Initially this involved the microinjection of x-rhodamine-conjugated vimentin which was used to monitor vimentin IF behavior and dynamics in live cells. Following microinjection the cells were incubated for ~ 8 hours to ensure that a steady state had been achieved. At this time all of the exogenous protein was incorporated into the endogenous IF network. After photobleaching bars across single filaments or multiple filaments, recovery of fluorescence was seen to take place equally all along the length of the bleach zone. Importantly these observations showed that IF were apolar in vivo and were capable of incorporating subunits anywhere along their long axes. Although it was difficult to determine the t1/2 for full recovery due to background photobleaching effects, we did determine that significant recovery of fluorescence required more time than could be accounted for by simple diffusion (Vikstrom et al., 1992). Several years later we carried out similar FRAP analyses on cells expressing GFP-vimentin. This permitted us to determine that the t1/2 for full recovery of vimentin was ~6 min (Yoon et al., 1998) (Fig. 6).
Fig. 6.
A region of a BHK-21 cell expressing GFP-vimentin before (top) and immediately following photobleaching (00); 6 min after bleaching (06) and 18 min following bleaching (18)). Figure is from reference: (Yoon et al., 1998).
Another benefit of expressing GFP-tagged IF proteins was that cells could be observed by time lapse imaging for longer periods. The results of such observations demonstrated that IF constantly alter their shape and that individual fibrils which are in close proximity can move in opposite directions. These movements required energy and most types of movements were dependent on microtubules or microfilaments. These live imaging studies also revealed that there were three distinct forms of vimentin in cells which we termed non-filamentous particles, short IF or squiggles and long IF. These three forms appeared to be interconvertible and most likely represented the different assembly states of IF in vivo and in fixed stained cells (Fig.7). Importantly we showed that both the particles and squiggles were capable of moving at high rates of speed in cells. These rapid movements (0.55 +/− 0.24 µm/sec) were bidirectional, typically tracking along microtubules. We also determined that these rapid movements required both kinesin and dynein (Helfand et al., 2002; Prahlad et al., 1998; Prahlad et al., 2000). These findings led us to propose that there is a mechanism for rapidly delivering and targeting IF precursors such as particles and squiggles to different regions of the cell for the assembly of IF networks. We speculated that this system might be responsible for regulating the structural and mechanical properties of different regions of cells (Helfand et al., 2003a). We were also able to show that a significant fraction of the non-filamentous particles of IF are formed co-translationally in a process we termed dynamic co-translation. This latter study demonstrated that multiple peripherin mRNAs (peripherin is a Type III IF protein) are clustered, typically at sites where peripherin is being synthesized. The synchronous synthesis of peripherin protein chains most likely reflects a mechanism for the formation of in-parallel and in-register coiled-coil dimers, the essential building blocks of IF (Chang et al., 2006).
Fig. 7.
A BHK21 cell fixed and processed for immunofluorescence with anti-vimentin. Shown are different structural forms of IF including vimentin particles which give rise to squiggles or short IF and, through end domain interactions, form long IF. From reference: (Prahlad et al., 1998)
The roles of IF in cell shape determination and maintenance
We have been interested in the roles of IF in cell shape determination and maintenance since the early 1970s. One of the most obvious experimental approaches for determining the role of IF in cell shape is to drive their disassembly in vivo. Unfortunately there were, and still are, no drugs available which specifically disassemble IF in vivo. Drugs such as colchicine and nocodazole for microtubules and cytochalasin B for microfilaments are not yet available for IF. This led us to explore a variety of alternative methods for causing their disassembly in cells. In collaboration with Peter Steinert we developed the use of a mimetic peptide derived from the helix initiation region of the vimentin central rod domain, termed 1A. In vitro this 1A peptide drives the disassembly of IF into small oligomers and monomers most likely by binding to the same 1A sequences in the intact IF causing destabilization of the IF structure. Furthermore we showed that the 1A peptide has no impact on the assembly state of microtubules and actin filaments. When the 1A peptide is microinjected into live cells, it rapidly causes the disassembly of IF and coincident with this disassembly, fibroblasts dramatically alter their shape as they retract their processes and completely round up. The cells do not die, and within a few hours IF networks begin to reform and cells re-spread into the elongated shapes of fibroblasts. These experimental results support the central role of IF in maintaining the shape and mechanical integrity of cells (Goldman et al., 1996). A significant role for neuronal IF proteins in maintaining cell shape was also demonstrated by silencing expression of peripherin (Helfand et al., 2003b) and the Type III IF glial fibrillary acidic protein (GFAP), which showed that this IF system was required for the formation of astrocytic processes (Weinstein et al., 1991).
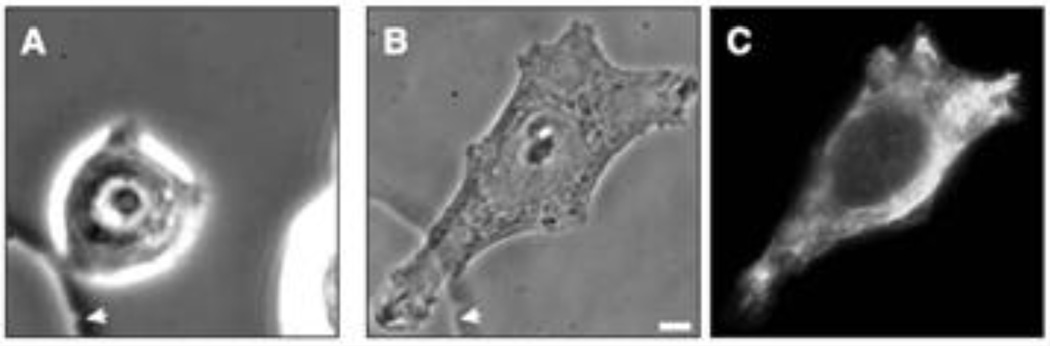
The cells of most adult tissues maintain well-defined shapes and positions with respect to their neighbors, and normally do not exhibit gross movements. Epithelial cells, for example, have specialized junctions holding them in tight apposition to form an effective barrier against mechanical stress. The keratin IF characteristic of these cells assembles into a network that is closely associated with specialized cellular adhesion sites (e.g. desmosomes, hemidesmosomes and focal adhesions) (Green and Goldman, 1986), and interacts with specific components of these structures (Bhattacharya et al., 2009; Gonzales et al., 2001). This network of keratin IF and cell-cell junctions contributes to the overall strength and stability of epithelial tissues (Fuchs, 1995; McLean and Lane, 1995). In contrast, various stages of morphogenesis are characterized by dramatic changes in the shape and motility of cells (Hay, 2005). This process, the epithelial to mesenchymal transition (EMT), involves a switch from expressing keratin IF to vimentin IF. Indeed vimentin expression in epithelial cells is a hallmark of the EMTs that take place both during development and in metastasis (Hay, 2005). We have long been intrigued by the expression of vimentin in epithelial cells and recently initiated studies to determine more precisely the functional significance of its expression in breast epithelial cells such as MCF-7 which express only keratin IF. We have found that the microinjection of vimentin into MCF-7 cells (or the expression of vimentin cDNA) caused rapid changes in cell shape (Fig. 8). Specifically, the more rounded epithelial cells became highly motile and mesenchymal in shape. This latter shape change involved the formation of a leading edge lamellipodium which typifies a locomoting mesenchymal cell (e.g., a motile fibroblast) (Mendez et al., 2010). When MCF-7 cells growing in colonies were transfected with vimentin, there was a rapid loss of desmosomes, a dramatic increase in focal adhesion dynamics and motility, as their epithelial morphology was converted into the typical leading-trailing edge polarity of mesenchymal cells. Conversely, silencing vimentin expression or altering the organization of VIF by the expression of a dominant negative mutant of vimentin in mesenchymal cells caused them to assume a more epithelial shape. These results demonstrate that the expression of vimentin IF in epithelial cells is much more than an epiphenomenon, but in fact helps to explain many of the morphological and behavioral changes seen during the EMT (Mendez et al., 2010).
Fig. 8.
An MCF-7 cell prior to (A) and following the microinjection of soluble vimentin. The cell was fixed and processed for immunofluorescence with vimentin antibody at 5 hrs. A, B are phase contrast images and C is the same cell as B, but observed with fluorescence optics). From reference: (Mendez et al., 2010).
Insights into the role of vimentin IF in cell motility
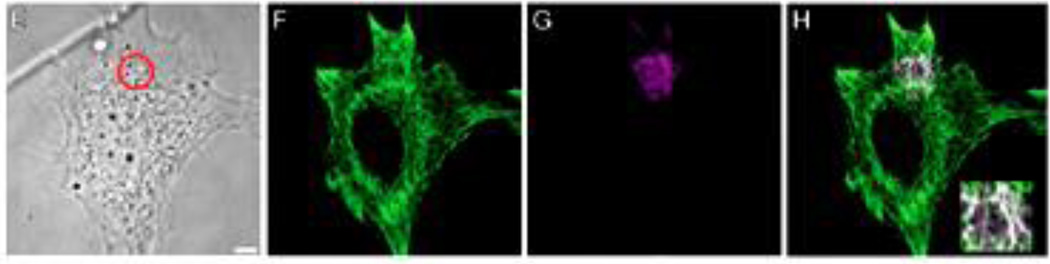
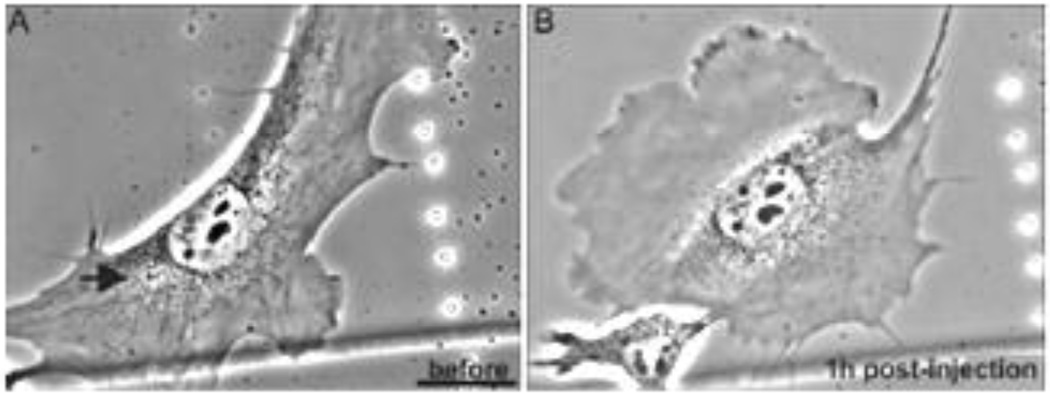
Based upon our insights into the role of vimentin IF in the EMT, we have recently initiated studies to delve more deeply into their specific roles in cell locomotion. These studies have been carried out in a close collaboration with the laboratories of Ueli Aebi and Harald Herrmann. The results to date demonstrate that vimentin IF play an important role in the formation of the lamellipodium, the locomotory structure located at the leading edge of migrating mesenchymal cells (e.g.,fibroblasts). In non-motile or serum starved fibroblasts in which there are no lamellipodia, vimentin IF networks extend to and are closely associated with the cell membrane. Following serum addition vimentin is phosphorylated (e.g., Ser38 in the N-terminal domain) within seconds and this is accompanied by the retraction and disassembly of IF networks only at sites where lamellipodia form (Helfand et al., 2011). Lamellipod formation can also be induced by the local activation of a photoactivatable form of Rac1 in serum deprived cells (PA-Rac1 (Wu et al., 2009)). This local activation of PA-Rac1 results in the rapid (i.e., within 5 sec) increase in vimentin phosphorylation only at the irradiation site (Fig. 9). Subsequently this leads to the local disassembly of vimentin IF where lamellipodia form (Helfand et al., 2011). We have also shown that the microinjection of a vimentin mimetic peptide (2B2) into serum deprived fibroblasts results in the local disassembly of vimentin IF, most likely into ULF, at sites of microinjection. Subsequently lamellipodia form at these same sites (Fig. 10). In addition, fibroblasts null for vimentin expression cannot engage in locomotory activity, despite the existence of lamellipodia over their entire surfaces (Helfand et al., 2011). Taken together these studies suggest that fully assembled VIF networks act as a ‘brake’ to inhibit membrane ruffling and the formation of lamellipodia. Conversely the disassembly of VIF appears to be required for the formation of lamellipodia.
Fig. 9.
A mouse embryo fibroblast (mEF) expressing PA-Rac1 before (E) and fixed within 5 sec. after spot irradiation. Subsequently this cell was processed for double label immunofluorescence with vimentin (green) and vimentin pSer38 (magenta) antibodies. Insert shows that this latter antibody stains vimentin IF only in the irradiated spot). Taken from reference: (Helfand et al., 2011).
Fig. 10.
Serum deprived live mEF before (A) and at 1hr following microinjection of peptide 2B2. The arrow shows site of injection where a lamellipodium formed (upper left)). Taken from reference: (Helfand et al., 2011)
Phosphorylation and the assembly states of vimentin IF
Our interest in the role of phosphorylation in regulating vimentin IF assembly and organization came from studies of BHK-21 cells undergoing mitosis. In this cell type the extensive networks of VIF present during prophase disassemble into aggregates or particles as cells transition from late prophase to metaphase. Coincident with this disassembly vimentin is hyperphosphorylated (Chou et al., 1989; Skalli et al., 1992). This phosphorylation is attributable to cdk1 (Chou et al., 1990) which phosphorylates vimentin at Ser55 (Chou et al., 1991). Interestingly, disassembly of the phosphorylated vimentin is dependent upon the presence of the intermediate filament protein nestin, which co-assembles with the vimentin (Chou et al., 2003). During interphase the turnover rate of vimentin phosphorylation appears to be very rapid since treating human fibroblasts or BHK-21 cells with phosphatase inhibitors such as okadaic acid, calyculin A and dinophysistoxin 1 (35-methylokadaic acid) results in a rapid increase in vimentin phosphorylation (Eriksson et al., 1992; Eriksson et al., 2004; Yatsunami et al., 1991). This is most likely attributable to the many kinases and phosphatases known to phosphorylate and dephosphorylate vimentin at numerous sites. A combination of in vivo and in vitro experiments have identified a large number of kinases including Protein Kinase A, Protein Kinase C, Calcium Calmodulin Kinase, p21-activated Kinase, Cdc2 Kinase, Rho-Kinase, Aurora-B, Polo-like Kinase and AKT Kinase which phosphorylate one or more of the 41 sites in vimentin identified to date (Eriksson et al., 2004; Goto et al., 1998; Izawa and Inagaki, 2006; Sihag et al., 2007).
In general, it appears that phosphorylation of vimentin subunits increases the off-rate and drives disassembly of IF, while dephosphorylation increases the on-rate and favors assembly of IF (Eriksson et al., 2004). The observed in vivo phosphorylation involving large numbers of sites provides for enormous complexity and very specific fine-tuning of the assembly states of vimentin. Likewise the kinases responsible for phosphorylation of vimentin are highly regulated and very sensitive to external chemical and mechanical stimuli like growth factors and shear stress (see below).
Mechanical properties of IF
The IF cytoskeleton plays an important role in the mechanical stability of cells and tissues that are subjected to external mechanical forces. We and our colleagues have studied the response of IF to shear stress in vascular endothelial and alveolar epithelial cells. Vascular endothelial cells modulate the physiological response to local changes in blood flow, or hemodynamic shear stress (Helmke, 2005; Helmke et al., 2000). Live cell imaging of GFP-vimentin expressing endothelial cells subjected to a shear stress of 12 dynes/cm2 revealed that within 3 min the movement or displacement of vimentin IF significantly increased, suggesting a role in intracellular mechanotransduction (Helmke et al., 2000; Helmke et al., 2001). We also found that keratin IF exhibit significant displacements following the onset of shear stress in PtK2 cells (Yoon et al., 2001). In alveolar epithelial cells, the mesh size delineated by the keratin IF network is smallest near the nucleus and largest at the cell perimeter. After subjecting these cells to moderate shear stress, the peripheral keratin IF network mesh size decreases to approximate that seen in the perinuclear region. Particle tracking microrheology (PTM) measurements before and after shear stress showed that there was a significant increase in the stiffness of the KIF network following shear stress, especially in the peripheral regions of the cytoplasm (Sivaramakrishnan et al., 2008). We also found that shear stress caused non-filamentous keratin particles to become incorporated into IF, resulting in the formation of thicker bundles (tonofibrils) and that this assembly was also regulated by phosphorylation (Flitney et al., 2009). These results showed that the keratin IF networks function as an “intracellular structural scaffold” which provides cells with a mechanism to rapidly respond to and withstand external forces. IF are uniquely suited to this mechanical role, as it has been shown that their rheological properties are distinctly different from microtubules and microfilaments. For example, IF are much more flexible than either actin filaments or microtubules, but at the same time they are also the only cytoskeletal system capable of withstanding strains of greater than 100%, which is similar to those experienced by soft tissue cells during the deformations accompanying mechanically induced stress (Janmey et al., 1991). Moreover, as the mechanical stress is increased, IF exhibit strain stiffening properties. Elegant work by Aebi and colleagues has extended our understanding of these special mechanical properties of IF down to the level of individual filaments. Using atomic force microscopy they showed that a single filament could be stretched up to 2.5–3.5 times its resting length, and that this is accompanied by a decrease in the filaments width of >60% (Kreplak and Fudge, 2007; Kreplak et al., 2008). These findings suggest that IF are major determinants of the mechanical properties of cells.
Acknowledgements
Thanks to Janine Aebi and Anne Goldman for tolerating long scientific dialogues between Ueli and myself, even while vacationing. This work has been supported by the National Institute of General Medical Sciences for over 30 years. Megan Cleland is supported by award number T32HL076139 from the National Heart, Lung, and Blood Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aebi U, Cohn J, Buhle L, Gerace L. The nuclear lamina is a meshwork of intermediate-type filaments. Nature. 1986;323:560–564. doi: 10.1038/323560a0. [DOI] [PubMed] [Google Scholar]
- Albers K, Fuchs E. The expression of mutant epidermal keratin cDNAs transfected in simple epithelial and squamous cell carcinoma lines. J Cell Biol. 1987;105:791–806. doi: 10.1083/jcb.105.2.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers K, Fuchs E. Expression of mutant keratin cDNAs in epithelial cells reveals possible mechanisms for initiation and assembly of intermediate filaments. J Cell Biol. 1989;108:1477–1493. doi: 10.1083/jcb.108.4.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aynardi MW, Steinert PM, Goldman RD. Human epithelial cell intermediate filaments: isolation, purification, and characterization. J Cell Biol. 1984;98:1407–1421. doi: 10.1083/jcb.98.4.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya R, Gonzalez AM, Debiase PJ, Trejo HE, Goldman RD, Flitney FW, Jones JC. Recruitment of vimentin to the cell surface by beta3 integrin and plectin mediates adhesion strength. J Cell Sci. 2009;122:1390–1400. doi: 10.1242/jcs.043042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Shav-Tal Y, Trcek T, Singer RH, Goldman RD. Assembling an intermediate filament network by dynamic cotranslation. J Cell Biol. 2006;172:747–758. doi: 10.1083/jcb.200511033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou YH, Rosevear E, Goldman RD. Phosphorylation and disassembly of intermediate filaments in mitotic cells. Proc Natl Acad Sci U S A. 1989;86:1885–1889. doi: 10.1073/pnas.86.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou YH, Ngai KL, Goldman R. The regulation of intermediate filament reorganization in mitosis. p34cdc2 phosphorylates vimentin at a unique N-terminal site. J Biol Chem. 1991;266:7325–7328. [PubMed] [Google Scholar]
- Chou YH, Bischoff JR, Beach D, Goldman RD. Intermediate filament reorganization during mitosis is mediated by p34cdc2 phosphorylation of vimentin. Cell. 1990;62:1063–1071. doi: 10.1016/0092-8674(90)90384-q. [DOI] [PubMed] [Google Scholar]
- Chou YH, Khuon S, Herrmann H, Goldman RD. Nestin promotes the phosphorylation-dependent disassembly of vimentin intermediate filaments during mitosis. Mol Biol Cell. 2003;14:1468–1478. doi: 10.1091/mbc.E02-08-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clubb BH, Chou YH, Herrmann H, Svitkina TM, Borisy GG, Goldman RD. The 300-kDa intermediate filament-associated protein (IFAP300) is a hamster plectin ortholog. Biochem Biophys Res Commun. 2000;273:183–187. doi: 10.1006/bbrc.2000.2916. [DOI] [PubMed] [Google Scholar]
- Dowling LM, Parry DA, Sparrow LG. Structural homology between hard alpha-keratin and the intermediate filament proteins desmin and vimentin. Bioscience reports. 1983;3:73–78. doi: 10.1007/BF01121573. [DOI] [PubMed] [Google Scholar]
- Eriksson A, Thornell LE. Intermediate (skeletin) filaments in heart Purkinje fibers. A correlative morphological and biochemical identification with evidence of a cytoskeletal function. J Cell Biol. 1979;80:231–247. doi: 10.1083/jcb.80.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson JE, Brautigan DL, Vallee R, Olmsted J, Fujiki H, Goldman RD. Cytoskeletal integrity in interphase cells requires protein phosphatase activity. Proc Natl Acad Sci U S A. 1992;89:11093–11097. doi: 10.1073/pnas.89.22.11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson JE, He T, Trejo-Skalli AV, Harmala-Brasken AS, Hellman J, Chou YH, Goldman RD. Specific in vivo phosphorylation sites determine the assembly dynamics of vimentin intermediate filaments. J Cell Sci. 2004;117:919–932. doi: 10.1242/jcs.00906. [DOI] [PubMed] [Google Scholar]
- Flitney EW, Kuczmarski ER, Adam SA, Goldman RD. Insights into the mechanical properties of epithelial cells: the effects of shear stress on the assembly and remodeling of keratin intermediate filaments. Faseb J. 2009;23:2110–2119. doi: 10.1096/fj.08-124453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E. Keratins and the skin. Annu Rev Cell Dev Biol. 1995;11:123–153. doi: 10.1146/annurev.cb.11.110195.001011. [DOI] [PubMed] [Google Scholar]
- Goldman AE, Maul G, Steinert PM, Yang HY, Goldman RD. Keratin-like proteins that coisolate with intermediate filaments of BHK-21 cells are nuclear lamins. Proc Natl Acad Sci U S A. 1986;83:3839–3843. doi: 10.1073/pnas.83.11.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R, Pollard TP, Rosenbaum JL. Cell Motility. Cold Spring Harbor, New York: Cold Spring Harbor Press; 1976. [Google Scholar]
- Goldman RD. The role of three cytoplasmic fibers in BHK-21 cell motility. I. Microtubules and the effects of colchicine. J Cell Biol. 1971;51:752–762. doi: 10.1083/jcb.51.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman RD, Follett EA. The structure of the major cell processes of isolated BHK21 fibroblasts. Exp Cell Res. 1969;57:263–276. doi: 10.1016/0014-4827(69)90150-5. [DOI] [PubMed] [Google Scholar]
- Goldman RD, Follett EA. Birefringent filamentous organelle in BHK-21 cells and its possible role in cell spreading and motility. Science. 1970;169:286–288. doi: 10.1126/science.169.3942.286. [DOI] [PubMed] [Google Scholar]
- Goldman RD, Khuon S, Chou YH, Opal P, Steinert PM. The function of intermediate filaments in cell shape and cytoskeletal integrity. J Cell Biol. 1996;134:971–983. doi: 10.1083/jcb.134.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales M, Weksler B, Tsuruta D, Goldman RD, Yoon KJ, Hopkinson SB, Flitney FW, Jones JC. Structure and function of a vimentin-associated matrix adhesion in endothelial cells. Mol Biol Cell. 2001;12:85–100. doi: 10.1091/mbc.12.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto H, Kosako H, Tanabe K, Yanagida M, Sakurai M, Amano M, Kaibuchi K, Inagaki M. Phosphorylation of vimentin by Rho-associated kinase at a unique amino-terminal site that is specifically phosphorylated during cytokinesis. J Biol Chem. 1998;273:11728–11736. doi: 10.1074/jbc.273.19.11728. [DOI] [PubMed] [Google Scholar]
- Green KJ, Goldman RD. Evidence for an interaction between the cell surface and intermediate filaments in cultured fibroblasts. Cell Motil Cytoskeleton. 1986;6:389–405. doi: 10.1002/cm.970060405. [DOI] [PubMed] [Google Scholar]
- Hay ED. The mesenchymal cell, its role in the embryo, and the remarkable signaling mechanisms that create it. Dev Dyn. 2005;233:706–720. doi: 10.1002/dvdy.20345. [DOI] [PubMed] [Google Scholar]
- Helfand BT, Chang L, Goldman RD. The dynamic and motile properties of intermediate filaments. Annu Rev Cell Dev Biol. 2003a;19:445–467. doi: 10.1146/annurev.cellbio.19.111401.092306. [DOI] [PubMed] [Google Scholar]
- Helfand BT, Mikami A, Vallee RB, Goldman RD. A requirement for cytoplasmic dynein and dynactin in intermediate filament network assembly and organization. J Cell Biol. 2002;157:795–806. doi: 10.1083/jcb.200202027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfand BT, Mendez MG, Pugh J, Delsert C, Goldman RD. A role for intermediate filaments in determining and maintaining the shape of nerve cells. Mol Biol Cell. 2003b;14:5069–5081. doi: 10.1091/mbc.E03-06-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfand BT, Mendez MG, Murthy SN, Shumaker DK, Grin B, Mahammad S, Aebi U, Wedig T, Wu YI, Hahn KM, Inagaki M, Herrmann H, Goldman RD. Vimentin organization modulates the formation of lamellipodia. Mol Biol Cell. 2011;22:1274–1289. doi: 10.1091/mbc.E10-08-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmke BP. Molecular control of cytoskeletal mechanics by hemodynamic forces. Physiology (Bethesda) 2005;20:43–53. doi: 10.1152/physiol.00040.2004. [DOI] [PubMed] [Google Scholar]
- Helmke BP, Goldman RD, Davies PF. Rapid displacement of vimentin intermediate filaments in living endothelial cells exposed to flow. Circ Res. 2000;86:745–752. doi: 10.1161/01.res.86.7.745. [DOI] [PubMed] [Google Scholar]
- Helmke BP, Thakker DB, Goldman RD, Davies PF. Spatiotemporal analysis of flow-induced intermediate filament displacement in living endothelial cells. Biophysical journal. 2001;80:184–194. doi: 10.1016/S0006-3495(01)76006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann H, Aebi U. Intermediate filament assembly: fibrillogenesis is driven by decisive dimer-dimer interactions. Current opinion in structural biology. 1998;8:177–185. doi: 10.1016/s0959-440x(98)80035-3. [DOI] [PubMed] [Google Scholar]
- Herrmann H, Aebi U. Intermediate filaments: molecular structure, assembly mechanism, and integration into functionally distinct intracellular Scaffolds. Annu Rev Biochem. 2004;73:749–789. doi: 10.1146/annurev.biochem.73.011303.073823. [DOI] [PubMed] [Google Scholar]
- Herrmann H, Haner M, Brettel M, Ku NO, Aebi U. Characterization of distinct early assembly units of different intermediate filament proteins. J Mol Biol. 1999;286:1403–1420. doi: 10.1006/jmbi.1999.2528. [DOI] [PubMed] [Google Scholar]
- Herrmann H, Bar H, Kreplak L, Strelkov SV, Aebi U. Intermediate filaments: from cell architecture to nanomechanics. Nat Rev Mol Cell Biol. 2007;8:562–573. doi: 10.1038/nrm2197. [DOI] [PubMed] [Google Scholar]
- Herrmann H, Haner M, Brettel M, Muller SA, Goldie KN, Fedtke B, Lustig A, Franke WW, Aebi U. Structure and assembly properties of the intermediate filament protein vimentin: the role of its head, rod and tail domains. J Mol Biol. 1996;264:933–953. doi: 10.1006/jmbi.1996.0688. [DOI] [PubMed] [Google Scholar]
- Hitchcock SE, Carisson L, Lindberg U. Depolymerization of F-actin by deoxyribonuclease I. Cell. 1976;7:531–542. doi: 10.1016/0092-8674(76)90203-8. [DOI] [PubMed] [Google Scholar]
- Izawa I, Inagaki M. Regulatory mechanisms and functions of intermediate filaments: a study using site- and phosphorylation state-specific antibodies. Cancer Sci. 2006;97:167–174. doi: 10.1111/j.1349-7006.2006.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janmey PA, Euteneuer U, Traub P, Schliwa M. Viscoelastic properties of vimentin compared with other filamentous biopolymer networks. J Cell Biol. 1991;113:155–160. doi: 10.1083/jcb.113.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreplak L, Fudge D. Biomechanical properties of intermediate filaments: from tissues to single filaments and back. Bioessays. 2007;29:26–35. doi: 10.1002/bies.20514. [DOI] [PubMed] [Google Scholar]
- Kreplak L, Herrmann H, Aebi U. Tensile properties of single desmin intermediate filaments. Biophysical journal. 2008;94:2790–2799. doi: 10.1529/biophysj.107.119826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieska N, Chen J, Maisel H, Romero-Herrera AE. Subunit characterization of lens intermediate filaments. Biochim Biophys Acta. 1980;626:136–153. doi: 10.1016/0005-2795(80)90205-6. [DOI] [PubMed] [Google Scholar]
- McLean WH, Lane EB. Intermediate filaments in disease. Curr Opin Cell Biol. 1995;7:118–125. doi: 10.1016/0955-0674(95)80053-0. [DOI] [PubMed] [Google Scholar]
- Meier M, Padilla GP, Herrmann H, Wedig T, Hergt M, Patel TR, Stetefeld J, Aebi U, Burkhard P. Vimentin coil 1A-A molecular switch involved in the initiation of filament elongation. J Mol Biol. 2009;390:245–261. doi: 10.1016/j.jmb.2009.04.067. [DOI] [PubMed] [Google Scholar]
- Mendez MG, Kojima SI, Goldman RD. Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. Faseb J. 2010 doi: 10.1096/fj.09-151639. (Ahead of print; PMID:20097873) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RK, Vikstrom K, Goldman RD. Keratin incorporation into intermediate filament networks is a rapid process. J Cell Biol. 1991;113:843–855. doi: 10.1083/jcb.113.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RK, Khuon S, Goldman RD. Dynamics of keratin assembly: exogenous type I keratin rapidly associates with type II keratin in vivo. J Cell Biol. 1993;122:123–135. doi: 10.1083/jcb.122.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolet S, Herrmann H, Aebi U, Strelkov SV. Atomic structure of vimentin coil 2. J Struct Biol. 2010;170:369–376. doi: 10.1016/j.jsb.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Parry DA, Steven AC, Steinert PM. The coiled-coil molecules of intermediate filaments consist of two parallel chains in exact axial register. Biochem Biophys Res Commun. 1985;127:1012–1018. doi: 10.1016/s0006-291x(85)80045-0. [DOI] [PubMed] [Google Scholar]
- Parry DA, Conway JF, Steinert PM. Structural studies on lamin. Similarities and differences between lamin and intermediate-filament proteins. Biochem J. 1986;238:305–308. doi: 10.1042/bj2380305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry DA, Strelkov SV, Burkhard P, Aebi U, Herrmann H. Towards a molecular description of intermediate filament structure and assembly. Exp Cell Res. 2007;313:2204–2216. doi: 10.1016/j.yexcr.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Parysek LM, Goldman RD. Characterization of intermediate filaments in PC12 cells. J Neurosci. 1987;7:781–791. doi: 10.1523/JNEUROSCI.07-03-00781.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plodinec M, Loparic M, Suetterlin R, Herrmann H, Aebi U, Schoenenberger CA. The nanomechanical properties of rat fibroblasts are modulated by interfering with the vimentin intermediate filament system. J Struct Biol. 2011;174:476–484. doi: 10.1016/j.jsb.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Portet S, Mucke N, Kirmse R, Langowski J, Beil M, Herrmann H. Vimentin intermediate filament formation: in vitro measurement and mathematical modeling of the filament length distribution during assembly. Langmuir. 2009;25:8817–8823. doi: 10.1021/la900509r. [DOI] [PubMed] [Google Scholar]
- Prahlad V, Yoon M, Moir RD, Vale RD, Goldman RD. Rapid movements of vimentin on microtubule tracks: kinesin-dependent assembly of intermediate filament networks. J Cell Biol. 1998;143:159–170. doi: 10.1083/jcb.143.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prahlad V, Helfand BT, Langford GM, Vale RD, Goldman RD. Fast transport of neurofilament protein along microtubules in squid axoplasm. J Cell Sci. 2000;113(Pt 22):3939–3946. doi: 10.1242/jcs.113.22.3939. [DOI] [PubMed] [Google Scholar]
- Sihag RK, Inagaki M, Yamaguchi T, Shea TB, Pant HC. Role of phosphorylation on the structural dynamics and function of types III and IV intermediate filaments. Exp Cell Res. 2007;313:2098–2109. doi: 10.1016/j.yexcr.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaramakrishnan S, DeGiulio JV, Lorand L, Goldman RD, Ridge KM. Micromechanical properties of keratin intermediate filament networks. Proc Natl Acad Sci U S A. 2008;105:889–894. doi: 10.1073/pnas.0710728105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalli O, Chou YH, Goldman RD. Cell cycle-dependent changes in the organization of an intermediate filament-associated protein: correlation with phosphorylation by p34cdc2. Proc Natl Acad Sci U S A. 1992;89:11959–11963. doi: 10.1073/pnas.89.24.11959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soellner P, Quinlan RA, Franke WW. Identification of a distinct soluble subunit of an intermediate filament protein: tetrameric vimentin from living cells. Proc Natl Acad Sci U S A. 1985;82:7929–7933. doi: 10.1073/pnas.82.23.7929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starger JM, Goldman RD. Isolation and preliminary characterization of 10-nm filaments from baby hamster kidney (BHK-21) cells. Proc Natl Acad Sci U S A. 1977;74:2422–2426. doi: 10.1073/pnas.74.6.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starger JM, Brown WE, Goldman AE, Goldman RD. Biochemical and immunological analysis of rapidly purified 10-nm filaments from baby hamster kidney (BHK-21) cells. J Cell Biol. 1978;78:93–109. doi: 10.1083/jcb.78.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert PM, Idler WW, Zimmerman SB. Self-assembly of bovine epidermal keratin filaments in vitro. J Mol Biol. 1976;108:547–567. doi: 10.1016/s0022-2836(76)80136-2. [DOI] [PubMed] [Google Scholar]
- Steinert PM, Zimmerman SB, Starger JM, Goldman RD. Ten-nanometer filaments of hamster BHK-21 cells and epidermal keratin filaments have similar structures. Proc Natl Acad Sci U S A. 1978;75:6098–6101. doi: 10.1073/pnas.75.12.6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strelkov SV, Herrmann H, Aebi U. Molecular architecture of intermediate filaments. Bioessays. 2003;25:243–251. doi: 10.1002/bies.10246. [DOI] [PubMed] [Google Scholar]
- Strelkov SV, Herrmann H, Geisler N, Wedig T, Zimbelmann R, Aebi U, Burkhard P. Conserved segments 1A and 2B of the intermediate filament dimer: their atomic structures and role in filament assembly. Embo J. 2002;21:1255–1266. doi: 10.1093/emboj/21.6.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strelkov SV, Herrmann H, Geisler N, Lustig A, Ivaninskii S, Zimbelmann R, Burkhard P, Aebi U. Divide-and-conquer crystallographic approach towards an atomic structure of intermediate filaments. J Mol Biol. 2001;306:773–781. doi: 10.1006/jmbi.2001.4442. [DOI] [PubMed] [Google Scholar]
- Sun TT, Green H. Immunofluorescent staining of keratin fibers in cultured cells. Cell. 1978;14:469–476. doi: 10.1016/0092-8674(78)90233-7. [DOI] [PubMed] [Google Scholar]
- Vikstrom KL, Borisy GG, Goldman RD. Dynamic aspects of intermediate filament networks in BHK-21 cells. Proc Natl Acad Sci U S A. 1989;86:549–553. doi: 10.1073/pnas.86.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikstrom KL, Lim SS, Goldman RD, Borisy GG. Steady state dynamics of intermediate filament networks. J Cell Biol. 1992;118:121–129. doi: 10.1083/jcb.118.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein DE, Shelanski ML, Liem RK. Suppression by antisense mRNA demonstrates a requirement for the glial fibrillary acidic protein in the formation of stable astrocytic processes in response to neurons. J Cell Biol. 1991;112:1205–1213. doi: 10.1083/jcb.112.6.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson EB. The cell in development and heredity. 3rd ed. New York, USA: Macmillan Company; 1928. [Google Scholar]
- Winheim S, Hieb AR, Silbermann M, Surmann EM, Wedig T, Herrmann H, Langowski J, Mucke N. Deconstructing the late phase of vimentin assembly by total internal reflection fluorescence microscopy (TIRFM) PLoS One. 2011;6:e19202. doi: 10.1371/journal.pone.0019202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YI, Frey D, Lungu OI, Jaehrig A, Schlichting I, Kuhlman B, Hahn KM. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature. 2009;461:104–108. doi: 10.1038/nature08241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsunami J, Fujiki H, Suganuma M, Yoshizawa S, Eriksson JE, Olson MO, Goldman RD. Vimentin is hyperphosphorylated in primary human fibroblasts treated with okadaic acid. Biochem Biophys Res Commun. 1991;177:1165–1170. doi: 10.1016/0006-291x(91)90662-q. [DOI] [PubMed] [Google Scholar]
- Yoon KH, Yoon M, Moir RD, Khuon S, Flitney FW, Goldman RD. Insights into the dynamic properties of keratin intermediate filaments in living epithelial cells. J Cell Biol. 2001;153:503–516. doi: 10.1083/jcb.153.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon M, Moir RD, Prahlad V, Goldman RD. Motile properties of vimentin intermediate filament networks in living cells. J Cell Biol. 1998;143:147–157. doi: 10.1083/jcb.143.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zackroff RV, Goldman RD. In vitro assembly of intermediate filaments from baby hamster kidney (BHK-21) cells. Proc Natl Acad Sci U S A. 1979;76:6226–6230. doi: 10.1073/pnas.76.12.6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zackroff RV, Goldman RD. In vitro reassembly of squid brain intermediate filaments (neurofilaments): purification by assembly-disassembly. Science. 1980;208:1152–1155. doi: 10.1126/science.7189605. [DOI] [PubMed] [Google Scholar]
- Zackroff RV, Idler WW, Steinert PM, Goldman RD. In vitro reconstitution of intermediate filaments form mammalian neurofilament triplet polypeptides. Proc Natl Acad Sci U S A. 1982;79:754–757. doi: 10.1073/pnas.79.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zackroff RV, Goldman AE, Jones JC, Steinert PM, Goldman RD. Isolation and characterization of keratin-like proteins from cultured cells with fibroblastic morphology. J Cell Biol. 1984;98:1231–1237. doi: 10.1083/jcb.98.4.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]