Abstract
Much of the current understanding about the pathogenesis of altered mood, impaired concentration and neurovegetative symptoms in major depression has come from animal models. However, because of the unique and complex features of human depression, the generation of valid and insightful depression models has been less straightforward than modeling other disabling diseases like cancer or autoimmune conditions. Today’s popular depression models creatively merge ethologically valid behavioral assays with the latest technological advances in molecular biology and automated video-tracking. This chapter reviews depression assays involving acute stress (e.g., forced swim test), models consisting of prolonged physical or social stress (e.g., social defeat), models of secondary depression, genetic models, and experiments designed to elucidate the mechanisms of antidepressant action. These paradigms are critically evaluated in relation to their ease, validity and replicability, the molecular insights that they have provided, and their capacity to offer the next generation of therapeutics for depression.
Keywords: Animal models, Antidepressants, Behavioral testing, Depression Resilience, Stress, Vulnerability
1 Introduction
Major depressive disorder (MDD) or depression is a heritable neuropsychiatric syndrome characterized by relatively subtle cellular and molecular alterations distributed across a circuit of neural substrates (Krishnan and Nestler 2008). This disease claims a malignant toll on health: a 2007 World Health Organization study of over 200,000 adults across the world showed that depression produces the greatest decrement in health when compared with chronic diseases like diabetes and arthritis (Moussavi et al. 2007). In spite of a large variety of available antidepressant medications and alternative therapeutic modalities including several forms of psychotherapy (e.g., cognitive behavioral therapy) and several other approaches such as yoga, exercise, and sleep deprivation, depression suffers a huge treatment gap worldwide, whereby large numbers of individuals who require care do not receive treatment (Kohn et al. 2004). Depressive disorders cause morbidity across the entire age spectrum (Kessler et al. 2005): they can be difficult to diagnose and treat in the pediatric and adolescent period (Prager 2009), complicate the course of patients with chronic illness (Evans et al. 2005), and increase overall medical burden in the elderly (Lyness et al. 2006).
Over and above this alarming public health problem, shortfalls in treatment pose a grave concern. Even if major depression is accurately diagnosed and treated in all individuals with perfect treatment compliance, the best remission rates with standard antidepressants are only 30–40% (Rapaport et al. 2003; Trivedi et al. 2006). This is in stark contrast with other chronic disorders such as diabetes mellitus (Krishnan and Nestler 2008), where the correct combination of medications ultimately can ensure normoglycemia and prevent diabetic complications in a large majority of patients. Several explanations have been put forth for this discrepancy between the treatment of depression and other chronic disabling conditions. First, the diagnosis of depressive episodes is made when patients display a certain number of vaguely defined clinical symptoms (e.g., depressed mood, anhedonia, sleep changes, appetite changes, guilt, etc.) for a 2-week period. In the absence of more objective diagnostics such as neuroimaging, genetic variations, biomarkers, or biopsies, this rudimentary “symptom-counting” approach creates obvious limitations for the development of animal models, clinical trials, and neuropathological investigations (Krishnan and Nestler 2008). While the symptomatic heterogeneity of depression (atypical vs. melancholic vs. psychotic, etc.) is well recognized (Rush 2007), little insight has been gained into the etiological and pathophysiological distinctions between these subtypes. Drug efficacy trials are seldom conducted on subtype-segregated groups, thereby increasing the chance of abandoning therapies that may be subtype-specific. Since all available pharmacological treatments for depression work through altering monoaminergic transmission (Berton and Nestler 2006), it is possible that only one type of depression is being treated (“monoamine-responsive”). Due to high placebo response rates (Brunoni et al. 2009) and side effect concerns, monoamine-based agents still constitute a significant proportion of “new” antidepressants being tested in clinical trials (Mathew et al. 2008). And finally, given that genetic, neuroimaging, postmortem analyses and laboratory investigations (e.g., markers in serum or cerebrospinal fluid) have yielded limited insight into the neurobiology underlying depression (Krishnan and Nestler 2008), most current theories of depression are based largely on animal models of the disease, which are also inherently limited.
2 Can Depression Be Modeled in Laboratory Animals?
If the full psychiatric syndrome of depression cannot be recapitulated in rodents or nonhuman primates, then is it worthwhile to infer anything at all from animal models of depression? While symptoms such as guilt, suicidality and sad mood are likely to be purely human features, other aspects of the depressive syndrome have been replicated in laboratory animals, and in several instances ameliorated with antidepressant treatment. These include measures of helplessness, anhedonia, behavioral despair and other neurovegetative changes such as alterations in sleep and appetite patterns. From an evolutionary perspective, depression has been proposed to be an analog of the involuntary defeat strategy (IDS), which is triggered when an animal perceives defeat in a hierarchical struggle for resources (Sloman 2008). Features of psychomotor retardation, hyperarousal, anhedonia and sleep disturbances in the setting of losing such a struggle are postulated to have an adaptive advantage in that they serve to protect losers from further attack and focus cognitive assets on planning ways out of complex social problems (Nesse 2000; Watson and Andrews 2002). Most, if not all, animal models of depression aim to quantitatively assay some form of experimentally induced defeat or despair, even though this aspect of mammalian behavior is likely physiological (i.e., adaptive) rather than pathological. In addition, while despair behavior is often extrapolated as being depression-like, the application of stress to rodents also produces anxiety-like changes that are manifestations of the fight or flight response (reduced exploration, freezing, stress-induced hyperthermia, etc.). Just as anxiety and depression often overlap clinically, the distinction between stress-induced depression-like and anxiety-like behaviors is difficult to ascertain, particularly since both types of behaviors respond to antidepressants. Thus, an important challenge of the field has been to produce a long-lasting state of depressive pathology in laboratory animals, which has seldom been achieved.
Today’s depression models are often evaluated by fulfilling three main criteria (a) face validity (the requirement for a reasonable degree of symptomatic homology), (b) construct (or etiological) validity (the requirement for similar causative factors), and (c) pharmacological validity (which requires the reversal of depressive symptoms by available antidepressants). These criteria serve as guides to compare models against each other, but each criterion suffers basic flaws (Nestler and Hyman 2010). For instance, in the olfactory bulbectomy model of depression, surgically bulbectomized adult rats display increased locomotor activity, increased aggression, and spatial memory impairments that are all reversed by the chronic administration of a diverse array of antidepressants (Song and Leonard 2005). While this model may appear to be weak in construct and face validity, its pharmacological validity is excellent: virtually all classes of available antidepressants reverse these behavioral changes with a therapeutic delay. Of course, people with depression do not have olfactory lesions. Nevertheless, our assessment of poor construct validity is of limited value, since the etiology of depression is incompletely understood. Strict applications of face validity pose the risk of excessive anthropomorphization, particularly when assessing rodents such as mice, rats or tree shrews, which each have their own distinct behavioral repertoires (Crawley 2000). Since candidate models of depression are often assessed for reversibility with known monoamine-based antidepressants, there exists the alarming possibility that the most popular models of depression may, by design, be insensitive to the antidepressant effects of nonmonoamine-based agents (Berton and Nestler 2006). A potential fourth criterion is pathological validity, whereby animal models are validated by their recapitulation of known postmortem pathological or serological changes found in human depressed patients. Given our current state of knowledge, this is a very difficult requirement, but with increasing efforts in this field stemming from more widespread access to human postmortem tissue, the elucidation of pathological validity criteria may potentially eliminate the circular arguments that lie at the core of modeling depression.
This chapter evaluates the current status of animal models in depression and highlights certain novel neurobiological insights which have been generated using these models. Given the emphasis on molecular perspectives, we focus on data from rodent studies. Preclinical studies in nonhuman primates have largely focused on the behavioral and endocrinological impacts of early life stress (Gilmer and McKinney 2003) and, while this is clearly a critical research avenue, this field has been limited by a variety of factors which restrict nonhuman primate research. Instead of attempting to be comprehensive, this review highlights key methodological strengths and limitations and provides recommendations for further experimentation. The reader is referred elsewhere for a recent systematic and concise description of the neurobiology of depression (Krishnan and Nestler 2010).
3 Animal Models of Depression and Molecular Insights
3.1 Models of Acute Stress
3.1.1 Forced Swim Test and Tail Suspension Test
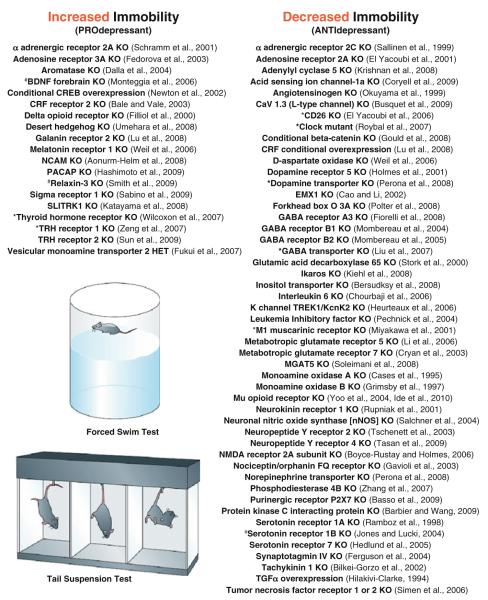
The forced swim test (FST) and tail suspension test (TST) are the most widely used tests of antidepressant action and are also used to infer “depression-like” behavior. In the Porsolt test (Porsolt et al. 1977), also known as the FST test, a mouse or rat is placed in an inescapable cylinder of water and, following an initial period of struggling, swimming and climbing, the animal eventually displays a floating or immobile posture. In the TST, immobility is scored while mice are suspended by their tails. Since water is not required, the TST is not confounded by challenges to thermoregulation (Cryan and Mombereau 2004). FST or TST immobility has been interpreted as an expression of behavioral despair or entrapment (Cryan et al. 2005; Lucki et al. 2001), and is reversed by the acute administration of almost all available antidepressants. This poses a problem for the model, since antidepressants restore mood in depressed humans only after many weeks of administration. Numerous agents that act independently of monoamine signaling have also been shown to reduce immobility time, such as recombinant ghrelin (Lutter et al. 2008), ketamine (Maeng et al. 2008), and estradiol (Dhir and Kulkarni 2008), to name a few. The foremost strength of these models is their ability to rapidly screen novel agents and phenotype genetically manipulated mice, and both paradigms have been successfully automated to reduce errors in subjective scoring. As shown in Fig. 1, a large number of mutant mice have been screened through the FST or TST. There appear to be a much larger number of “antidepressant-like” knockouts (KO), i.e., those mice that exhibit reduced immobility, compared with the number of KOs that exhibit increased immobility, but this may reflect a constraint of the model since it was originally designed to capture antidepressant effects. These studies illustrate the number and diversity of genes that may play a role in regulating stress-induced immobility, including transcription factors, growth factors, endocrine hormones, immune signaling molecules, and numerous genes encoding proteins required for synaptic neurotransmission.
Fig. 1.
The forced swim test (FST) and tail suspension test (TST) have been utilized to phenotype a large number of genetically manipulated mice, illustrating the sheer diversity of genes and pathways potentially involved in depression-related behavior. Knockout mice (“KO”), transgenic overexpressors or other types of mutants have been segregated into those that display increased immobility in either the FST or TST (“pro-depressant”), or reduced immobility (antidepressant-like). Hash indicates gender differences in the phenotype; asterisk indicates that results may be confounded by locomotor behavior. Shown are examples of genetic mutant mice examined to date; studies utilizing virally mediated gene transfer are not included. Abbreviations: HET heterozygote; BDNF brain-derived neurotrophic factor; TRH thyrotropin-releasing hormone; CREB cyclic adenosine monophosphate response element binding protein; CRF corticotropin-releasing factor; NCAM neuronal cell adhesion molecule; PACAP pituitary adenylyl cyclase activating peptide; SLITRK slit and NTRK-like family member 1; GABA gamma aminobutyric acid; NMDA N-methyl d aspartate; TGF transforming growth factor; EMX empty spiracles homolog; MGAT mannosyl glycoprotein acetylglucosaminyl transferase. Images obtained from Cryan and Holmes (2005)
Since the majority of mutants phenotyped thus far are constitutive KOs, their phenotype could be confounded by developmental compensatory effects, e.g., biochemical and anatomical alterations which are secondary to the loss of the gene of interest [see also Gondo et al. (2011); O’Tuathaigh et al. (2011) for further discussion]. These compensatory effects may, nevertheless, be relevant to the study of depression. For example, the profound antidepressant-like phenotype of TREK1 (Twik-related K Channel 1) KO mice is associated with markedly altered 5HT1A-receptor-mediated excitation in the hippocampus (Heurteaux et al. 2006), a change that is also observed following chronic treatment with a variety of antidepressants (Haddjeri et al. 1998). Performance on the FST and TST is also dependent on the background strain of the animals used: systematic comparisons of inbred mice reveal greater than a tenfold range of immobility (Liu and Gershenfeld 2003; Lucki et al. 2001; see also Gondo et al. 2011; O’Tuathaigh et al. 2011 for further discussion). While the effects of background strain tend to complicate phenotypic analysis of mutant mice, such variation has been exploited for QTL (quantitative trait loci) analyses, which have implicated genes in certain broad chromosomal regions in this type of behavioral response (Jacobson and Cryan 2007; Tomida et al. 2009).
The complexities of “simple” immobility testing are exemplified by data from serotonin transporter (SERT) KO mice. Since SERT is inhibited by many available antidepressants, one might expect SERT KO mice to display a robust antidepressant-like phenotype. However, they display increased FST immobility and decreased TST immobility on a 129S6 or 129S6/SvEV mixed background, have increased TST immobility on a CD1 background, and yet have no phenotype on a C57BL/6J background (Alexandre et al. 2006; Holmes et al. 2002; Lira et al. 2003). Subsequently, a SERT KO rat has been generated through random ENU (N-ethyl-N-nitrosurea) mutagenesis, which displays increased immobility on the FST (Olivier et al. 2008). Thus, while SERT inhibition is required for the antidepressant effects of SSRIs (selective serotonin-reuptake inhibitors) (Holmes et al. 2002), it appears that the developmental loss of SERT produces a complex phenotype that is clearly dependent on background strain. While the precise mechanistic details remain unclear, the observed pro-depressant-like phenotypes may be related to pathologically elevated synaptic serotonin levels during development causing a decrease in the number and firing rate of serotonergic neurons (Lira et al. 2003) as well as disorganized limbic cortical development (Olivier et al. 2008).
3.1.2 The Learned Helplessness Model
Following an uncontrollable and inescapable stress such as exposure to inescapable electric shocks, animals develop a state of “helplessness” such that when re-exposed to the same shocks, now with an easy escape route, animals will either display increased escape latency or completely fail to escape (Seligman et al. 1975). Following one or more sessions of inescapable shock, rats have been shown to develop persistent changes including weight loss, alterations in sleep patterns and HPA axis activity and loss of spine synapses in hippocampal regions (Cryan and Mombereau 2004; Haddjeri etal. 1998; Nestler et al. 2002). In mice, the learned helplessness (LH) syndrome appears to be short-lived (2–3 days), and several mutant lines of mice have been phenotyped on the LH assay, with results largely compatible with their corresponding FST data. Like the FST or TST, both mice and rats display a considerable degree of interstrain variation, and escape deficits are reversed by a variety of antidepressants (Henn and Vollmayr 2005).
One distinctive feature of LH is the considerable degree of variability in the expression of helplessness: anywhere from 10 to 80% of animals simply fail to develop escape deficits. While this may be a disadvantage in certain scenarios, this variability has been exploited to devise selective inbreeding strategies to create of helpless and nonhelpless strains of rats which differ across a variety of other indices, including measures of anhedonia, activity and sleep behavior (Henn and Vollmayr 2005). DNA microarray analyses performed on hippocampal tissues reveal that nonhelpless rats activate a distinct pattern of gene expression compared with helpless or stress-naïve rats, suggesting that their passive responsiveness may be due to distinct neurobiological changes (Kohen et al. 2005). In mice, the development of helpless behavior is inversely related to the activation of the transcription factor ΔFosB (a stable splice variant of FosB) in the periaqueductal gray (PAG) of the midbrain. The virally mediated overexpression of ΔFosB in PAG neurons protects against developing an escape deficit partly through the transcriptional repression of substance P, a neuropeptide known to modulate the physiology of serotonergic and other neurons (Berton et al. 2007).
Today, these acute stress models make up the first line of behavioral tests utilized to phenotype transgenic mice and are also exploited as tools to rapidly screen putative antidepressant compounds. Even though direct links to human depression may be weak since they use acute stressors and test acute antidepressant responses, these tests have directed the field toward a number of previously unappreciated molecular players (Fig. 1). Of course, to truly implicate these targets in the pathophysiology of depression without false positives and to shed light on complex relationships such as those observed in the case of the SERT KOs, positive hits on these screens require much further validation through a more diverse set of molecular and behavioral assays, ideally in conjunction with postmortem validation (Covington et al. 2009; Hunsberger et al. 2007; Krishnan et al. 2008; Svenningsson et al. 2006). Furthermore, the FST, TST and LH are highly sensitive to manipulations which impair motor function, and the LH model is particularly sensitive to alterations in central and peripheral pain sensitivity (Cryan and Mombereau 2004). Therefore, these screening assays should be followed up with tests of motor function or pain sensitivity.
3.2 Models of Secondary or Iatrogenic Depression
3.2.1 Hormones of the HPA Axis
The hypothalamic–pituitary–adrenal (HPA) axis is activated by a wide variety of stressful stimuli, and resultant increases in serum glucocorticoids serve an immediate adaptive role through increases in gluconeogenesis and lipolysis. The “cortisol” hypothesis suggests that certain symptoms of depression may be mediated by a persistently overactive HPA axis, brought about through (1) increased production of hypothalamic corticotropin-releasing factor (CRF) and (2) reduced negative feedback at the level of centrally expressed glucocorticoid receptors (Holsboer and Ising 2009). Clinical studies have demonstrated HPA axis dysregulation in some depressed individuals, mainly those with severe depression and psychotic symptoms (Gold and Chrousos 2002), and these patients may uniquely benefit clinically from pharmacological antagonists of the glucocorticoid receptor (Krishnan and Nestler 2008). In contrast, atypical depression (associated with increased sleep and appetite), posttraumatic stress disorder, chronic fatigue syndrome, and fibromyalgia are associated with reduced circulating glucocorticoid concentrations and heightened negative feedback (Krishnan and Nestler 2008), demonstrating that alterations in HPA axis activity in either direction can result in depressive features. A significant amount of preclinical effort has been devoted to generating animal models of impaired glucocorticoid function. Perhaps the most syndromically accurate model of melancholic depression is the forebrain glucocorticoid receptor (GR) knockout mouse, derived through conditional deletion of the GR allele via cre-recombinase loxP technology: these mice display enhanced basal serum glucocorticoid levels, dexamethasone nonsuppression, increased FST and TST immobility, and these changes are all reversible with chronic antidepressants (Boyle et al. 2005). Interestingly, the forebrain overexpression of GR leads to an identical behavioral phenotype (Wei et al. 2007), which suggests that the mood altering properties of glucocorticoid signaling are more complex than simple increases or decreases in steroid or receptor levels.
Depression is also commonly observed as an iatrogenic side effect of chronic glucocorticoid administration and is a key psychiatric symptom of Cushing’s syndrome which is characterized by hypercortisolemia secondary to adrenal or pituitary corticotrophic hyperplasia. Thus, the negative consequences of heightened HPA axis activity are at least partially related to the adverse effects of glucocorticoids themselves (McEwen 2007; Pittenger and Duman 2008). Consistent with this hypothesis, mice exposed to 20 days of corticosterone dissolved in their drinking water to develop decreased responding for food pellets in an operant conditioning task (an anhedonic phenotype) and increased TST immobility, both of which are reversible by chronic amitriptyline (a tricyclic antidepressant) (Gourley et al. 2008). Such corticosterone exposure decreases activation of ERK1/2 (extracellular signal regulated kinase 1/2) in the dentate gyrus, which is itself sufficient to increase FST immobility and antagonize the action of antidepressants (Duman et al. 2007).
Increases in circulating serum cortisol in depression may also be secondary to increased CRF synthesis and secretion (Nemeroff et al. 1984). Many of CRF’s strong effects on behavior occur through centrally mediated processes independent of adrenal function, i.e., are not reversed by adrenalectomy (Muller and Holsboer 2006). To tease out the behavioral significance of brain CRF signaling, numerous transgenic and knockout lines have been generated. While the loss of brain CRF has negligible behavioral consequences, the transient overexpression of CRF during development leads to reduced exploratory behavior (increased anxiety) and FST/ TST immobility during adulthood, and constitutive CRFR1KO mice display increased exploration (anxiolysis) (Kolber et al. 2010; Muller and Holsboer 2006). These data, combined with postmortem evidence of enhanced CRF levels in depression, have encouraged pharmaceutical companies to invest in the development of a safe and effective CRFR1 antagonist to be used in depression and anxiety disorders (Mathew et al. 2008). However, despite decades of study and numerous pharmacological prototypes, this hypothesis remains to be tested effectively in humans. An important challenge in this field has been to selectively antagonize brain CRF signaling without altering natural HPA axis responsiveness.
3.2.2 Retinoic Acid Derivatives
Isotretinoin (Accutane ©), a retinoic acid derivative used as a highly effective treatment of severe acne, has been associated with an increased risk for depression and suicide (Bremner and McCaffery 2008). Mice chronically treated with isotretinoin develop increases in FST and TST immobility which have thus far been correlated with decreased hippocampal metabolism and neuronal proliferation (Crandall et al. 2004; O’Reilly et al. 2006). Isotretinoin is known to bind and activate retinoic acid receptors (RARs) which are widely distributed in the adult brain (Bremner and McCaffery 2008). RARs belong to the nuclear hormone receptor family of transcription factors, and the transcriptional consequences of isotretinoin exposure within limbic brain regions remain unexplored.
3.2.3 Cytokines and Immune System Dysregulation
Proinflammatory cytokines such as interferon-α are used in humans to treat several disease states. Many of these recombinantly derived proteins produce clinically significant depression as a side effect (Loftis and Hauser 2004). A large body of preclinical evidence suggests a bidirectional association between immune activation and depressive symptoms: certain cytokines have been shown to induce depression-like behavior in rodents and primates (Dunn et al. 2005; Felger et al. 2007), and several models of chronic stress produce significant changes in immune function (Miller et al. 2009). One such example is IL-1β (interleukin-1β): increases in IL-1β signaling in the hippocampus play a role in mediating the anhedonic and antineurogenic effects of chronic stress through the actions of the transcription factor NFκB (nuclear factor-κB) (Koo and Duman 2008; Koo et al. 2010). A key priority in this field will be to progress from focusing on the sickness behavior induced by strong immune stimuli such as LPS (lipopolysaccharide) (O’Connor et al. 2009) to the behavioral consequences of more elegant manipulations of specific cytokine signaling axes, as well as defining the therapeutic relevance of a whole host of antiinflammatory therapeutics popularly prescribed for autoimmune conditions. Clearly, the answer is not simply decreasing inflammation. The immunization of rats with an altered version of MBP (myelin basic protein) activates weakly self-reactive T-cells and has been shown to render rats immune to the anhedonic effects of chronic unpredictable stress (CUS) (Lewitus et al. 2009), suggesting that specific activators of immune function may in fact promote stress resilience. Understanding the immunology of depression is particularly applicable to autoimmune diseases such as multiple sclerosis (MS) where up to 50% of patients experience clinically significant depression. Murine MS models display depression-like changes such as weight loss, anorexia and reduced social exploration well before the onset of neurologic deficits (Ghaffar and Feinstein 2007; Gold and Irwin 2009), suggesting the presence of shared pathogenic mechanisms.
Depression which is secondary to medical conditions (e.g., stroke, pancreatic cancer, hypothyroidism, hypercortisolemia, etc.) is clinically indistinguishable from so-called endogenous or primary depression. Without clear knowledge of the etiology of endogenous depression, models that are designed based on the direct application of clinical observations are positioned to play a critical role due to their strong construct validity. A direct comparison of the molecular changes associated with corticosterone, cytokine and/or isotretinoin exposures versus stress models are likely to provide insight into shared and distinct pathophysiological mechanisms between stress-induced, endogenous, and iatrogenic forms of depression. One obvious path of investigation would be to employ genome-wide transcriptional profiling techniques to look for shared patterns of molecular plasticity in both animal models and patient samples. These “common denominator” patterns could identify potential targets for antidepressant drug discovery, and such agents would likely be active against all forms of depression.
3.3 Chronic Stress Models
While acute stress paradigms are used broadly for their ease, automation, and rapid phenotyping abilities, they offer singular readouts that often cannot be unambiguously interpreted. For instance, increased immobility in the FST is often anthropomorphized as an expression of despair. However, it can also be understood as a successful and adaptive behavioral response that functions to conserve energy. Today’s chronic stress models are distinguished by their remarkable ability to simultaneously produce a set of behavioral alterations with strong face validity for depression. However, this enhanced face validity often comes at the cost of low throughput: the precise application of these chronic stress models requires more space and time and greater sample sizes and are consequently significantly more expensive than other models. Thus, fewer laboratories have experienced consistent success. Furthermore, even with their known pharmacological validity, their low throughput makes them poorly suited for the pharmacological validation of novel compounds. In essence, these models are composed of repeated applications of an uncontrollable and unpredictable stress that is coupled with a quantifiable assay of depression-like behavior. They are based on clinical evidence that stressful life events that significantly increase the risk of depressive episodes are generally of a chronic nature (divorce, financial problems, and sexual abuse) (Krishnan and Nestler 2008). As is discussed below, their main strengths lie in their ability to characterize the neuroplasticity associated with chronic stress or antidepressant exposures.
3.3.1 Chronic Mild Stress
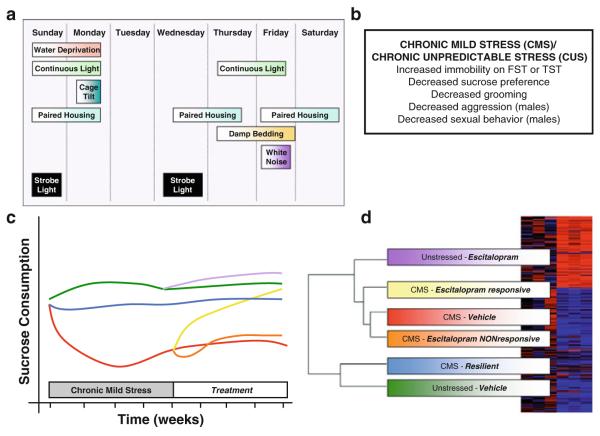
Chronic mild stress (CMS), better described as CUS, paradigms involve the application of varied intermittent physical stresses applied over a relatively prolonged time period (between 1 and 7 weeks, Fig. 2). Sucrose drinking is the most commonly utilized assay to assess the impact of CUS and CUS-exposed rats or mice show deficits in their motivation to consume a dilute (1–2%) solution of sucrose measured either as total sucrose intake or as a preference against water (Willner 2005). CUS has also been shown to result in a number of other “emotional” changes that are difficult to objectively quantify, such as grooming deficits and changes in aggressive and sexual behavior. Many of these phenotypes are reversed by chronic antidepressants applied either during the stress or as a poststress treatment (Strekalova et al. 2006). This model has been the subject of considerable controversy related to poor reproducibility (Argyropoulos and Nutt 1997; Broekkamp 1997; Willner 2005), and while some groups have had consistent success in repeatedly generating anhedonic mice/rats with a given paradigm, others have not experienced the same reliability. It would appear that this model is particularly sensitive to subtle variations in design (the various permutations of stressors) and numerous other sources of variability endemic to behavioral research (e.g., time of testing, vendor differences, etc.) and has accordingly faded in popularity. While it may not have the pharmacological screening capabilities of the FST, when performed reproducibly and reliably, it has clear potential to generate important molecular insights into depression.
Fig. 2.
The chronic mild stress (CMS)/chronic unpredictable stress (CUS) model of depression relies on a series of mostly physical stresses that are presented over 1–6 weeks (Willner 2005). (a) One example of a rat CUS protocol (Grippo 2009). (b) CUS paradigms in rats and mice produce a variety of behavioral changes. (c, d) The most popular assay for the effects of CUS is sucrose preference or sucrose intake whereby reductions in the consumption of a palatable sweet solution are interpreted as anhedonia. CUS has been applied to the study of stress resilience (CUS-resilient mice do not display a reduction in sucrose intake) and antidepressant resistance (escitalopram treated mice do not recover impairments in sucrose drinking). The key for the colored lines is provided in Panel d. DNA microarray technology combined with gene expression cluster analysis can aid in correlating behavioral groups with their gene expression patterns. For example, in this study examining total hippocampal tissue, genes modulated in CUS-resilient and vehicle-treated unstressed control rats were strongly overlapping, and these gene expression patterns were quite distant from unstressed rats treated with escitalopram (Bergstrom et al. 2007)
Aside from being a tool to study the physiological consequences of chronic stress, CUS has been applied recently to phenotype mouse mutants, study gender differences in stress responses, and validate novel antidepressants (Kong et al. 2009; LaPlant et al. 2009; Vitale et al. 2009). Like LH, CUS studies have reported significant individual differences. In one mouse study, decreased sucrose preference (anhedonia) was only observed in 61% of mice and was uniquely associated with increased immobility in the FST. In contrast, all CUS-exposed mice developed changes in locomotor behavior and decreased exploration, suggesting that segregating a subgroup of anhedonic mice identifies a unique susceptible population that displays stress-induced depressive features (Strekalova et al. 2004). A similar degree of variability has been observed in rats and when CUS-sensitive (i.e., vulnerable) rats were treated with antidepressants two distinct populations emerged: antidepressant-sensitive and antidepressant-resistant (Jayatissa et al. 2006). This ability of CUS to model two poorly understood human phenomena, stress resilience and antidepressant resistance has inspired a series of microarray studies aimed at exploring the molecular signatures associated with these phenomena. Resistance to the antidepressant effects of escitalopram, an SSRI, is associated with the upregulation of proapoptotic genes including APP (amyloid precursor protein) and TNF (tumor necrosis factor) in hippocampus, while vulnerability to CUS-induced anhedonia is associated with reduced expression of genes required for cellular proliferation and differentiation (Bergstrom et al. 2007). Similar experiments have been conducted in other brain regions including the amygdala and cingulate and frontal cortices (Orsetti et al. 2008; Sibille et al. 2009; Surget etal. 2009), each revealing unique region-specific molecular signatures associated with vulnerability to CUS.
At this stage, cellular heterogeneity represents a key limitation in the interpretation of these data: microarray studies performed on mixed samples of neuronal, glial, endothelial and immune cells are likely to result in poor reproducibility and low signal/noise ratio. Two important developments that are likely to address this problem are (1) laser capture microdissection techniques, which allow for precise isolation of limbic nuclei and subnuclei, and (2) mutant mice where subpopulations of neurons or other cell types are fluorescently labeled, allowing precise sorting of cells of interest through fluorescence-mediated techniques (Pollak et al. 2008; Sugino et al. 2006). As technological advances in DNA and protein array analysis allow for the rapid, reliable, and cost-effective genome-wide analysis of transcriptional regulation, these studies set the stage for an understanding of the complex gene network interactions involved in the pathophysiology of depression and antidepressant responsiveness.
3.3.2 Psychosocial Stress Models
One caveat with CUS is its questionable construct validity since certain routinely employed CUS stressors are physical (e.g., strobe lights, restraint or swim stress, or abrupt circadian disruptions) and are unlikely to be encountered by rats or mice in the wild. At least in this respect, models of psychosocial stress display their greatest strength since they entirely rely on innate social behavior. The central theme in these models (Fig. 3), whether they are conducted in rats, mice, or tree shrews, is to allow two or more subjects to socially and physically interact (an agonistic encounter) such that one achieves dominant status (alpha) and the others remain subordinate (omega). While some groups identify subordinates between age- and strain-matched pairs of mice or dyads (Avgustinovich et al. 2005; Malatynska and Knapp 2005), others employ a “forced subordination” strategy whereby reliably aggressive rodents (usually larger and/or of a more aggressive strain) are employed to consistently subordinate other subjects (Berton et al. 2006; Covington and Miczek 2005). In addition to the intense and unpredictable physical stress during social encounters, several laboratories add on the psychological stress of prolonged “sensory contact” through which subordinate mice are housed in the same cage as their dominant counterparts across a partition that prevents all but sensory interaction (Martinez et al. 1998). Following multiple defeat encounters, rodents display reduced social interaction, decreased exploration and locomotor behavior, anhedonia (e.g., decreased sucrose preference and sexual behavior), increased stress-induced immobility and alterations in HPA axis and autonomic function (Avgustinovich et al. 2005; Krishnan et al. 2007), many of which are reversed by chronic but not acute antidepressant administration (Becker et al. 2008; Rygula et al. 2008). Like CUS, the establishment and validation of such social stress models can be cumbersome and expensive. Reliable expression of aggressive behavior can be easily disrupted by minor procedural variations such as changes in bedding or cage size. In addition, laboratory personnel performing social defeat experiments must attain a sense for the correct “quantity” of aggressive behavior: while excessively injurious physical interactions are both unethical and irrelevant to the study of depression, weakly aggressive encounters pose the risk of producing mild and short-lived phenotypes that may affect molecular analyses.
Fig. 3.
Psychosocial stress models rely on innate social behavior among pairs or groups of male rodents allowing for the formation of stable dominant/subordinate relationships. (a) In the sensory contact adaptation of rodent social defeat, an intruder is periodically subordinated by a territorially aggressive resident mouse and is forced to spend the remainder of the day across a partition that permits sensory contact without fighting. (b) The main behavioral consequences of repeated bouts of such social subordination. (c) Aside from face, construct, and pharmacological validity, one can further validate animal models by demonstrating the presence of identical molecular changes in human postmortem tissue. Here, 10 days of social defeat in C57Bl/6 mice increases BDNF protein levels (by immunoblot) in the nucleus accumbens such that vulnerable or susceptible mice (S) display the greatest increases in BDNF (C controls, U unsusceptible), with the inset demonstrating a significant inverse correlation between interaction scores and BDNF levels. This molecular change is also observed in postmortem accumbens samples from male depressed individuals (Krishnan et al. 2007). (d) ChIP–chip analyses (chromatin immunoprecipitation followed by DNA promoter arrays) examining genome-wide patterns of a repressive form of histone H3 methylation in the nucleus accumbens. The region of Venn overlap (“275”) corresponds to 275 genes that are upregulated in susceptible animals and that are also reversed by imipramine and not seen in resilient animals (Wilkinson et al. 2009). Some examples of genes that fall within this overlap include CNK1D (casein kinase 1 delta), FGF1 (fibroblast growth factor 1) and HDAC4 (histone deacetylase 4). These results suggest that inhibiting the stress-induced histone methylation at these genes through inhibitors of histone methyltransferases constitutes a potential novel target for antidepressant development
The decreased sociability following such defeats can be quantifiably assessed with automated tests of social interaction that permit an assessment of individual differences among defeated mice. By combining this type of highly quantitative behavioral analysis with standard molecular and cellular techniques, this model has shed light on a number of mechanistic hypotheses related to variability in stress responsiveness. These include the role of activity-dependent BDNF (brain-derived neurotrophic factor) signaling within the mesolimbic dopamine circuit (Feder et al. 2009; Krishnan and Nestler 2008), endogenous kappa-opioid signaling (McLaughlin et al. 2006), the contribution of adult hippocampal neurogenesis (Lagace et al. 2010) and the role of peripherally derived mediators of energy homeostasis (Chuang et al. 2010). Such significant variability even among age-matched members of an inbred strain suggests that this heterogeneity occurs independently of DNA sequence variations. One possibility is that epigenetic modifications of the genome, which occur stochastically during development, may contribute to this variability seen among inbred mice raised in near identical environmental conditions. These epigenetic mechanisms include covalent modifications to histones (e.g., histone acetylation, methylation or phosphorylation) or DNA (e.g., DNA methylation) (Krishnan and Nestler 2008; Bountra et al. 2011).
Social defeat itself has a powerful impact on the epigenome: defeated mice display increases in repressive histone methylation in the hippocampus and nucleus accumbens (Tsankova et al. 2006) and increases in histone acetylation in the NAc (Covington et al. 2009). ChIP–chip techniques (chromatin immunoprecipitation combined with promoter array chips) have allowed for an appreciation of epigenetic profiles associated with the expression of susceptible or resilient behavior and antidepressant exposure (Wilkinson et al. 2009). This latter approach has illustrated a significant degree of overlap in patterns of epigenetic regulation between antidepressant-treated susceptible mice and vehicle-treated resilient mice, suggesting that certain individuals may avoid the deleterious effects of stress by naturally mounting an endogenous antidepressant-like response (Wilkinson et al. 2009). Furthermore, with the advent of pharmacological inhibitors of epigenetic enzymes such as histone deacetylase inhibitors (HDAC inhibitors; see Bountra et al. 2011), one can directly test epigenetic hypotheses in a more precise manner. For example, the antidepressant effects of systemically administered weakly selective HDAC inhibitors such as sodium butyrate and valproic acid (Gundersen and Blendy 2009; Schroeder et al. 2006; Tsankova et al. 2006) can be recapitulated by a localized infusion of more specific and selective drugs in the NAc (Covington et al. 2009). Similar strides have been made in understanding the behavioral impact of DNA methylation (LaPlant et al. 2010). Microarray analyses comparing the effects of systemic fluoxetine and localized HDAC inhibitor infusions reveal significant overlap in patterns of transcriptional activation and repression (Covington et al. 2009). On the other hand, genes influenced by HDAC inhibitors, and not by fluoxetine, may prove even more interesting in terms of identifying truly novel approaches for more effective antidepressant treatments.
Other forms of social stress are worth mentioning. Prolonged social isolation during adulthood results in reduced sucrose drinking and alterations in sexual reward behavior. While this model has received less recent attention, it displays excellent construct validity and requires minimal sophistication (Wallace et al. 2009; Wilkinson et al. 2009). Early life stress, typically applied in the form of maternal separation during early postnatal developmental periods, has been shown to result in cognitive and emotional changes that persist through adulthood. These phenotypes, such as altered HPA axis function, increased immobility, weakened prepulse inhibition, spatial learning deficits, etc., have been linked to a variety of neuropsychiatric syndromes with strong developmental hypotheses including schizophrenia (Fumagalli et al. 2007; Lupien et al. 2009). While studies in this field have traditionally almost exclusively emphasized the role of the HPA axis, more recent ventures have demonstrated how maternal separation paradigms are quite aptly designed to study epigenetic forms of neuroplasticity (Murgatroyd et al. 2009) as well as mechanisms by which early life stress can in fact promote resiliency during adulthood (Lyons et al. 2009). Since social defeat models rely on differences in intermale aggression, they cannot be directly applied to females. However, females do display depression-like features following other social stressors such as intermittent crowding or isolation (Herzog et al. 2009). Given the twofold preponderance of depression in females, further studies of pathophysiological mechanisms in female rodent models are a very high priority for the field and these psychosocial stress models, in their ability to directly compare across sexes, are ideal candidates for such studies.
4 Insights from Models of Antidepressant Action
While the molecular targets of current antidepressant agents are known, there still remain large gaps in understanding their neuroanatomical sites of action and why these agents are associated with a significant therapeutic delay. Most of the current knowledge of these mechanisms has come from animal studies examining neurobiological changes following chronic antidepressant administration, voluntary exercise (through the exposure to a running wheel), or the application of ECT. More recent reports have exploited other strategies in rodents such as repetitive transcranial magnetic stimulation (rTMS) using a noninvasive cortical stimulating device (Vieyra-Reyes et al. 2008) as well as more creative cognitive paradigms such as learned safety, where a benign environmental stimulus that signals “safety” produces antidepressant-like effects (Pollak et al. 2008).
The most compelling and reproducible biological findings from these approaches are focused largely on the hippocampus, perhaps due to its well-understood anatomy. These studies have contributed to the development of neurotrophic model of depression, whereby stressful experiences through glucocorticoid signaling and other mechanisms reduce the level of neurotrophic factors such as BDNF in the hippocampus resulting in atrophic morphological changes. Antidepressants, by activating cellular signaling cascades that culminate in the activation of CREB (cyclic-AMP response element binding protein), function to enhance levels of BDNF and other growth factors like VEGF (vascular endothelial growth factor) and VGF (nonacronymic), which promote the proliferation and differentiation of hippocampal progenitors and alter monoaminergic synaptic transmission (Balu and Lucki 2009; Krishnan and Nestler 2008; Pittenger and Duman 2008). Other key molecular mediators have been identified, such as p11, a scaffolding protein induced by antidepressants that binds and enhances the surface expression and activity of the serotonin 1B (5-HT1B) receptor, promoting an antidepressant-like response in laboratory assays (Svenningsson et al. 2006). p11 also enhances the activity of serotonin receptor type 4 (5-HT4) (Warner-Schmidt et al. 2009), which is of particular significance since 5-HT4 receptor agonists have rapid antidepressant-like activity. In the CUS model, while only 3–4 days of daily injections of RS67333 (a prototypical 5-HT4 receptor agonist) alleviated the reduced sucrose intake in CUS-vulnerable rats: citalopram-treated controls required greater than 14 days of treatment to observe a significant improvement (Lucas et al. 2007). This study illustrates a key point related to pharmacological validity. Even though acute stress models are often criticized for their acute responses to antidepressants, efforts should nevertheless still be devoted to identifying novel agents that do not exhibit a therapeutic delay. The identification of such rapidly acting agents offers hope that antidepressants of the future will no longer be limited by their therapeutic delay. A clinically validated example of one such a rapidly acting agent is ketamine (aan het Rot et al. 2010), and recent preclinical experiments reveal that ketamine’s antidepressant effects may be mediated through rapid forms of glutamatergic synaptic plasticity (Li et al. 2010).
The neurotrophic hypothesis described above is consistent with the observation that certain subpopulations of depressed patients display small reductions in total hippocampal volume with consequent ventricular enlargement (Savitz and Drevets 2009). Aside from these correlative data, there is little direct clinical evidence that alterations in hippocampal activity alter mood per se. Functional neuroimaging studies designed specifically to reveal the neuroanatomical substrates of altered emotional processing in depression have indicated roles for the amygdala and frontal cortical regions such as the subgenual cingulate cortex (area cg25), where the application of deep brain stimulation (DBS) produces long-lasting antidepressant effects in treatment-resistant depression (Mayberg 2009). Such profound effects of DBS applied to Cg25 or the NAc (Bewernick et al. 2010) constitute not only an important therapeutic development, but also provide unequivocal evidence regarding neural substrates that participate in improving mood symptoms. Of interest, patients in these studies were noted to have an immediate and intolerable worsening of depressive symptoms when DBS stimulators were turned off (Bewernick et al. 2010), illustrating how the antidepressant effects of nucleus accumbens DBS are profound and yet short-lived. In the future, we can expect refinements in stimulation parameters and localization of DBS thanks to rodent studies which have begun to explore the effects of DBS and optogenetic stimulation (a spatiotemporally precise technique that relies on light-mediated activation of cation or anion channels) on circuit-level neurophysiology and molecular mediators (Gradinaru et al. 2009; McCracken and Grace 2009; Temel et al. 2007). While this approach is still in its infancy, it promises to improve understanding of the dispersed neurocircuitry involved in complex psychiatric symptoms such as anhedonia and may offer insight into how DBS may one day be combined with pharmacological interventions to enhance antidepressant efficacy.
5 Conclusions
Sadly, in spite of almost 40 years of research into depression’s mechanisms, the newest agents released on to markets today only vary from their predecessors in side-effect profile, with negligible improvements in efficacy. Therefore, in addition to combining pharmacotherapy with psychotherapy, clinicians are often forced to initiate multiple antidepressant medications simultaneously, or rely on adjunct medications like thyroid hormone, antipsychotic agents or psychostimulants to boost the antidepressant response, with each additional medication coming at the expense of new off-target effects. From the examples discussed above, there is a diverse array of useful animal models that can expand our understanding of mechanisms in depression. Rather than advocate for a single “best” model, investigators must realize the relative strengths and limitations of each paradigm and always aim to utilize tools that advance our understanding of the disease. While there are examples of “simple” tests that have provided key molecular insights (Berton et al. 2007; Svenningsson et al. 2006), there have been other instances when more “sophisticated” models have only provided behavioral minutiae (Avgustinovich et al. 2005). To increase the likelihood that these models will provide the next generation of effective antidepressants, the approach to the utilization of animal models must mature.
The overarching goal should be to narrow the gap between basic and clinical fields of investigation, and this can be executed at several different levels. Neuroplastic changes that reliably occur in rodents following stress or antidepressant exposures can be explored in human postmortem samples, with replications providing a further validation and increasing knowledge of biomarkers in depression. When examining genes of interest, instead of focusing on behavioral phenotypes in constitutive knockout mice, efforts should be focused on recapitulating human polymorphisms in those genes, understanding their cellular and physiological consequences and advancing models to tease out more subtle phenotypes. Moreover, given the significance of gene × environment interactions in the pathogenesis of virtually all psychiatric disorders (Caspi and Moffitt 2006; see also Lesch 2011), we can gain insight into their neurobiological basis by recapitulating such interactions in animal models (Carola et al. 2008). Rather than emphasizing the more traditional “treatment versus control” approach, focusing on individual differences will increase the understanding of biological mechanisms underlying such variability, including a role for epigenetic mechanisms. Finally, while clinicians continue to refine novel experimental treatments for depression such as intravenous ketamine or DBS, basic scientists must complement their efforts by exploring the neurobiological mechanisms underlying those treatments; such translational approaches will further narrow the gap between human depression and the theoretical formulations of its mechanisms.
Acknowledgments
Acknowledgements and Financial Disclosures Preparation of this review was supported by grants from the National Institute of Mental Health. EJN reports consulting income from Merck Research Laboratories and PsychoGenics, and a research alliance with AstraZeneca.
Abbreviations
- 5HT
5-Hydroxytryptamine or serotonin
- BDNF
Brain-derived neurotrophic factor
- CRF
Corticotropin-releasing factor
- CUS
Chronic unpredictable stress
- DBS
Deep brain stimulation
- DNA
Deoxyribonucleic acid
- ECT
Electroconvulsive therapy
- FST
Forced swim test
- GR
Glucocorticoid receptor
- HPA
Hypothalamic–pituitary–adrenal
- KO
Knockout
- LH
Learned helplessness
- SERT
Serotonin transporter TST Tail suspension test
Contributor Information
Vaishnav Krishnan, Departments of Internal Medicine, Psychiatry and Neuroscience, The University of Texas Southwestern Medical Center, Dallas, TX, USA.
Eric J. Nestler, Fishberg Department of Neuroscience, Mount Sinai School of Medicine, New York, NY, USA
References
- aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67:139–145. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- Alexandre C, Popa D, Fabre V, Bouali S, Venault P, Lesch KP, Hamon M, Adrien J. Early life blockade of 5-hydroxytryptamine 1A receptors normalizes sleep and depression-like behavior in adult knock-out mice lacking the serotonin transporter. J Neurosci. 2006;26:5554–5564. doi: 10.1523/JNEUROSCI.5156-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyropoulos SV, Nutt DJ. Anhedonia and chronic mild stress model in depression. Psychopharmacology (Berl) 1997;134:333–336. doi: 10.1007/s002130050458. discussion 371–377. [DOI] [PubMed] [Google Scholar]
- Avgustinovich DF, Kovalenko IL, Kudryavtseva NN. A model of anxious depression: persistence of behavioral pathology. Neurosci Behav Physiol. 2005;35:917–924. doi: 10.1007/s11055-005-0146-6. [DOI] [PubMed] [Google Scholar]
- Balu DT, Lucki I. Adult hippocampal neurogenesis: regulation, functional implications, and contribution to disease pathology. Neurosci Biobehav Rev. 2009;33:232–252. doi: 10.1016/j.neubiorev.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker C, Zeau B, Rivat C, Blugeot A, Hamon M, Benoliel JJ. Repeated social defeat-induced depression-like behavioral and biological alterations in rats: involvement of cholecystokinin. Mol Psychiatry. 2008;13:1079–1092. doi: 10.1038/sj.mp.4002097. [DOI] [PubMed] [Google Scholar]
- Bergstrom A, Jayatissa MN, Thykjaer T, Wiborg O. Molecular pathways associated with stress resilience and drug resistance in the chronic mild stress rat model of depression: a gene expression study. J Mol Neurosci. 2007;33:201–215. doi: 10.1007/s12031-007-0065-9. [DOI] [PubMed] [Google Scholar]
- Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond mono-amines. Nat Rev Neurosci. 2006;7:137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Berton O, Covington HE, 3rd, Ebner K, Tsankova NM, Carle TL, Ulery P, Bhonsle A, Barrot M, Krishnan V, Singewald GM, Singewald N, Birnbaum S, Neve RL, Nestler EJ. Induction of deltaFosB in the periaqueductal gray by stress promotes active coping responses. Neuron. 2007;55:289–300. doi: 10.1016/j.neuron.2007.06.033. [DOI] [PubMed] [Google Scholar]
- Bewernick BH, Hurlemann R, Matusch A, Kayser S, Grubert C, Hadrysiewicz B, Axmacher N, Lemke M, Cooper-Mahkorn D, Cohen MX, Brockmann H, Lenartz D, Sturm V, Schlaepfer TE. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry. 2010;67:110–116. doi: 10.1016/j.biopsych.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Bountra C, Oppermann U, Heightman TD. Current topics in behavioural neuroscience. Springer; Heidelberg: 2011. Animal models of epigenetic regulation in neuropsychiatric disorders. doi: 10.1007/7854_2010_104. [DOI] [PubMed] [Google Scholar]
- Boyle MP, Brewer JA, Funatsu M, Wozniak DF, Tsien JZ, Izumi Y, Muglia LJ. Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior. Proc Natl Acad Sci USA. 2005;102:473–478. doi: 10.1073/pnas.0406458102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, McCaffery P. The neurobiology of retinoic acid in affective disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:315–331. doi: 10.1016/j.pnpbp.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekkamp C. Predictive validity and the robustness criterion for animal models. Psychopharmacology (Berl) 1997;134:341–343. doi: 10.1007/s002130050461. discussion 371–377. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Lopes M, Kaptchuk TJ, Fregni F. Placebo response of non-pharmacological and pharmacological trials in major depression: a systematic review and meta-analysis. PLoS One. 2009;4:e4824. doi: 10.1371/journal.pone.0004824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carola V, Frazzetto G, Pascucci T, Audero E, Puglisi-Allegra S, Cabib S, Lesch KP, Gross C. Identifying molecular substrates in a mouse model of the serotonin transporter x environment risk factor for anxiety and depression. Biol Psychiatry. 2008;63:840–846. doi: 10.1016/j.biopsych.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE. Gene-environment interactions in psychiatry: joining forces with neuroscience. Nat Rev Neurosci. 2006;7:583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- Chuang JC, Krishnan V, Yu HG, Mason B, Cui H, Wilkinson MB, Zigman JM, Elmquist JK, Nestler EJ, Lutter M. A beta(3)-adrenergic-leptin-melanocortin circuit regulates behavioral and metabolic changes induced by chronic stress. Biol Psychiatry. 2010;67(11):1075–1082. doi: 10.1016/j.biopsych.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE, 3rd, Miczek KA. Intense cocaine self-administration after episodic social defeat stress, but not after aggressive behavior: dissociation from corticosterone activation. Psychopharmacology (Berl) 2005;183:331–340. doi: 10.1007/s00213-005-0190-5. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Maze I, LaPlant QC, Vialou VF, Ohnishi YN, Berton O, Fass DM, Renthal W, Rush AJ, 3rd, Wu EY, Ghose S, Krishnan V, Russo SJ, Tamminga C, Haggarty SJ, Nestler EJ. Antidepressant actions of histone deacetylase inhibitors. J Neurosci. 2009;29:11451–11460. doi: 10.1523/JNEUROSCI.1758-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall J, Sakai Y, Zhang J, Koul O, Mineur Y, Crusio WE, McCaffery P. 13-Cis-retinoic acid suppresses hippocampal cell division and hippocampal-dependent learning in mice. Proc Natl Acad Sci USA. 2004;101:5111–5116. doi: 10.1073/pnas.0306336101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. What’s wrong with my mouse?: Behavioral phenotyping of transgenic and knockout mice. Wiley-Liss; New York: 2000. [Google Scholar]
- Cryan JF, Mombereau C. In search of a depressed mouse: utility of models for studying depression-related behavior in genetically modified mice. Mol Psychiatry. 2004;9:326–357. doi: 10.1038/sj.mp.4001457. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Dhir A, Kulkarni SK. Antidepressant-like effect of 17beta-estradiol: involvement of dopaminergic, serotonergic, and (or) sigma-1 receptor systems. Can J Physiol Pharmacol. 2008;86:726–735. doi: 10.1139/y08-077. [DOI] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Kodama M, Russell DS, Duman RS. A role for MAP kinase signaling in behavioral models of depression and antidepressant treatment. Biol Psychiatry. 2007;61:661–670. doi: 10.1016/j.biopsych.2006.05.047. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH, de Beaurepaire R. Cytokines as mediators of depression: what can we learn from animal studies? Neurosci Biobehav Rev. 2005;29:891–909. doi: 10.1016/j.neubiorev.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Evans DL, Charney DS, Lewis L, Golden RN, Gorman JM, Krishnan KR, Nemeroff CB, Bremner JD, Carney RM, Coyne JC, Delong MR, Frasure-Smith N, Glassman AH, Gold PW, Grant I, Gwyther L, Ironson G, Johnson RL, Kanner AM, Katon WJ, Kaufmann PG, Keefe FJ, Ketter T, Laughren TP, Leserman J, Lyketsos CG, McDonald WM, McEwen BS, Miller AH, Musselman D, O’Connor C, Petitto JM, Pollock BG, Robinson RG, Roose SP, Rowland J, Sheline Y, Sheps DS, Simon G, Spiegel D, Stunkard A, Sunderland T, Tibbits P, Jr, Valvo WJ. Mood disorders in the medically ill: scientific review and recommendations. Biol Psychiatry. 2005;58:175–189. doi: 10.1016/j.biopsych.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nat Rev Neurosci. 2009;10:446–457. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Alagbe O, Hu F, Mook D, Freeman AA, Sanchez MM, Kalin NH, Ratti E, Nemeroff CB, Miller AH. Effects of interferon-alpha on rhesus monkeys: a nonhuman primate model of cytokine-induced depression. Biol Psychiatry. 2007;62:1324–1333. doi: 10.1016/j.biopsych.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli F, Molteni R, Racagni G, Riva MA. Stress during development: Impact on neuroplasticity and relevance to psychopathology. Prog Neurobiol. 2007;81:197–217. doi: 10.1016/j.pneurobio.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Ghaffar O, Feinstein A. The neuropsychiatry of multiple sclerosis: a review of recent developments. Curr Opin Psychiatry. 2007;20:278–285. doi: 10.1097/YCO.0b013e3280eb10d7. [DOI] [PubMed] [Google Scholar]
- Gilmer WS, McKinney WT. Early experience and depressive disorders: human and non-human primate studies. J Affect Disord. 2003;75:97–113. doi: 10.1016/s0165-0327(03)00046-6. [DOI] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry. 2002;7:254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- Gold SM, Irwin MR. Depression and immunity: inflammation and depressive symptoms in multiple sclerosis. Immunol Allergy Clin North Am. 2009;29:309–320. doi: 10.1016/j.iac.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondo Y, Murata T, Makino S, Fukumura R, Ishitsuka Y. Current topics in behavioral neurosciences. Springer; Heidelberg: 2011. Mouse mutagenesis and disease models for neuropsychiatric disorders. doi: 10.1007/7854_2010_106. [DOI] [PubMed] [Google Scholar]
- Gourley SL, Kiraly DD, Howell JL, Olausson P, Taylor JR. Acute hippocampal brain-derived neurotrophic factor restores motivational and forced swim performance after corticosterone. Biol Psychiatry. 2008;64:884–890. doi: 10.1016/j.biopsych.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324:354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ. Mechanisms underlying altered mood and cardiovascular dysfunction: the value of neurobiological and behavioral research with animal models. Neurosci Biobehav Rev. 2009;33:171–180. doi: 10.1016/j.neubiorev.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen BB, Blendy JA. Effects of the histone deacetylase inhibitor sodium butyrate in models of depression and anxiety. Neuropharmacology. 2009;57:67–74. doi: 10.1016/j.neuropharm.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddjeri N, Blier P, de Montigny C. Long-term antidepressant treatments result in a tonic activation of forebrain 5-HT1A receptors. J Neurosci. 1998;18:10150–10156. doi: 10.1523/JNEUROSCI.18-23-10150.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henn FA, Vollmayr B. Stress models of depression: forming genetically vulnerable strains. Neurosci Biobehav Rev. 2005;29:799–804. doi: 10.1016/j.neubiorev.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Herzog CJ, Czeh B, Corbach S, Wuttke W, Schulte-Herbruggen O, Hellweg R, Flugge G, Fuchs E. Chronic social instability stress in female rats: a potential animal model for female depression. Neuroscience. 2009;159:982–992. doi: 10.1016/j.neuroscience.2009.01.059. [DOI] [PubMed] [Google Scholar]
- Heurteaux C, Lucas G, Guy N, El Yacoubi M, Thummler S, Peng XD, Noble F, Blondeau N, Widmann C, Borsotto M, Gobbi G, Vaugeois JM, Debonnel G, Lazdunski M. Deletion of the background potassium channel TREK-1 results in a depression-resistant phenotype. Nat Neurosci. 2006;9:1134–1141. doi: 10.1038/nn1749. [DOI] [PubMed] [Google Scholar]
- Holmes A, Yang RJ, Murphy DL, Crawley JN. Evaluation of antidepressant-related behavioral responses in mice lacking the serotonin transporter. Neuropsychopharmacology. 2002;27:914–923. doi: 10.1016/S0893-133X(02)00374-3. [DOI] [PubMed] [Google Scholar]
- Holsboer F, Ising M. Stress hormones and stress hormone regulation: biological role and behavioral effects. Annu Rev Psychol. 2009;61:81–109. doi: 10.1146/annurev.psych.093008.100321. [DOI] [PubMed] [Google Scholar]
- Hunsberger JG, Newton SS, Bennett AH, Duman CH, Russell DS, Salton SR, Duman RS. Antidepressant actions of the exercise-regulated gene VGF. Nat Med. 2007;13:1476–1482. doi: 10.1038/nm1669. [DOI] [PubMed] [Google Scholar]
- Jacobson LH, Cryan JF. Feeling strained? Influence of genetic background on depression-related behavior in mice: a review. Behav Genet. 2007;37:171–213. doi: 10.1007/s10519-006-9106-3. [DOI] [PubMed] [Google Scholar]
- Jayatissa MN, Bisgaard C, Tingstrom A, Papp M, Wiborg O. Hippocampal cytogenesis correlates to escitalopram-mediated recovery in a chronic mild stress rat model of depression. Neuropsychopharmacology. 2006;31:2395–2404. doi: 10.1038/sj.npp.1301041. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kohen R, Kirov S, Navaja GP, Happe HK, Hamblin MW, Snoddy JR, Neumaier JF, Petty F. Gene expression profiling in the hippocampus of learned helpless and nonhelpless rats. Pharmacogenomics J. 2005;5:278–291. doi: 10.1038/sj.tpj.6500322. [DOI] [PubMed] [Google Scholar]
- Kohn R, Saxena S, Levav I, Saraceno B. The treatment gap in mental health care. Bull World Health Organ. 2004;82:858–866. [PMC free article] [PubMed] [Google Scholar]
- Kolber BJ, Boyle MP, Wieczorek L, Kelley CL, Onwuzurike CC, Nettles SA, Vogt SK, Muglia LJ. Transient early-life forebrain corticotropin-releasing hormone elevation causes long-lasting anxiogenic and despair-like changes in mice. J Neurosci. 2010;30:2571–2581. doi: 10.1523/JNEUROSCI.4470-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong H, Sha LL, Fan Y, Xiao M, Ding JH, Wu J, Hu G. Requirement of AQP4 for antidepressive efficiency of fluoxetine: implication in adult hippocampal neurogenesis. Neuropsychopharmacology. 2009;34:1263–1276. doi: 10.1038/npp.2008.185. [DOI] [PubMed] [Google Scholar]
- Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci USA. 2008;105:751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS. Nuclear factor-{kappa}B is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc Natl Acad Sci USA. 2010;107(6):2669–2674. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. Linking molecules to mood: new insight into the biology of depression. Am J Psychiatry. 2010;167:1305–1320. doi: 10.1176/appi.ajp.2009.10030434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Mazei-Robison M, Iniguez SD, Ables JL, Vialou V, Berton O, Ghose S, Covington HE, Wiley MD, Henderson RP, Neve RL, Eisch AJ, Tamminga CA, Russo SJ, Bolanos CA, Nestler EJ. AKT signaling within the ventral tegmental area regulates cellular and behavioral responses to stressful stimuli. Biol Psychiatry. 2008;64:691–700. doi: 10.1016/j.biopsych.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace DC, Donovan MH, Decarolis NA, Farnbach LA, Malhotra S, Berton O, Nestler EJ, Krishnan V, Eisch AJ. Adult hippocampal neurogenesis is functionally important for stress-induced social avoidance. Proc Natl Acad Sci USA. 2010;107(9):4436–4441. doi: 10.1073/pnas.0910072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPlant Q, Chakravarty S, Vialou V, Mukherjee S, Koo JW, Kalahasti G, Bradbury KR, Taylor SV, Maze I, Kumar A, Graham A, Birnbaum SG, Krishnan V, Truong HT, Neve RL, Nestler EJ, Russo SJ. Role of nuclear factor kappaB in ovarian hormone-mediated stress hypersensitivity in female mice. Biol Psychiatry. 2009;65(10):874–880. doi: 10.1016/j.biopsych.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPlant Q, Vialou V, Covington HE, 3rd, Dumitriu D, Feng J, Warren BL, Maze I, Dietz DM, Watts EL, Iniguez SD, Koo JW, Mouzon E, Renthal W, Hollis F, Wang H, Noonan MA, Ren Y, Eisch AJ, Bolanos CA, Kabbaj M, Xiao G, Neve RL, Hurd YL, Oosting RS, Fan G, Morrison JH, Nestler EJ. Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nat Neurosci. 2010;13:1137–1143. doi: 10.1038/nn.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch K-P. Current topics in behavioral neurosciences. Springer; Heidelberg: 2011. When the serotonin transporter gene meets adversity: the contribution of animal models to understanding epigenetic mechanisms in affective disorders and resilience. doi: 10.1007/7854_2010_109. [DOI] [PubMed] [Google Scholar]
- Lewitus GM, Wilf-Yarkoni A, Ziv Y, Shabat-Simon M, Gersner R, Zangen A, Schwartz M. Vaccination as a novel approach for treating depressive behavior. Biol Psychiatry. 2009;65:283–288. doi: 10.1016/j.biopsych.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lira A, Zhou M, Castanon N, Ansorge MS, Gordon JA, Francis JH, Bradley-Moore M, Lira J, Underwood MD, Arango V, Kung HF, Hofer MA, Hen R, Gingrich JA. Altered depression-related behaviors and functional changes in the dorsal raphe nucleus of serotonin transporter-deficient mice. Biol Psychiatry. 2003;54:960–971. doi: 10.1016/s0006-3223(03)00696-6. [DOI] [PubMed] [Google Scholar]
- Liu X, Gershenfeld HK. An exploratory factor analysis of the tail suspension test in 12 inbred strains of mice and an F2 intercross. Brain Res Bull. 2003;60:223–231. doi: 10.1016/s0361-9230(03)00033-9. [DOI] [PubMed] [Google Scholar]
- Loftis JM, Hauser P. The phenomenology and treatment of interferon-induced depression. J Affect Disord. 2004;82:175–190. doi: 10.1016/j.jad.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Lucas G, Rymar VV, Du J, Mnie-Filali O, Bisgaard C, Manta S, Lambas-Senas L, Wiborg O, Haddjeri N, Pineyro G, Sadikot AF, Debonnel G. Serotonin(4) (5-HT(4)) receptor agonists are putative antidepressants with a rapid onset of action. Neuron. 2007;55:712–725. doi: 10.1016/j.neuron.2007.07.041. [DOI] [PubMed] [Google Scholar]
- Lucki I, Dalvi A, Mayorga AJ. Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology (Berl) 2001;155:315–322. doi: 10.1007/s002130100694. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, Jung S, Birnbaum S, Yanagisawa M, Elmquist JK, Nestler EJ, Zigman JM. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci. 2008;11:752–753. doi: 10.1038/nn.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyness JM, Niculescu A, Tu X, Reynolds CF, 3rd, Caine ED. The relationship of medical comorbidity and depression in older, primary care patients. Psychosomatics. 2006;47:435–439. doi: 10.1176/appi.psy.47.5.435. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Parker KJ, Katz M, Schatzberg AF. Developmental cascades linking stress inoculation, arousal regulation, and resilience. Front Behav Neurosci. 2009;3:32. doi: 10.3389/neuro.08.032.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Malatynska E, Knapp RJ. Dominant-submissive behavior as models of mania and depression. Neurosci Biobehav Rev. 2005;29:715–737. doi: 10.1016/j.neubiorev.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Martinez M, Calvo-Torrent A, Pico-Alfonso MA. Social defeat and subordination as models of social stress in laboratory rodents: a review. Aggress Behav. 1998;24:241–256. [Google Scholar]
- Mathew SJ, Manji HK, Charney DS. Novel drugs and therapeutic targets for severe mood disorders. Neuropsychopharmacology. 2008;33(9):2080–2092. doi: 10.1038/sj.npp.1301652. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Targeted electrode-based modulation of neural circuits for depression. J Clin Invest. 2009;119:717–725. doi: 10.1172/JCI38454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken CB, Grace AA. Nucleus accumbens deep brain stimulation produces region-specific alterations in local field potential oscillations and evoked responses in vivo. J Neurosci. 2009;29:5354–5363. doi: 10.1523/JNEUROSCI.0131-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology. 2006;31:1241–1248. doi: 10.1038/sj.npp.1300872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370:851–858. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- Muller MB, Holsboer F. Mice with mutations in the HPA-system as models for symptoms of depression. Biol Psychiatry. 2006;59:1104–1115. doi: 10.1016/j.biopsych.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Murgatroyd C, Patchev AV, Wu Y, Micale V, Bockmuhl Y, Fischer D, Holsboer F, Wotjak CT, Almeida OF, Spengler D. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat Neurosci. 2009;12:1559–1566. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, Vale W. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226:1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- Nesse RM. Is depression an adaptation? Arch Gen Psychiatry. 2000;57:14–20. doi: 10.1001/archpsyc.57.1.14. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Gould E, Manji H, Buncan M, Duman RS, Greshenfeld HK, Hen R, Koester S, Lederhendler I, Meaney M, Robbins T, Winsky L, Zalcman S. Preclinical models: status of basic research in depression. Biol Psychiatry. 2002;52:503–528. doi: 10.1016/s0006-3223(02)01405-1. [DOI] [PubMed] [Google Scholar]
- O’Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2, 3-dioxygenase activation in mice. Mol Psychiatry. 2009;14:511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly KC, Shumake J, Gonzalez-Lima F, Lane MA, Bailey SJ. Chronic administration of 13-cis-retinoic acid increases depression-related behavior in mice. Neuropsychopharmacology. 2006;31:1919–1927. doi: 10.1038/sj.npp.1300998. [DOI] [PubMed] [Google Scholar]
- O’Tuathaigh CMP, Desbonnet L, Moran PM, Kirby BP, Waddington JL. Current topics in behavioral neurosciences. Springer; Heidelberg: 2011. Molecular genetic models related to schizophrenia and psychotic illness: heuristics and challenges. doi: 10.1007/7854_2010_111. [DOI] [PubMed] [Google Scholar]
- Olivier JD, Van Der Hart MG, Van Swelm RP, Dederen PJ, Homberg JR, Cremers T, Deen PM, Cuppen E, Cools AR, Ellenbroek BA. A study in male and female 5-HT transporter knockout rats: an animal model for anxiety and depression disorders. Neuroscience. 2008;152:573–584. doi: 10.1016/j.neuroscience.2007.12.032. [DOI] [PubMed] [Google Scholar]
- Orsetti M, Di Brisco F, Canonico PL, Genazzani AA, Ghi P. Gene regulation in the frontal cortex of rats exposed to the chronic mild stress paradigm, an animal model of human depression. Eur J Neurosci. 2008;27:2156–2164. doi: 10.1111/j.1460-9568.2008.06155.x. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- Pollak DD, Monje FJ, Zuckerman L, Denny CA, Drew MR, Kandel ER. An animal model of a behavioral intervention for depression. Neuron. 2008;60:149–161. doi: 10.1016/j.neuron.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Prager LM. Depression and suicide in children and adolescents. Pediatr Rev. 2009;30:199–205. doi: 10.1542/pir.30-6-199. quiz 206. [DOI] [PubMed] [Google Scholar]
- Rapaport MH, Schneider LS, Dunner DL, Davies JT, Pitts CD. Efficacy of controlled-release paroxetine in the treatment of late-life depression. J Clin Psychiatry. 2003;64:1065–1074. doi: 10.4088/jcp.v64n0912. [DOI] [PubMed] [Google Scholar]
- Rush AJ. The varied clinical presentations of major depressive disorder. J Clin Psychiatry. 2007;68(Suppl 8):4–10. [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Havemann-Reinecke U, Ruther E, Hiemke C, Zernig G, Fuchs E, Flugge G. Pharmacological validation of a chronic social stress model of depression in rats: effects of reboxetine, haloperidol and diazepam. Behav Pharmacol. 2008;19:183–196. doi: 10.1097/FBP.0b013e3282fe8871. [DOI] [PubMed] [Google Scholar]
- Savitz J, Drevets WC. Bipolar and major depressive disorder: neuroimaging the developmental-degenerative divide. Neurosci Biobehav Rev. 2009;33:699–771. doi: 10.1016/j.neubiorev.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder FA, Lin CL, Crusio WE, Akbarian S. Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse. Biol Psychiatry. 2006;62:55–64. doi: 10.1016/j.biopsych.2006.06.036. [DOI] [PubMed] [Google Scholar]
- Seligman ME, Rosellini RA, Kozak MJ. Learned helplessness in the rat: time course, immunization, and reversibility. J Comp Physiol Psychol. 1975;88:542–547. doi: 10.1037/h0076431. [DOI] [PubMed] [Google Scholar]
- Sibille E, Wang Y, Joeyen-Waldorf J, Gaiteri C, Surget A, Oh S, Belzung C, Tseng GC, Lewis DA. A molecular signature of depression in the amygdala. Am J Psychiatry. 2009;166:1011–1024. doi: 10.1176/appi.ajp.2009.08121760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloman L. A new comprehensive evolutionary model of depression and anxiety. J Affect Disord. 2008;106:219–228. doi: 10.1016/j.jad.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Song C, Leonard BE. The olfactory bulbectomised rat as a model of depression. Neurosci Biobehav Rev. 2005;29:627–647. doi: 10.1016/j.neubiorev.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Strekalova T, Spanagel R, Bartsch D, Henn FA, Gass P. Stress-induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacology. 2004;29:2007–2017. doi: 10.1038/sj.npp.1300532. [DOI] [PubMed] [Google Scholar]
- Strekalova T, Gorenkova N, Schunk E, Dolgov O, Bartsch D. Selective effects of citalopram in a mouse model of stress-induced anhedonia with a control for chronic stress. Behav Pharmacol. 2006;17:271–287. doi: 10.1097/00008877-200605000-00008. [DOI] [PubMed] [Google Scholar]
- Sugino K, Hempel CM, Miller MN, Hattox AM, Shapiro P, Wu C, Huang ZJ, Nelson SB. Molecular taxonomy of major neuronal classes in the adult mouse forebrain. Nat Neurosci. 2006;9:99–107. doi: 10.1038/nn1618. [DOI] [PubMed] [Google Scholar]
- Surget A, Wang Y, Leman S, Ibarguen-Vargas Y, Edgar N, Griebel G, Belzung C, Sibille E. Corticolimbic transcriptome changes are state-dependent and region-specific in a rodent model of depression and of antidepressant reversal. Neuropsychopharmacology. 2009;34:1363–1380. doi: 10.1038/npp.2008.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Chergui K, Rachleff I, Flajolet M, Zhang X, El Yacoubi M, Vaugeois JM, Nomikos GG, Greengard P. Alterations in 5-HT1B receptor function by p11 in depression-like states. Science. 2006;311:77–80. doi: 10.1126/science.1117571. [DOI] [PubMed] [Google Scholar]
- Temel Y, Boothman LJ, Blokland A, Magill PJ, Steinbusch HW, Visser-Vandewalle V, Sharp T. Inhibition of 5-HT neuron activity and induction of depressive-like behavior by high-frequency stimulation of the subthalamic nucleus. Proc Natl Acad Sci USA. 2007;104:17087–17092. doi: 10.1073/pnas.0704144104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomida S, Mamiya T, Sakamaki H, Miura M, Aosaki T, Masuda M, Niwa M, Kameyama T, Kobayashi J, Iwaki Y, Imai S, Ishikawa A, Abe K, Yoshimura T, Nabeshima T, Ebihara S. Usp46 is a quantitative trait gene regulating mouse immobile behavior in the tail suspension and forced swimming tests. Nat Genet. 2009;41(6):688–695. doi: 10.1038/ng.344. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- Vieyra-Reyes P, Mineur YS, Picciotto MR, Tunez I, Vidaltamayo R, Drucker-Colin R. Antidepressant-like effects of nicotine and transcranial magnetic stimulation in the olfactory bulbectomy rat model of depression. Brain Res Bull. 2008;77:13–18. doi: 10.1016/j.brainresbull.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale G, Ruggieri V, Filaferro M, Frigeri C, Alboni S, Tascedda F, Brunello N, Guerrini R, Cifani C, Massi M. Chronic treatment with the selective NOP receptor antagonist [Nphe(1), Arg (14), Lys (15)]N/OFQ-NH (2) (UFP-101) reverses the behavioural and biochemical effects of unpredictable chronic mild stress in rats. Psychopharmacology (Berl) 2009;207(2):173–189. doi: 10.1007/s00213-009-1646-9. [DOI] [PubMed] [Google Scholar]
- Wallace DL, Han MH, Graham DL, Green TA, Vialou V, Iniguez SD, Cao JL, Kirk A, Chakravarty S, Kumar A, Krishnan V, Neve RL, Cooper DC, Bolanos CA, Barrot M, McClung CA, Nestler EJ. CREB regulation of nucleus accumbens excitability mediates social isolation-induced behavioral deficits. Nat Neurosci. 2009;12:200–209. doi: 10.1038/nn.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Flajolet M, Maller A, Chen EY, Qi H, Svenningsson P, Greengard P. Role of p11 in cellular and behavioral effects of 5-HT4 receptor stimulation. J Neurosci. 2009;29:1937–1946. doi: 10.1523/JNEUROSCI.5343-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson PJ, Andrews PW. Toward a revised evolutionary adaptationist analysis of depression: the social navigation hypothesis. J Affect Disord. 2002;72:1–14. doi: 10.1016/s0165-0327(01)00459-1. [DOI] [PubMed] [Google Scholar]
- Wei Q, Hebda-Bauer EK, Pletsch A, Luo J, Hoversten MT, Osetek AJ, Evans SJ, Watson SJ, Seasholtz AF, Akil H. Overexpressing the glucocorticoid receptor in forebrain causes an aging-like neuroendocrine phenotype and mild cognitive dysfunction. J Neurosci. 2007;27:8836–8844. doi: 10.1523/JNEUROSCI.0910-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson MB, Xiao G, Kumar A, LaPlant Q, Renthal W, Sikder D, Kodadek TJ, Nestler EJ. Imipramine treatment and resiliency exhibit similar chromatin regulation in the mouse nucleus accumbens in depression models. J Neurosci. 2009;29:7820–7832. doi: 10.1523/JNEUROSCI.0932-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]