Abstract
Significance: Neurodegenerative diseases are characterized by progressive loss of neurons. A common feature is oxidative stress, which arises when reactive oxygen species (ROS) and/or reactive nitrogen species (RNS) exceed amounts required for normal redox signaling. An imbalance in ROS/RNS alters functionality of cysteines and perturbs thiol–disulfide homeostasis. Many cysteine modifications may occur, but reversible protein mixed disulfides with glutathione (GSH) likely represents the common steady-state derivative due to cellular abundance of GSH and ready conversion of cysteine-sulfenic acid and S-nitrosocysteine precursors to S-glutathionylcysteine disulfides. Thus, S-glutathionylation acts in redox signal transduction and serves as a protective mechanism against irreversible cysteine oxidation. Reversal of protein-S-glutathionylation is catalyzed specifically by glutaredoxin which thereby plays a critical role in cellular regulation. This review highlights the role of oxidative modification of proteins, notably S-glutathionylation, and alterations in thiol homeostatic enzyme activities in neurodegenerative diseases, providing insights for therapeutic intervention. Recent Advances: Recent studies show that dysregulation of redox signaling and sulfhydryl homeostasis likely contributes to onset/progression of neurodegeneration. Oxidative stress alters the thiol–disulfide status of key proteins that regulate the balance between cell survival and cell death. Critical Issues: Much of the current information about redox modification of key enzymes and signaling intermediates has been gleaned from studies focused on oxidative stress situations other than the neurodegenerative diseases. Future Directions: The findings in other contexts are expected to apply to understanding neurodegenerative mechanisms. Identification of selectively glutathionylated proteins in a quantitative fashion will provide new insights about neuropathological consequences of this oxidative protein modification. Antioxid. Redox Signal. 16, 543–566.
-
IV. Inflammation, Oxidative Stress, and Neurodegenerative Diseases
-
VIII. S-Glutathionylation of Proteins Involved with Mitochondrial Respiration
IX. Potential Approaches to Therapy of the Neurodegenerative Diseases
I. Introduction
Controlled reversible S-glutathionylation of protein cysteine residues regulated by the glutaredoxin enzyme plays two essential roles in maintaining cell health. First, S-glutathionylation participates in redox-activated signal transduction; second, it protects against irreversible protein damage from oxidative stress. Other post-translational modifications of cysteine residues (S-nitrosylation and sulfenic acid formation) are also potential intermediates in redox signaling or protein damage. Dysregulation of redox signaling and sulfhydryl homeostasis are important considerations in the context of neurodegenerative diseases. Uncontrolled oxidative stress can alter the thiol–disulfide status of key protein intermediates that regulate the balance between cell survival and cell death.
This review highlights how disruption of normal thiol–disulfide status and S-glutathionylation of proteins or networks of proteins could contribute to the onset or progression of neurodegenerative diseases. Brief descriptions of the prominent neurodegenerative diseases and oxidative posttranslational modifications that may participate in neurodegenerative diseases are considered.
II. Neurodegenerative Diseases
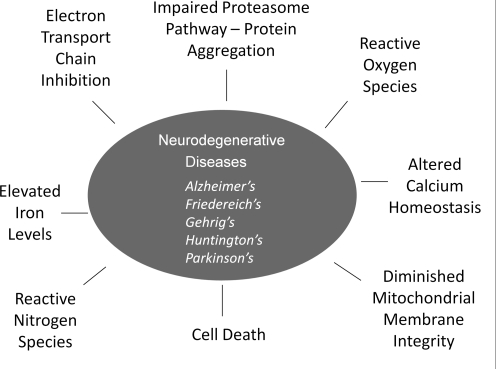
Neurodegenerative diseases comprise an increasing proportion of the debilitating illnesses that confront the growing population of elderly people worldwide, and they present an ever increasing economic burden due to the need for extensive long-term health care. Multiple types of neurodegeneration occur (Fig. 1); however, all are characterized by progressive loss of neurons. The classical diseases discussed in this review include Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease (HD), amyotrophic lateral sclerosis (ALS, also known as Lou Gehrig's disease), and Friedreich's ataxia (FRDA). These diseases manifest a number of complications, including impaired cognition, motor function, and dementia. Protein misfolding, excitotoxicity, activation of cell death pathways, mitochondrial dysfunction, increased iron deposition, and oxidative stress through the overexposure to reactive nitrogen species (RNS) and reactive oxygen species (ROS) have all been implicated in neuronal cell death (23, 128).
FIG. 1.
Schematic summary of the neurodegenerative diseases and their common molecular features. The five common neurodegenerative diseases are listed along with their common feature of neuronal cell death that is mediated by oxidative and nitrosative stress conditions and disruption of vital functions, including mitochondrial respiration and proteasomal degradation of altered proteins.
Although some genetic mutations have been implicated in neurodegenerative diseases, the major risk factor, particularly for AD and PD, is advanced age (106). Aging is marked by many changes, including telomere shortening and telomerase dysfunction, increases in mitochondrial DNA mutations, poor mitochondrial membrane integrity, impaired electron transport with diminished levels of ATP, as well as dysregulated cytosolic calcium (128). Together, these disturbances lead to an environment of elevated oxidative stress that strains the existing cellular antioxidants. The brain is particularly vulnerable to oxidative stress due to high energy needs (high oxygen consumption), paucity of antioxidants compared to other organs, and large lipid content (57). ROS molecules are generated from mitochondrial dysfunction, which is prevalent in these diseases (106). Increased oxidative damage to biological macromolecules such as proteins, lipids, and nucleic acids in neurodegenerative tissue is a common neuropathological feature, and is often accompanied by dysregulation of antioxidant scavenging systems (nonenzymatic and enzymatic). Brain tissue from PD patients has shown depletion of GSH in substantia nigra neurons (113, 142, 167) due to oxidation to glutathione disulfide (GSSG), formation and extrusion of conjugates of glutathione with electrophilic byproducts of lipid oxidation, and S-glutathionylation of proteins. However, inhibition of GSH synthesis was not found.
The autoimmune disease multiple sclerosis (MS) primarily involves deterioration of myelin in the spinal cord and brain, leading to interruption of neural transmission and eventual neuron degeneration. Although often included among the neurodegenerative diseases, MS occurs via a primary inflammatory insult, distinguishing it from the other common neurodegenerative diseases where inflammation is typically viewed as a secondary insult that exacerbates the problem rather than initiating it. In MS, focal demyelinated lesions are created from activated microglia as well as systemic infiltrates of activated macrophages. These activated immune modulating cells are the major source of ROS in this disease (183). Thus, as with the other neurodegenerative disease, the roles of ROS and RNS are important in the progression of MS (70). Recent emphasis has been focused on nitric oxide as a mediator of cysteine-based protein modifications in MS and other neurodegenerative diseases, as reviewed recently (26, 176); however, protein S-nitrosylation may serve as a precursor to protein S-glutathionylation, as discussed below.
It is remarkable that clinical symptoms for each of the neurodegenerative diseases occur as a result of loss of neurons from specific locations in the brain at different rates and with different cell types dependent on disease type. For example, Alzheimer's disease involves memory loss caused by loss of pyramidal neurons and β-amyloid deposition in the frontal, temporal, and parietal cortices. Parkinson's disease affects mainly dopaminergic neurons in the ventral substania nigra pars compacta and not dorsal dopaminergic neurons in the same region. Selective loss of neurons is thought to be due to a number of factors associated with the brain region most affected by the disease. Differences in protein expression, metal storage, receptor expression, neurotrophin factors, and antioxidant systems may all contribute to the selective loss. In order to address these challenging questions in the case of PD, microarray analysis and RTPCR of the substantia nigra has been done, comparing the dorsal and ventral regions. Changes seen in the ventral region that could account for greater susceptibility to death include diminished expression of antioxidant genes and increased presence of dopamine transporters (VMAT2 and DAT) that allow for reuptake of dopamine and increased exposure time to dopamine-derived oxidants formed within the cell. In addition, changes in other protein levels could also contribute to selective neuron loss, including increased transferrin in the ventral SNpc, but not the dorsal, resulting in increased exposure to iron (49). Taken together, it appears that localized differences in expression of particular proteins may result in increased oxidative stress in those neurons, likely accounting for locally selective neurodegeneration.
Each of the typical neurodegenerative diseases is described briefly below, and specific proteins are described whose oxidative modification, including S-glutathionylation, may contribute to the onset or progression of disease. Proteins which are glutathionylated are also listed according to the different neurodegenerative diseases (Table 1).
Table 1.
S-Glutathionylation Targets Implicated in Neurodegenerative Diseases
| Disease/Symptom | Protein | Organism/Material | Function | Effect of S-glutathionylation | Detection method | Citation |
|---|---|---|---|---|---|---|
| Parkinson's disease | Mitochondrial NADP+-dependent isocitrate dehydrogenase | MPTP-treated mice model | An enzyme converts isocitrate to ketoglutarate and produces NADPH | Inhibition of catalytic activity | Anti-GSH Ab | (94) |
| E1 ubiquitin enzyme | PC12 cells, RPE cells | Conjugates ubiquitin tag to targeted protein directing it to the proteasome | Inhibits E1 ligase activity and interrupts the ubiquitin-proteasome degradation pathway | Radiolabel [35S] Cysteine | (84; 86) | |
| Alzheimer's disease | p53 | AD patients | A pro-apoptotic protein responding to DNA damage, cell arrest and activation of apoptosis | Regulates protein oligomerization | Anit-GSH Ab | (47) |
| GAPDH | AD patients | A glycolytic enzyme to oxidize Glyceraldehyde-3-phosphate | Inhibition of catalytic activity | Anti-GSH Ab | (126) | |
| Tau protein | Neuronal tau proteins stabilize microtubules | Altered polymerization of 3-repeat tau | Mass spectrometry | (46) | ||
| α-Ketoglutarate dehydrogenase | HEK293 cells | An enzyme involved in the TCA cycle | Inhibition of catalytic activity | Anti-GSH Ab |
(164) | |
| Friedreich's ataxia | Actin | Fibroblasts from patients | An major protein of cytoskeleton maintains the infrastructure of the cytoplasmic matrix | Disarranges the polymerization of actin filaments and cytoskeletal reorganization | Anti-GSH Ab |
(140) |
| Neuroinflammation | Adenine Nucleotide Translocase (ANT) | Astrocytes | Maintains mitochondrial permeability and catalyzes ATP to ADP conversion | Decreases mitochondrial permeability and increases ATP/ADP translocation | Anti-GSH Ab |
(149) |
A. Alzheimer's disease
Alzheimer's disease is the major neurodegenerative disease affecting the aging adult population. Approximately 9% of adults over the age of 85 have AD (27). With increasing life expectancy, AD is quickly becoming a major public health issue. AD is characterized by progressive memory loss, changes in personality, and loss of cognition due to neuronal death within the limbic system and associated areas. Along with neuronal death, senile plaques, formed mainly from the protein amyloid beta (Aβ), and neurofibrillary tangles, formed from the protein tau, accumulate. Oxidative stress is an early event associated with AD (112), attributed to excess heavy metal, impaired respiration, and accumulation of Aβ. Aβ has also been associated with mitochondrial impairment, elevated calcium levels, and disruption of membranes. These actions by Aβ further exacerbate the oxidative stress on the neuron (181).
B. Parkinson's disease
Parkinson's disease (PD), the second most common neurodegenerative disease, affects approximately 1% of the population over the age of 65, and 5% over the age of 85. PD patients are characterized by bradykinesia, resting tremor, rigidity, and postural instability, due to loss of catecholaminergic neurons in the substantia nigra (152). Both genetic predisposition and environmental exposures (e.g., pesticides) have been implicated in the etiology of PD but most cases are of unknown origin. Increased oxidative stress, mitochondrial dysfunction, and protein aggregation are common features of PD (109). Oxidative stress has been attributed to increased dopamine turnover, deficient content of reduced glutathione (GSH), and increased iron in the substantia nigra (133). Importantly, GSH loss is thought to be one of the early changes associated with onset of PD (59).
Mutations in a number of genes have been identified with rare familial forms of PD, including those for PINK1, parkin, α-synuclein, UCH-L1, DJ-1, and LRRK2 (140). These proteins participate in regulation of cellular signaling pathways involving respiration, transport, mitochondrial fission-fusion, calcium homeostasis, ROS production, autophagy and apoptosis (40, 195, 196). For example, PINK1 kinase and phospho-parkin are mitochondria-associated proteins that appear to be anti-apoptotic factors, serving to maintain mitochondrial integrity; α-synuclein, parkin, and UCH-L1 are all involved in ubiquitin-dependent proteolysis.
Although mutations in these proteins are the basis for rare inherited PD, post-translational oxidative modifications of the proteins may contribute more broadly to sporadic Parkinson's disease where oxidative stress appears to be the common factor. For example, the rate of aggregation of α-synuclein is accelerated by GSSG (101); and neuronal death associated with α-synuclein expression in Drosophila can be overcome by interventions that increase glutathione (GSH) synthesis (130). Parkin is an E3 ubiquitin ligase whose active site cysteine residue is subject to oxidative modification. DJ-1 provides neuroprotection by serving as a redox sensor and anti-apoptotic agent under reducing conditions. The PINK1 protein kinase, like many other protein kinases (e.g., PKA, PKC, MAPK) appears susceptible to deactivation by cysteine modification (161). The LRRK2 protein contains both Ras GTPase-like and kinase (MAPKKK) domains (39). LRRK2 analogs (e.g., hRas and ASK1) are known to be altered functionally under oxidative stress conditions (118), implying LRRK2 is also redox sensitive.
C. Huntington's disease
Huntington's disease (HD) is characterized by a progressive dyskinesia coupled with cognitive and psychiatric impairment. HD is associated with a genetic mutation caused by polyglutamate repeats in the huntingtin protein, leading to progressive brain atrophy which is regionally accentuated in the striatum and cerebral cortex (100). It is thought that mutant huntingtin protein inhibits mitochondrial oxidative phosphorylation mainly through impairment of Complex II and Complex III and results in increased ROS production and diminished ATP levels (141). Furthermore, diminished mitochondrial membrane integrity within the neurons (111), and elevated cytosolic Ca2+ (128) have also been identified as contributing to increased oxidative challenge.
D. Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS; Lou Gehrig's disease) is associated with progressive loss of motor neurons within the ventral horn of the spinal cord, brainstem, and motor cortex. Loss of these neurons results in progressive skeletal muscle weakness, muscle atrophy, paralysis, and ultimately death within 2–5 years of onset. Approximately 20% of patients have a mutation in the superoxide dismutase 1 (SOD1) gene which implies decreased defense against superoxide-induced oxidative stress; however, the majority of patients have normal SOD1 activity. Other cellular characteristics of ALS include a loss of mitochondrial membrane potential and increased cytosolic calcium (128). These combined characteristics, along with alterations in protein folding and degradation (125), are interpreted to lead to excessive oxidative stress and cell death via impairment of autophagy and commitment to apoptosis. Although potential mediators of cell death have been identified in models of ALS (194), specific mechanisms of cell death have not been fully delineated. However, a role for reversible S-glutathionylation in ALS is implicated by model studies in which overexpression of Grx1 was found to increase the solubility of mutant SOD1 in the cytosol and overexpression of Grx2 increased its solubility in mitochondria and protected neuronal cells from apoptosis (54). These observations are consistent with the knowledge that in some forms of ALS, SOD-1 has been shown to form protein aggregates in the intermembrane space of mitochondria, leading to mitochondrial swelling, increase in ROS formation and dysfunction (90).
E. Friedreich's ataxia
FRDA, the most common recessive ataxia, is due to a trinucleotide extension (GAA) in the frataxin gene resulting in gene silencing and reduced protein expression of mitochondrial frataxin. Frataxin is generally thought to be an essential mediator of iron homeostasis, promoting synthesis of heme- and iron–sulfur cluster-containing proteins. Loss of frataxin impedes mitochondrial removal of cytosolic iron, leading to a buildup of free iron and increased oxidative stress concomitant with loss of large sensory neurons and spinocerebellar tracts. Symptoms of FRDA usually occur prior to early adulthood and lead to progressive limb and gait ataxia, along with secondary symptoms which may include scoliosis, cardiomyopathy, deafness, and diabetes, depending on severity of the disease (159). Blood samples from FRDA patients showed decreased abundance of free GSH and an increase in glutathione bound to hemoglobin, with very little change in total glutathione content (145). A common theme among the neurodegenerative diseases is cellular changes which increase the oxidant load on the cell above that controlled by normal homeostatic mechanisms.
III. Production of Oxidants Within the Brain
A. Cytoplasmic sources of ROS
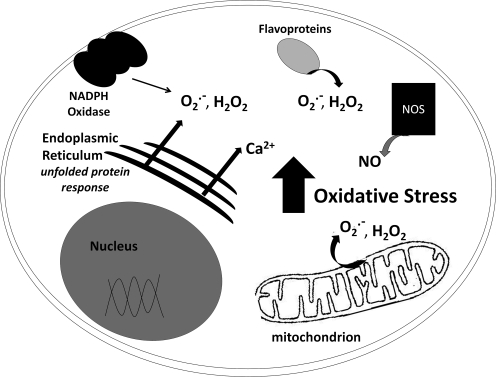
Most of the brain's ROS production comes as a side-product of mitochondrial respiration (discussed below); however, ROS also emanate from cytoplasmic sources (Fig. 2). For example, NADPH oxidases (NOX), first characterized in immune cells as generators of ROS to kill foreign organisms, are now known to function more broadly in most cell types to produce superoxide and function to mediate signal transduction (192). Thus, besides host defense, the NOX enzymes participate in post-translational processing of proteins, regulation of gene expression, and cell differentiation. In the central nervous system (CNS), normal NOX function appears to be required for neuronal signaling, cardiovascular homeostasis, and memory; whereas overproduction of ROS by NOX contributes to neurodegeneration (17, 48, 80). In particular, NOX accumulation at the cell surface has been noted in postmortem brain tissue of AD patients (165). Nevertheless, the contribution of NOX activity in the neurodegenerative diseases relative to other sources of ROS, including cytosolic, mitochondrial, and ER flavoprotein oxidases, requires further study.
FIG. 2.
Schematic representation of cellular sources of reactive species. Shown schematically are the primary sources of reactive oxgen species (ROS) and reactive nitrogen species (RNS), including NADPH oxidase (NOX) at the cellular membrane, oxidases of the endoplasmic reticulum, flavoenzymes (cytosol and ER), nitric oxide synthetase (NOS), and the mitochondria.
Unbound ionized calcium is exquisitely regulated in healthy cells. In aging neurons, however, calcium fluxes become less controlled, leading to calcium overload (115). This excess calcium is associated with increased ROS/RNS production, mitochondrial dysfunction, and ultimately cell death (178). Many neurodegenerative diseases implicate loss of calcium regulation as a molecular basis of disease.
B. Mitochondrial sources of ROS
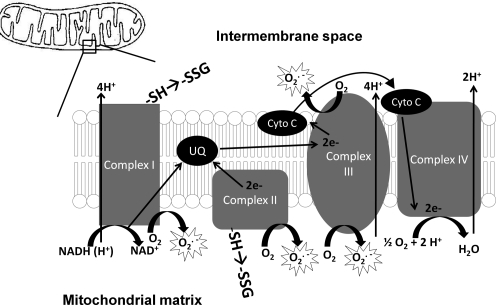
Despite its relatively small mass, the brain requires approximately 20% of total body oxygen consumption to produce sufficient ATP. The mitochondrial electron transport chain is comprised of multiple polyprotein complexes, including Complex I (NADH ubiquinone oxidoreductase), Complex II (succinate ubiquinone oxidoreductase), Complex III (ubiquinone-cytochrome C oxidase), Complex IV (cytochrome C oxidase), and Complex V (ATP synthase). Superoxide is produced as electrons escape from the respiratory chain and react with oxygen, mainly at Complex I and Complex III, but also at Complex II. The amount of ROS generated can be altered by reversible S-glutathionylation of components of Complex I (18, 78) and Complex II (3, 35, 168) (Fig. 3).
FIG. 3.
Mitochondrial generation of ROS. A schematic representation of the electron transport chain (ETC) is depicted here. Complex I, Complex II, and Complex III have all been implicated as generators of superoxide, leading to hydrogen peroxide formation.
Besides ROS from the electron transport chain in brain mitochondria, monoamine oxidase on the mitochondrial outer membrane produces H2O2 as a byproduct of its catalysis of oxidative deamination (106, 150). This may be of particular importance in PD where increased dopamine metabolism by monoamine oxidase B produces more hydrogen peroxide (133).
Another source of ROS within brain mitochondria occurs in the Krebs cycle, specifically through α-ketoglutarate dehydrogenase (α-KGDH) which is regulated by calcium, NADH/NAD ratio, dihydrolipoate/lipoate ratio, and ADP. When active, α-KGDH produces superoxide and hydrogen peroxide with maximum production occurring during times of maximum respiration. The ROS production by α-KGDH is stimulated by calcium, and the enzyme itself is susceptible to oxidative inactivation via S-glutathionylation (3). Thus, glutathionylation of α-KGDH might serve to limit ROS production.
IV. Inflammation, Oxidative Stress, and Neurodegenerative Diseases
A common feature of many neurodegenerative diseases is inflammation. It consists of a number of molecular and cellular mechanisms contributing to the cascade of events that can either promote or inhibit neurodegenerative processes (67, 193). During the previous decade, evidence has accumulated that neuroinflammation contributes to neurodegenerative diseases, including PD, ALS, and MS.
Inflammation is a normal response of the immune system (75, 160) against multiple extra/intracellular stimuli (e.g., infections, dying neurons, and accumulation of insoluble proteins). The response may result in generation of factors such as cytokines, reactive oxygen species (ROS), and reactive nitrogen species (RNS) that serve as “weapons” to combat pathogen invasion. However, the invasion of the immune cells and overproduction of molecules that induce cytotoxic effects and edema may amplify the underlying disease state by injury to host cells in the brain, propagating neurodegeneration (Fig. 4).
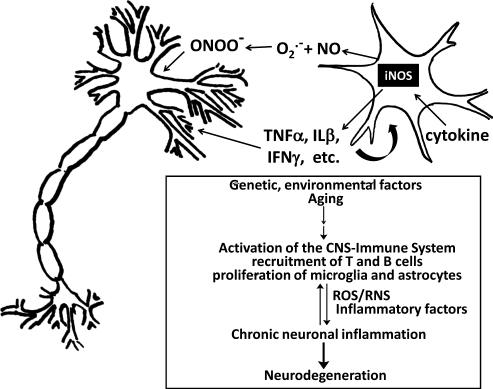
FIG. 4.
Activated microglia promote inflammatory assault on neurons. A schematic representation of an activated microglial cell and a neuron is depicted here. Microglia, the resident macrophages of the central nervous system (CNS) are activated by and produce cytokines and RNS and ROS that mediate external oxidative stress impacting the neuronal cells. Different insults, such as genetic and environmental factors or aging, affecting the CNS can lead to activation of the CNS–immune system, including lymphocyte recruitment, microglia activation, and astrocyte proliferation. These may in turn induce the production of reactive oxygen species (ROS) or reactive nitrogen species (RNS) and drive the expression of inflammatory factors. Those inflammatory mediators can influence the fate of neurons and stimulate the CNS-immune cells to amplify proinflammatory signals and induce neurotoxic effects. Uncontrolled or chronic inflammation can result in loss of neurons and progression of neurodegenerative diseases.
Understanding immune responses in the brain must include consideration of the special cellular features that comprise the “blood-brain barrier” (BBB) that limits access. Studies of postmortem brains from humans and animals with neuropathology indicate that immune cells can penetrate the BBB in pursuit of target antigens. (24, 74, 117). However, neuroinflammation is not solely dependent on lymphocytic infiltration into the CNS, as findings using animal models clearly indicate involvement of astrocytes and microglia—resident innate immune cells in the CNS. Astrocytes form part of the BBB and contribute to nutritional homeostasis, as well as participating in local immune responses to various insults (52). Microglia represent the resident macrophages of the CNS for immune surveillance. In response to various insults such as brain injury and disease, microglia can exhibit various activated phenotypes (143).
Activation of brain microglia and astrocytes appears to be a common aspect of inflammation in neurodegenerative diseases. For example, senile plaques in AD are associated with activated microglia and astrocytes. Activated microglia are also found in the substantia nigra of PD patient brains (106), and activation of microglia has been implicated as an exacerbating problem in ALS (68) Activation of microglia results in the production of ROS from NADPH oxidase or RNS from inducible nitric oxide synthase (NOS). Indeed, inhibition of NOS has been shown to be neuroprotective (82). Although it is difficult to determine whether oxidative stress initiates protein aggregation in neurodegenerative processes, a feedback loop appears to be in operation whereby brain immune cells activated by misfolded proteins to secrete neuronal toxic molecules, including ROS and RNS, in turn promote further loss of neurons. Thus, persistent dysregulation of the ubiquitin proteasome system (see below) under oxidative stress could become a driving force of chronic neuroinflammation through excessive activation of brain immune cells.
Neuronal immune cells express a subset of Toll-Like Receptors (TLR) whose activation promotes neurodegeneration, for example, in AD patients (132). Inflammation of neurons can cause lipid oxidation, perhaps including the highly reactive ω-2-carboxyethyl pyrole (CEP) which can irreversibly modify proteins. CEP adducts and their derivatives are uniquely generated from the oxidation of docosahexaenoate, which is the most abundant lipid in brain and retina. Clinical studies have suggested accumulation of CEP-protein adducts contribute to the pathogenesis of age-related macular degeneration (39). CEP-adducted proteins also are able to stimulate Toll-like receptor 2 (TLR2) specifically, promoting angiogenic responses (191). Therefore, it is conceivable that during neuroinflammation CEP accumulation in neurodegenerative regions may promote further loss of neurons.
A. Inflammation and Parkinson's disease
Overt inflammation in the substantia nigra and striatum is characteristic of Parkinson's disease and correlates with dopaminergic neuron cell death. Residing mainly in gray matter, the microglia may become activated and release cytokines, chemokines, and trophic factors launching an immune response implicated in neurodegeneration (62). PD patients exhibit elevated cytokines (TNF-α, IL-1B, and IL-6) in peripheral blood mononuclear cells (PBMC) compared to control; as well as in cerebrospinal fluid, and in postmortem substantia nigra brain tissue (148). TNF-α is considered a major controller of neuroinflammatory events that ultimately lead to neuronal cell death. Importantly, a mutation in the TNF-α gene elicits increased production of the cytokine and is associated with early onset PD (175). The potential impact of the immune response is evident also from in vitro studies. Thus, in co-cultures of microglial cells with neurons, lipopolysaccharide (LPS) activates microglia that then kill the neuronal cells; but LPS does not induce cell death in neuronal cultures alone.
B. Potential roles of glutaredoxin in inflammatory responses
It is conceivable that Grx1 and reversible S-glutathionylation may play a role in neuroinflammation based on analogy to previous work in other contexts. Thus, in studies of models of diabetic retinopathy in rat retinal glial cells (rMC-1), Grx1 was selectively upregulated in response to high glucose, similar to the two-fold increase in Grx activity observed in homogenates of retinal tissue from diabetic rats compared to controls (163). Coincident with Grx1 upregulation in the retinal glial cells, we observed a corresponding increase in NF-κB activation and expression of the pro-inflammatory intercelluIar adhesion molecule-1 (ICAM-1); these results were mimicked by adenoviral-directed upregulation of Grx1 in the cells in normal glucose. Remarkably, knockdown of Grx1 in rMC-1 cells in high glucose blocked the increase in ICAM-1, suggesting Grx1 regulates the S-glutathionylation status and corresponding activity of NF-κB and/or its upstream signaling mediators. Indeed, mass spectrometric analysis showed IKKβ is glutathionylated site-specifically (Cys-179), implicating it as a Grx-regulatable control point in NF-κB-mediated ICAM-1 expression. Furthermore conditioned media from rMC-1 cells overexpressing Grx1 contained an increased amount of IL-6, and it elicited induction of ICAM-1 and Grx1 in freshly cultured rMC-1 cells and in TRiBRB rat endothelial cells, simulating autocrine and paracrine propagation of the pro-inflammatory response (162). Analogous regulation of NF-κB-dependent inflammation via S-glutathionylation of IKKβ has been demonstrated in lung epithelial cells (151). Consistent with these findings, a study of cigarette smoke-induced inflammation in lungs of Grx1-knockout mice found that the level of glutathionylated IKKβ was increased and its kinase activity was inhibited; however, more, instead of less, inflammation was observed. This unexpected result was attributed to S-glutathionylation and concomitant activation of IKKβ leading to activation of NF-κB via a noncanonical pathway and resulting in phospho-acetylation of histone H3 (38). Thus, alternative mechanisms of glutaredoxin regulation of NF-κB activity involving S-glutathionylation of different IKK isoforms appear to control inflammatory responses in different cell types under different conditions. Future studies will examine whether Grx1 and S-glutathionylation play a regulatory role in neuroinflammation.
Tightly controlled oxidative stress in affected neuronal cells might neutralize ROS/RNS or reverse oxidative damage through scavenging enzymes or thiol repair enzymes, providing protective mechanisms for neuronal survival (see V. Cellular Oxidant Defense Mechanisms, below). This brief consideration of inflammation as a contributory factor to neurodegeneration highlights that more research is necessary to understand how oxidative stress accelerates the progression of disease pathogenesis and which specific alterations in cell signaling pathways occur during neuroinflammation. This insight will inform approaches to impede the progression of neurodegeneration.
V. Cellular Oxidant Defense and Sulfhydryl Homeostasis
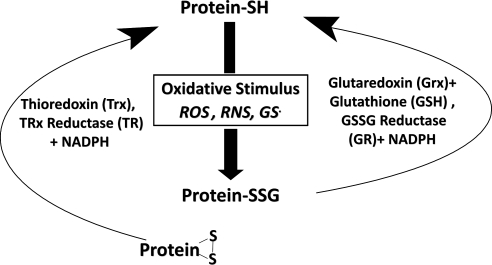
Oxidant defense mechanisms include enzymes such as catalase, peroxiredoxin, and superoxide dismutase that scavenge reactive oxygen species. Other antioxidant mechanisms depend in large part on sulfur-containing amino acids (cysteine and methionine) in proteins and nonprotein cofactors, especially the Cys-containing tripeptide GSH that is abundant within cells. Thus, sulfur redox status plays a very important role in the regulation of cell functions including signaling, growth, survival, and cell death. Reversible or irreversible modifications of Cys and Met residues alter protein function or stability and perturb intracellular sulfhydryl homeostasis. Hence, perturbations of the antioxidant systems and disruption of sulfur redox status is linked to abnormal cell functioning, aging, and many diseases, including arthritis, cancer, cardiovascular disorders, diabetes, and neurodegenerative diseases (202). Reversible thiol modifications, including intramolecular disulfides, intermolecular disulfides, protein sulfenic acids, and protein–glutathione mixed disulfides (protein–SSG) protect proteins from irreversible overoxidation, allowing for a return to normal function after the oxidant challenge has subsided. Irreversible oxidations, such as sulfinic (RSO2H) and sulfonic (RSO3H) acid formation, usually lead to protein degradation. In the case of certain forms of the peroxiredoxins, hyperoxidation to the sulfinic acid form is reversible by sulfiredoxin (108). In addition, sulfiredoxin has been shown to catalyze protein deglutathionylation in special cases (56, 139), although glutaredoxin is the principal catalyst of protein-SSG reduction (see below).
In neurodegenerative disease, levels of GSH have been reported to be diminished. Thus, in postmortem brain samples from patients with AD versus age matched controls, significantly lower levels of GSH were seen in post-mitochondrial supernatant fractions and synaptosomal fractions; and increased levels of GSSG were seen in mitochondrial fractions, post-mitochondrial supernatant fractions, and synaptosomal fractions (6). Likewise with PD samples, similar changes have been seen where levels of GSH are decreased and GSSG is increased (85, 167). The GSH loss is thought to be one of the early changes responsible for the increased oxidative stress associated with onset of PD (59, 113). Thus, changes in the GSH/GSSG ratio and conditions that produce ROS and RNS can promote modifications on protein cysteine residues and on glutathione that serve as precursors to the formation of protein mixed disulfides with glutathione (protein–SSG). The various ways that protein thiol moieties could be converted to protein–SSG include thiol disulfide exchange if the GSSG concentration is sufficiently high; reaction of GSH with protein–sulfenic acid or protein–SNO precursors, or recombination of protein- and glutathione-thiyl radicals. These mechanisms and the potential for enzymatic catalysis of protein–SSG formation have been discussed in detail previously (64, 118). Reversibly oxidized cysteine residues on proteins are repaired principally by thiol–disulfide oxidoreductase enzymes (TDORs) that catalyze thiol–disulfide exchange reactions. The two principal TDORs involved in sulfhydryl homeostasis are thioredoxin (Trx) and glutaredoxin (Grx), which catalyze the reduction of disulfide bonds in both protein and nonprotein substrates (119, 202). Each of these TDOR enzymes has displayed substrate selectivities and redox potentials indicative of distinct physiological functions. Trx primarily reduces intramolecular and intermolecular disulfide bonds (83). In contrast, Grx specifically reduces protein–glutathione mixed disulfides (119). The special characteristics of the thioredoxin and glutaredoxin enzyme systems suggest different, but complimentary functions for them, likely acting synergistically to maintain the thiol status in various types of cells (Fig. 5).
FIG. 5.
Glutaredoxin and thioredoxin systems contribute synergistically to sulfhydryl homeostasis. Besides scavenging of reactive species, the other important aspect of thiol homeostasis is repair of sulfhydryl modifications. This function is performed by the thiol-disulfide oxidoreductase (TDOR) enzyme systems. Glutaredoxin (Grx, also known as thioltransferase), coupled to GSH and GSSG reductase, and the thioredoxin (Trx)–thioredoxin reductase system catalyze disulfide reduction and reactivation of oxidatively-modified sulfhydryl proteins. The Trx system favors reduction of intramolecular disulfides via a Trx-(S-S) intramolecular disulfide intermediate, and it is indiscriminate with mixed disulfides substrates. Grx is highly selective for glutathione-containing mixed disulfides (i.e., protein-SSG). Thus, reversible protein–SSG formation by Grx may protect vital proteins from irreversible damage and serve as a regulatory mechanism. As described in the text, many of the proteins characteristically associated with neurodegenerative diseases are subject to oxidative modification and potential regulation via this mechanism.
Inconsistencies are evident in studies of antioxidant enzymes in the context of neurodegenerative diseases. Compared to thioredoxin, thioredoxin reductase exhibits diminished activity in brain samples from AD patients (107), whereas superoxide dismutase, catalase, and glutathione reductase have been found increased several-fold in samples from patients with AD (137, 200). Many of these oxidative stress-responsive enzymes contain redox-active cysteines. Thus, increases in their activities due to activation of antioxidant genes may be countered by oxidative post-translational modifications which deactivate the enzymes or otherwise alter function. For example, S-glutathionylation can alter the function of eNOS to switch it from production of NO to production of superoxide (32); also inhibition of NADPH oxidase by S-nitrosylation in neutrophils turns off the major source of superoxide generation during nitrosative stress (61). Taken together, these findings support the concept that disruption of intracellular redox homeostasis contributes to neurodegeneration.
A. Cellular functions of Grx
The main function of Grx is to deglutathionylate proteins and restore the reduced state (protein-SH), thereby contributing to sulfhydryl homeostasis and serving to regulate the steady-state levels of protein–SSG in redox signaling networks (65). Increased levels of protein–SSG due to loss of Grx activity has been implicated in many diseases (118), including neurodegenerative diseases (70, 126), the focus of this review. While the list of proteins that are potentially S-glutathionylated in various contexts has grown remarkably, the physiological relevance of the post-translational modification requires scrutiny in each case, according to key criteria; namely: does S-glutathionylation (1) alter the function of the protein? (2) occur in intact cells as a response to a physiological stimulus or a pathophysiological insult and elicit a physiological/pathophysiological response (64, 161)? There are a number of proteins that fulfill these criteria and illustrate how S-glutathionylation alters function. For example, protein tyrosine phosphatase-1B (PTP1B) is inactivated (15, 16), whereas hRas is activated (146), and eNOS undergoes a change of function (32). Proteomic studies showing protein modifications represent a starting point for more detailed mechanistic investigation. Since the chief function of Grx is to catalyze deglutathionylation, manipulation of its content in cells has been used effectively to document regulatory pathways that involve S-glutathionylated intermediates. For example, IKK-SSG and p-65-SSG have been identified as regulatory intermediates in the NF-κB signaling pathway in different contexts (147, 151, 162). Regulation by Grx also has been shown for other proteins representing a broad range of cellular functions, namely the transcription factor NF-1 (14), the signaling intermediate Ras (1), the scaffold protein actin (188), the apoptosis mediators Akt (122, 144) and cJun (98), and the ion transporters CFTR chloride channel (189) and K(ATP) channel (197). In each case, Grx was shown to restore the original functional state of the modified protein. These examples convey the breadth of regulation of cell functions by reversible S-glutathionylation and highlight the potential for dysregulation if the activity of glutaredoxin activity is altered.
B. Glutaredoxin and neurodegeneration
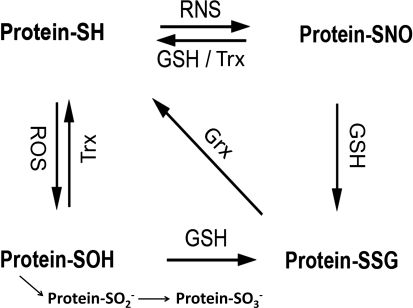
Oxidative and nitrosative stress conditions promote sulfhydryl oxidation, and various intermediate forms of protein–cysteine modification like sulfenic acid (S-OH) and S-NO are readily converted to protein–SSG in the cellular environment where GSH abounds (64) (Fig. 6). The formation of protein–SOH and its conversion to protein–SSG are shown as sequential spontaneous chemical reactions. It is conceivable that sulfenic acid formation could be catalyzed by a peroxidase (69), and there is evidence for glutathione-S-transferase-mediated catalysis of protein–SSG formation from protein–SOH (reviewed in ref. (180)). However, more study is necessary before these reactions may become viewed as typically enzyme-catalyzed reactions. In the case of the protein–SNO reaction with GSH, two different outcomes are depicted, namely denitrosylation or S-glutathionylation. Both types of reactions have been reported (153, 190) and further study is necessary to understand the molecular basis for why one or the other reaction is favored with particular proteins and conditions (64). Glutaredoxin and thioredoxin function to repair the oxidized sulfyhydryls and prevent irreversible modification, aggregation, and degradation. However, in many disease states, alterations in activity of these enzymes can lead to deleterious outcomes such as dysregulation of signaling pathways, inhibition of transcription factors, and interference with protein degradation, all of which may initiate commitment to apoptosis.
FIG. 6.
Interconversion of forms of modified cysteine residues on proteins. According to the relative reactivity and relative abundance of ROS, RNS, and GSH (GSSG) in cells under conditions of redox signaling or oxidative stress, the expected sequence of events is initial formation of protein-SOH which is readily converted to Protein-SSG; or initial formation of Protein-SNO which is readily converted to Protein-SSG. Thus, Grx plays a key role in determining the steady-state level of Protein-SSG under various conditions (64). Certain proteins that sequester the S-NO or S-OH moieties may be resistant to conversion to Protein-SSG. Also, Trx is reported to catalyze denitrosylation (20). In addition, the S-OH moiety may undergo additional oxidation to sulfinic (-SO2H) and sulfonic (-SO3H) acid forms which are essentially irreversible (see text).
Both Grx1 and Grx2 are expressed throughout the brain, and the Grx1 activity in brain is similar to that found in the liver (51, 89). However, differences in content of Grx appear to reflect relative neuron viability in Alzheimer disease samples. Thus, in postmortem AD brain tissue, immunohistochemical analysis revealed elevated Grx in healthy neurons, but Grx was diminished in neurons showing signs of neurodegeneration (5). Loss of Grx could enhance neuronal cell death not only through loss of its deglutathionylase function, but also through loss of its ability to bind pro-apoptotic protein, apoptosis signaling kinase 1 (ASK1) (see below).
In a mouse model of PD involving acute treatment with MPTP, Grx activity was observed to increase in the brains, and this was correlated with restoration of activity of mitochondrial Complex I, the target of MPTP-induced inhibition. When Grx1 was knocked down by antisense oligonucleotides, inactivation of Complex I by MPTP was not reversed (91).
Although it is not established whether inhibition of Complex I in PD patients can be attributed to S-glutathionylation, there is evidence in favor of regulation by glutaredoxin that supports this hypothesis. A study of GSH depletion in rat N27 neurons as a model of PD reported inhibition of mitochondrial Grx2 and disruption of the iron–sulfur centers of Complex I and aconitase. Furthermore, knockdown of Grx2 gave analogous results (102). These findings suggest that mitochondrial Grx2 may play a more important role in iron–sulfur homeostasis than in sulfhydryl homeostasis. Thus, under stress conditions, depletion of GSH should lead to dissociation of the dimeric Grx2-Fe2S2-(GSH)2-Grx2 complex (105), thereby inactivating iron–sulfur transferase activity. In contrast, dissociation of the dimeric Grx2 complex would activate the deglutathionylase activity of Grx2. Hence, diminution in GSH content paradoxically should protect against Complex I glutathionylation if Grx2 were the primary deglutathionylation catalyst. Alternatively, Grx1 in the mitochondrial inner membrane space (136) may serve to deglutathionylate Complex I.
C. Paradoxical pro-oxidant effects of therapy of Parkinson's disease
As introduced above, PD, characterized by dopaminergic neuronal loss, is attributed to oxidative stress, diminished GSH, mitochondrial dysfunction, and protein aggregation. Treatment of PD to replenish dopamine and alleviate symptoms involves chronic administration of Levodopa (L-DOPA) which itself is a pro-oxidant. Therefore, we examined the effects of L-DOPA in a model akin to PD, [i.e., immortalized dopaminergic neurons (SHSY5Y cells) with diminished GSH content (154)]. These neurons exhibited hypersensitivity to L-DOPA-induced cell death, attributable to concomitant loss of activity of the intracellular thiol disulfide oxidoreductases. Grx was deactivated, but its content was unchanged. GR activity was not altered. Decreased activities of Trx and TR corresponded to diminution of their cellular contents. To distinguish the mechanism for L-DOPA-induced apoptosis of SHSY-5Y neurons, we focused on cell death pathways implicated in PD with potential regulatory roles for both Grx and Trx (155), NF-κB activity was not altered, and the selective mixed lineage kinase (MLK) inhibitor (CEP-1347) did not protect the cells from L-DOPA. In contrast, ASK1 was activated and knockdown of ASK1 protected from L-DOPA induced neuronal cell death, identifying ASK1 as the main pro-apoptotic pathway activated in response to L-DOPA treatment. The next section focuses on oxidative stress and the key role of ASK1 in apoptosis of neuronal cells pertinent to neurodegeneration.
VI. Oxidative Stress and Apoptosis
As noted above, oxidative stress is inherent in neurodegenerative disease and increased oxidative stress has been shown in many instances to lead to apoptosis. Here, we focus on apoptotic mediators that are activated by oxidative stress. Moreover, diminution of Grx or Trx levels or loss of function impedes protein reduction and disrupts thiol homeostasis. Below are examples where deactivation of Grx or Trx could promote apoptosis in both cell and animal models of neurodegenerative diseases.
A. Apoptosis signaling kinase 1 may be regulated directly or indirectly by Grx1, Trx1, and other effectors
ASK1 has been reported to be activated in many models of PD including treatment of cells and animals with 6-hydroxydopamine (6-OHDA) or MPTP. In SHSY5Y cells, 6-OHDA led to ASK1 activation, resulting in JNK activation and apoptosis (135). For a general review of the ASK1-MAP kinase pathway in stress signaling, see Ref. (114). In MPTP-treated mice, the thiol antioxidant α lipoic acid prevented ASK1 activation implying a thiol–disulfide oxidative mechanism of ASK1 activation (88). In another context, it was shown that Grx1 remains bound to the C terminus of ASK1, maintaining ASK1 in its inactive state, so long as the active site cysteines of Grx1 are in the reduced state (171, 172). Various oxidative stimuli, including glucose deprivation and hydrogen peroxide treatment, lead to ASK1 activation. This activation was prevented by pretreatment with excess catalase or N-acetyl cysteine (171, 172). Trx1 binds the N terminus of ASK1 and is also shown to regulate ASK1 activation, and its dissociation can also be oxidatively regulated. Trx, like Grx, contains two active site cysteine residues that must be maintained in the reduced state. Upon oxidation, Trx no longer binds to ASK1 (158).
Alternatively, ASK1 activation could occur through dissociation of other ASK1-bound negative effectors. The negative effectors described below have been shown in other contexts to be subject to oxidative modifications that may interfere with their binding to ASK1, analogous to Grx and Trx. Upon dissociation of negative effectors, ASK1 is activated, leading to the propagation of an apoptosis cascade. Figure 7 provides a simplified scheme of the ASK1 signalosome showing various effectors of ASK1 that are subject to modification under oxidative stress conditions that may alter the activities of the Grx and/or Trx enzyme systems, the redox status of the effectors, and consequently the activity of ASK1.
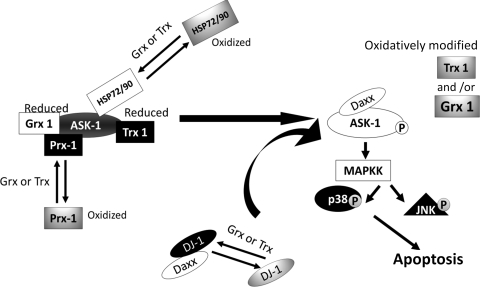
FIG. 7.
Schematic representation of potential modes of ASK1 activation under oxidative stress. On the left, ASK1 is represented in its inactive form in the cytosol, bound to some of its multiple negative regulators. Upon oxidative stimulus (e.g., inflammatory mediators, L-DOPA treatment), these negative regulators become oxidized or otherwise modified (e.g., glutathionylation, dopaquinone adduction) and dissociate from ASK1. Concomitantly, ASK 1 is activated wherein autophosphorylated (activated) ASK1 initiates a phosphorylation cascade of downstream mediators, which induces apoptosis (shown at the right). L-DOPA treatment has been shown to cause loss of Grx1 and Trx1 activities (154), which would impede reduction of other oxidized negative regulators of ASK1, resulting in prolonged ASK1 activation. Details of this model, including the potential involvement of Daxx and DJ-1, are presented in the text.
1. Oxidation of negative and positive effectors of ASK1
ASK1 (also known as “the apoptosome”) interacts with many proteins, including heat shock proteins 72 and 90, peroxiredoxin, Grx, and Trx, among others. The protein effectors presented here have been shown in other contexts to be oxidatively modified.
Heat shock proteins 72 and 90 (HSP 72, HSP 90) bind ASK1 and prevent its activation through different mechanisms. Bound HSP 72 inhibits ASK1 homo-oligomerization and activation (138), whereas HSP 90 stabilizes the association of Akt and ASK1, resulting in inhibition of ASK1 signaling (201). Furthermore, both proteins have been shown to be oxidatively modified with 4-hydroxynoneal treatment inhibiting their ATPase activities (28, 29). It would be informative to learn whether oxidative modifications of the HSPs occur in neurodegeneration.
DJ-1, a protein associated with genetic forms of PD, is an atypical peroxiredoxin, molecular chaperone, and transcription factor. DJ-1 is mutated with a loss of function or deleted in autosomal recessive PD (73). In another context, oxidation of DJ-1 was interpreted to target it for degradation (186). DJ-1 null mice show increased sensitivity to oxidative stress (95); however, it is currently unknown if DJ-1 is oxidized in sporadic PD and if such oxidation of DJ-1 would change its function or accelerate its degradation. In cell culture studies, oxidation of DJ-1 within the nucleus was reported to result from downregulation of Grx1, and this DJ-1 oxidation allowed dissociation of Daxx and translocation of Daxx to the cytosol (157). Daxx, a positive regulator of ASK1, binds and activates ASK1 and its apoptotic pathway (87). Taken together, these findings suggest that Grx1 may regulate the redox state of DJ-1 within the nucleus and/or alter the ability of DJ-1 to translocate freely between nuclear and cytosolic compartments. Thus, oxidation of DJ-1 could activate ASK1 indirectly by promoting the positive effector action of Daxx. In contrast, other studies have reported that oxidized DJ-1 is a negative regulator of ASK1 [i.e., it binds and inhibits ASK1 only when oxidized (186)]. Since the results/interpretation of these two studies are conflicting, further experimentation is necessary to clarify the role(s) of redox regulation of DJ-1 in modulating the apoptotic action of ASK1.
Peroxiredoxin 1 (Prx1), a small nonseleno peroxidase, functions to reduce peroxides within the cell via a 2-Cys redox cycling mechanism, and Prx1 is reduced to its original, active form by the Trx system (10). Prx1 has been implicated in both positive and negative regulation of ASK1 (34, 50, 170). In the context of neurodegeneration and oxidative stress, treatment of catecholaminergic PC12 cells with MPP+ (Complex I inhibitor) was reported to lead to diminution of Prx1 levels, concomitant with increased apoptosis (34). In another study, Prx1 was inactivated in dopaminergic MN9D cells after treatment with the pro-oxidant 6-hydroxydopamine (103). In a third study, Prx1 was reported to bind at the N-terminus of ASK1 and prevents its activation (96). Together, these three studies characterize Prx1 as a negative regulator of ASK1, so that dissociation of the Prx1-ASK1 complex due to Prx1 oxidative modification and/or degradation would lead to ASK1 activation and apoptosis. However, instead of oxidative stress leading to Prx1 dissociation, the study by Kim et al. (96) reported that Prx1 association with ASK1 was driven by hydrogen peroxide treatment, requiring 5 mM H2O2 to be effective. Hence, under the molecular mechanism of regulation of ASK1 activity by Prx1 under oxidative stress conditions requires further investigation.
Involvement of Prx1 in degeneration of neurons in the clinical setting or in animal models is unknown currently; however, Prx2, the most abundant neuronal peroxiredoxin, has been implicated as an indirect negative effector of ASK1 in a study of 6-OHDA-induced dopaminergic neurodegeneration in mice (and cultured cells) (76). Here Prx2 overexpression was reported to lead to overoxidation of Prx2 and concomitant maintenance of Trx1 in its reduced state, thereby facilitating Trx1 binding to and inactivation of ASK1. This proposed mechanism is very unusual, because the typical interaction between Trx1 and Prx operates in the opposite direction, namely after Prx is oxidized by peroxide Trx reduces the Prx; and the resultant oxidized Trx is recycled by TR. Hence, it must be questioned how well the studies of overexpressed Prx2 may reflect actual functional interactions involving endogenous levels of Prx2.
The various negative regulators of ASK1 may be subject to S-glutathionylation under oxidative stress conditions (Fig. 7). Thus, the ASK1 apoptosis pathway may be regulated by reversible glutathionylation and controlled by the deglutathionylase activity of Grx. Alternatively, the functional activities of multiple other cytosolic and mitochondrial proteins are reported to be regulated by Grx which may have impact on the sensitivity of neurons to oxidant-induced apoptosis. As discussed individually below, oxidation of these proteins resulting in a change in their native function could contribute to cell death through non-ASK1 mechanisms. In this context, it is interesting to note that cerebral granular neurons can be protected from dopamine-induced apoptosis by addition of E. coli Grx2 which can penetrate into the cells (41, 42). The protective effect of the Grx2 was attributed to activation of expression of Ref1 which in turn activated akt/NF-κB and AP-1.
B. Redox sensitivity of cytosolic proteins implicated in neuronal cell death
1. Glyceraldehyde-3-phosphate dehydrogenase
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) functions within the glycolytic cycle to generate glyceraldehyde-3-phosphophate and NADH. However, GAPDH is remarkably versatile, displaying regulatory roles in membrane fusion, calcium homeostasis, transcription, and cell viability. Recently, GAPDH has received increased attention in neurodegenerative research as it has been found to accumulate in neurofibrillary tangles, Lewy bodies, and other detergent-insoluble plaques associated with neurodegenerative disease (123). Furthermore, oxidative modification of GADPH has been linked to loss of its enzymatic activity (121); thus, treatment of isolated aortic endothelial cells with GSNO led to loss of GAPDH activity concomitant with its S-glutathionylation. Regarding neurodegeneration, isolated dermal fibroblasts from HD and AD patients compared to age-matched controls showed a decrease in GAPDH activity within the nucleus (116). Using anti-GSH antibodies, increased glutathionylated GAPDH was also observed via proteomic studies of postmortem brain sections from AD patients (126).
2. Tyrosine hydroxylase
Tyrosine hydroxylase (TH), the rate limiting enzyme in synthesis of dopamine, is a biomarker for neurons involved in Parkinson's disease. TH contains 7 Cys residues, however none have been shown to be required for catalytic function. These Cys residues have been reported to be glutathionylated when PC12 cells were treated with diamide, resulting in a diminution of TH enzymatic function (21). Furthermore, it has been reported that TH function decreases to a greater extent than the loss of dopaminergic neurons in a MPTP model of PD (7). Loss of TH activity via oxidation was not examined in that study, so it remains unknown if TH is glutathionylated in models of degenerative diseases.
3. p53
p53 responds to DNA damage through repair, cell cycle arrest, or activation of apoptosis. In regard to neurodegenerative diseases, p53 has been reported to be elevated both in mild cognitive impairment, in AD, and in PD (31, 120). Brain samples from AD patients showed increased oxidation of p53 compared to controls (31). More recently, p53 was found to be glutathionylated in samples from AD patients compared to age-matched controls. Immunoprecipitation of p53 from the inferior parietal lobule of postmortem samples showed increased reactivity to the anti-glutathione antibody, and increased monomer and dimer forms of p53 were observed. A lack of tetramerization was attributed to increased S-glutathionylation of p53, however functional consequences were not examined (47). Consistent with these findings, glutathionylation of p53 has been reported also in cancer cells to be coincident with inhibition of its oligomerization and transcriptional activation (184).
C. Apoptosis and modification of mitochondrial permeability pore proteins
Mitochondrial membrane permeability is tightly controlled. When compromised, a series of adverse effects occur, including loss of transmembrane potential, uncoupled electron transport, increased production of ROS, decreased ATP synthesis, and ultimately mitochondrial initiation of the apoptosis cascade (149). The mitochondrial permeability pore (MPP) is a conformationally dynamic complex likely comprised mainly by three proteins, voltage-dependent anion channel (VDAC), adenosine nucleotide transporter (ANT), and cyclophilin D (CypD). MPP opening in liver mitochondria from CypD knockout mice was much less sensitive to calcium than normal mitochondria, and MPP opening was no longer inhibited by the CypD antagonist cyclosporine A (CsA) (12). In contrast, knockout of all isoforms of ANT and of VDAC in separate studies (13, 99) revealed that neither of these components is essential for calcium-dependent, CsA-inhibitable mitochondrial permeability transition. However, a preponderance of evidence indicates that ANT, and likely VDAC, play regulatory roles in the mitochondrial permeability transition (71, 156, 198), and both are susceptible to modulation by oxidative stress. Both VDAC and ANT have been shown to be oxidatively modified in multiple contexts as discussed below.
1. Voltage-dependent anion channel
Proteomic studies have reported oxidative modification of voltage-dependent anion channel (VDAC) in post-traumatic brain injury (134) and in human AD brains compared to controls (177). VDAC resides on the outer mitochondrial membrane and functions as an interface between the mitochondrion and the cytosol through its ability to exchange both adenosine nucleotides and calcium. According to studies with yeast, VDAC functions as a redox sensor, modulating the cellular redox state (63). VDAC has two conserved cysteine residues, Cys 127 and Cys 232, which appear to be oriented differently (i.e., one faces the VDAC pore while the other faces the lipid bilayer). While thiol alkylation with fluorescein-NEM altered VDAC conductance, mutation of the conserved Cys residues to Ala did not alter channel activity, oligomerization, or apoptosis (9). In Neuro2a cells (model of mouse neurons), diminished Grx1 led to increased oxidized VDAC and mitochondrial dysfunction, suggesting S-glutathionylation of VDAC may alter its function; however, glutathionylation per se was not documented (156). The apparently conflicting results regarding the functional importance of the VDAC Cys residues revealed by these two studies could reflect the difference between replacing the cysteines by neutral isosteric Ala residues versus modification by the charged and bulky glutathionyl moiety. This type of distinction has been documented for other proteins regulated by reversible S-glutathionylation [e.g., NF-1 (14) and HIV1 protease (43)].
2. Adenosine nucleotide transporter
Adenosine nucleotide transporter (ANT) functions to maintain mitochondrial permeability and facilitates the exchange of ATP and ADP at the inner mitochondrial membrane. This protein also has been reported to interact with both pro-apoptotic and pro-survival Bcl-2 family members (19). ANT contains multiple oxidant-sensitive Cys residues that are critical for ANT function and pore forming properties. C-56, C-159, and C-256 reside on the matrix side of the inner mitochondrial membrane and oxidation of these residues results in permeability pore opening and increased pore forming activity of ANT (149). In brain cells, different effects on ANT were observed dependent on the extent of oxidative stress (i.e.m GSSG concentration). In astrocytes exposed to carbon monoxide, low levels of GSSG were associated with S-glutathionylation of ANT and decreased pore forming activity, but increased translocation of ADP/ATP (149). However, at higher GSSG concentrations in both astrocytes and cerebral granule neurons, glutathionylation of ANT was correlated again with decreased permeability but ADP/ATP translocation was also decreased in this case (149, 185), suggesting a different array of Cys residues modified by S-glutathionylation. It remains to be determined whether glutathione adduction of ANT is site-specific and functionally diverse.
3. Redox sensitivity of calcium transporters
Alterations in calcium homeostasis have been implicated in neurodegenerative disease (Fig. 8). Elevated cytosolic calcium promotes cellular oxidative stress (115). Mice treated with MPTP to induce PD leads to elevated intracellular calcium concentrations and cell death (104). Similarly, calcium levels rise following excitotoxic injury, leading to cell death. This increase in apoptosis is prevented through inhibition of the IP3 receptor or the ryanodine receptor (RyR) (115). RyR type 1 has been shown to be glutathionylated in vitro in response to several different treatments, where S-glutathionylation caused opening and calcium release from the endoplasmic reticulum (8). Recently, in a cerebral ischemic rat model, RyR type 2 was shown to be glutathionylated under ischemic conditions compared to control. This glutathionylation also resulted in increased activity of the channel, suggesting that this modification of RYR2 may contribute to neuronal death (25). SERCA2 is the chief regulator of Ca2+ reuptake from the cytoplasm to the SER, and its activity is known to be altered by reversible S-glutathionylation in other contexts (2). However, we are unaware of studies that have examined the glutathionylation status of the various calcium pumps in neurodegeneration models.
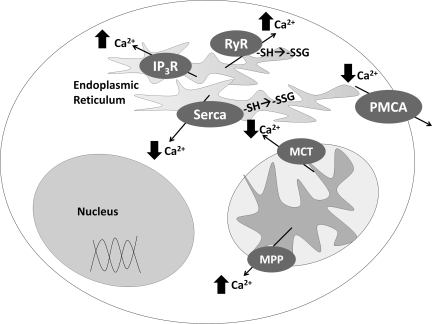
FIG. 8.
Regulation of intracellular ionized calcium. This scheme provides a representation of the various calcium channels and their effects on intracellular ionized calcium (increase or decrease) and identifies those which have been reported to be modified by S-glutathionylation. Ca2+ flux is a critical and tightly controlled aspect of normal cell activity. Dysregulation of intracellular ionized calcium concentration occurs with aging and oxidative stress, resulting in decreased cell viability. Several transporters that control intracellular calcium are subject to functional change by oxidation, [e.g., reversible S-glutathionylation (see text)]. Ca2+ flux is regulated by several transporters including two calcium ATPases associated with the smooth endoplasmic reticulum and the plasma membrane (SERCA and PMCA), two receptors located on the SER membrane (RyR, and IP3 receptor), and two transporters located within mitochondria (MPTP and MCT). The two Ca2+ ATPases extrude cytosolic calcium into sequestered compartments: SERCA moves most of the Ca2+ from the cytoplasm into the SER, while the plasma membrane calcium ATPase (PMCA) pumps Ca2+ into the extracellular milieu. RyR and IP3R release Ca2+ from the SER into the cytosol. The mitochondrial calcium transporter (MCT) promotes calcium reuptake, while the mitochondrial permeability pore (MPP) extrudes calcium.
D. Oxidative modifications affecting the proteasome system, protein aggregation, and mitochondrial dynamics in neurodegeneration
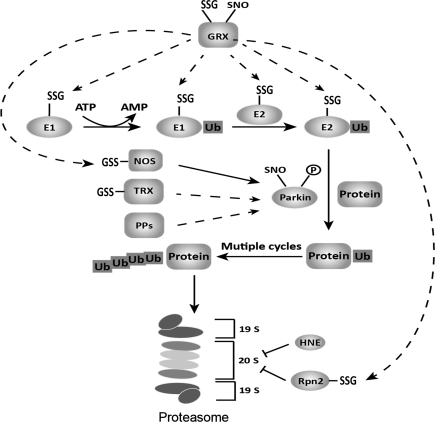
In previous reports, the ubiquitin ligases E1 and E2 have been identified as susceptible to deactivation by oxidative modification, including S-glutathionylation (84, 130). Activity of the 26 S proteasome also can be diminished indirectly by S-glutathionylation, specifically on its regulatory subunit Rpn2 (203). Such modification may alter its protein structure and impair interaction with the proteasome catalytic domain 20S CP. In the context of neurodegeneration, GSH was depleted in cultured catecholaminergic PC12 cells to serve as a model of PD (86). Under these conditions the ubiquitin-proteasome system was inhibited, resulting in decreased ubiquitination of misfolded and damaged proteins and appearance of Lewy bodies. In particular, the activity of the E1 ligase was impaired (86).
Other components of the proteasomal complex are also subject to oxidative modification. The 26S/20S proteasomes, which selectively eliminate oxidatively modified proteins, can be inhibited by oxidative stress (131), and the 20S proteasome is inhibited by S-glutathionylation in yeast (44). Such modifications may also contribute to disruption of intracellular protein folding homeostasis and thereby promote the progression of neurodegeneration.
The 20S proteasome under oxidative stress is subject to irreversible modification and deactivation by adduction of a major end product of lipid peroxidation, 4-hydroxy-2-nonenal (HNE) (60, 131), which modifies the catalytic cysteine (55). Based on the examples above, different types of protein modification may lead to a combinatorial regulation of the intracellular protein degradation system, controlling protein accumulation, and phosphatases may collaborate with the thiol homeostatic enzymes glutaredoxin and thioredoxin to regulate the steady-state levels of modified proteins. This is a key issue, since human cytosolic Trx, Grx, and a number of kinases and phosphatases are known to undergo Cys-based redox modifications, including intramolecular disulfide, S-glutathionylation, and S-nitrosylation (30, 58, 72, 118). In concert, these redox modifications may facilitate dynamic fine-tuning of the reactivity of the enzymes in response to different stress conditions. Figure 9 depicts the various sites of potential regulation via cysteine modifications within the proteasome pathway.
FIG. 9.
Post-translational redox modifications in the ubiquitin–proteasome system. Various oxidative modifications, mainly Cys-based (glutathionylation, nitrosylation), have been identified in proteins involved in the ubiquitin proteasome system (UPS). Previous studies have defined a putative role for each modification, for example, S-nitrosylation of Parkin (Parkin-SNO) triggered by generation of NO from nitric oxide synthase (NOS) can cause neuronal cell death through the accumulation of aberrant proteins; S-glutathionylation of ubiquitin ligase E1, 2 and 20S proteasome regulator Rpn2 can downregulate the intracellular proteasome degradation system. Recently, NOS itself was found to undergo glutathionylation, altering its function from NO production to superoxide production (see text). The Cys-based modifications are controlled by the oxidoreductases glutaredoxin (Grx) and thioredoxin (Trx), which mediate deglutathionylation and denitrosylation, respectively. Furthermore, human Grx and Trx have been reported to undergo glutathionylation or nitrosylation themselves, leading to inactivation in a context-dependent manner. It is still unclear whether the –SNO and –SSG modifications work in concert and if the modifications can influence each other. It appears that such modifications at various points in the UPS pathway may determine the accumulation of misfolded or aberrant proteins in neurodegeneration.
As noted above, the protein Parkin is implicated in familial PD. Lately, data based on both in vitro and in vivo analyses of animal and human brain samples have identified Parkin, a member of the family of ubiquitin E3 ligases, to be responsible for ubiquitinating aberrant proteins targeted for proteasomal degradation. S-Nitrosylation of the active-site cysteine of Parkin triggered by NO/ROS can inhibit selectively its ligase activity and therefore result in dysfunction of the ubiquitin proteasome pathway (37, 199). The zinc-finger protein PARIS (ZNF746), a recently identified Parkin substrate, is found accumulated in human PD brain and in animals where Parkin has been knocked out at the adult stage (166). Thus, Parkin inactivation by either redox modification or gene ablation leads to PARIS accumulation and loss of neuronal cells, consistent with PD pathogenesis. Furthermore, Parkin can be tyrosine-phosphorylated and thereby deactivated by the stress-signaling kinase c-Abl, which itself is activated by oxidative stress in cultured neuronal cells and in striatum of mice. In addition, activated c-Abl, elevated phospho-Parkin, and accumulated Parkin substrates were found in postmortem striatal samples from PD patients relative to controls (79).
How inactivation of Parkin ultimately leads to neuronal death is an active area of investigation, including studies of overactivation of autophagy and dysregulation of mitochondrial fusion and fission. For example, in primary cortical neurons, overexpression of mutant A53T α-synuclein, which is implicated in familial PD, led to massive loss of mitochondria and neuronal degeneration. These effects were blocked when the Parkin or autophagy-related genes were silenced (36).
In models of Huntington's disease, GTPase dynamin-related protein-1 (DRP1) has been reported to be activated by binding to mutant huntingtin protein, promoting dysregulation of mitochondrial fission and mitochondrial fragmentation (173). In addition, S-nitrosylation of DRP1 has been reported in several contexts; however, a coherent picture has not yet evolved. Whereas Bossy et al. (22) reported that S-nitrosylation of DRP1 does not affect its enzymatic activity, Nakamura et al. reported (124) that S-nitrosylation of DRP1 induces oligomerization and activation of its GTPase function, contributing to abnormal mitochondrial fragmentation and synaptic damage analogous to the effect of huntingtin protein binding (173). Perhaps this discrepancy is related to the amount of conversion of DRP1-SNO to DRP1-SSG in the different studies.
VII. S-Glutathionylation and Plaque Formation
Insoluble plaques are associated with neurodegenerative disease as described above. Plaques have been studied extensively for any characteristic marker that would help define their existence. Oxidized proteins, detailed below, have been shown to accumulate in plaques.
A. Actin
Actin is an abundant cellular protein involved in cell growth, movement, and division through its maintenance of cellular infrastructure. Actin function has been shown to be redox regulated by reversible S-glutathionylation, dependent on Grx. Under control conditions for A431 epithelial cells and NIH3T3 fibroblasts, actin is largely glutathionylated at Cys 374. Stimulation by growth factors leads to Grx-dependent deglutathionylation and increased polymerization and rearrangement of the cellular actin (187, 188). Moreover, actin glutathionylation appears to play a role in axonal and dendrite stability as well as neuronal survival in normal brain (174). Actin glutathionylation has been implicated in a number of neuronal diseases. For example, fibroblasts from Freidreich's ataxia patients showed increased actin glutathionylation, coincident with enlarged cells and cytoplasm; immunocytochemical visualization showed a disarrangement of actin filaments as a consequence of the glutathionylation (140). AD brain samples have also revealed increased actin oxidation (4). Accordingly, fine control of the extent and reversibility of actin glutathionylation may differentiate normal function from participation in disease progression.
B. Tau
Tau protein aids in neurite extension and axonal growth via microtubule assembly and maintenance; its dysregulation is implicated in AD. Tau has a required cysteine residue (Cys 322) which facilitates its binding to microtubules. Cys322 has been reported to be oxidized in vitro, altering tau's ability to form dimers (45, 46). In particular, polymerization of the S-glutathionylated form of three-repeat tau was interpreted as evidence that tau-S-S-tau dimer formation is not required for filament assembly (36). Further studies are necessary, however, to distinguish whether such glutathionylation of tau protects tau from aggregation into neurofibrillary tangles or promotes this deleterious effect associated with AD.
VIII. S-Glutathionylation of Proteins Involved with Mitochondrial Respiration
Mitochondria are a major site of ROS production within the brain, mainly generated from the electron transport chain, as described above. Mitochondria limit excess ROS through their own system of antioxidant enzymes and pool of GSH which equilibrates only slowly with the cytosol. As aging occurs, control of ROS becomes dysregulated, resulting in modification of proteins within mitochondria. The oxidative modifications of sulfhydryl groups have consequences not only to protein function but also to cellular functions and viability. A number of examples are described below. The uncoupling proteins UCP2 and UCP3 are known to abate oxidative stress and participate in a range of cellular functions, including dissipation of heat and regulation of satiety. The UCPs have reactive cysteine residues that are subject to S-glutathionylation (110); therefore, it would be informative to examine whether reversible glutathionylation of the UCPs plays a role in neurodegenerative diseases, especially because impairment of mitochondrial functions has been implicated broadly in these diseases.
A. α-Ketoglutarate dehydrogenase
Analysis of Alzheimer brains shows inhibition of the tricarboxylic acid (TCA) cycle which is the source of reducing equivalents for the ETC: specifically diminution of α-ketoglutarate dehydrogenase (α-KGDH) activity is observed. However, the mechanism of α-KGDH deactivation in this case has not been characterized. Oxidants, including hydrogen peroxide and peroxynitrite, are known to inhibit the function of α-KGDH. For example, α-KGDH was inhibited in isolated mitochondria treated with hydrogen peroxide, and incubation with Grx restored activity (129). Likewise, peroxynitrite treatment of isolated α-KGDH inactivated the enzyme via tyrosine nitration and this was restored by reaction with GSH (164). Given the association of α-KGDH deactivation with AD and the potential for deactivation of the enzyme by S-glutathionylation, it is pertinent to consider whether glutathionylation of α-KGDH may be a contributing factor in neurodegeneration. This question is worthy of investigation in light of recent studies of selective knockdown of the E2K subunit of α-KGDH, deactivating the enzyme in mouse brain and altering glucose utilization. These results imply that diminished activity of the α-KGDH complex has the potential to impair glucose utilization as seen in several neurodegenerative diseases (127).
B. Mitochondrial NADP+-dependent isocitrate dehydrogenase
NADP+-dependent isocitrate dehydrogenase (IDPm) catalyzes the oxidative decarboxylation of isocitrate to form α-ketoglutarate and generate NADPH. Furthermore, IDPm has been reported to protect against oxidative stress, likely by generation of NADPH as substrate for the antioxidant enzymes such as GR and TR which support the Grx, Trx, GPx, and peroxiredoxin systems. This enzyme contains a critical Cys residue within its active site, shown previously to be oxidatively modified (53, 93, 169). In particular, glutathionylation of Cys 269 by GSSG (or other oxidants) deactivates IDPm, and the isolated enzyme can be reactivated by either DTT or Grx2 plus GSH (94). In the same study, mice were treated with MPTP to simulate Parkinson's disease, resulting in a dose-dependent increase in glutathionylation of IDPm (94). Additional studies are needed to determine the consequence of glutathionylation of IDPm in the actual context of neurodegenerative disease.
C. Complex I
NADH-ubiquinone oxidoreductase, Complex I, is the first enzyme in the mitochondrial electron transport chain, responsible for the majority of cellular ATP production (77). In PD, inhibition of Complex I by as little as 25% is sufficient to impair oxidative phosphorylation. Modeling this effect, Complex I inhibitors, such as MPTP and rotenone, induce Parkinsonism in mice, impede ATP production, and stimulate ROS formation. These combined effects result in excessive oxidative stress and ultimately cell death (182). Grx 1 protein was elevated in mice acutely treated with MPTP potentially due to a protective antioxidant response. In separate studies, knocking down either Grx1 or Grx2 led to decreased Complex I activity. These findings implicate both Grx1 and Grx2 in the redox regulation of Complex I (89, 91). Consistent with this hypothesis, treatment of mitochondria with excess GSSG resulted in S-glutathionylation of Complex I and loss of its function; however, addition of GSH and Grx2 only partially reversed the inhibition (18, 179). Further characterization showed Complex I is glutathionylated on the 75kDa subunit at Cys 531 and Cys 704 resulting in loss of activity (78). It remains to be determined whether Grx2 in the mitochondrial matrix or Grx1 in the intermembrane space (136), or both, are responsible for regulating the activity of Complex I in situ.
D. Complex II
Complex II mediates the second step in the electron transport chain. This enzyme, succinate quinone reductase, couples oxidation of succinate to the reduction of ubiquinone. Recent studies examining ischemia in heart tissue reported a decrease in activity of Complex II under ischemic conditions. This loss of activity was correlated to an increase in S-glutathionylation of the 70 kDa subunit at Cys 90 as determined by anti-glutathione antibody reactivity. Under more intense treatment conditions, other Cys residues became glutathionylated as well (35). Interestingly, S-glutathionylation of the isolated protein resulted in protection from tyrosine nitration by peroxynitrite and preserved enzyme function (33). Remarkably, unlike Complex I, where glutathionylation deactivates its electron transfer activity and increases ROS production, isolated Complex II-SSG displays increased activity and decreased ROS generation. Since these changes have not been examined in neurodegeration models, directed studies are needed to determine if glutathionylation on Complex II plays a role.
E. ATP synthase
ATP synthase is responsible for converting ADP to ATP and is powered by the proton gradient established by the electron transport chain. In studies involving isolated brain and liver mitochondria from rats, ATP synthase was shown to be glutathionylated according to Western blot analysis with anti-GSH antibodies and mass spectrometry. Glutathionylation of ATP synthase also resulted in loss of enzyme function. Remarkably, brain mitochondria showed pronounced glutathionylation of ATP synthase compared to isolated liver mitochondria, appearing to be the major mitochondrial protein that becomes glutathionylated under oxidative stress (66). Loss of activity would mean diminished levels of ATP for the brain cells. In addition to ATP synthase, studies of young and aged rats showed an increase in nNOS content with age as well as increased nitration of ATP synthase (101). Further studies need to be performed to determine whether inhibition of ATP synthase occurs in samples from patients with neurodegerative disease and contributes to neuronal cell death.
F. Succinyl CoA transferase
Succinyl CoA transferase has been shown in two separate studies to be modified with age and increased oxidative stress. This enzyme catalyzes the rate limiting step in ketolysis, converting ketone bodies to acetoacetyl CoA which enters the Kreb's cycle to produce energy. This important pathway provides an alternative source of energy in the brain during times of low glucose. In studies of aging rats, succinyl CoA transferase was nitrated and this was attributed to elevated nNOS (101). In isolated rat brain mitochondria, this enzyme also was shown to be glutathionylated, resulting in diminished enzyme activity (66). Whether deactivation of succinyl CoA transferase by glutathionylation contributes to neurodegeneration remains to be investigated.
IX. Potential Approaches to Therapy of the Neurodegenerative Diseases
The preceding descriptions of the neurodegenerative diseases have highlighted common features, including their multifactorial nature and involvement of oxidative stress, emanating both from within the neurons and from external inflammation. Thus, therapy is aimed at the local immune responses as well as the neurons. This is an especially difficult task because brain inflammation is necessary for brain repair, but chronic inflammation is neuropathogenic. Accordingly, one recent study has focused on the selective cytokine action of IL-10 which can suppress neuroinflammation. Using a mouse model of Alzheimer's disease, adeno-associated virus-mediated expression of the IL-10 gene in neurons was found to improve cognitive function, but direct administration of the IL-10 did not (97). Another evolving approach involves inhibitors of histone deacetylase (HDAC), which have been reported to diminish oxidative stress and inflammation in models of Parkinson's disease. Co-administration with MPTP of the HDAC inhibitor valproic acid protected against dopaminergic cell loss in the substantia nigra (92). Valproic acid has the advantage of being an already useful drug for chronic disease, namely epilepsy. Another promising strategy for PD which is currently in clinical trials involves administration of adenosine receptor (AdR) antagonists along with L-DOPA, because the AdR antagonists appear to stimulate dopamine receptors as well as diminishing neurodegeneration (11). Ultimately, the goals of therapy for the neurodegenerative diseases include not only alleviation of symptoms and interference with disease progression, but also stimulation of neuronal regeneration (81).
X. Conclusions
This review provides an overview of the role of oxidative stress in initiation/propagation of neurodegenerative diseases and highlights multiple protein targets whose oxidative modification, in particular S-glutathionylation, may contribute to the disease process. Much of the current information has been garnered from studies that were focused on oxidative stress and redox signaling situations other than neurodegeneration. Nevertheless, the analogous contexts of oxidative stress and disruption of thiol homeostasis support the proposition that the findings are likely to apply also to neurodegenerative processes. As techniques continue to evolve to selectively identify specific glutathionylated proteins in a quantitative fashion related to changes in cellular functions, new insights about neuropathological consequences of this oxidative protein modification will emerge, informing novel approaches to therapeutic development.
Abbreviations Used
- Aβ
amyloid beta
- AD
Alzheimer's disease
- Akt
serine/threonine protein kinase (aka protein kinase B)
- ALS
amyotrophic lateral sclerosis
- ANT
adenosine nucleotide transporter
- ASK1
apoptosis signal regulating kinase 1
- BBB
blood-brain barrier
- CEP
ω-2-carboxyethyl pyrole
- CFTR
cystic fibrosis transmembrane conductance regulator
- CNS
central nervous system
- DRP1
dynamin-related protein-1
- DTT
dithiothreitol
- ER
endoplasmic reticulum
- ETC
electron transport chain
- FRDA
Friedriech's ataxia
- GR
GSSG reductase
- Grx
glutaredoxin
- GSH
glutathione
- GSSG
glutathione disulfide
- HD
Huntington's disease
- HSP
heat shock protein
- ICAM-1
intercelluIar adhesion molecule-1
- IDPm
NADP+-dependent isocitrate dehydrogenase
- IKKβ
I-kappaB kinase-beta
- IL-1B
interleukin-1B
- IL-6
interleukin-6
- α-KGDH
α-ketoglutarate dehydrogenase
- L-DOPA
L-dihydroxyphenylalanine
- LPS
lipopolysaccharide
- LRRK2
leucine-rich repeat kinase 2
- MAPK
mitogen activated protein kinase
- MAPKKK
mitogen activated protein kinase kinase kinase
- MPP
mitochondrial permeability pore
- MPP+
1-methyl-4-phenylpyridinium
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NF-1
nuclear factor-1
- NF-κB
nuclear factor kappaB
- eNOS
endothelial nitrogen oxide synthase
- iNOS
inducible nitrogen oxide synthetase
- NOX
NADPH oxidase
- 6-OHDA
6-hydroxydopamine
- p53
protein 53 tumor suppressor
- p65
NFκB subunit protein (65 kDa)
- PBMC
peripheral blood mononuclear cells
- PD
Parkinson's disease
- PINK1
PTEN-induced putative kinase 1
- PKA
protein kinase A
- PKC
protein kinase C
- Protein-SNO
S-nitrosylated protein
- Protein-SOH
protein-sulfenic acid
- Protein-SSG
S-glutathionylated protein
- Prx
peroxiredoxin
- rMC-1
rat retinal glial cells
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- RYR
ryanodine receptor
- SERCA
sarcoplasmic reticulum calcium ATPase
- SOD
superoxide dismutase
- TDOR
thiol-disulfide oxidoreductase
- TH
tyrosine hydroxylase
- TLR
Toll-like receptor
- TNF-α
tumor necrosis factor-alpha
- TR
thioredoxin reductase
- Trx
thioredoxin
- UCH-L1
ubiquitin carboxyl-terminal esterase L1
- VDAC
voltage-dependent anion channel
Acknowledgments
This effort was supported in part by National Institutes of Health Grant PO1 AG 15885 (JJM) and a Department of Veterans Affairs Merit Review Grant (JJM), and NIH Training Grants T32 GM008803 (EAS) and T32 DK007319 (EAS). The authors are grateful to Dr. Ruth Siegel for critical review of the manuscript.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Adachi T. Pimentel DR. Heibeck T. Hou X. Lee YJ. Jiang B. Ido Y. Cohen RA. S-glutathiolation of Ras mediates redox-sensitive signaling by angiotensin II in vascular smooth muscle cells. J Biol Chem. 2004;279:29857–29862. doi: 10.1074/jbc.M313320200. [DOI] [PubMed] [Google Scholar]
- 2.Adachi T. Weisbrod RM. Pimentel DR. Ying J. Sharov VS. Schoneich C. Cohen RA. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat Med. 2004;10:1200–1207. doi: 10.1038/nm1119. [DOI] [PubMed] [Google Scholar]
- 3.Adam-Vizi V. Production of reactive oxygen species in brain mitochondria: Contribution by electron transport chain and non-electron transport chain sources. Antioxid Redox Signal. 2005;7:1140–1149. doi: 10.1089/ars.2005.7.1140. [DOI] [PubMed] [Google Scholar]
- 4.Aksenov MY. Aksenova MV. Butterfield DA. Geddes JW. Markesbery WR. Protein oxidation in the brain in Alzheimer's disease. Neuroscience. 2001;103:373–383. doi: 10.1016/s0306-4522(00)00580-7. [DOI] [PubMed] [Google Scholar]
- 5.Akterin S. Cowburn RF. Miranda-Vizuete A. Jimenez A. Bogdanovic N. Winblad B. Cedazo-Minguez A. Involvement of glutaredoxin-1 and thioredoxin-1 in beta-amyloid toxicity and Alzheimer's disease. Cell Death Differ. 2006;13:1454–1465. doi: 10.1038/sj.cdd.4401818. [DOI] [PubMed] [Google Scholar]
- 6.Ansari MA. Scheff SW. Oxidative stress in the progression of Alzheimer disease in the frontal cortex. J Neuropathol Exp Neurol. 2010;69:155–167. doi: 10.1097/NEN.0b013e3181cb5af4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ara J. Przedborski S. Naini AB. Jackson-Lewis V. Trifiletti RR. Horwitz J. Ischiropoulos H. Inactivation of tyrosine hydroxylase by nitration following exposure to peroxynitrite and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Proc Natl Acad Sci USA. 1998;95:7659–7663. doi: 10.1073/pnas.95.13.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aracena-Parks P. Goonasekera SA. Gilman CP. Dirksen RT. Hidalgo C. Hamilton SL. Identification of cysteines involved in S-nitrosylation, S-glutathionylation, and oxidation to disulfides in ryanodine receptor type 1. J Biol Chem. 2006;281:40354–40368. doi: 10.1074/jbc.M600876200. [DOI] [PubMed] [Google Scholar]
- 9.Aram L. Geula S. Arbel N. Shoshan-Barmatz V. VDAC1 cysteine residues: Topology and function in channel activity and apoptosis. Biochem J. 2010;427:445–454. doi: 10.1042/BJ20091690. [DOI] [PubMed] [Google Scholar]
- 10.Aran M. Ferrero DS. Pagano E. Wolosiuk RA. Typical 2-Cys peroxiredoxins—Modulation by covalent transformations and noncovalent interactions. FEBS J. 2009;276:2478–2493. doi: 10.1111/j.1742-4658.2009.06984.x. [DOI] [PubMed] [Google Scholar]
- 11.Armentero MT. Pinna A. Ferre S. Lanciego JL. Muller CE. Franco R. Past, present and future of A(2A) adenosine receptor antagonists in the therapy of Parkinson's disease. Pharmacol Ther. 2011;132:280–299. doi: 10.1016/j.pharmthera.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baines CP. Kaiser RA. Purcell NH. Blair NS. Osinska H. Hambleton MA. Brunskill EW. Sayen MR. Gottlieb RA. Dorn GW. Robbins J. Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 13.Baines CP. Kaiser RA. Sheiko T. Craigen WJ. Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bandyopadhyay S. Starke DW. Mieyal JJ. Gronostajski RM. Thioltransferase (glutaredoxin) reactivates the DNA-binding activity of oxidation-inactivated nuclear factor I. J Biol Chem. 1998;273:392–397. doi: 10.1074/jbc.273.1.392. [DOI] [PubMed] [Google Scholar]
- 15.Barrett WC. DeGnore JP. Keng YF. Zhang ZY. Yim MB. Chock PB. Roles of superoxide radical anion in signal transduction mediated by reversible regulation of protein-tyrosine phosphatase 1B. J Biol Chem. 1999;274:34543–34546. doi: 10.1074/jbc.274.49.34543. [DOI] [PubMed] [Google Scholar]
- 16.Barrett WC. DeGnore JP. Konig S. Fales HM. Keng YF. Zhang ZY. Yim MB. Chock PB. Regulation of PTP1B via glutathionylation of the active site cysteine 215. Biochemistry. 1999;38:6699–6705. doi: 10.1021/bi990240v. [DOI] [PubMed] [Google Scholar]
- 17.Bedard K. Krause KH. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 18.Beer SM. Taylor ER. Brown SE. Dahm CC. Costa NJ. Runswick MJ. Murphy MP. Glutaredoxin 2 catalyzes the reversible oxidation and glutathionylation of mitochondrial membrane thiol proteins: Implications for mitochondrial redox regulation and antioxidant DEFENSE. J Biol Chem. 2004;279:47939–47951. doi: 10.1074/jbc.M408011200. [DOI] [PubMed] [Google Scholar]
- 19.Belzacq AS. Vieira HL. Verrier F. Vandecasteele G. Cohen I. Prevost MC. Larquet E. Pariselli F. Petit PX. Kahn A. Rizzuto R. Brenner C. Kroemer G. Bcl-2 and Bax modulate adenine nucleotide translocase activity. Cancer Res. 2003;63:541–546. [PubMed] [Google Scholar]
- 20.Benhar M. Forrester MT. Stamler JS. Protein denitrosylation: Enzymatic mechanisms and cellular functions. Nat Rev Mol Cell Biol. 2009;10:721–732. doi: 10.1038/nrm2764. [DOI] [PubMed] [Google Scholar]
- 21.Borges CR. Geddes T. Watson JT. Kuhn DM. Dopamine biosynthesis is regulated by S-glutathionylation. Potential mechanism of tyrosine hydroxylase inhibition during oxidative stress. J Biol Chem. 2002;277:48295–48302. doi: 10.1074/jbc.M209042200. [DOI] [PubMed] [Google Scholar]
- 22.Bossy B. Petrilli A. Klinglmayr E. Chen J. Lutz-Meindl U. Knott AB. Masliah E. Schwarzenbacher R. Bossy-Wetzel E. S-Nitrosylation of DRP1 does not affect enzymatic activity and is not specific to Alzheimer's disease. J Alzheimers Dis. 2010;20(Suppl 2):S513–S526. doi: 10.3233/JAD-2010-100552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bredesen DE. Rao RV. Mehlen P. Cell death in the nervous system. Nature. 2006;443:796–802. doi: 10.1038/nature05293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brochard V. Combadiere B. Prigent A. Laouar Y. Perrin A. Beray-Berthat V. Bonduelle O. Varez-Fischer D. Callebert J. Launay JM. Duyckaerts C. Flavell RA. Hirsch EC. Hunot S. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest. 2009;119:182–192. doi: 10.1172/JCI36470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bull R. Finkelstein JP. Galvez J. Sanchez G. Donoso P. Behrens MI. Hidalgo C. Ischemia enhances activation by Ca2+ and redox modification of ryanodine receptor channels from rat brain cortex. J Neurosci. 2008;28:9463–9472. doi: 10.1523/JNEUROSCI.2286-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calabrese V. Cornelius C. Rizzarelli E. Owen JB. Dinkova-Kostova AT. Butterfield DA. Nitric oxide in cell survival: A janus molecule. Antioxid Redox Signal. 2009;11:2717–2739. doi: 10.1089/ars.2009.2721. [DOI] [PubMed] [Google Scholar]
- 27.Candore G. Bulati M. Caruso C. Castiglia L. Colonna-Romano G. Di BD. Duro G. Lio D. Matranga D. Pellicano M. Rizzo C. Scapagnini G. Vasto S. Inflammation, cytokines, immune response, apolipoprotein E, cholesterol, and oxidative stress in Alzheimer disease: Therapeutic implications. Rejuvenation Res. 2010;13:301–313. doi: 10.1089/rej.2009.0993. [DOI] [PubMed] [Google Scholar]
- 28.Carbone DL. Doorn JA. Kiebler Z. Ickes BR. Petersen DR. Modification of heat shock protein 90 by 4-hydroxynonenal in a rat model of chronic alcoholic liver disease. J Pharmacol Exp Ther. 2005;315:8–15. doi: 10.1124/jpet.105.088088. [DOI] [PubMed] [Google Scholar]
- 29.Carbone DL. Doorn JA. Kiebler Z. Sampey BP. Petersen DR. Inhibition of Hsp72-mediated protein refolding by 4-hydroxy-2-nonenal. Chem Res Toxicol. 2004;17:1459–1467. doi: 10.1021/tx049838g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Casagrande S. Bonetto V. Fratelli M. Gianazza E. Eberini I. Massignan T. Salmona M. Chang G. Holmgren A. Ghezzi P. Glutathionylation of human thioredoxin: A possible crosstalk between the glutathione and thioredoxin systems. Proc Natl Acad Sci USA. 2002;99:9745–9749. doi: 10.1073/pnas.152168599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cenini G. Sultana R. Memo M. Butterfield DA. Effects of oxidative and nitrosative stress in brain on p53 proapoptotic protein in amnestic mild cognitive impairment and Alzheimer disease. Free Radic Biol Med. 2008;45:81–85. doi: 10.1016/j.freeradbiomed.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen CA. Wang TY. Varadharaj S. Reyes LA. Hemann C. Talukder MA. Chen YR. Druhan LJ. Zweier JL. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature. 2010;468:1115–1118. doi: 10.1038/nature09599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen CL. Chen J. Rawale S. Varadharaj S. Kaumaya PP. Zweier JL. Chen YR. Protein tyrosine nitration of the flavin subunit is associated with oxidative modification of mitochondrial complex II in the post-ischemic myocardium. J Biol Chem. 2008;283:27991–28003. doi: 10.1074/jbc.M802691200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen VT. Huang CL. Lee YC. Liao WC. Huang NK. The roles of the thioredoxin system and peroxiredoxins in 1-methyl-4-phenyl-pyridinium ion-induced cytotoxicity in rat pheochromocytoma cells. Toxicol In Vitro. 2010:1577–1583. doi: 10.1016/j.tiv.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 35.Chen YR. Chen CL. Pfeiffer DR. Zweier JL. Mitochondrial complex II in the post-ischemic heart: oxidative injury and the role of protein S-glutathionylation. J Biol Chem. 2007;282:32640–32654. doi: 10.1074/jbc.M702294200. [DOI] [PubMed] [Google Scholar]
- 36.Choubey V. Safiulina D. Vaarmann A. Cagalinec M. Wareski P. Kuum M. Zharkovsky A. Kaasik A. Mutant A53T alpha-synuclein induces neuronal death by increasing mitochondrial autophagy. J Biol Chem. 2011;286:10814–10824. doi: 10.1074/jbc.M110.132514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chung KK. Thomas B. Li X. Pletnikova O. Troncoso JC. Marsh L. Dawson VL. Dawson TM. S-nitrosylation of parkin regulates ubiquitination and compromises parkin's protective function. Science. 2004;304:1328–1331. doi: 10.1126/science.1093891. [DOI] [PubMed] [Google Scholar]
- 38.Chung S. Sundar IK. Yao H. Ho YS. Rahman I. Glutaredoxin 1 regulates cigarette smoke-mediated lung inflammation through differential modulation of I{kappa}B kinases in mice: Impact on histone acetylation. Am J Physiol Lung Cell Mol Physiol. 2010;299:L192–L203. doi: 10.1152/ajplung.00426.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crabb JW. Miyagi M. Gu X. Shadrach K. West KA. Sakaguchi H. Kamei M. Hasan A. Yan L. Rayborn ME. Salomon RG. Hollyfield JG. Drusen proteome analysis: An approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci USA. 2002;99:14682–14687. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dagda RK. Chu CT. Mitochondrial quality control: Insights on how Parkinson's disease related genes PINK1, parkin, and Omi/HtrA2 interact to maintain mitochondrial homeostasis. J Bioenerg Biomembr. 2009;41:473–479. doi: 10.1007/s10863-009-9255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daily D. Vlamis-Gardikas A. Offen D. Mittelman L. Melamed E. Holmgren A. Barzilai A. Glutaredoxin protects cerebellar granule neurons from dopamine-induced apoptosis by activating NF-kappa B via Ref-1. J Biol Chem. 2001;276:1335–1344. doi: 10.1074/jbc.M008121200. [DOI] [PubMed] [Google Scholar]
- 42.Daily D. Vlamis-Gardikas A. Offen D. Mittelman L. Melamed E. Holmgren A. Barzilai A. Glutaredoxin protects cerebellar granule neurons from dopamine-induced apoptosis by dual activation of the ras-phosphoinositide 3-kinase and jun n-terminal kinase pathways. J Biol Chem. 2001;276:21618–21626. doi: 10.1074/jbc.M101400200. [DOI] [PubMed] [Google Scholar]
- 43.Davis DA. Newcomb FM. Starke DW. Ott DE. Mieyal JJ. Yarchoan R. Thioltransferase (glutaredoxin) is detected within HIV-1 and can regulate the activity of glutathionylated HIV-1 protease in vitro. J Biol Chem. 1997;272:25935–25940. doi: 10.1074/jbc.272.41.25935. [DOI] [PubMed] [Google Scholar]
- 44.Demasi M. Silva GM. Netto LE. 20 S proteasome from Saccharomyces cerevisiae is responsive to redox modifications and is S-glutathionylated. J Biol Chem. 2003;278:679–685. doi: 10.1074/jbc.M209282200. [DOI] [PubMed] [Google Scholar]
- 45.Di Noto L. Biochemical Investigation of Tau and MAP2: Polymerization implications for neuropathic filament assembly in Alzheimer's disease (Dissertation) University of Florida; Gainsville, FL: 2002. [Google Scholar]
- 46.Di Noto L. Deture MA. Purich DL. Structural insights into Alzheimer filament assembly pathways based on site-directed mutagenesis and S-glutathionylation of three-repeat neuronal Tau protein. Microsc Res Tech. 2005;67:156–163. doi: 10.1002/jemt.20195. [DOI] [PubMed] [Google Scholar]
- 47.Di DF. Cenini G. Sultana R. Perluigi M. Uberti D. Memo M. Butterfield DA. Glutathionylation of the pro-apoptotic protein p53 in Alzheimer's disease brain: Implications for AD pathogenesis. Neurochem Res. 2009;34:727–733. doi: 10.1007/s11064-009-9924-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dickinson BC. Peltier J. Stone D. Schaffer DV. Chang CJ. Nox2 redox signaling maintains essential cell populations in the brain. Nat Chem Biol. 2011;7:106–112. doi: 10.1038/nchembio.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Double KL. Todd G. Duma SR. Pathophysiology of transcranial sonography signal changes in the human substantia nigra. Int Rev Neurobiol. 2010;90:107–120. doi: 10.1016/S0074-7742(10)90008-1. [DOI] [PubMed] [Google Scholar]
- 50.Du ZX. Yan Y. Zhang HY. Liu BQ. Gao YY. Niu XF. Guan Y. Meng X. Wang HQ. Suppression of MG132-mediated cell death by peroxiredoxin 1 through influence on ASK1 activation in human thyroid cancer cells. Endocr Relat Cancer. 2010;17:553–560. doi: 10.1677/ERC-09-0269. [DOI] [PubMed] [Google Scholar]
- 51.Ehrhart J. Gluck M. Mieyal J. Zeevalk GD. Functional glutaredoxin (thioltransferase) activity in rat brain and liver mitochondria. Parkinsonism Relat Disord. 2002;8:395–400. doi: 10.1016/s1353-8020(02)00020-2. [DOI] [PubMed] [Google Scholar]
- 52.Farina C. Aloisi F. Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28:138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 53.Fatania HR. al-Nassar KE. Thomas N. Chemical modification of rat liver cytosolic NADP(+)-linked isocitrate dehydrogenase by N-ethylmaleimide. Evidence for essential sulphydryl groups. FEBS Lett. 1993;322:245–248. doi: 10.1016/0014-5793(93)81579-o. [DOI] [PubMed] [Google Scholar]
- 54.Ferri A. Fiorenzo P. Nencini M. Cozzolino M. Pesaresi MG. Valle C. Sepe S. Moreno S. Carri MT. Glutaredoxin 2 prevents aggregation of mutant SOD1 in mitochondria and abolishes its toxicity. Hum Mol Genet. 2010;19:4529–4542. doi: 10.1093/hmg/ddq383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferrington DA. Kapphahn RJ. Catalytic site-specific inhibition of the 20S proteasome by 4-hydroxynonenal. FEBS Lett. 2004;578:217–223. doi: 10.1016/j.febslet.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 56.Findlay VJ. Townsend DM. Morris TE. Fraser JP. He L. Tew KD. A novel role for human sulfiredoxin in the reversal of glutathionylation. Cancer Res. 2006;66:6800–6806. doi: 10.1158/0008-5472.CAN-06-0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Floyd RA. Antioxidants, oxidative stress, and degenerative neurological disorders. Proc Soc Exp Biol Med. 1999;222:236–245. doi: 10.1046/j.1525-1373.1999.d01-140.x. [DOI] [PubMed] [Google Scholar]
- 58.Foster MW. Hess DT. Stamler JS. Protein S-nitrosylation in health and disease: A current perspective. Trends Mol Med. 2009;15:391–404. doi: 10.1016/j.molmed.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Franco R. Cidlowski JA. Apoptosis and glutathione: Beyond an antioxidant. Cell Death Differ. 2009;16:1303–1314. doi: 10.1038/cdd.2009.107. [DOI] [PubMed] [Google Scholar]
- 60.Friguet B. Szweda LI. Inhibition of the multicatalytic proteinase (proteasome) by 4-hydroxy-2-nonenal cross-linked protein. FEBS Lett. 1997;405:21–25. doi: 10.1016/s0014-5793(97)00148-8. [DOI] [PubMed] [Google Scholar]
- 61.Fujii H. Ichimori K. Hoshiai K. Nakazawa H. Nitric oxide inactivates NADPH oxidase in pig neutrophils by inhibiting its assembling process. J Biol Chem. 1997;272:32773–32778. doi: 10.1074/jbc.272.52.32773. [DOI] [PubMed] [Google Scholar]
- 62.Fuxe KG. Tarakanov AO. Goncharova LB. Agnati LF. A new road to neuroinflammation in Parkinson's disease? Brain Res Rev. 2008;58:453–458. doi: 10.1016/j.brainresrev.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 63.Galganska H. Karachitos A. Wojtkowska M. Stobienia O. Budzinska M. Kmita H. Communication between mitochondria and nucleus: Putative role for VDAC in reduction/oxidation mechanism. Biochim Biophys Acta. 2010;1797:1276–1280. doi: 10.1016/j.bbabio.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 64.Gallogly MM. Mieyal JJ. Mechanisms of reversible protein glutathionylation in redox signaling and oxidative stress. Curr Opin Pharmacol. 2007;7:381–391. doi: 10.1016/j.coph.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 65.Gallogly MM. Starke DW. Mieyal JJ. Mechanistic and kinetic details of catalysis of thiol-disulfide exchange by glutaredoxins and potential mechanisms of regulation. Antioxid Redox Signal. 2009;11:1059–1081. doi: 10.1089/ars.2008.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garcia J. Han D. Sancheti H. Yap LP. Kaplowitz N. Cadenas E. Regulation of mitochondrial glutathione redox status and protein glutathionylation by respiratory substrates. J Biol Chem. 2010;285:39646–39654. doi: 10.1074/jbc.M110.164160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Glass CK. Saijo K. Winner B. Marchetto MC. Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goos M. Zech WD. Jaiswal MK. Balakrishnan S. Ebert S. Mitchell T. Carri MT. Keller BU. Nau R. Expression of a Cu,Zn superoxide dismutase typical for familial amyotrophic lateral sclerosis increases the vulnerability of neuroblastoma cells to infectious injury. BMC Infect Dis. 2007;7:131. doi: 10.1186/1471-2334-7-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gutscher M. Sobotta MC. Wabnitz GH. Ballikaya S. Meyer AJ. Samstag Y. Dick TP. Proximity-based protein thiol oxidation by H2O2-scavenging peroxidases. J Biol Chem. 2009;284:31532–31540. doi: 10.1074/jbc.M109.059246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haider L. Fischer MT. Frischer JM. Bauer J. Hoftberger R. Botond G. Esterbauer H. Binder CJ. Witztum JL. Lassmann H. Oxidative damage in multiple sclerosis lesions. Brain. 2011;134:1914–1924. doi: 10.1093/brain/awr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Halestrap AP. Clarke SJ. Khaliulin I. The role of mitochondria in protection of the heart by preconditioning. Biochim Biophys Acta. 2007;1767:1007–1031. doi: 10.1016/j.bbabio.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hashemy SI. Johansson C. Berndt C. Lillig CH. Holmgren A. Oxidation and S-nitrosylation of cysteines in human cytosolic and mitochondrial glutaredoxins: Effects on structure and activity. J Biol Chem. 2007;282:14428–14436. doi: 10.1074/jbc.M700927200. [DOI] [PubMed] [Google Scholar]
- 73.Heutink P. PINK-1 and DJ-1–new genes for autosomal recessive Parkinson's disease. J Neural Transm Suppl. 2006:215–219. doi: 10.1007/978-3-211-45295-0_33. [DOI] [PubMed] [Google Scholar]
- 74.Hickey WF. Basic principles of immunological surveillance of the normal central nervous system. Glia. 2001;36:118–124. doi: 10.1002/glia.1101. [DOI] [PubMed] [Google Scholar]
- 75.Hirsch EC. Hunot S. Neuroinflammation in Parkinson's disease: A target for neuroprotection? Lancet Neurol. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- 76.Hu X. Weng Z. Chu CT. Zhang L. Cao G. Gao Y. Signore A. Zhu J. Hastings T. Greenamyre JT. Chen J. Peroxiredoxin-2 protects against 6-hydroxydopamine-induced dopaminergic neurodegeneration via attenuation of the apoptosis signal-regulating kinase (ASK1) signaling cascade. J Neurosci. 2011;31:247–261. doi: 10.1523/JNEUROSCI.4589-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hurd TR. Costa NJ. Dahm CC. Beer SM. Brown SE. Filipovska A. Murphy MP. Glutathionylation of mitochondrial proteins. Antioxid Redox Signal. 2005;7:999–1010. doi: 10.1089/ars.2005.7.999. [DOI] [PubMed] [Google Scholar]
- 78.Hurd TR. Requejo R. Filipovska A. Brown S. Prime TA. Robinson AJ. Fearnley IM. Murphy MP. Complex I within oxidatively stressed bovine heart mitochondria is glutathionylated on Cys-531 and Cys-704 of the 75-kDa subunit: Potential role of CYS residues in decreasing oxidative damage. J Biol Chem. 2008;283:24801–24815. doi: 10.1074/jbc.M803432200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Imam SZ. Zhou Q. Yamamoto A. Valente AJ. Ali SF. Bains M. Roberts JL. Kahle PJ. Clark RA. Li S. Novel regulation of parkin function through c-Abl-mediated tyrosine phosphorylation: Implications for Parkinson's disease. J Neurosci. 2011;31:157–163. doi: 10.1523/JNEUROSCI.1833-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Infanger DW. Sharma RV. Davisson RL. NADPH oxidases of the brain: Distribution, regulation, and function. Antioxid Redox Signal. 2006;8:1583–1596. doi: 10.1089/ars.2006.8.1583. [DOI] [PubMed] [Google Scholar]
- 81.Iqbal K. Grundke-Iqbal I. Opportunities and challenges in developing Alzheimer disease therapeutics. Acta Neuropathol. 2011;122:543–549. doi: 10.1007/s00401-011-0878-z. [DOI] [PubMed] [Google Scholar]
- 82.Ischiropoulos H. Beckman JS. Oxidative stress and nitration in neurodegeneration: Cause, effect, or association? J Clin Invest. 2003;111:163–169. doi: 10.1172/JCI17638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jacob C. Knight I. Winyard PG. Aspects of the biological redox chemistry of cysteine: From simple redox responses to sophisticated signalling pathways. Biol Chem. 2006;387:1385–1397. doi: 10.1515/BC.2006.174. [DOI] [PubMed] [Google Scholar]
- 84.Jahngen-Hodge J. Obin MS. Gong X. Shang F. Nowell TR., Jr. Gong J. Abasi H. Blumberg J. Taylor A. Regulation of ubiquitin-conjugating enzymes by glutathione following oxidative stress. J Biol Chem. 1997;272:28218–28226. doi: 10.1074/jbc.272.45.28218. [DOI] [PubMed] [Google Scholar]
- 85.Jenner P. Dexter DT. Sian J. Schapira AH. Marsden CD. Oxidative stress as a cause of nigral cell death in Parkinson's disease and incidental Lewy body disease. The Royal Kings and Queens Parkinson's Disease Research Group. Ann Neurol. 1992;32(Suppl):S82–S87. doi: 10.1002/ana.410320714. [DOI] [PubMed] [Google Scholar]
- 86.Jha N. Kumar MJ. Boonplueang R. Andersen JK. Glutathione decreases in dopaminergic PC12 cells interfere with the ubiquitin protein degradation pathway: Relevance for Parkinson's disease? J Neurochem. 2002;80:555–561. doi: 10.1046/j.0022-3042.2001.00009.x. [DOI] [PubMed] [Google Scholar]
- 87.Junn E. Taniguchi H. Jeong BS. Zhao X. Ichijo H. Mouradian MM. Interaction of DJ-1 with Daxx inhibits apoptosis signal-regulating kinase 1 activity and cell death. Proc Natl Acad Sci USA. 2005;102:9691–9696. doi: 10.1073/pnas.0409635102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Karunakaran S. Diwakar L. Saeed U. Agarwal V. Ramakrishnan S. Iyengar S. Ravindranath V. Activation of apoptosis signal regulating kinase 1 (ASK1) and translocation of death-associated protein, Daxx, in substantia nigra pars compacta in a mouse model of Parkinson's disease: Protection by alpha-lipoic acid. FASEB J. 2007;21:2226–2236. doi: 10.1096/fj.06-7580com. [DOI] [PubMed] [Google Scholar]
- 89.Karunakaran S. Saeed U. Ramakrishnan S. Koumar RC. Ravindranath V. Constitutive expression and functional characterization of mitochondrial glutaredoxin (Grx2) in mouse and human brain. Brain Res. 2007;1185:8–17. doi: 10.1016/j.brainres.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 90.Kawamata H. Manfredi G. Import, maturation, and function of SOD1 and its copper chaperone CCS in the mitochondrial intermembrane space. Antioxid Redox Signal. 2010;13:1375–1384. doi: 10.1089/ars.2010.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kenchappa RS. Ravindranath V. Glutaredoxin is essential for maintenance of brain mitochondrial complex I: Studies with MPTP. FASEB J. 2003;17:717–719. doi: 10.1096/fj.02-0771fje. [DOI] [PubMed] [Google Scholar]
- 92.Kidd SK. Schneider JS. Protective effects of valproic acid on the nigrostriatal dopamine system in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease. Neuroscience. 2011;194:189–194. doi: 10.1016/j.neuroscience.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kil IS. Chung KH. Park JW. Silencing of mitochondrial NADP+ -dependent isocitrate dehydrogenase gene enhances selenite-induced apoptosis. Free Radic Res. 2010;44:332–339. doi: 10.3109/10715760903494184. [DOI] [PubMed] [Google Scholar]
- 94.Kil IS. Park JW. Regulation of mitochondrial NADP+ -dependent isocitrate dehydrogenase activity by glutathionylation. J Biol Chem. 2005;280:10846–10854. doi: 10.1074/jbc.M411306200. [DOI] [PubMed] [Google Scholar]
- 95.Kim RH. Smith PD. Aleyasin H. Hayley S. Mount MP. Pownall S. Wakeham A. You-Ten AJ. Kalia SK. Horne P. Westaway D. Lozano AM. Anisman H. Park DS. Mak TW. Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative stress. Proc Natl Acad Sci USA. 2005;102:5215–5220. doi: 10.1073/pnas.0501282102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim SY. Kim TJ. Lee KY. A novel function of peroxiredoxin 1 (Prx-1) in apoptosis signal-regulating kinase 1 (ASK1)-mediated signaling pathway. FEBS Lett. 2008;582:1913–1918. doi: 10.1016/j.febslet.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 97.Kiyota T. Ingraham KL. Swan RJ. Jacobsen MT. Andrews SJ. Ikezu T. AAV serotype 2/1-mediated gene delivery of anti-inflammatory interleukin-10 enhances neurogenesis and cognitive function in APP+PS1 mice. http://www.ncbi.nim.nih.gov/pubmed/21918553. Gene Ther. 2011 Sep 15; doi: 10.1038/gt.2011.126. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Klatt P. Molina EP. Lamas S. Nitric oxide inhibits c-Jun DNA binding by specifically targeted S-glutathionylation. J Biol Chem. 1999;274:15857–15864. doi: 10.1074/jbc.274.22.15857. [DOI] [PubMed] [Google Scholar]
- 99.Kokoszka JE. Waymire KG. Levy SE. Sligh JE. Cai J. Jones DP. MacGregor GR. Wallace DC. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427:461–465. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kumar P. Kalonia H. Kumar A. Huntington's disease: Pathogenesis to animal models. Pharmacol Rep. 2010;62:1–14. doi: 10.1016/s1734-1140(10)70238-3. [DOI] [PubMed] [Google Scholar]
- 101.Lam PY. Yin F. Hamilton RT. Boveris A. Cadenas E. Elevated neuronal nitric oxide synthase expression during ageing and mitochondrial energy production. Free Radic Res. 2009;43:431–439. doi: 10.1080/10715760902849813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee DW. Kaur D. Chinta SJ. Rajagopalan S. Andersen J. A Disruption in iron-sulfur center biogenesis via inhibition of mitochondrial dithiol glutaredoxin 2 may contribute to mitochondrial and cellular iron dysregulation in mammalian glutathione-depleted dopaminergic cells: Implications for Parkinsons disease. Antioxid Redox Signal. 2009;9:2083–2094. doi: 10.1089/ars.2009.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee YM. Park SH. Shin DI. Hwang JY. Park B. Park YJ. Lee TH. Chae HZ. Jin BK. Oh TH. Oh YJ. Oxidative modification of peroxiredoxin is associated with drug-induced apoptotic signaling in experimental models of Parkinson disease. J Biol Chem. 2008;283:9986–9998. doi: 10.1074/jbc.M800426200. [DOI] [PubMed] [Google Scholar]
- 104.Leist M. Volbracht C. Fava E. Nicotera P. 1-Methyl-4-phenylpyridinium induces autocrine excitotoxicity, protease activation, and neuronal apoptosis. Mol Pharmacol. 1998;54:789–801. doi: 10.1124/mol.54.5.789. [DOI] [PubMed] [Google Scholar]
- 105.Lillig CH. Berndt C. Vergnolle O. Lonn ME. Hudemann C. Bill E. Holmgren A. Characterization of human glutaredoxin 2 as iron-sulfur protein: A possible role as redox sensor. Proc Natl Acad Sci USA. 2005;102:8168–8173. doi: 10.1073/pnas.0500735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lin MT. Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 107.Lovell MA. Xie C. Gabbita SP. Markesbery WR. Decreased thioredoxin and increased thioredoxin reductase levels in Alzheimer's disease brain. Free Radic Biol Med. 2000;28:418–427. doi: 10.1016/s0891-5849(99)00258-0. [DOI] [PubMed] [Google Scholar]
- 108.Lowther WT. Haynes AC. Reduction of cysteine sulfinic acid in eukaryotic, typical 2-Cys peroxiredoxins by sulfiredoxin. Antioxid Redox Signal. 2011;15:99–109. doi: 10.1089/ars.2010.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Maguire-Zeiss KA. Short DW. Federoff HJ. Synuclein, dopamine and oxidative stress: Co-conspirators in Parkinson's disease? Brain Res Mol Brain Res. 2005;134:18–23. doi: 10.1016/j.molbrainres.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 110.Mailloux RJ. Seifert EL. Bouillaud F. Aguer C. Collins S. Harper ME. Glutathionylation acts as a control switch for uncoupling proteins UCP2 and UCP3. J Biol Chem. 2011;286:21865–21875. doi: 10.1074/jbc.M111.240242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mancuso M. Coppede F. Migliore L. Siciliano G. Murri L. Mitochondrial dysfunction, oxidative stress and neurodegeneration. J Alzheimers Dis. 2006;10:59–73. doi: 10.3233/jad-2006-10110. [DOI] [PubMed] [Google Scholar]
- 112.Markesbery WR. Oxidative stress hypothesis in Alzheimer's disease. Free Radic Biol Med. 1997;23:134–147. doi: 10.1016/s0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- 113.Martin HL. Teismann P. Glutathione—A review on its role and significance in Parkinson's disease. FASEB J. 2009;23:3263–3272. doi: 10.1096/fj.08-125443. [DOI] [PubMed] [Google Scholar]
- 114.Matsuzawa A. Ichijo H. Redox control of cell fate by MAP kinase: Physiological roles of ASK1-MAP kinase pathway in stress signaling. Biochim Biophys Acta. 2008;1780:1325–1336. doi: 10.1016/j.bbagen.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 115.Mattson MP. Calcium and neurodegeneration. Aging Cell. 2007;6:337–350. doi: 10.1111/j.1474-9726.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- 116.Mazzola JL. Sirover MA. Reduction of glyceraldehyde-3-phosphate dehydrogenase activity in Alzheimer's disease and in Huntington's disease fibroblasts. J Neurochem. 2001;76:442–449. doi: 10.1046/j.1471-4159.2001.00033.x. [DOI] [PubMed] [Google Scholar]
- 117.McGeer PL. Itagaki S. Boyes BE. McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- 118.Mieyal JJ. Gallogly MM. Qanungo S. Sabens EA. Shelton MD. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxid Redox Signal. 2008;10:1941–1988. doi: 10.1089/ars.2008.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mieyal JJ. Srinivasan U. Starke DW. Gravina SA. Mieyal PA. Packer L. Cadenas E. Biothiols in Health and Disease. Ref Type: Serial (Book, Monograph) Marcel Dekker, Inc.; 1995. Glutathionyl specificity of the thioltransferases: Mechanistic and physiological implications; pp. 305–372. [Google Scholar]
- 120.Mogi M. Kondo T. Mizuno Y. Nagatsu T. p53 protein, interferon-gamma, and NF-kappaB levels are elevated in the parkinsonian brain. Neurosci Lett. 2007;414:94–97. doi: 10.1016/j.neulet.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 121.Mohr S. Hallak H. de Boitte A. Lapetina EG. Brune B. Nitric oxide-induced S-glutathionylation and inactivation of glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1999;274:9427–9430. doi: 10.1074/jbc.274.14.9427. [DOI] [PubMed] [Google Scholar]
- 122.Murata H. Ihara Y. Nakamura H. Yodoi J. Sumikawa K. Kondo T. Glutaredoxin exerts an antiapoptotic effect by regulating the redox state of Akt. J Biol Chem. 2003;278:50226–50233. doi: 10.1074/jbc.M310171200. [DOI] [PubMed] [Google Scholar]
- 123.Nakajima H. Amano W. Fujita A. Fukuhara A. Azuma YT. Hata F. Inui T. Takeuchi T. The active site cysteine of the proapoptotic protein glyceraldehyde-3-phosphate dehydrogenase is essential in oxidative stress-induced aggregation and cell death. J Biol Chem. 2007;282:26562–26574. doi: 10.1074/jbc.M704199200. [DOI] [PubMed] [Google Scholar]
- 124.Nakamura T. Lipton SA. Redox modulation by S-nitrosylation contributes to protein misfolding, mitochondrial dynamics, and neuronal synaptic damage in neurodegenerative diseases. Cell Death Differ. 2011;18:1478–1486. doi: 10.1038/cdd.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nassif M. Hetz C. Targeting autophagy in ALS: A complex mission. Autophagy. 2011;7:450–453. doi: 10.4161/auto.7.4.14700. [DOI] [PubMed] [Google Scholar]
- 126.Newman SF. Sultana R. Perluigi M. Coccia R. Cai J. Pierce WM. Klein JB. Turner DM. Butterfield DA. An increase in S-glutathionylated proteins in the Alzheimer's disease inferior parietal lobule. A proteomics approach. J Neurosci Res. 2007;85:1506–1514. doi: 10.1002/jnr.21275. [DOI] [PubMed] [Google Scholar]
- 127.Nilsen LH. Shi Q. Gibson GE. Sonnewald U. Brain [U-(13) C]glucose metabolism in mice with decreased alpha-ketoglutarate dehydrogenase complex activity. J Neurosci Res. 2011;89:1997–2007. doi: 10.1002/jnr.22606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Norenberg MD. Rao KV. The mitochondrial permeability transition in neurologic disease. Neurochem Int. 2007;50:983–997. doi: 10.1016/j.neuint.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nulton-Persson AC. Starke DW. Mieyal JJ. Szweda LI. Reversible inactivation of alpha-ketoglutarate dehydrogenase in response to alterations in the mitochondrial glutathione status. Biochemistry. 2003;42:4235–4242. doi: 10.1021/bi027370f. [DOI] [PubMed] [Google Scholar]
- 130.Obin M. Shang F. Gong X. Handelman G. Blumberg J. Taylor A. Redox regulation of ubiquitin-conjugating enzymes: Mechanistic insights using the thiol-specific oxidant diamide. FASEB J. 1998;12:561–569. doi: 10.1096/fasebj.12.7.561. [DOI] [PubMed] [Google Scholar]
- 131.Okada K. Wangpoengtrakul C. Osawa T. Toyokuni S. Tanaka K. Uchida K. 4-Hydroxy-2-nonenal-mediated impairment of intracellular proteolysis during oxidative stress. Identification of proteasomes as target molecules. J Biol Chem. 1999;274:23787–23793. doi: 10.1074/jbc.274.34.23787. [DOI] [PubMed] [Google Scholar]
- 132.Okun E. Griffioen KJ. Lathia JD. Tang SC. Mattson MP. Arumugam TV. Toll-like receptors in neurodegeneration. Brain Res Rev. 2009;59:278–292. doi: 10.1016/j.brainresrev.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Olanow CW. Tatton WG. Etiology and pathogenesis of Parkinson's disease. Annu Rev Neurosci. 1999;22:123–144. doi: 10.1146/annurev.neuro.22.1.123. [DOI] [PubMed] [Google Scholar]
- 134.Opii WO. Nukala VN. Sultana R. Pandya JD. Day KM. Merchant ML. Klein JB. Sullivan PG. Butterfield DA. Proteomic identification of oxidized mitochondrial proteins following experimental traumatic brain injury. J Neurotrauma. 2007;24:772–789. doi: 10.1089/neu.2006.0229. [DOI] [PubMed] [Google Scholar]
- 135.Ouyang M. Shen X. Critical role of ASK1 in the 6-hydroxydopamine-induced apoptosis in human neuroblastoma SH-SY5Y cells. J Neurochem. 2006;97:234–244. doi: 10.1111/j.1471-4159.2006.03730.x. [DOI] [PubMed] [Google Scholar]
- 136.Pai HV. Starke DW. Lesnefsky EJ. Hoppel CL. Mieyal JJ. What is the functional significance of the unique location of glutaredoxin 1 (GRx1) in the intermembrane space of mitochondria? Antioxid Redox Signal. 2007;9:2027–2033. doi: 10.1089/ars.2007.1642. [DOI] [PubMed] [Google Scholar]
- 137.Pappolla MA. Omar RA. Kim KS. Robakis NK. Immunohistochemical evidence of oxidative [corrected] stress in Alzheimer's disease. Am J Pathol. 1992;140:621–628. [PMC free article] [PubMed] [Google Scholar]
- 138.Park HS. Cho SG. Kim CK. Hwang HS. Noh KT. Kim MS. Huh SH. Kim MJ. Ryoo K. Kim EK. Kang WJ. Lee JS. Seo JS. Ko YG. Kim S. Choi EJ. Heat shock protein hsp72 is a negative regulator of apoptosis signal-regulating kinase 1. Mol Cell Biol. 2002;22:7721–7730. doi: 10.1128/MCB.22.22.7721-7730.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Park JW. Mieyal JJ. Rhee SG. Chock PB. Deglutathionylation of 2-Cys peroxiredoxin is specifically catalyzed by sulfiredoxin. J Biol Chem. 2009;284:23364–23374. doi: 10.1074/jbc.M109.021394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pastore A. Tozzi G. Gaeta LM. Bertini E. Serafini V. Di Cesare S. Bonetto V. Casoni F. Carrozzo R. Federici G. Piemonte F. Actin glutathionylation increases in fibroblasts of patients with Friedreich's ataxia: A potential role in the pathogenesis of the disease. J Biol Chem. 2003;278:42588–42595. doi: 10.1074/jbc.M301872200. [DOI] [PubMed] [Google Scholar]
- 141.Patten DA. Germain M. Kelly MA. Slack RS. Reactive oxygen species: Stuck in the middle of neurodegeneration. J Alzheimers Dis. 2010;20(Suppl 2):S357–S367. doi: 10.3233/JAD-2010-100498. [DOI] [PubMed] [Google Scholar]
- 142.Pearce RK. Owen A. Daniel S. Jenner P. Marsden CD. Alterations in the distribution of glutathione in the substantia nigra in Parkinson's disease. J Neural Transm. 1997;104:661–677. doi: 10.1007/BF01291884. [DOI] [PubMed] [Google Scholar]
- 143.Perry VH. Nicoll JA. Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol. 2010;6:193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- 144.Pham FH. Sugden PH. Clerk A. Regulation of protein kinase B and 4E-BP1 by oxidative stress in cardiac myocytes. Circ Res. 2000;86:1252–1258. doi: 10.1161/01.res.86.12.1252. [DOI] [PubMed] [Google Scholar]
- 145.Piemonte F. Pastore A. Tozzi G. Tagliacozzi D. Santorelli FM. Carrozzo R. Casali C. Damiano M. Federici G. Bertini E. Glutathione in blood of patients with Friedreich's ataxia. Eur J Clin Invest. 2001;31:1007–1011. doi: 10.1046/j.1365-2362.2001.00922.x. [DOI] [PubMed] [Google Scholar]
- 146.Pimentel DR. Adachi T. Ido Y. Heibeck T. Jiang B. Lee Y. Melendez JA. Cohen RA. Colucci WS. Strain-stimulated hypertrophy in cardiac myocytes is mediated by reactive oxygen species-dependent Ras S-glutathiolation. J Mol Cell Cardiol. 2006;41:613–622. doi: 10.1016/j.yjmcc.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 147.Qanungo S. Starke DW. Pai HV. Mieyal JJ. Nieminen AL. Glutathione supplementation potentiates hypoxic apoptosis by S-glutathionylation of p65-NFkappaB. J Biol Chem. 2007;282:18427–18436. doi: 10.1074/jbc.M610934200. [DOI] [PubMed] [Google Scholar]
- 148.Qian L. Flood PM. Microglial cells and Parkinson's disease. Immunol Res. 2008;41:155–164. doi: 10.1007/s12026-008-8018-0. [DOI] [PubMed] [Google Scholar]
- 149.Queiroga CS. Almeida AS. Martel C. Brenner C. Alves PM. Vieira HL. Glutathionylation of adenine nucleotide translocase induced by carbon monoxide prevents mitochondrial membrane permeabilization and apoptosis. J Biol Chem. 2010;285:17077–17088. doi: 10.1074/jbc.M109.065052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Reddy PH. Mitochondrial dysfunction in aging and Alzheimer's disease: Strategies to protect neurons. Antioxid Redox Signal. 2007;9:1647–1658. doi: 10.1089/ars.2007.1754. [DOI] [PubMed] [Google Scholar]
- 151.Reynaert NL. van der Vliet A. Guala AS. McGovern T. Hristova M. Pantano C. Heintz NH. Heim J. Ho YS. Matthews DE. Wouters EF. Janssen-Heininger YM. Dynamic redox control of NF-kappaB through glutaredoxin-regulated S-glutathionylation of inhibitory kappaB kinase beta. Proc Natl Acad Sci USA. 2006;103:13086–13091. doi: 10.1073/pnas.0603290103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Riedlerer PF. Views on neurodegeneration as a basis for neuroprotective strategies. Med Sci Monit. 2004;10:RA287–RA290. [PubMed] [Google Scholar]
- 153.Romero JM. Bizzozero OA. Intracellular glutathione mediates the denitrosylation of protein nitrosothiols in the rat spinal cord. J Neurosci Res. 2009;87:701–709. doi: 10.1002/jnr.21897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sabens EA. Distler AM. Mieyal JJ. Levodopa deactivates enzymes that regulate thiol-disulfide homeostasis and promotes neuronal cell death: Implications for therapy of Parkinson's disease. Biochemistry. 2010;49:2715–2724. doi: 10.1021/bi9018658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sabens E. Stellar K. Mieyal JJ. Levodopa activates apoptosis activating kinase 1 (ASK1) and promotes apoptosis in a neuronal model: Implications for Parkinson's disease. Chem Res in Toxicol. 2011;24:1644–1652. doi: 10.1021/tx200082h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Saeed U. Durgadoss L. Valli RK. Joshi DC. Joshi PG. Ravindranath V. Knockdown of cytosolic glutaredoxin 1 leads to loss of mitochondrial membrane potential: Implication in neurodegenerative diseases. PLoS ONE. 2008;3:e2459. doi: 10.1371/journal.pone.0002459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Saeed U. Ray A. Valli RK. Kumar AM. Ravindranath V. DJ-1 Loss by glutaredoxin but not glutathione depletion triggers Daxx translocation and cell death. Antioxid Redox Signal. 2010;13:127–144. doi: 10.1089/ars.2009.2832. [DOI] [PubMed] [Google Scholar]
- 158.Saitoh M. Nishitoh H. Fujii M. Takeda K. Tobiume K. Sawada Y. Kawabata M. Miyazono K. Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Santos R. Lefevre S. Sliwa D. Seguin A. Camadro JM. Lesuisse E. Friedreich ataxia: Molecular mechanisms, redox considerations, and therapeutic opportunities. Antioxid Redox Signal. 2010;13:651–690. doi: 10.1089/ars.2009.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Schwartz M. Shechter R. Systemic inflammatory cells fight off neurodegenerative disease. Nat Rev Neurol. 2010;6:405–410. doi: 10.1038/nrneurol.2010.71. [DOI] [PubMed] [Google Scholar]
- 161.Shelton MD. Chock PB. Mieyal JJ. Glutaredoxin: Role in reversible protein S-glutathionylation and regulation of redox signal transduction and protein translocation. Antioxid Redox Signal. 2005;7:348–366. doi: 10.1089/ars.2005.7.348. [DOI] [PubMed] [Google Scholar]
- 162.Shelton MD. Distler AM. Kern TS. Mieyal JJ. Glutaredoxin regulates autocrine and paracrine proinflammatory responses in retinal glial (Muller) cells. J Biol Chem. 2009;284:4760–4766. doi: 10.1074/jbc.M805464200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shelton MD. Kern TS. Mieyal JJ. Glutaredoxin regulates nuclear factor kappa-B and intercellular adhesion molecule in Muller cells: Model of diabetic retinopathy. J Biol Chem. 2007;282:12467–12474. doi: 10.1074/jbc.M610863200. [DOI] [PubMed] [Google Scholar]
- 164.Shi Q. Xu H. Yu H. Zhang N. Ye Y. Estevez AG. Deng H. Gibson GE. Inactivation and reactivation of the mitochondrial {alpha}-ketoglutarate dehydrogenase complex. J Biol Chem. 2011;286:17640–17648. doi: 10.1074/jbc.M110.203018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shimohama S. Tanino H. Kawakami N. Okamura N. Kodama H. Yamaguchi T. Hayakawa T. Nunomura A. Chiba S. Perry G. Smith MA. Fujimoto S. Activation of NADPH oxidase in Alzheimer's disease brains. Biochem Biophys Res Commun. 2000;273:5–9. doi: 10.1006/bbrc.2000.2897. [DOI] [PubMed] [Google Scholar]
- 166.Shin JH. Ko HS. Kang H. Lee Y. Lee YI. Pletinkova O. Troconso JC. Dawson VL. Dawson TM. PARIS (ZNF746) repression of PGC-1alpha contributes to neurodegeneration in Parkinson's disease. Cell. 2011;144:689–702. doi: 10.1016/j.cell.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sian J. Dexter DT. Lees AJ. Daniel S. Agid Y. Javoy-Agid F. Jenner P. Marsden CD. Alterations in glutathione levels in Parkinson's disease and other neurodegenerative disorders affecting basal ganglia. Ann Neurol. 1994;36:348–355. doi: 10.1002/ana.410360305. [DOI] [PubMed] [Google Scholar]
- 168.Smith MA. Nunomura A. Zhu X. Takeda A. Perry G. Metabolic, metallic, and mitotic sources of oxidative stress in Alzheimer disease. Antioxid Redox Signal. 2000;2:413–420. doi: 10.1089/15230860050192198. [DOI] [PubMed] [Google Scholar]
- 169.Smyth GE. Colman RF. Cysteinyl peptides of pig heart NADP-dependent isocitrate dehydrogenase that are modified upon inactivation by N-ethylmaleimide. J Biol Chem. 1991;266:14918–14925. [PubMed] [Google Scholar]
- 170.Song IS. Kim SU. Oh NS. Kim J. Yu DY. Huang SM. Kim JM. Lee DS. Kim NS. Peroxiredoxin I contributes to TRAIL resistance through suppression of redox-sensitive caspase activation in human hepatoma cells. Carcinogenesis. 2009;30:1106–1114. doi: 10.1093/carcin/bgp104. [DOI] [PubMed] [Google Scholar]
- 171.Song JJ. Lee YJ. Differential role of glutaredoxin and thioredoxin in metabolic oxidative stress-induced activation of apoptosis signal-regulating kinase 1. Biochem J. 2003;373:845–853. doi: 10.1042/BJ20030275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Song JJ. Rhee JG. Suntharalingam M. Walsh SA. Spitz DR. Lee YJ. Role of glutaredoxin in metabolic oxidative stress. Glutaredoxin as a sensor of oxidative stress mediated by H2O2. J Biol Chem. 2002;277:46566–46575. doi: 10.1074/jbc.M206826200. [DOI] [PubMed] [Google Scholar]
- 173.Song W. Chen J. Petrilli A. Liot G. Klinglmayr E. Zhou Y. Poquiz P. Tjong J. Pouladi MA. Hayden MR. Masliah E. Ellisman M. Rouiller I. Schwarzenbacher R. Bossy B. Perkins G. Bossy-Wetzel E. Mutant huntingtin binds the mitochondrial fission GTPase dynamin-related protein-1 and increases its enzymatic activity. Nat Med. 2011;17:377–382. doi: 10.1038/nm.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sparaco M. Gaeta LM. Tozzi G. Bertini E. Pastore A. Simonati A. Santorelli FM. Piemonte F. Protein glutathionylation in human central nervous system: Potential role in redox regulation of neuronal defense against free radicals. J Neurosci Res. 2006;83:256–263. doi: 10.1002/jnr.20729. [DOI] [PubMed] [Google Scholar]
- 175.Sriram K. O'Callaghan JP. Divergent roles for tumor necrosis factor-alpha in the brain. J Neuroimmune Pharmacol. 2007;2:140–153. doi: 10.1007/s11481-007-9070-6. [DOI] [PubMed] [Google Scholar]
- 176.Steinert JR. Chernova T. Forsythe ID. Nitric oxide signaling in brain function, dysfunction, and dementia. Neuroscientist. 2010;16:435–452. doi: 10.1177/1073858410366481. [DOI] [PubMed] [Google Scholar]
- 177.Sultana R. Poon HF. Cai J. Pierce WM. Merchant M. Klein JB. Markesbery WR. Butterfield DA. Identification of nitrated proteins in Alzheimer's disease brain using a redox proteomics approach. Neurobiol Dis. 2006;22:76–87. doi: 10.1016/j.nbd.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 178.Supnet C. Bezprozvanny I. Neuronal calcium signaling, mitochondrial dysfunction, and Alzheimer's disease. J Alzheimers Dis. 2010;20(Suppl 2):S487–S498. doi: 10.3233/JAD-2010-100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Taylor ER. Hurrell F. Shannon RJ. Lin TK. Hirst J. Murphy MP. Reversible glutathionylation of complex I increases mitochondrial superoxide formation. J Biol Chem. 2003;278:19603–19610. doi: 10.1074/jbc.M209359200. [DOI] [PubMed] [Google Scholar]
- 180.Tew KD. Townsend DM. Regulatory functions of glutathione S-transferase P1-1 unrelated to detoxification. Drug Metab Rev. 2011;43:179–193. doi: 10.3109/03602532.2011.552912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tillement L. Lecanu L. Papadopoulos V. Alzheimer's disease: Effects of beta-amyloid on mitochondria. Mitochondrion. 2011;11:13–21. doi: 10.1016/j.mito.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 182.Tretter L. Sipos I. Adam-Vizi V. Initiation of neuronal damage by complex I deficiency and oxidative stress in Parkinson's disease. Neurochem Res. 2004;29:569–577. doi: 10.1023/b:nere.0000014827.94562.4b. [DOI] [PubMed] [Google Scholar]
- 183.van HJ. Witte ME. Schreibelt G. de Vries HE. Radical changes in multiple sclerosis pathogenesis. Biochim Biophys Acta. 2011;1812:141–150. doi: 10.1016/j.bbadis.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 184.Velu CS. Niture SK. Doneanu CE. Pattabiraman N. Srivenugopal KS. Human p53 is inhibited by glutathionylation of cysteines present in the proximal DNA-binding domain during oxidative stress. Biochemistry. 2007;46:7765–7780. doi: 10.1021/bi700425y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Vesce S. Jekabsons MB. Johnson-Cadwell LI. Nicholls DG. Acute glutathione depletion restricts mitochondrial ATP export in cerebellar granule neurons. J Biol Chem. 2005;280:38720–38728. doi: 10.1074/jbc.M506575200. [DOI] [PubMed] [Google Scholar]
- 186.Waak J. Weber SS. Gorner K. Schall C. Ichijo H. Stehle T. Kahle PJ. Oxidizable residues mediating protein stability and cytoprotective interaction of DJ-1 with apoptosis signal-regulating kinase 1. J Biol Chem. 2009;284:14245–14257. doi: 10.1074/jbc.M806902200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wang J. Boja ES. Tan W. Tekle E. Fales HM. English S. Mieyal JJ. Chock PB. Reversible glutathionylation regulates actin polymerization in A431 cells. J Biol Chem. 2001;276:47763–47766. doi: 10.1074/jbc.C100415200. [DOI] [PubMed] [Google Scholar]
- 188.Wang J. Tekle E. Oubrahim H. Mieyal JJ. Stadtman ER. Chock PB. Stable and controllable RNA interference: Investigating the physiological function of glutathionylated actin. Proc Natl Acad Sci USA. 2003;100:5103–5106. doi: 10.1073/pnas.0931345100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wang W. Oliva C. Li G. Holmgren A. Lillig CH. Kirk KL. Reversible silencing of CFTR chloride channels by glutathionylation. J Gen Physiol. 2005;125:127–141. doi: 10.1085/jgp.200409115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.West MB. Hill BG. Xuan YT. Bhatnagar A. Protein glutathiolation by nitric oxide: An intracellular mechanism regulating redox protein modification. FASEB J. 2006;20:1715–1717. doi: 10.1096/fj.06-5843fje. [DOI] [PubMed] [Google Scholar]
- 191.West XZ. Malinin NL. Merkulova AA. Tischenko M. Kerr BA. Borden EC. Podrez EA. Salomon RG. Byzova TV. Oxidative stress induces angiogenesis by activating TLR2 with novel endogenous ligands. Nature. 2010;467:972–976. doi: 10.1038/nature09421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wu DC. Teismann P. Tieu K. Vila M. Jackson-Lewis V. Ischiropoulos H. Przedborski S. NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease. Proc Natl Acad Sci USA. 2003;100:6145–6150. doi: 10.1073/pnas.0937239100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wyss-Coray T. Mucke L. Inflammation in neurodegenerative disease—Aa double-edged sword. Neuron. 2002;35:419–432. doi: 10.1016/s0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
- 194.Xu R. Wu C. Zhang X. Zhang Q. Yang Y. Yi J. Yang R. Tao Y. Linking hypoxic and oxidative insults to cell death mechanisms in models of ALS. Brain Res. 2011;1372:133–144. doi: 10.1016/j.brainres.2010.11.056. [DOI] [PubMed] [Google Scholar]
- 195.Yang Q. Mao Z. Regulation of MEF2s by chaperone-mediated autophagy. Cell Cycle. 2009;8:1105. doi: 10.4161/cc.8.8.8235. [DOI] [PubMed] [Google Scholar]
- 196.Yang Q. Mao Z. Parkinson disease: A role for autophagy? Neuroscientist. 2010;16:335–341. doi: 10.1177/1073858409357118. [DOI] [PubMed] [Google Scholar]
- 197.Yang Y. Shi W. Cui N. Wu Z. Jiang C. Oxidative stress inhibits vascular K(ATP) channels by S-glutathionylation. J Biol Chem. 2010;285:38641–38648. doi: 10.1074/jbc.M110.162578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yang Z. Cheng W. Hong L. Chen W. Wang Y. Lin S. Han J. Zhou H. Gu J. Adenine nucleotide (ADP/ATP) translocase 3 participates in the tumor necrosis factor induced apoptosis of MCF-7 cells. Mol Biol Cell. 2007;18:4681–4689. doi: 10.1091/mbc.E06-12-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yao D. Gu Z. Nakamura T. Shi ZQ. Ma Y. Gaston B. Palmer LA. Rockenstein EM. Zhang Z. Masliah E. Uehara T. Lipton SA. Nitrosative stress linked to sporadic Parkinson's disease: S-nitrosylation of parkin regulates its E3 ubiquitin ligase activity. Proc Natl Acad Sci USA. 2004;101:10810–10814. doi: 10.1073/pnas.0404161101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zemlan FP. Thienhaus OJ. Bosmann HB. Superoxide dismutase activity in Alzheimer's disease: Possible mechanism for paired helical filament formation. Brain Res. 1989;476:160–162. doi: 10.1016/0006-8993(89)91550-3. [DOI] [PubMed] [Google Scholar]
- 201.Zhang R. Luo D. Miao R. Bai L. Ge Q. Sessa WC. Min W. Hsp90-Akt phosphorylates ASK1 and inhibits ASK1-mediated apoptosis. Oncogene. 2005;24:3954–3963. doi: 10.1038/sj.onc.1208548. [DOI] [PubMed] [Google Scholar]
- 202.Ziegler DM. Role of reversible oxidation-reduction of enzyme thiols-disulfides in metabolic regulation. Annu Rev Biochem. 1985;54:305–329. doi: 10.1146/annurev.bi.54.070185.001513. [DOI] [PubMed] [Google Scholar]
- 203.Zmijewski JW. Banerjee S. Abraham E. S-glutathionylation of the Rpn2 regulatory subunit inhibits 26 S proteasomal function. J Biol Chem. 2009;284:22213–22221. doi: 10.1074/jbc.M109.028902. [DOI] [PMC free article] [PubMed] [Google Scholar]