Abstract
Background
Breastfeeding has numerous maternal and infant benefits. Progesterone contraception after birth is frequently recommended, but because a decrease in progesterone is required to initiate lactation, early postpartum progesterone contraception use could inhibit lactation. The purpose of this article is to critically evaluate the scientific basis for conflicting clinical recommendations related to postpartum medroxyprogesterone use among breastfeeding women.
Methods
Relevant peer-reviewed literature was identified through a comprehensive search of PubMed through December 2010. The search was restricted to clinical trials, randomized clinical trials, or comparative studies written in English and conducted among humans. The studies included in this review addressed the effect of medroxyprogesterone administration at <6 weeks postpartum on breastfeeding exclusivity and/or duration and measured breastfeeding outcomes at ≥6 weeks postpartum.
Results
Of the 20 articles identified, only three studies satisfied the inclusion criteria. However, all three studies were of low-quality methodological rigor, and none accounted for potential confounders.
Conclusion
Current evidence is methodologically weak and provides an inadequate basis for inference about a possible causal relationship between early postpartum medroxyprogesterone use and poor breastfeeding outcomes. However, given the presence of a strong biological model describing the potential deleterious effect of postpartum medroxyprogesterone use on lactation, further research that improves on current literature is warranted. Meanwhile, we recommend that potential breastfeeding risks associated with early (<6 weeks) postpartum medroxyprogesterone use be disclosed to allow for a fully informed consent and decision-making process.
Background
The World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC) appointed experts to review the literature and generate clinical recommendations for the WHO's 2009 Medical Eligibility Criteria for Contraceptive Use1 (WHO MEC) and the “U.S. Medical Eligibility Criteria for Contraceptive Use, 2010”2 (U.S. MEC), respectively. The WHO and CDC expert panels utilized the same studies and produced divergent conclusions and conflicting recommendations regarding the use of early postpartum medroxyprogesterone among breastfeeding women; relative to the WHO MEC, the U.S. MEC endorsed less restricted use of medroxyprogesterone.1,2 In response to the U.S. MEC, the Academy of Breastfeeding Medicine issued a press release; the Academy's president Gerald Calnen argued “[t]he new guidelines ignore basic facts about how breastfeeding works. Mothers start making milk due to the natural fall in progesterone after birth. An injection of artificial progesterone could completely derail this process.”3
Medroxyprogesterone (Depo Provera®, Pfizer, New York, NY) is a synthetic progestin-only hormonal contraceptive injection given intramuscularly to women in doses of 150 mg every 3 months to prevent ovulation.4 The package insert recommends medroxyprogesterone be administered to the non-breastfeeding mother within 5 postpartum days and only after postpartum week 6 to exclusively breastfeeding mothers.4 The Food and Drug Administration approved medroxyprogesterone for contraceptive usage in 1992.5 Data regarding medroxyprogesterone use in the early postpartum period (<6 weeks) were not available for inclusion in the Food and Drug Administration application, and consequently no such data are included in the package insert.6,7 Moreover, the package insert does not provide guidance for postpartum use among non–exclusively breastfeeding women.
There are biological concerns regarding the appropriateness of early postpartum medroxyprogesterone administration among lactating women. Mechanistically, maintenance of elevated estrogen and progesterone levels during pregnancy inhibits milk production.8,9 The decline in progesterone levels (within 72 hours postpartum) initiates the development and secretion of copious milk and signals the onset of secretory activation.8,10 The action of infant suckling increases prolactin levels, causing alveolar cells to produce milk.6,9 The combination of suckling and heightened prolactin levels prompts oxytocin levels to increase, causing contractions around the alveoli that release milk into the ductal system.9,11 If a breastfeeding woman receives medroxyprogesterone in the immediate (prior to hospital discharge) or early (<6 weeks) postpartum period, the artificially elevated progesterone levels may prevent the homeostatic increase in prolactin levels required to establish lactation and ultimately may interfere with milk production prior to the transition from an endocrine to an autocrine process.7,12 Therefore, administration of medroxyprogesterone could delay the onset of secretory activation and hinder the creation, secretion, or volume of breastmilk. Immediate or early postpartum medroxyprogesterone receipt may limit breastfeeding duration, thus negating the short- and/or long-term maternal/infant benefits associated with breastfeeding (e.g., reduction in maternal risk of breast cancer and ovarian cancer; reduction in infant risk of allergies, asthma, and obesity).13
WHO MEC
Published in 2009, the fourth edition of the WHO MEC evaluated the effect of various contraceptive methods used in the presence of certain maternal characteristics (e.g., age, breastfeeding, or smoking status) or medical conditions (e.g., lupus, migraines, epilepsy) and provided over 1,800 clinical recommendations regarding the use of each contraceptive method in specific situations.1 WHO categorized medroxyprogesterone given prior to 6 weeks as risk level 3 (“a condition where the theoretical or proven risks usually outweigh the advantages of using the method”) and classified medroxyprogesterone administration ≥6 weeks postpartum as risk level 1 (“a condition for which there is no restriction for the use of the contraceptive method”) (Table 1).1
Table 1.
World Health Organization Ranking Categories
| Category | Definition | Recommended use with clinical judgment |
|---|---|---|
| 1 | A condition for which there is no restriction for the use of the contraceptive method | Use method in any circumstance |
| 2 | A condition for which the advantages of using the method generally outweigh the theoretical or proven risks | Generally use method |
| 3 | A condition where the theoretical or proven risks usually outweigh the advantages of using the method | Use of method not usually recommended unless more appropriate methods are not available or not acceptable |
| 4 | A condition that represents an unacceptable health risk if the contraception method is used | Method not to be used |
Adapted from the World Health Organization publication.1
U.S. MEC
WHO MEC recommendations provide the basis for international family planning programs, but not all 1,800 recommendations are relevant to family planning in the United States.14 In February 2009, the CDC convened 31 invited experts, including obstetrician/gynecologists, pediatricians, family physicians, nurse-midwives, nurse practitioners, and epidemiologists, to adapt the WHO MEC into a contextually specific document for utilization by U.S. practitioners.2,14
Details of the CDC's modification to the WHO MEC have been discussed in detail elsewhere.14,15 In brief, data were analyzed using the United States Preventive Services Task Force Scale and presented via systematic reviews.14,16 The systematic review of Kapp et al.17 evaluated the use of progesterone contraceptive methods among breastfeeding women. Each qualifying observational study in their review received a United States Preventive Services Task Force Scale grade of “fair” or “poor,” and the authors concluded there were no adverse effects of postpartum progesterone-only contraceptive methods on maternal ability to breastfeed.16,17
The U.S. MEC lowered the WHO MEC risk classification for medroxyprogesterone on breastfeeding in the early postpartum period from a 3 to a risk level 2: “a condition for which the advantages of using the method generally outweigh the theoretical or proven risks” (Table 1).2 The U.S. MEC include the following justification of this decision:2
Despite anecdotal clinical reports that POCs [Progestin-Only Contraceptives] might diminish milk production, direct evidence from available clinical studies demonstrates no significant negative effect of POCs on breastfeeding performance or on the health of the infant. In general, these studies are of poor quality, lack standard definitions of breastfeeding or outcome measures, and have not included premature or ill infants.
Recommendations for later postpartum medroxyprogesterone use remained a risk level 1.2 The latter recommendation is consistent with the package insert.
The U.S. MEC review process also identified gaps in contraceptive safety research, specifically, 15 conditions that require further research.15 Relative to breastfeeding, the report's authors noted the following unanswered research question: “[w]hat are the effects of maternal use of…progestin-only contraceptives on infant health and breastfeeding performance when contraception is initiated less than 6 weeks postpartum, particularly immediately postpartum?”15 The committee acknowledged current research regarding progestin-only contraceptive methods is fairly consistent and has not shown any effect on breastfeeding outcomes but observed several consistent methodological flaws, including the lack of standardized outcome measure and adjustment for any confounding variables.15
To be useful in meta-analyses or systematic reviews, methodologically rigorous studies must be transparent in the presentation of the study design, data collection, and statistical analysis.18 Similarly, articles reporting high-quality research should contain clear descriptions/definitions of study design, study participants, eligibility criteria, outcome(s), exposure(s), potential confounders or effect modifiers, sample size/power calculation, and bias minimization; results should include both crude and adjusted point estimates with a corresponding measure of precision.18,19
Drawing statistical inferences from methodologically nonrigorous studies is of questionable validity. These inferences contribute to conclusions of dubious value that should not be added to the existing evidence base. Given the U.S. MEC revised Depo Provera recommendations were derived from admittedly “fair”- to “poor”-quality studies, but no formal assessment of the available epidemiologic data's methodological rigor exists, the evidence is inadequate to either accept or reject a causal relationship between early postpartum administration of medroxyprogesterone use and poor breastfeeding outcomes. In the presence of flawed epidemiologic studies and a strong biologic model describing the potential deleterious effect of postpartum medroxyprogesterone use on lactation, a systematic study that evaluates the methodological rigor of the existing evidence base is needed.
The overall purpose of the present article is to critically evaluate the contradiction between the WHO MEC and the U.S. MEC related to early medroxyprogesterone use among breastfeeding women. Specifically, we (1) conducted a systematic review of the literature to analyze the quality of the evidence utilized for the U.S. MEC review by examining the internal validity of individual studies that evaluated the effect of off-label medroxyprogesterone postpartum use on early breastfeeding cessation and (2) investigated whether there was new evidence published after the U.S. MEC release.
Methods
A systematic search of all manuscripts indexed in PubMed through December 2010 was performed using two separate searches including the following keywords: (1) postpartum Depo Provera and breastfeeding and (2) postpartum contraception and lactation. Two separate searches were conducted in order to optimize the number of studies eligible for inclusion. To prevent bias by inadvertently eliminating studies from non–English-speaking countries, these results were cross-referenced with a search of postpartum medroxyprogesterone and breastfeeding. This search was restricted to clinical trials, randomized clinical trials, or comparative studies written in English and conducted among humans. The purpose was to identify all primary epidemiological studies that evaluated the association of interest. The same search terms were entered into ProQuest to identify relevant dissertations. Reference lists of qualifying articles were hand-searched for additional studies, and experts in the field were contacted to identify any additional published or unpublished studies to include in this systematic review. Reviews, letters to the editor, case reports, and case series were not included. Because of brevity of available information on methods, conference abstracts were excluded. Two individuals (E.A.B. and I.D.F.) independently reviewed PubMed abstracts and evaluated articles; disagreements were resolved by consensus.
The following inclusion criteria were applied to identify eligible studies for inclusion in this systematic review: (1) it was a primary study; (2) the research question addressed the effect of medroxyprogesterone administration at <6 weeks postpartum on breastfeeding exclusivity or duration; and (3) breastfeeding outcomes were measured at ≥6 weeks postpartum.
The CONSORT guidelines provided the framework to evaluate the quality of randomized clinical trials.19 The CONSORT checklist includes items to assess reporting transparency regarding randomized clinical trial methods and results, and it contains 25 items.19 To be consistent with the Agency for Research on Healthcare Quality breastfeeding report,13 we reviewed all items but paid particular attention to those addressing randomization methods, blinding, presence of intent-to-treat analysis, participant dropout rate, and description of primary and secondary outcome results.13,18
The Newcastle–Ottawa Scale (NOS) was used to assess the quality of nonrandomized observational studies in meta-analyses criteria.20 The NOS has been used previously in large-scale systematic reviews specifically addressing breastfeeding initiation, duration, and exclusivity.13,18 The NOS evaluates three categories of methodological rigor: Selection, Comparability, and Outcome.20 It awards a “star” for addressing specific items. Selection (ranging from 0 to 4 “stars”) assesses (1) the representativeness of the exposed cohort in the community, (2) selection process of the nonexposed cohort, (3) ascertainment of the exposure, and (4) demonstration that the outcome of interest was not present at the start of the study. Comparability (0–2 “stars”) measures the comparability of the cohorts on the basis of the design (matching) or the analysis (adjustment for confounders). Outcome (0–3 “stars”) evaluates (1) outcome assessment, (2) appropriateness of length of follow-up relative to outcome occurrence, and (3) adequacy of cohort follow-up (ensuring losses are not related to either the exposure or the outcome).
To translate the number of NOS “stars” into categories measuring quality (e.g., high vs. low), the definition of Roffey et al.21 was applied. To qualify as a methodologically high quality, each study included multivariable analysis or other methodologies to account for potential confounding and attained at least 5 cumulative NOS “stars.”21
Results
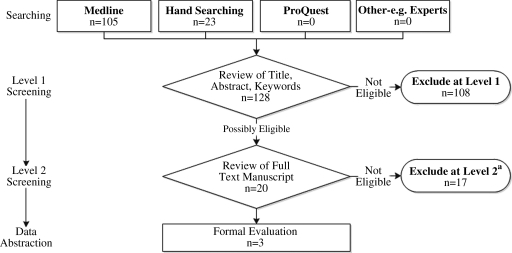
The search terms yielded 105 abstracts to review (Fig. 1). Ninety-seven abstracts were excluded because none included early postpartum medroxyprogesterone as an exposure or breastfeeding duration/cessation as an outcome. Conservatively, articles with abstracts containing unclear presentation of either the exact timing of medroxyprogesterone administration or specific details regarding the measurements of breastfeeding outcomes were included in the formal evaluation; eight articles qualified.22–29 For completeness, the literature represented in the U.S. MEC report was cross-checked with the PubMed literature search, and this produced an additional three articles.30–32 Lastly, after we read those 11 documents and hand-searched references, an additional nine articles were identified that potentially met the study's inclusion criteria.33–41 In total, 20 articles were evaluated, and three articles22,29,32 satisfied the systematic review inclusion criteria; the reasons for exclusion included review article (n=3), medroxyprogesterone not administered in the immediate postpartum period (n=9), medroxyprogesterone not administered ever (n=4), and no measure of breastfeeding cessation (n=1).
FIG. 1.
Study flow diagram. Adapted from Roffey et al.21 aReasons for exclusions: review article (n=3); no medroxyprogesterone administered in the immediate postpartum period (n=9); no administration of medroxyprogesterone (n=4); no measure of breastfeeding cessation (n=1).
Specific statistical results of the qualifying studies have been presented and reviewed in detail elsewhere (Table 2).16,42,43 Guiloff et al.22 observed that medroxyprogesterone recipients in the early postpartum period demonstrated a decreased risk of early breastfeeding cessation. The authors assessed breastfeeding duration in Chilean women using a randomized clinical trial/case-only design in which 696 women were randomized to receive various hormonal contraceptive methods. All participants were multiparous. The research group established a control group of 385 participants who reported breastfeeding duration from a previous pregnancy. The authors considered this to be “reliable retrospective information…representative from all treatment groups.” Randomized participants either received medroxyprogesterone immediately postpartum (n=80) or one of several other hormonal contraceptive methods given 1 month postpartum (n=33 medroxyprogesterone recipients). The authors concluded women who received medroxyprogesterone immediately postpartum had a statistically significant longer median breastfeeding duration (6.7 vs. 4.8 median months) relative to the control group, and women who received medroxyprogesterone 1 month postpartum also breastfeed their infants significantly longer than the control group (9.3 vs. 5.3 months). Major methodological weaknesses include utilization and subjective selection of non-concurrent controls and lack of stratified or multivariable analysis. Conclusions drawn from these data may not be valid.
Table 2.
Specific Evidence by Study
| Reference | Study design | Population | Exposure | Outcomes | Account for confounders | Results | Conclusion |
|---|---|---|---|---|---|---|---|
| Guiloff et al.22 | Randomized trial using historic controls (Chile) | n=969 15–40-year-old multiparous women | n=80 E+ 1–2 days postpartum; n=33 E+ 1 day+1 month postpartum | Median duration of lactation; 12 months follow-up | None (differences at baseline not presented) | Median duration lactation: E+ 6.7 months (95% CI 5.2–8.7; E– 4.8 months (95% CI 4.1–5.3); p=0.05 | Breastfeeding duration was significantly longer among E+ |
| Hannon et al.32 | Prospective cohort (United States) | n=393; intended to breastfeed, primarily Hispanic | n=102 E+ at hospital discharge | Breastfeeding patterns at 2, 4, and 6 weeks postpartum | None (baseline differences: age, previous breastfeeding experience, delivery mode) | Combining E+ and progestin-only groups demonstrated an increased risk of breastfeeding cessation at 4 weeks; no differences observed at 2 or 6 weeks | No deleterious effect on breastfeeding with any progestin-only method when administered early postpartum |
| Halderman and Nelson29 | Prospective cohort (United States) | n=95; intended to breastfeed postpartum, breastfeeding at hospital discharge, and agreed to telephone follow-up | n=43 E+ ≤11 days postpartum | Breastfeeding frequency, duration, and exclusivity at 1–8, 12, or 16 weeks postpartum | None (baseline differences: age, marital status) | Median breastfeeding duration: E+ 10.14 weeks (95% CI 0.71–19.57); E– 6.57 weeks (95% CI 3.43–9.71); p=0.19). No differences in frequency or exclusivity | Postpartum E+ has no detrimental effect on breastfeeding frequency, duration, or exclusivity within 16 weeks postpartum |
CI, confidence interval; E+, exposed (medroxyprogesterone recipients); E–, unexposed (medroxyprogesterone nonrecipients).
Hannon et al.32 reported no significant difference in early breastfeeding cessation between early postpartum medroxyprogesterone recipients and nonrecipients, but they discussed a nonstatistically significant trend toward favorable breastfeeding outcomes in the medroxyprogesterone group. The authors conducted a prospective cohort study of 95 women in Baltimore, MD to assess the effect of medroxyprogesterone administered prior to hospital discharge (compared with nonhormonal contraceptive methods) on breastfeeding duration and exclusivity. The study population included women of all parity, breastfeeding experience, income, and educational levels; statistically significant differences in age and marital status existed between exposure groups. Using χ2 analyses, the authors did not detect statistically significant differences between groups but did observe a nonsignificant trend towards favorable breastfeeding outcomes among the medroxyprogesterone group. The sample size calculation used the log rank test to detect a 20% difference using a one-sided α of 0.05. Using a one-sided (verses a two-sided) α could have favorably powered the study, but nonetheless the results were not statistically significant. Additionally, despite baseline differences, their analyses did not include stratified or multivariable methods to control for potential confounders. With the heterogeneous population that differed between groups, the lack of effect may have been attributable to confounding factors.
Halderman and Nelson29 also concluded there were no significant differences in breastfeeding cessation rates between nonhormonal and progestin-only contraceptive groups. The authors conducted a prospective cohort study among 319 women in Los Angeles, CA. Women of all ages, parity, and previous breastfeeding experience either self-selected to receive medroxyprogesterone (n=102) prior to hospital discharge or a prescription for progestin-only pills (n=77); a nonhormonal group agreed to use either male condoms or abstinence (n=138). The groups differed significantly in mothers' ages, prior breastfeeding experience, and delivery method. χ2 analyses grouped medroxyprogesterone with oral progestin-only users and compared hormonal versus the nonhormonal contraception users. Women using hormonal contraception were significantly more likely to cease breastfeeding at 4 weeks (relative to nonhormonal methods), but no differences between groups were observed at 2 or 6 weeks. The results are potentially confounded because of lack of statistically controlling for between-group differences in study heterogeneity. The difference in findings at 4 versus 2 and 6 weeks may have been a spurious finding due to the multiple analyses conducted.
Detailed results regarding the evaluation of qualifying studies are presented in Table 2. Guiloff et al.22 received 2/9 total stars: 1/4 for Selection, 0/2 for Comparability, and 1/3 for Outcome. Halderman and Nelson29 received 3.5/9 total stars: 2/4 for Selection, 0/2 for Comparability, and 1.5/3 for Outcome. Hannon et al.32 received 5/9 total stars: 3/4 for Selection, 0/2 for Comparability, and 2/3 for Outcome. There were several consistent strengths and weaknesses across all three studies. Regarding strengths, all studies drew the nonexposed cohort from the same community as the exposed cohort and included sufficient follow-up time to evaluate the outcome of interest. Common weaknesses included (1) no assessment of the representativeness of the exposed population in the community, (2) absence of a clear explanation describing utilization of secure records (e.g., medical records) to evaluate the exposure, (3) lack of complete follow-up data (two studies [Guiloff et al.22 and Halderman and Nelson29 did not address whether subjects lost to follow-up were likely to introduce bias]), and (4) no accounting for potential confounding variables via stratified or multivariable analysis. The unadjusted point estimates and any conclusions drawn from such estimates may be biased. Because of the ≤5 cumulative “star” ranking and lack of consideration of potential confounders, all three studies are of low quality (Table 3).
Table 3.
Newcastle–Ottawa Scale Methodological Quality Results
| Guiloff et al.22 | Halderman and Nelson29 | Hannon et al.32 | |
|---|---|---|---|
| Selection (maximum of 4 stars) | 1 star | 2 stars | 3 stars |
| 1. Representativeness of the exposed cohort | |||
| (a) Truly representative of the average population in the community | — | — | — |
| (b) Somewhat representative of the average population in the community | — | — | — |
| 2. Selection of the nonexposed cohort | |||
| (a) Drawn from the same community as the exposed cohort | Yes | Yes | Yes |
| 3. Ascertainment of exposure | |||
| (a) Secure record (e.g., surgical record) | — | — | — |
| (b) Structured interview | — | — | Yes |
| 4. Demonstration that outcome of interest was not present at start of study | |||
| (a) Yes | — | Yes | Yes |
| Comparabilitya (maximum of 2 stars) | 0 stars | 0 stars | 0 stars |
| 1. Comparability of cohorts on the basis of the design or analysis | |||
| (a) Study controls for confounding factors | — | — | — |
| Outcome (maximum of 3 stars) | 1 star | 1.5 stars | 2 stars |
| 1. Assessment of outcome | |||
| (a) Independent blind assessment | — | (0.5 star) | — |
| (b) Record linkage | — | — | — |
| 2. Was follow-up long enough for outcomes to occur? | |||
| (a) Yes | Yes | Yes | Yes |
| 3. Adequacy of follow-up of cohorts | |||
| (a) Complete follow-up: all subjects accounted for | — | — | — |
| (b) Subjects lost to follow-up unlikely to introduce bias | — | — | Yes |
| Total (maximum of 9 stars) | ★★ | ★★★★ | ★★★★★ |
| Methodological quality rankb | Low | Low | Low |
No study was awarded stars for any aspect of comparability.
Studies ranked “high” methodological quality if the sum of stars was ≥5 and application was multivariable or alternate statistical methods were used to adjust for potential confounders (Newcastle–Ottawa Scale Comparability section) and “low” if the sum of stars was <5 and the analysis does not account for potential confounders.
Discussion
The article systematically reviewed qualifying studies addressing early medroxyprogesterone use among breastfeeding women, conducted a methodological evaluation, and ranked each study as “low”-quality methodological rigor. Thus, each reviewed study's respective results may not be valid, and inferences made from such studies should not form the basis for evidence-based clinical recommendations. No additional primary studies published after the U.S. MEC release were identified.
These findings highlight the existing gap in the current evidence base regarding the relationship between early postpartum medroxyprogesterone use and early breastfeeding cessation. Of the studies that qualified for inclusion in this analysis, no research group earned a maximum amount of “stars” per category. Additionally, no study assessed the NOS Comparability of cohorts on the basis of design or analysis (matching or adjustment for potential confounders), thus producing results susceptible to criticism of low internal validity due to bias and unmeasured confounding. There are many content reviews of this topic, but only one methodological critique exists; Chantry43 reviewed the cohort of Halderman and Nelson29 and identified the following concerns:
overstatement of findings, statistical errors, inadequate power, non-randomization, lack of control for known confounders, undefined volume and frequency of supplementation, use of maternal perception of inadequate milk supply and conclusions stating a time frame for ‘early’ which was not utilized in this study
Some of the identified issues are specific to breastfeeding research (and would not be captured in the NOS), but the general methodological principles Chantry mentioned are consistent with this analysis.
Methodologically rigorous evidence demonstrating a positive or negative association between early postpartum medroxyprogesterone and early breastfeeding cessation does not exist; of note is that this lack of evidence is not equivalent to evidence failing to demonstrate an association between postpartum medroxyprogesterone and early breastfeeding cessation. Despite acknowledging that the association of interest was inconclusive and required additional research, the CDC committee revised the more restrictive WHO MEC Depo Provera recommendations.14 Given the methodologically low quality of current studies, their inconsistent results, and a strong biologic model describing the potential deleterious effect of postpartum medroxyprogesterone use on lactation, it seems premature to revise the more conservative WHO MEC recommendations regarding postpartum medroxyprogesterone use.
This systematic review has several important strengths. First, both the literature review results and methodological rigor assessment were consistent with previous research.17 Using an alternate scale (NOS verses the United States Preventive Services Task Force System scale) and an independent literature review, this systematic review identified the same three studies previously identified and observed the evidence to be suboptimal.17 Second, the NOS scale includes an assessment of Comparability, which has not been previously evaluated among studies of this topic; although Kapp et al.17 do suggest that their results may be due to differences at baseline, the importance (or nonimportance) of unadjusted results is not accounted for in the existing evidence. Third, previous research made oversimplified conclusions regarding the effect of all progestin-only methods on a woman's ability to breastfeed; these conclusions were general and without regard to mode of progestin only contraceptive or specific postpartum timing of use.17 Because this study evaluated medroxyprogesterone specifically, these results and conclusions are more precise and thus translatable to clinical practice.
These findings are subject to several limitations. First, decisions regarding postpartum contraception require weighing the cost of unintended pregnancy and potential postpartum lost-to-follow-up for contraception with the maternal/infant benefits attributable to breastfeeding. Randomized clinical trials evaluating the effect of postpartum medroxyprogesterone use on breastfeeding outcomes have not been published, and so none was included in this analysis. In the absence of such studies, it is difficult to evaluate the evidence and make clinical recommendations. Second, the abstractors were not blinded to the purpose of this study. However, given the consistency of these results (in regard to both manuscript identification and quality assessment) with previous research, minimal bias as a result of these methods is expected. Third, the NOS does not include evaluation of the sample size calculation (e.g., the use of one versus two-sided p values); Hannon et al.32 used a one-sided p value for their sample size calculation, and this is not accounted for in the assessment of methodological rigor with this scale. Lastly, the NOS does not include any items measuring potential confounders or biases specific to breastfeeding research (e.g., consistency of breastfeeding outcome definitions across studies, timing and frequency of supplementation), and such items were therefore not included in these analyses.
Conclusions
Because of methodological flaws either in study design or analyses, previous empirical research is inconclusive regarding whether medroxyprogesterone given in the early postpartum period is related to early breastfeeding cessation. Statistical inferences drawn from these studies are of questionable validity. Although authors of the U.S. MEC report acknowledged the overall lack of evidence, utilization of the conclusions drawn from these three methodologically weak studies was not sufficient justification to oppose the existing (and more conservative) WHO MEC recommendations. Given the presence of a convincing biologic mechanism describing the potential deleterious effect of early postpartum medroxyprogesterone use on lactation and the overall absence of methodologically rigorous studies, further research to evaluate this association should be designed, conducted, and reported according to the highest epidemiologic standards (especially using methods to control for potential confounders). Until such research consistently demonstrates either a positive or a negative impact on lactation, potential breastfeeding risks associated with early postpartum medroxyprogesterone use should be disclosed to breastfeeding mothers, thus allowing for a fully informed consent and decision-making process.
Acknowledgments
The study was funded through National Institutes of Health grant RO1 1R01HD055191 (P.I. A.M.D.). The authors declare no conflicts of interest. This project would not have been possible without manuscript assistance from Alice Nelson.
Disclosure Statement
All authors listed on this article contributed substantially to either the conception and design or analysis and interpretation of data. They were also involved in the drafting or revision of its intellectual content and approved the final version submitted for publication. No competing financial interests exist.
References
- 1.World Health Organization. Medical Eligibility Criteria for Contraceptive Use. 4th. World Health Organization; Geneva: 2009. [Google Scholar]
- 2.Centers for Disease Control and Prevention. U.S. medical eligibility criteria for contraceptive use, 2010. MMWR Recomm Rep. 2010;59:1–86. [PubMed] [Google Scholar]
- 3.Changes to guidelines for contraceptive use could compromise a woman's ability to breastfeed [press release] Academy of Breastfeeding Medicine. Jun 24, 2010.
- 4.Pfizer. DEPO-PROVERA® contraceptive injection medroxyprogesterone acetate injectable suspension. USP. Physician information; Pfizer, New York: 2006. [Google Scholar]
- 5.Leary WE. U.S. Approves Injectable Drug as Birth Control. The New York Times. 1992 Oct 30; [PubMed] [Google Scholar]
- 6.Kennedy KI. Short RV. Tully MR. Premature introduction of progestin-only contraceptive methods during lactation. Contraception. 1997;55:347–350. doi: 10.1016/s0010-7824(97)00042-5. [DOI] [PubMed] [Google Scholar]
- 7.Queenan JT. Contraception and breastfeeding. Clin Obstet Gynecol. 2004;47:734–739. doi: 10.1097/01.grf.0000139710.63598.b1. [DOI] [PubMed] [Google Scholar]
- 8.Hartmann PE. Cregan MD. Ramsay DT, et al. Physiology of lactation in preterm mothers: Initiation and maintenance. Pediatr Ann. 2003;32:351–355. doi: 10.3928/0090-4481-20030501-11. [DOI] [PubMed] [Google Scholar]
- 9.Mohrbacher N. Stock J. La Leche League International: The Breastfeeding Answer Book. 3rd. La Leche League International; Schaumburg, IL: 2003. [Google Scholar]
- 10.Pang WW. Hartman PE. Initiation of human lactation: Secretory differentiation and secretory activation. J Mammary Gland Biol Neoplasia. 2007;12:211–221. doi: 10.1007/s10911-007-9054-4. [DOI] [PubMed] [Google Scholar]
- 11.Biancuzzo M. Breastfeeding the Newborn: Clinical Strategies for Nurses. 2nd. Mosby; St. Louis: 2003. [Google Scholar]
- 12.Buhimschi CS. Endocrinology of lactation. Obstet Gynecol Clin North Am. 2004;31:963–979. doi: 10.1016/j.ogc.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Ip S. Chung M. Raman G, et al. Breastfeeding and Maternal and Infant Health Outcomes in Developed Countries. Agency for Healthcare Research and Quality; Rockville, MD: 2007. Evidence Report/Technology Assessment No. 153. [PMC free article] [PubMed] [Google Scholar]
- 14.Curtis KM. Jamieson DJ. Peterson HB, et al. Adaptation of the World Health Organization's medical eligibility criteria for contraceptive use for use in the United States. Contraception. 2010;82:3–9. doi: 10.1016/j.contraception.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 15.Folger SG. Curtis KM. Tepper NK, et al. Guidance on medical eligibility criteria for contraceptive use: Identification of research gaps. Contraception. 2010;82:113–118. doi: 10.1016/j.contraception.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 16.Harris RP. Helfand M. Woolf SH, et al. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med. 2001;20:21–35. doi: 10.1016/s0749-3797(01)00261-6. [DOI] [PubMed] [Google Scholar]
- 17.Kapp N. Curtis K. Nanda K. Progestogen-only contraceptive use among breastfeeding women: A systematic review. Contraception. 2010;82:17–37. doi: 10.1016/j.contraception.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 18.von Elm E. Altman DG. Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: The guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 19.Schulz KF. Altman DG. Moher D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. J Pharmacol Pharmacother. 2010;1:100–107. doi: 10.4103/0976-500X.72352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wells G. Shea B. O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. www.ohri.ca/programs/clinical_epidemiology/oxford.htm. [Mar 28;2011 ]. www.ohri.ca/programs/clinical_epidemiology/oxford.htm
- 21.Roffey DM. Wai EK. Bishop P, et al. Causal assessment of occupational pushing or pulling and low back pain: Results of a systematic review. Spine J. 2010;10:544–553. doi: 10.1016/j.spinee.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 22.Guiloff E. Ibarra-Polo A. Zanartu J, et al. Effect of contraception on lactation. Am J Obstet Gynecol. 1974;118:42–45. doi: 10.1016/s0002-9378(16)33643-2. [DOI] [PubMed] [Google Scholar]
- 23.Zanartu J. Aguilera E. Munoz-Pinto G. Maintenance of lactation by means of continuous low-dose progestogen given post-partum as a contraceptive. Contraception. 1976;13:313–318. doi: 10.1016/s0010-7824(76)80041-8. [DOI] [PubMed] [Google Scholar]
- 24.Tankeyoon M. Dusitsin N. Chalapati S, et al. Effects of hormonal contraceptives on milk volume and infant growth. WHO Special Programme of Research, Development and Research Training in Human Reproduction Task force on oral contraceptives. Contraception. 1984;30:505–522. doi: 10.1016/0010-7824(84)90001-5. [DOI] [PubMed] [Google Scholar]
- 25.Joshi UM. Virkar KD. Amatayakul K, et al. Impact of hormonal contraceptives vis-a-vis non-hormonal factors on the vitamin status of malnourished women in India and Thailand. World Health Organization: Special Programme of Research, Development and Research Training in Human Reproduction. Task Force on Oral Contraceptives. Hum Nutr Clin Nutr. 1986;40:205–220. [PubMed] [Google Scholar]
- 26.Zacharias S. Aguilera E. Assenzo JR, et al. Effects of hormonal and nonhormonal contraceptives on lactation and incidence of pregnancy. Contraception. 1986;33:203–213. doi: 10.1016/0010-7824(86)90014-4. [DOI] [PubMed] [Google Scholar]
- 27.Effects of hormonal contraceptives on breast milk composition and infant growth. World Health Organization (WHO) Task Force on Oral Contraceptives. Stud Fam Plann. 1988;19:361–369. [PubMed] [Google Scholar]
- 28.Costa TH. Dorea JG. Concentration of fat, protein, lactose and energy in milk of mothers using hormonal contraceptives. Ann Trop Paediatr. 1992;12:203–209. doi: 10.1080/02724936.1992.11747569. [DOI] [PubMed] [Google Scholar]
- 29.Halderman LD. Nelson AL. Impact of early postpartum administration of progestin-only hormonal contraceptives compared with nonhormonal contraceptives on short-term breast-feeding patterns. Am J Obstet Gynecol. 2002;186:1250–1256. doi: 10.1067/mob.2002.123738. discussion 1256–1258. [DOI] [PubMed] [Google Scholar]
- 30.Karim M. Ammar R. el-Mahgoub S, et al. Injected progestogen and lactation. Br Med J. 1971;1:200–203. doi: 10.1136/bmj.1.5742.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McEwan JA. Joyce DN. Tothill AU, et al. Early experience in contraception with a new progestogen. Contraception. 1977;16:339–350. doi: 10.1016/0010-7824(77)90045-2. [DOI] [PubMed] [Google Scholar]
- 32.Hannon PR. Duggan AK. Serwint JR, et al. The influence of medroxyprogesterone on the duration of breast-feeding in mothers in an urban community. Arch Pediatr Adolesc Med. 1997;151:490–496. doi: 10.1001/archpedi.1997.02170420060010. [DOI] [PubMed] [Google Scholar]
- 33.Koetsawang S. Effects of oral contraceptives on lactation. Fertil Steril. 1972;23:24–28. doi: 10.1016/s0015-0282(16)38704-0. [DOI] [PubMed] [Google Scholar]
- 34.Abdel Kader MM. Abdel Aziz MT. Bahgat R, et al. Effect of some progestational steroids on lactation in Egyptian women. II. Chemical composition of milk during the first year of lactation. J Biosoc Sci. 1976;8:49–51. doi: 10.1017/s0021932000010452. [DOI] [PubMed] [Google Scholar]
- 35.Multinational comparative clinical evaluation of two long-acting injectable contraceptive steroids: Norethisterone oenanthate and medroxyprogesterone acetate. Contraception. 1977;15:513–533. doi: 10.1016/0010-7824(77)90102-0. [DOI] [PubMed] [Google Scholar]
- 36.West CP. Factors influencing the duration of breast-feeding. J Biosoc Sci. 1980;12:325–331. doi: 10.1017/s0021932000012864. [DOI] [PubMed] [Google Scholar]
- 37.Benagiano G. The Depo-Provera debate; commentary on the article ‘Depo-Provera, a critical analysis.’. Contraception. 1981;24:493–528. doi: 10.1016/0010-7824(81)90056-1. [DOI] [PubMed] [Google Scholar]
- 38.Badraoui MH. Hefnawi F. Bahgat R, et al. Contraception during lactation. Reproduccion. 1982;6:9–18. [PubMed] [Google Scholar]
- 39.Feinstein JM. Berkelhamer JE. Gruszka ME, et al. Factors related to early termination of breast-feeding in an urban population. Pediatrics. 1986;78:210–215. [PubMed] [Google Scholar]
- 40.Laukaran VH. The effects of contraceptive use on the initiation and duration of lactation. Int J Gynecol Obstet. 1987;25:129–142. doi: 10.1016/0020-7292(87)90402-4. [DOI] [PubMed] [Google Scholar]
- 41.World Health Organization. Contraception during the postpartum period and during lactation: The effects on women's health. Int J Gynecol Obstet. 1987;25:13–26. [PubMed] [Google Scholar]
- 42.Rodriquez MI. Kaunitz AM. An evidence-based approach to postpartum use of depot medroxyprogesterone acetate in breastfeeding women. Contraception. 2009;80:4–6. doi: 10.1016/j.contraception.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 43.Chantry CJ. Does “Depo” given prior to hospital discharge affect milk supply? A unanswered question. Acad Breastfeed Med News Views. 2003;9:18–22. [Google Scholar]