Abstract
We studied adaptations to acute precipitated opioid withdrawal of spinal μ-opioid receptor (MOR)-coupled regulation of the release of endomorphin 2 (EM2). The release of this highly MOR-selective endogenous opioid from opioid-naive spinal tissue of male rats is subjected to MOR-coupled positive as well as negative modulation via cholera toxin-sensitive Gs and pertussis toxin-sensitive Gi/Go, respectively. The net effect of this concomitant bidirectional modulation is inhibitory. MOR-coupled pleiotropic regulation of EM2 release is retained in opioid-withdrawn spinal tissue of male rats, but the balance of MOR-coupled inhibitory and facilitatory regulation shifted such that facilitatory regulation predominates. Augmented coupling of MOR to Gs is causally associated with this change. Strikingly, pleiotropic characteristics of MOR-coupled regulation of spinal EM2 release and adaptations thereof to opioid withdrawal are male-specific. In females, MOR-coupled regulation of EM2 release from opioid-naive and -withdrawn spinal tissue does not have a significant Gs-coupled facilitatory component, and MOR-coupled inhibition of EM2 release persists unabated in withdrawn preparations. The male-specific adaptations to chronic morphine that shift the relative predominance of opposing dual G protein-coupled MOR pathways provides a mechanism for mitigating inhibitory MOR signaling without losing MOR-coupled feedback regulation. These adaptations enable using endogenous EM2 as a substitute for morphine that had been precipitously removed. The sexually dimorphic functionality and regulation of spinal EM2/MOR-coupled signaling suggest the clinical utility of using sex-specific treatments for addiction that harness the activity of endogenous opioids.
Introduction
The interplay between exogenously administered opioids and endogenous opioid systems remains largely unaddressed. Many studies have investigated the effects of morphine on endogenous opioid peptides, opioid receptor gene expression (Uhl et al., 1988; Brodsky et al., 1995; Romualdi et al., 1995; Rønnekleiv et al., 1996; Fang et al., 1998; Yeh et al., 1998), and opioid receptor trafficking (Whistler et al., 1999; Cahill et al., 2001; Eisinger et al., 2002). Nevertheless, the relevance of tolerance mechanisms recruited by exogenous opiates, e.g., morphine, to the functionality of endogenous opioid systems has not been elucidated. This could provide a window into the physiological recruitment of endogenous opioids to mitigate withdrawal sequelae.
The effects of acute and chronic administration of morphine, the most commonly used opiate, are predominantly mediated via the μ-opioid receptor (MOR), for which there are several endogenous substrates. Of these, endomorphin (EM) 1 and EM2 not only have the highest affinity and selectivity for MOR over the δ-opioid receptor (DOR) and κ-opioid receptor (KOR) but are also potent endogenous antinociceptive substrates (Zadina et al., 1997). We selected EM2 for the current study because it is the predominant spinal EM (Martin-Schild et al., 1998) and its release not only exhibits sexual dimorphism, e.g., magnitude, modulation by ovarian sex steroids, but is also subjected to MOR-coupled regulation (Gupta et al., 2007).
Activation of spinal MOR inhibits evoked spinal EM2 release, whereas neither spinal KOR nor DOR activation does so (Gupta et al., 2007). Moreover, in vitro blockade of spinal opioid receptors augments basal as well as evoked release of spinal EM2 (Gupta et al., 2007). These data indicate that release of spinal EM2 is subject to MOR-coupled negative feedback (Gupta et al., 2007) and underscore the importance of tonic endogenous MOR-coupled presynaptic inhibition to regulating spinal EM2 release. The reciprocal relationship between spinal MOR activation and spinal EM2 release makes it an ideal system for exploring adaptations to chronic morphine in a system that uses MOR-coupled regulation to maintain homeostasis.
Chronic morphine elicits multiple cellular adaptations. In addition to adaptations that result in diminished MOR functionality (Sim et al., 1996), we have identified multiple cellular adaptations that shift acute MOR-coupled adenylyl cyclase signaling from inhibitory to stimulatory (Chakrabarti et al., 1998, 2001, 2005a,b; Rivera and Gintzler, 1998; Chakrabarti and Gintzler, 2003, 2007). These postreceptor adaptations would be particularly advantageous to neurons using EM2 given their role not only in mitigating the persistent inhibition of EM2 release imposed by the sustained presence of morphine but also in preserving MOR-coupled regulation of that release, which would be eliminated by down-regulation/uncoupling of opioid receptors.
This study was designed to test the hypothesis that withdrawal from chronic systemic opioid treatment, which is sufficient to induce analgesic tolerance, elicits adaptive cellular mechanisms that use the pleiotropy inherent in receptor G protein coupling to maintain MOR-coupled regulation of EM2 release. We also investigated the sex dependence of these adaptations because spinal morphine recruits sexually dimorphic antinociceptive mechanisms (Liu et al., 2007; Chakrabarti et al., 2010). Using acute in vitro withdrawal to reflect changes that had occurred during chronic systemic morphine exposure, our findings indicate that chronic morphine shifts MOR-coupled regulation of EM2 release from inhibitory to stimulatory, which results from augmented coupling of MOR to Gs (Gsα). It is noteworthy that these adaptations occur in male rats, but not females. These findings provide physiological relevance for the previously reported chronic morphine-induced alteration of MOR G protein coupling and suggest that the recruitment of endogenous EM2 to cope with opioid withdrawal is sexually dimorphic.
Materials and Methods
Experimental Animals.
Sprague-Dawley male and female rats (250–300 g; Charles River Breeding Laboratories, Kingston, NY), used in experiments, were maintained in an approved controlled environment with food and water ad libitum. All surgeries were performed under sodium pentobarbital (Anpro Pharmaceutical, Arcadia, CA) anesthesia (40 and 50 mg/kg i.p. in females and males, respectively) and all experimental procedures were reviewed and approved by the Animal Care and Use Committee of the State University of New York Downstate Medical Center.
Implantation of Intrathecal Cannulae.
A permanent indwelling cannula was inserted into the lumbar spinal cord subarachnoid space as used previously (Liu et al., 2011). In brief, in anesthetized animals, a saline-filled catheter (PE-10; Clay Adams, Parsippany, NJ) was introduced through a small incision in the atlanto-occipital membrane, slowly inserted into the subarachnoid space, and secured in place. The cephalic portion of the catheter was externalized through the skin above the skull area where it was relatively inaccessible to the paws. All animals seemed to be free of infection upon gross inspection. Integrity of the motor system was assessed in all groups by using the righting reflex and the inclined plane test. Those exhibiting motor impairment after surgery were eliminated from the study.
Spinal Application of Pertussis Toxin and Cholera Toxin.
Bacterial toxins, pertussis toxin (PTX) and/or cholera toxin (CTX) (List Biological Laboratories Inc., Campbell, CA, for both), were injected via the intrathecal cannula to block Gi/Go and Gs proteins, respectively. PTX (10 μg/5 μl) was injected every other day (days 1, 3, and 5) during the last 5 days before sacrifice. CTX (5 μg/5 μl) was injected once 24 h before sacrifice.
Systemic Administration of Morphine.
Morphine or placebo were administered via the subcutaneous implantation of morphine base or placebo pellets (Cicero and Meyer, 1973) (one pellet on day 1, two pellets on day 3, and three pellets on day 5, six pellets in total; each morphine pellet contained 75 mg of morphine base). The gradual escalating dosing of systemic morphine is analogous to many medicinal settings where increasing doses of morphine are required for severe unremitting and/or escalating pain. On day 7, animals were sacrificed according to institutional guidelines by decapitation, after which cervical through lumbar spinal tissue was quickly expelled by injecting ice-cold saline into the rostral end. Spinal cord obtained from animals treated chronically with morphine and equilibrated for 40 min in vitro in the absence of morphine was used to model opioid withdrawal. Because experiments were designed to test adaptations to opioid withdrawal of MOR, we determined effects elicited by sufentanil (a highly MOR-selective alkaloid) instead of morphine (which activates DOR and KOR as well as MOR). Furthermore, epidural sufentanil or fentanyl is frequently used for medicinal purposes.
Spinal Tissue Preparation for Superfusion.
The spinal vertebral column was sectioned at the intervertebral spaces above vertebrae T11 and below L1. The lumbar spinal cord contained within this segment (L1-L5; 200–250 mg) was quickly expelled by injecting ice-cold saline into the caudal end, minced by using a Mcllwain Tissue Chopper (Mickle Laboratory Engineering Co., Guilford, Surrey, UK; 0.3-mm thickness), placed into a chamber (0.35 ml), and superfused (Brandel Superfusion System; Brandel Inc., Gaithersburg, MD). The Krebs' solution used for superfusion contained 118 mM NaCl, 4.7 mM KCl, 1.2 mM NaH2PO4, 25 mM NaHCO3, 1.2 mM MgCl2, 2.5 mM CaCl2, 11.1 mM dextrose, and gelatin (saturated with 4 g/l) and was gassed with 95% O2/5% CO2. In addition, the Krebs' superfusate used to assess basal and stimulated EM2 release contained the protease inhibitors captopril (10 μM), thiorphan (0.3 μM), bestatin (10 μM), and l-leucyl-l-leucine (2 mM) to protect peptides against the degradation resulting from the actions of the tissue proteases (Gupta et al., 2007).
Superfusion Paradigm.
The basal release of EM2 was defined as EM2 content in spinal superfusate obtained over 12 min (7.2 ml) in the absence of high potassium. The magnitude of evoked EM2 release was determined over a 3-min period (1.8 ml) by quantifying the content of EM2 in spinal superfusate that contained high potassium (K+; 50 mM; the content of sodium was proportionally reduced to maintain osmolarity). This constituted the first cycle of release. After stimulation with high K+, a 15-min rest period ensued before redetermining basal release and assessing evoked EM2 release in the presence of sufentanil (μ-opioid receptor agonist) or naloxone (opioid receptor antagonist) (1 μM). In all cases, the magnitude of K+-evoked release in the presence of drug (cycle 2) was compared with the magnitude of release observed in its absence (cycle 1). Determination of EM2 release in the presence of selected pharmacological agents constituted the second release cycle. Basal release [B] was subtracted from the total release while in the presence of high K+, stimulated release [S], to calculate the increment in evoked release [S − B]. The percentage increment in evoked release = [(S − B)/B] × 100, which was determined in the absence (cycle 1) and presence (cycle 2) of drug. A third cycle of release was obtained in which the conditions of cycle 1 were repeated to ensure that changes in basal and/or stimulated release of EM2 were attributable to the presence of drug and not simply to the passage of time. Drug effects were always reversed after washout and re-equilibration (cycle 3). Basal and stimulated superfusate were collected into prechilled tubes on ice. Superfusate containing basal release and evoked release were desalted and concentrated by using reverse-phase C-18 cartridges (Sep-Pak; Waters, Milford, MA). EM2 peptide was eluted, lyophilized to dryness, and stored at 4°C until quantified using radioimmunoassay (RIA).
EM2 Quantification Using RIA.
EM2 was assayed by using RIA that used a rabbit antibody (1:10,000) raised against EM2 (provided by J. E. Zadina) and highly specific for this peptide (Martin-Schild et al., 1997). Samples were incubated with anti-EM2 antibody (1:10,000; 2 h; room temperature) before adding radiolabeled 125I-EM2 (specific activity 1190 Ci/mmol; Phoenix Pharmaceuticals, Belmont, CA), which was incubated overnight. Antibody-bound radioactivity was determined by using scintillation proximity (Gupta et al., 2007). The reaction mixture was transferred from tubes to 96-well scintillation impregnated plates that were coated with anti-rabbit antibodies. The radiolabeled antigen-antibody complexes bound to secondary antibody were in proximity to scintillation bound to the plates and counted. In contrast, the nonbound 125I-EM2 did not come into proximity with the secondary antibody, and scintillation was not counted. The plates were counted in a Microbeta plate reader (PerkinElmer Life and Analytical Sciences, Waltham, MA). A standard curve was generated (0.5–16 pg/tube) in which the percentage of inhibition of binding was plotted against the log concentrations of nonlabeled peptide. Values from samples were calculated from the standard curve generated with nonlabeled EM2 by using the forecast function of Excel (Microsoft, Redmond, WA).
Membrane Preparation and Immunoprecipitation.
Spinal cord membranes were prepared and solubilized as described previously (Chakrabarti et al., 2005a). In brief, animals were sacrificed, and spinal cord tissues were isolated as described above. Tissues were homogenized in 20 mM HEPES, pH 7.4, containing 10% sucrose, 5 mM EDTA, 1 mM EGTA, 2 mM dithiothreitol, and multiple protease inhibitors [1 mM benzamidine, 0.2 g/liter bacitracin, 2 mg/liter aprotinin, and 3.2 mg/liter each of leupeptin and trypsin inhibitor from soybean; 20 mg/liter each of N-p-tosyl-l-phenylalanine chloromethyl ketone, Nα-tosyl-l-lysine chloromethyl ketone, and phenylmethylsulfonyl fluoride (Sigma, St. Louis, MO)] and complete cocktail inhibitor tablet/50 ml (Roche Molecular Biochemicals, Indianapolis, IN). Supernatants from a low-speed spin (1000g for 10 min) were centrifuged at a higher speed (30,000g) for 40 min to obtain membrane pellets. Immunoprecipitates were obtained from membranes solubilized in the above buffer containing 150 mM NaCl, 1% Nonidet P-40, 0.5% Na-deoxycholate, 0.1% Na-dodecyl sulfate, and 10% glycerol, agitated 60 min at 4°C, and centrifuged (16,000g for 40 min at 4°C). Gsα was immunoprecipitated as described previously (Chakrabarti et al., 2005a). In brief, immunoprecipitation was initiated with 50 μg of solubilized membrane and a rabbit polyclonal antibody generated against the C terminus of the Gsα subunit (amino acids 385–394; BD Biosciences, San Jose, CA). Prewashed protein A-agarose (50 μl; Roche Molecular Biochemicals) was used for immunoprecipitation overnight at 4°C. The beads were washed in 20 mM HEPES buffer, pH 7.4, containing 2 mM dithiothreitol, 5 mM EDTA, 150 mM NaCl, 0.05% Nonidet P-40, and the same protease inhibitors as mentioned above. Immunoprecipitates were eluted by heating the samples in 30 μl of sample buffer (15 min at 85°C). Samples separated on 4 to 12% gradient Bis-Tris gels (Invitrogen, Carlsbad, CA) were electro-transferred onto nitrocellulose membranes and used for Western analyses.
Western Analysis.
Standard procedures were used for Western analyses as described previously (Chakrabarti et al., 2005a). Gsα protein was visualized by using a 1:10,000 dilution of a rabbit polyclonal anti- Gsα antibody generated against the C terminus of Gsα (generous gift from Dr. John Hildebrandt, Medical University of South Carolina, Charleston, SC). MOR protein was visualized by using a 1:8000 dilution of a rabbit polyclonal antibody generated against the C-terminal 50 amino acids of MOR (Chalecka-Franaszek et al., 2000) (generously provided by Dr. Thomas Cote, Uniformed Services University of the Health Sciences, Bethesda, MD). The secondary antibody used was a peroxidase-labeled donkey anti-rabbit antibody (1:20,000; GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK). Antibody-substrate complex was visualized by using a Supersignal West Dura Chemiluminescence detection kit (Thermo Fisher Scientific, Waltham, MA). Specificity of Western signals was demonstrated previously via their diminution/elimination after incubation with antibodies while in the presence of a 3- to 5-fold excess of their respective blocking peptides (Chakrabarti et al., 2005a). Sample pairs obtained from opioid-naive and chronic morphine-withdrawal spinal cords were processed, electrophoresed, and blotted in parallel after which they were exposed concomitantly to a GBox (charge-coupled device camera; Syngene, Frederick, MD). Intensity of signals was quantified by using Genetools software (Syngene).
Statistical Analysis.
Kruskal-Wallis and Mann Whitney nonparametric tests were used to assess the significance of differences among groups. Significance of within-group differences was assessed by using Student's t test.
Results
Opioid Withdrawal Shifts Sufentanil Modulation of Evoked EM2 Release from Inhibition to Facilitation.
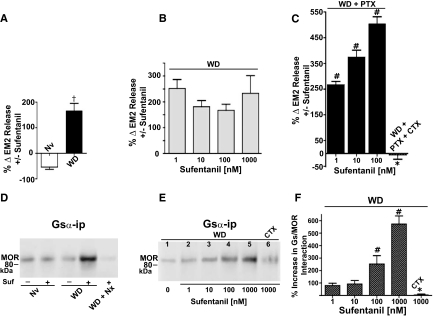
Figure 1A illustrates that sufentanil (100 nM) inhibited K+-evoked EM2 release from the spinal cord of opioid-naive rats (−54 ± 9%; n = 3; p < 0.05), as reported previously (Gupta et al., 2007). In contrast, the same concentration of sufentanil stimulated K+-evoked EM2 release (167 ± 11%; n = 3; p < 0.02) from spinal cord obtained from chronic morphine-treated male rats that had been equilibrated in vitro in the absence of morphine (withdrawn, spinal cord) (Fig. 1A). Statistical analyses confirmed apparent differences between sufentanil effects in opioid-naive versus -withdrawn spinal tissues (Fig. 1A, Nv versus WD; p < 0.05). Qualitative changes in MOR-coupled modulation of EM2 release from in vitro-withdrawn spinal tissue were not evident from similarly treated spinal tissue obtained from female rats (see below).
Fig. 1.
In spinal cord of male rats, opioid withdrawal augments MOR-coupled facilitation of evoked EM2 release and MOR coupling to Gs. A, sufentanil inhibited EM2 release from naive spinal tissue (Nv). In contrast, in withdrawn spinal tissue, sufentanil enhanced evoked EM2 release (WD) (n = 3–4). %Δ represents the difference of the percentage increment in K+-evoked EM2 release, calculated as [(evoked − basal)/basal] × 100, in the absence and presence of sufentanil. B, sufentanil (1–1000 nM) enhances evoked EM2 release from opioid-withdrawn spinal tissues but this modulation was not dose-dependent (n = 3–5). C, intrathecal PTX unmasks the dose dependence of the facilitation by sufentanil (WD+PTX; 1–100 nM; n = 3), which was abolished by pretreatment of intrathecal CTX in addition to PTX (WD+PTX+CTX; n = 3). D, MOR Western analysis of immunoprecipitates obtained from opioid naive (Nv) and withdrawn (WD) spinal cord using anti-Gsα antibodies. Immunoprecipitation was performed in the absence (−) and presence (+) of 1 μM sufentanil. Sufentanil stimulation of the CoIP of MOR with Gsα was abolished by naloxone (1 μM; WD+Nx). The first two lanes and last three lanes were processed and blotted in parallel. E, dose-dependent stimulation by sufentanil of MOR in Gsα immunoprecipitate (lanes 1–5) and its abolishment by intrathecal CTX (lane 6). All lanes were processed and blotted in parallel. F, quantification of the sufentanil-stimulated increment in MOR Gsα CoIP illustrated in E (n = 3). Opioid withdrawal augments facilitatory MOR-coupled modulation of EM2 release, which is causally associated with increased coupling of MOR with Gs. †, p < 0.05 for withdrawal effect; #, p < 0.05 for sufentanil dose effect; *, p < 0.05 for CTX effect.
Dose Dependence of Sufentanil Facilitation of Evoked Spinal EM2 Release during Opioid Withdrawal.
Figure 1B illustrates that sufentanil stimulation of K+-evoked EM2 release from withdrawn spinal tissue of male rats was observed at all concentrations tested (1–1000 nM). Strikingly, however, the magnitude of sufentanil stimulation of EM2 release was not dose-dependent (Fig. 1B; 252–233%; p > 0.05; n = 3–5 for each concentration). Because MOR can couple to Gs as well as to Gi/Go G proteins, the proportion of which is influenced by chronic morphine (Chakrabarti et al., 2005a; Chakrabarti and Gintzler, 2007), we investigated whether the apparent lack of sufentanil dose dependence resulted from the concomitant activation of opposing MOR-coupled G proteins by determining sufentanil dose responsiveness after chemically uncoupling spinal MOR from Gi/Go.
PTX treatment unmasked a significant dose dependence of sufentanil facilitation of EM2 release from withdrawn spinal tissues of male rats (p < 0.05). Intrathecal PTX not only unmasked dose dependence of sufentanil stimulation of evoked EM2 release from withdrawn spinal tissue (267–505%; n = 3; Fig. 1C) but also substantially increased the magnitude of that stimulation [505% (Fig. 1C) versus 167% (Fig. 1A) for with and without PTX treatment, respectively, at 100 nM sufentanil; p < 0.05]. This indicates that stimulatory and inhibitory MOR signaling can concomitantly regulate EM2 release from in vitro-withdrawn spinal tissue, but the net effect of MOR activation in these preparations is facilitation. It is noteworthy that facilitation by sufentanil (100 nM) of EM2 release from withdrawn spinal tissues was completely abolished by the intrathecal pretreatment with CTX in addition to PTX, strongly suggesting that sufentanil facilitation of evoked EM2 release from withdrawn spinal tissue is mediated via Gs (Fig. 1C; p < 0.01; n = 4).
MOR Gs Interaction Is Augmented in Opioid-Withdrawn Spinal Tissues of Male Rats.
The coimmunoprecipitation (CoIP) of MOR with Gsα was used to reflect changes in their interaction, as reported previously (Chakrabarti et al., 2005a; Chakrabarti and Gintzler, 2007). Sufentanil (1 μM) did not produce any detectable increase in the MOR content of Gsα immunoprecipitate obtained from opioid-naive spinal tissues. In contrast, the same concentration of sufentanil increased 5-fold (p < 0.05; n = 3) the MOR content of Gsα immunoprecipitate obtained from withdrawn spinal tissues (Fig. 1D; compare third and fourth lanes; n = 3). It is noteworthy that the withdrawal-associated increment in MOR Gsα CoIP was completely abolished by the addition of the opioid receptor antagonist naloxone concomitant with sufentanil (Fig. 1D, compare fourth and fifth lanes; n = 2), indicating that the ability of sufentanil to increase MOR Gsα CoIP was opioid receptor-mediated.
To validate the inferred causal association between the emergent sufentanil facilitation of K+-evoked EM2 release and the augmented MOR Gs coupling in withdrawn spinal tissue, we investigated the dose dependence and CTX reversibility of sufentanil stimulation of MOR Gsα CoIP. Sufentanil dose-dependently increased MOR Gs CoIP (Fig. 1, E and F; 80–572%; n = 3–6), paralleling its ability to facilitate evoked EM2 release. The sufentanil-stimulated increase in Gs-MOR interaction in withdrawn spinal cord was also abolished after intrathecal treatment with CTX (Fig. 1E, lane 6 versus lane 5 and Fig. 1F, with vs. without CTX, n = 3). These data, in combination with those described above, underscore the relevance of enhanced MOR Gs signaling to the emergent predominance of CTX-sensitive sufentanil facilitatory modulation of EM2 release that occurs in opioid-withdrawn spinal tissue of males.
Inhibition of Protein Phosphatase 2A Blocks Both the Withdrawal-Induced Emergence of Sufentanil Facilitation of Evoked EM2 Release and Augmented MOR-Gs Coupling.
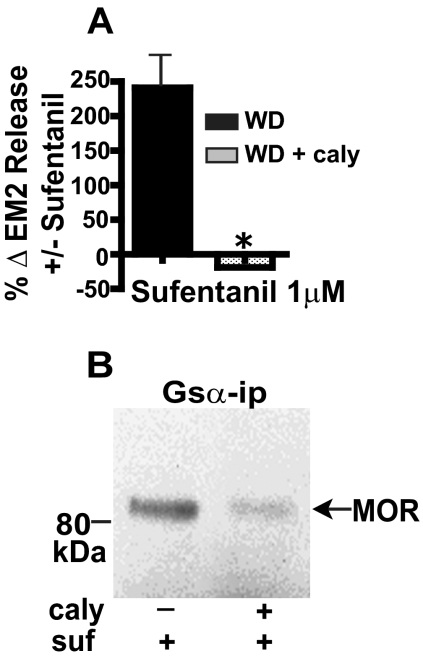
We demonstrated previously that dephosphorylation of Gsα by PP2A is essential for the enhanced MOR-Gs interaction in spinal cord membranes obtained from chronic morphine-treated rats (Chakrabarti et al., 2007). To further validate the role of MOR Gs signaling in the switch from MOR-coupled inhibition to facilitation of evoked EM2 release, we investigated the effect of inhibiting spinal PP2A on the withdrawal-associated emergence of sufentanil facilitation of evoked EM2 release and MOR Gs coupling. Intrathecal administration of the PP2A inhibitor calyculin A (5 pmoles) not only abolished sufentanil facilitation of evoked EM2 release (Fig. 2A; n = 3) but also eliminated sufentanil-stimulated MOR Gsα CoIP (Fig. 2B; n = 3) from withdrawn spinal tissue of male rats.
Fig. 2.
Inhibition of PP2A activity blocks both the stimulation of evoked EM2 release by sufentanil and its enhancement of MOR Gsα coupling in opioid-withdrawn spinal tissue of male rats. A, intrathecal pretreatment with the PP2A inhibitor calyculin A (caly) abolished the sufentanil (1 μM) stimulation of evoked EM2 release from opioid-withdrawn spinal tissue of males (WD + caly; n = 3; p < 0.01). Sufentanil (1 μM) stimulation of evoked EM2 release (WD) is replicated from Fig. 1B to facilitate comparison. B, CoIP of MOR with Gsα was determined in parallel from withdrawn spinal tissue in the absence of intrathecal treatment (left) and after intrathecal treatment (right) with calyculin A (n = 3). Inhibition of spinal PP2A abrogated both the withdrawal-associated augmented facilitatory modulation of EM2 release by sufentanil as well as the enhanced coupling of MOR to Gs.
Opioid Withdrawal Enhances Gs-Coupled MOR Regulation of EM2 Release That Is Present in the Spinal Cord of Opioid-Naive Male Rats.
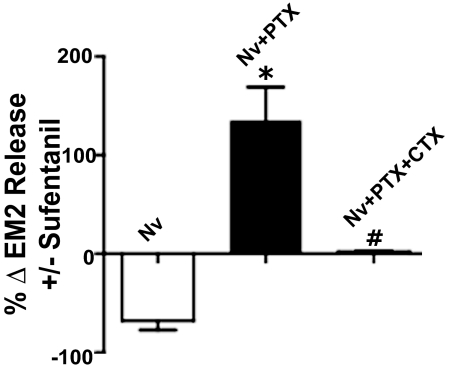
The switch from MOR-coupled inhibition to facilitation of EM2 release from opioid-withdrawn spinal cord could result from either the chronic morphine-induced de novo emergence of a novel MOR-coupled signaling pathway or the amplification of a pathway that is present but masked in opioid-naive tissue. To distinguish between these possibilities, we investigated whether or not intrathecal PTX unmasked facilitation by sufentanil of EM2 release from opioid-naive spinal tissue. Intrathecal PTX did unmask the presence of a MOR-coupled facilitation of EM2 release from opioid-naive spinal tissue (Fig. 3; compare 68 ± 9% inhibition versus 135 ± 34% facilitation by 1 μM sufentanil without and with PTX, respectively; n = 3; Fig. 3, Nv vs. Nv + PTX), which was abolished after intrathecal treatment with CTX concomitant with PTX (Fig. 3, Nv+PTX+CTX versus Nv+PTX). This indicates that MOR Gs-coupled regulation of EM2 release is indeed present in opioid-naive spinal tissue. However, it is present at a greatly reduced level [compare enhanced EM2 release produced by 100 nM (76%; data not shown) or 1 μM sufentanil (120%; Fig. 3) with the 505% increment produced by 100 nM sufentanil in withdrawn tissue (Fig. 1C)].
Fig. 3.
MOR-coupled regulation of EM2 release from the spinal cord of opioid-naive male rats exhibits pleiotropy. Inhibition of evoked EM2 release by sufentanil (1 μM; Nv) reverses to a facilitation after intrathecal PTX (Nv+PTX; *, p < 0.05), all of which was eliminated by concomitant intrathecal treatment with PTX and CTX (compare Nv+PTX+CTX and Nv+PTX; #, p < 0.05). n = 3 for all experimental groups. Results indicate that facilitatory MOR-coupled modulation of spinal EM2 release is present in opioid-naive spinal cord, albeit at a greatly reduced level.
Opioid Withdrawal-Induced Switch from Inhibitory to Facilitatory MOR-Coupled Modulation of Evoked EM2 Release Is Sexually Dimorphic.
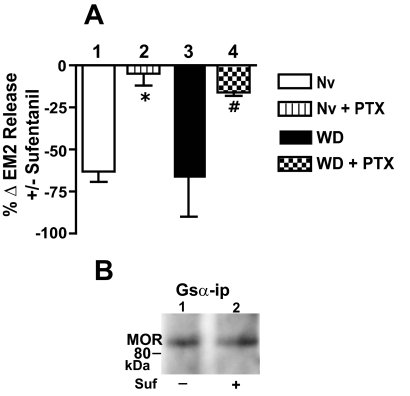
In contrast to opioid withdrawn spinal tissue of male rats, 1 μM sufentanil continued to inhibit evoked EM2 release from withdrawn spinal tissue of females (Fig. 4A, bar 3; − 66 ± 24%; n = 3). Moreover, although intrathecal PTX essentially abolished the inhibition of EM2 release produced by 1 μM sufentanil, PTX did not unmask any facilitation of evoked EM2 release (Fig. 4A, bar 4; n = 3), as was observed in withdrawn tissues of males (compare Fig. 4A, bar 4 with Fig. 1C).
Fig. 4.
Absence of augmented MOR-coupled facilitation of evoked EM2 release and MOR Gs coupling in opioid-withdrawn spinal cord of female rats. A, the magnitude of sufentanil (1 μM) inhibition of EM2 release from opioid-naive spinal tissue (bar 1) was comparable with that observed from opioid-withdrawn spinal preparations (bar 3). Intrathecal PTX essentially abolished the inhibition by sufentanil of EM2 release from both opioid-naive and -withdrawn preparations (bars 2 and 4, respectively; *, p < 0.05 for effect of PTX in naive; #, p < 0.05 for effect of PTX in withdrawn tissue; n = 3 for both). B, MOR Western analysis of Gsα immunoprecipitate obtained from opioid-withdrawn spinal tissue in the absence (−) or presence (+) of 1 μM sufentanil. Neither opioid-naive nor -withdrawn spinal tissue of females manifests facilitatory MOR-coupled modulation of EM2 release. Enhanced MOR Gs coupling was also absent in withdrawn tissue of females.
Pleiotropic MOR-Coupled Regulation of Evoked EM2 Release from Opioid-Naive Spinal Tissue Is Sexually Dimorphic.
MOR activation by 1 μM sufentanil inhibited evoked EM2 release from opioid naive spinal tissue of female rats (Fig. 4A, bar 1; −63 ± 6%; n = 3), as reported previously (Gupta et al., 2007). This inhibition, as expected, was essentially eliminated by intrathecal PTX (Fig. 4A, bar 2; n = 3). However, intrathecal PTX did not unmask any sufentanil facilitation of evoked EM2 release, as occurred in similarly treated spinal tissue of males (compare Fig. 4A, bar 2 with Fig. 3, Nv+PTX). It is noteworthy that neither the magnitude of sufentanil inhibition of EM2 release nor the effects thereon of intrathecal PTX differed between opioid-naive and -withdrawn spinal tissue of females (compare Fig. 4A, bars 1 and 3 and bars 2 and 4, respectively; p > 0.05 for both comparisons).
Sufentanil Fails to Augment MOR Gs Association in Withdrawn Spinal Tissue of Female Rats.
In contrast to the sufentanil-induced enhancement of MOR-Gs association in withdrawn spinal tissue from male rats, sufentanil failed to stimulate MOR-Gs association in corresponding tissue from females (Fig. 4B, lane 2 versus 1; p > 0.05). These results are in keeping with the absence of qualitative changes in MOR-coupled regulation of evoked EM2 release from withdrawn spinal tissues of females.
Opioid Withdrawal Shifts Endogenous MOR-Coupled Regulation of Spinal EM2 Release from Inhibitory to Facilitatory in Male Rats, But Not Females.
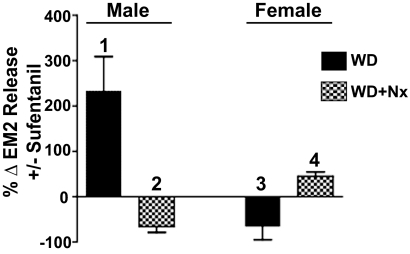
Naloxone (1 μM) enhances the evoked release of EM2 from the spinal tissue of opioid-naive males as well as females (Gupta et al., 2007), presumably reflecting blockade of the inhibitory effects of endogenous opioids on MOR autoreceptors. Strikingly, however, the same treatment with naloxone inhibits evoked EM2 release from withdrawn spinal tissue of males (Fig. 5, bar 2), presumably reflecting the blockade of facilitatory, Gs-mediated effects of endogenous opioids on MOR autoreceptors. In contrast to these observations, opioid withdrawal failed to reverse the direction of sufentanil or naloxone modulation in the spinal cord of females (Fig. 5). In withdrawn spinal tissue of females, sufentanil continued to inhibit (Fig. 5, bar 3) and naloxone continued to facilitate evoked release of EM2 (Fig. 5, bar 4; 31.5%; n = 3; p < 0.05). Thus, in both males and females, MOR-coupled endogenous regulation of spinal EM2 release parallels its regulation by sufentanil (Fig. 5, bars 1 and 3, respectively). The findings with naloxone also support the concept of a shift in coupling from Gi/Go to Gs in males, but not in females.
Fig. 5.
Endogenous MOR-coupled regulation of evoked EM2 release shifts from inhibitory to facilitatory in withdrawn spinal tissue of male rats, but not female rats. In vitro MOR blockade via naloxone (1 μM) inhibits evoked EM2 release from withdrawn spinal tissue of males (bar 2), whereas similar treatment enhances evoked EM2 release from withdrawn spinal tissue from females (bar 4) (n = 3 for both). Effects of 1 μM sufentanil on evoked EM2 release from withdrawn spinal tissue of males (bar 1) and females (bar 3) were reproduced from Figs. 1B and 4A, respectively, to facilitate comparison with the directionality of effects of naloxone. In withdrawn tissues of both males and females, modulation of EM2 release by naloxone was inversely proportional to the effects of MOR activation by sufentanil. Enhancement versus inhibition of evoked EM2 release by MOR blockade in withdrawn spinal cord of females and males, respectively, reveals the predominance of endogenous MOR-coupled positive modulation of spinal EM2 release in males but the persistence of negative modulation in spinal cord of females.
Discussion
Results of this study reveal that sexually dimorphic spinal adaptations are elicited by acute opioid withdrawal despite its largely sexually monomorphic behavioral presentation. In addition, using the same preparation to study biochemical and functional sequelae of opioid withdrawal enabled assessment of the physiological implications of specific cellular adaptations.
Specific findings in male rats include: 1) evoked EM2 release from opioid-naive and -withdrawn spinal cord is concomitantly subjected to negative as well as positive MOR-coupled modulation; 2) in opioid-withdrawn spinal tissue, the balance of MOR-coupled regulation of EM2 release shifts from predominantly inhibitory to facilitatory; 3) the qualitative shift in MOR-coupled regulation of EM2 release results from augmented MOR Gs coupling; and 4) MOR-coupled regulation of spinal EM2 release and changes thereof after opioid withdrawal exhibits striking sexual dimorphism. In female rats, MOR-coupled regulation of EM2 release from opioid-naive spinal tissue does not exhibit bimodal MOR-coupled regulation; opioid withdrawal neither augments MOR-coupled facilitation of EM2 release nor MOR Gs coupling. MOR-coupled regulation of spinal EM2 release in males exhibits physiological-state dependence that is not manifest in the spinal cord of females.
Pleiotropic bidirectional MOR-coupled regulation of EM2 release is masked in the spinal cord of opioid-naive male rats. This is revealed by the ability of intrathecal PTX to not only eliminate inhibition of evoked EM2 release by sufentanil but to also unmask its ability to facilitate that release. Neither inhibition nor facilitation was observed after intrathecal PTX and CTX.
Abolition by CTX of the facilitatory sufentanil modulation strongly suggests Gs mediation. Thus, it was surprising that we were not able to detect increased MOR in Gsα immunoprecipitation after acute sufentanil. This apparent dichotomy most likely results from much greater sensitivity of the EM2 release mechanism to small increases in Gsα versus the ability of the Western method used to detect them. In addition, the CTX sensitivity of sufentanil facilitated EM2 release used PTX-treated tissue, which could have facilitated detecting MOR Gs coupling, whereas MOR Gsα CoIP was investigated in the absence of PTX.
The newly emerged predominance of MOR-coupled facilitatory regulation of EM2 release from opioid-withdrawn spinal tissues of male rats did not exhibit any sufentanil dose dependence over three orders of magnitude. However, intrathecal PTX not only dramatically augmented the magnitude of facilitatory regulation of EM2 release by sufentanil, but also unmasked its dose dependence, all of which was eliminated by intrathecal CTX. The most parsimonious interpretation of these data is that sufentanil concomitantly activates MOR Gs- and MOR Gi/Go-coupled regulation of EM2 release, the relative contributions of which do not significantly vary over the range of sufentanil concentrations used.
The effects of intrathecal PTX and CTX underscore that pleiotropic MOR-coupled modulation of EM2 release that was present in opioid-naive spinal tissue from male rats persisted in opioid-withdrawn spinal tissue, but the balance between MOR-coupled inhibition and facilitation shifted. Whereas in opioid-naive preparations the net effect of the bidirectional MOR-coupled control was inhibitory, facilitatory MOR-coupled modulation predominated in opioid-withdrawn tissue. This plasticity of MOR-coupled bidirectional modulation of EM2 release enables the sensory MOR system to accommodate changing physiological demand.
In withdrawn spinal cord of male rats, cross-validating biochemical and pharmacological observations indicate that augmented MOR Gs coupling is causally associated with the switch from MOR-coupled inhibition to facilitation of EM2 release. 1) Intrathecal CTX eliminated the facilitation of evoked EM2 release by sufentanil. 2) Sufentanil stimulation of MOR Gs association (reflected by MOR Gsα CoIP) was dramatically elevated in opioid-withdrawn spinal tissue. 3) Stimulation of MOR Gs association by sufentanil was dose-dependent, paralleling the sufentanil facilitation of evoked EM2 release that was manifest after eliminating the contributions of Gi/Go activation. 4) Naloxone eliminated sufentanil stimulation of MOR Gs coupling, indicating that it was triggered by the activation of MOR. This conclusion is supported by the high selectivity of sufentanil for MOR and the inability of KOR and DOR to modulate evoked EM2 release (Gupta et al., 2007). 5) Inhibition of spinal PP2A (via calyculin), which blocks Gsα dephosphorylation that is essential for chronic morphine-induced augmented MOR Gs coupling (Chakrabarti and Gintzler, 2007), eliminated the withdrawal-induced emergence of sufentanil facilitation of evoked EM2 release as well as augmented MOR-Gs coupling.
We had reported previously that chronic morphine increases MOR Gs coupling in cell lines as well as spinal cord (Chakrabarti et al., 2005a; Chakrabarti and Gintzler, 2007; Shy et al., 2008). The present results reaffirm those findings and put them into a physiological context, i.e., enhanced MOR Gs signaling during withdrawal enables opioid facilitation of EM2 release, which in turn contributes to withdrawal-coping mechanisms. The demonstrated importance of adaptations in MOR Gs signaling to the predominance of MOR-coupled facilitatory regulation of EM2 release in withdrawn spinal cord does not eliminate the possibility that the qualitative shift in MOR regulation of EM2 release is multifactorial, i.e., it could result from additional putative sources such as alterations in MOR Gi/Go signaling. It should be noted, however, that PTX would not be expected to have the profound effect that it did on sufentanil facilitatory modulation of EM2 release (Fig. 1C) if the Gi-coupled MOR that is relevant to spinal EM2 release was substantially uncoupled during withdrawal.
Of critical relevance to the in vivo regulation of spinal EM2 release during opioid withdrawal in male rats is that the shift from negative to positive opioid regulation of EM2 release manifested in response to sufentanil is also evident in response to an endogenously released opioid. We reported previously (Gupta et al., 2007) and confirmed in the present study that spinal opioid receptor blockade in naive males enhances evoked EM2 release. However, opioid receptor blockade inhibited evoked EM2 release from opioid-withdrawn spinal tissues of males. This indicates that under opioid naive conditions spinal EM2 release is subject to ongoing endogenous negative feedback, but during withdrawal EM2 release is subjected to positive feedback. Colocalization of MOR and EM2 in fibers of the outer laminae of the spinal dorsal horn (Martin-Schild et al., 1997; Abbadie et al., 2002; Aicher et al., 2003) provides an anatomical basis for MOR-coupled regulation of spinal EM2 release.
Strikingly, MOR-coupled dual regulation of spinal EM2 release as well as its adaptation to opioid withdrawal is sexually dimorphic. MOR-coupled inhibition of evoked EM2 release was comparable in spinal cord of males and females. However, in contrast to the emergence of MOR-coupled facilitation of evoked EM2 release from withdrawn spinal tissue of males, MOR-coupled inhibition of evoked EM2 release from withdrawn spinal tissue of females persisted unabated and did not revert to a facilitation even after intrathecal PTX. Consistent with these observations, naloxone modulation of evoked EM2 release was not qualitatively different between opioid-naive versus opioid-withdrawn spinal preparations obtained from females.
The regulation of spinal EM2 release by EM2/MOR-mediated feedback inhibition (Gupta et al., 2007) indicates that spinal synaptic levels of EM2 are very tightly regulated by a closed loop in which MOR functions as an EM2 sensor. This places particular restrictions on the types of adaptive mechanisms to chronic morphine that would be advantageous to spinal EM2-transmitting neurons. If negative MOR Gi/Go-coupled modulation of spinal EM2 release persisted during long-term systemic morphine administration, spinal EM2 utilization would be greatly suppressed. MOR desensitization (uncoupling) would eliminate or substantially reduce the continual opioid inhibition of release but this adaptation would also eliminate EM2 feedback regulation of its own release (which is MOR-coupled), an important regulatory parameter. Thus, there is an imperative for additional or alternative adaptations to chronic morphine.
Adaptations (in males) to chronic morphine that involve shifting the relative predominance of opposing dual G protein-coupled MOR pathways that are concomitantly activated provides a mechanism for mitigating inhibitory MOR signaling without losing MOR-coupled feedback regulation. Moreover, a shift in the equilibrium from predominantly MOR Gi/Go-negative to MOR Gs-positive modulation of EM2 release would have a much greater dynamic range than would just uncoupling MOR Gi/Go inhibitory signaling because the magnitude of any increased EM2 utilization resulting from disinhibition (uncoupling) alone would be limited by the extent of ongoing presynaptic inhibition.
The male-specific enhanced utilization of the spinal EM2/MOR-coupled system during opioid withdrawal is consistent with other reports of MOR-related sexual dimorphism, e.g., 1) greater antinociceptive responsiveness to morphine of male versus female rats (Cicero et al., 1996, 1997; Wang et al., 2006; Loyd et al., 2008), 2) greater K+ -induced release of EM2 from spinal cord of male versus female rats (Gupta et al., 2007), 3) exclusive mediation of spinal morphine antinociception by spinal MOR in males but KOR as well as MOR in females (Liu et al., 2007), and 4) the almost 5-fold greater expression of MOR heterodimerized with KOR in the spinal cord of females than males (Chakrabarti et al., 2010). All suggest the predominance and perhaps the exclusivity of MOR-mediated events in males versus the importance in females of alternative opioid systems, alone or in combination with MOR.
Sexually dimorphic adaptations of the spinal EM2 system to chronic morphine indicate that males and females differentially use endogenous spinal EM2 to cope with the precipitous removal of morphine after its chronic systemic administration. In withdrawn spinal cord of males, augmented MOR Gs signaling and the resultant increase in EM2 release enable using endogenous EM2 to substitute for morphine that had been precipitously removed. The absence of this adaptation in the spinal cord of females suggests that alternative coping strategies, yet to be determined, are used.
The demonstrated sexually dimorphic spinal EM2/MOR-coupled signaling suggests the clinical utility of using sex-specific treatments for addiction that harness the activity of endogenous opioids. The predominance of MOR Gs-coupled facilitative modulation of EM2 release from withdrawn spinal tissue of males, but not females, could suggest that clinical management of opioid dependence in women would benefit (more than in men) from adjunctive pharmacotherapies that enhance spinal EM2 release.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- MOR
- μ-opioid receptor
- EM
- endomorphin
- DOR
- δ-opioid receptor
- KOR
- κ-opioid receptor
- PTX
- pertussis toxin
- CTX
- cholera toxin
- RIA
- radioimmunoassay
- CoIP
- coimmunoprecipitation
- PP2A
- protein phosphatase 2A.
Authorship Contributions
Participated in research design: Chakrabarti, Liu, Sharma, and Gintzler.
Conducted experiments: Chakrabarti, Liu, and Sharma.
Contributed new reagents or analytic tools: Zadina.
Performed data analysis: Chakrabarti, Liu, Zadina, Sharma, and Gintzler.
Wrote or contributed to the writing of the manuscript: Chakrabarti, Liu, Zadina, Sharma, and Gintzler.
References
- Abbadie C, Rossi GC, Orciuolo A, Zadina JE, Pasternak GW. (2002) Anatomical and functional correlation of the endomorphins with μ opioid receptor splice variants. Eur J Neurosci 16:1075–1082 [DOI] [PubMed] [Google Scholar]
- Aicher SA, Mitchell JL, Swanson KC, Zadina JE. (2003) Endomorphin-2 axon terminals contact μ-opioid receptor-containing dendrites in trigeminal dorsal horn. Brain Res 977:190–198 [DOI] [PubMed] [Google Scholar]
- Brodsky M, Elliott K, Hynansky A, Inturrisi CE. (1995) CNS levels of μ opioid receptor (MOR-1) mRNA during chronic treatment with morphine or naltrexone. Brain Res Bull 38:135–141 [DOI] [PubMed] [Google Scholar]
- Cahill CM, Morinville A, Lee MC, Vincent JP, Collier B, Beaudet A. (2001) Prolonged morphine treatment targets δ opioid receptors to neuronal plasma membranes and enhances δ-mediated antinociception. J Neurosci 21:7598–7607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S, Gintzler AR. (2003) Phosphorylation of Gβ is augmented by chronic morphine and enhances Gβγ stimulation of adenylyl cyclase activity. Mol Brain Res 119:144–151 [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Gintzler AR. (2007) Phosphorylation of Gαs influences its association with the μ-opioid receptor and is modulated by chronic morphine. Mol Pharmacol 72:753–760 [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Liu NJ, Gintzler AR. (2010) Formation of μ-/κ-opioid receptor heterodimer is sex-dependent and mediates female-specific opioid analgesia. Proc Natl Acad Sci U S A 107:20115–20119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S, Oppermann M, Gintzler AR. (2001) Chronic morphine induces the concomitant phosphorylation and altered association of multiple signaling proteins: a novel mechanism for modulating cell signaling. Proc Natl Acad Sci U S A 98:4209–4214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S, Regec A, Gintzler AR. (2005a) Biochemical demonstration of μ-opioid receptor association with Gsα: enhancement following morphine exposure. Mol Brain Res 135:217–224 [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Regec A, Gintzler AR. (2005b) Chronic morphine acts via a protein kinase Cγ-Gβ-adenylyl cyclase complex to augment phosphorylation of Gβ and Gβγ stimulatory adenylyl cyclase signaling. Mol Brain Res 138:94–103 [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Wang L, Tang WJ, Gintzler AR. (1998) Chronic morphine augments adenylyl cyclase phosphorylation: relevance to altered signaling during tolerance/dependence. Mol Pharmacol 54:949–953 [DOI] [PubMed] [Google Scholar]
- Chalecka-Franaszek E, Weems HB, Crowder AT, Cox BM, Côté TE. (2000) Immunoprecipitation of high-affinity, guanine nucleotide-sensitive, solubilized μ-opioid receptors from rat brain: coimmunoprecipitation of the G proteins Gαo, Gαi1, and Gαi3. J Neurochem 74:1068–1078 [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Meyer ER. (1973) Morphine pellet implantation in rats: quantitative assessment of tolerance and dependence. J Pharmacol Exp Ther 184:404–408 [PubMed] [Google Scholar]
- Cicero TJ, Nock B, Meyer ER. (1996) Gender-related differences in the antinociceptive properties of morphine. J Pharmacol Exp Ther 279:767–773 [PubMed] [Google Scholar]
- Cicero TJ, Nock B, Meyer ER. (1997) Sex-related differences in morphine's antinociceptive activity: relationship to serum and brain morphine concentrations. J Pharmacol Exp Ther 282:939–944 [PubMed] [Google Scholar]
- Eisinger DA, Ammer H, Schulz R. (2002) Chronic morphine treatment inhibits opioid receptor desensitization and internalization. J Neurosci 22:10192–10200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Kelly MJ, Rønnekleiv OK. (1998) Proopiomelanocortin (POMC) mRNA expression: distribution and region-specific down-regulation by chronic morphine in female guinea pig hypothalamus. Brain Res Mol Brain Res 55:1–8 [DOI] [PubMed] [Google Scholar]
- Gupta DS, von Gizycki H, Gintzler AR. (2007) Sex-/ovarian steroid-dependent release of endomorphin 2 from spinal cord. J Pharmacol Exp Ther 321:635–641 [DOI] [PubMed] [Google Scholar]
- Liu NJ, Schnell SA, Schulz S, Wessendorf MW, Gintzler AR. (2011) Regulation of spinal dynorphin 1–17 release by endogenous pituitary adenylyl cyclase-activating polypeptide in the male rat: relevance of excitation via disinhibition. J Pharmacol Exp Ther 336:328–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu NJ, von Gizycki H, Gintzler AR. (2007) Sexually dimorphic recruitment of spinal opioid analgesic pathways by the spinal application of morphine. J Pharmacol Exp Ther 322:654–660 [DOI] [PubMed] [Google Scholar]
- Loyd DR, Wang X, Murphy AZ. (2008) Sex differences in micro-opioid receptor expression in the rat midbrain periaqueductal gray are essential for eliciting sex differences in morphine analgesia. J Neurosci 28:14007–14017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Schild S, Gerall AA, Kastin AJ, Zadina JE. (1998) Endomorphin-2 is an endogenous opioid in primary sensory afferent fibers. Peptides 19:1783–1789 [DOI] [PubMed] [Google Scholar]
- Martin-Schild S, Zadina JE, Gerall AA, Vigh S, Kastin AJ. (1997) Localization of endomorphin-2-like immunoreactivity in the rat medulla and spinal cord. Peptides 18:1641–1649 [DOI] [PubMed] [Google Scholar]
- Rivera M, Gintzler AR. (1998) Differential effect of chronic morphine on mRNA encoding adenylyl cyclase isoforms: relevance to physiological sequela of tolerance/dependence. Mol Brain Res 54:165–169 [DOI] [PubMed] [Google Scholar]
- Romualdi P, Lesa G, Donatini A, Ferri S. (1995) Long-term exposure to opioid antagonists up-regulates prodynorphin gene expression in rat brain. Brain Res 672:42–47 [DOI] [PubMed] [Google Scholar]
- Rønnekleiv OK, Bosch MA, Cunningham MJ, Wagner EJ, Grandy DK, Kelly MJ. (1996) Down-regulation of μ-opioid receptor mRNA in the mediobasal hypothalamus of the female guinea pig following morphine treatment. Neurosci Lett 216:129–132 [PubMed] [Google Scholar]
- Shy M, Chakrabarti S, Gintzler AR. (2008) Plasticity of adenylyl cyclase-related signaling sequelae after long-term morphine treatment. Mol Pharmacol 73:868–879 [DOI] [PubMed] [Google Scholar]
- Sim LJ, Selley DE, Dworkin SI, Childers SR. (1996) Effects of chronic morphine administration on μ opioid receptor-stimulated [35S]GTPγS autoradiography in rat brain. J Neurosci 16:2684–2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl GR, Ryan JP, Schwartz JP. (1988) Morphine alters preproenkephalin gene expression. Brain Res 459:391–397 [DOI] [PubMed] [Google Scholar]
- Wang X, Traub RJ, Murphy AZ. (2006) Persistent pain model reveals sex difference in morphine potency. Am J Physiol Regul Integr Comp Physiol 291:R300–R306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whistler JL, Chuang HH, Chu P, Jan LY, von Zastrow M. (1999) Functional dissociation of μ opioid receptor signaling and endocytosis: implications for the biology of opiate tolerance and addiction. Neuron 23:737–746 [DOI] [PubMed] [Google Scholar]
- Yeh GC, Chen HL, Wang TL, Tao PL. (1998) Alteration in transcripting the gene encoding the δ-opioid receptor in rat brain is not underlying the development of tolerance to [d-Ala2,d-Leu5] enkephalin. Chin J Physiol 41:217–221 [PubMed] [Google Scholar]
- Zadina JE, Hackler L, Ge LJ, Kastin AJ. (1997) A potent and selective endogenous agonist for the μ-opiate receptor. Nature 386:499–502 [DOI] [PubMed] [Google Scholar]