Abstract
BACKGROUND
An impaired glomerular filtration rate (GFR) leads to end-stage renal disease and increases the risks of cardiovascular disease and death. Persons with type 1 diabetes are at high risk for kidney disease, but there are no interventions that have been proved to prevent impairment of the GFR in this population.
METHODS
In the Diabetes Control and Complications Trial (DCCT), 1441 persons with type 1 diabetes were randomly assigned to 6.5 years of intensive diabetes therapy aimed at achieving near-normal glucose concentrations or to conventional diabetes therapy aimed at preventing hyperglycemic symptoms. Subsequently, 1375 participants were followed in the observational Epidemiology of Diabetes Interventions and Complications (EDIC) study. Serum creatinine levels were measured annually throughout the course of the two studies. The GFR was estimated with the use of the Chronic Kidney Disease Epidemiology Collaboration formula. We analyzed data from the two studies to determine the long-term effects of intensive diabetes therapy on the risk of impairment of the GFR, which was defined as an incident estimated GFR of less than 60 ml per minute per 1.73 m2 of body-surface area at two consecutive study visits.
RESULTS
Over a median follow-up period of 22 years in the combined studies, impairment of the GFR developed in 24 participants assigned to intensive therapy and in 46 assigned to conventional therapy (risk reduction with intensive therapy, 50%; 95% confidence interval, 18 to 69; P = 0.006). Among these participants, end-stage renal disease developed in 8 participants in the intensive-therapy group and in 16 in the conventional-therapy group. As compared with conventional therapy, intensive therapy was associated with a reduction in the mean estimated GFR of 1.7 ml per minute per 1.73 m2 during the DCCT study but during the EDIC study was associated with a slower rate of reduction in the GFR and an increase in the mean estimated GFR of 2.5 ml per minute per 1.73 m2 (P<0.001 for both comparisons). The beneficial effect of intensive therapy on the risk of an impaired GFR was fully attenuated after adjustment for glycated hemoglobin levels or albumin excretion rates.
CONCLUSIONS
The long-term risk of an impaired GFR was significantly lower among persons treated early in the course of type 1 diabetes with intensive diabetes therapy than among those treated with conventional diabetes therapy. (Funded by the National Institute of Diabetes and Digestive and Kidney Diseases and others; DCCT/EDIC ClinicalTrials.gov numbers, NCT00360815 and NCT00360893.)
An impaired glomerular filtration rate (GFR) is the final common pathway of diabetic kidney disease. Once the GFR is impaired, cardiovascular disease events and progression to end-stage renal disease occur at unacceptably high rates, even with proven medical management.1–3 This underscores the need for the primary prevention of impaired GFR in persons with diabetes.
The Diabetes Control and Complications Trial (DCCT) and the observational study that followed it, the Epidemiology of Diabetes Interventions and Complications (EDIC) study, showed that intensive diabetes therapy that lowered glycated hemoglobin levels reduced the risk of microalbuminuria and macroalbuminuria among persons with type 1 diabetes.4–6 Albuminuria is a sensitive marker of diabetic kidney disease that usually develops before the GFR is impaired and increases the risk that the GFR will fall.7 Moreover, albuminuria and an impaired GFR are strong additive risk factors for cardiovascular disease and death.8,9 The prevention of albuminuria by means of intensive diabetes therapy is therefore a cornerstone of recommendations that encourage tight glycemic control in patients with type 1 diabetes.10,11 Nonetheless, albuminuria is not universally accepted as a clinical or surrogate outcome.12 In the current study, we tested the effects of intensive diabetes therapy in the DCCT on the development of an impaired GFR, with a total follow-up period of 22 years.
METHODS
STUDY OVERSIGHT
The data were collected by the DCCT/EDIC research group. The writing group designed this study, analyzed the data, wrote the manuscript, and made the decision to submit it for publication. In addition, the members of the writing group vouch for the completeness and accuracy of the data and analyses and for the fidelity of the study to the protocol (which is available with the full text of this article at NEJM.org). All DCCT/EDIC procedures were approved by the institutional review board at each participating center, and all participants provided written informed consent.
STUDY POPULATION
The DCCT was a multicenter clinical trial, involving patients with type 1 diabetes mellitus, that examined the effects of intensive diabetes therapy aimed at lowering blood glucose to a level as close to the nondiabetic range as safely possible.4–6 The trial included two cohorts. Patients in the primary-prevention cohort had had diabetes for 1 to 5 years and had an albumin excretion rate of less than 40 mg per 24 hours, with no retinopathy as assessed with the use of fundus photography. Patients in the secondary-intervention cohort had had diabetes for 1 to 15 years and had an albumin excretion rate of 200 mg or less per 24 hours and at least one microaneurysm in either eye (but no more than moderate nonproliferative retinopathy). Other inclusion criteria in both cohorts were a serum creatinine level of less than 1.2 mg per deciliter (106.1 μmol per liter) or a creatinine clearance of more than 100 ml per minute per 1.73 m2 of body-surface area. A total of 1441 participants, 13 to 39 years of age, were enrolled between 1983 and 1989.
STUDY DESIGN OF THE DCCT
In the DCCT, participants were randomly assigned to receive intensive diabetes therapy or conventional diabetes therapy, as described previously.4 Briefly, intensive therapy consisted of three or more injections of insulin daily or the use of an insulin pump, with the aim of achieving a glycated hemoglobin level of less than 6.05% (which was considered to be the upper limit of the normal range). The goal of conventional therapy was the prevention of symptoms of hyperglycemia and hypoglycemia with the use of one or two injections of insulin daily. The DCCT was terminated in 1993, after a mean follow-up period of 6.5 years. Subsequently, participants who had been receiving intensive treatment were encouraged to continue intensive treatment, and participants who had been receiving conventional treatment were offered instruction in intensive therapy; all the participants returned to their own health care providers for ongoing diabetes care. All DCCT participants were invited to join the EDIC study, which was an observational extension of the DCCT, and 1375 (96% of the surviving cohort) agreed to participate.
IMPAIRED GFR
An impaired GFR was defined as an estimated GFR of less than 60 ml per minute per 1.73 m2 at two consecutive study visits, usually 1 year apart. This definition differs from the original DCCT definitions of renal impairment (a doubling of the serum creatinine concentration or a serum creatinine level of 2 mg per deciliter [176.8 μmol per liter]) and was modified to reflect contemporary guidelines issued by the American Diabetes Association and the National Kidney Foundation.10,11 Serum creatinine levels were measured yearly throughout the course of the two studies at the DCCT/EDIC Central Biochemistry Laboratory, University of Minnesota. The overall interassay coefficient of variation was less than 3%, and the overall coefficient of reliability was greater than 0.98.
Until 2007, serum creatinine was measured with the use of the Jaffe procedure. Thereafter, creatinine was measured by an enzymatic method that produced values traceable to the isotope dilution mass spectrometry (IDMS) values assigned by the National Institute of Standards and Technology. We calibrated creatinine results generated before 2007 to the IDMS-traceable values obtained with the enzymatic method (see the Supplementary Appendix, available at NEJM.org). Data on serum creatinine levels, age, sex, and race were then used to calculate the estimated GFR with the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.13 Iothalamate GFR measurements were obtained in subsets of participants at baseline of the DCCT, at closeout of the DCCT (i.e., baseline of the EDIC), and at either year 1 or year 2 of the EDIC.5
Events of end-stage renal disease, which were defined as the need for kidney transplantation or the initiation of maintenance dialysis, were assessed yearly by means of questionnaires and were adjudicated by the DCCT/EDIC mortality and morbidity committee, whose members were unaware of the DCCT treatment assignments. The analyses described herein include data from the baseline period of the DCCT study (1983 through 1989) through year 16 of the EDIC study (September 2008 through April 2010).
COVARIATES
Body-mass index, blood pressure, and glycated hemoglobin levels were measured quarterly during the DCCT and yearly during the EDIC study. Glycated hemoglobin was measured with the use of high-performance ion-exchange liquid chromatography.14 The albumin excretion rate was measured yearly during the DCCT and every 2 years during the EDIC study.5 All laboratory measurements were performed at the DCCT/EDIC Central Biochemistry Laboratory. The use of angiotensin-converting–enzyme (ACE) inhibitors was strongly discouraged during the DCCT unless clearly required for clinical reasons. The use of inhibitors of the renin–angiotensin–aldosterone system was assessed yearly on the basis of self-report during the EDIC study.
STATISTICAL ANALYSIS
Characteristics of the participants were summarized at baseline of the DCCT, at closeout of the DCCT (i.e., baseline of the EDIC study), and at year 16 of the EDIC study and were compared with the use of the Wilcoxon rank-sum test or the chi-square test. The cumulative incidence of an impaired GFR according to DCCT treatment assignment was estimated by means of Gray’s method, with death as a competing risk.15 The effects of intensive diabetes therapy and other covariates on the cause-specific hazard of an impaired estimated GFR were tested with the use of Cox proportional-hazards models adjusted for the estimated GFR at baseline of the DCCT. Data were right-censored when no impaired GFR occurred before year 16 of the EDIC study (1240 participants) or because of loss to follow-up (68 participants, including 10 during the course of the DCCT) or death as a competing risk (63 participants). The robust estimation of the covariance matrix according to the method of Lin and Wei was used to compute confidence limits and P values that are valid when proportional-hazards assumptions are violated.16 The results were virtually identical when we used the Fine–Gray generalization of the proportional-hazards model that accounted for death as a competing risk.17 An impaired GFR or death, whichever occurred first, was also examined as a secondary composite outcome.
Generalized linear mixed models were used to summarize and test the between-group differences in the mean estimated GFR and the rate of change in the estimated GFR over time according to the DCCT treatment group. Separate analyses were performed with data from the DCCT and with data from the EDIC study. Baseline estimated GFR values from each study (DCCT and EDIC) were included as covariates in each model. For the analyses of data from the EDIC study, age, sex, duration of diabetes, and DCCT cohort (primary prevention or secondary intervention) were also included in the model. All analyses were performed according to the intention-to-treat principle, with the use of SAS software, version 9.2, and the R statistical program, version 2.13.
RESULTS
CHARACTERISTICS OF THE PARTICIPANTS
At baseline in the DCCT (1983 through 1989), the mean age of the participants was 27 years, and the mean duration of diabetes was 6 years (Table 1). None of the participants were taking antihypertensive medications, including inhibitors of the renin–angiotensin–aldosterone system. A total of 157 participants (11%) had albumin excretion rates in the range of 30 to 200 mg per 24 hours.
Table 1.
Demographic and Clinical Characteristics of the Participants at Baseline and at Closeout of the Diabetes Control and Complications Trial (DCCT) and at Year 16 of the Epidemiology of Diabetes Interventions and Complications (EDIC) Study, According to DCCT Treatment Group.*
| Variable | DCCT at Baseline (1983–1989) (N = 1441) | End of DCCT (1993) (N = 1415) | EDIC at Year 16 (2008–2010) (N = 1222) | |||
|---|---|---|---|---|---|---|
| Intensive Therapy (N = 711) | Conventional Therapy (N = 730) | Intensive Therapy (N = 698) | Conventional Therapy (N = 717) | Intensive Therapy (N = 618) | Conventional Therapy (N = 604) | |
|
Demographic characteristics
| ||||||
| Age (yr) | 27.1±7.1 | 26.5±7.1 | 33.4±7.0 | 32.8±7.0 | 50.4±6.9 | 49.4±6.9† |
|
| ||||||
| Female sex (%) | 48.5 | 45.9 | 49.0 | 45.9 | 48.4 | 46.9 |
|
| ||||||
|
Medical history
| ||||||
| Duration of diabetes (yr) | 6.0±4.2 | 5.7±4.1 | 12.1±4.9 | 11.7±4.8 | 28.7±5.0 | 28.2±4.9 |
|
| ||||||
| DCCT primary cohort (%)‡ | 49.0 | 51.8 | 49.1 | 51.7 | 48.7 | 51.5 |
|
| ||||||
| Hypertension (%)§ | 0 | 0.3 | 0.7 | 1.8 | 53.7 | 51.2 |
|
| ||||||
| Hyperlipidemia (%)¶ | 22.8 | 23.3 | 26.0 | 29.7 | 65.7 | 65.2 |
|
| ||||||
| Current smoking (%) | 20.5 | 21.6 | 23.1 | 23.2 | 13.3 | 11.8 |
|
| ||||||
| Current alcohol use (%) | 37.8 | 39.9 | 36.3 | 38.9 | 42.9 | 44.7 |
|
| ||||||
|
Medical treatment
| ||||||
| Glucose management (%)
| ||||||
| Insulin pump or ≥3 daily insulin injections | 0 | 0 | 97.4 | 5.0|| | 97.6 | 96.2 |
|
| ||||||
| Glucose monitoring ≥4 times/day | 0 | 0 | 52.8 | 3.8|| | 65.4 | 70.3 |
|
| ||||||
| Use of antihypertensive medication (%)**
| ||||||
| Any | 0 | 0 | — | — | 56.2 | 59.3 |
|
| ||||||
| ACE inhibitor or ARB | 0 | 0 | — | — | 53.1 | 57.0 |
|
| ||||||
|
Physical examination findings
| ||||||
| Body-mass index†† | 23.4±2.7 | 23.5±2.9 | 26.6±4.3 | 25.0±3.1|| | 29.4±13.0 | 28.2±4.8 |
|
| ||||||
| Blood pressure (mm Hg)
| ||||||
| Systolic | 114.5±11.3 | 114.6±11.4 | 116.6±11.5 | 116.6±11.9 | 122.1±14.6 | 121.2±15.2 |
|
| ||||||
| Diastolic | 73.1±8.2 | 72.9±8.7 | 74.8±8.7 | 74.4±8.9 | 72.5±9.1 | 72.2±8.8 |
|
| ||||||
| Mean arterial pressure (mm Hg) | 86.9±8.2 | 86.8±8.6 | 88.8±8.7 | 88.5±8.8 | 89.0±9.6 | 88.5±9.6 |
|
| ||||||
|
Laboratory values
| ||||||
| Glycated hemoglobin (%)‡‡ | 9.1±1.6 | 9.1±1.6 | 7.3±0.9 | 9.1±1.3|| | 7.9±1.1 | 8.0±1.0 |
|
| ||||||
| Albumin excretion rate
| ||||||
| Median (mg/24 hr) | 11.5 | 11.5 | 8.6 | 10.1|| | 11.5 | 13.0† |
|
| ||||||
| Interquartile range (mg/24 hr) | 7.2–17.3 | 7.2–18.7 | 5.8–14.1 | 5.8–20.2 | 7.2–20.2 | 7.2–28.8 |
|
| ||||||
| ≥30 mg/24 hr (%) | 11.7 | 10.1 | 10.2 | 17.7|| | 19.4 | 22.6 |
|
| ||||||
| ≥300 mg/24 hr (%) | 0 | 0 | 1.4 | 3.2† | 3.2 | 7.3|| |
|
| ||||||
| Serum creatinine (mg/dl) | 0.68±0.14 | 0.68±0.14 | 0.73±0.14 | 0.72±0.18 | 0.85±0.33 | 0.89±0.59 |
|
| ||||||
| Plasma lipids (mg/dl)
| ||||||
| Total cholesterol | 177.1±32.8 | 175.7±33.6 | 180.3±30.5 | 184.0±37.3 | 175.1±36.1 | 172.2±37.4 |
|
| ||||||
| HDL cholesterol | 50.8±12.3 | 50.3±12.3 | 51.0±12.9 | 51.8±13.1 | 61.0±18.7 | 60.6±17.5 |
|
| ||||||
| LDL cholesterol | 110.3±28.7 | 109.1±29.4 | 112.5±27.1 | 114.6±31.9 | 97.4±30.1 | 94.9±30.1 |
|
| ||||||
| Triglycerides | 80.8±43.3 | 81.8±51.3 | 84.2±52.6 | 88.1±50.8† | 84.1±50.8 | 82.1±58.3† |
Plus–minus values are means ± SD. There were no significant differences between the study groups in characteristics at DCCT baseline. To convert the values for serum creatinine to micromoles per liter, multiply by 88.4. To convert the values for cholesterol to millimoles per liter, multiply by 0.02586. To convert the values for triglycerides to millimoles per liter, multiply by 0.01129. ARB denotes angiotensin II– receptor blocker, HDL high-density lipoprotein, and LDL low-density lipoprotein.
P<0.05 for the comparison between the conventional-therapy group and the intensive-therapy group, with the use of the Wilcoxon rank-sum test or the chi-square test.
The primary cohort comprised patients who had had diabetes for 1 to 5 years and had an albumin excretion rate of less than 40 mg per 24 hours and no retinopathy as assessed with the use of fundus photography.
Patients were considered to have hypertension if they had a systolic pressure of 140 mm Hg or higher or a diastolic pressure of 90 mm Hg or higher or if they were taking antihypertensive medications.
Patients were considered to have hyperlipidemia if they had an LDL cholesterol level of 130 mg per deciliter (3.4 mmol per liter) or higher or were taking lipid-lowering agents.
P<0.01 for the comparison between the conventional-therapy group and the intensive-therapy group, with the use of the Wilcoxon rank-sum test or the chi-square test.
Data on the use of medications were not collected during the DCCT. Angiotensin-converting–enzyme (ACE) inhibitors were prohibited, and other classes of antihypertensive medication were allowed only after a diagnosis of hypertension. At year 1 of the EDIC, 8.7% of the participants in the intensive-therapy group and 10.1% of those in the conventional-therapy group were taking antihypertensive agents, and 5.6% and 6.9% in the two groups, respectively, were taking ACE inhibitors.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
The glycated hemoglobin values at the end of the DCCT are time-averaged mean values throughout the DCCT; the values at year 16 of the EDIC study are time-averaged mean values throughout the EDIC study. The mean levels of glycated hemoglobin time-averaged through the combined period of the two studies were 7.8±0.9% in the participants assigned to intensive diabetes therapy in the DCCT and 8.3±1.0% in the participants assigned to conventional therapy.
The mean glycated hemoglobin level during the DCCT (1983 through 1993), time-averaged throughout the study, was 7.3% in the intensive-therapy group and 9.1% in the conventional-therapy group. During the EDIC study (including data from 1994 through April 2010), the time-averaged mean glycated hemoglobin level in participants who had been in the DCCT intensive-therapy group was similar to the level in participants who had been in the DCCT conventional-therapy group (Table 1).
At year 16 of the EDIC study (2008–2010), serum creatinine was measured in 1222 participants (84.8% of all participants who underwent randomization). At that time, the mean age of the participants was 50 years, and the mean duration of diabetes was 28 years. A total of 56.2% of the participants who had been assigned to intensive therapy in the DCCT and 59.3% of those who had been assigned to conventional therapy were taking antihypertensive medication; 53.1% and 57.0%, respectively, were taking inhibitors of the renin–angiotensin–aldosterone system (Table 1). The albumin excretion rate was 30 mg or more per 24 hours in 19.4% of the participants who had been in the DCCT intensive-therapy group and in 22.6% of those who had been in the DCCT conventional-therapy group.
IMPAIRED GFR
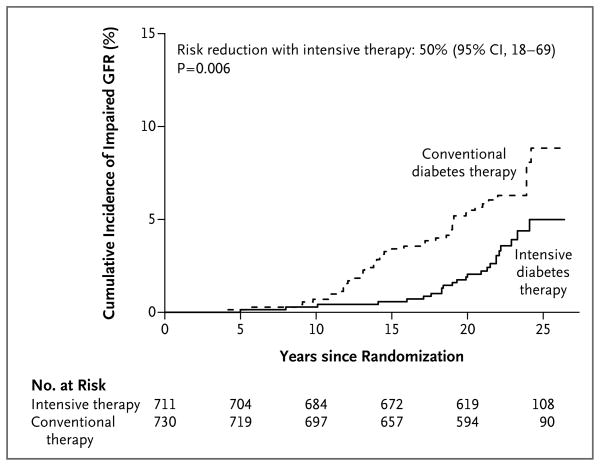
The median follow-up period for the two studies combined was 22 years (interquartile range, 21 to 24). During this time, impairment of the GFR developed in 70 participants, of whom 24 had been assigned to DCCT intensive therapy and 46 to DCCT conventional therapy. The large majority of these cases occurred during the EDIC study period (Table 2). Intensive diabetes therapy reduced the risk of an impaired GFR by 50% (95% confidence interval [CI], 18 to 69; P = 0.006) (Fig. 1). The cumulative incidence of an impaired GFR 20 years after randomization was 2.0% among participants who had been assigned to intensive therapy and 5.5% among participants who had been assigned to conventional therapy, representing an absolute difference in risk of 3.5 percentage points.
Table 2.
Incidence of an Impaired Glomerular Filtration Rate (GFR) and Secondary Outcomes.*
| Outcome | Intensive Diabetes Therapy | Conventional Diabetes Therapy | Risk Reduction with Intensive Therapy† | P Value | ||
|---|---|---|---|---|---|---|
| No. of Events | Incidence Rate/1000 Person-Yr | No. of Events | Incidence Rate/1000 Person-Yr | % (95% CI) | ||
| Impaired GFR‡ | 24 | 1.6 | 46 | 3.0 | 50 (18 to 69) | 0.006 |
|
| ||||||
| Onset during DCCT | 1 | 3 | ||||
|
| ||||||
| Onset during EDIC | 23 | 43 | ||||
|
| ||||||
| Estimated GFR <45 ml/min/1.73 m2 | 24 | 1.6 | 39 | 2.5 | 40 (1 to 64) | 0.045 |
|
| ||||||
| Estimated GFR <30 ml/min/1.73 m2§ | 13 | 0.8 | 23 | 1.5 | 44 (−9 to 72) | 0.09 |
|
| ||||||
| End-stage renal disease§ | 8 | 0.5 | 16 | 1.1 | 51 (−14 to 79) | 0.10 |
|
| ||||||
| Combined outcome of impaired GFR or death¶ | 53 | 3.4 | 80 | 5.2 | 37 (10 to 55) | 0.01 |
Separate models were created to assess the effect of intensive therapy in the DCCT on the risk of each outcome, with the use of Cox proportional-hazards models, with robust estimation of the covariance matrix according to the method of Lin and Wei16 to compute confidence limits and P values that are valid when proportional-hazards assumptions are violated; models were adjusted for the estimated GFR at baseline in the DCCT.
The reduction in risk associated with intensive diabetes therapy was calculated as (1 − hazard ratio with intensive versus conventional diabetes therapy) × 100.
An impaired GFR, defined as a sustained estimated GFR of less than 60 ml per minute per 1.73 m2 of body-surface area, was the primary study outcome.
All cases of an estimated GFR that was less than 30 ml per minute per 1.73 m2 and all cases of end-stage renal disease occurred during the course of the follow-up EDIC study. There were two participants (both assigned to conventional therapy) in whom end-stage renal disease developed without a documented sustained estimated GFR of less than 60 ml per minute per 1.73 m2; it was assumed for the purposes of the analyses that the impaired GFR developed midway between the last available measurement of the estimated GFR and the time of onset of end-stage renal disease.
This analysis included all 70 instances of renal impairment that were observed (of which 24 occurred among participants in the intensive-therapy group and 46 among participants in the conventional-therapy group), plus an additional 63 deaths that occurred among participants who were still free of renal impairment (of which 29 occurred in the intensive-therapy group and 34 in the conventional-therapy group). There were additional deaths (data not shown) that occurred after renal impairment developed in a participant; these deaths did not constitute a competing risk for renal impairment, and the difference between the groups in the rate of these deaths is not reported. The number of such deaths was insufficient to permit a reliable analysis.
Figure 1. Cumulative Incidence of an Impaired Glomerular Filtration Rate, According to Treatment Group.
An impaired glomerular filtration rate (GFR) was defined as a sustained estimated GFR of less than 60 ml per minute per 1.73 m2 of body-surface area. The cumulative incidence of an impaired GFR is shown according to the group to which the participants had been randomly assigned in the Diabetes Control and Complications Trial, with death accounted for as a competing risk. The hazard ratio and P value were calculated with the use of a Cox proportional-hazards model with a robust estimate of confidence limits according to the method of Lin and Wei16 and the robust Wald test.
The relative risk reductions for the secondary outcomes of an estimated GFR of less than 45 ml per minute per 1.73 m2, an estimated GFR of less than 30 ml per minute per 1.73 m2, and end-stage renal disease were similar in magnitude to those for the primary study outcome, but only the risk reduction for an estimated GFR of less than 45 ml per minute per 1.73 m2 was significant at the alpha level of 0.05, probably owing to the small numbers of events (Table 2). Intensive therapy reduced the risk of the composite outcome of an impaired GFR or death by 37% (95% CI, 10 to 55; P=0.01).
Higher glycated hemoglobin levels, a higher albumin excretion rate, higher blood pressure, and the use of antihypertensive medications and inhibitors of the renin–angiotensin–aldosterone system were each strongly associated with an increased risk of an impaired GFR when they were evaluated as time-dependent covariates in separate models (Table 3). The beneficial effect of intensive diabetes therapy on the risk of an impaired GFR was fully attenuated after adjustment for between-group differences in the mean glycated hemoglobin level or albumin excretion rate, each evaluated separately (Table 3). However, the effect of intensive diabetes therapy remained significant after separate adjustment for between-group differences in blood pressure, the body-mass index, the use of antihypertensive agents, or the use of inhibitors of the renin–angiotensin–aldosterone system.
Table 3.
Association of Covariates with Risk of an Impaired GFR and Effect of Intensive Diabetes Therapy after Adjustment for Covariates.*
| Covariate | Risk Associated with the Covariate | Effect of DCCT Intensive Diabetes Therapy, Adjusted for the Covariate | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI)† | P Value | Risk Reduction‡ | P Value | |
| % (95% CI) | ||||
| None | 50 (18 to 69) | 0.006 | ||
|
| ||||
| Glycated hemoglobin§ | 2.73 (2.31 to 3.23) | <0.001 | −23 (−106 to 27)¶ | 0.44 |
|
| ||||
| Albumin excretion
| ||||
| Mean rate§ | 1.11 (1.09 to 1.13) | <0.001 | 10 (−63 to 50) | 0.73 |
|
| ||||
| Sustained rate ≥30 mg/24 hr | 23.8 (13.5 to 42.2) | <0.001 | 23 (−26 to 52) | 0.30 |
|
| ||||
| Rate ≥300 mg/24 hr | 50.4 (29.0 to 87.6) | <0.001 | 10 (−53 to 47) | 0.70 |
|
| ||||
| Mean arterial blood pressure | 2.52 (2.06 to 3.08) | <0.001 | 51 (20 to 70) | 0.004 |
|
| ||||
| Body-mass index | 1.16 (0.74 to 1.82) | 0.51 | 50 (19 to 70) | 0.005 |
|
| ||||
| Use of RAAS inhibitors | 7.6 (3.8 to 15.1) | <0.001 | 44 (8 to 66) | 0.02 |
|
| ||||
| Use of antihypertensive medications | 13.9 (6.3 to 30.7) | <0.001 | 42 (5 to 65) | 0.03 |
An impaired GFR was defined as a sustained estimated GFR that was less than 60 ml per minute per 1.73 m2 of body-surface area. Each covariate was modeled separately and was updated throughout the combined course of the DCCT and EDIC studies. Separate Cox proportional-hazards models were used to evaluate the associations of each time- dependent covariate with the risk of an impaired estimated GFR to generate each covariate hazard ratio. Separate Cox proportional-hazards models were then used to evaluate the effect of DCCT treatment (intensive vs. conventional diabetes therapy) on the risk of an impaired estimated GFR, with adjustment for one time-dependent covariate at a time. The Lin–Wei robust covariance estimation method16 was used for each model, and all models were adjusted for the estimated GFR at baseline in the DCCT. RAAS denotes renin–angiotensin–aldosterone system.
The hazard ratio for covariates was evaluated according to every 10% increment in glycated hemoglobin, every 10% increment in albumin excretion rate, the presence or absence of albuminuria (a sustained rate of ≥30 mg per 24 hours or a rate of ≥300 mg per 24 hours) at the time of ascertainment, or increments of 1 SD in mean arterial blood pressure (10 mm Hg) or body-mass index (9 units).
The reduction in risk associated with intensive diabetes therapy was calculated as (1 − hazard ratio with intensive versus conventional diabetes therapy) × 100.
The glycated hemoglobin level and mean albumin excretion rate were time-weighted mean values up to each study visit in the DCCT and EDIC studies.
The risk-reduction estimate of −23% corresponds to a nonsignificant increase in risk of 23%.
RATES OF DECREASE IN GFR
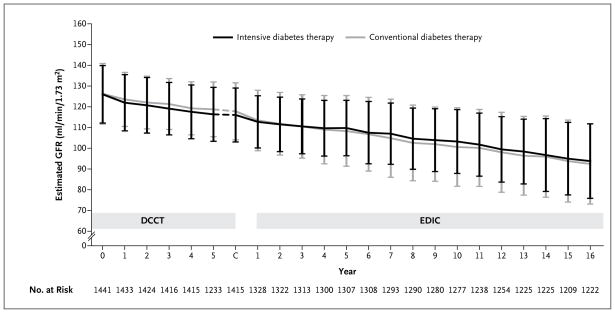
From baseline to year 1 of the DCCT, the mean estimated GFR in the intensive-therapy group fell from 126.0 to 121.8 ml per minute per 1.73 m2, which was a decrease that was greater by 1.4 ml per minute per 1.73 m2 than that in the conventional-therapy group (95% CI, 0.6 to 2.2; P<0.001) (Fig. 2, and Table S1 in the Supplementary Appendix). During the DCCT, the estimated GFR in the two treatments group declined in parallel after the first year. The overall mean estimated GFR was lower by 1.7 ml per minute per 1.73 m2 in the intensive-therapy group than in the conventional-therapy group.
Figure 2. Estimated GFR over Time.
The simple means of the estimated GFR are shown over time in the Diabetes Control and Complications Trial (DCCT) and the Epidemiology of Diabetes Interventions and Complications (EDIC) study, according to the group to which the participants had been randomly assigned (intensive diabetes therapy or conventional diabetes therapy) in the DCCT. I bars indicate interquartile ranges. C denotes the DCCT closeout visit.
From baseline in the EDIC study (i.e., the end of the DCCT) to year 1 of the EDIC study, the mean estimated GFR in the conventional-therapy group fell from 117.8 to 113.0 ml per minute per 1.73 m2, crossing below the mean estimated GFR in the intensive-therapy group for the first time. Throughout the remainder of the EDIC study, the estimated GFR in participants who had been assigned to intensive therapy in the DCCT was higher than the estimated GFR in the participants assigned to conventional therapy in the DCCT (difference, 2.5 ml per minute per 1.73 m2; 95% CI, 1.4 to 3.6; P<0.001) and declined more slowly (difference in slope, 0.23 ml per minute per 1.73 m2 per year; 95% CI, 0.05 to 0.41; P = 0.01). Over the course of the combined studies, the average decrease in the estimated GFR was 1.27 ml per minute per 1.73 m2 per year (95% CI, 1.20 to 1.35) with intensive therapy, as compared with 1.56 ml per minute per 1.73 m2 per year (95% CI, 1.48 to 1.63) with conventional therapy (P<0.001).
Iothalamate GFR measurements obtained in subsets of participants showed changes that were in the same direction as the changes in the estimated GFR, but they were of larger magnitude. From DCCT baseline to DCCT closeout, the decrease in the mean iothalamate GFR was greater by 6.2 ml per minute per 1.73 m2 in the intensive-therapy group than in the conventional-therapy group (95% CI, 2.2 to 10.2; P = 0.003) (Table S2 in the Supplementary Appendix). From EDIC baseline (DCCT closeout) to EDIC year 1 or 2, the decrease in the mean iothalamate GFR was greater by 5.4 ml per minute per 1.73 m2 in the conventional-therapy group than in the intensive-therapy group (95% CI, 2.9 to 7.9; P<0.001).
DISCUSSION
The long-term risk of an impaired GFR was lower by 50% among persons treated for an average of 6.5 years with DCCT intensive diabetes therapy than among those treated with conventional diabetes therapy. This effect was not evident until more than 10 years after randomization, beyond the period of the DCCT treatment intervention.
By design, participants were enrolled in the DCCT early in the course of type 1 diabetes, when they had mild microvascular complications or none that were apparent. Following this population provides an opportunity to test the effects of intensive diabetes therapy on the primary prevention of an impaired GFR. The absolute incidence of an impaired GFR was low. This probably reflects contemporary incidence rates of an impaired GFR in patients with type 1 diabetes, since the rates of other microvascular complications among participants assigned to DCCT conventional therapy were similar to those in a community-based population with type 1 diabetes.18 Higher incidence rates of advanced renal complications reported in earlier cohorts with type 1 diabetes suggest that the incidence of kidney disease in patients with type 1 diabetes may have decreased over time.19,20
Our data suggest that giving approximately 29 persons with type 1 diabetes intensive diabetes therapy for 6.5 years prevents one case of an impaired GFR over a total follow-up period of 20 years. The observed beneficial effect of intensive diabetes therapy on the risk of an impaired GFR reinforces the beneficial effects of such therapy on albuminuria outcomes in previous studies.4–6 Moreover, along with congruent salutary effects on retinopathy, neuropathy, and cardiovascular disease,4,21 these effects reinforce current recommendations to target a glycated hemoglobin level of less than 7% in patients with type 1 diabetes.10,11
The beneficial effects of intensive diabetes therapy on the risk of an impaired GFR were fully attenuated by statistical adjustment for the glycated hemoglobin level or albumin excretion rate. A previous study showed that the beneficial effects of intensive diabetes therapy on the risk of albuminuria were also negated after adjustment for the glycated hemoglobin level.6 Although the mechanism of the effect cannot be definitively determined, together these results suggest that hyperglycemia contributes to the pathogenesis of both albuminuria and an impaired GFR in patients with type 1 diabetes and that the biologic pathways through which intensive diabetes therapy prevents impairment of the GFR are reflected by a reduction in albuminuria. In this regard, our findings support, but cannot on their own validate, the use of albuminuria as a surrogate marker of an impairment of GFR in patients with type 1 diabetes.12
Extensive longitudinal measurements allowed us to explore the time course for the effects of intensive diabetes therapy on the estimated GFR. Because most of the study participants did not have a substantial decrease in the estimated GFR and because measurement of the estimated GFR in the normal range is imprecise, changes in the mean estimated GFR in the treatment groups were expected to be small and were not intended to gauge clinical relevance. During active treatment in the DCCT, intensive diabetes therapy, as compared with conventional therapy, reduced the estimated GFR and iothalamate GFR to levels that were still within the normal ranges. This effect may represent a mitigation of hyperglycemia-induced hyperfiltration, since acute hyperglycemia is known to increase the GFR by increasing renal plasma flow and the filtration fraction, and correction of hyperglycemia has been shown to lower the GFR in the short term.22–24 During the EDIC study, the mean estimated GFR was higher and declined less rapidly among participants who had been assigned to intensive diabetes therapy in the DCCT than among those who had been assigned to conventional therapy. These beneficial long-term effects of intensive diabetes therapy, extending beyond the period of the DCCT intervention, are reminiscent of the “metabolic memory” effect described previously with respect to reduction in albuminuria.6
Because death occurring before impairment of the GFR is a potential competing risk, the reduction in the risk of an impaired GFR with intensive therapy could have been an artifact of an increased risk of death with intensive therapy (before renal impairment). However, intensive therapy also provided a significant reduction (37%) in the risk of the combined outcome of an impaired GFR or death (whichever occurred first). Thus, the between-group difference in the risk of an impaired GFR was not influenced by the number of deaths in the study.
The strengths of the current study include the randomly assigned original DCCT treatment intervention, the long duration of follow-up, the use of multiple longitudinal measurements of estimated GFR analyzed at a central laboratory to identify sustained reductions in the estimated GFR, and the use of multiple longitudinal measurements of key biomarkers to explore covariates associated with the treatment effect.
One limitation of our study was the nonrandomized use of medications other than insulin. The use of antihypertensive medications or inhibitors of the renin–angiotensin–aldosterone system was associated with a markedly increased risk of an impaired GFR, probably because these medications were prescribed for participants who were already at high risk for impaired GFR owing to hypertension or albuminuria. However, adjustment for the use of antihypertensive medications or inhibitors of the renin–angiotensin–aldosterone system did not explain the beneficial effect of intensive diabetes therapy on the risk of an impaired GFR.
These and other findings of the combined DCCT and EDIC studies show the benefit of intensive diabetes therapy, which resulted in a mean glycated hemoglobin level of 7.3%, as compared with conventional therapy, which resulted in a mean glycated hemoglobin level of 9.1%, but do not directly assess intermediate or more extreme strategies.4–6,21 It is also not clear whether the beneficial effects of intensive diabetes therapy on the risk of an impaired GFR will be applicable to patients with type 2 diabetes. Treatment strategies targeting very low glycated hemoglobin levels may cause harm in patients with long-standing type 2 diabetes.25
Given the long time that elapsed between the treatment intervention and the observed clinical effect, persons with advanced complications of diabetes who are at imminent risk for a progressive decrease in the GFR may not derive the same benefit as did the study participants, who were treated early in their disease course.26 In addition, the use of inhibitors of the renin–angiotensin–aldosterone system was discouraged during the DCCT, and the effects of intensive diabetes therapy could vary when the intensive therapy is added to treatment with inhibitors of the renin–angiotensin–aldosterone system, a practice that has become more common since the initiation of the DCCT.27 Finally, impairment of the GFR is an intermediate outcome. Although the reduction in the relative risk of end-stage renal disease was not significant, it was of similar magnitude.
In conclusion, the long-term risk of an impaired GFR was significantly lower among persons treated early in the course of type 1 diabetes with intensive diabetes therapy than among those treated with conventional diabetes therapy. We believe that this study provides strong evidence that impairment of the GFR may be prevented in patients with type 1 diabetes and reinforces the importance of early glycemic control.
Supplementary Material
Acknowledgments
Supported by contracts with the Division of Diabetes, Endocrinology, and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Eye Institute, National Institute of Neurological Disorders and Stroke, and the General Clinical Research Centers Program and by funding from the Clinical and Translational Science Awards Program, National Center for Research Resources, and Genentech through a Cooperative Research and Development Agreement with the NIDDK. Additional support for this study came from grants (R01DK087726, R01DK088762, and RC4DK090766) from the NIDDK. Free or discounted supplies or equipment were contributed by Abbott, Animas, Aventis, Bayer, Becton Dickinson, Can Am, Eli Lilly, LifeScan, Medtronic, MiniMed, Omron, OmniPod, Roche, and Sanofi-Aventis. These companies had no role in the design of the study or in the analysis of the data.
Dr. de Boer reports that his institution, the University of Washington, received grants from Abbott Laboratories; Dr. Lachin reports receiving consulting fees from Reata Pharmaceuticals, Eli Lilly, and Novartis Pharmaceuticals; Dr. Molitch reports receiving consulting fees from Abbott Laboratories, Novo Nordisk, and CVS Caremark; Dr. Molitch reports that his institution, Northwestern University, received grants from Sanofi-Aventis and Eli Lilly; and Dr. Steffes reports receiving consulting fees from Pharma Diagnostic.
We thank the technologists for efficient and accurate completion of the assays used in this study.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
No other potential conflict of interest relevant to this article was reported.
References
- 1.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting–enzyme inhibition on diabetic nephropathy. N Engl J Med. 1993;329:1456–62. doi: 10.1056/NEJM199311113292004. [Erratum, N Engl J Med 1993;330:152.] [DOI] [PubMed] [Google Scholar]
- 2.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–60. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 3.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–9. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 4.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 5.Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. Kidney Int. 1995;47:1703–20. doi: 10.1038/ki.1995.236. Idem. [DOI] [PubMed] [Google Scholar]
- 6.Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA. 2003;290:2159–67. doi: 10.1001/jama.290.16.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molitch ME, Steffes M, Sun W, et al. Development and progression of renal insufficiency with and without albuminuria in adults with type 1 diabetes in the Diabetes Control and Complications Trial and the Epidemiology of Diabetes Interventions and Complications study. Diabetes Care. 2010;33:1536–43. doi: 10.2337/dc09-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Boer IH, Katz R, Cao JJ, et al. Cystatin C, albuminuria, and mortality among older adults with diabetes. Diabetes Care. 2009;32:1833–8. doi: 10.2337/dc09-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ninomiya T, Perkovic V, de Galan BE, et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol. 2009;20:1813–21. doi: 10.1681/ASN.2008121270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis. 2007;49(Suppl):S12–S154. doi: 10.1053/j.ajkd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 11.American Diabetes Association. Standards of medical care in diabetes — 2011. Diabetes Care. 2011;34(Suppl 1):S11–S61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levey AS, Cattran D, Friedman A, et al. Proteinuria as a surrogate outcome in CKD: report of a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis. 2009;54:205–26. doi: 10.1053/j.ajkd.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steffes M, Cleary P, Goldstein D, et al. Hemoglobin A1c measurements over nearly two decades: sustaining comparable values throughout the Diabetes Control and Complications Trial and the Epidemiology of Diabetes Interventions and Complications study. Clin Chem. 2005;51:753–8. doi: 10.1373/clinchem.2004.042143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–54. [Google Scholar]
- 16.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84:1074–8. [Google Scholar]
- 17.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 18.Nathan DM, Zinman B, Cleary PA, et al. Modern-day clinical course of type 1 diabetes mellitus after 30 years’ duration: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications and Pittsburgh Epidemiology of Diabetes Complications experience (1983–2005) Arch Intern Med. 2009;169:1307–16. doi: 10.1001/archinternmed.2009.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krolewski AS, Warram JH, Christlieb AR, Busick EJ, Kahn CR. The changing natural history of nephropathy in type I diabetes. Am J Med. 1985;78:785–94. doi: 10.1016/0002-9343(85)90284-0. [DOI] [PubMed] [Google Scholar]
- 20.Finne P, Reunanen A, Stenman S, Groop PH, Grönhagen-Riska C. Incidence of end-stage renal disease in patients with type 1 diabetes. JAMA. 2005;294:1782–7. doi: 10.1001/jama.294.14.1782. [DOI] [PubMed] [Google Scholar]
- 21.Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–53. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christiansen JS, Frandsen M, Parving HH. Effect of intravenous glucose infusion on renal function in normal man and in insulin-dependent diabetics. Diabetologia. 1981;21:368–73. doi: 10.1007/BF00252683. [DOI] [PubMed] [Google Scholar]
- 23.Wiseman MJ, Mangili R, Alberetto M, Keen H, Viberti G. Glomerular response mechanisms to glycemic changes in insulin-dependent diabetics. Kidney Int. 1987;31:1012–8. doi: 10.1038/ki.1987.100. [DOI] [PubMed] [Google Scholar]
- 24.Wiseman MJ, Saunders AJ, Keen H, Viberti G. Effect of blood glucose control on increased glomerular filtration rate and kidney size in insulin-dependent diabetes. N Engl J Med. 1985;312:617–21. doi: 10.1056/NEJM198503073121004. [DOI] [PubMed] [Google Scholar]
- 25.Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–59. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ismail-Beigi F, Moghissi E, Tiktin M, Hirsch IB, Inzucchi SE, Genuth S. Individualizing glycemic targets in type 2 diabetes mellitus: implications of recent clinical trials. Ann Intern Med. 2011;154:554–9. doi: 10.7326/0003-4819-154-8-201104190-00007. [DOI] [PubMed] [Google Scholar]
- 27.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305:2532–9. doi: 10.1001/jama.2011.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.