Abstract
Unilateral vestibular lesions cause marked asymmetry in the horizontal vestibulo-ocular reflex (VOR) during rapid head rotations, with VOR gain being lower for head rotations toward the lesion than for rotations in the opposite direction. Reducing this gain asymmetry by enhancing ipsilesional responses would be an important step toward improving gaze stability following vestibular lesions. To that end, there were two goals to this study. First, we wanted to determine whether we could selectively increase VOR gain in only one rotational direction in normal monkeys by exposing them to a training session comprised of a 3 hr series of rotations in only one direction (1000°/s2 acceleration to a plateau of 150°/s for 1 s) while they wore 1.7x magnifying spectacles. Second, in monkeys with unilateral vestibular lesions, we designed a paradigm intended to reduce the gain asymmetry by rotating the monkeys toward the side of the lesion in the same way as above but without spectacles. There were three main findings: 1) unidirectional rotations with magnifying spectacles result in gain asymmetry in normal monkeys, 2) gain asymmetry is reduced when animals are rotated towards the side of the labyrinthectomy via the ipsilesional rotation paradigm, 3) repeated training causes lasting reduction in VOR gain asymmetry.
Introduction
Permanent loss of function in one labyrinth can occur for a variety of reasons, including vestibular neuritis (Nadol 1995; Palla et al. 2004), vestibular schwannoma resection (Minor et al. 1999a), and intratympanic gentamicin delivery for treatment of intractable Meniere's disease (Carey et al. 2002). Individuals with reduced or absent function in one labyrinth transiently suffer vestibular symptoms including spontaneous nystagmus, head tilt and postural dysfunction due to the imbalance in tonic input to the brainstem vestibular nuclei (Baloh et al. 1984; Paige 1989). The brain compensates well to these static imbalances in that the majority of these symptoms and signs disappear over time (Fetter and Zee 1988). The response of the angular vestibulo-ocular reflex (VOR) to rotations of low-frequency and velocity also returns toward normal after unilateral vestibular lesions through process of vestibular compensation. However, a marked asymmetry in the response to high-frequency, high-acceleration rotations persists (Halmagyi and Curthoys 1988; Halmagyi et al. 1990; Aw et al. 1996; Cremer et al. 1998; Carey et al. 2007). VOR gain is markedly reduced when the head moves in the direction towards the lesion (ipsilesional) but is closer to normal when the head moves away from the lesion (contralesional). This asymmetry in gain causes oscillopsia, decreased visual acuity and a feeling of imbalance during ipsilesional head rotation.
Interestingly, evidence suggests that animals with stable asymmetric VOR gain deficits after unilateral labyrinthectomy (UL) retain sufficient “adaptive reserve” to raise the gain of the VOR if they are exposed to a training regimen comprising repeated whole body rotations in both directions while viewing a patterned background (Maioli and Precht 1985) or while wearing magnifying spectacles (Clendaniel et al. 2003). This raises a question: If VOR gain for ipsilesional rotations can be increased through selective adaptation training, then why does this not occur spontaneously through recovery processes? One reason could be that during normal visual conditions and natural head movements typical of daily life, VOR gain adaptation predominantly acts on semicircular canal inputs after a difference signal between the two labyrinths has been computed, so that gain increases that would bring the deficient ipsilesional VOR up toward normal do not occur because they would be accompanied by retinal slip in the opposite direction causing an undesirable change in contralesional VOR gain to above normal. If this is the case, then a training session that introduces retinal slip only for ipsilesional head rotations and never for contralesional head rotations might decouple ipsilesional and contralesional VOR gain adaptation sufficiently to allow selective enhancement of ipsilesional VOR gain.
Constrained learning paradigms have been successfully employed to overcome other asymmetric motor deficits, such as when a healthy appendage is constrained from use so that selective use of the unhealthy side facilitates compensation (Taub et al. 1993; Taub et al. 1994; Wittenberg and Schaechter 2009; Helveston 2010). Because the VOR shows a robust capacity to adapt to optically-induced changes (Gonshor and Melvill Jones 1976; Clendaniel et al. 2001; Clendaniel et al. 2002; Schubert et al. 2008) we hypothesized that an analogous asymmetric VOR training paradigm might selectively increase the ipsilesional gain of the VOR. We first tested this hypothesis by rotating monkeys in only one direction (1000°/s2 leftward acceleration to a plateau of 150°/s for 1 s, repeated for 3 hrs) while they wore 1.7x magnifying spectacles. This was to determine whether normal monkeys exposed to this training regimen could increase VOR gain asymmetrically. Second, we tested an analogous paradigm in monkeys with stable asymmetric VOR gains after unilateral labyrinthine lesions, by rotating the monkeys towards the side of the lesion in the same way as above but without spectacles.
Methods
Surgical preparation
All animal procedures were performed in accordance with a protocol approved by the Johns Hopkins University School of Medicine Animal Care and Use Committee. Surgery was done under sterile conditions in three rhesus monkeys (1 Macaca mulatta and 2 M. fasicularis) anesthetized with inhalation of halothane/nitrous oxide/oxygen. A head bolt was cemented to the skull at a position such that the animal was pitched 15° nose-down relative to the horizontal stereotaxic plane when in the upright position. The horizontal semicircular canals are approximately in the earth-horizontal plane when the head is in this position (Blanks et al. 1985; Minor and Goldberg 1990; Minor et al. 1999b). A prefabricated search coil was implanted in the frontal plane about the limbus of each eye (Minor and Goldberg 1991; Paige and Tomko 1991; Lasker et al. 1999), and the leads were soldered to plugs cemented to the skull. Labyrinthectomy was performed on the left side in each animal by making a postauricular incision and removing the mastoid bone with an otologic drill and curettes to expose the horizontal and posterior semicircular canals. The petrous bone was removed further anteriorly and superiorly to visualize the superior canal near its union with the common crus. Each of the semicircular canals then was obliterated with removal of the ampulla. The vestibule was entered, and the utriculus and sacculus were removed. The internal auditory canal was opened next, and the distal ends of the ampullary nerve branches were removed. The space created by the labyrinthectomy was packed with muscle and fascia and the postauricular incision was closed (Lasker et al. 2000).
Eye movement recording and rotational testing
Each animal was seated in a plastic chair with its head restrained by securing the implanted bolt to a chair-mounted clamp. The chair was connected to a superstructure that was mounted to the top surface of a servo-controlled rotation table capable of generating a peak torque of 375 N-m (Acutronic, Pittsburgh, PA). The horizontal angular vestibulo-ocular reflex (VOR) was tested with the animal seated in the upright position in the superstructure and aligned such that the horizontal canals were approximately in the earth-horizontal plane ofrotation. The VOR was measured in darkness before and after adaptation by using a step stimulus consisting of 1,000°/s2 accelerations to a peak velocity of 150°/s followed by a plateau of head velocity lasting 0.45 s and then deceleration at 1,000°/s2 to rest (Fig. 1). Eye movement trials were removed if the animal blinked or made a saccade during the initial head acceleration. In order to keep the animals alert during the testing sessions, D-amphetamine (0.2-0.3 mg/kg) was given orally 15 min before the beginning of a session (Raphan et al. 1979, Minor et al. 1999b)
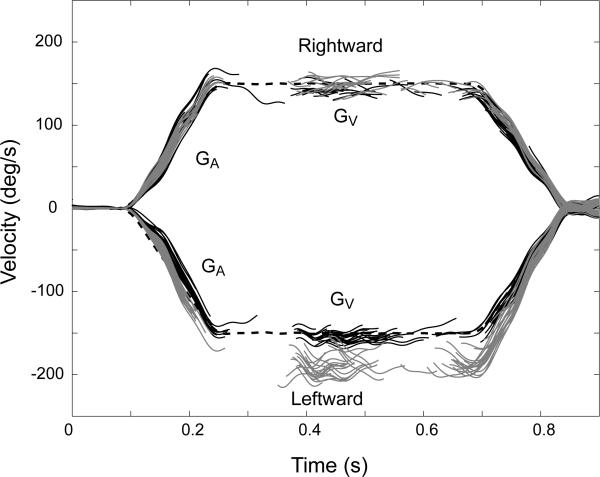
Figure 1.
Responses to rightward (positive) and leftward (negative) yaw head rotations (dashed, black) (1000°/s2 to a peak head velocity of 150°/s). Eye responses are shown with the fast phases removed. Eye responses in the dark before training are in black. Monkey was then fitted with 1.7 X magnifying spectacles and trained with the unidirectional training paradigm in the counterclockwise direction. Post training eye responses are shown in gray.
Adaptation paradigms
We have three training paradigms each of which was made up of multiple sessions. The first training paradigm was performed in 3 monkeys before vestibular lesions. The second and third training paradigms were performed in monkeys who have had a unilateral labyrinthectomy on the left side.
Paradigm 1 - Training in normal animals
Before each training session each animal was rotated in the dark with the step stimuli randomly in the leftward (n=30) and rightward (n=30) directions (Fig. 1). To induce adaptation, monkeys were first fit with magnifying (1.7X) spectacles that were attached to their acrylic skullcap. Each session for each animal consisted of a series of steps in the leftward direction (described above) for 3 hours. Animals were kept stationary for 1.1 s between each rotation, to allow for the horizontal semicircular canal cupullae to go back to their resting position. This minimized development of left-beating nystagmus due to velocity storage (Raphan et al. 1979). Because each step stimulus took a total of 2 seconds, each animal was rotated 5400 times during the 3 hour adaptation session. After the training session was completed each animal was rotated in the dark with the step stimuli in the leftward (n =30) and rightward (n = 30) directions (Fig. 1). In normal animals this training session was repeated 1 week later in the rightward direction.
Training after Unilateral Labyrinthectomy
Paradigm 2 (Ipsilesional training)
All three monkeys underwent the following procedure. First a unilateral labyrinthectomy was performed on the left side of each animal as described above. Sadeghi et al. (2006) have shown that VOR gain reaches a steady-state level within 25 days after the lesion; therefore, we allowed animals to recover for 4-5 weeks in normal diurnal lighting conditions without exposure to any training sessions. The VOR was tested 3-4 times in the dark during this time to obtain baseline data before the training sessions were started. During the second month after the labyrinthectomy the animals began the ipsilesional direction training paradigm. The ipsilesional direction training paradigm consisted of a series (3 or 4) of training sessions over the next month that were similar to the training sessions described for normal animals.
Before each training session each animal was rotated in the dark with the step stimuli randomly in the leftward (n = 30) and rightward (n = 30) directions (Fig. 2) in order to determine the gain of the VOR. In the training sessions after labyrinthectomy, animals were not fitted with magnifying spectacles but instead were rotated without spectacles in the ipsilesional direction. The gain was already low in this direction and we wanted to determine the effects of adaptation without additional magnification. Each session for each animal consisted of a series of steps in the ipsilesional direction for 3 hours. A total of 5400 ipsilesional rotations were performed on each animal during each adaptation session. The VOR was then tested in each animal in the dark to determine the acute changes in gain after the adaptation session. Each animal was tested in the dark again 3 days later (returning to their normal cages and lighting conditions in the interim) to determine whether changes persisted. A training session was not performed at this point, just the testing of the VOR. Four days later, animals were then exposed to another identical training session. This procedure was repeated in each monkey 2-3 additional times.
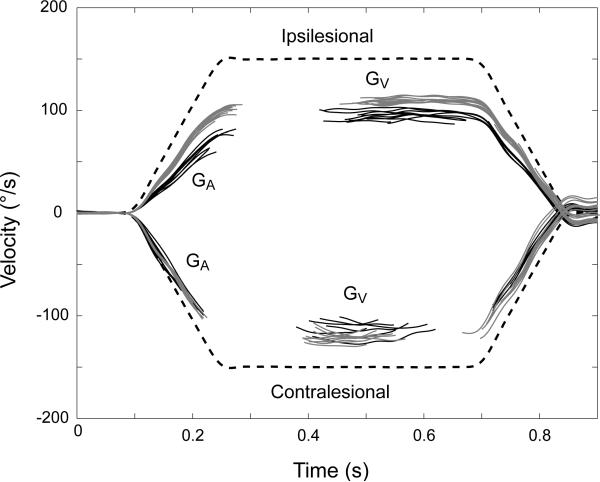
Figure 2.
Responses to ipsilesional (positive) and contralesional (negative) yaw head rotations (dashed, black) (1000°/sec2 to a peak head velocity of 150°/sec). Eye responses are shown with the fast phases removed. Eye responses in the dark before the ipsilesional training paradigm are in black. Monkey 1 was trained with the ipsilesional training paradigm in the ipsilesional direction (training paradigm 2). Post training eye responses are shown in gray.
Paradigm 3 - Bidirectional training
After Experiment 2 was concluded, animals were tested with a bidirectional training session. Before each training session each animal was rotated in the dark with the step stimuli randomly in the leftward (n = 30) and rightward (n = 30) directions in order to determine the gain of the VOR. In the training sessions after labyrinthectomy, animals were not fitted with magnifying spectacles but instead were rotated without spectacles. Each session for each animal consisted of a series of steps randomly in the ipsilesional and contralesional directions for 3 hours. A total of 5400 rotations (2700 contralesional and 2700 ipsilesional) were performed on each animal during each adaptation session. The VOR was then tested in each animal in the dark to determine the acute changes in gain after the adaptation session. Animals were then exposed to a final training session approximately 2 weeks later for a total of 2 sessions in each animal
Data Analysis
Gains were measured during the 1000°/s2 to 150°/s steps by the mean ratio of eye velocity divided by head velocity during the constant-acceleration portion of the stimulus (GA) and during the constant-velocity plateau of the step (GV) (Fig. 1). GA was measured during the initial 20 – 120 msec after the start of the stimulus and GV was measured during 500-700 msec after the start of the stimulus. Fast phases were removed prior to the measuring of gains. We quantified gain asymmetry for GA (SGA) and GV (SGV) by computing the ratio between the contra- vs ipsilesional difference in gain and the average gain, SGx = 200*(Gxcontra – Gxipsi)/ (Gxcontra + Gxipsi).
Results were described as means ± SD. Data from two groups were compared with an unpaired t-test. ANOVA was used to compare data from more than two groups.
Results
Normal animals (Before and after magnifying spectacles)
Fig. 1 shows the individual responses in monkey 1 before and after spectacle-induced adaptation for steps of yaw head velocity in the rightward and leftward directions. All VOR training and testing was done with this stimulus. Three monkeys were tested before and after 1.7 X spectacle-induced adaptation. The average change in GA, GV and the values for SGA and SGV are shown in Table 1. On average GA increased by 21 ± 5% (mean ± SD, p < 0.001, n = 6) and GV increased by 18 ± 5% (p < 0.001, n = 6) when pooling together all rotations in the direction of the adapting stimulus compared to no change for rotations in the opposite direction. When gain was measured one week after the first training session there was no difference from pre-adaptation values from a week before (p > 0.35).
Table 1.
Mean ± SD percentage change in gain from before to after the unidirectional training paradigm using 1.7 X magnifying lenses in normal monkeys. A negative change in asymmetry SGA denotes a change in the rightward direction. SGA and SGV refer to the total asymmetry of the response.
| Training Paradigm | Change in GA | Change in GV | ||||
|---|---|---|---|---|---|---|
| CW | CCW | SGA | CW | CCW | SGV | |
| Rightward | 0.24 ± 0.07 | -0.02 ± 0.06 | -0.23 ± 0.07a | 0.17 ± 0.05 | -0.02 ± 0.09 | -0.18 ± 0.07a |
| Leftward | -0.04 ± 0.04 | 0.19 ± 0.03 | 0.21 ± 0.03a | -0.03 ± 0.04 | 0.19 ± 0.06 | 0.20 ± 0.03a |
p < 0.008
Responses after labyrinthectomy
Unilateral labyrinthectomy resulted in an enduring asymmetry in VOR gain during step rotations that persisted throughout the testing. Figure 2 shows an example of an increase in the ipsilesional gain and a reduction of asymmetry after performing the ipsilesional adaptation paradigm.
Before ipsilesional training
After the labyrinthectomy there was a significant asymmetry with SGA measuring 0.62 ± 0.09 and SGV measured 0.54 ± 0.01, 1 week after the labyrinthectomy. By 4-5 weeks after labyrinthectomy (and prior to any training), SGA had remained diminished (0.50 ± 0.05, p > 0.1) but SGV had decreased to 0.28 ± 0.01 (p < 0.015). These findings agree with the general trend that the high-frequency asymmetry (SGA) does not recover after unilateral vestibular loss whereas the low-frequency asymmetry (SGV) shows significant recovery.
Acute effects of ipsilesional and bidirectional training paradigms
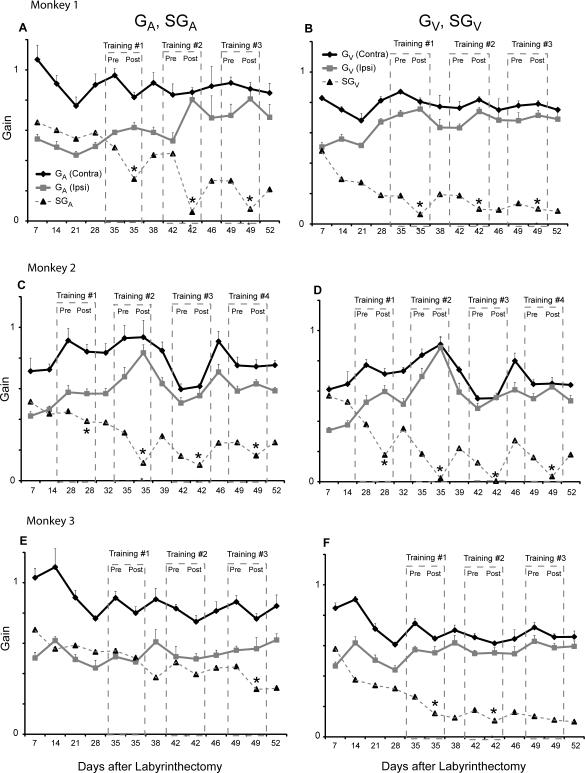
In order to study the specific effects of training for each individual monkey, we show the changes in SGA and SGV before and after each ipsilesional training paradigm in Figure 3. In monkeys 1 and 2 a reduction in SGA and SGV was evident after each training session. In monkey 3 a reduction in SGA was only evident after the third training session, and a reduction of SGV only occurred after the first and second training sessions (p <0.05). For the first training session in monkeys 1 and 2, the reduction in SGA was due to a decrease in contralesional gain with no change in ipsilesional gain (p < 0.05 for GAcontra, p > 0.05 for GAipsi). In contrast, the reduction of SGA in the subsequent sessions in monkeys 1 and 2 was due to an increase in the ipsilesional gain (p < 0.05 for GAipsi, p > 0.05 for GAcontra). For each of the training sessions, reduction in SGV was due to an increase in ipsilesional gain (p < 0.05 for GAipsi, p > 0.05 for GAcontra). Monkey 3 showed no increase in ipsilesional gain after each ipsilesional training session for either GA or GV (p > 0.05). We pooled all of the training sessions from each monkey before and after ipsilesional training (Table 2). After the ipsilesional training paradigm, SGA decreased an average of 15 ± 10 % (p < 0.013) and SGV decreased an average of 11 ± 5% (p < 0.018).
Figure 3.
Data after the labyrinthectomy from each of the 3 animals before and after ipsilesional training. A (monkey 1), C (monkey 2) and E (monkey 3) show the contralesional GA, ipsilesional GA and SGA for each monkey over the first 2 months after the labyrinthectomy. B (monkey 1), D (monkey 2) and F (monkey 3) show the contralesional GV, ipsilesional GV and SGV for each monkey. Contralesional gain is denoted in black and ipsilesional gain is denoted in gray. SGA and SGV are shown with a dashed line. Dashed boxes denote the gains tested immediately before and after each ipsilesional training session. Pre refers to the gain tested before the ipsilesional training session and post refers to the gain values tested immediately after the ipsilesional training session. In every training session animals were trained by rotating them in the ipsilesional direction without magnifying spectacles. An asterisk denotes if there was a reduction in asymmetry after each ipsilesional training session (p < 0.05). In all animals there is was a general decline in the asymmetry over time.
Table 2.
The Mean ± SD percentage change in gain from before and after all of the ipsilesional and bidirectional training sessions in the unilateral labyrinthectomized monkeys. We refer to the % change in SGA and SGV with a negative change in asymmetry denoting a decrease in the asymmetry of the response and positive value denoting an increase in the asymmetry. Ipsilesional training sessions caused a decrease in the average asymmetry while bidirectional rotations caused no change in the asymmetry.
| Training Paradigm | Change in GA and SGA | Change in GV and SGV | ||||
|---|---|---|---|---|---|---|
| Ipsi | Contra | SGA | Ipsi | Contra | SGV | |
| Ipsilesional | 0.10 ± 0.16 | -0.06 ± 0.07 | -0.15 ± 0.10a | 0.09 ± 0.10 | 0.01 ± 0.06 | -0.11 ± 0.05a |
| Bidirectional | -0.01 ± 0.14 | 0.05 ± 0.11 | 0.06 ± 0.19b | 0.06 ± 0.10 | 0.06 ± 0.07 | 0.00 ± 0.05b |
The p values for the comparisons indicated by superscripts are as follows:
p < 0.018
p > 0.38
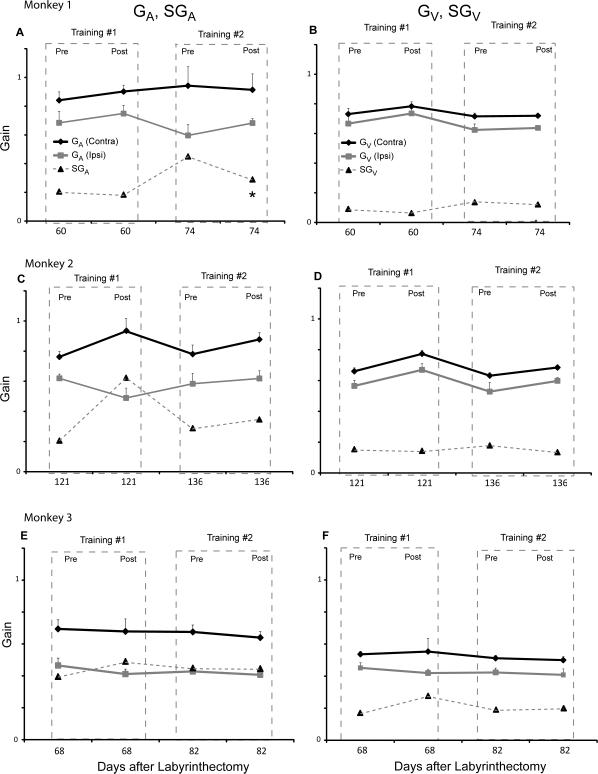
After the bidirectional training paradigm (Figure 4), SGA and SGV decreased in only 1 of the 6 training sessions (monkey 1, training session 2). When we pooled the bidirectional training sessions we found no significant change in gain or asymmetry in SGA (p > 0.38) or SGV (p > 0.97).
Figure 4.
Responses before and after the bidirectional training for each monkey. A (monkey 1), C (monkey 2) and E (monkey 3) show the contralesional GA, ipsilesional GA and SGA for each monkey during the 3rd month after the labyrinthectomy. B (monkey 1), D (monkey 2) and F (monkey 3) show the contralesional GV, ipsilesional GV and SGV for each monkey. Contralesional gain is denoted in black and ipsilesional gain is denoted in gray. SGA and SGV are shown with a dashed line. Dashed boxes denote the gains tested immediately before and after each bidirectional training session. Pre refers to the gain tested before the bidirectional training session and post refers to the gain values tested immediately after the bidirectional training session. In every training session animals were trained by rotating them randomly in the ipsilesional and contralesional directions without magnifying spectacles. An asterisk denotes if there was a reduction in asymmetry after each bidirectional training session (p < 0.05).
Long-term changes after ipsilesional training
Figure 5 shows the average contra and ipsilesional gain at four specific time points during the first 2 months after unilateral labyrinthectomy before and after the ipsilesional training sessions. After the last ipsilesional training session, SGA was 0.18 ± 0.11 [p < 0.01 when compared to SGA (0.50 ± 0.05) from 1 month prior] and SGV decreased to 0.08 ± 0.04 [p < 0.04 when compared to SGV (0.28 ± 0.01) from 1 month prior]. After waiting 3 days after the last ipsilesional learning paradigm the value for SGA was 0.26 ± 0.05 (p < 0.01, compared to SGA from 1 month prior). SGV was unchanged at 0.13 ± 0.05 (p > 0.07 compared to SGV from 1 month prior).
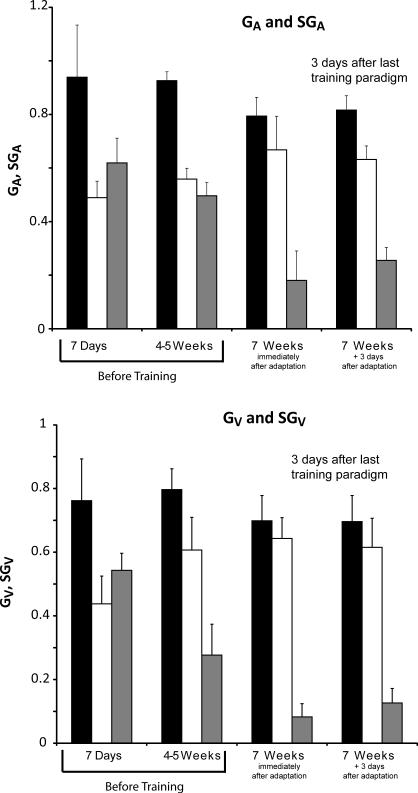
Figure 5.
Average long-term change in GA (A) and GV (B) for each labyrinthectomized monkey after the ipsilesional adaptation paradigm. Bars in black represent the gains in the contralesional direction. White bars represent the gain in the ipsilesional direction. Gray bars represent SGA in A and SGV in B. Before ipsilesional training there is a marked asymmetry in SGA 7 days after the lesion. This asymmetry is still evident 4-5 weeks after the lesion (p > 0.1). At 7 weeks after the lesion, after 3-4 ipsilesional adaptation sessions, there is a marked reduction in SGA (p < 0.01). This reduction in SGA persists when tested 3 days later (p < 0.01). Before ipsilesional training there is a marked asymmetry in SGV 7 days after the lesion. This asymmetry is still evident but markedly reduced 4-5 weeks after the lesion (p < 0.015). At 7 weeks after the lesion, after 3-4 ipsilesional adaptation sessions, there is a reduction in SGV (p < 0.01). This reduction in SGV, however, is not different from SGV before the ipsilesional training sessions when tested 3 days later (p > 0.07).
Discussion
Normal animals (Before and after magnifying spectacles)
Prior studies that have varied the gain of the VOR through asymmetrical adaptation paradigms have involved low-frequency sinusoidal rotations paired with asymmetric optokinetic stimulation such that the gain would increase in one direction and not change and/or decrease in the other (Aoki et al. 1998; Hirata et al. 2002; Maruyama et al. 2004; Marti et al. 2006). In the present study, we showed that we could increase VOR gain for one direction of rotation by placing magnifying spectacles on monkeys during high acceleration head rotations. This is important because fast head rotations have dramatically reduced gains in the ipsilesional direction (Halmagyi and Curthoys 1988; Halmagyi et al. 1990; Lasker et al. 1999; Lasker et al. 2000; Carey et al. 2007; Sadeghi et al. 2007; Minor and Lasker 2010). In normal animals, gains increased by about 20 % after three hours of continuous unidirectional adaptation with 1.7 X spectacles. Although a significant increase, 20% seems small considering that the animals were adapting for 3 hours and similar short-term adaptation experiments using bidirectional stimulation paired with gain enhancing adaptation paradigms in monkeys have yielded increases of 40 % or more (Raymond and Lisberger 1996; Ushio et al. 2009). There are at least two possible reasons for these findings. First, the animals were not trained or rewarded in our experiments. Second, Hirata et al. (2002) showed that vertical gain change during conflicting visual optokinetic stimulation (X0 in one direction and X2 in the opposite direction) changed with an overall lower magnitude than in monkeys that had the same visual command in both head directions. We hypothesize that while it is possible to alter the gain of the VOR differentially, depending on head direction, the lower efficacy may result from an incomplete dissociation between the error signal driving the change and the direction of the head motion.
Acute effects of ipsilesional and bidirectional training paradigms
Previous studies have attributed the reduced gain in the ipsilesional direction to an inherent asymmetry in the neurons that supply the signals to the VOR. One hypothesis is that high acceleration rotations almost solely stimulate the excitatory side of the vestibular end organ because the neurons on the inhibitory side go rapidly in to cutoff (negative saturation). It has been suggested, that rapid head rotations are a form of unilateral stimulation (Halmagyi et al. 1990). If that is the case then adaptation paradigms seeking to increase the gain of the VOR in the ipsilesional direction should be ineffective due to this inhibitory cutoff. We were, however, able to increase the gain of the VOR in the ipsilesional direction after the ipsilesional adaptation paradigm.
There are at least two reasons that could account for the average increase in ipsilesional gain being relatively modest (~10%). First, the improvement in ipsilesional gain may have persisted from one adaptation session to the next. Such persistence in the adapted change could reduce increases resulting from subsequent training sessions. Results presented in Figure 5 show that reductions in gain asymmetry did persist for at least three days after the final training paradigm. Another possible reason for the modest increase in ipsilesional gain is that one animal (monkey 3), showed little improvement in its ipsilesional gains. We do not have an explanation for the reduced adaptation in this animal. It does not appear to be species related as monkey 1 and monkey 3 were the cynomolgus monkeys and monkey 1 showed the largest amount of adaptation. It is interesting to note that this monkey also had the smallest change in gain when using magnifying lenses before the labyrinthectomy and so may not have been as susceptible to adaption-induced changes in VOR gain. These differences in the absolute magnitude of changes in gain lead us to conclude that asymmetry is more useful as a measure than is the magnitude of the gain.
The fundamental finding from this study is that the asymmetry in GA after unilateral labyrinthectomy did not improve until we had the monkeys undergo the ipsilesional adaptation paradigm. This is in contrast to GV, which showed marked improvement during the first month after the labyrinthectomy (Figure 3). There are two reasons that may account for this finding. There is some evidence that patients who suffer from vestibular dysfunction may change their movement strategies such that they move less (Herdman 1998; Cromwell et al. 2004; Kvale et al. 2008). One possibility is that the monkeys are moving less toward the side of the lesion, which prevents an increase in gain due to the scarcity of retinal slip error in that direction. Clendaniel et al. (2002) showed that adaptation of gain during rapid head rotations could only occur if the retinal slip error signal was paired with head rotations that were of similar frequency and velocity to the test stimuli. It may be that these animals are not moving their heads rapidly enough during everyday activity to induce the necessary gain increases in the ipsilesional direction. Another possible reason underlying the lack of improvement in ipsilesional gain is that the animals are using non-vestibular compensatory mechanisms to supplement the VOR, such as compensatory saccades. (Peng et al. 2005; Tian et al. 2007). Although we do not see evidence of these types of eye movements, it is important to remember we are using passive whole-body rotations. Other mechanisms of compensation might be evident for head-on-body or active head rotations.
Another possible explanation for improvement in the ipsilesional gain during the ipsilesional adaptation paradigm but not during everyday activity is that gain adaptation during rapid head rotations is partially constrained such that the retinal error signal causes a change in gain to occur for both leftward and rightward directions (Hirata et al. 2002). This could occur, for instance, if the gain changing circuitry is downstream of neurons that compute the difference between inputs from the two labyrinths. Because in everyday activity the animal moves its head in both directions and never repeatedly in one direction, there may be a conflict in the error signal induced by motion in the contralesional and ipsilesional directions. This error signal could result because the gain is normal for rotations in the contralesional direction. Therefore, an increase in gain would cause an error signal opposite to the error signal resulting from the low gain in the ipsilesional direction. Rotating the animal exclusively in one direction overcomes this limitation because the animal receives only an error signal to increase the gain. The fact that the asymmetry reduced only after the ipsilesional adaptation paradigm but did not reduce after the bidirectional adaptation paradigm supports this theory.
If plasticity remains in the ipsilesional direction why does such a large asymmetry in gain between the ipsi- and contralesional directions occur after a unilateral lesion? One explanation comes from our two pathway model of the VOR (Minor et al. 1999b; Lasker et al. 1999; Lasker et al. 2000; Clendaniel et al. 2001; Lasker et al. 2002; Migliaccio et al. 2004, Minor and Lasker 2010). In this model, one component has phasic dynamics which primarily encode head acceleration and velocity. The second component has tonic dynamics which encode head velocity over a broad range of frequencies and velocities. In the model inhibitory cutoff is mainly occurring in the phasic pathway and is the main cause of the reduced responses to ipsilesional head rotation. The tonic pathway provides linear encoding of head velocity with little susceptibility to inhibitory cutoff. VOR adaptation occurs when the lesioned animal is allowed to see and receive retinal slip error. Initially, the gain increases dramatically in the phasic pathway and is what is responsible for the initial x2 increase in the contralesional gain immediately after the lesion. Because the neurons in the phasic pathway are highly asymmetric (i.e. susceptible to inhibitory cutoff) the gain in the ipsilesional direction does not increase very much.
We hypothesize, therefore, that the gain adaptation occurs in the phasic pathway much more rapidly than adaptation in the tonic pathway. Once the contralesional gain recovers to normal further adaptation is prevented because of conflicting error signal between ipsilesional and contralesional head movements. Repeated ipsilesional training may increase the gain in those neurons that are still performing in their linear operating range, the majority of which probably reside in the tonic pathway. Interestingly, in squirrel monkeys (Clendaniel et al. 2001) and rhesus monkeys (Ushio et al. 2008), GA increased substantially more than GV after adaptation with magnification spectacles. This preferential increase of GA over GV was thought to be due to adaptation in the phasic pathway. However, in this study following the ipsilesional adaptation paradigm, unlesioned animals with magnifying spectacles had both GA and GV increased by the same amount (~20 %). Identical increases of GA and GV lends further support to the hypothesis that unidirectional stimulation preferentially increased the gain of the tonic pathway in comparison to the phasic pathway.
Long-term changes after unidirectional training
In normal animals, GA and GV exhibited no retention in adaptation-induced changes when tested 1 week after the unidirectional training paradigm. It is not surprising that a maladaptive gain asymmetry does not persist during normal activity. Retinal slip error would presumably return the adapted gain to normal once the lenses were removed. There was a significant change in SGA and SGV immediately following the last ipsilesional adaptation paradigm in animals after unilateral labyrinthectomy; however, the change persisted in SGA but not in SGV when tested 3 days after the last paradigm (Figure 5). One possible reason for this finding is that there is an optimal gain based on both visual and vestibular information. Because optokinetic and pursuit signals can contribute to the gain of the VOR during low-frequency head movements (GV), it may be that these visual signals make up for the deficiency of VOR gain in the ipsilesional direction rather than vestibular signals. The reduced role of visual following mechanisms in responses to rapid head movements may account for the retention of changes in SGA compared to SGV. These results show that repeated ipsilesional training may be a potential rehabilitative technique for increasing the gain of the VOR during rapid head movements in the ipsilesional direction for patients that have unilateral hypofunction.
Acknowledgements
This work was supported by NIH RO1 DC02390 and NIH RO1 DC009255
Reference List
- Aoki M, Burchill P, Ito Y, Gresty M. Asymmetry of vestibular function induced by unidirectional visual-vestibular conflict. Acta Otolaryngol. 1998;118:628–634. doi: 10.1080/00016489850183098. [DOI] [PubMed] [Google Scholar]
- Aw ST, Halmagyi GM, Haslwanter T, Curthoys IS, Yavor RA, Todd MJ. Three-dimensional vector analysis of the human vestibulo-ocular reflex in response to high acceleration head rotations II.Responses in subjects with unilateral vestibular loss and selective semicircular canal occlusion. J Neurophysiol. 1996;76:4021–4030. doi: 10.1152/jn.1996.76.6.4021. [DOI] [PubMed] [Google Scholar]
- Baloh RW, Honrubia V, Yee RD, Hess K. Changes in the human vestibulo-ocular reflex after loss of peripheral sensitivity. Ann Neurol. 1984;16:222–228. doi: 10.1002/ana.410160209. [DOI] [PubMed] [Google Scholar]
- Carey JP, Migliaccio AA, Minor LB. Semicircular canal function before and after surgery for superior canal dehiscence. Otol Neurotol. 2007;28:356–364. doi: 10.1097/01.mao.0000253284.40995.d8. [DOI] [PubMed] [Google Scholar]
- Carey JP, Minor LB, Peng GC, Della Santina CC, Cremer PD, Haslwanter T. Changes in the three-dimensional angular vestibulo-ocular reflex following intratympanic gentamicin for Meniere's disease. J Assoc Res Otolaryngol. 2002;3:430–443. doi: 10.1007/s101620010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clendaniel RA, Lasker DM, Minor LB. Horizontal vestibuloocular reflex evoked by high-acceleration rotations in the squirrel monkey. IV. Responses after spectacle-induced adaptation. J Neurophysiol. 2001;86:1594–1611. doi: 10.1152/jn.2001.86.4.1594. [DOI] [PubMed] [Google Scholar]
- Clendaniel RA, Lasker DM, Minor LB. Differential adaptation of the linear and nonlinear components of the horizontal vestibuloocular reflex in squirrel monkeys. J Neurophysiol. 2002;88:3534–3540. doi: 10.1152/jn.00404.2002. [DOI] [PubMed] [Google Scholar]
- Clendaniel RA, Schubert MC, Minor LB. Decreased VOR asymmetry in unilateral vestibular hypofunction post spectacle-induced adaptation. Soc Neurosci Abstr. 2003 Program #593.15. [Google Scholar]
- Cremer PD, Halmagyi GM, Aw ST, Curthoys IS, McGarvie LA, Todd MJ, Black RA, Hannigan IP. Semicircular canal plane head impulses detect absent function of individual semicircular canals. Brain. 1998;121:699–716. doi: 10.1093/brain/121.4.699. [DOI] [PubMed] [Google Scholar]
- Cromwell R, Schurter J, Shelton S, Vora S. Head stabilization strategies in the sagittal plane during locomotor tasks. Physiother Res Int. 2004;9:33–42. doi: 10.1002/pri.298. [DOI] [PubMed] [Google Scholar]
- Fetter M, Zee DS. Recovery from unilateral labyrinthectomy in rhesus monkey. J Neurophysiol. 1988;59:370–393. doi: 10.1152/jn.1988.59.2.370. [DOI] [PubMed] [Google Scholar]
- Gonshor A, Melvill Jones G. Extreme vestibulo-ocular adaptation induced by prolonged optical reversal of vision. J Physiol (London) 1976;256:381–414. doi: 10.1113/jphysiol.1976.sp011330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halmagyi GM, Curthoys IS. A clinical sign of canal paresis. Arch Neurol. 1988;45:737–739. doi: 10.1001/archneur.1988.00520310043015. [DOI] [PubMed] [Google Scholar]
- Halmagyi GM, Curthoys IS, Cremer PD, Henderson CJ, Todd MJ, Staples MJ, D'Cruz DM. The human horizontal vestibulo-ocular reflex in response to high-acceleration stimulation before and after unilateral vestibular neurectomy. Exp Brain Res. 1990;81:479–490. doi: 10.1007/BF02423496. [DOI] [PubMed] [Google Scholar]
- Helveston EM. Understanding, detecting, and managing strabismus. Community Eye Health. 2010 Mar;23(72):12–4. 2010. 23:12-14. [PMC free article] [PubMed] [Google Scholar]
- Herdman SJ. Role of vestibular adaptation in vestibular rehabilitation. Otalaryngol Head Neck Surg. 1998;119:49–54. doi: 10.1016/S0194-5998(98)70195-0. [DOI] [PubMed] [Google Scholar]
- Hirata Y, Lockard JM, Highstein SM. Capacity of vertical VOR adaptation in squirrel monkey. J Neurophysiol. 2002;88:3194–3207. doi: 10.1152/jn.00698.2001. [DOI] [PubMed] [Google Scholar]
- Kvale A, Wilhelmsen K, Fiske HA. Physical findings in patients with dizziness undergoing a group exercise programme. Physiother Res Int. 2008;13:162–175. doi: 10.1002/pri.402. [DOI] [PubMed] [Google Scholar]
- Lasker DM, Backous DD, Lysakowski A, Davis GL, Minor LB. Horizontal vestibuloocular reflex evoked by high-acceleration rotations in the squirrel monkey. II. Responses after canal plugging. J Neurophysiol. 1999;82:1271–1285. doi: 10.1152/jn.1999.82.3.1271. [DOI] [PubMed] [Google Scholar]
- Lasker DM, Hullar TE, Minor LB. Horizontal vestibuloocular reflex evoked by high-acceleration rotations in the squirrel monkey. III. Responses after labyrinthectomy. J Neurophysiol. 2000;83:2482–2496. doi: 10.1152/jn.2000.83.5.2482. [DOI] [PubMed] [Google Scholar]
- Lasker DM, Ramat S, Carey JP, Minor LB. Vergence-mediated modulation of the human horizontal angular VOR provides evidence of pathway-specific changes in VOR dynamics. Ann N Y Acad Sci. 2002;956:324–337. doi: 10.1111/j.1749-6632.2002.tb02831.x. [DOI] [PubMed] [Google Scholar]
- Maioli C, Precht W. On the role of vestibulo-ocular reflex plasticity in recovery after unilateral peripheral vestibular lesions. Exp Brain Res. 1985;59:267–272. doi: 10.1007/BF00230906. [DOI] [PubMed] [Google Scholar]
- Marti S, Bockisch CJ, Straumann D. Asymmetric short-term adaptation of the vertical vestibulo-ocular reflex in humans. Exp Brain Res 2006 Jul. 2006;172(3):343–50. doi: 10.1007/s00221-005-0341-2. Epub 2006 Jan 26 172:343-350. [DOI] [PubMed] [Google Scholar]
- Maruyama M, Fushiki H, Yasuda K, Watanabe Y. Asymmetric adaptive gain changes of the vertical vestibulo-ocular reflex in cats. Brain Res. 2004;1023:302–308. doi: 10.1016/j.brainres.2004.07.049. [DOI] [PubMed] [Google Scholar]
- Migliaccio AA, Schubert MC, Jiradejvong P, Lasker DM, Clendaniel RA, Minor LB. The three-dimensional vestibulo-ocular reflex evoked by high-acceleration rotations in the squirrel monkey. Exp Brain Res. 2004;159:433–446. doi: 10.1007/s00221-004-1974-2. [DOI] [PubMed] [Google Scholar]
- Minor LB, Goldberg JM. Vestibular-nerve inputs to the vestibulo-ocular reflex: A functional-ablation study in the squirrel monkey. J Neurosci. 1991;11:1636–1648. doi: 10.1523/JNEUROSCI.11-06-01636.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor LB, Haslwanter T, Straumann D, Zee DS. Hyperventilation-induced nystagmus in patients with vestibular schwannoma. Neurology. 1999a;53:2158–2168. doi: 10.1212/wnl.53.9.2158. [DOI] [PubMed] [Google Scholar]
- Minor LB, Lasker DM, Backous DD, Hullar TE. Horizontal vestibuloocular reflex evoked by high-acceleration rotations in the squirrel monkey. I. Normal responses. J Neurophysiol. 1999b;82:1254–1270. doi: 10.1152/jn.1999.82.3.1254. [DOI] [PubMed] [Google Scholar]
- Minor L, Lasker D. Tonic and phasic contributions to the pathways mediating compensation and adaptation of the vestibulo-ocular reflex. J Vestib Res. 2010;19:159–170. doi: 10.3233/VES-2009-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadol JB. Vestibular neuritis. Otalaryngol Head Neck Surg. 1995;112:162–72. doi: 10.1016/S0194-59989570316-0. [DOI] [PubMed] [Google Scholar]
- Paige GD. Nonlinearity and asymmetry in the human vestibulo-ocular reflex. Acta Otolaryngol (Stockh) 1989;108:1–8. doi: 10.3109/00016488909107385. [DOI] [PubMed] [Google Scholar]
- Paige GD, Tomko DL. Eye movement responses to linear head motion in the squirrel monkey I. Basic characteristics. J Neurophysiol. 1991;65:1170–1182. doi: 10.1152/jn.1991.65.5.1170. [DOI] [PubMed] [Google Scholar]
- Palla A, Straumann D. Recovery of the high acceleration vestibulo-ocular reflex after vestibular neuritis. J Assoc Res Otolaryngol. 2004;5:427–435. doi: 10.1007/s10162-004-4035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng GC, Minor LB, Zee DS. Gaze position corrective eye movements in normal subjects and in patients with vestibular deficits. Ann N Y Acad Sci. 2005;1039:337–48. doi: 10.1196/annals.1325.032. [DOI] [PubMed] [Google Scholar]
- Raphan T, Matsuo V, Cohen B. Velocity storage in the vestibulo-ocular reflex arc (VOR). Exp Brain Res. 1979;35:229–248. doi: 10.1007/BF00236613. [DOI] [PubMed] [Google Scholar]
- Raymond JL, Lisberger SG. Behavioral analysis of signals that guide learned changes in the amplitude and dynamics of the vestibulo-ocular reflex. J Neurosci. 1996;16:7791–7802. doi: 10.1523/JNEUROSCI.16-23-07791.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi SG, Minor LB, Cullen KE. Dynamics of the horizontal vestibuloocular reflex after unilateral labyrinthectomy: response to high frequency, high acceleration, and high velocity rotations. Exp Brain Res. 2006;175:471–484. doi: 10.1007/s00221-006-0567-7. [DOI] [PubMed] [Google Scholar]
- Sadeghi SG, Minor LB, Cullen KE. Response of vestibular-nerve afferents to active and passive rotations under normal conditions and after unilateral labyrinthectomy. J Neurophysiol. 2007;97:1503–1514. doi: 10.1152/jn.00829.2006. [DOI] [PubMed] [Google Scholar]
- Schubert MC, Migliaccio AA, Minor LB, Clendaniel RA. Retention of VOR gain following short-term VOR adaptation. Exp Brain Res. 2008;187:117–127. doi: 10.1007/s00221-008-1289-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub E, Crago JE, Burgio LD, Groomes TE, Cook EW, III, DeLuca SC, Miller NE. An operant approach to rehabilitation medicine: overcoming learned nonuse by shaping. J Exp Anal Behav. 1994;61:281–293. doi: 10.1901/jeab.1994.61-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub E, Miller NE, Novack TA, Cook EW, III, Fleming WC, Nepomuceno CS, Connell JS, Crago JE. Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil. 1993;74:347–354. [PubMed] [Google Scholar]
- Tian JR, Crane BT, Ishiyama A, Demer JL. Three dimensional kinematics of rapid compensatory eye movements in humans with unilateral vestibular deafferentation. Exp Brain Res. 2007;182:143–155. doi: 10.1007/s00221-007-0977-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushio M, Lasker DM, Minor LB. Specificity and generalization of spectacle-induced adaptation in the vestibulo-ocular reflex (VOR). ARO Midwinter Meeting Abstract. 2008:774. [Google Scholar]
- Wittenberg GF, Schaechter JD. The neural basis of constraint-induced movement therapy. Curr Opin Neurol. 2009;22:582–588. doi: 10.1097/WCO.0b013e3283320229. [DOI] [PubMed] [Google Scholar]