Abstract
One of the most promising methods to treat neurodegeneration is noninvasive transcranial near-infrared laser therapy (NILT), which appears to promote acute neuroprotection by stimulating mitochondrial function, thereby increasing cellular energy production. NILT may also promote chronic neuronal function restoration via trophic factor-mediated plasticity changes or possibly neurogenesis. Clearly, NILT is a treatment that confers neuroprotection or neurorestoration using pleiotropic mechanisms. The most advanced application of NILT is for acute ischemic stroke based upon extensive preclinical and clinical studies. In laboratory settings, NILT is also being developed to treat traumatic brain injury, Alzheimer’s disease and Parkinson’s disease. There is some intriguing data in the literature that suggests that NILT may be a method to promote clinical improvement in neurodegenerative diseases where there is a common mechanistic component, mitochondrial dysfunction and energy impairment. This article will analyze and review data supporting the continued development of NILT to treat neurodegenerative diseases.
Keywords: acute ischemic stroke, Alzheimer’s disease, LLLT, mitochondria, neuroprotection, NILT, Parkinson’s disease, photobiomodulation, photobiostimulation, TLT, traumatic brain injury
Photobiostimulation or photobiomodulation is a novel noninvasive method used to promote neuroprotection and repair of injured neuronal pathways by activating endogenous mechanisms that are involved in both processes. Currently, we hypothesize that near-infrared laser therapy (NILT) efficacy requires at least a two-step process, an acute phase response followed by a chronic phase response that requires activation of survival and plasticity elements [1]. The wavelength-specific two-step process, which is not thermal based [2–4], appears to be effective when near-infrared (NIR) irradiation with 808 nm infrared light is used [5–13], but lower wavelengths of approximately 630 nm have also been shown to have therapeutic efficacy [14]. Photobiostimulation directly affects cellular metabolic activity that is regulated by energy production within mitochondria. Three studies have now shown that NILT otherwise known as either transcranial laser therapy (TLT) or low-level laser therapy (LLLT) increases ATP formation after energy (photon or light) absorption by mitochondrial chromophores [15]. There is accumulating evidence suggesting that the primary mitochondrial chromophore for NILT is cytochrome-c-oxidase (COX) [5,15–17], a terminal enzyme in cellular respiration that is located in the inner mitochondrial membrane. COX is an enzyme complex that contains two copper centers, CuA and CuB. The CuA center has a broad absorption peak at approximately 830 nm (near-infrared) in its oxidized form and another peak approximately 665 nm (red) [18]. The type of infrared laser used in preclinical or clinical studies delivers energy at either 630–665 or 808 nm, which is within this absorption spectra of CuA. The stimulation of COX activity regulates cellular bioenergetics by delivering protons across the inner membrane, and drives the formation of ATP by oxidative phosphorylation. In addition to resulting in increased mitochondrial ATP formation, photobiostimulation may also initiate secondary cell-signaling pathways [19,20].
A few additional clues to the mechanisms of action of NILT in CNS neurodegenerative diseases are also presented. In addition to the energy hypothesis proposed above, for which there is growing evidence, there are also studies that show that NILT decreases apoptosis [21] and may induce neurogenesis [22]. More importantly, NILT may also enhance the production of endogenous neurotrophic factors to remodel the neuronal environment following injury or long-term neurodegeneration [23] via well known effects of trophic factors on synaptic plasticity. Since both neurotrophism and neurogenesis are possibly important clues involved in the efficacy of NILT, both mechanisms are being studied.
Primarily pertinent to stroke and diseases where increased cerebral blood flow (CBF) may attenuate clinical deficits, Uozumi et al. showed that there was a correlation between laser-induced changes in nitric oxide synthase (NOS), nitric oxide (NO) and CBF studied using a variety of power densities between 0.8 and 3.2 W/cm at a wavelength of 808 nm [24]. The study showed that NIR laser irradiation (1.6 W/cm2 for 15–45 min) significantly increased CBF, which was correlated with increased cortical NO levels. Importantly, because of safety concerns, the study showed that the physiological effects on CBF and NO were temperature independent. The authors did measure a small increase in brain temperature, similar to that previously described [25]; however, taken together, the data indicate that the minimal heating effect of NILT is not a critical mechanism involved in CBF regulation or NILT effects. Recent studies by De Taboada et al. [26] have also demonstrated that NILT has widespread effects beyond that on mitochondrial function, and CBF and NO as discussed above. The authors showed that chronic NILT suppressed inflammatory processes, specifically IL-1b, TNF-a and TGF-b. Unfortunately, the significance of the changes were not discussed in detail in the context of the pathology observed in the disease model.
Therefore, currently there are multiple mechanisms hypothesized to be involved in the beneficial effects of NILT photobiostimulation, including increased mitochondrial function and enhanced ATP production, resulting in improved cellular function and homeostasis, increased cortical perfusion, which ultimately allows for cells to receive increased levels of oxygen and nutrients. The observation that trophic factors are regulated by NILT suggests either a direct or indirect effects on synaptic plasticity and possibly neurogenesis. Regardless of the initiating mechanism, NILT is neuroprotective and can restore function following various brain insults. It has previously been postulated that pleiotropic therapies will be necessary to treat neurodegenerative diseases [27] such as acute ischemic stroke (AIS) owing to the complexity of mechanisms involved in neurodegeneration and behavioral dysfunction. NILT appears to be a prime candidate and example of a noninvasive therapy that is pleiotropic. The clinical uses of NILT will be discussed in the following four sections.
Development of NILT for AIS
NILT has been under development for the treatment of AIS for almost 11 years [1]. AIS is usually characterized by motor function, somatosensory, linguistic (or aphasia) and visual–spatial/attentional (or neglect) deficits, and are the main clinical functions scored on the National Institutes of Health Stroke Scale (NIHSS) and modified Rankin Scale (mRS) [28–31]. NILT has been tested in preclinical stroke models in two different species, the rat and rabbit, with differing results. The initial seminal studies that lead to the NEST-1 clinical trial were conducted in New Zealand white rabbits using the rabbit small clot embolic stroke model (RSCEM) [25]. The RSCEM has multiple advantages over most other stroke models used in preclinical or translational research. These include the use of nonautologous blood clots for embolization, embolization in the absence of anesthetics, inclusion of a heterogeneous population of stroke subjects and a well-defined clinically relevant behavioral end point [32,33].
Safety
The original rabbit embolic stroke study by Lapchak et al. was exploratory in nature owing to the absence of NILT development information in the literature [25]. The initial phase of the study determined NILT safety by measuring cortical temperature. The study showed that 10-min NILT treatment of the rabbit brain at midline using high continuous wave (CW) energy (15 J/cm2) at 808 nm wavelength, through the overlaying shaved skin and skull increased the surface skin temperature below the laser probe by 3°C. However, the focal cortical brain temperature directly under the laser probe was increased by 0.8–1.8°C during treatment. There were no short- or long-term detrimental effects of NILT treatment using the regimen indicated above.
Additional safety studies have been documented for a second species [11]. The researchers directly tested the effects of increasing energy density between 0.9, 9 and 90 J/cm2 (7.5, 75 and 750 mW/cm2, respectively) on neuronal damage using CW treatment with an 808 nm wavelength. They found that there was no discernable tissue damage, either using light or electron microscopic methodology between 0.9 and 9 J/cm2. However, at the highest dose, there was significant behavioral, and histological or neuronal damage. The study did not test NILT at 15 J/cm2, which was used in the rabbit study. Thus, CW NILT below 9 J/cm2 (75 mW/cm2) appears to be safe and well tolerated.
Preclinical efficacy
NILT has been shown to be efficacious as a method to promote neuronal function following a stroke based upon translational studies measuring clinical function. This novel method of neuroprotection was first demonstrated in the RSCEM [25] using the laser in CW mode, which according to recent advances in the field, may now not be the optimal method to promote neuroprotection and behavioral recovery following a stroke [1,19,26,34] owing to the involvement of cortical and subcortical structures. The need to use a pulse wave (PW) mode will be discussed in that context and will be a recurring theme when Alzheimer’s disease (AD), Parkinson’s disease (PD) and traumatic brain injury (TBI) are discussed in their respective sections.
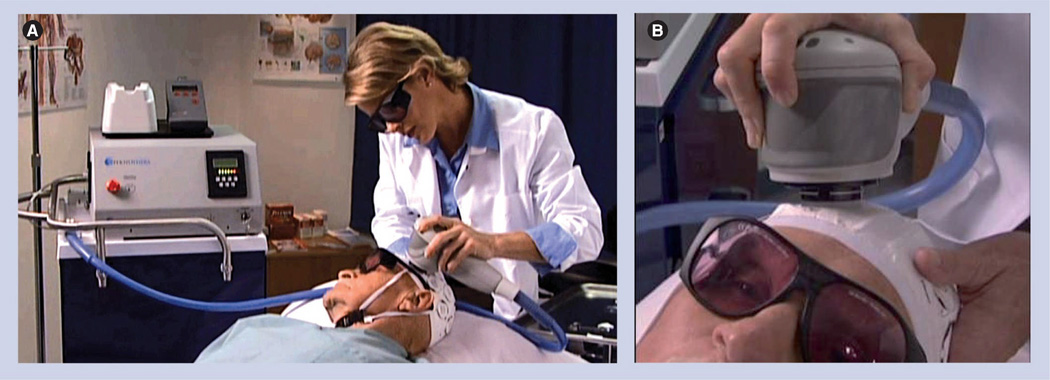
Initial studies to provide proof-of-principle efficacy showed that NILT was effective when applied transcranially following embolization [25]. Figure 1A is a photograph showing the original Acculaser® device (Photothera Inc.; Carlsbad, CA, USA) that was used to provide proof-of-concept data for the NEST trials. Figure 1B shows the newest generation of laser device, which the clinical device was based upon. The figure shows the treatment design of an experimental subject with the laser device. The therapeutic window in embolized rabbits was quite wide; efficacy on the primary end point, motor function, was noted when NILT was applied between 5 min and 6 h after embolization and there was also a trend toward efficacy in a subset population of rabbits as long as 12 h after embolization, but there was variability in the response in the experimental group, suggesting that not all stroke animals responded to the treatment. It is hypothesized that the variability and differential response could be due to heterogeneous distribution, because of the technical nature of the RSCEM [32,35]. When a NILT-induced clinical improvement was measured, the effect was durable and could be measured up to 21 days after a single treatment [25,34]. The therapeutic window of NILT in the RSCEM should be compared with the therapeutic window of Alteplase (tPA), the only US FDA-approved pharmacological treatment for AIS. In the RSCEM, the original model that was used in the development of tPA, the thrombolytic effectively improves function when given 1–1.5 h following embolization and in patients, tPA is effective within 3–4.5 h after stroke onset [36–38]. Thus, NILT has a therapeutic window four- to six-times longer than tPA in the RSCEM. This is a significant finding suggesting that a large population of stroke patients may be enrolled in clinical trials, and receive potentially useful therapy.
Figure 1. Preclinical laser device.
(A) The figure shows the 2001 Photothera Inc. (previously Acculaser Inc.) research laser used in the original translational stroke studies [19,25,34]. The device is a Gallium–Aluminum–Arsenide (Ga-AI-As) diode laser with a wavelength of 808 ± 5 nm. The laser was coupled to a female SMA-905 adapted, 2-cm diameter probe via an OZ Optics Ltd. fiber optic (step index fiber with a 550-µm core diameter and a numerical aperture of 0.22). The probe utilized specially designed optics to generate a divergent, diffused, 5-mm diameter beam. (B) The figure shows the current 2011 Photothera Inc. translational multipulse wave mode research laser used in the translational development of noninvasive transcranial near-infrared laser therapy to treat stroke. The probe is also designed to generate a divergent, diffused, 5-mm diameter beam. The figure shows noninvasive transcranial near-infrared laser therapy treatment of a New Zealand white rabbit. Note the position of the laser probe central to the rabbit’s head positioned over the shaved pink area. Transmission studies in the rabbit demonstrated that when the laser probe is placed on the skin surface posterior to bregma on the midline [25], the laser beam covers the complete cortex. The laser was not turned on for this demonstration.
Since it was hypothesized that there is sufficient skull penetration of the laser light to the cortex followed by subsequent activation of COX, which may be critical to produce a response, NILT was studied in the RSCEM with a fixed treatment time of 6 h using two pulse modes with a common power density of 7.5 mW/cm2 and a fixed cortical fluence of 0.9 J/cm2. In the studies, a comparison was made using 300 µs pulses at 1 kHz, 30 % duty cycle, cortical fluence 0.9 J/cm2 and 2 ms pulses at 100Hz, 20 % duty cycle, cortical fluence 0.9 J/cm2 to 7.5 mW/cm2, cortical fluence 0.9 J/cm2, 100% duty cycle [34]. With these specific settings, PW NILT modes produced statistically significant increases in clinical performance (p < 0.05), a significance level not achieved by CW NILT at the same time point. The original RSCEM studies described above used 10 min NILT with a power density of 25 mW/cm2 (cortical fluence 15 J/cm2) to provide therapeutic efficacy data with a 6-h delay [25]. Thus, taken together, the studies suggested that PW NILT treatment may be more beneficial to treat stroke than CW NILT. The enhanced effectiveness of PW NILT may be due to increased penetration of photons through the brain, since at the pulsed peaks, the PW mode will deposit more photons deeper into the brain. Table 1 summarizes preclinical efficacy results.
Table 1.
Correlation between power density, treatment regimen and clinical outcome in neurodegenerative disease: 808-nm laser light.
| Disease indication |
Preclinical treatment regimen & outcome | Clinical treatment regimen & outcome | Ref. |
|---|---|---|---|
| AIS | CW: 0.9 J/cm2 Rabbit: neuroprotection behavioral improvement |
CW: 1.2 J/cm2 Human: clinical improvement NIHSS and mRS (caveat NIHSS <16) |
[12,19,22,25,47] |
| CW: 0.9 J/cm2 Rat: neurogenesis, delayed behavioral improvement | |||
| PW: 4.5–31.5 J/cm2 Rabbit: increased ATP levels | |||
| TBI | CW: 36 J/cm2 Rat: behavioral improvement; reduced infarct volume |
CW: 0.4 J/cm2 Human: executive function and memory improvement (caveat required repeated treatment) |
[14,55,58] |
| CW: 1.2–2.4 J/cm2 Rat: behavioral improvement; reduced infarct volume | |||
| AD | CW: 0.9 J/cm2 Mouse: memory improvement; no effect on amyloid load; small reduction of inflammatory markers |
Early development | [26] |
| PW: 0.9–9 J/cm2 Mouse: memory improvement; decreased amyloid load; reduced inflammation and increased ATP levels |
Early development | [26] | |
| PD | CW: 6 J/cm2 PD cybrid cell: increased motility and mitochondrial function |
Attenuated development of PD symptoms (behavioral deficits) correlating with increased MAO B and Cu/Zn-SOD activity | [93–95] |
| Mouse: increased TH+ cell survival in the substantia nigra pars compacta |
AD: Alzheimer’s disease; AIS: Acute ischemic stroke; CW: Continuous wave; MAO B: Monoamine oxidase B; mRS: Modified Rankin Scale; NIHSS: National Institutes of Health Stroke Scale; PD: Parkinson’s disease; PW: Pulse wave; SOD: Superoxide dismutase; TBI: Traumatic brain injury.
The mechanism of action of NILT has been studied in the RSCEM. The studies tested the hypothesis that COX is as important as a chromophore or light acceptor to initiate neuroprotective or neurorestorative pathways. Thus, correlative data exist from RSCEM studies showing that there is a relationship between cortical surface energy density (J/cm2), behavioral performance and mitochondrial function measured using cellular ATP content. In the studies, the authors showed that an energy density of 0.9 J/cm2, which produced a statistically significant behavioral improvement after an embolic stroke, only increased cortical ATP content by 40%, an increase that was not statistically significant and did not approach baseline control levels. This NILT energy appeared to be below the threshold required for normalization (i.e., back to control levels) of cortical ATP levels. For normalization of ATP levels, it has been estimated that 2.86 J/cm2 of NILT energy is required [19] using a wavelength of 808 nm. The study also showed that PW NILT is more efficacious than CW NILT. In gene array studies designed to identify mechanisms of NILT-induced neuroprotection, the expression of mRNA for neurotrophic factors and angiogenesis markers [39]. NILT enhanced cortical brain-derived neurotrophic factor, neurotrophin-3 and bone morphogenetic protein-7, factors previously shown to be involved in cell survival, plasticity and behavioral improvement [40–45]. Interestingly, NILT also elevated brain-specific angiogenesis inhibitor-1, a molecule that contains thrombospondin (5 TSP-Type 1 repeats) and TSP1 levels [46]. Based upon this intriguing data, it was hypothesized that NILT may improve long-term function by enhancing synaptic plasticity within the cortex and may also downregulate angiogenesis (Table 1).
Additional preclinical studies with NILT (CW, 0.9 J/cm2) have been performed in a rat suture stroke model, where the middle cerebral artery is artificially occluded by a nylon suture while the rat is under anesthesia [22]. Unlike the neuroprotective effects of NILT, demonstrated in the RSCEM, the authors found that NILT was not neuroprotective in the rat when treatment was initiated 4 h after a stroke. In the rat, there was no acute response to NILT, the research group found that NILT was only effective when applied 24 h after the stroke. Similar findings using CW NILT (7.5 mW/cm2) and the same filament model were published by De Taboada et al. [10]. This difference may be related to technical aspects of the rat model, the use of a suture to induce ischemia and the presence of neuroprotective anesthetics in the animals during suture placement. Moreover, the model used a fixed population of ischemic rats (i.e., nonheterogeneous population) chosen by excluding large numbers of outliers that are behaviorally different during preliminary screens.
Clinical efficacy
Two randomized double-blind clinical trials with NILT (10 mW/cm2, delivering an estimated cortical fluence of 1.2 J/cm2 at a wavelength of 808 nm) have already been completed in stroke patients [12,47]. Figure 2 provides a photograph of the clinical laser device and how a patient receives transcranial NILT treatment.
Figure 2. Clinical noninvasive transcranial near-infrared laser therapy treatment for stroke.
The figure shows a Photothera Inc. clinical laser used in the NEST-1–3 clinical trials [12]. (A) Overall view including a Photothera Inc. continuous wave clinical laser. Essentially, the laser is similar to the device shown in Figure 1B but has been programmed for continuous wave delivery of 10 mW/cm2 (1.2 J/cm2).
(B) Close-up view of a patient with the wearable head apparatus receiving near-infrared laser therapy. Note the use of protective goggles during noninvasive transcranial near-infrared laser therapy treatment.
Figure courtesy of C Tedford (Photothera Inc., Carlsbad, CA, USA).
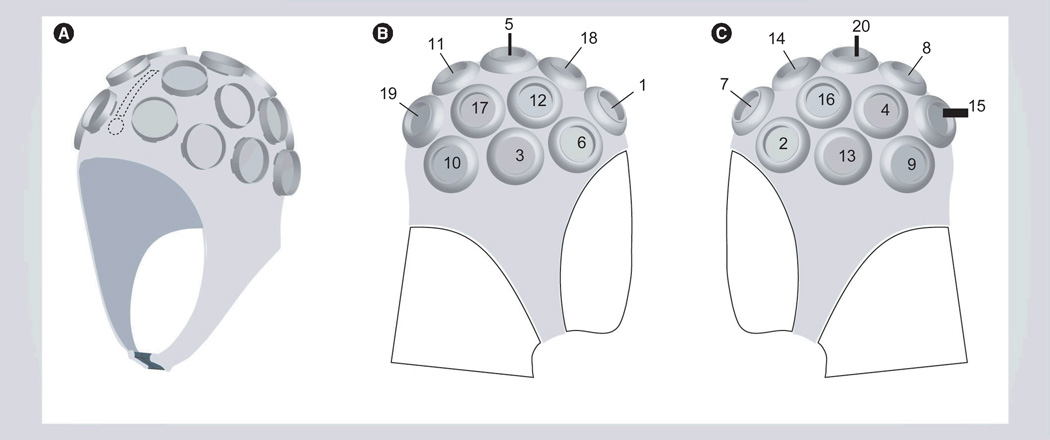
Photothera Inc. the developers of the NILT device also developed head gear to allow investigators to irradiate 20 different positions on the head for 2 min at each position to attempt to encompass the complete brain. Figure 3 provides three different views of the head gear required to provide laser therapy to stroke patients. Each numbered circle on the head gear represents a specific laser position that is computer tracked to ensure maximum treatment.
Figure 3. Patient noninvasive transcranial near-infrared laser therapy treatment therapy headgear.
The apparatus has positions for 20 independent 2-min applications of noninvasive transcranial near-infrared laser therapy treatment to cover the complete brain. The number system on the gear indicates all 20 treatment positions.
Modified from [301].
NEST-1 was a Phase II double-blind, skewed randomized (2:1) study of patients 40–85 years of age treated within 24 h of stroke onset (mean time to treatment: 16 h 56 min). The trial was inclusive of stroke patients with an NIHSS baseline score of 7–22 (mean score: 10) [12]. The primary end points were two standard stroke scales commonly used in clinical trials; NIHSS and mRS [36,48–51]. For the trial, the NILT probe was applied to 20 points on the skull for a 2 min duration at each spot, using a power density of 10 mW/cm2 delivering an energy density of 1.2 J/cm2. In the clinical trial, Lampl and colleagues found that NILT-treated patients showed greater improvement from baseline to 90 days (p = 0.021) than the sham-treated group did, apparently with the laser probe applied but not delivering any energy [12]. In the NILT group, 59% of patients had successful outcomes compared with 44% in the control group measured at 90 days as a binary mRS score of 0 to 2 (p = 0.034 stratified by severity and time to treatment; p = 0.043 stratified only by severity). NILT was also shown to be safe since there were no differences in mortality rates (placebo 9.8% vs NILT 8.9%) or serious adverse events (placebo 36.6% vs NILT 25.3%) between the two groups (Table 1).
NEST-2 was a larger Phase III double-blind, equal randomized clinical trial that enrolled 660 stroke patients [47]. The trial was nearly identical to NEST-1, but used mRS as the primary end point and NIHSS was used for additional analysis when results were stratified according to enrollment baseline. In NEST-2 the time to treatment was 14 h 38 min. Unlike NEST-1, the trial was not overwhelmingly positive and did not reach statistical significance (p = 0.094) for all enrolled patients (mean NIHSS: 13.1). Additional post hoc analysis with severity stratification indicated that AIS patients with NIHSS scores of 7–15 upon enrollment actually did achieve better performance at 90 days (p = 0.044) (Table 1).
Currently, the NEST-3 clinical trial is enrolling stroke patients within 24 h of a stroke [201] with an NIHSS baseline score of 7–17, the range where beneficial effects of NILT were observed in the NEST-1 and NEST-2 [12,47] clinical trials. Moreover, the trial is attempting to enroll patients with superficial cortical strokes, rather than subcortical strokes owing to the limitations of CW NILT described below. Efficacy results are pending and should be available by 2013–2014. Lack of efficacy or a statistically significant result (p > 0.05) in the NEST-3 trial would not be too surprising given the fact that AIS is an extremely difficult disease to treat and that the same CW NILT regimen that was used in NEST-1 and NEST-2 is being used once again. Even though the NEST trials are novel and ground breaking, they have not taken into account many important factors such as dosimetry, PW utilization and adequate tissue coverage.
NILT penetration profiles
Using the RSCEM, NILT penetration of the rabbit skull and brain is achieved to a depth of 25–30 mm, almost the complete thickness, and the NILT beam would encompass the majority of the brain if placed on the skin surface posterior to bregma at midline [25]. In the NEST-2 trial [47], the investigators also indicate that NILT will only penetrate the brain to approximately 20 mm in depth [47] using the CW design (10 mW/cm2 1.2 J/cm2). Previous calculations [10] indicate an estimated power density drop from 10 mW/cm2 (1.2 J/cm2) to 7.5 µW/cm2 (0.9 mJ/cm2) at 18 mm of depth from the cortical surface. Given the fact that the average thickness of a human skull is 7–10 mm [52] and the distance from the interior skull surface to the brain is an additional 1–2 mm, then the NILT beam must penetrate 12 mm before contacting tissue, resulting in a maximum penetration depth of 8 mm. For example, if maximum penetration is 20 mm from skin surface to brain tissue, after subtraction of 12 mm for skull and dura penetration, NILT will penetrate 8 mm of brain tissue. Thus, maximal photons will be delivered to that surface and, thereafter, there is a gradient of photon effect between 0.2 and 3% (see below and [10]). This suggests the hypothesis that a limited amount of cortical tissue receives sufficient NILT for maximal photobiostiumulation.
Since the average thickness of the human cortex is 1–4.5 mm [53] and because of the complex pattern of sulci in the human brain, there will be differential NILT penetration when the probe is placed on the skin surface overlaying the skull. There may be insufficient coverage of all structures underlying the cortex with CW NILT currently being used in the NEST-3 clinical trial. This is further emphasized in a paper by Wan et al. [54] and discussed by Naeser et al. [14]. Wan et al. calculated that an energy density of 8 J/cm2 applied to the head will allow for penetration of 2–3% of NIR photons up to 1 cm from the scalp surface [54]. Furthermore, only 0.2–0.3% of NIR photons will reach 2 cm depth. It is interesting to note that Naeser et al. used a mixed wavelength diode in their TBI study; clusters of 870 nm and 633 nm diodes, suggesting that wavelengths other than 808 nm may also produce efficacious photobiomodulation [14].
Future generations of laser devices will have to incorporate PW modes to provide optimal photobiostimulation based upon exciting data from translational research studies in multiple species [19,26,34]. It is hypothesized that PW NILT will have greater efficacy owing to increased penetration of photons through the brain layers, primarily because PW NILT will deposit more photons deeper into the brain and overcome the large gradient effects noted for CW NILT.
Traumatic brain injury
The use of NILT to treat TBI is an obvious extension of the wealth of knowledge gained from translational and clinical studies aimed at treating stroke. There are two preclinical reports and one clinical report in the literature describing the development of NILT for TBI. The first scientific report from Oron and colleagues used a laser device with a wavelength of 808 nm, a power density of 10–20 mW/cm2 and a single treatment with a duration of 2 min [55]. The TBI model used by Oron was previously described in the literature [56,57]. Basically, for the closed-head injury model, 25–35 g male Sabra mice were used and a 94 g weight was dropped on the surface of the skull (3 mm lateral to midline, 1 mm caudal to the left coronal suture) to produce cortical injury and subsequent behavioral deficits and primarily motor function deficits. The drop distance was not reported. Table 1 summarizes the preclinical efficacy results. Behavioral analysis was measured using a composite neurological score (NSS) comprised of ten measures. In this preclinical study, TBI resulted in a mean NSS of 4.8 when measured 1 h after injury. In the control group, there was consistent improvement on the NSS scale up to 28 days post-TBI, which reached a mean decrease of 1.6 points and this was further decreased by NILT (~0.8 points), which was statistically different from the control improvement (p < 0.05). Overall, there was a 26% decrease in NSS at day 28 in the NILT-treated group. In NILT-treated mice, there was also a small infarct volume (~1.4%) compared with the control group (12.1%). The most interesting aspect of the study is the observation that NILT effects were not observed within 2 days but required at least 5 days. This is suggestive of NILT-induced neurogenesis and not neuroprotection; however, data were not presented to support the hypothesis.
The second LLLT study also used mice (male BALB/c 20–25 g) and a closed-head injury weight-drop technique [58]. However, the authors reduced the drop weight to 69 g rather than 94 g and the drop distance was 15 cm. The authors also used behavioral analysis as a primary measure using NSS and infarct volume as a secondary end point. LLLT was applied using a laser device (manufacturer not indicated). This study, unlike the Oron study [55], investigated three different wavelengths, 660 nm (also described as 655, 666 or 670 nm in the paper), 810 nm and 980 nm, but the specific rationale for the three different wavelengths was not provided. The study also used a power density 7.5–15-fold higher (i.e., 150 mW/cm2; 36 J/cm2) than the Oron study. LLLT at a wavelength in the mid 600 nm range (655–670) and at 810 nm appeared to produce decreased NSS scores; there was no effect of 980 nm LLLT treatment. It should be noted that in the control group, there was greater than a 60% decrease in NSS by day 28, indicating a large extent of spontaneous behavioral improvement in the model. The study also showed that both wavelengths that were effective at reducing NSS also reduced relative lesion volume by 50–60%. Unfortunately, the authors did not correlate behavioral improvement with any molecular mechanism.
Clinical efficacy
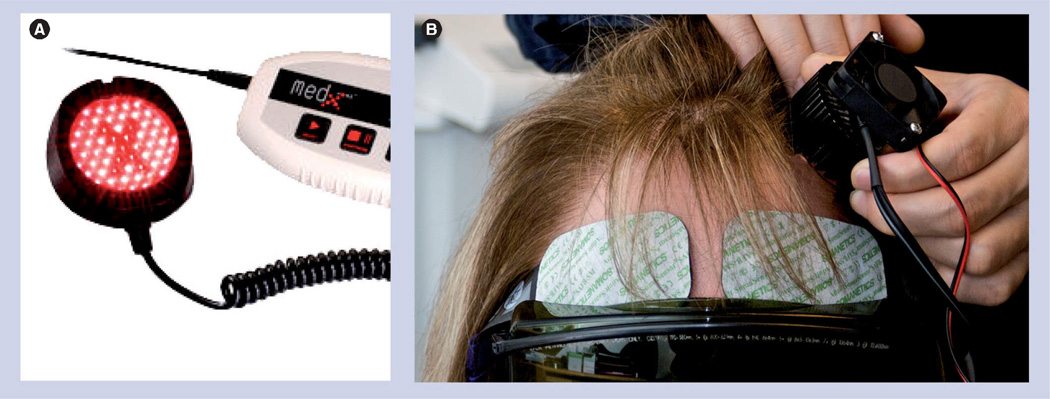
The translational studies described above used motor function ratings as the end point. The next logical step in the translation of NILT or LLLT to the clinic would be a measure of efficacy on motor deficits in TBI patients, if the preclinical studies were truly translational? However, in humans, TBI is a condition where there can be bilateral neurodegenerative changes in the form of axonal injury in both the frontal and temporal lobes, resulting in altered working memory and memory retrieval [59–61], in addition to motor deficits. However, a recent report by Naeser et al., which measured the effects of light in patients, used cognition and memory function, rather than motor function [14]. There were two patients included in the study. The first patient had a closed-head TBI resulting from a motor vehicle accident, while the second was a patient with multiple concussions resulting from sports injuries and recreational accidents. In the study, two different near-infrared light emitting diode (LED) clusters were used. The first was a combination of 40–870 nm and 9–633 nm diodes. This CW device had a total power of 500 mW (±20%) and a power density of 25.8 mW/cm2. The second was a combination of 52–870 nm and 9–633 nm diodes. This CW device had a total power of 500 mW (±20%) and a power density of 22.2 mW/cm2. In both patients, with chronic 2–4 month intermittent treatment using different devices, there were significant clinical improvements using a variety of measures such as attention span, memory function and ‘executive functions’ (Table 1). The efficacy and durability of LED treatment in two patients with different causes of TBI suggests that the clinical improvement is not due to sustained neurogenesis, because both patients regressed without repeated treatment at 1–2-week intervals, after establishing a baseline of significant clinical improvement. Figure 4 shows the LED device used in the TBI trials as well as a patient having the LED applied to her forehead to stimulate the frontal lobe.
Figure 4. Clinical noninvasive transcranial near-infrared laser therapy treatment (light emitting diode) for traumatic brain injury.
The figure shows a MedX Health Corp. (Mississauga, ON, Canada) light emitting diode (LED). (A) The LED has one circular-shaped cluster head, with a diameter of 5.35 cm (2.1 inches). Treatment area coverage is 22.48 cm2; each cluster head contained 61 diodes (52 near-infrared 870-nm diodes and nine red 633-nm diodes, 12–15 mW each). Total optical output power was 500 mW (+20%) continuous wave; power density was 22.2 mW/cm2 (+20%). (B) Close-up view of a patient receiving LED treatment to the left temporal lobe. Note the use of protective goggles during LED treatment.
Photographs courtesy of M Hamblin, Harvard University (Boston, MA, USA).
A personal communication from M Naeser (Harvard University, Boston, MA, USA) described further advances made by their research group. Since the preliminary studies were published [14], five additional chronic, mild TBI cases were treated with a series of 18 transcranial LED treatments for 6 weeks (Monday, Wednesday and Friday). All TBI patients showed significant improvement (p = 0.04–0.05) on executive function and verbal memory. Thus, for TBI, chronic, repeated administration of LED treatment is necessary to initiate and sustain clinical improvement.
AD (amyloid transgenic mouse)
AD with hallmarks of amyloid plaques and tangles [62] is usually characterized as a chronic neurodegenerative disease with multiple risk factors, including vascular and metabolic dysfunction [63], oxidative damage [64] chronic inflammation [65,66], mitochondrial dysfunction and impaired energy production [67,68], and a cholinergic deficit [69] leading to memory loss. There is one interesting article in the literature from the Photothera Inc. research group [26], including De Taboada who designed the NILT device for preclinical rat, mouse and rabbit studies, and was integral in the development of the human NILT device.
The AD study used a standard amyloid-b (Ab) precursor protein [70] transgenic mouse that overexpresses Ab and has spatial learning deficits, which are apparent in the Morris Water Maze [71]. Ab deposition is still one of the primary hypotheses underlying neuronal dysfunction and degeneration is AD [62], but this has always been controversial [72–74]. De Taboada applied NILT treatment to 3-month-old Ab precursor protein transgenic mice [26]. In this transgenic animal, brain amyloid deposits are not usually observed until 5–6 months of age, and as the authors show, there were significant levels of Ab peptide measured in CSF and plasma at 3 and 6 months. Thus, because of the temporal profile of amyloid deposition in this mouse model, the study in essence is not an AD treatment study, rather it is a study to show that NILT may slow the progression of biochemical, histological and behavioral abnormalities, before pathological changes related to amyloid are present (Table 1).
Nevertheless, the study showed that NILT treatment using CW at a wavelength of 808 nm with a cortical power density of 10 mW/cm2 improved behavior in the Morris Water Maze (p < 0.05), without affecting amyloid load in brain. Moreover, CW NILT did not have any significant effect on CSF Ab or plasma Ab levels at 90 days, but did produce a decrease in Ab in plasma at 180 days. PW NILT at cortical power densities of 50–100 mW/cm2 produced larger more statistically significant changes in behavioral improvement, amyloid load and both CSF and plasma Ab levels. Mechanistically, the effects of NILT, using the 50 mW/cm2 PW mode were associated with improved mitochondrial function since there was normalization of ATP levels and mitochondrial oxygen consumption, a finding consistent with a previous study [19]. This amyloid mouse study also found that NILT, primarily PW modes with high power densities and greater penetration, significantly reduced select markers of inflammation in brain tissue 6 months post-treatment initiation, including IL-1b, TNF-a and TGF-b, suggesting that NILT may chronically reduce inflammatory mechanisms. The reason for differential effect of PW modes in a mouse brain is not clear from the publication. There appeared to be variability in the response depending on which marker was measured (Table 1).
How can NILT be applied in the context of an AD clinical trial? It is apparent from the studies that specific PW mode regimens were more efficacious than CW NILT for a chronic neurodegenerative disease involving amyloid plaque deposition, when treatment is initiated before brain pathology is observed. However, in AD, clinicians will not be able to treat patients before there is brain pathology, even with current advances with AD diagnosis and detection [75–80]. As discussed by Khachaturian, there is a temporal lag between the initiating pathological event and the first appearance of symptoms and diagnosis of AD remains a challenge [75]. Thus, the timing of NILT utilization to treat AD, or a disease where there is significant amyloid pathology remains undefined. Preclinical and/or translational studies in animals models with already established AD pathology and clinical deficits are required before NILT should be advanced to clinical trial status, especially if NILT treatment will be required for the remainder of a patient’s life span (see the discussion in the TBI section related to chronic treatment).
Parkinson’s disease
PD is a chronic neurodegenerative disease commonly known as a movement disorder disease because symptoms are movement related, including shaking, rigidity, slowness of movement, and difficulty with walking and gait [81–83]. However, in many mid–late stage PD patients, there are also cognitive deficits and dementia [84–86] reminiscent of AD. The primary neuronal deficit in PD is within the nigrostriatal pathway owing to the death of dopaminergic fibers in the pathway. As is the case in AIS and TBI tissues, in PD tissues researchers have also found several lines of evidence for mitochondrial dysfunction [87–92]. For example, specific gene mutations that directly affect mitochondrial functions have been identified. These changes include impaired function of the mitochondrial electron transport chain, specifically mitochondrial complex I and damage to mitochondrial DNA [88,89,91].
There are few scientific studies related to the use of laser therapy to treat PD. One such study by Trimmer et al. used cybrid neuronal cells as a model [93]. Basically, a cybrid cell line is a fusion product using platelets from patients [93] to incorporate genetic abnormalities of PD patients. Hinging on the observation that there is mitochondrial dysfunction in PD patients, the study first determined the velocity of mitochondrial movement in cybrids from PD patients compared with control. There was significantly reduced mitochondrial velocity and reduced total distance traveled in PD cybrids. With LLLT (810 nm and 50 mW/cm2) of PD cybrids, there was increased velocity (Table 1). It is difficult to speculate on the clinical use of LLLT to treat PD based upon the artificially produced cybrids. However, because PD patients do show significant mitochondrial deficits [88,89,91], the hypothesis that LLLT may repair mitochondrial damage should be further tested.
A recent report used BALB/c albino mice to demonstrate that NILT treatment (670 nm light, Quantum Devices WARP 10 system, Scalp intensity 40 mW/cm2 with energy inside skull measured at 5.3 mW/cm2; 90 s duration repeated four times over 30 h) was neuroprotective following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced nigro-striatal degeneration [94]. The investigators quantified dopaminergic cells in the substantia nigra pars compacta (SNc) and the zona incerta-hypothalamus using standard tyrosine hydroxylase immunochemistry. In the study, NILT differentially promoted cell survival in the SNc by approximately 35–45%, compared with no significant effect of NILT on TH+ cell survival in the zona incerta-hypothalamus (Table 1).
Last, a report from Komel’kova et al. documented that laser therapy (He–Ne) can augment the time course of behavioral deficits associated with nigrostriatal degeneration in PD [95]. More importantly, they correlated behavioral improvement with enhanced or normalized enzyme activity in blood, specifically monoamine oxidase B, which catalyzes the oxidative deamination of dopamine, copper/zinc superoxide dismutase, which catalyze the superoxide free radical (O2•−) to form hydrogen peroxide and dioxygen and catalase, which converts hydrogen peroxide into water and molecular oxygen. The authors propose that laser therapy may reduce free radical damage in PD patients, and attenuate behavioral manifestations associated with dopaminergic cell loss.
Contraindications & precautions
There is currently one possible contraindication for NILT based upon the pharmacology of NILT administration and the effect of NILT to produce large increases in CBF [24]. This is a possible contraindication in a specific patient population, such as ischemic stroke patients with possible vascular damage (i.e., a hemorrhagic transformation or other type of brain bleed) [96]. NILT-induced changes in CBF may be detrimental to an ischemic stroke patient if there is expansion of a hemorrhage. Moreover, in a hemorrhagic stroke patient, NILT may cause excessive and uncontrollable brain bleeding and subsequent ischemic tissue death. However, a preclinical study in rabbits did show that NILT was safe and did not significantly affect hemorrhage [97]. Nevertheless, precautions should be taken in patients until safety has been shown.
There are also precautionary measures that should be taken when receiving NILT therapy in any setting. It is well known that photons generated by a laser using a wavelength in the NIR range (IR-A range of 700–1400 nm), such as those used in translational and clinical development, may cause retinal damage if the laser probe is directed toward the eye or the beam is reflected off of a surface [98–100]. However, this type of damage depends upon a variety of factors including power density. While this is not a contraindication for treating diseases, it is a technical concern for patients and technicians involved in laser treatment. It is also quite possibly a limiting step in the widespread use of NILT or LED devices.
According to the exclusion profile for the NEST trials [201], there are also a series of other contraindications for laser use including seizure at stroke onset or within the 7 days prior to stroke onset, sustained high or low blood glucose (>300 or <60 mg/dl), sustained hypertension (systolic blood pressure >220 mmHg or diastolic blood pressure >140 mmHg) or hypotension (systolic blood pressure <80 mmHg or diastolic blood pressure <50 mmHg), history of vascular disease and use of an intravenous or intra-arterial thrombolytic [201].
Expert commentary
The principal aim of this article was to address the possible use of NILT (TLT or LLLT) to treat acute and chronic neurodegenerative diseases to determine if and how NILT can be applied to improve clinical function. Based upon the scientific justification presented in this review and the efficacy profile of NILT in multiple preclinical translational models and in two randomized clinical trials for AIS, NILT should be pursued as a possible important neuroprotective or neurorestorative treatment for AIS, TBI, AD and PD. Preclinical data suggest that NILT has pleiotropic effects in the CNS that promote behavioral recovery. Specifically, in AIS, NILT regulates mitochondrial function to promote ATP formation, which allows cells to maintain functional machinery. Secondly, NILT may promote the production of neurotrophic factors to further improve cellular status and induce changes in plasticity that allow for recognition of long-term functional recovery.
While current data support the use of NILT to treat CNS diseases, there are three primary concerns that need to be addressed by the research community to continue to advance NILT.
First, can NILT be used to treat neurodegenerative disease that are not primarily cortical surface-based? The NEST-2 clinical data [47], showing efficacy only in mild stroke patients, suggest that the CW treatment regimen currently being used in the NEST-3 [201] trial is not optimal for AIS, and furthermore may not be transferable to other diseases such as AD. The exclusion criteria for the NEST-3 trials include infarcts located exclusively in the brainstem, cerebellum, or small deep infarctions or massive hemispheric strokes. This infers that enrollment may be limited to patients with small superficial cortical infarcts to optimize low power density CW treatment efficacy. By contrast, the recent clinical data for TBI suggest that a 2–2.5-fold increase in power density [14] over that used in the NEST-3 trial [201] may be useful to treat cortical-based executive function and memory deficits in TBI patients.
Second, the data supporting the use of NILT in AD is based upon using a high PW power density in a mouse brain, a brain that is miniscule in size compared with a human brain [26]. CW NILT was not very effective when measuring behavior or biochemical end points. Thus, even though there was significant efficacy in a mouse, there are still significant questions concerning development and optimization of laser devices to promote maximal stimulation in the human brain. With an increasing emphasis on PW NILT mode, further studies are warranted in larger animals to determine if NILT can be used to treat AD, where neuronal degeneration is widespread including degeneration in the temporal and parietal lobes, the frontal cortex, cingulate gyrus, thalamus and hippocampal gyrus.
Third, is a single treatment sufficient to sustain a durable effect in all neurodegenerative diseases? The preclinical stroke data show that a single CW treatment can promote behavioral recovery for 21 days [25]. It is interesting to note that even though preclinical data only supported the use of CW NILT out to 21 days following an ischemic event, the NEST trials used a single CW treatment and measured clinical efficacy 3 months thereafter. Nevertheless, the trial demonstrated single CW dose efficacy in a specific stroke population [12,47]. However, the preclinical AD data presented in this article suggests that chronic treatment is required [26]. Clinical TBI data point to the same conclusion [14], that repeated administration will be necessary to maintain significant clinical benefit.
Five-year view
The rapid advancement of NILT may occur over the next 4–5 years if NILT does not prove to be negative in the NEST-3 AIS clinical trial. In the event that substantial efficacy is not demonstrated in the NEST-3 trial, the device should be reconfigured to provide dosing and delivery regimens based upon translational research. It is becoming obvious that efficacy for diseases involving cortical and subcortical brain regions, including stroke, will ultimately require the development of devices that can administer PW NILT treatment regimens. Even if NILT is effective in the NEST-3 trial, efficacy in the limited patient population enrolled may not be applicable to AIS patients presenting with severe strokes involving subcortical brain regions. Thus, additional trials will eventually be required so that all patients can receive the beneficial treatment.
Moreover, as emphasized in the TBI work, because repeated treatment may be necessary to maintain clinical efficacy, devices will have to be compact and mobile so that repeated treatments could be made either in a clinical or nonclinical environment. In addition, further development of laser therapy for AD and PD may require a new generation of devices that can deliver energy to deep brain structures and they will also require repetitive use, similar to that described above for TBI.
Key issues.
Acute ischemic stroke (AIS) translational research 1: noninvasive transcranial near-infrared laser therapy (NILT) promotes recovery of function following embolic strokes, an effect correlated with enhanced mitochondrial ATP formation.
AIS translational research 2: pulse wave NILT is more effective than continuous wave NILT at enhancing mitochondrial function and behavior.
AIS clinical 3 study: NILT reduced clinical deficits in AIS patients with National Institutes of Health Stroke Scale scores of <15.
Traumatic brain injury (TBI) translational research 4: NILT improved neurological function and reduced infarct volume following TBI.
TBI clinical trial 5: NILT (low level laser therapy via light emitting diode) improved executive function and memory in TBI patients, but repeated administration was necessary.
Alzheimer’s disease translational research 6: NILT improved memory function in amyloid-b transgenic mice, an effect correlated with enhanced mitochondrial function and reduced inflammation. Chronic administration was required.
Parkinson’s disease translational research 7: NILT enhanced mitochondrial transport in an in vitro model of Parkinson’s disease and specifically increased the survival of dopaminergic cells in the substantia nigra pars compacta following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine treatment in mice.
NILT is a promising noninvasive method that should be further pursued to treat acute and chronic neurological diseases.
Acknowledgments
PA Lapchak is supported by a U01 Translational research grant NS060685 from the National Institute of Neurological Disorders and Stroke.
Footnotes
Financial & competing interests disclosure
The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
PA Lapchak is Director of Translational Research at Cedars-Sinai Medical Center and is on the Scientific Advisory Boards of Photothera Inc. He has no financial interest in Photothera Inc. Photothera Inc. did not pay the author to contribute this article to the scientific literature and had no editorial influence on the scientific content or opinions in this article.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Lapchak PA. Taking a light approach to treating acute ischemic stroke patients: transcranial near-infrared laser therapy translational science. Ann. Med. 2010;42(8):576–586. doi: 10.3109/07853890.2010.532811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anders JJ, Borke RC, Woolery SK, Van de Merwe WP. Low power laser irradiation alters the rate of regeneration of the rat facial nerve. Lasers Surg. Med. 1993;13(1):72–82. doi: 10.1002/lsm.1900130113. [DOI] [PubMed] [Google Scholar]
- 3.Castro-e-Silva O, Jr, Zucoloto S, Marcassa LG, et al. Spectral response for laser enhancement in hepatic regeneration for hepatectomized rats. Lasers Surg. Med. 2003;32(1):50–53. doi: 10.1002/lsm.10141. [DOI] [PubMed] [Google Scholar]
- 4.Mochizuki-Oda N, Kataoka Y, Cui Y, Yamada H, Heya M, Awazu K. Effects of near-infra-red laser irradiation on adenosine triphosphate and adenosine diphosphate contents of rat brain tissue. Neurosci. Lett. 2002;323(3):207–210. doi: 10.1016/s0304-3940(02)00159-3. [DOI] [PubMed] [Google Scholar]
- 5.Desmet KD, Paz DA, Corry JJ, et al. Clinical and experimental applications of NIR-LED photobiomodulation. Photomed. Laser Surg. 2006;24(2):121–128. doi: 10.1089/pho.2006.24.121. [DOI] [PubMed] [Google Scholar]
- 6.Nissan M, Rochkind S, Razon N, Bartal A. HeNe laser irradiation delivered transcutaneously: its effect on the sciatic nerve of rats. Lasers Surg. Med. 1986;6(5):435–438. doi: 10.1002/lsm.1900060502. [DOI] [PubMed] [Google Scholar]
- 7.Byrnes KR, Waynant RW, Ilev IK, et al. Light promotes regeneration and functional recovery and alters the immune response after spinal cord injury. Lasers Surg. Med. 2005;36(3):171–185. doi: 10.1002/lsm.20143. [DOI] [PubMed] [Google Scholar]
- 8.Oron U, Yaakobi T, Oron A, et al. Low-energy laser irradiation reduces formation of scar tissue after myocardial infarction in rats and dogs. Circulation. 2001;103(2):296–301. doi: 10.1161/01.cir.103.2.296. [DOI] [PubMed] [Google Scholar]
- 9.Ad N, Oron U. Impact of low level laser irradiation on infarct size in the rat following myocardial infarction. Int. J. Cardiol. 2001;80(2–3):109–116. doi: 10.1016/s0167-5273(01)00503-4. [DOI] [PubMed] [Google Scholar]
- 10.De Taboada L, Ilic S, Leichliter-Martha S, Oron U, Oron A, Streeter J. Transcranial application of low-energy laser irradiation improves neurological deficits in rats following acute stroke. Lasers Surg. Med. 2006;38(1):70–73. doi: 10.1002/lsm.20256. [DOI] [PubMed] [Google Scholar]
- 11. Ilic S, Leichliter S, Streeter J, Oron A, DeTaboada L, Oron U. Effects of power densities, continuous and pulse frequencies, and number of sessions of low-level laser therapy on intact rat brain. Photomed. Laser Surg. 2006;24(4):458–466. doi: 10.1089/pho.2006.24.458. • Important safety data study.
- 12.Lampl Y, Zivin JA, Fisher M, et al. Infrared laser therapy for ischemic stroke: a new treatment strategy: results of the NeuroThera Effectiveness and Safety Trial-1 (NEST-1) Stroke. 2007;38(6):1843–1849. doi: 10.1161/STROKEAHA.106.478230. [DOI] [PubMed] [Google Scholar]
- 13.Lapchak PA, Araujo DM. Advances in ischemic stroke treatment: neuroprotective and combination therapies. Expert Opin. Emerg. Drugs. 2007;12(1):97–112. doi: 10.1517/14728214.12.1.97. [DOI] [PubMed] [Google Scholar]
- 14. Naeser MA, Saltmarche A, Krengel MH, Hamblin MR, Knight JA. Improved cognitive function after transcranial, light-emitting diode treatments in chronic, traumatic brain injury: two case reports. Photomed. Laser Surg. 2011;29(5):351–358. doi: 10.1089/pho.2010.2814. • Study reporting efficacy in traumatic brain injury.
- 15.Eells JT. Therapeutic photobiomodulation for methanol-induced retinal toxicity. Proc. Natl Acad. Sci. USA. 2003;100:3439. doi: 10.1073/pnas.0534746100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Karu T. Mitochondrial mechanisms of photobiomodulation in context of new data about multiple roles of ATP. Photomed. Laser Surg. 2010;28(2):159–160. doi: 10.1089/pho.2010.2789. •• Key paper describing mechanisms of action.
- 17.Drochioiu G. Laser-induced ATP formation: mechanism and consequences. Photomed. Laser Surg. 2010;28(4):573–574. doi: 10.1089/pho.2009.2651. [DOI] [PubMed] [Google Scholar]
- 18. Karu TI, Pyatibrat LV, Kolyakov SF, Afanasyeva NI. Absorption measurements of a cell monolayer relevant to phototherapy: reduction of cytochrome c oxidase under near IR radiation. J. Photochem. Photobiol. B Biol. 2005;81(2):98–106. doi: 10.1016/j.jphotobiol.2005.07.002. • Classic study showing relationship between transcranial near-infrared laser treatment (NILT) and cytochrome c oxidase.
- 19.Lapchak PA, De Taboada L. Transcranial near infrared laser treatment (NILT) increases cortical adenosine-5´-triphosphate (ATP) content following embolic strokes in rabbits. Brain Res. 2010;1306:100–105. doi: 10.1016/j.brainres.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 20.Huang YY, Chen AC, Carroll JD, Hamblin MR. Biphasic dose response in low level light therapy. Dose Response. 2009;7(4):358–383. doi: 10.2203/dose-response.09-027.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang HL, Whelan HT, Eells JT, Wong-Riley MT. Near-infrared light via light-emitting diode treatment is therapeutic against rotenone- and 1-methyl-4-phenylpyridinium ion-induced neurotoxicity. Neuroscience. 2008;153(4):963–974. doi: 10.1016/j.neuroscience.2008.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oron A, Oron U, Chen J, et al. Low-level laser therapy applied transcranially to rats after induction of stroke significantly reduces long-term neurological deficits. Stroke. 2006;37(10):2620–2624. doi: 10.1161/01.STR.0000242775.14642.b8. [DOI] [PubMed] [Google Scholar]
- 23.Leung MC, Lo SC, Siu FK, So KF. Treatment of experimentally induced transient cerebral ischemia with low energy laser inhibits nitric oxide synthase activity and up-regulates the expression of transforming growth factor-beta 1. Lasers Surg. Med. 2002;31(4):283–288. doi: 10.1002/lsm.10096. [DOI] [PubMed] [Google Scholar]
- 24.Uozumi Y, Nawashiro H, Sato S, Kawauchi S, Shima K, Kikuchi M. Targeted increase in cerebral blood flow by transcranial near-infrared laser irradiation. Lasers Surg. Med. 2010;42(6):566–576. doi: 10.1002/lsm.20938. [DOI] [PubMed] [Google Scholar]
- 25. Lapchak PA, Wei J, Zivin JA. Transcranial infrared laser therapy improves clinical rating scores after embolic strokes in rabbits. Stroke. 2004;35(8):1985–1988. doi: 10.1161/01.STR.0000131808.69640.b7. •• Key study supporting the initiation of NEST-1.
- 26. De Taboada L, Yu J, El-Amouri S, et al. Transcranial laser therapy attenuates amyloid-b peptide neuropathology in amyloid-b protein precursor transgenic mice. J. Alzheimer’s Dis. 2011;23(3):521–535. doi: 10.3233/JAD-2010-100894. • Study showing that pretreatment can alter the Alzheimer’s disease time-course in transgenic mice.
- 27.Lapchak PA. Emerging therapies: pleiotropic multi-target drugs to treat stroke victims. Transl. Stroke Res. 2011;2(2):129–135. doi: 10.1007/s12975-011-0074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lyden P, Lu M, Jackson C, et al. underlying structure of the national institutes of health stroke scale: results of a factor analysis. Stroke. 1999;30:2347. doi: 10.1161/01.str.30.11.2347. [DOI] [PubMed] [Google Scholar]
- 29.Payabvash S, Kamalian S, Fung S, et al. Predicting language improvement in acute stroke patients presenting with aphasia: a multivariate logistic model using location-weighted atlas-based analysis of admission CT perfusion scans. AJNR Am. J. Neuroradiol. 2010;31(9):1661–1668. doi: 10.3174/ajnr.A2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kasner SE. Clinical interpretation and use of stroke scales. Lancet Neurol. 2006;5(7):603–612. doi: 10.1016/S1474-4422(06)70495-1. [DOI] [PubMed] [Google Scholar]
- 31.Singer OC, Dvorak F, du Mesnil de Rochemont R, Lanfermann H, Sitzer M, Neumann-Haefelin T. A simple 3-item stroke scale: comparison with the National Institutes of Health Stroke Scale and prediction of middle cerebral artery occlusion. Stroke. 2005;36(4):773–776. doi: 10.1161/01.STR.0000157591.61322.df. [DOI] [PubMed] [Google Scholar]
- 32.Lapchak PA. Translational stroke research using a rabbit embolic stroke model: a correlative analysis hypothesis for novel therapy development. Transl. Stroke Res. 2010;1(2):96–107. doi: 10.1007/s12975-010-0018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turner R, Jickling G, Sharp F. Are underlying assumptions of current animal models of human stroke correct: from STAIRS to high hurdles? Transl. Stroke Res. 2011;2(2):138–143. doi: 10.1007/s12975-011-0067-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lapchak PA, Salgado KF, Chao CH, Zivin JA. Transcranial near-infrared light therapy improves motor function following embolic strokes in rabbits: an extended therapeutic window study using continuous and pulse frequency delivery modes. Neuroscience. 2007;148(4):907–914. doi: 10.1016/j.neuroscience.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Lapchak PA. A clinically relevant rabbit embolic stroke model for acute ischemic stroke therapy development: mechanisms and targets. In: Lapchak PA, Zhang JH, editors. Translational Stroke Research: From Target Selection to Clinical Trials. New York, NY, USA: Springer; 2012. [Google Scholar]
- 36.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N. Engl. J. Med. 2008;359(13):1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 37.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N. Engl. J. Med. 1995;333(24):1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 38.Del Zoppo GJ, Saver JL, Jauch EC, Adams HP., Jr Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator. A science advisory from the American Heart Association/American Stroke Association. Stroke. 2009;40(8):2945–2948. doi: 10.1161/STROKEAHA.109.192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lapchak PA, Zivin J. Transcranial near-infrared laser therapy improves behavior and differentially regulates the expression of rapid response elements in rabbits following embolic strokes. Stroke. 2009;49:P268. [Google Scholar]
- 40.Helm GA, Alden TD, Sheehan JP, Kallmes D. Bone morphogenetic proteins and bone morphogenetic protein gene therapy in neurological surgery: a review. Neurosurgery. 2000;46(5):1213–1222. doi: 10.1097/00006123-200005000-00038. [DOI] [PubMed] [Google Scholar]
- 41.Marini AM, Jiang X, Wu X, et al. Preconditioning and neurotrophins: a model for brain adaptation to seizures, ischemia and other stressful stimuli. Amino Acids. 2007;32(3):299–304. doi: 10.1007/s00726-006-0414-y. [DOI] [PubMed] [Google Scholar]
- 42.Reichardt LF. Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006;361(1473):1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patapoutian A, Reichardt LF. Trk receptors: mediators of neurotrophin action. Curr. Opin. Neurobiol. 2001;11(3):272–280. doi: 10.1016/s0959-4388(00)00208-7. [DOI] [PubMed] [Google Scholar]
- 44.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lapchak PA, Beck KD, Araujo DM, Irwin I, Langston JW, Hefti F. Chronic intranigral administration of brain-derived neurotrophic factor produces striatal dopaminergic hypofunction in unlesioned adult rats and fails to attenuate the decline of striatal dopaminergic function following medial forebrain bundle transection. Neuroscience. 1993;53(3):639–650. doi: 10.1016/0306-4522(93)90612-j. [DOI] [PubMed] [Google Scholar]
- 46.Tabruyn SP, Griffioen AW. Molecular pathways of angiogenesis inhibition. Biochem. Biophys. Res. Commun. 2007;355(1):1–5. doi: 10.1016/j.bbrc.2007.01.123. [DOI] [PubMed] [Google Scholar]
- 47.Zivin JA, Albers GW, Bornstein N, et al. Effectiveness and safety of transcranial laser therapy for acute ischemic stroke. Stroke. 2009;40(4):1359–1364. doi: 10.1161/STROKEAHA.109.547547. [DOI] [PubMed] [Google Scholar]
- 48.Lyden P, Lu M, Jackson C, et al. Underlying structure of the National Institutes of Health Stroke Scale: results of a factor analysis. NINDS tPA Stroke Trial Investigators. Stroke. 1999;30(11):2347–2354. doi: 10.1161/01.str.30.11.2347. [DOI] [PubMed] [Google Scholar]
- 49.Bluhmki E, Chamorro A, Davalos A, et al. Stroke treatment with alteplase given 3.0–4.5 h after onset of acute ischaemic stroke (ECASS III): additional outcomes and subgroup analysis of a randomised controlled trial. Lancet Neurol. 2009;8(12):1095–1102. doi: 10.1016/S1474-4422(09)70264-9. [DOI] [PubMed] [Google Scholar]
- 50.Saver JL, Gornbein J, Grotta J, et al. Number needed to treat to benefit and to harm for intravenous tissue plasminogen activator therapy in the 3- to 4.5-hour window. Joint outcome table analysis of the ECASS 3 trial. Stroke. 2009;40:2433–2437. doi: 10.1161/STROKEAHA.108.543561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saver JL, Gornbein J. Treatment effects for which shift or binary analyses are advantageous in acute stroke trials. Neurology. 2009;72(15):1310–1315. doi: 10.1212/01.wnl.0000341308.73506.b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lynnerup N, Astrup JG, Sejrsen B. Thickness of the human cranial diploe in relation to age, sex and general body build. Head Face Med. 2005;1:13. doi: 10.1186/1746-160X-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl Acad. Sci. USA. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wan S, Parrish JA, Anderson RR, Madden M. Transmittance of nonionizing radiation in human tissues. Photochem. Photobiol. 1981;34(6):679–681. doi: 10.1111/j.1751-1097.1981.tb09063.x. [DOI] [PubMed] [Google Scholar]
- 55. Oron A, Oron U, Streeter J, Alexandrovich A, Trembovler V, Shohami E. Low-level laser therapy applied transcranially to mice following traumatic brain injury significantly reduces long-term neurological deficits. J. Neurotrauma. 2007;24(4):651–656. doi: 10.1089/neu.2006.0198. • First traumatic brain injury study showing that NILT has benefit.
- 56.Yatsiv I, Morganti-Kossmann MC, Perez D, et al. Elevated intracranial IL-18 in humans and mice after traumatic brain injury and evidence of neuroprotective effects of IL-18-binding protein after experimental closed head injury. J. Cereb. Blood Flow Metab. 2002;22(8):971–978. doi: 10.1097/00004647-200208000-00008. [DOI] [PubMed] [Google Scholar]
- 57.Chen Y, Constantini S, Trembovler V, Weinstock M, Shohami E. An experimental model of closed head injury in mice: pathophysiology, histopathology, and cognitive deficits. J. Neurotrauma. 1996;13(10):557–568. doi: 10.1089/neu.1996.13.557. [DOI] [PubMed] [Google Scholar]
- 58.Wu Q, Huang Y-Y, Dhital S, et al. Low level laser therapy for traumatic brain injury. SPIE Proceedings. 2010;7552:755206–755208. [Google Scholar]
- 59.Ashman TA, Gordon WA, Cantor JB, Hibbard MR. Neurobehavioral consequences of traumatic brain injury. Mt Sinai J. Med. 2006;73(7):999–1005. [PubMed] [Google Scholar]
- 60.Helmy A, Vizcaychipi M, Gupta AK. Traumatic brain injury: intensive care management. Br. J. Anaesth. 2007;99(1):32–42. doi: 10.1093/bja/aem139. [DOI] [PubMed] [Google Scholar]
- 61.Kiraly M, Kiraly SJ. Traumatic brain injury and delayed sequelae: a review – traumatic brain injury and mild traumatic brain injury (concussion) are precursors to later-onset brain disorders, including early-onset dementia. Sci. World J. 2007;7:1768–1776. doi: 10.1100/tsw.2007.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Selkoe DJ. The molecular pathology of Alzheimer’s disease. Neuron. 1991;6(4):487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- 63.Murray IV, Proza JF, Sohrabji F, Lawler JM. Vascular and metabolic dysfunction in Alzheimer’s disease: a review. Exp. Biol. Med. (Maywood) 2011;236(7):772–782. doi: 10.1258/ebm.2011.010355. [DOI] [PubMed] [Google Scholar]
- 64.Sims NR. Energy metabolism, oxidative stress and neuronal degeneration in Alzheimer’s disease. Neurodegeneration. 1996;5(4):435–440. doi: 10.1006/neur.1996.0059. [DOI] [PubMed] [Google Scholar]
- 65.Grammas P. Neurovascular dysfunction, inflammation and endothelial activation: implications for the pathogenesis of Alzheimer’s disease. J. Neuroinflammation. 2011;8:26. doi: 10.1186/1742-2094-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marchesi VT. Alzheimer’s dementia begins as a disease of small blood vessels, damaged by oxidative-induced inflammation and dysregulated amyloid metabolism: implications for early detection and therapy. FASEB J. 2011;25(1):5–13. doi: 10.1096/fj.11-0102ufm. [DOI] [PubMed] [Google Scholar]
- 67.Atamna H, Frey WH., II Mechanisms of mitochondrial dysfunction and energy deficiency in Alzheimer’s disease. Mitochondrion. 2007;7(5):297–310. doi: 10.1016/j.mito.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 68.Parnetti L, Gaiti A, Brunetti M, et al. Increased CSF pyruvate levels as a marker of impaired energy metabolism in Alzheimer’s disease. J. Am. Geriatr. Soc. 1995;43(3):316–318. doi: 10.1111/j.1532-5415.1995.tb07351.x. [DOI] [PubMed] [Google Scholar]
- 69.Araujo DM, Lapchak PA, Robitaille Y, Gauthier S, Quirion R. Differential alteration of various cholinergic markers in cortical and subcortical regions of human brain in Alzheimer’s disease. J. Neurochem. 1988;50(6):1914–1923. doi: 10.1111/j.1471-4159.1988.tb02497.x. [DOI] [PubMed] [Google Scholar]
- 70.Hook VY, Kindy M, Reinheckel T, Peters C, Hook G. Genetic cathepsin B deficiency reduces beta-amyloid in transgenic mice expressing human wild-type amyloid precursor protein. Biochem. Biophys. Res. Commun. 2009;386(2):284–288. doi: 10.1016/j.bbrc.2009.05.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sutherland RJ, Whishaw IQ, Regehr JC. Cholinergic receptor blockade impairs spatial localization by use of distal cues in the rat. J. Comp. Physiol. Psychol. 1982;96(4):563–573. doi: 10.1037/h0077914. [DOI] [PubMed] [Google Scholar]
- 72.Armstrong RA. The pathogenesis of Alzheimer’s disease: a reevaluation of the ‘amyloid cascade hypothesis’. Int. J. Alzheimers Dis. 2011;2011 doi: 10.4061/2011/630865. 630865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saxena U. Alzheimer’s disease amyloid hypothesis at crossroads: where do we go from here? Expert Opin. Ther. Targets. 2010;14(12):1273–1277. doi: 10.1517/14728222.2010.528285. [DOI] [PubMed] [Google Scholar]
- 74.Gandy S. Testing the amyloid hypothesis of Alzheimer’s disease in vivo. Lancet Neurol. 2010;9(4):333–335. doi: 10.1016/S1474-4422(10)70055-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Khachaturian ZS. Revised criteria for diagnosis of Alzheimer’s disease: National Institute on Aging-Alzheimer’s Association diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):253–256. doi: 10.1016/j.jalz.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 76.Padovani A, Gilberti N, Borroni B. The usefulness of biological and neuroimaging markers for the diagnosis of early-onset Alzheimer’s disease. Int. J. Alzheimers Dis. 2011;2011 doi: 10.4061/2011/296374. 296374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Craig-Schapiro R, Kuhn M, Xiong C, et al. Multiplexed immunoassay panel identifies novel CSF biomarkers for Alzheimer’s disease diagnosis and prognosis. PLoS One. 2011;6(4):e18850. doi: 10.1371/journal.pone.0018850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vann AS. Ten things you should do when the diagnosis is Alzheimer’s. Am. J. Alzheimers Dis. Other Demen. 2011;26(2):93–96. doi: 10.1177/1533317510397332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wirdefeldt K, Adami HO, Cole P, Trichopoulos D, Mandel J. Epidemiology and etiology of Parkinson’s disease: a review of the evidence. Eur. J. Epidemiol. 2011;26 Suppl. 1:S1–S58. doi: 10.1007/s10654-011-9581-6. [DOI] [PubMed] [Google Scholar]
- 82.Hickey P, Stacy M. Available and emerging treatments for Parkinson’s disease: a review. Drug Des. Devel. Ther. 2011;5:241–254. doi: 10.2147/DDDT.S11836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lapchak PA, Gash DM, Jiao S, Miller PJ, Hilt D. Glial cell line-derived neurotrophic factor: a novel therapeutic approach to treat motor dysfunction in Parkinson’s disease. Exp. Neurol. 1997;144(1):29–34. doi: 10.1006/exnr.1996.6384. [DOI] [PubMed] [Google Scholar]
- 84.Williams-Gray CH, Foltynie T, Lewis SJ, Barker RA. Cognitive deficits and psychosis in Parkinson’s disease: a review of pathophysiology and therapeutic options. CNS Drugs. 2006;20(6):477–505. doi: 10.2165/00023210-200620060-00004. [DOI] [PubMed] [Google Scholar]
- 85.Dubois B, Pillon B. Cognitive deficits in Parkinson’s disease. J. Neurol. 1997;244(1):2–8. doi: 10.1007/pl00007725. [DOI] [PubMed] [Google Scholar]
- 86.Lees AJ, Smith E. Cognitive deficits in the early stages of Parkinson’s disease. Brain. 1983;106(Pt 2):257–270. doi: 10.1093/brain/106.2.257. [DOI] [PubMed] [Google Scholar]
- 87.Beal MF. Therapeutic approaches to mitochondrial dysfunction in Parkinson’s disease. Parkinsonism Relat. Disord. 2009;15 Suppl. 3:S189–S194. doi: 10.1016/S1353-8020(09)70812-0. [DOI] [PubMed] [Google Scholar]
- 88.Lin TK, Liou CW, Chen SD, et al. Mitochondrial dysfunction and biogenesis in the pathogenesis of Parkinson’s disease. Chang Gung Med. J. 2009;32(6):589–599. [PubMed] [Google Scholar]
- 89.Winklhofer KF, Haass C. Mitochondrial dysfunction in Parkinson’s disease. Biochim. Biophys. Acta. 2010;1802(1):29–44. doi: 10.1016/j.bbadis.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 90.Park J, Kim Y, Chung J. Mitochondrial dysfunction and Parkinson’s disease genes: insights from Drosophila. Dis. Model Mech. 2009;2(7–8):336–340. doi: 10.1242/dmm.003178. [DOI] [PubMed] [Google Scholar]
- 91.Malkus KA, Tsika E, Ischiropoulos H. Oxidative modifications, mitochondrial dysfunction, and impaired protein degradation in Parkinson’s disease: how neurons are lost in the Bermuda triangle. Mol. Neurodegener. 2009;4:24. doi: 10.1186/1750-1326-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Banerjee R, Starkov AA, Beal MF, Thomas B. Mitochondrial dysfunction in the limelight of Parkinson’s disease pathogenesis. Biochim. Biophys. Acta. 2009;1792(7):651–663. doi: 10.1016/j.bbadis.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Trimmer PA, Schwartz KM, Borland MK, De Taboada L, Streeter J, Oron U. Reduced axonal transport in Parkinson’s disease cybrid neurites is restored by light therapy. Mol. Neurodegener. 2009;4:26. doi: 10.1186/1750-1326-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Shaw VE, Spana S, Ashkan K, et al. Neuroprotection of midbrain dopaminergic cells in MPTP-treated mice after near-infrared light treatment. J. Comp. Neurol. 2010;518(1):25–40. doi: 10.1002/cne.22207. • Study providing data to support that NILT protects dopamine neurons.
- 95.Komel’kova LV, Vitreshchak TV, Zhirnova IG, et al. Biochemical and immunological induces of the blood in Parkinson’s disease and their correction with the help of laser therapy. Patol. Fiziol. Eksp. Ter. 2004;(1):15–18. [PubMed] [Google Scholar]
- 96.Lapchak PA, Wu Q. Vascular dysfunction in brain hemorrhage: translational pathways to developing new treatments from old targets. J. Neurol. Neurophysiol. 2011 doi: 10.4172/2155-9562.S1-e001. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lapchak PA, Han MK, Salgado KF, Streeter J, Zivin JA. Safety profile of transcranial near-infrared laser therapy administered in combination with thrombolytic therapy to embolized rabbits. Stroke. 2008;39(11):3073–3078. doi: 10.1161/STROKEAHA.108.516393. [DOI] [PubMed] [Google Scholar]
- 98.Ham WT, Jr, Clarke AM, Geeraets WJ, Cleary SF, Mueller HA, Williams RC. The eye problem in laser safety. Arch. Environ. Health. 1970;20(2):156–160. doi: 10.1080/00039896.1970.10665563. [DOI] [PubMed] [Google Scholar]
- 99.Denton ML, Foltz MS, Schuster KJ, Estlack LE, Thomas RJ. Damage thresholds for cultured retinal pigment epithelial cells exposed to lasers at 532 nm and 458 nm. J. Biomed. Opt. 2007;12(3) doi: 10.1117/1.2737394. 034030. [DOI] [PubMed] [Google Scholar]
- 100.Denton ML, Foltz MS, Estlack LE, et al. Damage thresholds for exposure to NIR and blue lasers in an in vitro RPE cell system. Invest. Ophthalmol. Vis. Sci. 2006;47(7):3065–3073. doi: 10.1167/iovs.05-1066. [DOI] [PubMed] [Google Scholar]
Website
- 201.NEST-3. http://clinicaltrials.gov/ct2/show/NCT01120301.
Patent
- 301.Streeter, et al. US2011/0144723 A1 2011