Abstract
The paraoxonase (PON) gene cluster contains three adjacent gene members, PON1, PON2, and PON3. Originating from the same fungus lactonase precursor, all of the three PON genes share high sequence identity and a similar β propeller protein structure. PON1 and PON3 are primarily expressed in the liver and secreted into the serum upon expression, whereas PON2 is ubiquitously expressed and remains inside the cell. Each PON member has high catalytic activity toward corresponding artificial organophosphate, and all exhibit activities to lactones. Therefore, all three members of the family are regarded as lactonases. Under physiological conditions, they act to degrade metabolites of polyunsaturated fatty acids and homocysteine (Hcy) thiolactone, among other compounds. By detoxifying both oxidized low-density lipoprotein and Hcy thiolactone, PONs protect against atherosclerosis and coronary artery diseases, as has been illustrated by many types of in vitro and in vivo experimental evidence. Clinical observations focusing on gene polymorphisms also indicate that PON1, PON2, and PON3 are protective against coronary artery disease. Many other conditions, such as diabetes, metabolic syndrome, and aging, have been shown to relate to PONs. The abundance and/or activity of PONs can be regulated by lipoproteins and their metabolites, biological macromolecules, pharmacological treatments, dietary factors, and lifestyle. In conclusion, both previous results and ongoing studies provide evidence, making the PON cluster a prospective target for the treatment of atherosclerosis. Antioxid. Redox Signal. 16, 597–632.
-
IV. PONs Protect Against Hcy Toxicity and Associated Atherogenesis
I. Introduction
The human paraoxonase (PON) enzyme was initially characterized as an organophosphate hydrolase, because it catalyzes the hydrolysis of paraoxon organophosphate insecticides and sarin nerve gases as well as other similar compounds (74). PON has also been demonstrated to detoxify oxidized low-density lipoprotein (oxLDL) and protect against protein modification (N-homocysteinylation) by detoxifying homocysteine (Hcy) thiolactone. PON has a protective effect on the cardiovascular system and can reduce the incidence cardiovascular diseases (CVD), especially atherosclerosis. Atherosclerosis is still considered to be the most prevalent cardiovascular cause of disabling illness and death in developed societies (106, 201), although the lives of countless individuals suffering from atherosclerosis-related disease have been improved and/or saved by the currently available interventions (244). Thus, new strategies for preventing and treating atherosclerosis based on an enhanced understanding of the contributing factors are very much needed. The notion that the human PON gene cluster could be a target for the treatment of atherosclerosis has shed some light on this challenge (276).
Studies exploring the relationship between PON and CVD (especially atherosclerosis) constitute a flourishing field that has accumulated abundant data that could be useful for developing PON-related therapeutic strategies for atherosclerosis. In this review, we examine the reasons to use the PON gene cluster as a target in atherosclerosis by reviewing the progress made toward understanding the roles of PON in atherosclerosis and the regulation of the PON cluster. A better understanding of the possibilities for use of the human PON gene cluster as a target for the treatment of atherosclerosis may enable us to design novel and more effective strategies to combat atherosclerosis, the leading cause of death in humans.
II. The PON Family
In 1946, Mazur described the enzymatic hydrolysis of organophosphorus compounds by animal tissues (227). The esterases identified were named PONs by Aldridge based on their capability to hydrolyze their canonical substrate paraoxon (10, 11). In 1996, it was established that the gene responsible for the PON/arylesterase activities is a member of a multigene family; three such esterases (PON1, PON2, and PON3) have been identified (268). They are named according to their order of discovery.
A. Evolution of the PON genes
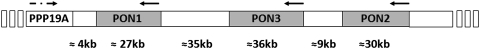
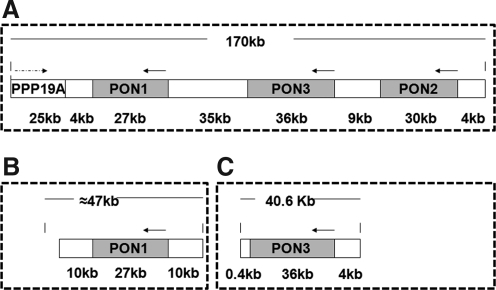
As a gene family, all three genes are located adjacent to one another in a cluster on the long arm of human chromosome 7 and on mouse chromosome 6, between q22.3 and q23.1 (268) (Fig. 1). The three gene members contain nine exons of approximately the same length in both species and share considerable structural homology. Within a given mammalian species, PON1, PON2, and PON3 share ∼70% identity at the nucleotide level and 60% identity at the amino acid level. However, each of the three genes share 81%–90% identity at the nucleotide level and 79%–90% identity at the amino acid level between mammalian species (206). Also, polymorphic variants are known to be common in at least the human and rabbit PONs (352). All PON1s have extra three additional nucleotide residues in exon 4, which code for amino acid 106 (lysine in human PON1), compared with PON2 and PON3 (268).
FIG. 1.
Genetic map of the human PON gene cluster. PON, paraoxonase.
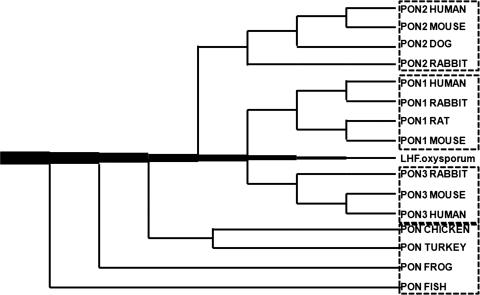
The whole gene cluster may have arisen from the tandem duplication of a common evolutionary precursor. The lactonase of the fungus Fusarium oxysporium, based on its appreciable structural homology and similar substrate spectrum (dihydrocoumarin and homogentisic acid lactone) with human serum PON1, is an attractive candidate for the common ancestor of this family (42, 169) (Fig. 2). PON-like genes can also be found in bacteria, plants, the worm Caenorhabditis elegans, the fruit fly (Drosophila melanogaster), zebrafish (Danio rerio), frogs (Xenopus laevis), chickens (Gallus gallus), turkeys (Meleagris gallopavo), and a number of mammals (86). Based on these findings, Draganov et al. came up with a phylogenetic tree of the vertebrate PONs (Fig. 2) (86). Of these three members in the gene cluster, PON2 appears to be the oldest based on the structural homology and predicted evolutionary distance among the family members. PON3 arose from PON2, followed by the appearance of PON1. (295) PON2 and PON3 also exhibit lactonase activity but not PON activity (88). The gene duplication suggests that the PONs have important, as-yet unknown physiological roles, hence the redundancy and the number of polymorphic variants (86). Lactonase activity may be the common role of the PON enzyme family, because lactones are commonly found in plants, natural flavoring agents, and many food products. This activity may protect the PON-carrying species against dietary and environmental lactones, which could be the selective forces responsible for maintaining the balanced polymorphisms in the PON enzymes in mammals (295).
FIG. 2.
Phylogenetic tree of vertebrate PONs. The tree begins at the fungal lactone hydrolase (LH) [for details, see ref. (86)].
B. Structure of the PONs
1. Primary structure
The human PON1 gene encodes a protein of 355 amino acids with a molecular mass of 43 kDa (210). The mature PON1 protein starts with an N-terminal hydrophobic sequence (125), which mediates PON1's association with high-density lipoprotein (HDL) (316, 317). There are three cysteine (Cys) residues at positions 42, 284, and 353 in the serum form of PON1. Cys42 and Cys353 form a disulfide linkage; Cys284, however, is free (178). The activity of PON1 can be abolished by mutating Cys42 or Cys353 to alanine; either of these mutations also significantly decreases the secretion of the protein (161). Mutation of Cys284 (to alanine or serine) decreases but does not abolish the PON and arylesterase activities (318). However, Cys284 has been demonstrated to be required for PON1's ability to protect LDL against copper-induced oxidation (22). Aviram et al. thus speculate that that PON1 possesses two catalytic sites, one that is required for the hydrolytic activity and another that is necessary for the antioxidant activity (21, 22). Two calcium-binding sites have been identified. The higher-affinity site is essential for enzyme stability, whereas the other is essential for the enzymatic hydrolytic activity. The activity and stability of PON1 are irreversibly destroyed when Ca2+ is removed by chelating agents. Some divalent ions, such as Zn, Mn, and Mg, however, can keep PON1 in a stable but inactive state (179). Several other amino acid residues (His115, His134, His155, His243, and Trp281) that are essential for PON1's esterase activity have also been identified using group-selective labeling and site-directed mutagenesis (160).
2. Three-dimensional structure
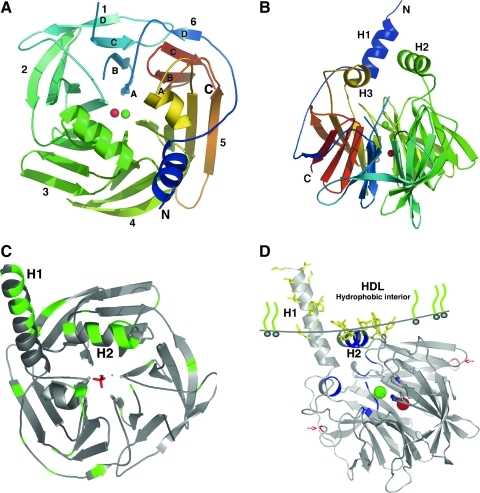
The three-dimensional structure of PON1 has been by resolved by Harel et al by crystallizing and analyzing a recombinant variant of rabbit PON1 at a resolution of 2.2 Å. This PON1 variant is highly similar to human PON1 (Fig. 3A, B) (121). PON was the first HDL-associated protein to be structurally elucidated (121). All the residues except the N-terminal residues (residues 1–15) and a surface loop (residues 72–79), two calcium ions, a phosphate ion, and 115 water molecules are shown in the structure (121). The overall architecture of PON1 is a β-propeller with six blades and a central tunnel; each blade consists of four β-sheets. A disulfide bridge between Cys42 and Cys353 forms a covalent closure between the N and C termini, which are conserved throughout the PON family (121). Two calcium ions, one at the top of the structure and one in the central section, are present in the central tunnel at a distance of 7.4 Å (121). The top one is considered to be the catalytic calcium and interacts with the side chain oxygens of Asn224, Asn270, Asn168, Asp269, and Glu53 (121, 179). The central calcium ion may contribute to the protein's structural stability (121, 179). PON1 is a glycosylated protein, and Asn253 and Asn324 have been proposed to be the two glycosylated sites (121). Throughout the whole gene cluster, the aforementioned residues that are important for maintaining the basic structure and catalytic activity are highly conserved, suggesting that all of the members maintained a common active site structure and catalytic machinery while diverging into three independent genes during evolution and expansion (121). PON1 and PON3 have additional N-terminal regions that are rich in hydrophobic residues and compatible with a transmembrane helix structure (at residues 7–18 and 19–28, respectively); these regions are hypothesized to contribute to HDL binding by acting as major hydrophobic interfaces, along with other hydrophobic residues in the second helix (121) (Fig. 3C, D).
FIG. 3.
PON1 protein structure and its docking to HDL. (A) Top view of the six-bladed propeller-like structure of PON1. The top of the propeller shows the face that carries the loops linking the outer strand of each blade (strand D) with the inner strand (A) of the next blade. The N and C termini and the two calcium atoms (Ca1, green; Ca2, red) are located in the central tunnel of the propeller. 1–6, six blades of PON1 molecule. (B) One of the sides of the propeller. H1, H2, and H3 helices appear at the top of the propeller. Residues 1–15 (at the N-terminus) and residues 72–79 (in a surface loop between strands 1B and 1C) are invisible in this structural model [adapted from ref. (121) with permission]. (C) Tertiary structure of rePON1. The hydrophobic surfaces are shown to be exposed. N-terminal residues 7–18 are predicted to be helical and are thus modeled as part of H1, which is actually not displayed in the crystal structure. All the hydrophobic residues appear with an accessible surface area of 20 Å2. (D) PON1 is anchored to HDL via its hydrophobic side chains (shown in yellow). The line models the interface between the hydrophobic interior and the exterior aqueous portions of HDL, which is defined by the side chains of Tyr 185, Phe 186, Tyr190, Trp194, and Trp202 on helix H2 and the adjacent loops and Lys21 on helix H1, respectively. The hydrophobic side chains of the leucine and phenylalanine residues of H1 are located in the apolar region. The active site and the selectivity-determining residues are shown in blue. Glycosylation sites on Asn253 and Asn324 are shown in red. The high-resolution images were kindly supplied by Prof. Joel L. Sussman from the Weizmann Institute of Science, Israel. HDL, high-density lipoprotein.
C. Tissue and cellular distribution of PONs
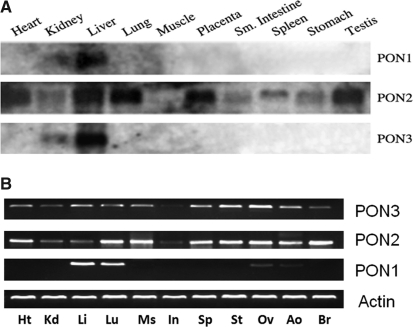
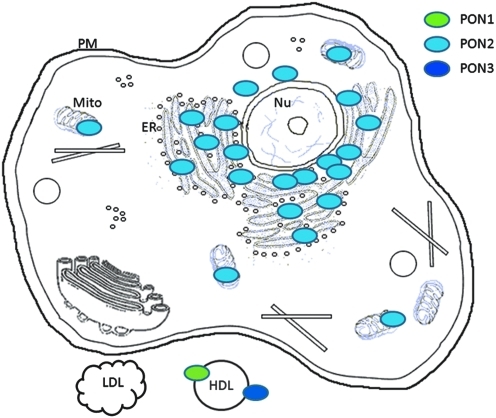
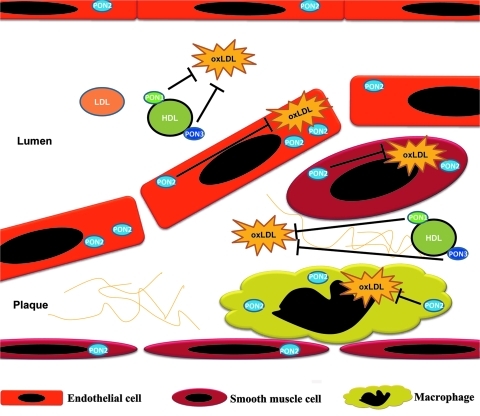
In humans, PON1 and PON3 are expressed primarily in the liver, though a low level of PON3 expression can also be found in the kidney (277). Human PON2, however, is expressed in many tissues including heart, kidney, liver, lung, placenta, small intestine, spleen, stomach, and testis (245, 268). Cells of the artery wall, including endothelial cells, smooth muscle cells (SMCs), and macrophages, can also express PON2 (245, 268) (Fig. 4A). In mice, we found that the expression of mouse PON1 is limited highly to the liver (high expression) and the lung (low expression). Mouse PON2 and PON3 are expressed more universally in a variety of tissues including the heart, kidney, liver, lung, muscle, intestine, spleen, stomach, ovary, aorta, and brain at various levels (301) (Fig. 4B). PON3 mRNA and protein are also present in murine but not in human macrophages (284). In rabbits (254) and rats (282), PON3 can be purified from liver microsomes. Upon expression, PON1 and PON3 associate with HDL in the circulation (Fig. 5) (245); PON2, however, localizes intracellularly, notably to the perinuclear region, where it associates with the endoplasmic reticulum and nuclear envelope instead of the plasma membrane (Fig. 5) (134). In atherosclerotic plaques, PON1 and PON3 are present due to their association with HDL; PON2 is synthesized and retained by the major cellular components of plaques, that is, endothelial cells, SMCs, and macrophages, to protect them against risk factors (Fig. 6).
FIG. 4.
Tissue distribution of the PON enzymes. (A) Northern blotting was used to detect the mRNA levels of the PONs in different human tissues using PON1, PON2, and PON3 probes [adapted from ref. (244) with permission]. Sm, small. (B) Reverse transcription–polymerase chain reaction was used to detect the expression of the PONs in mouse heart (Ht), kidney (Kd), liver (Li), lung (Lu), muscle (Ms), intestine (In), spleen (Sp), stomach (St), aorta (Ao), ovary (Ov), and brain (Br) with primers specific for mouse PON1, PON2, and PON3 messenger RNA [adapted from ref. (301) with permission]. β-Actin was used as a control.
FIG. 5.
Cellular distribution of the PONs. Upon expression, PON1 and PON3 are secreted from the cells and associate with HDL in the circulation (245); PON2 protein remains in the perinuclear region, where it associates with the ER and nuclear envelope rather than the PM (134). ER, endoplasmic reticulum; LDL, low-density lipoprotein; Mito, mitochondria; Nu, nuclear; PM, plasma membrane.
FIG. 6.
Distribution of PONs and their functions in blocking the effects of oxLDL in atherosclerotic plaques. PON1 and PON3 appear in atherosclerotic plaque by associating with HDL. PON2 is expressed in plaque endothelial cells, smooth muscle cells, and macrophages. PON1 and PON3 exert their effect at the extracellular level by associating with HDL in either the serum or the atherosclerotic plaque. PON2, however, protects plaque competent cells by acting intracellularly. oxLDL, oxidized LDL.
D. Substrates of PONs
1. Chemical substrates
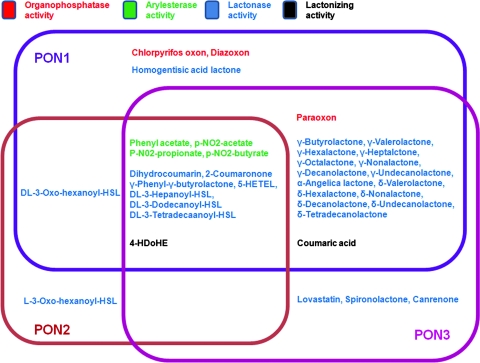
Human PON1, which is classified as an “A-esterase” (10), is able to hydrolyze various toxic oxon metabolites of insecticides with different efficiencies. Its activity toward paraoxon, parathion, diazinon, chlorpyriphos, and similar compounds is low (79, 86), but it exhibits much higher catalytic activity to other organophosphates, such as diazoxon (DZO) and chlorpyriphos oxon (198). Some nerve agents, such as sarin and soman, can also be hydrolyzed by PON1 (79, 86). In addition, phenylacetate, thiophenylacetate, 2-naphthylacetate, and other aromatic esters are substrates for PON1 (42, 86). PON1 has also been found to be able to hydrolyze a variety of aromatic and aliphatic lactones and cyclic carbonates, for example, homogentisic acid lactone, dihydrocoumarin, γ-butyrolactone, and Hcy thiolactone (41, 42, 145). Nevertheless, under nonphysiological conditions, PON1 is even reported to be able to catalyze the lactonization of γ- and δ-hydroxycarboxylic acids, which is thought to be a reverse reaction (328). Human PON2 and PON3 exhibit very limited or no PON and arylesterase activities. Like PON1, however, they can hydrolyze aromatic and long-chain aliphatic lactones, for example, dihydrocoumarin and 5-hydroxy-6E,8Z,11Z,14Z-eicosatetraenoic acid lactone (88). Using a baculoviral expression system, Draganov et al. characterized the catalytic spectrum of all three PON members. The results support the idea that the PONs are lactonases/lactonizing enzymes. The three members share some overlapping substrates (e.g., aromatic lactones) but also have distinctive substrate specificities (88) (Fig. 7). Nevertheless, some drugs containing lactone or cyclic carbonate moieties can also be metabolized by the PONs. For example, the unsaturated cyclic carbonate prodrug prulifloxacin can be hydrolyzed by PON1 to the active quinolone antibiotic NM394 (333). PON3 can hydrolyze some of the statin lactone drugs (lovastatin and simvastatin) and the diuretic spironolactone (86). However, none of these chemicals is present in the normal human body; therefore, none of them can be PON3's physiological substrate.
FIG. 7.
Specific enzymatic activities of the purified recombinant human PONs and relationships among the enzymatic activities of various PONs. Some chemicals are substrates for more than one PON. However, the catalytic efficiency sometimes dramatically differs among PON members. None of the substrates shown is the physiological substrate of PONs [see ref. (88) for details]. 5-HETEL, (±)5-hydroxy-6E,8Z,11Z,14Z-eicosatetraenoic acid 1,5-lactone; HSL, homoserine lactone.
2. Physiological substrates
Based on the substrate spectrum elucidated by many lines of research, the PONs are now proposed to be lactonase enzymes. Oxidized metabolites of polyunsaturated fatty acids (PUFAs) could be physiological substrates of PONs, because the structure of many of these molecules is similar to that of lactones (88, 128, 167). Purified PON1 can destroy the biologically active lipids in mildly oxLDL (351). Further experiments indicated that PON1 is capable of mediating the hydrolysis of 19% of the lipid peroxides and 90% of the cholesteryl linoleate hydroperoxides in oxidized HDL. Both HDL-associated PON1 and purified PONs were able to substantially hydrolyze hydrogen peroxide (H2O2) (27). PON1 was also confirmed to be able to cleave the ester bound between the cholesterol and the linoleic acid hydroperoxide or hydroxide (23).
Accumulated data have shown that Hcy-thiolactone is also very likely to be a natural substrate of PON1 (349). Jakubowski has demonstrated that a single specific enzyme, present in mammalian but not in avian sera, hydrolyzes thiolactone to Hcy. Thiolactonase, in the presence of the 45-kDa protein component of HDL in human serum, requires calcium for its activity and stability and is inhibited by isoleucine and penicillamine. Substrate specificity studies suggest that Hcy-thiolactone is a likely natural substrate of this enzyme. The unanticipated outcome of this study was that Hcy-thiolactonase is identical with serum PON (145). Hcy-thiolactone is a reactive metabolite that causes protein N-homocysteinylation through the formation of amide bonds with protein lysine residues, which impairs or alters protein function (147). It has also been demonstrated that the Hcy-thiolactonase activity of PON1 is a determinant of plasma N-Hcy-protein levels, leading to the conclusion that PON1 protects proteins against N-homocysteinylation in vivo (263). PONs can thus prevent protein homocysteinylation and related protein inactivation and cell damage.
In addition, PON1 has also been shown to have the ability to hydrolyze N-(3-oxododecanoyl)-l-homoserine lactone (3-OC12-HSL), which is an important quorum-sensing component of Pseudomonas aeruginosa (330). PON2 has also been demonstrated to be able to lower H2O2-induced intracellular oxidative stress (245), reduce 2,3-dimethoxy-1,4-naphthoquinone-induced reactive oxygen species (ROS) production in cells, and even decrease ROS levels during endoplasmic reticulum stress (134). Among the PON family, PON2 exhibits the greatest capability to degrade acylhomoserine lactones (88) and 3-OC12-HSL (330), which are related to pulmonary infections and cystic fibrosis (355).
The ability to hydrolyze oxidized metabolites and Hcy-thiolactone allows the PONs to protect against oxidative stress and inflammatory diseases, including atherosclerosis.
III. PONs Protect Against Atherosclerosis and Coronary Heart Disease by Decreasing the Toxicity of LDL
A. Atherosclerosis-related CVDs do great harm to human health
CVD has accounted for more than half of all deaths in the United States and other industrialized nations since the 1950s. Worldwide, CVD is projected to become the most common cause of death, with more than 36% of all deaths, for the first time in human history in 2020. This is more than twice the number of deaths from cancer (53). Thus, CVD has become one of the most serious health problems for the present and foreseeable future. Atherosclerosis, which is related to coronary artery disease (CAD), stroke, abdominal aortic aneurysm, and peripheral artery disease, constitutes the single most important contributor to this growing burden of CVD (201). Thus, developing effective strategies to treat atherosclerosis is among the most important ways scientists can work to overcome CVD.
B. OxLDL is one of the most important risk factors for atherogenesis
Numerous genetic and environmental risk factors for atherosclerosis have been revealed by epidemiological studies over the past 50 years (Fig. 8) (203). Increased levels of atherogenic lipoproteins, mainly LDL and very LDL (VLDL), are a prerequisite for most forms of the disease (202). As the major cholesterol carrier in human plasma, the surface of LDL is composed of a monolayer of 700 phospholipid molecules, consisting primarily of lecithin, small amounts of sphingomyelin and lysolecithin, and 600 molecules of cholesterol. The apolipoprotein (Apo)B-100 molecule embeds in the outer layer. Approximately 1600 molecules of cholesterol ester and 170 molecules of triglyceride reside in the central cores of LDL particles (111). Half of the fatty acids inside LDL are PUFAs that are mostly composed of linoleic acid but also include arachidonic acid and docosahexaenoic acid. All of these PUFAs are usually protected against free radical attack and oxidation by alpha-tocopherol and other antioxidants (272). Whenever there is an imbalance in the levels of antioxidants and the amount of PUFAs, LDL is oxidized. LDL can be oxidized by metal ions, lipoxygenases, myeloperoxidase, and reactive nitrogen species, mainly under the aorta intima; this process is mediated by the cells residing in the aorta wall (230). Based on the extent of modification, oxLDL can be classified as minimally modified LDL, mildly modified oxLDL, moderately modified oxLDL, heavily modified oxLDL, or extensively modified oxLDL. Transitional products of the oxidation process including aldehydes, for example, malondialdehyde (MDA), and 4-hydroxynonenal, interact with the positively charged ɛ-amino groups of lysine residues on apoB. This interaction not only changes the structure of LDL and decreases its affinity for liver LDL receptor but also renders the LDL more negatively charged and increases its affinity for scavenger receptors (SRs). OxLDL plays a pivotal role in triggering proinflammatory events that initiate and exacerbate atherogenesis (201, 320).
FIG. 8.
Risk factors for atherosclerosis. Susceptibility to atherosclerosis is determined by many genetic factors; some of these (typed in bold) are also related to PON gene cluster. Environmental factors can also affect the development of atherosclerosis; amongst, factors shown in bold have been indicated to affect PON status [modified from ref. (203)]. VLDL, very LDL.
OxLDL plays important roles in endothelial injury. First, oxLDL is toxic to cells; increased oxLDL levels can cause endothelial cell degeneration, necrosis, and detachment from the aorta wall. OxLDL was reported to dose-dependently promote the death of endothelial cells when added to an endothelial culture system (131, 173 199, 273). OxLDL can also induce increased production of intracellular ROS and apoptosis by binding to lectin-like oxLDL receptor-1 (65, 72). OxLDL also affects the expression of various key genes in endothelial cells, thus altering endothelial function (126). In addition, oxLDL can also induce cytoskeletal rearrangements such as F-actin distribution, cell contraction, and the formation of intercellular gaps (92, 364). Lastly, oxLDL can induce endothelial cells to express and secrete various inflammatory and adhesive molecules such as intercellular adhesion molecule 1 (ICAM-1) and monocyte chemotactic protein 1 (MCP-1), which facilitate the adherence and migration of monocytes and T lymphocytes to injured sites.
Recent studies have shown that oxLDL promotes morphological changes in cultured human vascular smooth muscle cells (VSMCs), suggesting that oxLDL prompts VSMCs to switch from the contractile to the synthetic type (279). In addition, oxLDL can dose-dependently stimulate SMC migration via a chemotactic mechanism; native LDL has no such activity (20). It has also been reported that oxLDL but not native LDL stimulates DNA synthesis in cultured SMCs and that alpha-tocopherol (vitamin E) inhibits this proliferative response (183). These effects are partly mediated by oxidative stress, which causes the release of fibroblast growth factor-2 and a subsequent autocrine or paracrine response (63).
In addition, Okura et al. found that oxLDL increases the susceptibility of VSMCs to apoptosis (251). At lower concentrations, oxLDL may stimulate VSMCs to proliferate and develop into foam cells; at higher concentrations, it may induce apoptosis in VSMCs (365). OxLDL has been shown to induce tissue factor gene expression mediated by both early growth response protein 1 (Egr-1) and specificity protein 1 (Sp1) in SMCs (77). Collagen production by SMCs is stimulated by oxLDL but not native LDL, suggesting that elevations in oxLDL could lead to collagenosis in atherosclerosis (159). OxLDL may also promote osteogenic differentiation of VSMCs and thus vascular calcification (35).
Minimally modified oxLDL (MM-LDL) stimulates the endothelium to express adhesion molecules such as ICAM-1 and vascular adhesion molecule 1, which mediate adhesion of monocytes to the injured endothelium (71). MM-LDL also induces endothelial cells to express monocyte colony stimulating factor (M-CSF), thus promoting monocyte proliferation and differentiation into macrophages (223, 271). Nevertheless, MCP-1 expression and secretion from endothelial cells and SMCs can also be stimulated by oxLDL, which induces the infiltration of monocytes into the subendothelial space (78, 195). Mertens and Holvoet hypothesized that, because it is a potent inhibitor of macrophage motility, oxLDL may also promote macrophage retention in the arterial wall (230).
Upon being taken up as a ligand of the macrophage surface receptor (319), oxLDL promotes foam cell formation. OxLDL, especially MM-LDL, can upregulate the expression of the macrophage SR by activating activator protein 1 (AP-1), nuclear factor kappa B (NF-κB), and other transcription factors (143, 226), cluster of differentiation 36 via peroxisome proliferator-activated receptor γ (PPAR-γ) activation (94, 119), and macrosialin (361). Upregulation of the oxLDL receptor leads to increased uptake of oxLDL by macrophages, so there is no negative feedback between oxLDL and its receptors. OxLDL is also very resistant to degradation by the lysosome. Because of these properties, oxLDL stimulation facilitates lipid accumulation in macrophages, leading to foam cell formation (16, 174, 230, 362).
It has been demonstrated that the growth of murine macrophages is induced by oxLDL (289). Many signal transduction pathways, including the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB) (136), extracellular signal-regulated kinase 1/2 (ERK1/2) or p38 mitogen-activated protein kinase (MAPK) (297), and adenosine monophosphate-activated protein kinase pathways (141), have been reported to be involved in the mitogenic effect of oxLDL. Oxidatively modified apoB is thought to be the main growth-stimulating component of oxLDL, although oxidized phospholipids may play a secondary role (222).
OxLDL can injure macrophages, especially when the levels of phagocytosed oxLDL exceed their degradation capability. Cell death can occur through necrosis or apoptosis depending on the oxidation level of oxLDL, the concentration of oxLDL, and the length of stimulation. Most of the toxicity is caused by the lipid fraction of oxLDL, which contains a wide variety of oxidized lipids (130, 239). OxLDL not only activates caspase-8 through the Fas-FasL pathway but also induces caspase-9 activation by promoting mitochondria to release cytochrome c (70, 197, 225, 246, 256).
When stimulated with oxLDL, macrophages present in atherosclerotic plaques become activated and release many cytokines, tissue factors, and inflammatory mediators. These molecules can induce and regulate repair, migration, mitosis, and synthesis of lipid and proteins in neighboring cells, thus affecting atherogenesis, vasomotion and blood coagulation. For example, interleukin (IL)-1 and tumor necrosis factor α (TNF-α) released by oxLDL-activated macrophages in atherosclerotic plaques induce VSMCs to express platelet-derived growth factor (PDGF), which promotes VSMC proliferation. TNF-α also induces VSMCs to express SRs (43, 166).
C. PON1 contributes to the anti-atherosclerotic functions of HDL
In contrast to LDL, HDL levels have been shown to be inversely related to the development of cardiovascular atherosclerosis (108). Gotto and Brinton reported that every 1 mg/dl increase in HDL is associated with up to 3% decrease in the risk of adverse cardiovascular events (110). HDL has been thought to be at least as important in the pathogenesis of atherosclerosis as LDL. Nevertheless, it has been shown that low serum levels of HDL more strongly predict future adverse cardiovascular events than elevated LDL levels (288). Even in patients with low or close to normal LDL levels, the serum levels of HDL are strongly predictive for coronary heart disease (CHD) (33, 108). In addition to being a useful predictor for atherosclerosis-related diseases, HDL has also been suggested to have a direct protective effect on atherosclerosis (59, 107, 187, 200).
HDL exerts its protective effect through multiple mechanisms. The first proposed mechanism is that HDL facilitates reverse cholesterol transport (RCT), through which free cholesterol produced by peripheral tissues and cells is transferred to the liver (54, 110), where cholesterol is converted to sterol metabolites and then excreted into the bile. HDL can also accept cholesterol and facilitate its efflux from macrophages and foam cells that reside within atherosclerotic plaques through this RCT mechanism (200).
In addition, HDL exerts protective effects by reducing systemic and local inflammation (34). This process is mostly accounted for by HDL's protection of LDL from oxidation, which prevents the recruitment and migration of inflammatory cells to the arterial wall (54, 212, 238). The fact that HDL is able to reduce LDL oxidation has been shown in vitro in various experimental models of LDL oxidation in many independent studies (236, 241), including our own (301). The induction of LDL oxidation was always shown to be significantly prevented or reduced when HDL particles were present in the incubated system compared with conditions without HDL present. In such cases, the HDL complex enzymatically hydrolyzes the oxidized phospholipid molecules of LDL particles and transfers the modified phospholipid particles to HDL itself. Reduction of the levels of oxLDL therefore attenuates the vicious atherogenic cycle originating from LDL oxidation. Many other antiatherogenic effects, such as reductions in endothelial death, SMC apoptosis (131), and macrophage foam cell formation (236), also result from reductions in LDL oxidation. The antiatherogenic and antioxidant effects of HDL should be attributed to HDL-associated proteins such as apoA I, lecithin cholesterol acyltransferase, and PON1, among others (211, 343, 348). Of these proteins, PON1 contributes significantly more antioxidant activity to HDL (90) than the others, because many of the lipid oxidative metabolites can be degraded by members of the PON family, as discussed earlier. However, several studies show that PON1 does not have intrinsic antioxidant activity and previous reports of such activity in vitro may have been due to contaminations present in PON1 preparations (73, 216, 329).
Moreover, HDL has the ability to hydrolyze Hcy-thiolactone and thus is hypothesized to protect against Hcy-thiolactone toxicity by preventing thiolactone formation and limiting the extent of protein modification (149). Earlier work by Jakubowski suggests that HDL is capable of hydrolyzing Hcy-thiolactone and blocking the accumulation of N-Hcy-modified proteins in vitro, and PON1 has the appropriate enzyme activity. The Hcy-thiolactonase activity of plasma is associated with PON1 polymorphic variants (Leu/Met 55 and Arg/Gln 192) (148). In the latest study by Perla-Kajan and Jakubowski, the use of new assays for the quantification of N-linked Hcy content in proteins and for the determination of Hcy-thiolactonase activity showed that the Hcy-thiolactonase activity of PON1 influences the serum N-Hcy-protein level and protects against the atherogenic effects of N-Hcy-protein accumulation in vivo. These findings provide evidence for atheroprotective roles for HDL in humans (263).
D. PON activity decreases atherosclerosis
1. PON1 and its protection against atherosclerosis
PON1, the first member of the PON gene cluster to be discovered, has been studied for many years and is by far the best understood member of the family. It is highly conserved in mammals but is absent in fish, birds, and invertebrates such as arthropods (181). Changes in the size and shape of HDL particles strongly influences the binding affinity and stability of PON1 and results in a reduced antioxidative capacity (90). PON1 is the main factor the nervous system uses to protect itself against neurotoxic organophosphates that enter the circulatory system (82).
In addition to its established important roles in organophosphate metabolism, PON has also been shown to play roles in lipid metabolism. A relatively new area of investigation, the study of the links between PON1 and LDL oxidation, led to an extensive investigation of the potential roles of PON1 in atherosclerosis. The capacity of PON1 to prevent LDL and HDL against oxidation by a variety of pro-oxidant factors, including cell-induced LDL oxidation, has been demonstrated in both in vitro and in vivo studies (82, 90, 181, 237, 351). It has been shown that the antioxidant activity of PON1, by blocking LDL oxidation, prevents a number of pathological characteristics associated with atherogenesis, monocyte recruitment, and gene activation (305). The strongest evidence for PON1's roles in hydrolyzing oxLDL and providing atheroprotection comes from studies of PON1-knockout mice (303, 305). Both HDL and LDL isolated from PON1 null mice are more susceptible to oxidation in the presence of cocultured cells than those from wild-type littermates. Also, HDL isolated from PON1 null mice was no longer able to prevent LDL oxidation in a cell coculture model of the artery wall. Thus, PON1 null mice are more susceptible to both lipoprotein oxidation and atherosclerosis than wild-type mice. The association between low plasma PON1 activity and features of atherosclerosis has been verified by additional studies in mice, humans, and other species. In apo E-knockout mice, LDL receptor-knockout mice, and apoAII-overexpressing transgenic mice, the plasma PON1 levels are greatly reduced, and the mice exhibit hypercholesterolemia and atherosclerosis (196).
As reported in 2002, Tward et al. constructed human PON1 transgenic mice to test whether high levels of PON1 protect against LDL oxidation and decrease atherosclerosis in vivo (338). The results indicated that transgenic overexpression of human PON1 dose-dependently protects mice against both early and late stages of atherogenesis. This study demonstrated, for the first time, that the human PON1 protein exhibits antioxidative and antiatherogenic functions in vivo. In the PON1 transgenic mice, HDL exhibited three times as much PON1 activity as wild-type HDL and was better able to protect LDL against oxidation. It has also been reported that PON1 preserves HDL function during oxidative stress (250). Using PON transgenic mice, Oda et al. found that overexpression of PON1 inhibits lipid hydroperoxide formation on HDL and protects HDL integrity and function. PON1 also reduces monocyte chemotaxis and adhesion to endothelial cells (7). The antioxidative functions of PON1 are attributable either to its phospholipase A2-like activity, which hydrolyzes biologically active oxidized phospholipids, and its peroxidase-like activity, which destroys lipid hydroperoxides and H2O2 (8, 23, 27, 155), or to the contamination of other enzymes during PON1 purification (73, 216, 329). In addition to preventing LDL oxidation, PON1 can also promote cellular cholesterol efflux, which is the first step in RCT. Thus, PON1 is hypothesized to affect the efficiency of lipid transfer between HDL cholesterol and LDL cholesterol (206). It has also been demonstrated that PON1 plays a role in protection against bacterial endotoxins, thus stabilizing cellular membranes during either acute or chronic exposure to oxidative agents and free radicals that challenge the selective permeability of the membrane (283). All of these biochemical functions contribute to PON1's protective effect in the development of atherosclerosis and CVDs.
At the same time, clinical investigations also support the idea that PON1 protects against atherosclerosis-related disease. The PON1 activity in patients with CHD is approximately half that in disease-free control participants (28). Low PON1 activity increases the development of atherosclerosis (353). The group of men with the highest levels of serum PON1 activity were nearly 60% less likely to have CHD than those with the lowest (205). These results show that serum PON1 concentration is inversely correlated with susceptibility to atherosclerosis. Other lines of evidence for PON1's atheroprotective effect include studies showing that purified PON1 reduces the uptake of oxLDL by macrophages by ∼68%. PON1 also inhibits cholesterol biosynthesis in macrophages (by ∼84%) and increases the efflux of cholesterol from macrophages (by ∼70%) (205).
2. PON2 and its protection against atherosclerosis
As the second member of the PON gene family, PON2 gene shares 79%–90% identity with PON1 (268). PON2 is thought to be able to lower intracellular oxidative stress and prevent cellular oxidation of LDL, although its aromatic ester and PON hydrolyzing activities are lower than those of PON1 (245). Cells overexpressing PON2 are less able to oxidize LDL and show significantly less intracellular oxidative stress when exposed to either H2O2 or oxidized phospholipids (245), suggesting that PON2 plays a protective role in atherosclerosis. Indeed, when PON2-knockout, apoE null mice were challenged with a high-fat diet, these mice developed significantly larger atherosclerotic lesions than their wild-type counterparts, although the serum levels of VLDL and LDL cholesterol were significantly lower in PON2-deficient mice compared with wild-type mice. Enhanced inflammatory signaling by LDL, an attenuated antiatherogenic capacity of HDL, and a heightened state of oxidative stress, along with an exacerbated inflammatory response in PON2-deficient macrophages, were also detected in the PON2-deficient mice (241). Conversely, adenoviral overexpression of PON2 in apoE null mice significantly enhances the efflux potential and antioxidant capacity of serum and increases the anti-inflammatory properties of HDL, thus protecting mice against atherogenesis in vivo (243). Further investigation showed that the antiatherogenic effects of PON2 are partly contributed by its protection of mitochondrial against oxidative stress (84). PON2 has been found to be able to prevent mitochondrial superoxide formation and apoptosis of cells, which is independent from its lactonase activity (12). These studies are sufficient to show that PON2 strongly inhibits the development of atherosclerosis.
3. PON3 and atherosclerosis
PON3, the third member of the PON gene family to be identified, encodes the PON3 protein, which is composed of 353 amino acid residues. Rabbit PON3 is more efficient at protecting LDL from copper-induced oxidation than rabbit PON1 (87). PON3 overexpression can prevent the formation of mildly modified oxLDL and reduce the concentration of previously formed mildly modified oxLDL in cultured human aortic endothelial cells (277). Interestingly, PON3 expression is not regulated by oxidized phospholipids in HepG2 cells or by high-fat atherogenic diet feeding in mouse liver (277). Further, infecting apoE-deficient mice with adenovirus expressing human PON3 resulted in a significant decrease in lesion formation compared with mice infected with a control adenovirus. Serum from mice overexpressing human PON3 contained significantly lower levels of lipid hydroperoxides and was better able to release cholesterol from cholesterol-loaded macrophages than serum from control mice. In addition, the LDL from these mice was less susceptible to oxidation, and their HDL was better able to protect against LDL oxidation (242). Human PON3 transgenic mice developed significantly smaller atherogenic diet-induced atherosclerotic lesions compared with their nontransgenic littermates either on a B6 background or an LDL receptor knockout background, although no significant differences in total plasma, HDL, VLDL/LDL cholesterol, triglyceride, or glucose levels were observed between the PON3 Tg and the control mice. Nevertheless, the aortic expression of monocyte chemoattractant protein-1 was also decreased compared with the control littermates. More impressively, decreased adiposity and lower circulating leptin levels were also observed in both lines of human PON3 transgenic mice compared with the nontransgenic mice. These results demonstrate that PON3 may play an important role in protection against not only atherosclerosis but also obesity (306).
4. Roles of the PON gene cluster in atherosclerosis
Based on the findings discussed earlier, Reddy et al. concluded that all of the three members of the PON gene family protect against atherosclerosis and could be used as therapeutic targets for the treatment of atherosclerosis (276). However, the role of the entire PON gene cluster in atherogenesis remains to be elucidated. Therefore, we generated human PON gene cluster transgenic mice to analyze the functions of the PON gene cluster in atherogenesis. We found that PON cluster transgenic apoE null mice formed significantly fewer atherosclerotic lesions than nontransgenic apoE null mice under a high-fat diet challenge, although there was no change in total plasma cholesterol, HDL cholesterol, VLDL/LDL cholesterol, triglycerides, or glucose levels. Contrary to our expectations, no observable additive effect on atherogenesis of overexpressing the entire gene cluster versus expressing only PON1 or PON3 was found. We hypothesize that overexpression of any single PON gene might saturate oxLDL inhibition, rendering overexpression of all three PONs redundant. This idea is partly supported by our results showing that sufficient amounts of wild-type HDL attenuate copper-induced lipid hydroperoxide production by human LDL to the same extent as an equal amount of HDL isolated from PON cluster transgenic mice (301). In addition, oxLDL, although important, is not the only risk factor for atherosclerosis. Thus, although the PON cluster potentiates most of the functions of oxLDL, it cannot completely inhibit atherogenesis. It is also possible that an additive effect of the PONs does exist but that we failed to find it because of differences between the experimental systems; the background of the mice, the diet, induction period, and the evaluation strategies all differ among our studies and the studies investigating the effects of single gene overexpression (Table 1). It would be better to compare the effect of overexpressing the PON cluster versus single PON genes on atherogenesis in the same experimental system. It is also possible that other genomic elements in our transgenic construct affected atherogenesis via unknown mechanisms, because the construct we used was much longer than those used in the PON1 and PON3 single transgenic mice (Fig. 9).
Table 1.
Comparison of Atherogenesis Phenotypes Among Mouse Strains with Various Genetic Modifications of Paraoxonase
| |
Diet |
|
|
||
|---|---|---|---|---|---|
| Mice model | Fat | CHO | Sodium cholate | Period | Plaque quantification |
| PON1 ko | 15.75% | 1.25% | 0.50% | 15 weeks | Cross-section |
| PON1 ko/ApoE ko | 42.00% | 0.15% | 0 | 16 weeks | En face |
| PON1 tg | 15.75% | 1.25% | 0.50% | 15 weeks | Cross-section |
| PON1 tg/ApoE ko | Regular chow | 28 weeks | Cross-section | ||
| PON2 ko | 15.80% | 1.25% | 0 | 15 weeks | Cross-section |
| PON3 tg | 15.75% | 1.25% | 0.50% | 15 weeks | Cross-section |
| PON3 tg/LDLR ko | 42.00% | 0.15% | 0 | 8 weeks | Cross-section |
| PON3 ad | Regular chow | 3 weeks | Cross-section | ||
| PC tg/ApoE ko | 10% | 1.25% | 0 | 10 or 16 weeks | En face and section |
CHO, cholesterol.
FIG. 9.
Comparison among PON1, PON3, and PON cluster transgenic mice. (A) Human BAC DNA fragment used to make human PON cluster transgenic mice. (B) Human BAC DNA fragment used to make human PON1 transgenic mice. (C) Human BAC DNA fragment used to make human PON3 transgenic mice. BAC, bacterial artificial chromosome.
Like transgenic overexpression of PON1 or PON3 alone, PON cluster overexpression enhanced the ability of HDL to protect LDL from oxidation in vitro. In addition, serum expression of ICAM-1 and monocyte chemoattractant protein-1 were also repressed by PON cluster overexpression, as were proatherogenic reactions (ROS generation, inflammation, matrix metalloproteinase-9 expression, and foam cell formation) by peritoneal macrophages induced by oxLDL. More importantly, we found that plaques from PON cluster transgenic apoE null mice exhibited increased levels of collagen and SMCs and reduced levels of macrophages and lipids compared with those from apoE null mice, indicating that the lesions of PON cluster transgenic apoE null mice more closely resemble stable plaques than those of apoE null mice. Thus, the PON cluster transgene not only represses atherogenesis but also promotes atherosclerotic plaque stability in vivo (301). It seems that, as a gene cluster, PONs exert their antiatherosclerotic functions at the following two levels: PON1 and PON3 exert their effect on HDL in the serum, and PON2 protects macrophages and possibly other cells by acting intracellularly (Fig. 6).
IV. PONs Protect Against Hcy Toxicity and Associated Atherogenesis
A. Hcy is an important risk factor in atherosclerosis
Hcy is a product of the methionine cycle that is generated by transfer of a methyl group to methionine. Methionine can also be converted to produce S-adenosyl methionine (SAM), which is the most important biological donor of methyl groups, by adenosyl transferase. SAM can then be demethylated to produce S-adenosyl Hcy, which is converted to Hcy after deadenylation. Endogenous Hcy can be metabolized via two pathways: the methionine cycle and transsulfurylation (218). This transsulfurylation reaction requires vitamin B6 as an essential cofactor. In cases of methionine deficiency, Hcy can be remethylated into methionine by methionine synthase (MS) using vitamin B12 as a cofactor and 5-methyltetrahydrofolate as a substrate (218). Deficiency of any of the enzymes or cofactors aforementioned will lead to an accumulation of Hcy. High levels of Hcy [>15 μM is considered “abnormal” (278)] are now considered to be an independent risk factor for CVD (50). The first report that homocysteinemia was related to the pathogenesis of arteriosclerosis was made by McCully in 1969 (228). Such a notion is supported by almost all of the large meta-analyses that have been conducted. The first large meta-analysis (reported in 1995) stated that Hcy is strongly associated with vascular disease and that Hcy accounts for up to 10% of the population's CAD risk (50). A 5 μM increment in total Hcy concentration is correlated with a 20 mg/dl plasma cholesterol increase (50). A recent meta-analysis indicated that a 3 μM reduction of the plasma Hcy concentration could lead to a reduction of the relative risk of 11% for CAD and 19% for stroke (131a). An increased plasma Hcy concentration in patients with acute coronary syndromes is an independent predictor for recurrent cardiovascular events (240, 322, 347). Roles for Hcy in atherosclerosis have been elucidated by these clinical studies and also by experimental investigations (68).
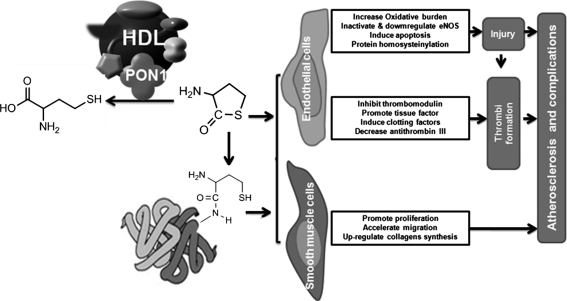
Impairment of endothelium-dependent dilation has been well documented in subjects with homocystinuria (85). In addition, high levels of Hcy can lead to vascular endothelial injury, especially when blood pressure is high. These effects could be accounted for by one of several possible mechanisms. First, homocysteinemia increases the vascular oxidative burden by inducing nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and inducible nitric oxide synthase activity (340), altering superoxide dismutase and glutathione peroxidase (354), elevating asymmetrical dimethylarginine levels (323), and uncoupling endothelial nitric oxide synthase (eNOS) (13, 14). Second, Hcy not only inactivates eNOS by inducing its phosphorylation at threonine 495 (158) but also downregulates eNOS by interfering with intracellular redox signaling in endothelial cells (231, 363). Third, high levels of Hcy induce apoptosis in endothelial cells by affecting the PI-3 kinase (36), Fas-FasL (325), p53 (188), and p38 MAPK (31) pathways, among others. Fourth, the level of Hcy is important for thiolactone formation and protein homocysteinylation, which is known to induce cell death (149, 264) and a variety of immunological consequences (264).
Hcy also dose-dependently promotes the proliferation of rat, human, and pig aortic VSMCs (64, 334). Hcy can also upregulate collagen synthesis and accumulation by arterial SMCs (213), which could lead to the increased collagen content of the atherosclerotic plaques observed in patients with untreated homocystinuria. In addition, Hcy indirectly promotes VSMC migration through a paracrine or endocrine effect involving adipocyte-derived resistin (157).
Severe homocysteinemia resulting from genetic homocystinuria has been reported to be closely related to recurrent vascular thrombosis (52, 229). Endothelial injury is thought to be the major mechanism for Hcy-mediated promotion of this prothrombotic state, because platelet aggregation and thrombi formation always occur at sites of injury endothelium in both human and animal homocysteinemia (122). Some studies suggest that, in humans, the plasma total Hcy level has effects on fibrin clot structure, representing a novel prothrombotic effect of Hcy (40, 146, 339). In addition, high levels of Hcy can shift the balance between procoagulation and antithrombus factors. For example, Hcy may be able to inhibit the expression of thrombomodulin and promote the expression of tissue factor in endothelial cells (99). Hcy can also induce the expression of clotting factors II, V, X, and XII (122) and attenuate the activation of protein C and antithrombin III (124). Hcy levels have been used as an independent predictor of thrombotic events in these individuals (40).
B. PONs protect against Hcy toxicity
Hcy metabolites are thought to be the major cause of Hcy toxicity. Elevated Hcy levels lead to protein N-homocysteinylation. Accumulating evidence has suggested that protein N-homocysteinylation can cause enzyme inactivation and protein aggregation and precipitation. Studies by Paoli et al. revealed how the low levels of protein N-homocysteinylation induce mild conformational changes leading to the formation of native-like aggregates that evolve over time to produce amyloid-like structures. (257) Hui and coworkers have hypothesized that plasma levels of Hcy-thiolactone adducts could be a more direct predictive index of CHD than plasma Hcy levels. They concluded that high plasma levels of Hcy-thiolactone adducts are independent of traditional risk factors and that Hcy-thiolactone (HTL) adducts might play a role in atherosclerotic vascular diseases (359). Given that Hcy is formed in all cell types (145) and that Hcy-thiolactone harms the body in many ways, the ability to detoxify Hcy is essential for biological integrity. Jakubowski and coworker demonstrated that human PON1 has Hcy-thiolactonase activity and provided evidence that Hcy-thiolactone is a physiological substrate of PON1. They also suggested that the plasma N-Hcy-protein levels are mainly determined by the Hcy thiolactonase activity of PON1 and that PON1 protects against accumulation of proatherogenic N-Hcy-proteins in vivo (263). This may be a novel mechanism for the atheroprotective role of PON1 (Fig. 10).
FIG. 10.
PON1 can protect against atherogenesis by detoxifying Hcy. Hcy can promote atherogenesis by inducing atherogenic alterations of vascular endothelial cells and smooth muscle cells, either by direct toxicity or by homocysteinylating intracellular protein. PON1 has been reported to be able to detoxify Hcy by hydrolyzing it to cysteine. eNOS, endothelial nitric oxide synthase; Hcy, homocysteine.
In clinical investigations, serum PON activity has been reported to be negatively associated with the serum Hcy concentration (118, 163, 165, 182, 185). One study reported that the hydrolytic activity of human PON1 toward Hcy-thiolactone is associated with the PON1-192-Arg/Arg and PON1-55-Leu/Leu genotypes and strongly correlated with PON1's hydrolytic activity toward organophosphate paraoxon substrates (182). The T allele of PON1/T(−107)C and the Ser allele of the PON2/Cys (311)Ser polymorphism were reported to be associated with lower plasma Hcy and Hcy thiolactone complex levels (148, 269). The Hcy thiolactonase activity of PON1 is negatively associated with the thickness of the carotid intima media in patients with type 2 diabetes mellitus. The Hcy thiolactonase activity of serum PON1 may thus reflect the status of atherosclerosis in patients with type 2 diabetes mellitus (172).
V. PONs Polymorphisms and Coronary Artery Disease
A. PON1 polymorphisms
1. PON1 polymorphisms and PON1 activity
Two major gene polymorphisms in the coding region of the human PON1 gene have been reported. One is at position 192 and leads to a glutamine to arginine substitution (Arg/Gln192). Another mutation encodes a leucine to methionine substitution at position 55 (Met/Leu55). Each has been reported being independently associated with PON1 activity and defined as the molecular basis for interindividual variability (4, 105, 135). These two polymorphisms are also hypothesized to be the major determinants of the well-known biochemical polymorphism in serum PON activity toward various organophosphates (Table 2).
Table 2.
Major PON1 Gene Polymorphisms
| Site | Amino acid/nucleotide | Effects on expression or activity | Association with atherosclerosis-related condition | References |
|---|---|---|---|---|
| 55 | Leu vs. Met | Leu/Leu: highest expression; highest PON, arylesterase, and lactonase activity | Met/Met: lowest total and LDL cholesterol and ApoB/ApoAI ratio. Leu/Leu: higher HDL and ApoAI. Leu/Leu: risk factor for CHD or no association. | (15, 44, 56, 74, 128, 189, 192, 214, 215, 270, 291, 358) |
| 192 | Gln vs. Arg | Gln/Gln: highest activity to phenylacetate, lactonase and sarinase activity; Arg/Arg: highest PON and arylesterase activity | Gln/Gln: lowest total and LDL cholesterol and ApoB/ApoAI ratio. Gln/Gln: lower oxLDL | (56, 79, 135, 137, 189, 270) |
| −108/−107 | C vs. T | −108C: two times higher activity; CC: highest serum concentration and activity to phenylacetate | (55, 56, 190, 324) | |
| −126 | No differences | (55) | ||
| −160/−162 | A vs. G | AA: highest activity to phenylacetate | (55, 56) | |
| −824/−832 | A vs. G | −824A: 1.7 times higher activity or no difference; AA: highest activity to phenylacetate | (55, 81, 190) | |
| −907/−909 | C vs. G | GG: highest activity to phenylacetate | (55, 56, 81, 190) |
apo, apolipoprotein; CHD, coronary heart disease; HDL, high-density lipoprotein; LDL, low-density lipoprotein; PON, paraoxonase.
PON1 activity levels vary widely among individuals, which may partly account for differences in susceptibility to organophosphate poisoning. The molecular basis for these differences was reported to be associated with PON gene polymorphisms. Purified PON1 from PON1 192 Arg/Arg and PON1 55 Leu/Leu individuals has the greatest hydrolytic activity toward paraoxon, whereas that from 192 Gln/Gln and PON1 55 Met/Met individuals has the least; heterozygotes have intermediate levels of activity (140, 352). A similar pattern of substrate specificity was observed when other oxons, such as methyl paraoxon, chlorthion-oxon, and amine, were used as substrates (352).
However, the capacity of PON1 to protect LDL against oxidation follows the opposite trend as that of paraoxon hydrolytic activity. PON1 from 55 Met/Met/-192 Gln/Gln individuals exhibits the greatest protective capacity for LDL oxidization. This type of PON1 is also able to best hydrolyze DZO and the nerve gases sarin and soman (352). For other substrates, such as phenyl acetate, chlorpyrifos oxon, and 2-naphthyl acetate, all polymorphic variants of PON1 have similar hydrolytic activities (352).
In addition to the coding region polymorphisms, five gene polymorphism sites have been reported in the PON1 upstream transcription regulatory region, at −107/−108, −126, −160/−162, −824/−832, and −907/−909 (55, 56, 102, 190, 191, 193, 194). Brophy et al. reported that the −108 polymorphism has a significant effect on PON1 transcription, whereas the −162 polymorphism has a lesser effect (56).
The −909 polymorphism is in linkage disequilibrium with the other sites and has no independent effect on PON1 activity. In addition, the −107T, −824G, and −907G genotypes are correlated with high PON1 concentration and activity, and −107T seems to have a dominant effect on PON1 gene expression (81).
2. PON1 polymorphisms and plasma lipoprotein levels
Because PON1 polymorphisms affect serum PON1 activity and serum PON1 activity is associated with variations in the concentrations of plasma lipoproteins including serum apoAI, LDL cholesterol, and HDL cholesterol, PON1 gene polymorphisms could affect plasma lipoprotein levels. This hypothesis has been tested in several studies. One study shows that PON1 polymorphisms were associated with variations in most plasma lipoproteins, including LDL and HDL cholesterol, in samples taken from the genetically isolated Alberta Hutterites and aboriginal Oji-Cree from northern Ontario (128).
PON1 Gln192 homozygotes exhibit a better plasma lipoprotein profile, with lower levels of total cholesterol and plasma apoB-related biochemical variables and lower apoB/apoA-I ratios than heterozygotes and PON1 Arg192 homozygotes. A significant difference was detected in the mean total cholesterol and LDL cholesterol levels between subjects with the PON1 Leu/Leu55 and Met/Met55 genotypes; PON1 Met/Met55 subjects have a better plasma lipoprotein profile (189). PON1 Gln192 patients have been found to have lower plasma oxLDL levels than control subjects (137). Mean plasma concentrations of total and LDL cholesterol and apoB are higher in PON1 Met55 carriers than those of noncarriers in samples from two Canadian aboriginal Oji-Cree and Inuit populations (93). Malin et al. reported that PON1 Leu/Leu55 homozygotes have increased basal HDL concentrations and also tended to have higher apoAI concentrations (215). They also found that the Arg/Gln192 polymorphism is a more powerful predictor of changes in HDL cholesterol and apoAI concentration during pravastatin therapy than the Met/Leu55 polymorphism.
However, some studies claim that no association between PON1 polymorphisms and plasma lipoproteins exists (48, 204, 353). Contradictions among the results from different studies could result from partially intrinsic differences in serum PON levels among populations. Thus, it is important to compare the results obtained from association studies in diverse genetic isolates with different ethnicities. Also, the mechanisms by which PON genotypes affect the total cholesterol, HDL cholesterol, and LDL cholesterol levels and the apoB/apoAI ratio remain to be elucidated.
3. PON1 polymorphisms and CHD
In recent years, a relationship between the PON1 192 polymorphism and CHD has been proposed (142, 253), although some studies have reported no association between PON genotype and CHD risk (128, 353). Such dichotomies often occur with association studies, which are thought to have several biases (196). Some recent studies that combine association studies with functional data have suggested that the PON activity/concentration, in addition to the PON genotype, contribute to the risk of CHD in vivo (215). One recent study that involved 1399 patients undergoing diagnostic coronary angiography systematically investigated the relationship between PON1 genotypes and functional activity with systemic oxidative stress and CVD risk in humans. The results indicated that (i) the PON1 genotype is dose-dependently associated with decreased serum PON1 activity (Gln/Gln192>Gln/Arg192>Arg/Arg192) and with increased levels of systemic oxidative stress (Gln/Gln192<Gln/Arg192<Arg/Arg192); (ii) participants with the Gln/Gln192 genotype, compared with participants with either the PON1 Arg/Arg192 or Gln/Arg192 genotype, demonstrated an increased risk of overall mortality and of major adverse cardiac events; and (iii) the incidence of major adverse cardiac events was significantly lower in participants with the highest PON1 activity compared with those with the lowest PON1 activity. (39) In addition, Blatter-Garin et al. reported that homozygosity for the 55Leu allele of PON1 is an independent risk factor for CHD in non–insulin-dependent diabetics (44), which is supported by other studies (74, 128). In contrast, some studies conducted in patient groups of different ethnicities found no significant association between the 55 polymorphism and the presence of CHD (15, 214, 291, 358).
These inconsistent results suggest that the association between the PON1 genotype and CHD might exist but be weak or variable and that it might be influenced by other polymorphisms or by environmental factors. Thus, the PON1 genotype cannot convincingly be used as a predictor of CHD. Instead, PON1 activity level appears to be a better predictor of CHD than genotype. Jarvik et al. showed low levels of PON1 activity in CHD subjects compared with age- and race-matched controls, although the PON1 192 (p=0.75) and PON1 55 (p=0.83) genotypes could not predict case–control status (154). PON1 activity, however, predicted CHD independently of traditional risk factors. These results influence the earlier report of decreased PON1 activity in 50 myocardial infarction survivors, making it clear that PON1 activity is a risk factor for, rather than a result of, infarction. It was also reported that PON1 activity was lower in type 2 diabetic patients with CHD than in those without CHD (152). Significantly lowered PON1 activity was detected in 417 CHD patients versus 282 controls (204).
B. PON2 polymorphisms
1. PON2 polymorphisms
The human PON2 gene has two common polymorphisms at residues 148 and 311, both of which lead to amino acid substitutions (245). The alleles encode either glycine or alanine at codon 148 and either Cys or serine at codon 311. Accordingly, these two polymorphisms are designated Gly/Ala148 and Cys/Ser311 (234). Almost total linkage disequilibrium exists between these two polymorphic sites in four different human populations, which indicate that the genotype at one site can be used as a surrogate for the genotype at the other site. In other words, the Ala148 and Ser311 variants form one common allelic haplotype, and the Gly148 and Cys311 variants form the second common allelic haplotype in white, South Asian, and African samples (128) (Table 3).
Table 3.
Major PON2 Gene Polymorphisms
| Site | Amino acid/nucleotide | Association with atherosclerosis | References |
|---|---|---|---|
| 148 | Gly vs. Ala | Ala/Ala: highest LDL cholesterol and ApoB; Lowest HDL cholesterol and ApoAI. Gly/Gly: highest HDL cholesterol and ApoAI. | (49, 128, 307) |
| 311 | Cys vs. Ser | Ser/Ser: highest LDL cholesterol and ApoB; Ser allele: risk for CHD, Cys allele: protect against CHD. | (66, 128, 189, 255, 307) |
2. PON2 polymorphisms and plasma lipoprotein levels
PON2 polymorphisms are associated with variations in plasma lipoprotein levels. By analyzing a sample of 334 nondiabetic Oji-Cree individuals, Hegele found that PON2 Ala148/Ser311 homozygotes exhibited significantly higher plasma total and LDL cholesterol and apoB than subjects with the other two genotypes (128), which was confirmed by an investigation by Shin et al. (307). Another study showed that individuals homozygous for PON2 Gly148 had the highest plasma concentrations of total and HDL cholesterol and apoAI, Ala148 homozygotes the lowest, and heterozygotes had an intermediate phenotype (49). Leus et al. reported that the common PON2 polymorphisms are associated with clinical manifestations of CVD in familial hypercholesterolemia patients. The results also indicated that PON2 Ser311 carriers seem to be at risk for CHD due to significantly higher total and LDL cholesterol; conversely, subjects with the Cys/Cys311 genotype seem to be protected against the development of premature CVD (189). The authors also claimed that the associations were not related to linkage disequilibrium between the two sites, because the associations for PON2 were independent of the associations for PON1. The finding of associations between PON2 polymorphic variants and plasma lipoprotein levels suggests a relationship between these polymorphisms and lipoprotein, although the corresponding mechanisms are still unknown.
3. PON2 polymorphisms and CHD
The PON2 Ser311 polymorphism is also consistently reported to be associated with CHD in various populations (66, 189, 255), leading to a speculation that PON2 is even more important for protection against CHD than PON1. Other interpretations are that an as-yet undiscovered functional mutation is in stronger linkage disequilibrium with the PON2 311 polymorphism than the PON1 polymorphism or that there are other polymorphisms of PON1 that are more strongly related to CHD risk than the 192 polymorphism. More extensive data from independent studies are needed to make a final conclusion about the associations between PON2 polymorphisms and CHD.
VI. Other Diseases Related to PONs
In addition to being directly related to atherosclerosis and CHD, PONs have also been shown to play roles in many other diseases, probably because of their multiple enzymatic capacities. Many of these diseases can also contribute to atherogenesis and CHD.
A. PONs' relations to diabetes mellitus and corresponding complications
Diabetic patients may overproduce ROS as a result of chronic hyperglycemia, hyperinsulinemia, elevated free fatty acids, and dyslipidemia (219). Their PON1 activities, however, have been found to be lower than normal (139). Glycation of the PON1 protein, rather than reduced expression, is considered to be the main reason for the low enzyme activity in these patients (127). Another possibility is that PON activity is inactivated by abnormally high levels of ROS, because it has been shown that components of ox-LDL are able to strongly downregulate PON1 activity (26).
The Met allele of the PON1 Leu/Met 55 polymorphism is more frequently in type 1 and 2 diabetes patients than in controls, whereas the Arg allele of the Gln/Arg192 polymorphism is less frequent in type 1 and 2 diabetics than healthy controls. Serum PON1 activity is significantly decreased in both type 1 and 2 diabetes patients compared with the controls. The Met/Met and Gln/Gln genotypes are more tightly associated with lower PON1 activity than the Leu/Leu and Arg/Arg genotypes. Also, PON levels are lower in diabetic patients with complications such as neuropathy, nephropathy, and retinopathy (98). Van den Berg et al. also found that the Arg/Arg genotype was found at a significantly higher frequency in newly diagnosed type 2 diabetes patients compared with subjects with normal glucose tolerance (341). An association between coding region polymorphisms in PON1 or PON2 and the presence of diabetic complications such as diabetic nephropathy and retinopathy in diabetes has also been reported. Pinizzotto et al. showed that PON2 polymorphisms are strongly and significantly associated with diabetic nephropathy in Swiss type 2 diabetes patients and were independent of traditional risk factors (266). A strong association between PON1 Met/Leu55, but not Gln/Arg192, and diabetic retinopathy was reported in Australian adolescents with type 1 diabetes (162). Young type 1 diabetes patients with the L/L polymorphism at position 55 of PON1 gene were found to be more susceptible to retinal complications (171). The 192Arg allele of PON1 predisposes to microangiopathy in type 2 diabetes mellitus patients, as shown in a case–control study with a total of 280 patients with type 2 diabetes with or without microangiopathies (235). The PON2-311 polymorphism is associated with the presence of microvascular complications in diabetes mellitus, although no association was found between the PON2-311 polymorphism and lipid or lipoprotein concentrations. In this study, over-representation of the Cys/Cys 310 genotype in patients with diabetes and microvascular complications was found (207). The presence of specific PON gene polymorphisms in diabetic patients provides evidence for a relationship between the PON gene family and diabetes.
Oxidative stress can accelerate the development of diabetes. PON1-overexpressing mice had decreased diabetes-induced macrophage oxidative stress, decreased risk of development of diabetes, and decreased mortality compared with wild-type mice; this phenotype was even more dramatic when compared with that of PON1 knockout mice. PON1 was therefore hypothesized to be able to attenuate the development of diabetes because of its antioxidative properties (287). Low PON1 activity is also related to higher CRP, which is independent of adipokines, obesity, and lipids. Decreased PON1 activity may enhance systemic low-grade inflammation and thus contribute to increased cardiovascular risk in type 2 diabetes mellitus (89).
A significantly increased incidence of CVD was found in diabetes patients who had a lower level of PON1 activity than the median value. Low concentration and enzymatic activity of PON1 may be used as independent predictors of cardiovascular events in diabetic patients (138). Decreased PON activity also accounts for the increased risk of cardiovascular events in diabetic patients.
B. PONs' associations with metabolic syndrome and obesity
Metabolic syndrome is a group of metabolic abnormalities including dyslipidemia, abdominal obesity, high blood pressure, and thrombotic and inflammatory states (235a) and has been recognized as a major risk factor for CVD mortality. Metabolic syndrome is considered to result primarily from insulin resistance (115), which may be partially caused by oxidative stress (258). Thus, because of their antioxidant activities, the PONs are hypothesized to protect against metabolic syndrome. In a study of 1364 randomly recruited subjects, of whom 285 were found to be suffering from metabolic syndrome, Senti et al. found that serum PON1 activity levels were significantly lower and lipid peroxide concentrations were significantly higher in subjects with metabolic syndrome compared with the unaffected subjects. They also reported that, in this population, as the number of metabolic disturbances increased, PON1 activity decreased significantly and lipid peroxide concentrations increased significantly. Although no differences in the prevalence of PON1 codon 192 genotypes were found among the subjects with various metabolic abnormalities, metabolic syndrome severity was hypothesized to be associated with increased oxidative stress and lower antioxidant PON1 enzymatic capacity (298). Rizos et al. also found that serum PON1 activities were lower in the metabolic syndrome group compared with the non-MS group (280). In studies by Garin et al., the metabolic syndrome was found to be characterized by the presence of smaller, denser lipoprotein particles, which are more susceptible to oxidative modifications and decreases in serum PON1 levels (103). The Leu/Met 55 polymorphism has effects on insulin resistance in healthy subjects. The presence of the Leu/Leu PON1 genotype is associated with a more severe degree of insulin resistance (32). A significant interaction was found between the metabolic syndrome and both the PON1 Leu/Met55 and Gln/Arg192 polymorphisms in determining the risk for coronary artery disease. Subjects who had metabolic syndrome and both the 55Leu and 192Arg alleles had significantly increased risk compared with subjects without metabolic syndrome and with the Met/Met55-Gln/Gln192 genotype (224). An investigation of the relationship between adiponectin and PON1 showed that PON1 activity correlated positively with HDL-C and adiponectin levels and negatively with body–mass index, waist circumference, systolic blood pressure, levels of HbA(1C), insulin, homeostatic model assessment–insulin resistance, and other markers of metabolic syndrome (29). Some studies, however, have failed to find a positive relationship between metabolic syndrome and serum PON activity (184, 327). In leptin- and LDL receptor-deficient mice, which serve as a model for metabolic syndrome, adenoviral overexpression of human PON1 significantly reduced the total plaque volume, the plaque macrophage volume, and plaque-associated oxLDL. It also increased the percentage of SMCs in the plaques. Expressing human PON1 lowered the titer of autoantibodies against MDA-modified LDL, although no effect on plasma total cholesterol and triglycerides was found (208). These results suggested that increasing PON1 activity could decrease the cardiovascular risk of metabolic syndrome patients.
As an independent risk factor for CVD, obesity has been shown to be related to low PON activity, because the activity of HDL-PON in obese subjects is significantly lower than that in controls (96, 170). The HDL composition is altered in obese individuals, exhibiting a decrease in protein content and an increase in the cholesterol levels and triglyceride/protein and cholesterol/protein ratios. These changes may decrease the binding of PON1 to the surface of HDL, resulting in inhibition of PON1 enzyme activity (97). PON1 arylesterase activity showed an inverse univariate correlation with leptin levels and a positive correlation with adiponectin levels (170). The high concentrations of leptin present in obese individuals may decrease plasma PON1 activity and induce oxidative stress, which may account for some of the proatherogenic effects of obesity (38). Also, transgenic overexpression of human PON3 in mice decreases adiposity and circulating leptin levels in addition to inhibiting atherogenesis, suggesting that PON3 may play an important role in protecting against obesity as well as atherosclerosis (306).
C. PONs and aging
Aging is thought to result partially from an imbalance between pro-oxidants and antioxidants, in which excess accumulation of pro-oxidants promotes aging (123).
It has been reported that plasma and HDL PON activities decrease significantly with aging (27, 67, 232). Serum PON1 activity was found to be significantly decreased with age, although its arylesterase activity and concentration in the serum exhibited no significant change. Decreased PON1 activity may contribute to the increased susceptibility of HDL to oxidation modification observed with aging (299). Jaouad et al. have suggested that age-related decreases in the antioxidant capacities of HDL and PON1 are due to alterations in PON1's free sulfhydryl groups (153).
Increased age is also negatively correlated with basal and stimulated PON activities of the PONs and the 192 (Gln/Arg) polymorphic variant of PON1. Thus, PON1 gene and activities may contribute to the aging process (357). An investigation of the PON1 polymorphisms at residues 192 and 55 showed that the frequency of the 192Arg allele was significantly increased in centenarians over young people, suggesting a small survival advantage for Arg allele carriers (48). A more extended follow-up study confirmed this result (275). These polymorphic variants have differences in PON activity, with Arg+ and Met–carriers having significant higher PON activities than their Gln and Leu counterparts. Thus, not only the levels of PON activity but also the PON1 genotype may significantly increase longevity (217). However, in a recent large association study, no significant association of the PON1 192 Gln/Arg genotype with longevity was observed. Moreover, a potential interaction of PON1 192 Gln/Arg with ApoE epsilon 4 was found to exist in one German population but not in the other three populations examined. These results do not exclude the possibility of population-specific effects of PON1 on longevity, however, which could result from differences in gene–environment interactions (57).
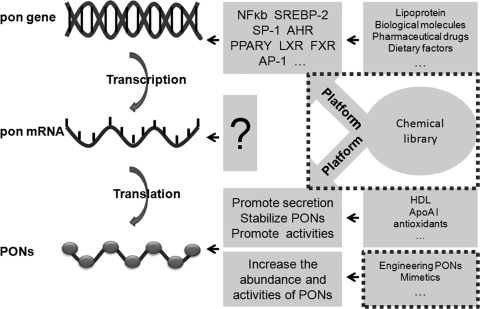
D. Other diseases related to PONs
Because oxidative stress is an important etiological factor for carcinogenesis (290) and PONs are important free-radical scavenging molecules, the relationships between PONs and many types of cancer have been extensively explored. PONs have been shown to be able to protect against many kinds of cancer, including prostate cancer, lung cancer, gastrointestinal tumors, glioma, ovarian malignancy, and breast cancer, among others. In addition, PONs are involved in some types of neurological disorders, such as autism, Alzheimer's disease, Parkinson's disease, schizophrenia, amyotrophic lateral sclerosis, depression, and acute ischemic stroke. Recently, mixed connective tissue disorder, Behchet's disease, systemic lupus erythematosus, rheumatoid arthritis, fibromyalgia, osteoarthritis, and other connective tissue diseases have also been reported to be influenced by PON1 polymorphisms. PONs are also reported to be associated with some gynecological disorders, such as endometriosis. Many liver disorders, such as viral hepatitis, nonalcoholic steatohepatitis, cirrhosis, hepatosteatosis, and even alcoholic hepatitis are also related to PONs. PONs have also been reported to protect against certain renal diseases and disorders. As the main means by which the nervous system protects itself against neurotoxicity arising from organophosphate exposure, PONs are a key for the response to organophosphorus and lead poisoning (109).
VII. Regulation of PONs
The results discussed earlier show that the PONs not only protect against atherosclerosis, atherosclerosis-related diseases, and many other diseases but also detoxify organophosphorus pesticides and nerve agents; therefore, PON regulation and the use of PONs to treat disease are worthy of further investigation.
Human serum PON1 activity increases from 15 to 25 months of age onwards and plateaus in adults (69). PON1 activity is theoretically determined by the genetic background of the individual, including the PON1 regulatory region polymorphisms. However, variations in environmental factors such as lipoproteins, cytokines, environmental chemicals, drugs, physiological and pathological states, diet, and lifestyle have been demonstrated to affect PON expression and activity.
A. Transcriptional regulation of PONs
Shih et al. have claimed that the expression of PON1 is under genetic control, based on the finding that a high-fat diet decreases the expression of PON1 in C57BL/6J mice but has no such effect in C3H/HeJ mice (302). Bioinformatic analysis of the sequence immediately upstream of the PON1 gene revealed that the PON1 promoter region contains neither a canonical TATA nor a CAAT box and is GC rich, which is typical of TATA-less promoters (55). Serial deletion analysis of 11.5 kb of the PON1 promoter region showed that cell-type-specific promoter elements for liver and kidney are present in the first 200 bp upstream of the coding sequence (190). The polymorphisms at positions −909 and −162 of upstream of coding region, which are potential NF-I transcription factor binding sites, have approximately a twofold effect on expression level, as does the −108 position. The effects of these three polymorphisms may depend on the effects of the surrounding sequences because they are not strictly additive (190). Other single-nucleotide polymorphisms (SNPs) in the PON1 promoter region that are able to affect the serum concentration of PON1 have been reported, namely, −107(T or C), −824(G or A), and −907(C or G). The latter residues are correlated with higher concentrations and activities than the former, as shown in a reporter system and by direct inspection (252). Of these three SNPs, −107 is a putative Sp1 binding site. Research has indicated that overexpression of full-length Sp1 dramatically enhances PON1 promoter activity. It had also been found that the GC box sequence located at residues −111 to −106 may be the most important factor for transcriptional regulation by Sp1. PKC can upregulate the expression of PON1, partially through its direct or indirect interaction with Sp1 (83). Because very few explorations of the transcriptional elements of PON2 and PON3 have been reported, we are now working in this area to attempt to elucidate the transcriptional regulation of PON2 and PON3.
B. Lipoproteins and their metabolic products regulate PONs expression
HDL is the main serum carrier of PON1 and plays important role in determining the enzyme concentration; this effect is clearly observed in HDL deficiency syndromes, in which PON1 levels are reduced (150). PON1 is synthesized in the liver and secreted into the serum. PON1's secretion is dramatically decreased if lipoproteins are absent, whereas the addition of phospholipid micelles or HDL promotes its secretion. However, LDL and lipid-free ApoA-I have no such effect. This suggests that an appropriate acceptor is required by PON1 for its release into serum. HDL appears to be the predominant physiological acceptor (82). Binding to HDL is necessary for PON1 to maintain optimum activity and stability (317). Human Apo A-I overexpression significantly increases the PON activity in both wild-type C57BL/6J and ApoE-deficient C57BL/6J mice, although adenoviral Apo A-I gene transfer does not have this effect, probably because of cytokine production in the liver (80). Thus, factors that increase the level of HDL may increase the enzyme activity of PON1. OxLDL and oxidized lipids can downregulate PON1 expression when administered to the HepG2 liver cell line (238, 344). When C57BL6 mice were fed a cholate-containing, high-fat, high-cholesterol diet for 15 weeks, the PON1 message levels in their livers were reduced compared with their chow-fed counterparts. Strangely, however, this effect was absent in ApoE null mice given the same challenge (244). More interestingly, the high-fat diet failed to decrease the expression of human PON1 in human PON1 transgenic mice (in which the transgene included the PON1 gene with 10 kb of the upstream and downstream regions), although endogenous mouse PON1 expression dramatically decreased by a mechanism that remains unclear (338). This result could be accounted for by differences in PON1 regulation between human and mouse, although it is also possible that the necessary regulatory elements were included in the transgenic construct. PON2 and PON3 have different responses to oxidative stress compared with PON1. Unlike PON1 expression, PON2 message and activity were upregulated upon exposure to oxidative stress (244), whereas PON3 expression is not significantly altered by oxidative stress (277). Treating mouse peritoneal macrophages with oxidative stress-inducing agents increases PON2 expression and lactonase activity but has no effect on PON3 mRNA levels, although the PON3 lactonase activity decreases (286). In vivo, C57BL6 or apoE null mice fed a high-fat, high-cholesterol, cholate-containing diet for 8 or 15 weeks, respectively, exhibit significantly higher levels of PON2 mRNA but no change in PON3 expression in their livers compared with their chow-fed counterparts, contrary to the changes observed in PON1 under the same conditions (244). However, in humans, PON2 message levels in monocyte-derived macrophages from hypercholesterolemic individuals are lower than in those derived from their normal counterparts (286). This effect can be reversed by the administration of atorvastatin (286). Oxidative stress was shown to inactivate serum PON1 and PON3, whereas macrophage PON2 expression increases under oxidative stress (24), which may be a compensatory mechanism.
C. Biological macromolecules modulate PONs expression
With increased research into PON regulation, various biological macromolecules and medications have been found to increase the expression of PON1. Incubating the liver cell line HepG2 with IL-6 has been shown to rapidly decrease PON1 expression (344). Treating HepG2 cells with IL-1beta and TNF-alpha decreases the relative promoter activities of the sequences at –589 to –6 upstream of PON1, resulting in a decrease in PON1 mRNA expression; however, the opposite effect was observed upon treatment with IL-6 (175). The discrepancy seen in the regulation of PON1 mRNA by IL-6 may be due to differences in the treatment protocols (100 ng/ml for 16 h vs. 10 ng/ml for 24 h) and methods of analysis (northern blot vs. reverse transcription–polymerase chain reaction) used (244) or to the complexity of PON1 regulation. These results suggest that PON1 mRNA expression in hepatocytes is regulated by proinflammatory cytokines and that proinflammatory cytokines secreted during disease may play a role in the development of atherosclerotic lesions via modification of PON1 mRNA expression and effects on the antioxidative status of HDL (175). In intestinal Caco-2/15 cells, expression of all three PONs is inhibited by oxidative stress induced by the addition of iron-ascorbate into the culture. However, this inhibition can be markedly attenuated by preincubation of Caco-2/15 cells with strong antioxidants such as butylated hydroxytoluene and Trolox. Lipopolysaccharides (LPS) also dose-dependently affect PON expression in Caco-2/15 cells; 200 μg/ml LPS decreases PON1 and PON3 mRNA but not PON2 mRNA levels compared with control cells. On the other hand, 50 μg/ml LPS increases PON2 mRNA levels without affecting PON1 and PON3 mRNA. When Caco-2/15 cells were treated with tumor necrosis factor-alpha (TNF-α) or interferon-γ, their PON1 and PON3 mRNA were reduced, with a significant increase in PON2 mRNA. Notably, these effects are partially mediated through the NF-κB pathway (267).
PON2 transcription is promoted by urokinase plasminogen activator (uPA) via its receptor uPAR through an integrated multistep pathway in macrophages (100). PON2 expression is regulated by cellular cholesterol content (285, 311) and cellular oxidative stress (309), and PPAR-γ, AP-1 activation (310), and NADPH oxidase activation (101, 308) all play important roles in stimulating PON2 mRNA expression. Fuhrman et al. identified the signal transduction pathway initiated by uPA that promotes PON2 transcription in macrophages (100). Following uPAR activation by uPA, PDGF receptor-β is activated through association with uPAR, leading to PI3K activation. NADPH oxidase is then activated, resulting in ROS production. ROS activate ERK1/2, which stimulate sterol regulatory binding protein-2 (SREBP-2) activity. SREBP-2 translocates into the nucleus and binds to transcriptional regulatory elements upstream of the PON2 gene, promoting its expression (100).
3OC12-HSL, one of the two virulence factors of P. aeruginosa, decreases PON2 activity in host cells by downregulating PON2 mRNA and protein levels and inhibiting PON2's hydrolytic activity, resulting in increased vulnerability of the cells to pyocyanin-induced oxidative stress. The effects are mediated by Ca2+, as the Ca2+ chelator 1,2-bis-(o-aminophenoxy)ethane-N,N,N′,N′-tetra-acetic acid etrakis(acetoxymethyl ester) diminishes the ability of 3OC12 to decrease PON2 and 3OC12 causes an increase in cytosolic Ca2+ levels. These results not only support a significant role for PON2 in defending against P. aeruginosa but also suggest that this bacterium may attenuate the protection presented by PON2 (133).
D. Pharmacological modulators of PONs
Various pharmaceutical drugs, particularly traditional lipid-lowering drugs such as statins and fibrates, have been tested for effects on PON expression and activity. These studies have yielded conflicting results, however (25, 58, 75).
1. Statins
Statins, which inhibit cholesterol synthesis by blocking 3-hydroxy-3-methyl-glutaryl-CoA reductase, are commonly used as hypolipidemic drugs. Short-term simvastatin administration does not improve PON activity; however, treatment of hyperlipoproteinemia patients with atorvastatin increases HDL-associated PON activity and exerts a favorable effect on the lipid profile (120, 164, 261). Orlistat also has a beneficial effect on the lipid profile and improves antioxidant status by promoting serum PON1 activity after 6 months of treatment in obese populations (19). Tsimihodimos et al. reported that atorvastatin does not change the serum PON activity toward paraoxon and phenylacetate but did improve the serum PON1 activity toward LDL cholesterol owing to dramatically lowered LDL cholesterol levels (336).
Simvastatin dose-dependently upregulates the promoter activity of the PON1 gene in expression cassettes including 1 kb of the upstream sequence transfected into HepG2 cells. This effect was blocked by administration of mevalonate and other intermediates of the cholesterol biosynthetic pathway. Simvastatin increases SREBP-2 levels, and this effect is also blocked by mevalonate. The consistency among the cellular studies and clinical application affirms the effects of simvastatin treatment, which has the potential to influence antiatherogenic mechanisms at the HDL level, thus providing evidence for one molecular mechanism by which PON expression could be regulated (83). In Huh7 cells, Arii et al. found that pitavastatin treatment activates PON1 gene expression and increases PON1 protein expression by increasing the phosphorylation of p44/42 MAP kinases, probably by activating SREBP-2 and Sp1 and increasing their binding to PON1 DNA (18). Pitavastatin can also increase Sp1 binding to PON1 DNA by activating PKC, especially the PKCzeta isoform (17). In apoE-deficient mice, pravastatin treatment increases the plasma PON activity by up to 80%; treatment of mice with the A-002 A secretory phospholipase A2 inhibitor also results in a profound increase in plasma PON activity. These compounds synergize with pravastatin to inhibit atherogenesis (300). These experimental animal results support the clinical evidence regarding the stimulatory effects of lipid-lowering medications on PON activity.
2. Fibrates
Durrington et al. investigated the effects of two fibric acid derivatives, bezafibrate mono and gemfibrozil, on serum PON activity and found no significant effects (91). Gemfibrozil administration has been shown to increase serum PON activity, improving antioxidant status and lowering lipid levels in type 2 diabetic patients with associated hypertriglyceridemia (30). Three months of treatment of CHD patients with micronized fenofibrate not only normalized the lipid profile but also improved antioxidant status by increasing serum PON activity (259). Fenofibrate increases PON activity, decreases the expression of inflammatory markers, and improves lipid and lipoprotein levels in patients with combined hyperlipidemia (360). Ciprofibrate has also been reported to favorably affect the lipid profile and increase the resistance of LDL to oxidation by increasing serum PON activity, thus improving the antioxidant status in patients with metabolic syndrome (260).
3. Other cardiovascular drugs
Traditionally used as a platelet aggregation inhibitor, low-dose aspirin (acetylsalicylic acid) was reported to increase serum PON1 activity (by ∼13%) in coronary patients (44), although administration of aspirin did not alter the serum PON1 activity of healthy volunteers (180). Aspirin was also found to increase PON1 activity in HuH7 cells (6). Some derivatives of aspirin, such as nitro-aspirin and the aspirin metabolite salicylic acid, were also able to increase PON1 expression and activity (292). Administration of aspirin (2 mg/day for 6 days) to atherogenic diet-fed LDLr–/– or C57BL6 mice resulted in a sevenfold increase in liver PON1 gene expression and a twofold increase in serum PON1 activity in an Ah receptor-dependent manner. Similar results were observed in HepG2 cells (144). Interestingly, PON1 had been reported to hydrolyze aspirin and nitro-aspirin (292).
Probucol, a drug used to lower cholesterol, was reported to increase serum PON activity and PON1 expression in the hepatocytes of hypercholesterolemic rabbits when given at 500 mg/kg/day for 14 days (132). The cholesterol absorption-inhibiting drug ezetimibe was also reported to increase serum PON1 activity when given to hyperlipidemic patients for 12 weeks (10 mg/day) (337).
4. Diabetic drugs
Rosiglitazone, a PPAR-γ agonist, is used in the treatment of type 2 diabetes. Recently, its effects on PON1 have been investigated. Administration of rosiglitazone (4 mg twice daily for 8 weeks) to diabetic patients caused an increase (9%–13%) in fasting and postprandial serum PON1 activity without changing PON1 expression (345). Rosiglitazone was also reported to increase serum PON1 activity by promoting the synthesis of apo AI and smaller HDL particles in rabbits (61). Similarly, treatment of rats with rosiglitazone was also found to reverse the decrease in hepatic PON1 activity induced by administration of a high-fructose diet (3). Eplerenone is a selective aldosterone blocker used as an antidiabetic drug. It was recently found that eplerenone increases hepatic PON1 activity in control and streptozotocin-treated diabetic mice (249). Oral hypoglycemic sulfonylureas used in the treatment of diabetes, such as glimepiride and glibenclamide, were reported to increase PON1 activity in the livers of control and streptozotocin-treated diabetic rats, although the plasma PON1 activity was decreased in control rats and unchanged in diabetic rats (356).
5. Other drugs
In addition to the aforementioned cardiovascular and antidiabetic drugs, many other pharmaceutical drugs have been investigated for effects on PON1 activity and/or expression.
The commonly used antibiotics clarithromycin and chloramphenicol were reported to inhibit the enzyme activity of purified human serum PON (serum PON1) and PON purified from human hepatoma (HepG2) cells (liver hPON1) (315). In addition, gentamycin sulfate and cefazolin sodium have been shown to decrease the activity of HepG2 cell PON, purified human serum PON1, and mouse serum PON1 (313). Other antibiotics, such as sodium ampicillin, ciprofloxacin, and clindamycin sulfate (but not rifamycin), also inhibit the PON activity of purified human PON1 (314). When tested in mice, these antibiotics induced increases or decreases in serum PON1 activity at various time points, with similar results observed in the liver (314).
The liver X receptor (LXR) receptor has been suggested to be involved in the modulation of PON activity (114), and the LXR agonist T0901317 has been documented to rescue the decrease in rat serum PON1 activity resulting from leptin administration (37). Bile acids, however, activate another nuclear hormone receptor, the farnesoid X receptor, and induce a decrease in serum PON1 activity and liver PON1 expression in mice (304).
Oral contraceptives (desogestrel or levonorgestrel in combination with ethinyl estradiol) are reported to increase serum PON1 activity in mice, although they significantly decrease mouse liver PON1 activity (168). Estrogen replacement therapy (ERT) with conjugated equine estrogen and medroxyprogesterone acetate increases the serum PON1 activity in postmenopausal women with type 2 diabetes. This effect is more evident in women with lower PON1 baseline levels and in those who have a concomitant increase in HDL cholesterol (326). In normal postmenopausal women, however, no significant effect on serum PON1 activity was observed after intranasal administration of estradiol (95). ERT can also rescue the reduction in PON1 and increase in plasma MDA resulting from estrogen ablation by surgical menopause (176). The anabolic steroid nandrolone decanoate, which is used as an adjuvant to erythropoietin in the treatment of anemia, has been reported to induce a mild but significant decrease in serum PON1 activity (104). The cholinergic muscarinic antagonist atropine has been documented to inhibit human plasma PON activity in vitro at high concentrations (76).
Several protein drugs have also been investigated with respect to their effects on PON. In predialysis patients with chronic renal disease and anemia, serum PON1 activity was increased by ∼20% by treatment with erythropoietin beta (221). The ApoA-I mimetic peptide D-4F, which was developed for use in CHD patients, has been shown to be able to slightly increase plasma PON1 activity in mice and nonhuman primates (58).
E. Dietary factors and lifestyle factors
It is believed that atherosclerosis is to some extent a “lifestyle disease,” although the extent to which it is affected by lifestyle depends on genetic background. Some lifestyle factors affect the development of atherosclerosis by modifying PONs.
1. Vitamin C and vitamin E
Administration of the dietary antioxidants vitamin C (ascorbic acid) and E (α-tocopherol) was reported to be associated with elevated PON activity in male Caucasian subjects (155). An in vitro study in human plasma showed that vitamin C administration reverses the decrease in serum PON activity induced by hypochlorite (177). In rats, it was reported that the decrease in serum PON activity caused by propylthiouracil-induced experimental hypothyroidism was reversed by vitamin E administration and that vitamin E also induced a small increase in serum PON1 activity in control animals (294). In high-cholesterol diet-fed rabbits, vitamin E was shown to increase serum and liver PON activity without affecting hepatic PON1 mRNA levels (156). A recent small study in humans showed that vitamin E supplementation prevented an exercise-induced reduction in serum PON1 activity (335).
2. Natural plant extracts
Eucommia ulmoides Oliver (Du-zhong) leaf extract significantly elevated plasma PON activity and lowered blood glucose concentrations when administered to type 2 diabetic (C57BL/6J-db/db) mice (262). When rats were fed with a diet containing 20% pistachio for 10 weeks, their HDL levels were increased, whereas their total cholesterol, LDL-cholesterol, and triglyceride levels were unaffected. Further, consumption of pistachio as 20% of the daily caloric intake also increased serum PON activity (35%) and arylesterase activity (60%). However, this effect was blunted when the pistachio intake was increased to 40% of daily caloric intake, for unknown reasons (9). When juice from wonderful variety pomegranates (WPJ) and pomegranate polyphenol extract (WPOMxl) were given to diabetic patients at doses of 50 ml/day for 4 weeks (WPJ) or 5 ml/day for 6 weeks (WPOMxl), there were no significant effects on fasting blood serum glucose or hemoglobin A1c levels. However, basal serum oxidative stress levels were significantly decreased, and HDL-associated PON1 arylesterase, PON, and lactonase activities were significantly increased by WPJ and WPOMxl consumption. PON1 binding to HDL was also significantly increased. Therefore, pomegranate consumption may retard the development of atherosclerosis via promoting serum PON1 stability and activity in diabetic patients (281).
3. Dietary polyphenol compounds
Some dietary polyphenol compounds, such as naringenin, flavones, quercetin, and catechin, increase PON-1 mRNA transcription when administered to HuH7 human hepatoma cells, although the effect of catechin is mild. These polyphenols must bind to and activate aryl hydrocarbon receptor (AhR) to exert their effects, because these effects are inhibited by specific AhR 7-keto-cholesterol and AhR-directed short interfering RNA. Upon ligand binding and activation, AhR translocates into the nucleus and forms a heterodimer with the AhR nuclear translocator (ARNT). The AhR/ARNT heterodimer complex then binds to xenobiotic responsive elements located in the PON1 promoter regions to promote PON1 transcription (113).
Low-dose red wine polyphenolic extract (PE) supplementation significantly attenuates the decreases in hepatic and plasma PON1 activity induced by chronic hyperhomocysteinemia in mice. PE treatment also resulted in a drastic reduction in plasma Hcy levels and in aortic expression of proinflammatory cytokines and adhesion molecules. (248). The phytoalexin resveratrol, which is considered to be the main mediator of the beneficial effect of wine (117, 265), has been shown to display antioxidant, antiplatelet, and anti-inflammatory effects, inhibit lipid peroxidation, and decrease serum triglycerides and LDL levels in vivo (233, 265). In addition to modulating gene expression by affecting NFκB, AP-1 (60), and estrogen receptor (ER) (51, 117), resveratrol has also been shown to bind to the AhR (62). Gouedard et al. found that resveratrol treatment increased PON-1 expression in both human hepatocytes and the HuH7 hepatoma cell line. Like other plant polyphenols, this effect of resveratrol was mediated by the AhR pathway rather than the ERalpha signal (112).
4. Dietary flavonoids
Quercetin, a flavonol frequently present in fruits and vegetables, has been testified to be able to induce moderate but significant upregulation of PON1 gene expression in mice but not in human (45, 46). To evaluate the effect of dietary flavonoids on PON2 expression, Boesch-Saadatmandi et al. treated cultured murine macrophages and overweight subjects with a high cardiovascular risk phenotype with quercetin. They found that treatment of murine RAW264.7 macrophages with quercetin or its methylated derivative isorhamnetin dose-dependently increased PON2 mRNA and protein levels compared with untreated controls. Quercetin's glucuronidated metabolite quercetin-3-glucuronide, however, did not affect PON2 gene expression in cultured macrophages. However, the investigation failed to find any effect of dietary quercetin supplementation on mRNA levels in human monocytes in vivo. (47) The discrepancy between in vitro and in vivo effect of dietary factors regarding the PON1 inducing activity may be mainly due to that most polyphenols shown to induce PON in cultured cells have a low bioavailability in vivo (296).
5. Dietary lipids
In rats, 8 weeks of high omega-3 PUFA and ethanol feed decreased liver PON1 mRNA expression, serum PON1 levels, and Hcy thiolactonase activity compared with a low omega-3 PUFA-fed group. However, betaine attenuated these effects when administered simultaneously. These results suggest that dietary betaine protects against atherosclerosis via promoting PON1 activity and quenching free radicals (346). Diets with a high trans-unsaturated fat content reduce PON1 activity, whereas consumption of olive acid (from olive oil) is associated with increased PON1 activity (332, 350).
6. Alcohol
In an early in vitro study, several aliphatic alcohols, including ethanol, were reported to inhibit human serum PON1 activity (76). Subsequent studies in humans, however, showed that moderate alcohol consumption increases serum PON1 activity (274, 312, 342), although one study found that a similar level of exposure did not alter serum PON activity (293). Similarly, moderate alcohol consumption for 8 weeks in rats also caused 20% and 25% increases in serum and liver PON1 activity, respectively, accompanied by an increase in hepatic PON1 mRNA levels (274). LDL−/− mice given a diet containing 18% ethanol for 4 weeks experienced a 31% increase in liver PON1 mRNA levels and a 64% increase in plasma PON1 activity (186). Heavy alcohol consumption in humans, in contrast to moderate alcohol consumption, leads to a significant decrease in PON1 activity (220, 274). In rats, heavy alcohol consumption also decreases serum and liver PON1 activity (186, 274).
7. Cigarettes
Cigarette smoke has been shown to inhibit PON1 activity in vitro (247) and in vivo (151), probably because of the acrolein present in cigarette smoke. It has been shown that acrolein inhibits HDL PON-1 activity in a time- and concentration-dependent fashion in studies where human HDL was incubated with 0–10 mM acrolein for 2 h (116).
8. Fasting
Fasting increases serum PON1 activity; effects can be seen within 6 h in rats. However, this is only a short-term adaptation designed to attenuate blood lipid peroxidation caused by fasting and cannot be sustained long term (331).
These results show that lifestyle factors, which can be readily modified, affect atherogenesis, partially through effects on PON1.
VIII. PONs Mimetics
In addition to upregulating or protecting endogenous PONs, PON activity can be modified by adding exogenous PONs or PON mimetics to the appropriate subjects. Reliable artificial molecules that can mimic PON's atheroprotective function would be extremely useful for such purposes. Using DNA family shuffling, investigators managed to express the first PON variants in a soluble and active form in Escherichia coli. These PON1 variants have 40-fold higher organic phosphate–hydrolyzing activities than wild-type PONs and a specificity switch of >2000-fold (5). These molecules open new prospects for improving PON catalytic activities and preventing atherosclerosis. For example, Mackness et al. used adenovirus-mediated PON1 gene transfer (AdPON1) to overexpress human PON1 in mice with metabolic syndrome induced by a combined deficiency of leptin and the LDL receptor. AdPON1 expression led to a 4.4-fold increase in serum PON1 activity in AdPON1 mice compared with mice injected with control virus. Overexpressing human PON1 also significantly reduced the total plaque volume and altered plaque composition by reducing the plaque macrophage volume and enhancing the SMC content. Accordingly, the levels of plaque-associated oxLDL and the titer of autoantibodies against MDA-modified LDL were also lowered upon AdPON1 overexpression. No effects on total plasma cholesterol or triglycerides were detected after adenovirus transfection. This report suggests that AdPON1 could be a useful strategy to prevent or retard atherosclerosis in humans by reducing oxLDL levels (208). Using a recombinant Escherichia coli gene expression system, Stevens et al. successfully produced a highly purified engineered recombinant human PON1 (rHuPON1K192) that protects against DZO. When injected into PON1-deficient mice, the nontoxic engineered PON1 molecules remain in the serum for at least 2 days and protected against at least three times the median lethal dose of DZO (321). Expression of active rHuPON1 in Escherichia coli or other recombinant gene expression systems could lead to the generation of PONs with high oxLDL catalytic efficiency that can protect against or treat atherosclerosis.
IX. Conclusions and Future Directions
Atherosclerosis has become the most prevalent cardiovascular cause of disabling illness and death in developed societies, and the current prevention and treatment strategies are unsatisfactory. As a multiple-factor chronic inflammatory disease, atherosclerosis results from interactions among blood flow, modified lipoproteins, monocyte-derived macrophages, T cells, and the normal cellular components of the vessel walls, such as endothelial cells and SMCs. OxLDL and Hcy play important roles in promoting initiation, progression, and complications of atherosclerosis by affecting almost all of the components of atherosclerotic plaques. HDL protects against atherosclerosis; one of the important mechanisms mediating this protection is HDL's ability to detoxify oxLDL and Hcy-thiolactone by virtue of its binding partner PON1. PON1 and its family members PON2 and PON3 have been shown to protect against atherosclerosis in mouse models and clinical studies. The PON family also provides protection against many other diseases. The expression and activity of the PONs are modulated by many factors, allowing for multiple strategies to target PON activity in atherosclerotic patients. Most of the currently available literature supports the notion of using PONs in the prevention and treatment of atherosclerosis. However, to pave the road for clinical interventions based on PON activity, the following concerns need to be addressed in future studies (Fig. 11).
FIG. 11.
Future directions required for targeting PONs to treat atherosclerosis. The indicated issues of PONs have to be addressed in future PON-related studies to facilitate clinical interventions of PONs activities in atherosclerosis treatment.
More convincing investigations need to be conducted to examine the association between PON polymorphisms and atherosclerosis-related CHD, because to date the results have not been consistent across different studies. In addition, several of the association studies conducted thus far suffer from several potential problems. For example, when the target populations are composed of multiple ethnic groups, a positive association could exist only due to differences in allelic frequency among ethnic groups. The appropriate analytical procedures, such as transmission-distortion analysis, should be used to minimize this problem. Also, probable associations have sometimes been dwarfed by insufficiently stringent thresholds when significant findings were established. Some results have been taken from separate studies in separate populations. Attempting to analyze these studies together is not reliable because of the conflicting nature of the documents, although the most convincing evidence for a PON–atherosclerosis association is the significant reproducibility among separate studies and separate populations (129).
The physiological roles and physiological substrates of PONs have to be yet confirmed. PONs are now considered lactonases based on their catalytic activity toward their common substrate lactone. However, the fact that the best PON substrates are artificial chemicals instead of natural materials is still difficult to understand. Why would such an evolutionarily well-conserved cluster of proteins lack an important physiological function? What survival advantages does the PON gene cluster confer? What kind of evolutionary pressure led to the duplication of the PON gene and preserved the three genes through evolution? Ablation of PON1 or PON2 alone resulted in increased susceptibility to several stresses (organic phosphate toxicity and atherosclerosis, among others); however, no lethality has been reported from PON1 or PON2 deletion. The effects of PON3 deletion are as yet unknown, as is the phenotype of the triple knockout. Answers to these questions will lead to an increased understanding of the physiological roles of the PON gene family. At the same time, there is an urgent need to explore the physiological substrates of the PONs. Elucidating the physiological substrates and functions of PONs may also facilitate the prediction of probable side effects of atherosclerosis treatments targeting PONs.
The biochemical mechanisms by which PONs degrade oxidized lipids, especially oxLDL, have not been clearly identified. As early as 1995, Watson et al. reported that PON1 bound to HDL could degrade the biologically active lipids present in mildly oxLDL, thus exerting an anti-inflammatory effect in the cells of the artery wall (351). Even purified PON1 significantly reduces the ability of MM-LDL to induce monocyte–endothelial cell interactions (26, 27, 209, 351). This function of PON1 is supported by other studies in PON1 knockout and transgenic mice, in which HDL from PON1 knockout mice was observed to lack most of its capability to degrade oxLDL (303, 305), and HDL from PON1 transgenic mice exhibited a much higher oxLDL detoxification activity (338). PON2 and PON3 also act to decrease LDL oxidation (87, 245). However, Draganov et al. found that baculovirus-mediated recombinant and purified PONs (PON1, PON2, and PON3) failed to protect LDL against copper-induced oxidation in vitro, although these enzymes hydrolyze many overlapping substrates and distinctive substrates (88). A possible explanation for these results is that the system used is not appropriate for examining physiologically relevant PON activity (88). It is also possible that HDL or other associating proteins are indispensable for the PONs, especially PON1, to protect lipoproteins against oxidation. Dissecting the specific mechanisms of PON-mediated protection against oxLDL toxicity will provide strong evidence as to the mechanism of the PONs' protection against atherosclerosis.
To control the expression of the PONs, we first need to fully understand their transcriptional regulation. The three PON member genes exist in the genome as an end-to-end cluster. Thus far, no other genes have been reported to localize to this cluster. As a gene cluster, are their expressions controlled in a spatial-temporal manner, like the hemoglobin gene cluster? Are there any coregulatory elements in this cluster that coordinate their expression? If these three members are not governed by a common transcriptional regulation module, are there any insulators or other cis-elements that interrupt the continuity of the genomic structure? If the three members are expressed independently, how are they individually regulated? Thus far, only the human PON1 promoter has been characterized. Characterization of the PON2 and PON3 promoters is necessary for a full understanding of the regulation of this gene cluster and its use for treatment.
Screening effective and safe medications that increase the protective effect of PONs on atherosclerosis is another leading direction for future PON research. Because the status (quantity and quality) of the enzyme in the serum is significantly related to an individual's risk for developing atherosclerosis-related diseases, it is important to elucidate the factors influencing the serum levels of PON1 and use them therapeutically to increase its activity. The concentration and activity of the PONs are highly variable among individuals. Genetic polymorphisms, gene expression levels, protein stability, protein secretion, association with HDL, and environmental factors all contribute to affecting the status of the PONs. Therefore, molecules that are able to improve these processes and/or increase these factors are potential therapeutic candidates. Setting up feasible and reliable high-throughput screening platforms will be very important for screening PON activators. Genetic engineering and DNA shuffle techniques could also be used to synthesize and optimize artificial PON gene, leading to expression of novel PON or PON-like proteins with higher catalytic activities toward oxLDL than natural PONs. These techniques will enable us to create and apply extrinsic PON mimetics to treat patients suffering from atherosclerosis (Fig. 12).
FIG. 12.
Strategies to target PONs. Biological and chemical molecules have been reported to be able to, on the one hand, promote expression of PONs via corresponding transcription factors at DNA level; on the other hand, improve PON status at protein level. More effective and safer chemicals can be screened using probable platform to increase PONs expression, to promote secretion and activities, or to stabilize PONs. Engineering PON mimetics are also prosperous to this end. AhR, aryl hydrocarbon receptor; AP-1, activator protein 1; apo, apolipoprotein; FXR, farnesoid X receptor; PPAR, peroxisome proliferator-activated receptors; SREBP, sterol regulatory element binding protein Sp1, specificity protein 1; LXR, liver X receptor.
In conclusion, previous results solidly support the protective roles of the PONs in atherosclerosis; if groundbreaking progress in the directions mentioned earlier is made, the PON gene cluster will be used as a prospective target for the treatment of atherosclerosis.
Abbreviations Used
- 3-OC12-HSL
N-(3-oxododecanoyl)-l-homoserine lactone
- 5-HETEL
5-hydroxy-6E,8Z,11Z,14Z eicosatetraenoic acid lactone
- AdPON1
PON1-expressing adenovirus
- AhR
aryl hydrocarbon receptor
- AP-1
activator protein 1
- apo
apolipoprotein
- ARNT
AhR nuclear translocator
- BAC
bacterial artificial chromosome
- CAD
coronary artery disease
- CHD
coronary heart disease
- CVD
cardiovascular disease
- Cys
cysteine
- eNOS
endothelial nitric oxide synthase
- ER
estrogen receptor
- ERK
extracellular signal-regulated kinase
- ERT
estrogen replacement therapy
- FXR
farnesoid X receptor
- Hcy
homocysteine
- HDL
high-density lipoprotein
- H2O2
hydrogen peroxide
- ICAM-1
intercellular adhesion molecule 1
- IL-1
interleukin 1
- LDL
low-density lipoprotein
- LPS
lipopolysaccharides
- LXR
liver X receptor
- MAPK
mitogen-activated protein kinases
- MCP-1
monocyte chemotactic protein-1
- MDA
malondialdehyde
- mm-LDL
minimally modified oxidized low-density lipoprotein
- MS
methionine synthase
- NADPH
nicotinamide adenine dinucleotide phosphate
- NFκB
nuclear factor kappa B
- oxLDL
oxidized low-density lipoprotein
- PDGF
platelet-derived growth factor
- PE
polyphenolic extract
- PI3K
phosphatidylinositol 3-kinase
- PKB
protein kinase B
- PKC
protein kinase C
- PON
paraoxonase
- PPAR
peroxisome proliferator-activated receptors
- PUFAs
polyunsaturated fatty acids
- RCT
reverse cholesterol transportation
- rHuPON1
recombined human PON1
- ROS
reactive oxygen species
- SAM
S-adenosyl methionine
- SMC
smooth muscle cell
- SNP
single-nucleotide polymorphism
- Sp1
specificity protein 1
- SRs
scavenger receptors
- SREBP
sterol regulatory element binding protein
- TNF-α
tumor necrosis factor α
- uPA
urokinase plasminogen activator
- uPAR
urokinase plasminogen activator receptor
- VLDL
very low-density lipoprotein
- VSMC
vascular smooth muscle cell
- WPJ
wonderful variety pomegranate juice
Acknowledgments
This work was supported by the National Basic Research Program of China (Grant No. 2011CB503902) and the Special Fund of the National Laboratory of China (Grant No. 2060204).
References
- 1–2.These references have been deleted
- 3.Ackerman Z. Oron-Herman M. Pappo O. Peleg E. Safadi R. Schmilovitz-Weiss H. Grozovski M. Hepatic effects of rosiglitazone in rats with the metabolic syndrome. Basic Clin Pharmacol Toxicol. 2010;107:663–668. doi: 10.1111/j.1742-7843.2010.00553.x. [DOI] [PubMed] [Google Scholar]
- 4.Adkins S. Gan KN. Mody M. La Du BN. Molecular basis for the polymorphic forms of human serum paraoxonase/arylesterase: glutamine or arginine at position 191, for the respective A or B allozymes. Am J Hum Genet. 1993;52:598–608. [PMC free article] [PubMed] [Google Scholar]
- 5.Aharoni A. Gaidukov L. Yagur S. Toker L. Silman I. Tawfik DS. Directed evolution of mammalian paraoxonases PON1 and PON3 for bacterial expression and catalytic specialization. Proc Natl Acad Sci U S A. 2004;101:482–487. doi: 10.1073/pnas.2536901100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmad S. Carter JJ. Scott JE. A homogeneous cell-based assay for measurement of endogenous paraoxonase 1 activity. Anal Biochem. 2010;400:1–9. doi: 10.1016/j.ab.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed Z. Babaei S. Maguire GF. Draganov D. Kuksis A. La Du BN. Connelly PW. Paraoxonase-1 reduces monocyte chemotaxis and adhesion to endothelial cells due to oxidation of palmitoyl, linoleoyl glycerophosphorylcholine. Cardiovasc Res. 2003;57:225–231. doi: 10.1016/s0008-6363(02)00659-4. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed Z. Ravandi A. Maguire GF. Emili A. Draganov D. La Du BN. Kuksis A. Connelly PW. Multiple substrates for paraoxonase-1 during oxidation of phosphatidylcholine by peroxynitrite. Biochem Biophys Res Commun. 2002;290:391–396. doi: 10.1006/bbrc.2001.6150. [DOI] [PubMed] [Google Scholar]
- 9.Aksoy N. Aksoy M. Bagci C. Gergerlioglu HS. Celik H. Herken E. Yaman A. Tarakcioglu M. Soydinc S. Sari I. Davutoglu V. Pistachio intake increases high density lipoprotein levels and inhibits low-density lipoprotein oxidation in rats. Tohoku J Exp Med. 2007;212:43–48. doi: 10.1620/tjem.212.43. [DOI] [PubMed] [Google Scholar]
- 10.Aldridge WN. Serum esterases. I. Two types of esterase (A and B) hydrolysing p-nitrophenyl acetate, propionate and butyrate, and a method for their determination. Biochem J. 1953;53:110–117. doi: 10.1042/bj0530110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aldridge WN. Serum esterases. II. An enzyme hydrolysing diethyl p-nitrophenyl phosphate (E600) and its identity with the A-esterase of mammalian sera. Biochem J. 1953;53:117–124. doi: 10.1042/bj0530117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altenhofer S. Witte I. Teiber JF. Wilgenbus P. Pautz A. Li H. Daiber A. Witan H. Clement AM. Forstermann U. Horke S. One enzyme, two functions: PON2 prevents mitochondrial superoxide formation and apoptosis independent from its lactonase activity. J Biol Chem. 2010;285:24398–24403. doi: 10.1074/jbc.M110.118604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antoniades C. Antonopoulos AS. Tousoulis D. Marinou K. Stefanadis C. Homocysteine and coronary atherosclerosis: from folate fortification to the recent clinical trials. Eur Heart J. 2009;30:6–15. doi: 10.1093/eurheartj/ehn515. [DOI] [PubMed] [Google Scholar]
- 14.Antoniades C. Shirodaria C. Warrick N. Cai S. de Bono J. Lee J. Leeson P. Neubauer S. Ratnatunga C. Pillai R. Refsum H. Channon KM. 5-methyltetrahydrofolate rapidly improves endothelial function and decreases superoxide production in human vessels: effects on vascular tetrahydrobiopterin availability and endothelial nitric oxide synthase coupling. Circulation. 2006;114:1193–1201. doi: 10.1161/CIRCULATIONAHA.106.612325. [DOI] [PubMed] [Google Scholar]
- 15.Arca M. Ombres D. Montali A. Campagna F. Mangieri E. Tanzilli G. Campa PP. Ricci G. Verna R. Pannitteri G. PON1 L55M polymorphism is not a predictor of coronary atherosclerosis either alone or in combination with Q192R polymorphism in an Italian population. Eur J Clin Invest. 2002;32:9–15. doi: 10.1046/j.1365-2362.2002.00935.x. [DOI] [PubMed] [Google Scholar]
- 16.Argmann CA. Sawyez CG. Li S. Nong Z. Hegele RA. Pickering JG. Huff MW. Human smooth muscle cell subpopulations differentially accumulate cholesteryl ester when exposed to native and oxidized lipoproteins. Arterioscler Thromb Vasc Biol. 2004;24:1290–1296. doi: 10.1161/01.ATV.0000131260.80316.37. [DOI] [PubMed] [Google Scholar]
- 17.Arii K. Suehiro T. Ikeda Y. Kumon Y. Inoue M. Inada S. Takata H. Ishibashi A. Hashimoto K. Terada Y. Role of protein kinase C in pitavastatin-induced human paraoxonase I expression in Huh7 cells. Metabolism. 2010;59:1287–1293. doi: 10.1016/j.metabol.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Arii K. Suehiro T. Ota K. Ikeda Y. Kumon Y. Osaki F. Inoue M. Inada S. Ogami N. Takata H. Hashimoto K. Terada Y. Pitavastatin induces PON1 expression through p44/42 mitogen-activated protein kinase signaling cascade in Huh7 cells. Atherosclerosis. 2009;202:439–445. doi: 10.1016/j.atherosclerosis.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 19.Audikovszky M. Pados G. Seres I. Harangi M. Fulop P. Katona E. Illyes L. Winkler G. Katona EM. Paragh G. Orlistat increases serum paraoxonase activity in obese patients. Nutr Metab Cardiovasc Dis. 2007;17:268–273. doi: 10.1016/j.numecd.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Autio I. Jaakkola O. Solakivi T. Nikkari T. Oxidized low-density lipoprotein is chemotactic for arterial smooth muscle cells in culture. FEBS Lett. 1990;277:247–249. doi: 10.1016/0014-5793(90)80857-f. [DOI] [PubMed] [Google Scholar]
- 21.Aviram M. Does paraoxonase play a role in susceptibility to cardiovascular disease? Mol Med Today. 1999;5:381–386. doi: 10.1016/s1357-4310(99)01546-4. [DOI] [PubMed] [Google Scholar]
- 22.Aviram M. Billecke S. Sorenson R. Bisgaier C. Newton R. Rosenblat M. Erogul J. Hsu C. Dunlop C. La Du B. Paraoxonase active site required for protection against LDL oxidation involves its free sulfhydryl group and is different from that required for its arylesterase/paraoxonase activities: selective action of human paraoxonase allozymes Q and R. Arterioscler Thromb Vasc Biol. 1998;18:1617–1624. doi: 10.1161/01.atv.18.10.1617. [DOI] [PubMed] [Google Scholar]
- 23.Aviram M. Hardak E. Vaya J. Mahmood S. Milo S. Hoffman A. Billicke S. Draganov D. Rosenblat M. Human serum paraoxonases (PON1) Q and R selectively decrease lipid peroxides in human coronary and carotid atherosclerotic lesions: PON1 esterase and peroxidase-like activities. Circulation. 2000;101:2510–2517. doi: 10.1161/01.cir.101.21.2510. [DOI] [PubMed] [Google Scholar]
- 24.Aviram M. Rosenblat M. Paraoxonases 1, 2, and 3, oxidative stress, and macrophage foam cell formation during atherosclerosis development. Free Radic Biol Med. 2004;37:1304–1316. doi: 10.1016/j.freeradbiomed.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 25.Aviram M. Rosenblat M. Paraoxonases and cardiovascular diseases: pharmacological and nutritional influences. Curr Opin Lipidol. 2005;16:393–399. doi: 10.1097/01.mol.0000174398.84185.0f. [DOI] [PubMed] [Google Scholar]
- 26.Aviram M. Rosenblat M. Billecke S. Erogul J. Sorenson R. Bisgaier CL. Newton RS. La Du B. Human serum paraoxonase (PON 1) is inactivated by oxidized low density lipoprotein and preserved by antioxidants. Free Radic Biol Med. 1999;26:892–904. doi: 10.1016/s0891-5849(98)00272-x. [DOI] [PubMed] [Google Scholar]
- 27.Aviram M. Rosenblat M. Bisgaier CL. Newton RS. Primo-Parmo SL. La Du BN. Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions. A possible peroxidative role for paraoxonase. J Clin Invest. 1998;101:1581–1590. doi: 10.1172/JCI1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ayub A. Mackness MI. Arrol S. Mackness B. Patel J. Durrington PN. Serum paraoxonase after myocardial infarction. Arterioscler Thromb Vasc Biol. 1999;19:330–335. doi: 10.1161/01.atv.19.2.330. [DOI] [PubMed] [Google Scholar]
- 29.Bajnok L. Csongradi E. Seres I. Varga Z. Jeges S. Peti A. Karanyi Z. Juhasz A. Mezosi E. Nagy EV. Paragh G. Relationship of adiponectin to serum paraoxonase 1. Atherosclerosis. 2008;197:363–367. doi: 10.1016/j.atherosclerosis.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Balogh Z. Seres I. Harangi M. Kovacs P. Kakuk G. Paragh G. Gemfibrozil increases paraoxonase activity in type 2 diabetic patients. A new hypothesis of the beneficial action of fibrates? Diabetes Metab. 2001;27:604–610. [PubMed] [Google Scholar]
- 31.Bao XM. Wu CF. Lu GP. Atorvastatin inhibits homocysteine-induced oxidative stress and apoptosis in endothelial progenitor cells involving Nox4 and p38MAPK. Atherosclerosis. 2010;210:114–121. doi: 10.1016/j.atherosclerosis.2009.11.032. [DOI] [PubMed] [Google Scholar]
- 32.Barbieri M. Bonafe M. Marfella R. Ragno E. Giugliano D. Franceschi C. Paolisso G. LL-paraoxonase genotype is associated with a more severe degree of homeostasis model assessment IR in healthy subjects. J Clin Endocrinol Metab. 2002;87:222–225. doi: 10.1210/jcem.87.1.8183. [DOI] [PubMed] [Google Scholar]
- 33.Barter P. Gotto AM. LaRosa JC. Maroni J. Szarek M. Grundy SM. Kastelein JJ. Bittner V. Fruchart JC. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med. 2007;357:1301–1310. doi: 10.1056/NEJMoa064278. [DOI] [PubMed] [Google Scholar]
- 34.Barter PJ. Nicholls S. Rye KA. Anantharamaiah GM. Navab M. Fogelman AM. Antiinflammatory properties of HDL. Circ Res. 2004;95:764–772. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 35.Bear M. Butcher M. Shaughnessy SG. Oxidized low-density lipoprotein acts synergistically with beta-glycerophosphate to induce osteoblast differentiation in primary cultures of vascular smooth muscle cells. J Cell Biochem. 2008;105:185–193. doi: 10.1002/jcb.21812. [DOI] [PubMed] [Google Scholar]
- 36.Bellas RE. Harrington EO. Sheahan KL. Newton J. Marcus C. Rounds S. FAK blunts adenosine-homocysteine-induced endothelial cell apoptosis: requirement for PI 3-kinase. Am J Physiol Lung Cell Mol Physiol. 2002;282:L1135–L1142. doi: 10.1152/ajplung.00174.2001. [DOI] [PubMed] [Google Scholar]
- 37.Beltowski J. Wojcicka G. Jakubowski H. Modulation of paraoxonase 1 and protein N-homocysteinylation by leptin and the synthetic liver X receptor agonist T0901317 in the rat. J Endocrinol. 2010;204:191–198. doi: 10.1677/JOE-09-0298. [DOI] [PubMed] [Google Scholar]
- 38.Beltowski J. Wojcicka G. Jamroz A. Leptin decreases plasma paraoxonase 1 (PON1) activity and induces oxidative stress: the possible novel mechanism for proatherogenic effect of chronic hyperleptinemia. Atherosclerosis. 2003;170:21–29. doi: 10.1016/s0021-9150(03)00236-3. [DOI] [PubMed] [Google Scholar]
- 39.Bhattacharyya T. Nicholls SJ. Topol EJ. Zhang R. Yang X. Schmitt D. Fu X. Shao M. Brennan DM. Ellis SG. Brennan ML. Allayee H. Lusis AJ. Hazen SL. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA. 2008;299:1265–1276. doi: 10.1001/jama.299.11.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bienvenu T. Ankri A. Chadefaux B. Montalescot G. Kamoun P. Elevated total plasma homocysteine, a risk factor for thrombosis. Relation to coagulation and fibrinolytic parameters. Thromb Res. 1993;70:123–129. doi: 10.1016/0049-3848(93)90153-f. [DOI] [PubMed] [Google Scholar]
- 41.Biggadike K. Angell RM. Burgess CM. Farrell RM. Hancock AP. Harker AJ. Irving WR. Ioannou C. Procopiou PA. Shaw RE. Solanke YE. Singh OM. Snowden MA. Stubbs RJ. Walton S. Weston HE. Selective plasma hydrolysis of glucocorticoid gamma-lactones and cyclic carbonates by the enzyme paraoxonase: an ideal plasma inactivation mechanism. J Med Chem. 2000;43:19–21. doi: 10.1021/jm990436t. [DOI] [PubMed] [Google Scholar]
- 42.Billecke S. Draganov D. Counsell R. Stetson P. Watson C. Hsu C. La Du BN. Human serum paraoxonase (PON1) isozymes Q and R hydrolyze lactones and cyclic carbonate esters. Drug Metab Dispos. 2000;28:1335–1342. [PubMed] [Google Scholar]
- 43.Binder CJ. Chang MK. Shaw PX. Miller YI. Hartvigsen K. Dewan A. Witztum JL. Innate and acquired immunity in atherogenesis. Nat Med. 2002;8:1218–1226. doi: 10.1038/nm1102-1218. [DOI] [PubMed] [Google Scholar]
- 44.Blatter-Garin MC. Kalix B. De Pree S. James RW. Aspirin use is associated with higher serum concentrations of the anti-oxidant enzyme, paraoxonase-1. Diabetologia. 2003;46:593–594. doi: 10.1007/s00125-003-1065-0. [DOI] [PubMed] [Google Scholar]
- 45.Boesch-Saadatmandi C. Egert S. Schrader C. Coumoul X. Barouki R. Muller MJ. Wolffram S. Rimbach G. Effect of quercetin on paraoxonase 1 activity—studies in cultured cells, mice and humans. J Physiol Pharmacol. 2010;61:99–105. [PubMed] [Google Scholar]
- 46.Boesch-Saadatmandi C. Niering J. Minihane AM. Wiswedel I. Gardeman A. Wolffram S. Rimbach G. Impact of apolipoprotein E genotype and dietary quercetin on paraoxonase 1 status in apoE3 and apoE4 transgenic mice. Atherosclerosis. 2010;211:110–113. doi: 10.1016/j.atherosclerosis.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 47.Boesch-Saadatmandi C. Pospissil RT. Graeser AC. Canali R. Boomgaarden I. Doering F. Wolffram S. Egert S. Mueller MJ. Rimbach G. Effect of quercetin on paraoxonase 2 levels in RAW264.7 macrophages and in human monocytes—role of quercetin metabolism. Int J Mol Sci. 2009;10:4168–4177. doi: 10.3390/ijms10094168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bonafe M. Marchegiani F. Cardelli M. Olivieri F. Cavallone L. Giovagnetti S. Pieri C. Marra M. Antonicelli R. Troiano L. Gueresi P. Passeri G. Berardelli M. Paolisso G. Barbieri M. Tesei S. Lisa R. De Benedictis G. Franceschi C. Genetic analysis of Paraoxonase (PON1) locus reveals an increased frequency of Arg192 allele in centenarians. Eur J Hum Genet. 2002;10:292–296. doi: 10.1038/sj.ejhg.5200806. [DOI] [PubMed] [Google Scholar]
- 49.Boright AP. Connelly PW. Brunt JH. Scherer SW. Tsui LC. Hegele RA. Genetic variation in paraoxonase-1 and paraoxonase-2 is associated with variation in plasma lipoproteins in Alberta Hutterites. Atherosclerosis. 1998;139:131–136. doi: 10.1016/s0021-9150(98)00071-9. [DOI] [PubMed] [Google Scholar]
- 50.Boushey CJ. Beresford SA. Omenn GS. Motulsky AG. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA. 1995;274:1049–1057. doi: 10.1001/jama.1995.03530130055028. [DOI] [PubMed] [Google Scholar]
- 51.Bowers JL. Tyulmenkov VV. Jernigan SC. Klinge CM. Resveratrol acts as a mixed agonist/antagonist for estrogen receptors alpha and beta. Endocrinology. 2000;141:3657–3667. doi: 10.1210/endo.141.10.7721. [DOI] [PubMed] [Google Scholar]
- 52.Brattstrom L. Wilcken DE. Homocysteine and cardiovascular disease: cause or effect? Am J Clin Nutr. 2000;72:315–323. doi: 10.1093/ajcn/72.2.315. [DOI] [PubMed] [Google Scholar]
- 53.Braunwald E. Shattuck lecture—cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med. 1997;337:1360–1369. doi: 10.1056/NEJM199711063371906. [DOI] [PubMed] [Google Scholar]
- 54.Brewer HB., Jr. Increasing HDL Cholesterol Levels. N Engl J Med. 2004;350:1491–1494. doi: 10.1056/NEJMp048023. [DOI] [PubMed] [Google Scholar]
- 55.Brophy VH. Hastings MD. Clendenning JB. Richter RJ. Jarvik GP. Furlong CE. Polymorphisms in the human paraoxonase (PON1) promoter. Pharmacogenetics. 2001;11:77–84. doi: 10.1097/00008571-200102000-00009. [DOI] [PubMed] [Google Scholar]
- 56.Brophy VH. Jampsa RL. Clendenning JB. McKinstry LA. Jarvik GP. Furlong CE. Effects of 5' regulatory-region polymorphisms on paraoxonase-gene (PON1) expression. Am J Hum Genet. 2001;68:1428–1436. doi: 10.1086/320600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Caliebe A. Kleindorp R. Blanche H. Christiansen L. Puca AA. Rea IM. Slagboom E. Flachsbart F. Christensen K. Rimbach G. Schreiber S. Nebel A. No or only population-specific effect of PON1 on human longevity: a comprehensive meta-analysis. Ageing Res Rev. 2010;9:238–244. doi: 10.1016/j.arr.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 58.Camps J. Marsillach J. Joven J. Pharmacological and lifestyle factors modulating serum paraoxonase-1 activity. Mini Rev Med Chem. 2009;9:911–920. doi: 10.2174/138955709788681591. [DOI] [PubMed] [Google Scholar]
- 59.Cardenas GA. Lavie CJ. Cardenas V. Milani RV. McCullough PA. The importance of recognizing and treating low levels of high-density lipoprotein cholesterol: a new era in atherosclerosis management. Rev Cardiovasc Med. 2008;9:239–258. [PubMed] [Google Scholar]
- 60.Carluccio MA. Siculella L. Ancora MA. Massaro M. Scoditti E. Storelli C. Visioli F. Distante A. De Caterina R. Olive oil and red wine antioxidant polyphenols inhibit endothelial activation: antiatherogenic properties of Mediterranean diet phytochemicals. Arterioscler Thromb Vasc Biol. 2003;23:622–629. doi: 10.1161/01.ATV.0000062884.69432.A0. [DOI] [PubMed] [Google Scholar]
- 61.Carreon-Torres E. Rendon-Sauer K. Monter-Garrido M. Toledo-Ibelles P. Gamboa R. Menjivar M. Lopez-Marure R. Luc G. Fievet C. Cruz D. Vargas-Alarcon G. Perez-Mendez O. Rosiglitazone modifies HDL structure and increases HDL-apo AI synthesis and catabolic rates. Clin Chim Acta. 2009;401:37–41. doi: 10.1016/j.cca.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 62.Casper RF. Quesne M. Rogers IM. Shirota T. Jolivet A. Milgrom E. Savouret JF. Resveratrol has antagonist activity on the aryl hydrocarbon receptor: implications for prevention of dioxin toxicity. Mol Pharmacol. 1999;56:784–790. [PubMed] [Google Scholar]
- 63.Chai YC. Howe PH. DiCorleto PE. Chisolm GM. Oxidized low density lipoprotein and lysophosphatidylcholine stimulate cell cycle entry in vascular smooth muscle cells. Evidence for release of fibroblast growth factor-2. J Biol Chem. 1996;271:17791–17797. doi: 10.1074/jbc.271.30.17791. [DOI] [PubMed] [Google Scholar]
- 64.Chen C. Halkos ME. Surowiec SM. Conklin BS. Lin PH. Lumsden AB. Effects of homocysteine on smooth muscle cell proliferation in both cell culture and artery perfusion culture models. J Surg Res. 2000;88:26–33. doi: 10.1006/jsre.1999.5756. [DOI] [PubMed] [Google Scholar]
- 65.Chen J. Mehta JL. Haider N. Zhang X. Narula J. Li D. Role of caspases in Ox-LDL-induced apoptotic cascade in human coronary artery endothelial cells. Circ Res. 2004;94:370–376. doi: 10.1161/01.RES.0000113782.07824.BE. [DOI] [PubMed] [Google Scholar]
- 66.Chen Q. Reis SE. Kammerer CM. McNamara DM. Holubkov R. Sharaf BL. Sopko G. Pauly DF. Merz CN. Kamboh MI. Association between the severity of angiographic coronary artery disease and paraoxonase gene polymorphisms in the National Heart, Lung, and Blood Institute-sponsored Women's Ischemia Syndrome Evaluation (WISE) study. Am J Hum Genet. 2003;72:13–22. doi: 10.1086/345312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cherki M. Berrougui H. Isabelle M. Cloutier M. Koumbadinga GA. Khalil A. Effect of PON1 polymorphism on HDL antioxidant potential is blunted with aging. Exp Gerontol. 2007;42:815–824. doi: 10.1016/j.exger.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 68.Christen WG. Ajani UA. Glynn RJ. Hennekens CH. Blood levels of homocysteine and increased risks of cardiovascular disease: causal or casual? Arch Intern Med. 2000;160:422–434. doi: 10.1001/archinte.160.4.422. [DOI] [PubMed] [Google Scholar]
- 69.Cole TB. Jampsa RL. Walter BJ. Arndt TL. Richter RJ. Shih DM. Tward A. Lusis AJ. Jack RM. Costa LG. Furlong CE. Expression of human paraoxonase (PON1) during development. Pharmacogenetics. 2003;13:357–364. doi: 10.1097/00008571-200306000-00007. [DOI] [PubMed] [Google Scholar]
- 70.Colles SM. Maxson JM. Carlson SG. Chisolm GM. Oxidized LDL-induced injury and apoptosis in atherosclerosis. Potential roles for oxysterols. Trends Cardiovasc Med. 2001;11:131–138. doi: 10.1016/s1050-1738(01)00106-2. [DOI] [PubMed] [Google Scholar]
- 71.Cominacini L. Garbin U. Pasini AF. Davoli A. Campagnola M. Contessi GB. Pastorino AM. Lo Cascio V. Antioxidants inhibit the expression of intercellular cell adhesion molecule-1 and vascular cell adhesion molecule-1 induced by oxidized LDL on human umbilical vein endothelial cells. Free Radic Biol Med. 1997;22:117–127. doi: 10.1016/s0891-5849(96)00271-7. [DOI] [PubMed] [Google Scholar]
- 72.Cominacini L. Pasini AF. Garbin U. Davoli A. Tosetti ML. Campagnola M. Rigoni A. Pastorino AM. Lo Cascio V. Sawamura T. Oxidized low density lipoprotein (ox-LDL) binding to ox-LDL receptor-1 in endothelial cells induces the activation of NF-kappaB through an increased production of intracellular reactive oxygen species. J Biol Chem. 2000;275:12633–12638. doi: 10.1074/jbc.275.17.12633. [DOI] [PubMed] [Google Scholar]
- 73.Connelly PW. Draganov D. Maguire GF. Paraoxonase-1 does not reduce or modify oxidation of phospholipids by peroxynitrite. Free Radic Biol Med. 2005;38:164–174. doi: 10.1016/j.freeradbiomed.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 74.Costa LG. Cole TB. Jarvik GP. Furlong CE. Functional genomic of the paraoxonase (PON1) polymorphisms: effects on pesticide sensitivity, cardiovascular disease, and drug metabolism. Annu Rev Med. 2003;54:371–392. doi: 10.1146/annurev.med.54.101601.152421. [DOI] [PubMed] [Google Scholar]
- 75.Costa LG. Giordano G. Furlong CE. Pharmacological and dietary modulators of paraoxonase 1 (PON1) activity and expression: the hunt goes on. Biochem Pharmacol. 2011;81:337–344. doi: 10.1016/j.bcp.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Costa LG. Vitalone A. Cole TB. Furlong CE. Modulation of paraoxonase (PON1) activity. Biochem Pharmacol. 2005;69:541–550. doi: 10.1016/j.bcp.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 77.Cui MZ. Penn MS. Chisolm GM. Native and oxidized low density lipoprotein induction of tissue factor gene expression in smooth muscle cells is mediated by both Egr-1 and Sp1. J Biol Chem. 1999;274:32795–32802. doi: 10.1074/jbc.274.46.32795. [DOI] [PubMed] [Google Scholar]
- 78.Cushing SD. Berliner JA. Valente AJ. Territo MC. Navab M. Parhami F. Gerrity R. Schwartz CJ. Fogelman AM. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc Natl Acad Sci U S A. 1990;87:5134–5138. doi: 10.1073/pnas.87.13.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Davies HG. Richter RJ. Keifer M. Broomfield CA. Sowalla J. Furlong CE. The effect of the human serum paraoxonase polymorphism is reversed with diazoxon, soman and sarin. Nat Genet. 1996;14:334–336. doi: 10.1038/ng1196-334. [DOI] [PubMed] [Google Scholar]
- 80.De Geest B. Stengel D. Landeloos M. Lox M. Le Gat L. Collen D. Holvoet P. Ninio E. Effect of overexpression of human apo A-I in C57BL/6 and C57BL/6 apo E-deficient mice on 2 lipoprotein-associated enzymes, platelet-activating factor acetylhydrolase and paraoxonase. Comparison of adenovirus-mediated human apo A-I gene transfer and human apo A-I transgenesis. Arterioscler Thromb Vasc Biol. 2000;20:E68–E75. doi: 10.1161/01.atv.20.10.e68. [DOI] [PubMed] [Google Scholar]
- 81.Deakin S. Leviev I. Brulhart-Meynet MC. James RW. Paraoxonase-1 promoter haplotypes and serum paraoxonase: a predominant role for polymorphic position −107, implicating the Sp1 transcription factor. Biochem J. 2003;372:643–649. doi: 10.1042/BJ20021670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Deakin S. Leviev I. Gomaraschi M. Calabresi L. Franceschini G. James RW. Enzymatically active paraoxonase-1 is located at the external membrane of producing cells and released by a high affinity, saturable, desorption mechanism. J Biol Chem. 2002;277:4301–4308. doi: 10.1074/jbc.M107440200. [DOI] [PubMed] [Google Scholar]
- 83.Deakin S. Leviev I. Guernier S. James RW. Simvastatin modulates expression of the PON1 gene and increases serum paraoxonase: a role for sterol regulatory element-binding protein-2. Arterioscler Thromb Vasc Biol. 2003;23:2083–2089. doi: 10.1161/01.ATV.0000096207.01487.36. [DOI] [PubMed] [Google Scholar]
- 84.Devarajan A. Bourquard N. Hama S. Navab M. Grijalva VR. Morvardi S. Clarke CF. Vergnes L. Reue K. Teiber JF. Reddy ST. Paraoxonase 2 deficiency alters mitochondrial function and exacerbates the development of atherosclerosis. Antioxid Redox Signal. 2011;14:341–351. doi: 10.1089/ars.2010.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dorup I. Sorensen KE. [Non-invasive assessment of endothelial function] Ugeskr Laeger. 1998;160:3376–3382. [PubMed] [Google Scholar]
- 86.Draganov DI. La Du BN. Pharmacogenetics of paraoxonases: a brief review. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:78–88. doi: 10.1007/s00210-003-0833-1. [DOI] [PubMed] [Google Scholar]
- 87.Draganov DI. Stetson PL. Watson CE. Billecke SS. La Du BN. Rabbit serum paraoxonase 3 (PON3) is a high density lipoprotein-associated lactonase and protects low density lipoprotein against oxidation. J Biol Chem. 2000;275:33435–33442. doi: 10.1074/jbc.M004543200. [DOI] [PubMed] [Google Scholar]
- 88.Draganov DI. Teiber JF. Speelman A. Osawa Y. Sunahara R. La Du BN. Human paraoxonases (PON1, PON2, and PON3) are lactonases with overlapping and distinct substrate specificities. J Lipid Res. 2005;46:1239–1247. doi: 10.1194/jlr.M400511-JLR200. [DOI] [PubMed] [Google Scholar]
- 89.Dullaart RP. de Vries R. Sluiter WJ. Voorbij HA. High plasma C-reactive protein (CRP) is related to low paraoxonase-I (PON-I) activity independently of high leptin and low adiponectin in type 2 diabetes mellitus. Clin Endocrinol (Oxf ) 2009;70:221–226. doi: 10.1111/j.1365-2265.2008.03306.x. [DOI] [PubMed] [Google Scholar]
- 90.Durrington PN. Mackness B. Mackness MI. Paraoxonase and atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:473–480. doi: 10.1161/01.atv.21.4.473. [DOI] [PubMed] [Google Scholar]
- 91.Durrington PN. Mackness MI. Bhatnagar D. Julier K. Prais H. Arrol S. Morgan J. Wood GN. Effects of two different fibric acid derivatives on lipoproteins, cholesteryl ester transfer, fibrinogen, plasminogen activator inhibitor and paraoxonase activity in type IIb hyperlipoproteinaemia. Atherosclerosis. 1998;138:217–225. doi: 10.1016/s0021-9150(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 92.Essler M. Retzer M. Bauer M. Heemskerk JW. Aepfelbacher M. Siess W. Mildly oxidized low density lipoprotein induces contraction of human endothelial cells through activation of Rho/Rho kinase and inhibition of myosin light chain phosphatase. J Biol Chem. 1999;274:30361–30364. doi: 10.1074/jbc.274.43.30361. [DOI] [PubMed] [Google Scholar]
- 93.Fanella S. Harris SB. Young TK. Hanley AJ. Zinman B. Connelly PW. Hegele RA. Association between PON1 L/M55 polymorphism and plasma lipoproteins in two Canadian aboriginal populations. Clin Chem Lab Med. 2000;38:413–420. doi: 10.1515/CCLM.2000.060. [DOI] [PubMed] [Google Scholar]
- 94.Feng J. Han J. Pearce SF. Silverstein RL. Gotto AM., Jr. Hajjar DP. Nicholson AC. Induction of CD36 expression by oxidized LDL and IL-4 by a common signaling pathway dependent on protein kinase C and PPAR-gamma. J Lipid Res. 2000;41:688–696. [PubMed] [Google Scholar]
- 95.Fenkci IV. Serteser M. Fenkci S. Akyol AM. Effects of intranasal estradiol treatment on serum paraoxonase and lipids in healthy, postmenopausal women. Gynecol Obstet Invest. 2006;61:203–207. doi: 10.1159/000091418. [DOI] [PubMed] [Google Scholar]
- 96.Ferretti G. Bacchetti T. Masciangelo S. Bicchiega V. HDL-paraoxonase and membrane lipid peroxidation: a comparison between healthy and obese subjects. Obesity (Silver Spring) 2010;18:1079–1084. doi: 10.1038/oby.2009.338. [DOI] [PubMed] [Google Scholar]
- 97.Ferretti G. Bacchetti T. Moroni C. Savino S. Liuzzi A. Balzola F. Bicchiega V. Paraoxonase activity in high-density lipoproteins: a comparison between healthy and obese females. J Clin Endocrinol Metab. 2005;90:1728–1733. doi: 10.1210/jc.2004-0486. [DOI] [PubMed] [Google Scholar]
- 98.Flekac M. Skrha J. Zidkova K. Lacinova Z. Hilgertova J. Paraoxonase 1 gene polymorphisms and enzyme activities in diabetes mellitus. Physiol Res. 2008;57:717–726. doi: 10.33549/physiolres.931285. [DOI] [PubMed] [Google Scholar]
- 99.Fryer RH. Wilson BD. Gubler DB. Fitzgerald LA. Rodgers GM. Homocysteine, a risk factor for premature vascular disease and thrombosis, induces tissue factor activity in endothelial cells. Arterioscler Thromb. 1993;13:1327–1333. doi: 10.1161/01.atv.13.9.1327. [DOI] [PubMed] [Google Scholar]
- 100.Fuhrman B. Gantman A. Khateeb J. Volkova N. Horke S. Kiyan J. Dumler I. Aviram M. Urokinase activates macrophage PON2 gene transcription via the PI3K/ROS/MEK/SREBP-2 signalling cascade mediated by the PDGFR-beta. Cardiovasc Res. 2009;84:145–154. doi: 10.1093/cvr/cvp184. [DOI] [PubMed] [Google Scholar]
- 101.Fuhrman B. Khateeb J. Shiner M. Nitzan O. Karry R. Volkova N. Aviram M. Urokinase plasminogen activator upregulates paraoxonase 2 expression in macrophages via an NADPH oxidase-dependent mechanism. Arterioscler Thromb Vasc Biol. 2008;28:1361–1367. doi: 10.1161/ATVBAHA.108.166041. [DOI] [PubMed] [Google Scholar]
- 102.Furlong CE. Cole TB. Jarvik GP. Costa LG. Pharmacogenomic considerations of the paraoxonase polymorphisms. Pharmacogenomics. 2002;3:341–348. doi: 10.1517/14622416.3.3.341. [DOI] [PubMed] [Google Scholar]
- 103.Garin MC. Kalix B. Morabia A. James RW. Small, dense lipoprotein particles and reduced paraoxonase-1 in patients with the metabolic syndrome. J Clin Endocrinol Metab. 2005;90:2264–2269. doi: 10.1210/jc.2004-1295. [DOI] [PubMed] [Google Scholar]
- 104.Ghorbanihaghjo A. Argani H. Rahbaninoubar M. Rashtchizadeh N. Effect of nandrolone decanonate on paraoxonase activity in hemodialysis patients. Clin Biochem. 2005;38:1076–1080. doi: 10.1016/j.clinbiochem.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 105.Ginsberg G. Neafsey P. Hattis D. Guyton KZ. Johns DO. Sonawane B. Genetic polymorphism in paraoxonase 1 (PON1): population distribution of PON1 activity. J Toxicol Environ Health B Crit Rev. 2009;12:473–507. doi: 10.1080/10937400903158409. [DOI] [PubMed] [Google Scholar]
- 106.Glass CK. Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 107.Glueck CJ. Gartside P. Fallat RW. Sielski J. Steiner PM. Longevity syndromes: familial hypobeta and familial hyperalpha lipoproteinemia. J Lab Clin Med. 1976;88:941–957. [PubMed] [Google Scholar]
- 108.Gordon DJ. Probstfield JL. Garrison RJ. Neaton JD. Castelli WP. Knoke JD. Jacobs DR., Jr. Bangdiwala S. Tyroler HA. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79:8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]
- 109.Goswami B. Tayal D. Gupta N. Mallika V. Paraoxonase: a multifaceted biomolecule. Clin Chim Acta. 2009;410:1–12. doi: 10.1016/j.cca.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 110.Gotto AM., Jr. and Brinton EA. Assessing low levels of high-density lipoprotein cholesterol as a risk factor in coronary heart disease: a working group report and update. J Am Coll Cardiol. 2004;43:717–724. doi: 10.1016/j.jacc.2003.08.061. [DOI] [PubMed] [Google Scholar]
- 111.Gotto AM., Jr. Pownall HJ. Havel RJ. Introduction to the plasma lipoproteins. Methods Enzymol. 1986;128:3–41. doi: 10.1016/0076-6879(86)28061-1. [DOI] [PubMed] [Google Scholar]
- 112.Gouedard C. Barouki R. Morel Y. Induction of the paraoxonase-1 gene expression by resveratrol. Arterioscler Thromb Vasc Biol. 2004;24:2378–2383. doi: 10.1161/01.ATV.0000146530.24736.ce. [DOI] [PubMed] [Google Scholar]
- 113.Gouedard C. Barouki R. Morel Y. Dietary polyphenols increase paraoxonase 1 gene expression by an aryl hydrocarbon receptor-dependent mechanism. Mol Cell Biol. 2004;24:5209–5222. doi: 10.1128/MCB.24.12.5209-5222.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gouedard C. Koum-Besson N. Barouki R. Morel Y. Opposite regulation of the human paraoxonase-1 gene PON-1 by fenofibrate and statins. Mol Pharmacol. 2003;63:945–956. doi: 10.1124/mol.63.4.945. [DOI] [PubMed] [Google Scholar]
- 115.Grundy SM. Hypertriglyceridemia, insulin resistance, and the metabolic syndrome. Am J Cardiol. 1999;83:25F–29F. doi: 10.1016/s0002-9149(99)00211-8. [DOI] [PubMed] [Google Scholar]
- 116.Gugliucci A. Lunceford N. Kinugasa E. Ogata H. Schulze J. Kimura S. Acrolein inactivates paraoxonase 1: changes in free acrolein levels after hemodialysis correlate with increases in paraoxonase 1 activity in chronic renal failure patients. Clin Chim Acta. 2007;384:105–112. doi: 10.1016/j.cca.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 117.Gusman J. Malonne H. Atassi G. A reappraisal of the potential chemopreventive and chemotherapeutic properties of resveratrol. Carcinogenesis. 2001;22:1111–1117. doi: 10.1093/carcin/22.8.1111. [DOI] [PubMed] [Google Scholar]
- 118.Hamelet J. Ait-Yahya-Graison E. Matulewicz E. Noll C. Badel-Chagnon A. Camproux AC. Demuth K. Paul JL. Delabar JM. Janel N. Homocysteine threshold value based on cystathionine beta synthase and paraoxonase 1 activities in mice. Eur J Clin Invest. 2007;37:933–938. doi: 10.1111/j.1365-2362.2007.01879.x. [DOI] [PubMed] [Google Scholar]
- 119.Han J. Hajjar DP. Febbraio M. Nicholson AC. Native and modified low density lipoproteins increase the functional expression of the macrophage class B scavenger receptor, CD36. J Biol Chem. 1997;272:21654–21659. doi: 10.1074/jbc.272.34.21654. [DOI] [PubMed] [Google Scholar]
- 120.Harangi M. Seres I. Varga Z. Emri G. Szilvassy Z. Paragh G. Remenyik E. Atorvastatin effect on high-density lipoprotein-associated paraoxonase activity and oxidative DNA damage. Eur J Clin Pharmacol. 2004;60:685–691. doi: 10.1007/s00228-004-0820-6. [DOI] [PubMed] [Google Scholar]
- 121.Harel M. Aharoni A. Gaidukov L. Brumshtein B. Khersonsky O. Meged R. Dvir H. Ravelli RB. McCarthy A. Toker L. Silman I. Sussman JL. Tawfik DS. Structure and evolution of the serum paraoxonase family of detoxifying and anti-atherosclerotic enzymes. Nat Struct Mol Biol. 2004;11:412–419. doi: 10.1038/nsmb767. [DOI] [PubMed] [Google Scholar]
- 122.Harker LA. Slichter SJ. Scott CR. Ross R. Homocystinemia. Vascular injury and arterial thrombosis. N Engl J Med. 1974;291:537–543. doi: 10.1056/NEJM197409122911101. [DOI] [PubMed] [Google Scholar]
- 123.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 124.Harpel PC. Zhang X. Borth W. Homocysteine and hemostasis: pathogenic mechanisms predisposing to thrombosis. J Nutr. 1996;126:1285S–1289S. doi: 10.1093/jn/126.suppl_4.1285S. [DOI] [PubMed] [Google Scholar]
- 125.Hassett C. Richter RJ. Humbert R. Chapline C. Crabb JW. Omiecinski CJ. Furlong CE. Characterization of cDNA clones encoding rabbit and human serum paraoxonase: the mature protein retains its signal sequence. Biochemistry. 1991;30:10141–10149. doi: 10.1021/bi00106a010. [DOI] [PubMed] [Google Scholar]
- 126.He F. Guo R. Wu SL. Sun M. Li M. Protective effects of ginsenoside Rb1 on human umbilical vein endothelial cells in vitro. J Cardiovasc Pharmacol. 2007;50:314–320. doi: 10.1097/FJC.0b013e3180cab12e. [DOI] [PubMed] [Google Scholar]
- 127.Hedrick CC. Thorpe SR. Fu MX. Harper CM. Yoo J. Kim SM. Wong H. Peters AL. Glycation impairs high-density lipoprotein function. Diabetologia. 2000;43:312–320. doi: 10.1007/s001250050049. [DOI] [PubMed] [Google Scholar]
- 128.Hegele RA. Paraoxonase genes and disease. Ann Med. 1999;31:217–224. doi: 10.3109/07853899909115981. [DOI] [PubMed] [Google Scholar]
- 129.Heinecke JW. Lusis AJ. Paraoxonase-gene polymorphisms associated with coronary heart disease: support for the oxidative damage hypothesis? Am J Hum Genet. 1998;62:20–24. doi: 10.1086/301691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hessler JR. Morel DW. Lewis LJ. Chisolm GM. Lipoprotein oxidation and lipoprotein-induced cytotoxicity. Arteriosclerosis. 1983;3:215–222. doi: 10.1161/01.atv.3.3.215. [DOI] [PubMed] [Google Scholar]
- 131.Hessler JR. Robertson AL., Jr. Chisolm GM., 3rd LDL-induced cytotoxicity and its inhibition by HDL in human vascular smooth muscle and endothelial cells in culture. Atherosclerosis. 1979;32:213–229. doi: 10.1016/0021-9150(79)90166-7. [DOI] [PubMed] [Google Scholar]
- 131a.Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA. 2002;288:2015–2022. doi: 10.1001/jama.288.16.2015. [DOI] [PubMed] [Google Scholar]
- 132.Hong SC. Zhao SP. Wu ZH. Probucol up-regulates paraoxonase 1 expression in hepatocytes of hypercholesterolemic rabbits. J Cardiovasc Pharmacol. 2006;47:77–81. doi: 10.1097/01.fjc.0000194687.19335.59. [DOI] [PubMed] [Google Scholar]
- 133.Horke S. Witte I. Altenhofer S. Wilgenbus P. Goldeck M. Forstermann U. Xiao J. Kramer GL. Haines DC. Chowdhary PK. Haley RW. Teiber JF. Paraoxonase 2 is down-regulated by the Pseudomonas aeruginosa quorumsensing signal N-(3-oxododecanoyl)-L-homoserine lactone and attenuates oxidative stress induced by pyocyanin. Biochem J. 2010;426:73–83. doi: 10.1042/BJ20091414. [DOI] [PubMed] [Google Scholar]
- 134.Horke S. Witte I. Wilgenbus P. Kruger M. Strand D. Forstermann U. Paraoxonase-2 reduces oxidative stress in vascular cells and decreases endoplasmic reticulum stress-induced caspase activation. Circulation. 2007;115:2055–2064. doi: 10.1161/CIRCULATIONAHA.106.681700. [DOI] [PubMed] [Google Scholar]
- 135.Humbert R. Adler DA. Disteche CM. Hassett C. Omiecinski CJ. Furlong CE. The molecular basis of the human serum paraoxonase activity polymorphism. Nat Genet. 1993;3:73–76. doi: 10.1038/ng0193-73. [DOI] [PubMed] [Google Scholar]
- 136.Hundal RS. Salh BS. Schrader JW. Gomez-Munoz A. Duronio V. Steinbrecher UP. Oxidized low density lipoprotein inhibits macrophage apoptosis through activation of the PI 3-kinase/PKB pathway. J Lipid Res. 2001;42:1483–1491. [PubMed] [Google Scholar]
- 137.Ikeda T. Obayashi H. Hasegawa G. Nakamura N. Yoshikawa T. Imamura Y. Koizumi K. Kinoshita S. Paraoxonase gene polymorphisms and plasma oxidized low-density lipoprotein level as possible risk factors for exudative age-related macular degeneration. Am J Ophthalmol. 2001;132:191–195. doi: 10.1016/s0002-9394(01)00975-8. [DOI] [PubMed] [Google Scholar]
- 138.Ikeda Y. Inoue M. Suehiro T. Arii K. Kumon Y. Hashimoto K. Low human paraoxonase predicts cardiovascular events in Japanese patients with type 2 diabetes. Acta Diabetol. 2009;46:239–242. doi: 10.1007/s00592-008-0066-3. [DOI] [PubMed] [Google Scholar]
- 139.Ikeda Y. Suehiro T. Inoue M. Nakauchi Y. Morita T. Arii K. Ito H. Kumon Y. Hashimoto K. Serum paraoxonase activity and its relationship to diabetic complications in patients with non-insulin-dependent diabetes mellitus. Metabolism. 1998;47:598–602. doi: 10.1016/s0026-0495(98)90246-3. [DOI] [PubMed] [Google Scholar]
- 140.Imai Y. Morita H. Kurihara H. Sugiyama T. Kato N. Ebihara A. Hamada C. Kurihara Y. Shindo T. Oh-hashi Y. Yazaki Y. Evidence for association between paraoxonase gene polymorphisms and atherosclerotic diseases. Atherosclerosis. 2000;149:435–442. doi: 10.1016/s0021-9150(99)00340-8. [DOI] [PubMed] [Google Scholar]
- 141.Ishii N. Matsumura T. Kinoshita H. Motoshima H. Kojima K. Tsutsumi A. Kawasaki S. Yano M. Senokuchi T. Asano T. Nishikawa T. Araki E. Activation of AMP-activated protein kinase suppresses oxidized low-density lipoprotein-induced macrophage proliferation. J Biol Chem. 2009;284:34561–34569. doi: 10.1074/jbc.M109.028043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ito T. Yasue H. Yoshimura M. Nakamura S. Nakayama M. Shimasaki Y. Harada E. Mizuno Y. Kawano H. Ogawa H. Paraoxonase gene Gln192Arg (Q192R) polymorphism is associated with coronary artery spasm. Hum Genet. 2002;110:89–94. doi: 10.1007/s00439-001-0654-6. [DOI] [PubMed] [Google Scholar]
- 143.Iwashima Y. Eto M. Hata A. Kaku K. Horiuchi S. Ushikubi F. Sano H. Advanced glycation end products-induced gene expression of scavenger receptors in cultured human monocyte-derived macrophages. Biochem Biophys Res Commun. 2000;277:368–380. doi: 10.1006/bbrc.2000.3685. [DOI] [PubMed] [Google Scholar]
- 144.Jaichander P. Selvarajan K. Garelnabi M. Parthasarathy S. Induction of paraoxonase 1 and apolipoprotein A-I gene expression by aspirin. J Lipid Res. 2008;49:2142–2148. doi: 10.1194/jlr.M800082-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jakubowski H. Calcium-dependent human serum homocysteine thiolactone hydrolase. A protective mechanism against protein N-homocysteinylation. J Biol Chem. 2000;275:3957–3962. doi: 10.1074/jbc.275.6.3957. [DOI] [PubMed] [Google Scholar]
- 146.Jakubowski H. The pathophysiological hypothesis of homocysteine thiolactone-mediated vascular disease. J Physiol Pharmacol. 2008;59(Suppl 9):155–167. [PubMed] [Google Scholar]
- 147.Jakubowski H. The role of paraoxonase 1 in the detoxification of homocysteine thiolactone. Adv Exp Med Biol. 2010;660:113–127. doi: 10.1007/978-1-60761-350-3_11. [DOI] [PubMed] [Google Scholar]
- 148.Jakubowski H. Ambrosius WT. Pratt JH. Genetic determinants of homocysteine thiolactonase activity in humans: implications for atherosclerosis. FEBS Lett. 2001;491:35–39. doi: 10.1016/s0014-5793(01)02143-3. [DOI] [PubMed] [Google Scholar]
- 149.Jakubowski H. Zhang L. Bardeguez A. Aviv A. Homocysteine thiolactone and protein homocysteinylation in human endothelial cells: implications for atherosclerosis. Circ Res. 2000;87:45–51. doi: 10.1161/01.res.87.1.45. [DOI] [PubMed] [Google Scholar]
- 150.James RW. Blatter Garin MC. Calabresi L. Miccoli R. von Eckardstein A. Tilly-Kiesi M. Taskinen MR. Assmann G. Franceschini G. Modulated serum activities and concentrations of paraoxonase in high density lipoprotein deficiency states. Atherosclerosis. 1998;139:77–82. doi: 10.1016/s0021-9150(98)00058-6. [DOI] [PubMed] [Google Scholar]
- 151.James RW. Leviev I. Righetti A. Smoking is associated with reduced serum paraoxonase activity and concentration in patients with coronary artery disease. Circulation. 2000;101:2252–2257. doi: 10.1161/01.cir.101.19.2252. [DOI] [PubMed] [Google Scholar]
- 152.James RW. Leviev I. Ruiz J. Passa P. Froguel P. Garin MC. Promoter polymorphism T(-107)C of the paraoxonase PON1 gene is a risk factor for coronary heart disease in type 2 diabetic patients. Diabetes. 2000;49:1390–1393. doi: 10.2337/diabetes.49.8.1390. [DOI] [PubMed] [Google Scholar]
- 153.Jaouad L. de Guise C. Berrougui H. Cloutier M. Isabelle M. Fulop T. Payette H. Khalil A. Age-related decrease in high-density lipoproteins antioxidant activity is due to an alteration in the PON1's free sulfhydryl groups. Atherosclerosis. 2006;185:191–200. doi: 10.1016/j.atherosclerosis.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 154.Jarvik GP. Rozek LS. Brophy VH. Hatsukami TS. Richter RJ. Schellenberg GD. Furlong CE. Paraoxonase (PON1) phenotype is a better predictor of vascular disease than is PON1(192) or PON1(55) genotype. Arterioscler Thromb Vasc Biol. 2000;20:2441–2447. doi: 10.1161/01.atv.20.11.2441. [DOI] [PubMed] [Google Scholar]
- 155.Jarvik GP. Tsai NT. McKinstry LA. Wani R. Brophy VH. Richter RJ. Schellenberg GD. Heagerty PJ. Hatsukami TS. Furlong CE. Vitamin C and E intake is associated with increased paraoxonase activity. Arterioscler Thromb Vasc Biol. 2002;22:1329–1333. doi: 10.1161/01.atv.0000027101.40323.3a. [DOI] [PubMed] [Google Scholar]
- 156.Jeon SM. Park YB. Kwon OS. Huh TL. Lee WH. Do KM. Park T. Choi MS. Vitamin E supplementation alters HDL-cholesterol concentration and paraoxonase activity in rabbits fed high-cholesterol diet: comparison with probucol. J Biochem Mol Toxicol. 2005;19:336–346. doi: 10.1002/jbt.20098. [DOI] [PubMed] [Google Scholar]
- 157.Jiang C. Zhang H. Zhang W. Kong W. Zhu Y. Xu Q. Li Y. Wang X. Homocysteine promotes vascular smooth muscle cell migration by induction of the adipokine resistin. Am J Physiol Cell Physiol. 2009;297:C1466–C1476. doi: 10.1152/ajpcell.00304.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jiang H. Wang XF. Fang L. Tang C. Zhu Y. Wang X. Upregulation of aldose reductase by homocysteine in type II alveolar epithelial cells. Biochem Biophys Res Commun. 2005;337:1084–1091. doi: 10.1016/j.bbrc.2005.09.160. [DOI] [PubMed] [Google Scholar]
- 159.Jimi S. Saku K. Uesugi N. Sakata N. Takebayashi S. Oxidized low density lipoprotein stimulates collagen production in cultured arterial smooth muscle cells. Atherosclerosis. 1995;116:15–26. doi: 10.1016/0021-9150(95)05515-x. [DOI] [PubMed] [Google Scholar]
- 160.Josse D. Xie W. Masson P. Lockridge O. Human serum paraoxonase (PON1): identification of essential amino acid residues by group-selective labelling and site-directed mutagenesis. Chem Biol Interact. 1999:119–120. doi: 10.1016/s0009-2797(99)00015-0. 71–78. [DOI] [PubMed] [Google Scholar]
- 161.Josse D. Xie W. Renault F. Rochu D. Schopfer LM. Masson P. Lockridge O. Identification of residues essential for human paraoxonase (PON1) arylesterase/organophosphatase activities. Biochemistry. 1999;38:2816–2825. doi: 10.1021/bi982281h. [DOI] [PubMed] [Google Scholar]
- 162.Kao YL. Donaghue K. Chan A. Knight J. Silink M. A variant of paraoxonase (PON1) gene is associated with diabetic retinopathy in IDDM. J Clin Endocrinol Metab. 1998;83:2589–2592. doi: 10.1210/jcem.83.7.5096. [DOI] [PubMed] [Google Scholar]
- 163.Karikas GA. Kriebardis A. Samara I. Schulpis K. Papachristodoulou M. Fytou-Pallikari A. Serum homocysteine levels and paraoxonase 1 activity in preschool aged children in Greece. Clin Chem Lab Med. 2006;44:623–627. doi: 10.1515/CCLM.2006.110. [DOI] [PubMed] [Google Scholar]
- 164.Kassai A. Illyes L. Mirdamadi HZ. Seres I. Kalmar T. Audikovszky M. Paragh G. The effect of atorvastatin therapy on lecithin:cholesterol acyltransferase, cholesteryl ester transfer protein and the antioxidant paraoxonase. Clin Biochem. 2007;40:1–5. doi: 10.1016/j.clinbiochem.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 165.Kerkeni M. Addad F. Chauffert M. Chuniaud L. Miled A. Trivin F. Maaroufi K. Hyperhomocysteinemia, paraoxonase activity and risk of coronary artery disease. Clin Biochem. 2006;39:821–825. doi: 10.1016/j.clinbiochem.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 166.Khan M. Pelengaris S. Cooper M. Smith C. Evan G. Betteridge J. Oxidised lipoproteins may promote inflammation through the selective delay of engulfment but not binding of apoptotic cells by macrophages. Atherosclerosis. 2003;171:21–29. doi: 10.1016/j.atherosclerosis.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 167.Khersonsky O. Tawfik DS. Structure-reactivity studies of serum paraoxonase PON1 suggest that its native activity is lactonase. Biochemistry. 2005;44:6371–6382. doi: 10.1021/bi047440d. [DOI] [PubMed] [Google Scholar]
- 168.Kiranoglu S. Sinan S. Gencer N. Kockar F. Arslan O. In vivo effects of oral contraceptives on paraoxonase, catalase and carbonic anhydrase enzyme activities on mouse. Biol Pharm Bull. 2007;30:1048–1051. doi: 10.1248/bpb.30.1048. [DOI] [PubMed] [Google Scholar]
- 169.Kobayashi M. Shinohara M. Sakoh C. Kataoka M. Shimizu S. Lactone-ring-cleaving enzyme: genetic analysis, novel RNA editing, and evolutionary implications. Proc Natl Acad Sci U S A. 1998;95:12787–12792. doi: 10.1073/pnas.95.22.12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Koncsos P. Seres I. Harangi M. Illyes I. Jozsa L. Gonczi F. Bajnok L. Paragh G. Human paraoxonase-1 activity in childhood obesity and its relation to leptin and adiponectin levels. Pediatr Res. 2010;67:309–313. doi: 10.1203/PDR.0b013e3181c9fb66. [DOI] [PubMed] [Google Scholar]
- 171.Kordonouri O. James RW. Bennetts B. Chan A. Kao YL. Danne T. Silink M. Donaghue K. Modulation by blood glucose levels of activity and concentration of paraoxonase in young patients with type 1 diabetes mellitus. Metabolism. 2001;50:657–660. doi: 10.1053/meta.2001.23291. [DOI] [PubMed] [Google Scholar]
- 172.Kosaka T. Yamaguchi M. Motomura T. Mizuno K. Investigation of the relationship between atherosclerosis and paraoxonase or homocysteine thiolactonase activity in patients with type 2 diabetes mellitus using a commercially available assay. Clin Chim Acta. 2005;359:156–162. doi: 10.1016/j.cccn.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 173.Kosugi K. Morel DW. DiCorleto PE. Chisolm GM. Toxicity of oxidized low-density lipoprotein to cultured fibroblasts is selective for S phase of the cell cycle. J Cell Physiol. 1987;130:311–320. doi: 10.1002/jcp.1041300302. [DOI] [PubMed] [Google Scholar]
- 174.Kume N. Kita T. Lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) in atherogenesis. Trends Cardiovasc Med. 2001;11:22–25. doi: 10.1016/s1050-1738(01)00079-2. [DOI] [PubMed] [Google Scholar]
- 175.Kumon Y. Suehiro T. Ikeda Y. Hashimoto K. Human paraoxonase-1 gene expression by HepG2 cells is downregulated by interleukin-1beta and tumor necrosis factor-alpha, but is upregulated by interleukin-6. Life Sci. 2003;73:2807–2815. doi: 10.1016/s0024-3205(03)00704-5. [DOI] [PubMed] [Google Scholar]
- 176.Kumru S. Aydin S. Aras A. Gursu MF. Gulcu F. Effects of surgical menopause and estrogen replacement therapy on serum paraoxonase activity and plasma malondialdehyde concentration. Gynecol Obstet Invest. 2005;59:108–112. doi: 10.1159/000082647. [DOI] [PubMed] [Google Scholar]
- 177.Kunes JP. Cordero-Koning KS. Lee LH. Lynch SM. Vitamin C attenuates hypochlorite-mediated loss of paraoxonase-1 activity from human plasma. Nutr Res. 2009;29:114–122. doi: 10.1016/j.nutres.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 178.Kuo CL. La Du BN. Comparison of purified human and rabbit serum paraoxonases. Drug Metab Dispos. 1995;23:935–944. [PubMed] [Google Scholar]
- 179.Kuo CL. La Du BN. Calcium binding by human and rabbit serum paraoxonases. Structural stability and enzymatic activity. Drug Metab Dispos. 1998;26:653–660. [PubMed] [Google Scholar]
- 180.Kurban S. Mehmetoglu I. Effects of acetylsalicylic acid on serum paraoxonase activity, Ox-LDL, coenzyme Q10 and other oxidative stress markers in healthy volunteers. Clin Biochem. 2010;43:287–290. doi: 10.1016/j.clinbiochem.2009.10.054. [DOI] [PubMed] [Google Scholar]
- 181.La Du BN. Structural and functional diversity of paraoxonases. Nat Med. 1996;2:1186–1187. doi: 10.1038/nm1196-1186. [DOI] [PubMed] [Google Scholar]
- 182.Lacinski M. Skorupski W. Cieslinski A. Sokolowska J. Trzeciak WH. Jakubowski H. Determinants of homocysteine-thiolactonase activity of the paraoxonase-1 (PON1) protein in humans. Cell Mol Biol (Noisy-le-grand) 2004;50:885–893. [PubMed] [Google Scholar]
- 183.Lafont AM. Chai YC. Cornhill JF. Whitlow PL. Howe PH. Chisolm GM. Effect of alpha-tocopherol on restenosis after angioplasty in a model of experimental atherosclerosis. J Clin Invest. 1995;95:1018–1025. doi: 10.1172/JCI117746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lagos KG. Filippatos TD. Tsimihodimos V. Gazi IF. Rizos C. Tselepis AD. Mikhailidis DP. Elisaf MS. Alterations in the high density lipoprotein phenotype and HDL-associated enzymes in subjects with metabolic syndrome. Lipids. 2009;44:9–16. doi: 10.1007/s11745-008-3251-9. [DOI] [PubMed] [Google Scholar]
- 185.Lakshman MR. Gottipati CS. Narasimhan SJ. Munoz J. Marmillot P. Nylen ES. Inverse correlation of serum paraoxonase and homocysteine thiolactonase activities and antioxidant capacity of high-density lipoprotein with the severity of cardiovascular disease in persons with type 2 diabetes mellitus. Metabolism. 2006;55:1201–1206. doi: 10.1016/j.metabol.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 186.Leckey LC. Garige M. Varatharajalu R. Gong M. Nagata T. Spurney CF. Lakshman RM. Quercetin and ethanol attenuate the progression of atherosclerotic plaques with concomitant up regulation of paraoxonase1 (PON1) gene expression and PON1 activity in LDLR-/- mice. Alcohol Clin Exp Res. 2010;34:1535–1542. doi: 10.1111/j.1530-0277.2010.01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lee JM. Choudhury RP. Prospects for atherosclerosis regression through increase in high-density lipoprotein and other emerging therapeutic targets. Heart. 2007;93:559–564. doi: 10.1136/hrt.2005.066050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lee SJ. Kim KM. Namkoong S. Kim CK. Kang YC. Lee H. Ha KS. Han JA. Chung HT. Kwon YG. Kim YM. Nitric oxide inhibition of homocysteine-induced human endothelial cell apoptosis by down-regulation of p53-dependent Noxa expression through the formation of S-nitrosohomocysteine. J Biol Chem. 2005;280:5781–5788. doi: 10.1074/jbc.M411224200. [DOI] [PubMed] [Google Scholar]
- 189.Leus FR. Zwart M. Kastelein JJ. Voorbij HA. PON2 gene variants are associated with clinical manifestations of cardiovascular disease in familial hypercholesterolemia patients. Atherosclerosis. 2001;154:641–649. doi: 10.1016/s0021-9150(00)00440-8. [DOI] [PubMed] [Google Scholar]
- 190.Leviev I. James RW. Promoter polymorphisms of human paraoxonase PON1 gene and serum paraoxonase activities and concentrations. Arterioscler Thromb Vasc Biol. 2000;20:516–521. doi: 10.1161/01.atv.20.2.516. [DOI] [PubMed] [Google Scholar]
- 191.Leviev I. Kalix B. Brulhart Meynet MC. James RW. The paraoxonase PON1 promoter polymorphism C(-107)T is associated with increased serum glucose concentrations in non-diabetic patients. Diabetologia. 2001;44:1177–1183. doi: 10.1007/s001250100610. [DOI] [PubMed] [Google Scholar]
- 192.Leviev I. Negro F. James RW. Two alleles of the human paraoxonase gene produce different amounts of mRNA. An explanation for differences in serum concentrations of paraoxonase associated with the (Leu-Met54) polymorphism. Arterioscler Thromb Vasc Biol. 1997;17:2935–2939. doi: 10.1161/01.atv.17.11.2935. [DOI] [PubMed] [Google Scholar]
- 193.Leviev I. Poirier O. Nicaud V. Evans A. Kee F. Arveiler D. Morrisson C. Cambien F. James RW. High expressor paraoxonase PON1 gene promoter polymorphisms are associated with reduced risk of vascular disease in younger coronary patients. Atherosclerosis. 2002;161:463–467. doi: 10.1016/s0021-9150(01)00668-2. [DOI] [PubMed] [Google Scholar]
- 194.Leviev I. Righetti A. James RW. Paraoxonase promoter polymorphism T(-107)C and relative paraoxonase deficiency as determinants of risk of coronary artery disease. J Mol Med. 2001;79:457–463. doi: 10.1007/s001090100240. [DOI] [PubMed] [Google Scholar]
- 195.Li D. Mehta JL. Upregulation of endothelial receptor for oxidized LDL (LOX-1) by oxidized LDL and implications in apoptosis of human coronary artery endothelial cells: evidence from use of antisense LOX-1 mRNA and chemical inhibitors. Arterioscler Thromb Vasc Biol. 2000;20:1116–1122. doi: 10.1161/01.atv.20.4.1116. [DOI] [PubMed] [Google Scholar]
- 196.Li HL. Liu DP. Liang CC. Paraoxonase gene polymorphisms, oxidative stress, and diseases. J Mol Med. 2003;81:766–779. doi: 10.1007/s00109-003-0481-4. [DOI] [PubMed] [Google Scholar]
- 197.Li HL. Wang AB. Zhang R. Wei YS. Chen HZ. She ZG. Huang Y. Liu DP. Liang CC. A20 inhibits oxidized low-density lipoprotein-induced apoptosis through negative Fas/Fas ligand-dependent activation of caspase-8 and mitochondrial pathways in murine RAW264.7 macrophages. J Cell Physiol. 2006;208:307–318. doi: 10.1002/jcp.20665. [DOI] [PubMed] [Google Scholar]
- 198.Li WF. Costa LG. Richter RJ. Hagen T. Shih DM. Tward A. Lusis AJ. Furlong CE. Catalytic efficiency determines the in-vivo efficacy of PON1 for detoxifying organophosphorus compounds. Pharmacogenetics. 2000;10:767–779. doi: 10.1097/00008571-200012000-00002. [DOI] [PubMed] [Google Scholar]
- 199.Liao L. Aw TY. Kvietys PR. Granger DN. Oxidized LDL-induced microvascular dysfunction. Dependence on oxidation procedure. Arterioscler Thromb Vasc Biol. 1995;15:2305–2311. doi: 10.1161/01.atv.15.12.2305. [DOI] [PubMed] [Google Scholar]
- 200.Libby P. Managing the risk of atherosclerosis: the role of high-density lipoprotein. Am J Cardiol. 2001;88:3N–8N. doi: 10.1016/s0002-9149(01)02145-2. [DOI] [PubMed] [Google Scholar]
- 201.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 202.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lusis AJ. Fogelman AM. Fonarow GC. Genetic basis of atherosclerosis: part I: new genes and pathways. Circulation. 2004;110:1868–1873. doi: 10.1161/01.CIR.0000143041.58692.CC. [DOI] [PubMed] [Google Scholar]
- 204.Mackness B. Davies GK. Turkie W. Lee E. Roberts DH. Hill E. Roberts C. Durrington PN. Mackness MI. Paraoxonase status in coronary heart disease: are activity and concentration more important than genotype? Arterioscler Thromb Vasc Biol. 2001;21:1451–1457. doi: 10.1161/hq0901.094247. [DOI] [PubMed] [Google Scholar]
- 205.Mackness B. Durrington P. McElduff P. Yarnell J. Azam N. Watt M. Mackness M. Low paraoxonase activity predicts coronary events in the Caerphilly Prospective Study. Circulation. 2003;107:2775–2779. doi: 10.1161/01.CIR.0000070954.00271.13. [DOI] [PubMed] [Google Scholar]
- 206.Mackness B. Durrington PN. Mackness MI. The paraoxonase gene family and coronary heart disease. Curr Opin Lipidol. 2002;13:357–362. doi: 10.1097/00041433-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 207.Mackness B. McElduff P. Mackness MI. The paraoxonase-2–310 polymorphism is associated with the presence of microvascular complications in diabetes mellitus. J Intern Med. 2005;258:363–368. doi: 10.1111/j.1365-2796.2005.01554.x. [DOI] [PubMed] [Google Scholar]
- 208.Mackness B. Quarck R. Verreth W. Mackness M. Holvoet P. Human paraoxonase-1 overexpression inhibits atherosclerosis in a mouse model of metabolic syndrome. Arterioscler Thromb Vasc Biol. 2006;26:1545–1550. doi: 10.1161/01.ATV.0000222924.62641.aa. [DOI] [PubMed] [Google Scholar]
- 209.Mackness MI. Arrol S. Durrington PN. Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Lett. 1991;286:152–154. doi: 10.1016/0014-5793(91)80962-3. [DOI] [PubMed] [Google Scholar]
- 210.Mackness MI. Mackness B. Durrington PN. Fogelman AM. Berliner J. Lusis AJ. Navab M. Shih D. Fonarow GC. Paraoxonase and coronary heart disease. Curr Opin Lipidol. 1998;9:319–324. doi: 10.1097/00041433-199808000-00006. [DOI] [PubMed] [Google Scholar]
- 211.Macphee CH. Nelson JJ. Zalewski A. Lipoprotein-associated phospholipase A2 as a target of therapy. Curr Opin Lipidol. 2005;16:442–446. doi: 10.1097/01.mol.0000174155.61307.5f. [DOI] [PubMed] [Google Scholar]
- 212.Maier JA. Barenghi L. Pagani F. Bradamante S. Comi P. Ragnotti G. The protective role of high-density lipoprotein on oxidized-low-density-lipoprotein-induced U937/endothelial cell interactions. Eur J Biochem. 1994;221:35–41. doi: 10.1111/j.1432-1033.1994.tb18712.x. [DOI] [PubMed] [Google Scholar]
- 213.Majors A. Ehrhart LA. Pezacka EH. Homocysteine as a risk factor for vascular disease. Enhanced collagen production and accumulation by smooth muscle cells. Arterioscler Thromb Vasc Biol. 1997;17:2074–2081. doi: 10.1161/01.atv.17.10.2074. [DOI] [PubMed] [Google Scholar]
- 214.Malin R. Knuuti J. Janatuinen T. Laaksonen R. Vesalainen R. Nuutila P. Jokela H. Laakso J. Jaakkola O. Solakivi T. Lehtimaki T. Paraoxonase gene polymorphisms and coronary reactivity in young healthy men. J Mol Med. 2001;79:449–458. doi: 10.1007/s001090100232. [DOI] [PubMed] [Google Scholar]
- 215.Malin R. Laaksonen R. Knuuti J. Janatuinen T. Vesalainen R. Nuutila P. Lehtimaki T. Paraoxonase genotype modifies the effect of pravastatin on high-density lipoprotein cholesterol. Pharmacogenetics. 2001;11:625–633. doi: 10.1097/00008571-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 216.Marathe GK. Zimmerman GA. McIntyre TM. Platelet-activating factor acetylhydrolase, and not paraoxonase-1, is the oxidized phospholipid hydrolase of high density lipoprotein particles. J Biol Chem. 2003;278:3937–3947. doi: 10.1074/jbc.M211126200. [DOI] [PubMed] [Google Scholar]
- 217.Marchegiani F. Marra M. Spazzafumo L. James RW. Boemi M. Olivieri F. Cardelli M. Cavallone L. Bonfigli AR. Franceschi C. Paraoxonase activity and genotype predispose to successful aging. J Gerontol A Biol Sci Med Sci. 2006;61:541–546. doi: 10.1093/gerona/61.6.541. [DOI] [PubMed] [Google Scholar]
- 218.Marinou K. Antoniades C. Tousoulis D. Pitsavos C. Goumas G. Stefanadis C. Homocysteine: a risk factor for coronary artery disease? Hellenic J Cardiol. 2005;46:59–67. [PubMed] [Google Scholar]
- 219.Maritim AC. Sanders RA. Watkins JB., 3rd Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 220.Marsillach J. Ferre N. Vila MC. Lligona A. Mackness B. Mackness M. Deulofeu R. Sola R. Pares A. Pedro-Botet J. Joven J. Caballeria J. Camps J. Serum paraoxonase-1 in chronic alcoholics: relationship with liver disease. Clin Biochem. 2007;40:645–650. doi: 10.1016/j.clinbiochem.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 221.Marsillach J. Martinez-Vea A. Marcas L. Mackness B. Mackness M. Ferre N. Joven J. Camps J. Administration of exogenous erythropoietin beta affects lipid peroxidation and serum paraoxonase-1 activity and concentration in predialysis patients with chronic renal disease and anaemia. Clin Exp Pharmacol Physiol. 2007;34:347–349. doi: 10.1111/j.1440-1681.2007.04552.x. [DOI] [PubMed] [Google Scholar]
- 222.Martens JS. Lougheed M. Gomez-Munoz A. Steinbrecher UP. A modification of apolipoprotein B accounts for most of the induction of macrophage growth by oxidized low density lipoprotein. J Biol Chem. 1999;274:10903–10910. doi: 10.1074/jbc.274.16.10903. [DOI] [PubMed] [Google Scholar]
- 223.Martens JS. Reiner NE. Herrera-Velit P. Steinbrecher UP. Phosphatidylinositol 3-kinase is involved in the induction of macrophage growth by oxidized low density lipoprotein. J Biol Chem. 1998;273:4915–4920. doi: 10.1074/jbc.273.9.4915. [DOI] [PubMed] [Google Scholar]
- 224.Martinelli N. Girelli D. Olivieri O. Cavallari U. Biscuola M. Trabetti E. Friso S. Pizzolo F. Tenuti I. Bozzini C. Villa G. Ceradini B. Sandri M. Cheng S. Grow MA. Pignatti PF. Corrocher R. Interaction between metabolic syndrome and PON1 polymorphisms as a determinant of the risk of coronary artery disease. Clin Exp Med. 2005;5:20–30. doi: 10.1007/s10238-005-0060-9. [DOI] [PubMed] [Google Scholar]
- 225.Martinet W. Kockx MM. Apoptosis in atherosclerosis: focus on oxidized lipids and inflammation. Curr Opin Lipidol. 2001;12:535–541. doi: 10.1097/00041433-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 226.Maziere C. Djavaheri-Mergny M. Frey-Fressart V. Delattre J. Maziere JC. Copper and cell-oxidized low-density lipoprotein induces activator protein 1 in fibroblasts, endothelial and smooth muscle cells. FEBS Lett. 1997;409:351–356. doi: 10.1016/s0014-5793(97)00545-0. [DOI] [PubMed] [Google Scholar]
- 227.Mazur A. An enzyme in animal tissues capable of hydrolyze the phosphorus-fluorine bond of alkyl fluorophosphates. J Biol Chem. 1946;164:271–289. [PubMed] [Google Scholar]
- 228.McCully KS. Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am J Pathol. 1969;56:111–128. [PMC free article] [PubMed] [Google Scholar]
- 229.McDonald L. Bray C. Field C. Love F. Davies B. Homocystinuria, Thrombosis, and the Blood-Platelets. Lancet. 1964;1:745–746. doi: 10.1016/s0140-6736(64)92852-1. [DOI] [PubMed] [Google Scholar]
- 230.Mertens A. Holvoet P. Oxidized LDL and HDL: antagonists in atherothrombosis. Faseb J. 2001;15:2073–2084. doi: 10.1096/fj.01-0273rev. [DOI] [PubMed] [Google Scholar]
- 231.Meye C. Schumann J. Wagner A. Gross P. Effects of homocysteine on the levels of caveolin-1 and eNOS in caveolae of human coronary artery endothelial cells. Atherosclerosis. 2007;190:256–263. doi: 10.1016/j.atherosclerosis.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 232.Milochevitch C. Khalil A. Study of the paraoxonase and platelet-activating factor acetylhydrolase activities with aging. Prostaglandins Leukot Essent Fatty Acids. 2001;65:241–246. doi: 10.1054/plef.2001.0320. [DOI] [PubMed] [Google Scholar]
- 233.Miura D. Miura Y. Yagasaki K. Hypolipidemic action of dietary resveratrol, a phytoalexin in grapes and red wine, in hepatoma-bearing rats. Life Sci. 2003;73:1393–1400. doi: 10.1016/s0024-3205(03)00469-7. [DOI] [PubMed] [Google Scholar]
- 234.Mochizuki H. Scherer SW. Xi T. Nickle DC. Majer M. Huizenga JJ. Tsui LC. Prochazka M. Human PON2 gene at 7q21.3: cloning, multiple mRNA forms, and missense polymorphisms in the coding sequence. Gene. 1998;213:149–157. doi: 10.1016/s0378-1119(98)00193-0. [DOI] [PubMed] [Google Scholar]
- 235.Murata M. Maruyama T. Suzuki Y. Saruta T. Ikeda Y. Paraoxonase 1 Gln/Arg polymorphism is associated with the risk of microangiopathy in Type 2 diabetes mellitus. Diabet Med. 2004;21:837–844. doi: 10.1111/j.1464-5491.2004.01252.x. [DOI] [PubMed] [Google Scholar]
- 235a.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 236.Navab M. Ananthramaiah GM. Reddy ST. Van Lenten BJ. Ansell BJ. Fonarow GC. Vahabzadeh K. Hama S. Hough G. Kamranpour N. Berliner JA. Lusis AJ. Fogelman AM. The oxidation hypothesis of atherogenesis: the role of oxidized phospholipids and HDL. J Lipid Res. 2004;45:993–1007. doi: 10.1194/jlr.R400001-JLR200. [DOI] [PubMed] [Google Scholar]
- 237.Navab M. Berliner JA. Subbanagounder G. Hama S. Lusis AJ. Castellani LW. Reddy S. Shih D. Shi W. Watson AD. Van Lenten BJ. Vora D. Fogelman AM. HDL and the inflammatory response induced by LDL-derived oxidized phospholipids. Arterioscler Thromb Vasc Biol. 2001;21:481–488. doi: 10.1161/01.atv.21.4.481. [DOI] [PubMed] [Google Scholar]
- 238.Navab M. Hama-Levy S. Van Lenten BJ. Fonarow GC. Cardinez CJ. Castellani LW. Brennan ML. Lusis AJ. Fogelman AM. La Du BN. Mildly oxidized LDL induces an increased apolipoprotein J/paraoxonase ratio. J Clin Invest. 1997;99:2005–2019. doi: 10.1172/JCI119369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Negre-Salvayre A. Lopez M. Levade T. Pieraggi MT. Dousset N. Douste-Blazy L. Salvayre R. Ultraviolet-treated lipoproteins as a model system for the study of the biological effects of lipid peroxides on cultured cells. II. Uptake and cytotoxicity of ultraviolet-treated LDL on lymphoid cell lines. Biochim Biophys Acta. 1990;1045:224–232. doi: 10.1016/0005-2760(90)90124-g. [DOI] [PubMed] [Google Scholar]
- 240.Nevado JB., Jr. Imasa MS. Homocysteine predicts adverse clinical outcomes in unstable angina and non-ST elevation myocardial infarction: implications from the folate intervention in non-ST elevation myocardial infarction and unstable angina study. Coron Artery Dis. 2008;19:153–161. doi: 10.1097/MCA.0b013e3282f52910. [DOI] [PubMed] [Google Scholar]
- 241.Ng CJ. Bourquard N. Grijalva V. Hama S. Shih DM. Navab M. Fogelman AM. Lusis AJ. Young S. Reddy ST. Paraoxonase-2 deficiency aggravates atherosclerosis in mice despite lower apolipoprotein-B-containing lipoproteins: anti-atherogenic role for paraoxonase-2. J Biol Chem. 2006;281:29491–29500. doi: 10.1074/jbc.M605379200. [DOI] [PubMed] [Google Scholar]
- 242.Ng CJ. Bourquard N. Hama SY. Shih D. Grijalva VR. Navab M. Fogelman AM. Reddy ST. Adenovirus-mediated expression of human paraoxonase 3 protects against the progression of atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:1368–1374. doi: 10.1161/ATVBAHA.106.134189. [DOI] [PubMed] [Google Scholar]
- 243.Ng CJ. Hama SY. Bourquard N. Navab M. Reddy ST. Adenovirus mediated expression of human paraoxonase 2 protects against the development of atherosclerosis in apolipoprotein E-deficient mice. Mol Genet Metab. 2006;89:368–373. doi: 10.1016/j.ymgme.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 244.Ng CJ. Shih DM. Hama SY. Villa N. Navab M. Reddy ST. The paraoxonase gene family and atherosclerosis. Free Radic Biol Med. 2005;38:153–163. doi: 10.1016/j.freeradbiomed.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 245.Ng CJ. Wadleigh DJ. Gangopadhyay A. Hama S. Grijalva VR. Navab M. Fogelman AM. Reddy ST. Paraoxonase-2 is a ubiquitously expressed protein with antioxidant properties and is capable of preventing cell-mediated oxidative modification of low density lipoprotein. J Biol Chem. 2001;276:44444–44449. doi: 10.1074/jbc.M105660200. [DOI] [PubMed] [Google Scholar]
- 246.Nicholson AC. Hajjar DP. CD36, oxidized LDL and PPAR gamma: pathological interactions in macrophages and atherosclerosis. Vascul Pharmacol. 2004;41:139–146. doi: 10.1016/j.vph.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 247.Nishio E. Watanabe Y. Cigarette smoke extract inhibits plasma paraoxonase activity by modification of the enzyme's free thiols. Biochem Biophys Res Commun. 1997;236:289–293. doi: 10.1006/bbrc.1997.6961. [DOI] [PubMed] [Google Scholar]
- 248.Noll C. Hamelet J. Matulewicz E. Paul JL. Delabar JM. Janel N. Effects of red wine polyphenolic compounds on paraoxonase-1 and lectin-like oxidized low-density lipoprotein receptor-1 in hyperhomocysteinemic mice. J Nutr Biochem. 2009;20:586–596. doi: 10.1016/j.jnutbio.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 249.Noll C. Messaoudi S. Milliez P. Samuel JL. Delcayre C. Janel N. Eplerenone administration has beneficial effect on hepatic paraoxonase 1 activity in diabetic mice. Atherosclerosis. 2010;208:26–27. doi: 10.1016/j.atherosclerosis.2009.06.038. [DOI] [PubMed] [Google Scholar]
- 250.Oda MN. Bielicki JK. Ho TT. Berger T. Rubin EM. Forte TM. Paraoxonase 1 overexpression in mice and its effect on high-density lipoproteins. Biochem Biophys Res Commun. 2002;290:921–927. doi: 10.1006/bbrc.2001.6295. [DOI] [PubMed] [Google Scholar]
- 251.Okura Y. Brink M. Itabe H. Scheidegger KJ. Kalangos A. Delafontaine P. Oxidized low-density lipoprotein is associated with apoptosis of vascular smooth muscle cells in human atherosclerotic plaques. Circulation. 2000;102:2680–2686. doi: 10.1161/01.cir.102.22.2680. [DOI] [PubMed] [Google Scholar]
- 252.Osaki F. Ikeda Y. Suehiro T. Ota K. Tsuzura S. Arii K. Kumon Y. Hashimoto K. Roles of Sp1 and protein kinase C in regulation of human serum paraoxonase 1 (PON1) gene transcription in HepG2 cells. Atherosclerosis. 2004;176:279–287. doi: 10.1016/j.atherosclerosis.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 253.Osei-Hyiaman D. Hou L. Mengbai F. Zhiyin R. Zhiming Z. Kano K. Coronary artery disease risk in Chinese type 2 diabetics: is there a role for paraxonase 1 gene (Q192R) polymorphism? Eur J Endocrinol. 2001;144:639–644. doi: 10.1530/eje.0.1440639. [DOI] [PubMed] [Google Scholar]
- 254.Ozols J. Isolation and complete covalent structure of liver microsomal paraoxonase. Biochem J. 1999;338(Pt 2):265–272. [PMC free article] [PubMed] [Google Scholar]
- 255.Pan JP. Lai ST. Chiang SC. Chou SC. Chiang AN. The risk of coronary artery disease in population of Taiwan is associated with Cys-Ser 311 polymorphism of human paraoxonase (PON)-2 gene. Zhonghua Yi Xue Za Zhi (Taipei) 2002;65:415–421. [PubMed] [Google Scholar]
- 256.Panini SR. Sinensky MS. Mechanisms of oxysterol-induced apoptosis. Curr Opin Lipidol. 2001;12:529–533. doi: 10.1097/00041433-200110000-00008. [DOI] [PubMed] [Google Scholar]
- 257.Paoli P. Sbrana F. Tiribilli B. Caselli A. Pantera B. Cirri P. De Donatis A. Formigli L. Nosi D. Manao G. Camici G. Ramponi G. Protein N-homocysteinylation induces the formation of toxic amyloid-like protofibrils. J Mol Biol. 2010;400:889–907. doi: 10.1016/j.jmb.2010.05.039. [DOI] [PubMed] [Google Scholar]
- 258.Paolisso G. Tagliamonte MR. Rizzo MR. Giugliano D. Advancing age and insulin resistance: new facts about an ancient history. Eur J Clin Invest. 1999;29:758–769. doi: 10.1046/j.1365-2362.1999.00522.x. [DOI] [PubMed] [Google Scholar]
- 259.Paragh G. Seres I. Harangi M. Balogh Z. Illyes L. Boda J. Szilvassy Z. Kovacs P. The effect of micronised fenofibrate on paraoxonase activity in patients with coronary heart disease. Diabetes Metab. 2003;29:613–618. doi: 10.1016/s1262-3636(07)70077-0. [DOI] [PubMed] [Google Scholar]
- 260.Paragh G. Seres I. Harangi M. Erdei A. Audikovszky M. Debreczeni L. Kovacsay A. Illyes L. Pados G. Ciprofibrate increases paraoxonase activity in patients with metabolic syndrome. Br J Clin Pharmacol. 2006;61:694–701. doi: 10.1111/j.1365-2125.2006.02565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Paragh G. Torocsik D. Seres I. Harangi M. Illyes L. Balogh Z. Kovacs P. Effect of short term treatment with simvastatin and atorvastatin on lipids and paraoxonase activity in patients with hyperlipoproteinaemia. Curr Med Res Opin. 2004;20:1321–1327. doi: 10.1185/030079904125004394. [DOI] [PubMed] [Google Scholar]
- 262.Park SA. Choi MS. Jung UJ. Kim MJ. Kim DJ. Park HM. Park YB. Lee MK. Eucommia ulmoides Oliver leaf extract increases endogenous antioxidant activity in type 2 diabetic mice. J Med Food. 2006;9:474–479. doi: 10.1089/jmf.2006.9.474. [DOI] [PubMed] [Google Scholar]
- 263.Perla-Kajan J. Jakubowski H. Paraoxonase 1 protects against protein N-homocysteinylation in humans. Faseb J. 2010;24:931–936. doi: 10.1096/fj.09-144410. [DOI] [PubMed] [Google Scholar]
- 264.Perla-Kajan J. Stanger O. Luczak M. Ziolkowska A. Malendowicz LK. Twardowski T. Lhotak S. Austin RC. Jakubowski H. Immunohistochemical detection of N-homocysteinylated proteins in humans and mice. Biomed Pharmacother. 2008;62:473–479. doi: 10.1016/j.biopha.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 265.Pervaiz S. Resveratrol: from grapevines to mammalian biology. Faseb J. 2003;17:1975–1985. doi: 10.1096/fj.03-0168rev. [DOI] [PubMed] [Google Scholar]
- 266.Pinizzotto M. Castillo E. Fiaux M. Temler E. Gaillard RC. Ruiz J. Paraoxonase2 polymorphisms are associated with nephropathy in Type II diabetes. Diabetologia. 2001;44:104–107. doi: 10.1007/s001250051586. [DOI] [PubMed] [Google Scholar]
- 267.Precourt LP. Seidman E. Delvin E. Amre D. Deslandres C. Dominguez M. Sinnett D. Levy E. Comparative expression analysis reveals differences in the regulation of intestinal paraoxonase family members. Int J Biochem Cell Biol. 2009;41:1628–1637. doi: 10.1016/j.biocel.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 268.Primo-Parmo SL. Sorenson RC. Teiber J. La Du BN. The human serum paraoxonase/arylesterase gene (PON1) is one member of a multigene family. Genomics. 1996;33:498–507. doi: 10.1006/geno.1996.0225. [DOI] [PubMed] [Google Scholar]
- 269.Qin Q. Li YL. Zhao FM. Wang H. Li Y. Cui RZ. Zhao BR. [Association of paraoxonase polymorphisms and serum homocysteine thiolactone complex with coronary heart disease] Zhonghua Xin Xue Guan Bing Za Zhi. 2006;34:803–807. [PubMed] [Google Scholar]
- 270.Rainwater DL. Rutherford S. Dyer TD. Rainwater ED. Cole SA. Vandeberg JL. Almasy L. Blangero J. Maccluer JW. Mahaney MC. Determinants of variation in human serum paraoxonase activity. Heredity. 2009;102:147–154. doi: 10.1038/hdy.2008.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Rajavashisth TB. Andalibi A. Territo MC. Berliner JA. Navab M. Fogelman AM. Lusis AJ. Induction of endothelial cell expression of granulocyte and macrophage colony-stimulating factors by modified low-density lipoproteins. Nature. 1990;344:254–257. doi: 10.1038/344254a0. [DOI] [PubMed] [Google Scholar]
- 272.Ramos P. Gieseg SP. Schuster B. Esterbauer H. Effect of temperature and phase transition on oxidation resistance of low density lipoprotein. J Lipid Res. 1995;36:2113–2128. [PubMed] [Google Scholar]
- 273.Rangaswamy S. Penn MS. Saidel GM. Chisolm GM. Exogenous oxidized low-density lipoprotein injures and alters the barrier function of endothelium in rats in vivo. Circ Res. 1997;80:37–44. doi: 10.1161/01.res.80.1.37. [DOI] [PubMed] [Google Scholar]
- 274.Rao MN. Marmillot P. Gong M. Palmer DA. Seeff LB. Strader DB. Lakshman MR. Light, but not heavy alcohol drinking, stimulates paraoxonase by upregulating liver mRNA in rats and humans. Metabolism. 2003;52:1287–1294. doi: 10.1016/s0026-0495(03)00191-4. [DOI] [PubMed] [Google Scholar]
- 275.Rea IM. McKeown PP. McMaster D. Young IS. Patterson C. Savage MJ. Belton C. Marchegiani F. Olivieri F. Bonafe M. Franceschi C. Paraoxonase polymorphisms PON1 192 and 55 and longevity in Italian centenarians and Irish nonagenarians. A pooled analysis. Exp Gerontol. 2004;39:629–635. doi: 10.1016/j.exger.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 276.Reddy ST. Devarajan A. Bourquard N. Shih D. Fogelman AM. Is it just paraoxonase 1 or are other members of the paraoxonase gene family implicated in atherosclerosis? Curr Opin Lipidol. 2008;19:405–408. doi: 10.1097/MOL.0b013e328304b64e. [DOI] [PubMed] [Google Scholar]
- 277.Reddy ST. Wadleigh DJ. Grijalva V. Ng C. Hama S. Gangopadhyay A. Shih DM. Lusis AJ. Navab M. Fogelman AM. Human paraoxonase-3 is an HDL-associated enzyme with biological activity similar to paraoxonase-1 protein but is not regulated by oxidized lipids. Arterioscler Thromb Vasc Biol. 2001;21:542–547. doi: 10.1161/01.atv.21.4.542. [DOI] [PubMed] [Google Scholar]
- 278.Refsum H. Smith AD. Ueland PM. Nexo E. Clarke R. McPartlin J. Johnston C. Engbaek F. Schneede J. McPartlin C. Scott JM. Facts and recommendations about total homocysteine determinations: an expert opinion. Clin Chem. 2004;50:3–32. doi: 10.1373/clinchem.2003.021634. [DOI] [PubMed] [Google Scholar]
- 279.Rensen SS. Doevendans PA. van Eys GJ. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth Heart J. 2007;15:100–108. doi: 10.1007/BF03085963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Rizos E. Tambaki AP. Gazi I. Tselepis AD. Elisaf M. Lipoprotein-associated PAF-acetylhydrolase activity in subjects with the metabolic syndrome. Prostaglandins Leukot Essent Fatty Acids. 2005;72:203–209. doi: 10.1016/j.plefa.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 281.Rock W. Rosenblat M. Miller-Lotan R. Levy AP. Elias M. Aviram M. Consumption of wonderful variety pomegranate juice and extract by diabetic patients increases paraoxonase 1 association with high-density lipoprotein and stimulates its catalytic activities. J Agric Food Chem. 2008;56:8704–8713. doi: 10.1021/jf801756x. [DOI] [PubMed] [Google Scholar]
- 282.Rodrigo L. Gil F. Hernandez AF. Lopez O. Pla A. Identification of paraoxonase 3 in rat liver microsomes: purification and biochemical properties. Biochem J. 2003;376:261–268. doi: 10.1042/BJ20030732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Rodrigo L. Hernandez AF. Lopez-Caballero JJ. Gil F. Pla A. Immunohistochemical evidence for the expression and induction of paraoxonase in rat liver, kidney, lung and brain tissue. Implications for its physiological role. Chem Biol Interact. 2001;137:123–137. doi: 10.1016/s0009-2797(01)00225-3. [DOI] [PubMed] [Google Scholar]
- 284.Rosenblat M. Draganov D. Watson CE. Bisgaier CL. La Du BN. Aviram M. Mouse macrophage paraoxonase 2 activity is increased whereas cellular paraoxonase 3 activity is decreased under oxidative stress. Arterioscler Thromb Vasc Biol. 2003;23:468–474. doi: 10.1161/01.ATV.0000059385.95664.4D. [DOI] [PubMed] [Google Scholar]
- 285.Rosenblat M. Hayek T. Hussein K. Aviram M. Decreased macrophage paraoxonase 2 expression in patients with hypercholesterolemia is the result of their increased cellular cholesterol content: effect of atorvastatin therapy. Arterioscler Thromb Vasc Biol. 2004;24:175–180. doi: 10.1161/01.ATV.0000104011.88939.06. [DOI] [PubMed] [Google Scholar]
- 286.Rozenberg O. Shih DM. Aviram M. Human serum paraoxonase 1 decreases macrophage cholesterol biosynthesis: possible role for its phospholipase-A2-like activity and lysophosphatidylcholine formation. Arterioscler Thromb Vasc Biol. 2003;23:461–467. doi: 10.1161/01.ATV.0000060462.35946.B3. [DOI] [PubMed] [Google Scholar]
- 287.Rozenberg O. Shiner M. Aviram M. Hayek T. Paraoxonase 1 (PON1) attenuates diabetes development in mice through its antioxidative properties. Free Radic Biol Med. 2008;44:1951–1959. doi: 10.1016/j.freeradbiomed.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 288.Sacks FM. Tonkin AM. Craven T. Pfeffer MA. Shepherd J. Keech A. Furberg CD. Braunwald E. Coronary heart disease in patients with low LDL-cholesterol: benefit of pravastatin in diabetics and enhanced role for HDL-cholesterol and triglycerides as risk factors. Circulation. 2002;105:1424–1428. doi: 10.1161/01.cir.0000012918.84068.43. [DOI] [PubMed] [Google Scholar]
- 289.Sakai M. Miyazaki A. Hakamata H. Kodama T. Suzuki H. Kobori S. Shichiri M. Horiuchi S. The scavenger receptor serves as a route for internalization of lysophosphatidylcholine in oxidized low density lipoprotein-induced macrophage proliferation. J Biol Chem. 1996;271:27346–27352. doi: 10.1074/jbc.271.44.27346. [DOI] [PubMed] [Google Scholar]
- 290.Sander CS. Chang H. Hamm F. Elsner P. Thiele JJ. Role of oxidative stress and the antioxidant network in cutaneous carcinogenesis. Int J Dermatol. 2004;43:326–335. doi: 10.1111/j.1365-4632.2004.02222.x. [DOI] [PubMed] [Google Scholar]
- 291.Sangvanich P. Mackness B. Gaskell SJ. Durrington P. Mackness M. The effect of high-density lipoproteins on the formation of lipid/protein conjugates during in vitro oxidation of low-density lipoprotein. Biochem Biophys Res Commun. 2003;300:501–506. doi: 10.1016/s0006-291x(02)02849-8. [DOI] [PubMed] [Google Scholar]
- 292.Santanam N. Parthasarathy S. Aspirin is a substrate for paraoxonase-like activity: implications in atherosclerosis. Atherosclerosis. 2007;191:272–275. doi: 10.1016/j.atherosclerosis.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 293.Sarandol E. Serdar Z. Dirican M. Safak O. Effects of red wine consumption on serum paraoxonase/arylesterase activities and on lipoprotein oxidizability in healthy-men. J Nutr Biochem. 2003;14:507–512. doi: 10.1016/s0955-2863(03)00099-8. [DOI] [PubMed] [Google Scholar]
- 294.Sarandol E. Tas S. Dirican M. Serdar Z. Oxidative stress and serum paraoxonase activity in experimental hypothyroidism: effect of vitamin E supplementation. Cell Biochem Funct. 2005;23:1–8. doi: 10.1002/cbf.1119. [DOI] [PubMed] [Google Scholar]
- 295.Satoh T. Taylor P. Bosron WF. Sanghani SP. Hosokawa M. La Du BN. Current progress on esterases: from molecular structure to function. Drug Metab Dispos. 2002;30:488–493. doi: 10.1124/dmd.30.5.488. [DOI] [PubMed] [Google Scholar]
- 296.Schrader C. Schiborr C. Frank J. Rimbach G. Curcumin induces paraoxonase 1 in cultured hepatocytes in vitro but not in mouse liver in vivo. Br J Nutr. 2011;105:167–170. doi: 10.1017/S0007114510004356. [DOI] [PubMed] [Google Scholar]
- 297.Senokuchi T. Matsumura T. Sakai M. Matsuo T. Yano M. Kiritoshi S. Sonoda K. Kukidome D. Nishikawa T. Araki E. Extracellular signal-regulated kinase and p38 mitogen-activated protein kinase mediate macrophage proliferation induced by oxidized low-density lipoprotein. Atherosclerosis. 2004;176:233–245. doi: 10.1016/j.atherosclerosis.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 298.Senti M. Tomas M. Fito M. Weinbrenner T. Covas MI. Sala J. Masia R. Marrugat J. Antioxidant paraoxonase 1 activity in the metabolic syndrome. J Clin Endocrinol Metab. 2003;88:5422–5426. doi: 10.1210/jc.2003-030648. [DOI] [PubMed] [Google Scholar]
- 299.Seres I. Paragh G. Deschene E. Fulop T., Jr. Khalil A. Study of factors influencing the decreased HDL associated PON1 activity with aging. Exp Gerontol. 2004;39:59–66. doi: 10.1016/j.exger.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 300.Shaposhnik Z. Wang X. Trias J. Fraser H. Lusis AJ. The synergistic inhibition of atherogenesis in apoE-/- mice between pravastatin and the sPLA2 inhibitor varespladib (A-002) J Lipid Res. 2009;50:623–629. doi: 10.1194/jlr.M800361-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.She ZG. Zheng W. Wei YS. Chen HZ. Wang AB. Li HL. Liu G. Zhang R. Liu JJ. Stallcup WB. Zhou Z. Liu DP. Liang CC. Human paraoxonase gene cluster transgenic overexpression represses atherogenesis and promotes atherosclerotic plaque stability in ApoE-null mice. Circ Res. 2009;104:1160–1168. doi: 10.1161/CIRCRESAHA.108.192229. [DOI] [PubMed] [Google Scholar]
- 302.Shih DM. Gu L. Hama S. Xia YR. Navab M. Fogelman AM. Lusis AJ. Genetic-dietary regulation of serum paraoxonase expression and its role in atherogenesis in a mouse model. J Clin Invest. 1996;97:1630–1639. doi: 10.1172/JCI118589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Shih DM. Gu L. Xia YR. Navab M. Li WF. Hama S. Castellani LW. Furlong CE. Costa LG. Fogelman AM. Lusis AJ. Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature. 1998;394:284–287. doi: 10.1038/28406. [DOI] [PubMed] [Google Scholar]
- 304.Shih DM. Kast-Woelbern HR. Wong J. Xia YR. Edwards PA. Lusis AJ. A role for FXR and human FGF-19 in the repression of paraoxonase-1 gene expression by bile acids. J Lipid Res. 2006;47:384–392. doi: 10.1194/jlr.M500378-JLR200. [DOI] [PubMed] [Google Scholar]
- 305.Shih DM. Xia YR. Wang XP. Miller E. Castellani LW. Subbanagounder G. Cheroutre H. Faull KF. Berliner JA. Witztum JL. Lusis AJ. Combined serum paraoxonase knockout/apolipoprotein E knockout mice exhibit increased lipoprotein oxidation and atherosclerosis. J Biol Chem. 2000;275:17527–17535. doi: 10.1074/jbc.M910376199. [DOI] [PubMed] [Google Scholar]
- 306.Shih DM. Xia YR. Wang XP. Wang SS. Bourquard N. Fogelman AM. Lusis AJ. Reddy ST. Decreased obesity and atherosclerosis in human paraoxonase 3 transgenic mice. Circ Res. 2007;100:1200–1207. doi: 10.1161/01.RES.0000264499.48737.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Shin BS. Oh SY. Kim YS. Kim KW. The paraoxonase gene polymorphism in stroke patients and lipid profile. Acta Neurol Scand. 2008;117:237–243. doi: 10.1111/j.1600-0404.2007.00929.x. [DOI] [PubMed] [Google Scholar]
- 308.Shiner M. Fuhrman B. Aviram M. Paraoxonase 2 (PON2) expression is upregulated via a reduced-nicotinamide-adenine-dinucleotide-phosphate (NADPH)-oxidase-dependent mechanism during monocytes differentiation into macrophages. Free Radic Biol Med. 2004;37:2052–2063. doi: 10.1016/j.freeradbiomed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 309.Shiner M. Fuhrman B. Aviram M. A biphasic U-shape effect of cellular oxidative stress on the macrophage anti-oxidant paraoxonase 2 (PON2) enzymatic activity. Biochem Biophys Res Commun. 2006;349:1094–1099. doi: 10.1016/j.bbrc.2006.08.150. [DOI] [PubMed] [Google Scholar]
- 310.Shiner M. Fuhrman B. Aviram M. Macrophage paraoxonase 2 (PON2) expression is up-regulated by pomegranate juice phenolic anti-oxidants via PPAR gamma and AP-1 pathway activation. Atherosclerosis. 2007;195:313–321. doi: 10.1016/j.atherosclerosis.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 311.Shiner M. Fuhrman B. Aviram M. Macrophage paraoxonase 2 (PON2) expression is upregulated by unesterified cholesterol through activation of the phosphatidylinositol 3-kinase (PI3K) pathway. Biol Chem. 2007;388:1353–1358. doi: 10.1515/BC.2007.145. [DOI] [PubMed] [Google Scholar]
- 312.Sierksma A. van der Gaag MS. van Tol A. James RW. Hendriks HF. Kinetics of HDL cholesterol and paraoxonase activity in moderate alcohol consumers. Alcohol Clin Exp Res. 2002;26:1430–1435. doi: 10.1097/01.ALC.0000030639.57507.60. [DOI] [PubMed] [Google Scholar]
- 313.Sinan S. Kockar F. Arslan O. Novel purification strategy for human PON1 and inhibition of the activity by cephalosporin and aminoglikozide derived antibiotics. Biochimie. 2006;88:565–574. doi: 10.1016/j.biochi.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 314.Sinan S. Kockar F. Gencer N. Yildirim H. Arslan O. Effects of some antibiotics on paraoxonase from human serum in vitro and from mouse serum and liver in vivo. Biol Pharm Bull. 2006;29:1559–1563. doi: 10.1248/bpb.29.1559. [DOI] [PubMed] [Google Scholar]
- 315.Sinan S. Kockar F. Gencer N. Yildirim H. Arslan O. Amphenicol and macrolide derived antibiotics inhibit paraoxonase enzyme activity in human serum and human hepatoma cells (HepG2) in vitro. Biochemistry (Mosc) 2006;71:46–50. doi: 10.1134/s0006297906010068. [DOI] [PubMed] [Google Scholar]
- 316.Sorenson RC. Aviram M. Bisgaier CL. Billecke S. Hsu C. La Du BN. Properties of the retained N-terminal hydrophobic leader sequence in human serum paraoxonase/arylesterase. Chem Biol Interact. 1999:119–120. doi: 10.1016/s0009-2797(99)00033-2. 243–249. [DOI] [PubMed] [Google Scholar]
- 317.Sorenson RC. Bisgaier CL. Aviram M. Hsu C. Billecke S. La Du BN. Human serum Paraoxonase/Arylesterase's retained hydrophobic N-terminal leader sequence associates with HDLs by binding phospholipids: apolipoprotein A-I stabilizes activity. Arterioscler Thromb Vasc Biol. 1999;19:2214–2225. doi: 10.1161/01.atv.19.9.2214. [DOI] [PubMed] [Google Scholar]
- 318.Sorenson RC. Primo-Parmo SL. Kuo CL. Adkins S. Lockridge O. La Du BN. Reconsideration of the catalytic center and mechanism of mammalian paraoxonase/arylesterase. Proc Natl Acad Sci U S A. 1995;92:7187–7191. doi: 10.1073/pnas.92.16.7187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Sparrow CP. Parthasarathy S. Steinberg D. A macrophage receptor that recognizes oxidized low density lipoprotein but not acetylated low density lipoprotein. J Biol Chem. 1989;264:2599–2604. [PubMed] [Google Scholar]
- 320.Steinberg D. Low density lipoprotein oxidation and its pathobiological significance. J Biol Chem. 1997;272:20963–20966. doi: 10.1074/jbc.272.34.20963. [DOI] [PubMed] [Google Scholar]
- 321.Stevens RC. Suzuki SM. Cole TB. Park SS. Richter RJ. Furlong CE. Engineered recombinant human paraoxonase 1 (rHuPON1) purified from Escherichia coli protects against organophosphate poisoning. Proc Natl Acad Sci U S A. 2008;105:12780–12784. doi: 10.1073/pnas.0805865105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Stubbs PJ. Al-Obaidi MK. Conroy RM. Collinson PO. Graham IM. Noble IM. Effect of plasma homocysteine concentration on early and late events in patients with acute coronary syndromes. Circulation. 2000;102:605–610. doi: 10.1161/01.cir.102.6.605. [DOI] [PubMed] [Google Scholar]
- 323.Suda O. Tsutsui M. Morishita T. Tasaki H. Ueno S. Nakata S. Tsujimoto T. Toyohira Y. Hayashida Y. Sasaguri Y. Ueta Y. Nakashima Y. Yanagihara N. Asymmetric dimethylarginine produces vascular lesions in endothelial nitric oxide synthase-deficient mice: involvement of renin-angiotensin system and oxidative stress. Arterioscler Thromb Vasc Biol. 2004;24:1682–1688. doi: 10.1161/01.ATV.0000136656.26019.6e. [DOI] [PubMed] [Google Scholar]
- 324.Suehiro T. Nakamura T. Inoue M. Shiinoki T. Ikeda Y. Kumon Y. Shindo M. Tanaka H. Hashimoto K. A polymorphism upstream from the human paraoxonase (PON1) gene and its association with PON1 expression. Atherosclerosis. 2000;150:295–298. doi: 10.1016/s0021-9150(99)00379-2. [DOI] [PubMed] [Google Scholar]
- 325.Suhara T. Fukuo K. Yasuda O. Tsubakimoto M. Takemura Y. Kawamoto H. Yokoi T. Mogi M. Kaimoto T. Ogihara T. Homocysteine enhances endothelial apoptosis via upregulation of Fas-mediated pathways. Hypertension. 2004;43:1208–1213. doi: 10.1161/01.HYP.0000127914.94292.76. [DOI] [PubMed] [Google Scholar]
- 326.Sutherland WH. Manning PJ. de Jong SA. Allum AR. Jones SD. Williams SM. Hormone-replacement therapy increases serum paraoxonase arylesterase activity in diabetic postmenopausal women. Metabolism. 2001;50:319–324. doi: 10.1053/meta.2001.20201. [DOI] [PubMed] [Google Scholar]
- 327.Tabur S. Torun AN. Sabuncu T. Turan MN. Celik H. Ocak AR. Aksoy N. Non-diabetic metabolic syndrome and obesity do not affect serum paraoxonase and arylesterase activities but do affect oxidative stress and inflammation. Eur J Endocrinol. 2010;162:535–541. doi: 10.1530/EJE-09-0732. [DOI] [PubMed] [Google Scholar]
- 328.Teiber JF. Draganov DI. La Du BN. Lactonase and lactonizing activities of human serum paraoxonase (PON1) and rabbit serum PON3. Biochem Pharmacol. 2003;66:887–896. doi: 10.1016/s0006-2952(03)00401-5. [DOI] [PubMed] [Google Scholar]
- 329.Teiber JF. Draganov DI. La Du BN. Purified human serum PON1 does not protect LDL against oxidation in the in vitro assays initiated with copper or AAPH. J Lipid Res. 2004;45:2260–2268. doi: 10.1194/jlr.M400213-JLR200. [DOI] [PubMed] [Google Scholar]
- 330.Teiber JF. Horke S. Haines DC. Chowdhary PK. Xiao J. Kramer GL. Haley RW. Draganov DI. Dominant role of paraoxonases in inactivation of the Pseudomonas aeruginosa quorum-sensing signal N-(3-oxododecanoyl)-L-homoserine lactone. Infect Immun. 2008;76:2512–2519. doi: 10.1128/IAI.01606-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Thomas-Moya E. Nadal-Casellas A. Gianotti M. Llado I. Proenza AM. Time-dependent modulation of rat serum paraoxonase 1 activity by fasting. Pflugers Arch. 2007;453:831–837. doi: 10.1007/s00424-006-0174-2. [DOI] [PubMed] [Google Scholar]
- 332.Tomas M. Senti M. Elosua R. Vila J. Sala J. Masia R. Marrugat J. Interaction between the Gln-Arg 192 variants of the paraoxonase gene and oleic acid intake as a determinant of high-density lipoprotein cholesterol and paraoxonase activity. Eur J Pharmacol. 2001;432:121–128. doi: 10.1016/s0014-2999(01)01482-0. [DOI] [PubMed] [Google Scholar]
- 333.Tougou K. Nakamura A. Watanabe S. Okuyama Y. Morino A. Paraoxonase has a major role in the hydrolysis of prulifloxacin (NM441), a prodrug of a new antibacterial agent. Drug Metab Dispos. 1998;26:355–359. [PubMed] [Google Scholar]
- 334.Tsai JC. Perrella MA. Yoshizumi M. Hsieh CM. Haber E. Schlegel R. Lee ME. Promotion of vascular smooth muscle cell growth by homocysteine: a link to atherosclerosis. Proc Natl Acad Sci U S A. 1994;91:6369–6373. doi: 10.1073/pnas.91.14.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Tsakiris S. Karikas GA. Parthimos T. Tsakiris T. Bakogiannis C. Schulpis KH. Alpha-tocopherol supplementation prevents the exercise-induced reduction of serum paraoxonase 1/arylesterase activities in healthy individuals. Eur J Clin Nutr. 2009;63:215–221. doi: 10.1038/sj.ejcn.1602918. [DOI] [PubMed] [Google Scholar]
- 336.Tsimihodimos V. Karabina SA. Tambaki AP. Bairaktari E. Goudevenos JA. Chapman MJ. Elisaf M. Tselepis AD. Atorvastatin preferentially reduces LDL-associated platelet-activating factor acetylhydrolase activity in dyslipidemias of type IIA and type IIB. Arterioscler Thromb Vasc Biol. 2002;22:306–311. doi: 10.1161/hq0202.102918. [DOI] [PubMed] [Google Scholar]
- 337.Turfaner N. Uzun H. Balci H. Ercan MA. Karter YH. Caner M. Sipahioglu F. Genc H. Ezetimibe therapy and its influence on oxidative stress and fibrinolytic activity. South Med J. 2010;103:428–433. doi: 10.1097/SMJ.0b013e3181d83374. [DOI] [PubMed] [Google Scholar]
- 338.Tward A. Xia YR. Wang XP. Shi YS. Park C. Castellani LW. Lusis AJ. Shih DM. Decreased atherosclerotic lesion formation in human serum paraoxonase transgenic mice. Circulation. 2002;106:484–490. doi: 10.1161/01.cir.0000023623.87083.4f. [DOI] [PubMed] [Google Scholar]
- 339.Undas A. Brozek J. Jankowski M. Siudak Z. Szczeklik A. Jakubowski H. Plasma homocysteine affects fibrin clot permeability and resistance to lysis in human subjects. Arterioscler Thromb Vasc Biol. 2006;26:1397–1404. doi: 10.1161/01.ATV.0000219688.43572.75. [DOI] [PubMed] [Google Scholar]
- 340.Ungvari Z. Csiszar A. Edwards JG. Kaminski PM. Wolin MS. Kaley G. Koller A. Increased superoxide production in coronary arteries in hyperhomocysteinemia: role of tumor necrosis factor-alpha, NAD(P)H oxidase, and inducible nitric oxide synthase. Arterioscler Thromb Vasc Biol. 2003;23:418–424. doi: 10.1161/01.ATV.0000061735.85377.40. [DOI] [PubMed] [Google Scholar]
- 341.van den Berg SW. Jansen EH. Kruijshoop M. Beekhof PK. Blaak E. van der Kallen CJ. van Greevenbroek MM. Feskens EJ. Paraoxonase 1 phenotype distribution and activity differs in subjects with newly diagnosed Type 2 diabetes (the CODAM Study) Diabet Med. 2008;25:186–193. doi: 10.1111/j.1464-5491.2007.02328.x. [DOI] [PubMed] [Google Scholar]
- 342.van der Gaag MS. van Tol A. Scheek LM. James RW. Urgert R. Schaafsma G. Hendriks HF. Daily moderate alcohol consumption increases serum paraoxonase activity; a diet-controlled, randomised intervention study in middle-aged men. Atherosclerosis. 1999;147:405–410. doi: 10.1016/s0021-9150(99)00243-9. [DOI] [PubMed] [Google Scholar]
- 343.Van Lenten BJ. Navab M. Shih D. Fogelman AM. Lusis AJ. The role of high-density lipoproteins in oxidation and inflammation. Trends Cardiovasc Med. 2001;11:155–161. doi: 10.1016/s1050-1738(01)00095-0. [DOI] [PubMed] [Google Scholar]
- 344.Van Lenten BJ. Wagner AC. Navab M. Fogelman AM. Oxidized phospholipids induce changes in hepatic paraoxonase and ApoJ but not monocyte chemoattractant protein-1 via interleukin-6. J Biol Chem. 2001;276:1923–1929. doi: 10.1074/jbc.M004074200. [DOI] [PubMed] [Google Scholar]
- 345.van Wijk J. Coll B. Cabezas MC. Koning E. Camps J. Mackness B. Joven J. Rosiglitazone modulates fasting and post-prandial paraoxonase 1 activity in type 2 diabetic patients. Clin Exp Pharmacol Physiol. 2006;33:1134–1137. doi: 10.1111/j.1440-1681.2006.04505.x. [DOI] [PubMed] [Google Scholar]
- 346.Varatharajalu R. Garige M. Leckey LC. Gong M. Lakshman MR. Betaine protects chronic alcohol and omega-3 PUFA-mediated down-regulations of PON1 gene, serum PON1 and homocysteine thiolactonase activities with restoration of liver GSH. Alcohol Clin Exp Res. 2010;34:424–431. doi: 10.1111/j.1530-0277.2009.01107.x. [DOI] [PubMed] [Google Scholar]
- 347.Vizzardi E. Nodari S. Fiorina C. Metra M. Dei Cas L. Plasma homocysteine levels and late outcome in patients with unstable angina. Cardiology. 2007;107:354–359. doi: 10.1159/000099050. [DOI] [PubMed] [Google Scholar]
- 348.Vohl MC. Neville TA. Kumarathasan R. Braschi S. Sparks DL. A novel lecithin-cholesterol acyltransferase antioxidant activity prevents the formation of oxidized lipids during lipoprotein oxidation. Biochemistry. 1999;38:5976–5981. doi: 10.1021/bi982258w. [DOI] [PubMed] [Google Scholar]
- 349.Vos E. Homocysteine levels, paraoxonase 1 (PON1) activity, and cardiovascular risk. JAMA. 2008;300:168–169. doi: 10.1001/jama.300.2.168. author reply 169. [DOI] [PubMed] [Google Scholar]
- 350.Wallace AJ. Sutherland WH. Mann JI. Williams SM. The effect of meals rich in thermally stressed olive and safflower oils on postprandial serum paraoxonase activity in patients with diabetes. Eur J Clin Nutr. 2001;55:951–958. doi: 10.1038/sj.ejcn.1601250. [DOI] [PubMed] [Google Scholar]
- 351.Watson AD. Berliner JA. Hama SY. La Du BN. Faull KF. Fogelman AM. Navab M. Protective effect of high density lipoprotein associated paraoxonase. Inhibition of the biological activity of minimally oxidized low density lipoprotein. J Clin Invest. 1995;96:2882–2891. doi: 10.1172/JCI118359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Watson CE. Draganov DI. Billecke SS. Bisgaier CL. La Du BN. Rabbits possess a serum paraoxonase polymorphism similar to the human Q192R. Pharmacogenetics. 2001;11:123–134. doi: 10.1097/00008571-200103000-00003. [DOI] [PubMed] [Google Scholar]
- 353.Watzinger N. Schmidt H. Schumacher M. Schmidt R. Eber B. Fruhwald FM. Zweiker R. Kostner GM. Klein W. Human paraoxonase 1 gene polymorphisms and the risk of coronary heart disease: a community-based study. Cardiology. 2002;98:116–122. doi: 10.1159/000066321. [DOI] [PubMed] [Google Scholar]
- 354.Weiss N. Heydrick SJ. Postea O. Keller C. Keaney JF., Jr. Loscalzo J. Influence of hyperhomocysteinemia on the cellular redox state—impact on homocysteine-induced endothelial dysfunction. Clin Chem Lab Med. 2003;41:1455–1461. doi: 10.1515/CCLM.2003.223. [DOI] [PubMed] [Google Scholar]
- 355.Willcox MD. Zhu H. Conibear TC. Hume EB. Givskov M. Kjelleberg S. Rice SA. Role of quorum sensing by Pseudomonas aeruginosa in microbial keratitis and cystic fibrosis. Microbiology. 2008;154:2184–2194. doi: 10.1099/mic.0.2008/019281-0. [DOI] [PubMed] [Google Scholar]
- 356.Wojcicka G. Jamroz-Wisniewska A. Marciniak A. Lowicka E. Beltowski J. The differentiating effect of glimepiride and glibenclamide on paraoxonase 1 and platelet-activating factor acetylohydrolase activity. Life Sci. 2010;87:126–132. doi: 10.1016/j.lfs.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 357.Xia Y. Gueguen R. Vincent-Viry M. Siest G. Visvikis S. Effect of six candidate genes on early aging in a French population. Aging Clin Exp Res. 2003;15:111–116. doi: 10.1007/BF03324487. [DOI] [PubMed] [Google Scholar]
- 358.Yamada M. Sodeyama N. Itoh Y. Otomo E. Matsushita M. Mizusawa H. No association of paraoxonase genotype or atherosclerosis with cerebral amyloid angiopathy. Stroke. 2002;33:896–900. doi: 10.1161/01.str.0000013673.70986.ab. [DOI] [PubMed] [Google Scholar]
- 359.Yang X. Gao Y. Zhou J. Zhen Y. Yang Y. Wang J. Song L. Liu Y. Xu H. Chen Z. Hui R. Plasma homocysteine thiolactone adducts associated with risk of coronary heart disease. Clin Chim Acta. 2006;364:230–234. doi: 10.1016/j.cccn.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 360.Yesilbursa D. Serdar A. Saltan Y. Serdar Z. Heper Y. Guclu S. Cordan J. The effect of fenofibrate on serum paraoxonase activity and inflammatory markers in patients with combined hyperlipidemia. Kardiol Pol. 2005;62:526–530. [PubMed] [Google Scholar]
- 361.Yoshida H. Quehenberger O. Kondratenko N. Green S. Steinberg D. Minimally oxidized low-density lipoprotein increases expression of scavenger receptor A, CD36, and macrosialin in resident mouse peritoneal macrophages. Arterioscler Thromb Vasc Biol. 1998;18:794–802. doi: 10.1161/01.atv.18.5.794. [DOI] [PubMed] [Google Scholar]
- 362.Zettler ME. Prociuk MA. Austria JA. Zhong G. Pierce GN. Oxidized low-density lipoprotein retards the growth of proliferating cells by inhibiting nuclear translocation of cell cycle proteins. Arterioscler Thromb Vasc Biol. 2004;24:727–732. doi: 10.1161/01.ATV.0000120373.95552.aa. [DOI] [PubMed] [Google Scholar]
- 363.Zhang JG. Wang LZ. Han XQ. Jiang YD. Zhang RM. Wang SR. [The pathogenic mechanism of homocysteine -induced endothelial nitric oxide synthase dysfunction and the antagonistic effects by folic acid] Fen Zi Xi Bao Sheng Wu Xue Bao. 2007;40:17–23. [PubMed] [Google Scholar]
- 364.Zhao B. Ehringer WD. Dierichs R. Miller FN. Oxidized low-density lipoprotein increases endothelial intracellular calcium and alters cytoskeletal f-actin distribution. Eur J Clin Invest. 1997;27:48–54. doi: 10.1046/j.1365-2362.1997.750628.x. [DOI] [PubMed] [Google Scholar]
- 365.Zhao GF. Seng JJ. Zhang H. She MP. Effects of oxidized low density lipoprotein on the growth of human artery smooth muscle cells. Chin Med J (Engl) 2005;118:1973–1978. [PubMed] [Google Scholar]