Abstract
Bilateral loss of vestibular sensation can disable individuals whose vestibular hair cells are injured by ototoxic medications, infection, Ménière’s disease or other insults to the labyrinth including surgical trauma during cochlear implantation. Without input to vestibulo-ocular and vestibulo-spinal reflexes that normally stabilize the eyes and body, affected patients suffer blurred vision during head movement, postural instability, and chronic disequilibrium. While individuals with some residual sensation often compensate for their loss through rehabilitation exercises, those who fail to do so are left with no adequate treatment options. An implantable neuroelectronic vestibular prosthesis that emulates the normal labyrinth by sensing head movement and modulating activity on appropriate branches of the vestibular nerve could significantly improve quality of life for these otherwise chronically dizzy patients.
This brief review describes the impact and current management of bilateral loss of vestibular sensation, animal studies supporting the feasibility of prosthetic vestibular stimulation, and a vestibular prosthesis designed to restore sensation of head rotation in all directions. Similar to a cochlear implant in concept and size, the Johns Hopkins Multichannel Vestibular Prosthesis (MVP) includes miniature gyroscopes to sense head rotation, a microcontroller to process inputs and control stimulus timing, and current sources switched between pairs of electrodes implanted within the vestibular labyrinth. In rodents and rhesus monkeys rendered bilaterally vestibular-deficient via treatment with gentamicin and/or plugging of semicircular canals, the MVP partially restores the vestibulo-ocular reflex for head rotations about any axis of rotation in 3-dimensional space. Our efforts now focus on addressing issues prerequisite to human implantation, including refinement of electrode designs and surgical technique to enhance stimulus selectivity and preserve cochlear function, optimization of stimulus protocols, and reduction of device size and power consumption.
Bilateral Vestibular Deficiency – Manifestations and Epidemiology
Normally, both eyes rotate opposite the direction of any head rotation in order to stabilize images on each retina. This compensatory rotation is driven by the angular vestibulo-ocular reflex (aVOR), for which sensory input is provided by three mutually orthogonal semicircular canals (SCC) in each inner ear’s vestibular labyrinth. Profound bilateral loss of vestibular sensation disrupts the aVOR and other vestibular reflexes. Affected individuals suffer illusory drift of visual fields during head movement, chronic disequilibrium and postural instability that interfere with otherwise routine activities such as walking or driving (Minor, 1998).
The severity of disability due to bilateral vestibular deficiency (BVD) depends on the degree of loss and one’s age at onset. Children with congenital BVD suffer delays in reaching developmental milestones for sitting, standing and walking (Abadie et al., 2000), but plasticity of the developing brain is such that many are nearly asymptomatic as long as they avoid rapid head movements and poorly lit places. In contrast, adults with acquired BVD may be dramatically affected by chronic disequilibrium, oscillopsia (illusory visual field movement during head movements), increased fall tendency and cognitive dysfunction due to the need for constant attention to normally automatic functions.
Accurate estimates of prevalence and incidence for bilateral vestibular deficiency (BVD) are difficult to generate, due to lack of central reporting mechanisms like those in place for deafness or blindness. However, useful data have recently become available through the 2008 United States National Health Interview Survey, which included detailed questions about balance disorders (NCHS 2008). Of 21,782 adults surveyed in a random national sampling, 26 respondents reported a history consistent with chronic, disabling, profound BVD (i.e., all of the following: dizzy in the past year, visual blur during head movement despite little/no problem reading a newspaper [presumably while still], unsteadiness, symptom duration >5 years, and severity of problem “big” or “very big”). This yields an estimated point prevalence of 120/100,000 adults, which equates to a prevalence of about 700,000 severe/profound BVD adults in the United States and European Union, or 6 million worldwide.
About 1.6% of the ~800 new dizzy patients evaluated per year in the Johns Hopkins vestibular clinic present with chief complaints ultimately attributed to profound BVD confirmed by history, exam and caloric electronystagmography. Assuming a referral population of ~3M and regional community otolaryngologists who (according to an informal survey) refer ~25% of their BVD cases to our center, we can roughly estimate an incidence of about 1.7 new cases per 100K adult population per year.
Since many cases are due to aminoglycoside toxicity, this rate of iatrogenic BVD may fall with increasing physician awareness and availability of alternate antibiotic regimens. However, iatrogenic BVD due to cochlear implantation (CI) may rise as binaural CI becomes more common. A recent study by our group revealed a 1/28 rate of new ipsilateral profound vestibular loss after CI, suggesting a ~3% risk when performing CI in a patient’s “only balancing ear” and a ~0.1% rate of iatrogenic BVD in simultaneous bilateral CI with preoperatively normal labyrinths (Melvin, et al. 2009).
Current and Future Management of Bilateral Vestibular Deficiency
After cessation of known causative agents (such as aminoglycoside antibiotics) and vestibular suppressant medications, the mainstay of current therapy for BVD is vestibular rehabilitation therapy, which involves gaze and posture stabilization exercises intended to enlist visual and proprioceptive cues to partly supplant missing vestibular sensation (Brown et al., 2001; Krebs et al., 1993). Traditional exercises help most BVD patients compensate adequately to resume normal daily activities, and novel paradigms involving incremental adjustment of desired VOR gain may improve outcomes further (Schubert et al., 2008). However, BVD patients who fail to compensate currently have no adequate treatment options.
Noninvasive approaches to supplanting missing vestibular sensation have included tactile stimulation of the torso,(Peterka et al., 2006; Kentala et al., 2003) sound presented via headphones,(Dozza et al., 2005; Hegeman et al., 2005)and even electrical stimulation of the tongue (Tyler et al., 2003). These may augment postural reflexes, but none can emulate SCC function with sufficient speed or fidelity to restore a normal aVOR. Transcutaneous galvanic vestibular stimulation (TCGVS) has been suggested as a noninvasive technique for modulating vestibular nerve activity via conducting pads applied to post-auricular skin, but even at currents that cause intolerable pain, TCGVS elicits only a slight horizontal-torsional nystagmus that is much too small, too slow and too limited in direction to stabilize gaze during head movements of everyday life (MacDougall et al., 2005).
In contrast to TCGVS, the striking success of cochlear implants suggests that a vestibular prosthesis implanted in the labyrinth could selectively stimulate individual vestibular nerve branches. By measuring head rotation using head-mounted or implanted sensors, decomposing it into components parallel to the planes of the SCCs, and encoding it via selective electrical stimulation of the corresponding ampullary branches of the vestibular nerve, such a device should restore a useful aVOR and reduce the chronic disequilibrium typical of BVD.
Drawing on classic experiments by Suzuki, Cohen and others who demonstrated the ability to evoke eye movements via the aVOR through electrical stimulation of ampullary nerves (e.g., Suzuki and Cohen, 1964), Merfeld, Gong and colleagues created a single-channel head-mounted vestibular prosthesis able to measure head movement in one direction and elicit eye movements in animals rendered BVD though SCC plugging (Gong and Merfeld 2000, 2002; Merfeld et al., 2006, 2007; Gong et al., 2008)
Current projects in the Johns Hopkins Vestibular Neuroengineering Lab are advancing prosthetic vestibular stimulation toward clinical application by (1) developing an animal model of BVD that accurately reflects the condition of human patients affected by aminoglycoside ototoxicity, (2) characterizing the electrically-evoked 3-dimensional [3D] aVOR in BVD animals, (3) creating a multichannel vestibular prosthesis able to restore the 3D aVOR for all directions of head rotation, (4) developing a model of electric current flow in the implanted labyrinth to facilitate optimal design of electrodes and stimulus protocols, (5) assessing hearing outcomes after vestibular implantation, and (6) extending our approach to nonhuman primates with anatomy similar to humans. In what follows, we briefly review the status and trajectory of these efforts.
Gentamicin Ototoxicity
To develop an assay of prosthesis performance, we used 3D scleral search coils and 3D binocular video-oculography to examine eye movements in response to head rotations in alert chinchillas (C. lanigera) in darkness. Normal chinchillas exhibited responses like those of normal humans, with gain (eye/head acceleration) near −1 in each canal plane (Migliaccio et al, 2007). Chinchillas treated unilaterally with intratympanic gentamicin exhibited findings typical of similarly treated humans, with decreased gain for head movements exciting each semicircular canal of the treated labyrinth (Della Santina et al., 2005). Immunofluorescent labelling of gentamicin delivered via intratympanic injection revealed selective uptake and retention of gentamicin in vestibular hair cells, with Type I hair cells mostly destroyed and Type II hair cells losing stereocilia (Lyford-Pike et al, 2007). However, single-unit recording and histologic exam confirmed persistence of viable vestibular nerve afferent fibers in treated cristae, likely supported by on-going neurotransmitter release from “hairless” Type II hair cell bodies (Della Santina et al., 2005; Hirvonen et al., 2005).
Electrically-Evoked 3-Dimensional Vestibulo-Ocular Reflex
In chinchillas treated with gentamicin to ablate vestibular sensation bilaterally, pulse-frequency-modulated prosthetic stimulation partially restored a 3D VOR with dynamics similar to that of normal animals (Della Santina et al., 2005, 2007). (See Figure 1.) Postural stability improved qualitatively, although otolith endorgans were not stimulated deliberately. As is true for multichannel cochlear implants, current spread beyond the intended target of a given electrode emerged as a key factor determining how well prosthetic stimulation can recreate a normal pattern of nerve stimulation for a given head movement. Ideally, different electrodes should stimulate each of the three ampullary nerves with high selectivity, minimizing spurious stimulation of other ampullary nerves and the utricular, saccular, cochlear and facial nerves. Optimizing stimulation selectivity is a key goal of our current research, and recent advances in electrode design, surgical technique and stimulus protocols are yielding continued improvement in outcomes (Davidovics et al., 2010; Fridman et al., 2010).
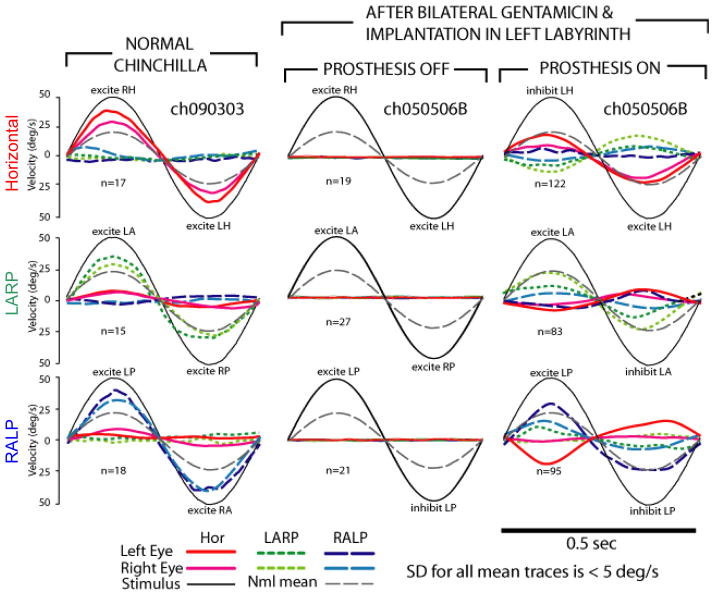
Figure 1.
In chinchillas treated with gentamicin to ablate vestibular sensation bilaterally, pulse-frequency-modulated prosthetic stimulation partially restored a 3D vestibulo-ocular reflex with gain similar to that of normal animals. (Column 1) Mean head rotation and eye rotations of a normal chinchilla during 2 Hz 50°/s head rotations about mean horizontal (top), left-anterior/right-posterior (LARP, middle), and right-anterior/left-posterior (RALP, bottom) semicircular canal (SCC) axes in darkness. Each component of the 3D response is shown. This animal’s gains were higher than the average responses of 5 normal animals (thin dotted line in each panel). Head or eye traces are inverted as required to facilitate visual comparison; n=number of cycles averaged. (Column 2) Responses for a chinchilla treated bilaterally with gentamicin and implanted with electrodes in the three left SCCs, with prosthetic stimulation off. (Column 3) Responses of same animal to same head rotations, 3.5 h after activation of the multichannel prosthesis. (Adapted with permission from Della Santina et al., 2007).
Multi-Channel Prosthesis Device Development
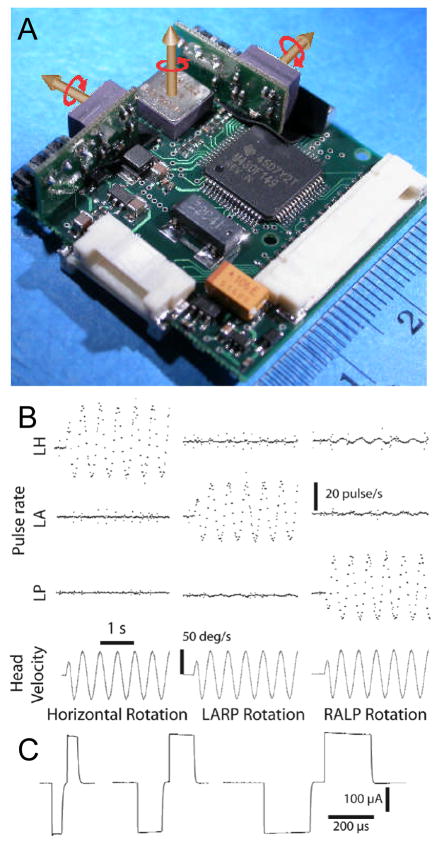
The first-generation Johns Hopkins Multichannel Vestibular Prosthesis (MVP1; see Figure 2) employed three mutually orthogonal, micromachined gyro sensors to emulate the transduction of 3D head angular velocity by the three SCCs of one labyrinth (Della Santina et al., 2007). The space and power required for the MVP1’s core circuitry (30×34×10 mm3 and ~140 mW) are adequate for research applications in which the processor and sensors are fixed to the skull externally and connected to implanted electrodes via a percutaneous connector. However, transition toward a wirelessly powered device implanted beneath periosteum (comparable to existing cochlear implants) has mandated a significant reduction in device thickness and power consumption. To address this need, we recently developed a second-generation device (Chiang et al., 2009). The MVP2’s core circuitry is small and thin enough (29×29×5 mm3) to fit within a hermetic container similar to the housings for cochlear implants currently in clinical use. Power consumption has been reduced ~50%, extending battery life and reducing battery size. Other enhancements include: an increase to 12 electrodes, multiple independent current sources allowing multipolar stimulation, a tri-axis linear accelerometer, control via a wireless interface, and circuitry for measurement of electrically-evoked compound action potentials.
Figure 2.
(A) The Johns Hopkins Multichannel Vestibular Prosthesis (version MVP1) uses three silicon gyroscopes to measure 3D head rotation and a microprocessor to emulate normal semicircular canal sensation and processing. Scale bar units = cm. (B) Rotation of the device about each semicircular canal axis modulates pulse rates on the electrodes implanted in that canal’s ampulla.(C) Stimuli are biphasic, charge-balanced constant-current pulses similar to those used in cochlear implants. (Adapted with permission from Della Santina et al., 2007)
Finite Element / Neuromorphic Computational Models of the Implanted Labyrinth
To better understand the biophysics of prosthetic vestibular nerve stimulation and thus facilitate design of electrode arrays with optimal selectivity, we constructed an anatomically precise finite element model of current flow in the implanted labyrinth (Hayden et al., 2008). Model geometry was created through segmentation of high-resolution microCT and microMRI images of normal and implanted chinchilla labyrinths. Meshing and finite element analysis for different electrode/stimulus conditions was performed using extensively customized versions of commercially available software packages. The model predicts potential field and current intensities through the labyrinth. Potential field values serve as input to a neuromorphic computational model incorporating well-established dynamics of vestibular afferents and axons. We tested the model by comparing its predictions against the axis of eye rotation observed during prosthetic electrical stimulation via different electrode pairs implanted in chinchilla labyrinths. The model’s predictions aligned fairly well with the actual axis of eye rotation, with mean misalignment of 20 ± 9°. We are currently extending this modelling and design approach to non-human primate and human anatomy.
Effects of Vestibular Prosthesis Electrode Implantation on the Cochlea
The size of the candidate patient population vestibular prosthesis implantation will depend on whether electrodes can be inserted into the vestibular labyrinth without cochlear injury. To estimate the risk of hearing loss due to vestibular implantation, we implanted the left SCCs of six chinchillas and compared ipsilateral hearing to contralateral ears that underwent the same mastoid surgery without SCC fenestration or implantation (Tang et al., 2009). Auditory brainstem response (ABR) thresholds to free-field clicks and tone pips at 2, 4, 6, and 8 kHz were measured bilaterally 3–9 weeks after implantation and compared between sides and against data from six normal chinchillas. Four implanted ears suffered severe hearing loss, with thresholds more than 5 standard deviations (SD) above the mean threshold for contralateral ears. However, two implanted ears retained normal hearing thresholds (within 1 SD of the contralateral mean). Eye movements could be elicited via all electrodes. Thus, implantation of functional vestibular prosthesis electrodes in chinchilla ampullae can be accomplished without significant loss of hearing, although the risk of hearing loss with the current surgical technique is high.
Extending the Approach Toward the Human Labyrinth
Although chinchillas are a useful species with which to develop and test vestibular stimulation paradigms, their labyrinths are much smaller than those of humans, so problems we encounter in chinchillas due to current spread and cochlear injury may well be less severe in humans. Prior to initial human trials, we have transitioned the studies described above to rhesus monkeys, which are closer to humans in labyrinth size and anatomy. Preliminary results from the four monkeys we have implanted to date demonstrate that prosthetic stimulation of the primate labyrinth evokes aVOR responses with amplitudes and selectivity similar to that observed in chinchillas (see Figure 3). Because monkeys can actively participate in rehabilitation exercise paradigms analogous to those we anticipate using with human subjects after implantation, we anticipate being able to improve responses over time through visual-vestibular integration exercises. ABR testing in these animals has yet to reveal any hearing loss due to implantation, suggesting that risk to the cochlea may be lower in humans than in rodents (Dai et al., 2010).
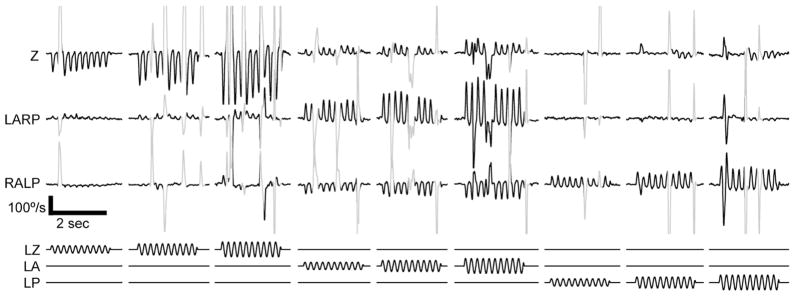
Figure 3.
Eye movement responses to pulse-rate modulated, biphasic constant-current pulses delivered via monopolar prosthesis electrodes implanted in the left labyrinth of a stationary alert rhesus monkey tested in darkness. Methods for stimulus delivery and scleral coil recording of eye movements are as described in (Dai et al., 2010). Lower traces show stimulus waveforms modulating pulse rates on electrodes in the left horizontal (LZ), left anterior (LA) and left posterior (LP) semicircular canal ampullary nerves. In each case, modulation frequency is 5 Hz (i.e., encoding a 5 Hz virtual head rotation about the axis of the stimulated canal), baseline pulse rate is 100 pulse/sec, and the three different stimulus intensities shown represent 40%, 60% and 80% of maximum possible pulse frequency modulation depth. (At 100% intensity, pulse rate would modulate down to 0 and up to 400 pulse/sec.) Upper traces show eye movements about the horizontal (Z), left-anterior/right-posterior (LARP), and right-anterior/left-posterior (RALP) axes of the head, which approximately align with the implanted semicircular canals. Slow phase nystagmus segments are black; saccades, quick phases and blinks are shown in gray. Stimulation via each canal’s electrode generates eye movements that approximately align with that canal.
Conclusion
Progress to date strongly suggests that a multichannel vestibular prosthesis designed to take the place of lost semicircular canal function is feasible and will be an effective tool for treatment for patients who are otherwise unable to compensate for profound loss of labyrinthine sensation due to hair cell injury.
Acknowledgments
Supported by the United States National Institute on Deafness and Other Communication Disorders (NIDCD) R01-DC009255, K08-DC006216 and R01-DC002390; a grant from the American Otological Society; and the Johns Hopkins School of Medicine. We thank Howard Hoffmann, of the NIDCD Translational Research Branch, for his invaluable contributions to epidemiology of BVD through his oversight of the balance segment of the National Health Interview Survey 2008.
References
- 1.Abadie V, Wiener-Vacher S, Morisseau-Durand MP, Poree C, Amiel J, Amanou L, Peigne C, Lyonnet S, Manac’h Y. Vestibular anomalies in CHARGE syndrome: investigations on and consequences for postural development. Eur J Pediatr. 2000;159:569–574. doi: 10.1007/s004319900409. [DOI] [PubMed] [Google Scholar]
- 2.Brown KE, Whitney SL, Wrisley DM, Furman JM. Physical therapy outcomes for persons with bilateral vestibular loss. Laryngoscope. 2001;111:1812–1817. doi: 10.1097/00005537-200110000-00027. [DOI] [PubMed] [Google Scholar]
- 3.Chiang B, Fridman GY, Della Santina CC. Enhancements to the Johns Hopkins Multi-Channel Vestibular Prosthesis yield reduced size, extended battery life, current steering and wireless control. Abst #213, Assoc for Research in Otolaryngology Ann Mtg (ARO); 2009.2009. [Google Scholar]
- 4.Dai C, Fridman GY, Della Santina CC. Effects of vestibular electrode implantation and prosthetic stimulation on hearing in rhesus monkeys. ARO; 2010; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davidovics NS, Fridman GY, Della Santina CC. Effects of Stimulus Pulse Parameters on Eye Movement Responses to Stimulation Delivered by a Vestibular Prosthesis. ARO; 2010.2010. [Google Scholar]
- 6.Della Santina CC, Migliaccio AA, Park HJ, Anderson IW, Jiradejvong P, Minor LB, Carey JP. 3D Vestibuloocular reflex, afferent responses and crista histology in chinchillas after unilateral intratympanic gentamicin.# 813. ARO; 2005.2005. [Google Scholar]
- 7.Della Santina CC, Migliaccio AA, Patel AH. Electrical Stimulation to Restore Vestibular Function – Development of a 3-D Vestibular Prosthesis. Conf Proc IEEE Eng Med Bio Soc. 2005;7:7380–5. doi: 10.1109/IEMBS.2005.1616217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Della Santina CC, Migliaccio AA, Patel AH. A multi-channel semicircular canal neural prosthesis using electrical stimulation to restore 3D vestibular sensation. IEEE Trans Biomed Eng. 2007;54(6 Pt 1):1016–30. doi: 10.1109/TBME.2007.894629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dozza M, Chiari L, Horak FB. Audio-biofeedback improves balance in patients with bilateral vestibular loss. Arch Phys Med Rehabil. 2005;86:1401–1403. doi: 10.1016/j.apmr.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 10.Fridman GY, Davidovics NS, Dai C, Migliaccio AA, Della Santina CC. Vestibulo-Ocular Reflex Responses to a Multichannel Vestibular Prosthesis Incorporating a 3D Coordinate Transformation for Correction of Misalignment. JARO. 2010 doi: 10.1007/s10162-010-0208-5. (accepted for publication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong W, Merfeld DM. Prototype neural semicircular canal prosthesis using patterned electrical stimulation. Ann Biomed Eng. 2000;28(5):572–81. doi: 10.1114/1.293. [DOI] [PubMed] [Google Scholar]
- 12.Gong W, Merfeld DM. System design and performance of a unilateral horizontal semicircular canal prosthesis. IEEE Trans Biomed Eng. 2002;49(2):175–81. doi: 10.1109/10.979358. [DOI] [PubMed] [Google Scholar]
- 13.Gong W, Haburcakova C, Merfeld DM. Vestibulo-ocular responses evoked via bilateral electrical stimulation of the lateral semicircular canals. IEEE Trans Biomed Eng. 2008;55(11):2608–19. doi: 10.1109/TBME.2008.2001294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayden RP, Mori S, Della Santina CC. An electroanatomical/neuromorphic model to guide electrode design for a multichannel vestibular prosthesis #792. ARO; 2008.2008. [Google Scholar]
- 15.Hegeman J, Honegger F, Kupper M, Allum JH. The balance control of bilateral peripheral vestibular loss subjects and its improvement with auditory prosthetic feedback. J Vestib Res. 2005;15:109–117. [PubMed] [Google Scholar]
- 16.Hirvonen TP, Minor LB, Hullar TE, Carey JP. Effects of intratympanic gentamicin on vestibular afferents and hair cells in the chinchilla. J Neurophysiol. 2005;93(2):643–55. doi: 10.1152/jn.00160.2004. [DOI] [PubMed] [Google Scholar]
- 17.Kentala E, Vivas J, Wall C., III Reduction of postural sway by use of a vibrotactile balance prosthesis prototype in subjects with vestibular deficits. Ann Otol Rhinol Laryngol. 2003;112:404–409. doi: 10.1177/000348940311200503. [DOI] [PubMed] [Google Scholar]
- 18.Krebs DE, Gill-Body KM, Riley PO, Parker SW. Double-blind, placebo-controlled trial of rehabilitation for bilateral vestibular hypofunction: preliminary report. Otolaryngol Head Neck Surg. 1993;109:735–741. doi: 10.1177/019459989310900417. [DOI] [PubMed] [Google Scholar]
- 19.Lyford-Pike S, Vogelheim C, Chu E, Della Santina CC, Carey JP. Gentamicin is primarily localized in vestibular type I hair cells after intratympanic administration. JARO8. 2007;(4):497–508. doi: 10.1007/s10162-007-0093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacDougall HG, Brizuela AE, Burgess AM, Curthoys IS, Halmagyi GM. Patient and normal three-dimensional eye-movement responses to maintained (DC) surface galvanic vestibular stimulation. Otol Neurotol. 2005;26:500–511. doi: 10.1097/01.mao.0000169766.08421.ef. [DOI] [PubMed] [Google Scholar]
- 21.Melvin TA, Della Santina CC, Carey JP, Migliaccio AA. The effects of cochlear implantation on vestibular function. Otol Neurotol. 2009;30(1):87–94. doi: 10.1097/mao.0b013e31818d1cba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merfeld DM, Gong W, Morrissey J, Saginaw M, Haburcakova C, Lewis RF. Acclimation to chronic constant-rate peripheral stimulation provided by a vestibular prosthesis. IEEE Trans Biomed Eng. 2006;53(11):2362–72. doi: 10.1109/TBME.2006.883645. [DOI] [PubMed] [Google Scholar]
- 23.Merfeld DM, Haburcakova C, Gong W, Lewis RF. Chronic vestibulo-ocular reflexes evoked by a vestibular prosthesis. IEEETrans Biomed Eng. 2007;54(6/1):1005–15. doi: 10.1109/TBME.2007.891943. [DOI] [PubMed] [Google Scholar]
- 24.Migliaccio AA, Minor LB, Della Santina CC. The 3-Dimensional angular vestibulo-ocular reflex evoked by high-acceleration rotations in normal chinchilla is conjugate, nonlinear and isotropic #944. ARO; 2007. [Google Scholar]
- 25.Minor LB. Gentamicin-induced bilateral vestibular hypofunction. JAMA. 1998;279(7):541–4. doi: 10.1001/jama.279.7.541. [DOI] [PubMed] [Google Scholar]
- 26.Natl Ctr for Health Statistics. National Health Interview Survey 2008. Hyattsville, Maryland: United States National Institute on Deafness and Other Communication Disorders and United States Public Health Service; 2008. [Google Scholar]
- 27.Peterka RJ, Wall C, III, Kentala E. Determining the effectiveness of a vibrotactile balance prosthesis. J Vestib Res. 2006;16:45–56. [PubMed] [Google Scholar]
- 28.Schubert MC, Della Santina CC, Shelhamer M. Incremental angularvestibulo-ocular reflex adaptation to active head rotation. Exp Brain Res. 2008;191(4):435–46. doi: 10.1007/s00221-008-1537-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki JI, Cohen B. Head, eye, body and limb movements from semicircular canal nerves. Exp Neurol. 1964;10:393–405. doi: 10.1016/0014-4886(64)90031-7. [DOI] [PubMed] [Google Scholar]
- 30.Tang S, Melvin TA, Della Santina CC. Effects of semicircular canal electrodeimplantation on hearing in chinchillas. Acta Otolaryngol. 2009;129(5):481–6. doi: 10.1080/00016480802252243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tyler M, Danilov Y, Bach YR. Closing an open-loop control system: vestibular substitution through the tongue. J Integr Neurosci. 2003;2:159–164. doi: 10.1142/s0219635203000263. [DOI] [PubMed] [Google Scholar]