Abstract
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) have become recognized as second messengers for initiating and/or regulating vital cellular signaling pathways, and they are known also as deleterious mediators of cellular stress and cell death. ROS and RNS, and their cross products like peroxynitrite, react primarily with cysteine residues whose oxidative modification leads to functional alterations in the proteins. In this Forum, the collection of six review articles presents a perspective on the broad biological impact of cysteine modifications in health and disease from the molecular to the cellular and organismal levels, focusing in particular on reversible protein-S-glutathionylation and its central role in transducing redox signals as well as protecting proteins from irreversible cysteine oxidation. The Forum review articles consider the role of S-glutationylation in regulation of the peroxiredoxin enzymes, the special redox environment of the mitochondria, redox regulation pertinent to the function of the cardiovascular system, mechanisms of redox-activated apoptosis in the pulmonary system, and the role of glutathionylation in the initiation, propagation, and treatment of neurodegenerative diseases. Several common themes emerge from these reviews; notably, the probability of crosstalk between signaling/regulation mechanisms involving protein-S-nitrosylation and protein-S-glutathionylation, and the need for quantitative analysis of the relationship between specific cysteine modifications and corresponding functional changes in various cellular contexts. Antioxid. Redox Signal. 16, 471–475.
Historical Perspective
Serious consideration of reversible protein glutathionylation as a mechanism of regulation has a relatively brief history, probably less than 30 years. Nevertheless, this focused area of research has captured the imagination of a growing number of biologists, most intensively during the last 10 years. This emergence has been fostered largely by the convergence of understanding about reactive oxygen and reactive nitrogen species as second messengers in signal transduction, the importance of posttranslational modification of cysteine residues, and the special properties of the glutaredoxin (Grx) (thioltransferase) enzyme as a specific catalyst of deglutathionylation of protein–glutathione (GSH) mixed disulfides. An historical perspective on evolution of this focal area of biology can be gleaned from considering a number of key reviews that have appeared over the past 25 years. In reverse chronology, 25 years ago Ziegler presented the point of view that considered protein glutathionylation strictly in the context of thermodynamic redox equilibria coupled to the GSH/glutathione disulfide (GSSG) ratio, and he concluded that reversible glutathionylation as a regulatory phenomenon was highly unlikely (31). According to his premise he was correct, because most cysteine residues have redox potentials that would require the intracellular GSH/GSSG ratio to change from about 100:1 to 1:1 in order for 50% of the protein-SH of interest to be converted to S-glutathionylated protein (protein-SSG) (12). Thus, there must be mechanisms for activating the protein-SH or GSH thiol groups to facilitate protein-SSG formation under normal GSH/GSSG redox conditions where redox signaling occurs (see below). Certainly, under overt oxidative stress conditions where GSSG concentration is very elevated, GSSG may serve as the proximal mediator of protein-SSG formation. Thomas, a pioneer in developing tools for evaluating protein thiolation and protein-SSG formation in particular (26), reviewed the role of enzymatic reversibility of protein disulfides in the context of oxidative stress. Besides homeostatic protection and repair as defenses against oxidative stress, Mieyal and coworkers (19) posed the concept of regulation via reversible S-glutathionylation, centering on the unique specificity of Grx (thioltransferase) for catalyzing reduction of glutathionyl mixed disulfides (13, 29).
The net reaction catalyzed by the Grxs is appropriately depicted as a thiol–disulfide exchange reaction involving sequential nucleophilic displacement reactions, rather than single-electron transfer reactions that would involve radical intermediates. Accordingly, the name “transhydrogenase,” which was applied originally to the enzyme activity from rat liver (23), was replaced by the name “thioltransferase” (3), because the latter more accurately describes the nature of the reaction that is catalyzed. Besides the original characterization of the mammalian thioltransferase enzyme (3), analogous catalytic activity was discovered in bacteria and attributed to an enzyme that promoted the GSH-dependent turnover of ribonucleotide reductase in a mutant of Escherichia coli that lacked thioredoxin. This E. coli enzyme was named “glutaredoxin” (15). Subsequent to these early studies, thioltransferase and glutaredoxin enzymes have been isolated from a variety of organisms, species, and tissues, and characterized as having homology of both amino acid sequence and three-dimensional structure. Consequently, it has been concluded that thioltransferase and glutaredoxin simply represent alternative names for the same family of enzymes. Although “thioltransferase” more accurately reflects the catalytic reaction, “glutaredoxin” has emerged internationally as the more commonly used name for this family of enzymes.
Documented by studies of various protein mixed disulfides and kinetic characterization (13), the exquisite specificity of the human Grx enzyme for the glutathionyl moiety was further demonstrated by mass spectrometric analysis (29). Thus, hGrx1 was reacted with cysteine-gluthathione mixed disulfide (Cys-SSG), which represents the prototype for all protein-Cys-SSG substrates. The Grx1 distinguished between the two sulfur atoms of Cys-SSG so that glutathionyl GRx mixed disulfide intermediate (Grx-SSG) was found to be the exclusive disulfide adduct. In contrast, the analogous form of human thioredoxin reacted with Cys-SSG to give Trx-SSCys and Trx-SSG in equal amounts. Focusing on the glutathionyl specificity of Grx, which was also described for E. coli Grx (4), Cotgreave and coworkers first demonstrated the proteomic approach of exploiting the deglutathionylation specificity of Grx to identify protein-SSG adducts (18). A review by Cotgreave and Gerdes (6) addressed the linkage between sulfhydryl modulation and cell proliferation, implicating the potential regulatory role of protein glutathionylation in cancer biology.
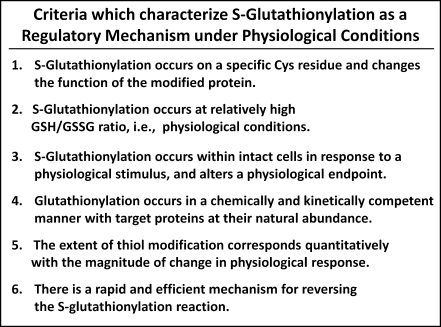
Klatt and Lamas presented a seminal review in 2000 (17), which identified protein S-glutathionylation specifically as an emerging candidate mechanism by which redox signals mediated through reactive oxygen species (ROS) and reactive nitrogen species might be transduced, introducing the concept of cross interactions among protein thiol modifications initiated by nitrosative and/or oxidative stress. Since that time there has been a proliferation of original research articles and reviews on the topic of protein-S-glutathionylation as a regulatory and/or protective mechanism, providing a variety of points of view [e.g., (8, 9, 11, 16, 25, 28)]. In particular, our previous review (24) first introduced criteria for evaluating whether reports of S-glutathionylation of specific proteins are indicative of cellular regulatory events. Figure 1 presents these criteria in their current formulation.
FIG. 1.
Criteria that characterize S-glutathionylation as a regulatory mechanism under physiological conditions.
The frequency of reviews on the topic of protein S-glutathionylation has been increasing remarkably, coincident with the burgeoning interest in redox-activated signal transduction as a mechanism of cellular regulation that can be perturbed by the oxidative/nitrosative stress associated with many diseases. A survey of PubMed in 2011 revealed cumulatively more than 1200 articles have been published on Grx and/or thioltransferase; more than 500 articles have been published on glutathionylation or glutathiolation; and more than 120 articles have been published on Grx (or thioltransferase) and glutathionylation (or glutathiolation). Thus, awareness of the pivotal role of Grx in regulating reversible glutathionylation has expanded. However, future studies of cellular redox regulation via S-glutathionylation are well advised to include examination of the role of Grx-mediated deglutathionylation in determining the steady-state protein-SSG status of specific proteins. Moreover, much more attention needs to be focused on potential enzymatic mechanisms of formation of protein-SSG, possibly involving glutathione-S-transferase or peroxidase enzymes (9, 14, 27).
Clarification of Nomenclature
More than two-thirds of published articles have referred to protein-Cys-S-S-glutathione mixed disulfide formation as “glutathionylation,” but less than one-third have used the term “glutathiolation.” “Glutathionylation” is the preferred term for several reasons, and it is used uniformly in this collection of reviews. “Thiolation” of proteins may be used as an inclusive term to describe protein mixed disulfide formation when the nature of the adducted thiol compound is unknown, referring broadly to attachment of cysteine (cysteinylation), cysteamine (cysteaminylation), GSH (glutathionylation), etc. Some authors may have chosen to use “glutathiolation” as a parallel construction. However, the name of the tripeptide moiety that is attached is “glutathione” and not “glutathiol,” so “glutathionylation” is the appropriate specific term. Some authors may have used “glutathiolation” to refer to a nonenzymatic reaction. However, the standard nomenclature for the attachment of a moiety to a protein does not differ whether the reaction is a spontaneous chemical reaction or an enzyme-mediated reaction. For example, chemical adduction of an acetyl group via acetic anhydride and enzymatic adduction of an acetyl group via acetyl-CoA both lead to “acetylation” of the protein, not “acetation.”
Limited Methodology for Characterization of Protein-SSG
As awareness of protein-S-glutathionylation as an important regulatory mechanism has grown, so have technological developments for its detection and characterization. However, future advances are necessary to make quantitative analysis of low abundant protein-SSG in physiological settings a routine research endeavor. Because specific glutathionylation amino acid sequence motifs have not been identified as yet, the proprietary anti-GSH antibodies are directed against the GS-moiety bound to an unknown antigenic protein at an unknown site; hence, false negatives as well as false positives (not reversible by DTT and/or Grx) may occur in western blot analyses of cell lysates or immunocytochemistry applications. The use of biotinylated glutathione ethyl ester and avidin beads to facilitate the capture of glutathionylated proteins is useful, but it may provide inaccurate estimates of the steady-state levels of the protein-SSGs. Modification of the glutathionyl moiety by the bulky biotin molecule likely interferes with the efficiency of deglutathionylationation by Grx, and it may also interfere with formation of protein-SSG if the reaction is enzymatically catalyzed. Use of radiolabeled precursors (cystine and methionine) to generate radiolabeled GSH within cells suffers from the necessity to inhibit protein synthesis and thereby perturb natural cellular homeostasis. The most promising approaches for detecting physiologically relevant specific protein-SSGs in situ in tissues or cells, or in cell lysates, involve trapping free thiols with alkylating agent (e.g., N-ethyl maleimide), followed treatment with Grx to specifically remove the glutathionyl moiety and expose the corresponding free thiols for modification by thiol-specific fluorescent labels or biotinylated thiol reagents to facilitate detection. Once detected, proteolytic digestion and mass spectrometric analysis is necessary to identify the specific proteins that are glutathionylated and the specific Cys-containing sequences where the glutathionylation occurs. Identifying specific glutathionylation sites on specific proteins will open the door for more effective in situ and quantitative analysis of the glutathionylation status of specific proteins. Thus, antibodies directed against specific glutathionylated peptides of key regulatory proteins would facilitate in situ analysis of specific signaling intermediates, and specific glutathionylated peptides could be used as internal standards for quantitative mass spectrometric analyses of changes in protein-SSG under different physiological or pathophysiological conditions. For other perspectives and more critical discussion of current methods, see this Forum (21, 30), as well as previous reviews (1, 7, 10, 22, 25).
In the following sections brief synopses are provided of the review articles that comprise this Forum.
S-glutathionylation in regulation of the oligomeric state and functions of peroxiredoxins
This review is focused on the family of enzymes called peroxiredoxins that perfom a versatile array of functions vital to cell regulation—namely, they serve as modulators of redox signal transduction by limiting and localizing steady-state levels of H2O2; they serve as antioxidants by scavenging excess H2O2; and they serve as molecular chaperones to stabilize protein structure [(5), this Forum]. Remarkably posttranslational modification by S-glutathionylation of particular Cys residues can regulate the functions of different isoforms of Prx in different ways. Thus, glutathionylation at the active site of certain Prxs is integral to the catalytic mechanism of their peroxidase activity and also provides protection from irreversible hyperoxidation. Alternatively, glutathionylation of typical 2-Cys Prx (e.g., Prx1) away from its active site modifies its quarternary structure, converting the decameric Prx to its dimers with concomitant loss of chaperone activity. Oligomeric Prx serves as a docking station that binds and modulates the activities of other effector proteins like PTEN which regulates cell cycle and mammalian MST1 kinase that activates apoptosis. Hence, the effects of glutathionylation of Prx may be transmitted to regulation of vital cellular signaling pathways.
S-glutathionylation in regulation of apoptosis
Continuing the theme of regulation of cellular signaling by reversible S-glutathionylation of specific effector proteins, this review article [(2), this Forum] focuses on mechanisms of control of apoptosis. Remarkable information has emerged from studies of apoptosis in various contexts over the recent past that implicates relative levels of GSH, modulation of the content and/or activity of Grx, and S-glutathionylation of cell death mediators at various stages of the apoptosis cascade as contributors to regulation of apoptosis. Cell membrane receptors (e.g., Fas receptor) and multiprotein complexes that initiate apoptosis are subject to regulation by reversible glutathionylation, as are signaling kinases (e.g., IκB kinase) and apoptosis execution factors (e.g., caspase-3). A picture emerges of competing mechanisms that may serve as checks and balances for commitment to apoptosis versus survival, or represent different mechanisms for different cell types under different circumstances. It is clear that S-glutathionylation of specific proteins likely plays an important role in regulation of cell fate at various control points; however, much remains to be learned about the relative contributions of the different mechanisms in different cellular and redox contexts.
S-glutathionylation in mitochondrial sulfhydryl homeostasis and redox regulation
In this review article [(20), this Forum], an insightful perspective is conveyed about the continuity from redox signal transduction to antioxidant defense within the mitochondrial matrix where production of ROS and related reactive species occurs continuously. S-glutathionylation is considered along with other forms of cysteine modification, and cogent kinetic and thermodynamic arguments are presented to distinguish between possible and probable antioxidant or signaling mechanisms in vivo. Integrated networks of homeostatic thiol-disulfide enzymes are discussed in terms of their signaling and/or protective roles, namely, the thioredoxin/peroxiredoxin/methionine sulfoxide reductase systems and the GSH/glutathione peroxidase/glutathione-S-transferase/Grx systems. In conjunction with reversible modifications of protein thiols, these enzyme systems prevent much of the potential oxidative damage and likely serve to relay redox signals throughout the mitochondrial matrix, regulating the activity of biochemical processes.
S-glutathionylation in homeostatic sulfhydryl regulation and pathogenesis of the cardiovascular system
In this review article [(20), this Forum] the authors consider regulation by reversible glutathionylation as an integral mechanism whereby reactive oxygen and nitrogen species contribute to homeostatic regulation of the cardiovascular system and to pathogenesis of various cardiovascular diseases, including atherosclerosis, hypertension, endothelial dysfunction, and cardiac hypertrophy. Various reports are discussed that illustrate how reversible oxidative modifications of cysteine residues of specific proteins (e.g., Ras, ryanodine receptor, smooth endoplasmic reticulum calcium ATPase, endothelial nitric oxide synthase, and Na+K+-ATPase) may modulate cardiovascular function and physiology; however, there is often a challenge in distinguishing redox signaling events from the consequences of oxidative stress. In common with several of the other reviews, the potentially controversial issue of S-nitrosylation as a precursor of S-glutathionylation is considered in light of recent evidence.
S-glutathionylation in neurodegenerative diseases
In this review article [(24), this Forum] the authors consider the role of oxidative modifications of proteins, notably S-glutathionylation, and alterations in thiol homeostatic enzyme activities in the initiation and/or progression of the major neurodegenerative diseases, including Alzheimer's, Friedreich's, Gehrig's, Huntington's, and Parkinson's. As conveyed also in other reviews in this Forum, controlled reversible S-glutathionylation is viewed as playing two essential roles in maintaining cell health—namely, participation in redox-activated signal transduction and protection against irreversible protein damage from oxidative stress. Other post-translational modifications of cysteine residues (S-nitrosylation and sulfenic acid formation) are also considered as potential intermediates in redox signaling or protein damage. The review highlights how disruption of normal thiol-disulfide status and S-glutathionylation of proteins or networks of proteins could contribute to the onset or progression of neurodegenerative diseases, using specific examples like the apoptosis signal regulating kinase 1 signaling pathway in neuronal cells to illustrate the concept and consider potential therapeutic interventions.
S-glutathionylation in oxidative stress and redox regulation in photosynthetic organisms
In this review [(30), this Forum] the authors highlight the emerging understanding that protein S-glutathionylation in photosynthetic organisms likely represents an important alternative redox regulation/homeostasis mechanism, controlled by the Grxs, which complements the well-established disulfide/dithiol exchange reactions specifically controlled by the thioredoxins. The review provides a global overview of protein glutathionylation, and considers the role of the numerous Grxs in photosynthetic organisms as potential regulators of cellular functions besides providing protection against irreversible protein damage from oxidative stress.
Abbreviations Used
- Cys-SSG
cysteine gluthathione mixed disulfide
- DTT
dithiothreitol
- Grx
glutaredoxin
- Grx-SSG
glutathionyl GRx mixed disulfide intermediate
- GSH
glutathione
- GSSG
glutathione disulfide
- Protein-SSG
S-glutathionylated protein
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
Acknowledgments
This effort was supported in part by NIH Grant PO1 AG 15885 (J.J.M.), a Department of Veterans Affairs Merit Review Grant (J.J.M.), and by the Intramural Research Program of NHLBI, NIH (P.B.C.).
References
- 1.Aesif SW. Janssen-Heininger YM. Reynaert NL. Protocols for the detection of s-glutathionylated and s-nitrosylated proteins in situ. Methods Enzymol. 2010;474:289–296. doi: 10.1016/S0076-6879(10)74017-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anathy V. Roberson EC. Guala AS. Godburn KE. Budd RC. Janssen-Heininger Y. Redox-based regulation of apoptosis: S-glutathionylation as a regulatory mechanism to control cell death. Antioxid Redox Signal. 2012;16:496–505. doi: 10.1089/ars.2011.4281. This issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Askelof P. Axelsson K. Eriksson S. Mannervik B. Mechanism of action of enzymes catalyzing thiol-disulfide interchange. Thioltransferases rather than transhydrogenases. FEBS Lett. 1974;38:263–267. doi: 10.1016/0014-5793(74)80068-2. [DOI] [PubMed] [Google Scholar]
- 4.Bushweller JH. Aslund F. Wuthrich K. Holmgren A. Structural and functional characterization of the mutant Escherichia coli glutaredoxin (C14——S) and its mixed disulfide with glutathione. Biochemistry. 1992;31:9288–9293. doi: 10.1021/bi00153a023. [DOI] [PubMed] [Google Scholar]
- 5.Chae HZ. Oubrahim H. Park JW. Rhee SG. Chock PB. Protein glutathionylation in the regulation of peroxiredoxins: A family of thiol-specific peroxidases function as antioxidants, molecular chaperones and signal modulators. Antioxid Redox Signal. 2012;16:506–523. doi: 10.1089/ars.2011.4260. This issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cotgreave IA. Gerdes RG. Recent trends in glutathione biochemistry—glutathione-protein interactions: a molecular link between oxidative stress and cell proliferation? Biochem Biophys Res Commun. 1998;242:1–9. doi: 10.1006/bbrc.1997.7812. [DOI] [PubMed] [Google Scholar]
- 7.Cuddihy SL. Baty JW. Brown KK. Winterbourn CC. Hampton MB. Proteomic detection of oxidized and reduced thiol proteins in cultured cells. Methods Mol Biol. 2009;519:363–375. doi: 10.1007/978-1-59745-281-6_23. [DOI] [PubMed] [Google Scholar]
- 8.Dalle-Donne I. Rossi R. Colombo G. Giustarini D. Milzani A. Protein S-glutathionylation: a regulatory device from bacteria to humans. Trends Biochem Sci. 2009;34:85–96. doi: 10.1016/j.tibs.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Gallogly MM. Mieyal JJ. Mechanisms of reversible protein glutathionylation in redox signaling and oxidative stress. Curr Opin Pharmacol. 2007;7:381–391. doi: 10.1016/j.coph.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Gao XH. Bedhomme M. Veyel D. Zaffagnini M. Lemaire SD. Methods for analysis of protein glutathionylation and their application to photosynthetic organisms. Mol Plant. 2009;2:218–235. doi: 10.1093/mp/ssn072. [DOI] [PubMed] [Google Scholar]
- 11.Ghezzi P. Di SP. Glutathionylation pathways in drug response. Curr Opin Pharmacol. 2007;7:398–403. doi: 10.1016/j.coph.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert HF. Molecular and cellular aspects of thiol-disulfide exchange. Adv Enzymol Relat Areas Mol Biol. 1990;63:69–172. doi: 10.1002/9780470123096.ch2. [DOI] [PubMed] [Google Scholar]
- 13.Gravina SA. Mieyal JJ. Thioltransferase is a specific glutathionyl mixed disulfide oxidoreductase. Biochemistry. 1993;32:3368–3376. doi: 10.1021/bi00064a021. [DOI] [PubMed] [Google Scholar]
- 14.Gutscher M. Sobotta MC. Wabnitz GH. Ballikaya S. Meyer AJ. Samstag Y. Dick TP. Proximity-based protein thiol oxidation by H2O2-scavenging peroxidases. J Biol Chem. 2009;284:31532–31540. doi: 10.1074/jbc.M109.059246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmgren A. Hydrogen donor system for Escherichia coli ribonucleoside-diphosphate reductase dependent upon glutathione. Proc Natl Acad Sci U S A. 1976;73:2275–2279. doi: 10.1073/pnas.73.7.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hurd TR. Costa NJ. Dahm CC. Beer SM. Brown SE. Filipovska A. Murphy MP. Glutathionylation of mitochondrial proteins. Antioxid Redox Signal. 2005;7:999–1010. doi: 10.1089/ars.2005.7.999. [DOI] [PubMed] [Google Scholar]
- 17.Klatt P. Lamas S. Regulation of protein function by S-glutathiolation in response to oxidative and nitrosative stress. Eur J Biochem. 2000;267:4928–4944. doi: 10.1046/j.1432-1327.2000.01601.x. [DOI] [PubMed] [Google Scholar]
- 18.Lind C. Gerdes R. Hamnell Y. Schuppe-Koistinen I. von Lowenhielm HB. Holmgren A. Cotgreave IA. Identification of S-glutathionylated cellular proteins during oxidative stress and constitutive metabolism by affinity purification and proteomic analysis. Arch Biochem Biophys. 2002;406:229–240. doi: 10.1016/s0003-9861(02)00468-x. [DOI] [PubMed] [Google Scholar]
- 19.Mieyal JJ. Srinivasan U. Starke DW. Gravina SA. Mieyal PA. Glutathionyl specificity of the thioltransferases: mechanistic and physiological implications. In: Packer L, editor; Cadenas E., editor. Biothiols in Health and Disease. New York: Marcel Dekker, Inc.; 1995. pp. 305–372. [Google Scholar]
- 20.Murphy M. Mitochondrial thiols in antioxidant protection and redox signalling: distinct roles for glutathionylation and other thiol modifications. Antioxid Redox Signal. 2012;16:476–495. doi: 10.1089/ars.2011.4289. This issue. [DOI] [PubMed] [Google Scholar]
- 21.Pimentel D. Haeussler DJ. Matsui R. Burgoyne J. Cohen RA. Bachschmid M. Regulation of cell physiology and pathology by protein S-glutathionylation. Lessons learned from the cardiovascular system. Antioxid Redox Signal. 2012;16:524–542. doi: 10.1089/ars.2011.4336. This issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Priora R. Coppo L. Salzano S. Di SP. Ghezzi P. Measurement of mixed disulfides including glutathionylated proteins. Methods Enzymol. 2010;473:149–159. doi: 10.1016/S0076-6879(10)73007-X. [DOI] [PubMed] [Google Scholar]
- 23.Racker E. Glutathione-homocystine transhydrogenase. J Biol Chem. 1955;217:867–874. [PubMed] [Google Scholar]
- 24.Sabens EA. Gao XH. Mieyal JJ. Mechanisms of altered redox regulation in neurodegenerative diseases—focus on S-glutathionylation. Antioxid Redox Signal. 2012;16:543–566. doi: 10.1089/ars.2011.4119. This issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shelton MD. Chock PB. Mieyal JJ. Glutaredoxin: role in reversible protein s-glutathionylation and regulation of redox signal transduction and protein translocation. Antioxid Redox Signal. 2005;7:348–366. doi: 10.1089/ars.2005.7.348. [DOI] [PubMed] [Google Scholar]
- 26.Thomas JA. Poland B. Honzatko R. Protein sulfhydryls and their role in the antioxidant function of protein S-thiolation. Arch Biochem Biophys. 1995;319:1–9. doi: 10.1006/abbi.1995.1261. [DOI] [PubMed] [Google Scholar]
- 27.Townsend DM. Manevich Y. He L. Hutchens S. Pazoles CJ. Tew KD. Novel role for glutathione S-transferase pi. Regulator of protein S-Glutathionylation following oxidative and nitrosative stress. J Biol Chem. 2009;284:436–445. doi: 10.1074/jbc.M805586200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiong Y. Uys JD. Tew KD. Townsend DM. S-glutathionylation: from molecular mechanisms to health outcomes. Antioxid Redox Signal. 2011;15:233–270. doi: 10.1089/ars.2010.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Y. Jao S. Nanduri S. Starke DW. Mieyal JJ. Qin J. Reactivity of the human thioltransferase (glutaredoxin) C7S, C25S, C78S, C82S mutant and NMR solution structure of its glutathionyl mixed disulfide intermediate reflect catalytic specificity. Biochemistry. 1998;37:17145–17156. doi: 10.1021/bi9806504. [DOI] [PubMed] [Google Scholar]
- 30.Zaffagnini M. Bedhomme M. Marchand CH. Morisse S. Trost P. Lemaire SD. Redox Regulation in Photosynthetic Organisms: Focus on Glutathionylation. Antioxid Redox Signal. 2012;16:567–586. doi: 10.1089/ars.2011.4255. This issue. [DOI] [PubMed] [Google Scholar]
- 31.Ziegler DM. Role of reversible oxidation-reduction of enzyme thiols-disulfides in metabolic regulation. Annu Rev Biochem. 1985;54:305–329. doi: 10.1146/annurev.bi.54.070185.001513. [DOI] [PubMed] [Google Scholar]