Habitual participation in physical activity results in many health benefits, including increased longevity, decreased risk of cardiorespiratory and metabolic diseases and some cancers (most notably colon and breast), maintenance of energy balance, and improved musculoskeletal, functional and mental health.1 The extensive evidence base demonstrating these beneficial effects suggests that they apply to the adult population as a whole, women as well as men and older as well as younger. However, the question of whether physical activity attenuates any of the adverse health effects that frequently accompany the menopausal transition, such as occurrence of vasomotor symptoms (VMS), increases in weight and body fat, decreases in bone density, and changes in mood and somatic symptoms, has not been fully explored. In this article, we review the existing literature relevant to this question, drawing on findings from the Study of Women’s Health Across the Nation (SWAN) and other studies of midlife women’s health.
PHYSICAL ACTIVITY AND VMS
Considerable uncertainty still exists regarding the role of physical activity in reducing the risk of developing hot flashes and night sweats and the efficacy of physical activity as a treatment for VMS. The first analysis of this question in SWAN found an inverse association between physical activity and the occurrence of VMS, independent of potential confounding variables, based on cross-sectional data from the initial screening survey of over 16,000 women, ages 40 to 55, of varying menopausal status.2 However, this result was not confirmed in 2 subsequent SWAN analyses conducted in the SWAN cohort, which found neither a cross-sectional relation at baseline between physical activity and VMS3 nor a longitudinal relation over 5 years of follow-up.4 In both of these studies, physical activity was associated with fewer VMS in bivariate analyses, but the association did not persist after adjustment for confounders, particularly body mass index (BMI) and overall health. Complicating the picture in SWAN even further, a report by Gold et al5 from the Daily Hormone Study, a substudy of SWAN, found that physical activity was associated cross-sectionally with increased risk of VMS, but only in the relatively small group of women (n = 134) who had low or medium levels of the progesterone metabolite, pregnanediol-glucuronide.5
Part of the explanation for the inconsistency in the SWAN findings is owing to differences in the assessment of physical activity. In the cross-sectional survey, physical activity was assessed by a single global question that asked respondents to compare their physical activity relative with others their age and gender. An evaluation of this question within the SWAN sample revealed that this question seemed to rank women reasonably accurately in terms of physical activity within their respective race/ethnic group, but did not result in the expected differences in physical activity across different race/ethnic groups.6 Because the reporting of VMS varies by race/ethnicity, this global question may have led to a biased finding owing to differential misclassification. In the cohort study, physical activity was assessed with the Kaiser Physical Activity Survey,7 which provides a much more detailed measure of activity across several domains, including household and care giving, recreational sports and exercise, and active living behaviors, such as walking to work. It is likely that the Kaiser Physical Activity Survey yields a less biased estimate, and, therefore, a more valid measure of association with any outcome of interest, including VMS.
On the other hand, the inconsistency in the SWAN findings regarding the relationship between physical activity and VMS reflects the inconsistency in the literature as a whole. Of the more than 30 studies that have addressed the question of physical activity and the risk of VMS, more than half have reported no association (Table 1 provides a description of selected studies). The remaining suggest a generally protective, inverse relation while a small number (n = 3)8-10 report increased VMS with higher levels of activity. The vast majority of these studies are observational and cross-sectional in design and suffer from many of the limitations common to this type of study, including heterogeneous study samples with regard to menopause status,11-14 too few women with frequent and severe symptoms,12,14,15 too few women participating in regular activity of at least moderate intensity,9,16 a lack of adequate control over confounding variables,8,11 and inability to establish temporality.17 In addition, establishing comparability across studies is challenging, given the assessments of physical activity that range from a single global question18,19 to detailed recalls of duration, frequency, and mode of activity that allow for creation of summary scores in terms of metabolic equivalent (MET)-hours or -minutes a week.9,13,16,20-22 The differences in the assessment of symptoms, with some studies considering frequency, severity and/or bother as separate domains,9,12 others combining those domains into a single measure,21,23 and still others considering only frequency,3,4,24 add to this challenge.
Table 1.
Selected studies of physical activity and vasomotor symptoms
| Observational Studies | ||||||
|---|---|---|---|---|---|---|
| Reference | Study Design | Sample | Physical Activity Measure | Symptom Measure | Other Variables | Main Findings |
| Collins et al, 199511 | Cross-sectional survey | Population-based sample of 1,324 Swedish women, 48 yrs old, varying menopausal status | Participation in regular exercise (yes/no) | Menopause Symptom Inventory (frequency of symptoms on scale of 1–5) | — | No relation between physical activity and vasomotor symptoms; inverse relation with negative mood, direct relation with well-being |
| Daley et al, 200715 | Cross-sectional survey | 1,206 British women, ages 46–55, from 10 general practices, based on purposeful sampling for location, level of deprivation and practice size | Regularly active or not based on stage of readiness for change in moderate intensity activity 3 or more times a week for 20 minutes of longer each time | Vasomotor symptoms and 8 other domains of heath related quality of life from Women’s Health Questionnaire | — | No relation between physical activity and vasomotor symptoms; inverse relation with depressed mood and somatic symptoms |
| Elavsky and McAuley, 200512 | Cross-sectional survey | 133 women, ages 44–60, varying menopausal status | Aerobics Center Longitudinal Study Physical Activity Survey | Menopause Symptom List (frequency and severity of 25 symptoms) | Self-esteem, life satisfaction | Significant inverse association between exercise frequency and frequency and severity of VMS, somatic and total symptoms |
| Gold et al, 20043, Gold et al, 20064 | Prospective cohort study | 3,302 racially/ethnically diverse women, ages 42–52, initially in pre- or early peri-menopause | Ordinal ranking of total activity, as measured by Kaiser Physical Activity Survey | Occurrence and frequency of VMS in past 2 weeks | BMI, health status, other confounders | No association between physical activity and VMS, either at baseline or over time |
| Guthrie et al, 199513 | Cross-sectional survey | 1,181 Australian women, ages 45–55, of varying menopausal status | MLTPA questionnaires, assessing frequency, duration and intensity of recreational activities in past year | Overall symptoms | BMI, self-rated health | No association between physical activity and vasomotor symptoms or psychological well-being; physical activity directly related to overall health |
| Guthrie et al, 200520 | Prospective cohort study | 438 Australian women, ages 45–55, pre-menopausal at baseline | Frequency of exercise on 7 point ordinal scale | Hot flash index based on frequency and severity in past 2 weeks, frequency and bother of somatic symptoms | Health status, BMI, menopausal status, other confounding variables | Daily exercise at baseline significantly associated with 49% lower risk of developing VMS during follow-up |
| Li and Holm 200324 | Cross-sectional survey | 239 post-menopausal women | Usual Physical Activity questionnaire | Women’s health Assessment scale | Use of hormone therapy | Non-significant trend for active women to report fewer symptoms than inactive women, within HT stratum (use/no use) |
| Moilanen et al, 201014 | Population-based cross-sectional survey based on a national health examination survey | 1,427 Finnish women, ages 45–64, varying menopausal status | Single ordinal question about level of recreational physical activity | Occurrence and bother of various vasomotor, somatic and mood symptoms | Lifestyle factors, medical conditions, other confounding variables | Low physical activity associated with significantly more psychological, somatic and vasomotor symptoms, relative to high physical activity, independently of confounders |
| Romani et al, 20098 | Population-based cross-sectional survey | 639 pre- or early-perimenopausal women from Baltimore | Three-level categorical variable regarding usual physical activity (light, moderate, or heavy) at work, home, and for recreation | Frequency, severity and duration of hot flashes | BMI | High level of physical activity associated with increased risk of moderate or severe hot flashes (OR = 2.88, 95% CI, 1.12-7.40 for moderate, OR = 4.16, 95% CI, 18.08, for heavy, p for trend = 0.02) relative to low level, and with non-significant increased risk for any hot flashes, daily hot flashes and hot flashes for more than a year |
| Slaven and Lee 1997106, Study I and Study II | Cross-sectional | 220 Australian women of varying menopausal status in Study I; 47 Australian women of varying menopausal status who were regular exercisers for Study II | Regular exercise defined as participation in aerobic activity at least twice a week for 30 minutes a time for last 3 months; exercisers assessed immediately prior to work-out | Women’s Health Questionnaire | Profile of Mood States | Study I: No relation between physical activity and vasomotor symptoms; inverse relation with depressed mood, anxiety, fears, fatigue, tension, problems with memory and concentration, sexual dysfunction, sleep problems; direct relation with vigor and perceived attractiveness Study II: Fewer vasomotor and somatic symptoms reported following exercise class, independent of change in mood |
| Sternfeld et al, 199916 | Case-control | Cases defined as women 48–52 years old, 3–12 months since LMP with frequent vasomotor symptoms (n = 82), controls same chronological and biological age without vasomotor symptoms (n = 89) | Activity score based on intensity of activity and frequency; separate scores for recreational, occupational and household activity | Case definition based on frequency of VMS | Psychological and somatic symptoms | No relation between physical activity and case status; activity attenuated relation between psychological and vasomotor symptoms |
| Van Poppel and Brown 200822 | Prospective cohort study | 3,300 mid-life women participating in 3rd and 4th surveys of the Australian Longitudinal Study on Women’s Health | Change in physical activity based on frequency, duration and intensity of usual physical activity | Vasomotor symptoms, somatic and psychological symptoms, total symptom score | Change in weight | No association between change in physical activity with total symptoms, fasomotor or psychological symptoms; significant, but modest, inverse association with somatic symptoms; weight gain associated with increased total, vasomotor and somatic symptoms and weight loss association with reduction in total and vasomotor symptoms |
| Whitcomb et al, 20079 | Cross-sectional survey | 512 peri- and post-menopausal women in Baltimore metropolitan area | Historical physical activity (frequency of participation in moderate and vigorous activities at different ages; activity at 35–39 used to examine long-term effects | History of frequency of hot flashes, summarized as any menopausal hot flashes, daily hot flashes and any moderate or severe hot flashes | History of HT, smoking, BMI | High activity associated with increased risk of moderate to severe hot flashes (adjusted OR = 1.77, p = 0.01) and daily hot flashes (OR = 1.77, p = 0.01), relative to minimal activity; those highly active during 5 year age period prior to LMP had increased risk for moderate to severe hot flashes and for daily hot flashes compared to minimal activity |
| Wilbur et al, 1990128 | Cross-sectional | 386 Australian women between ages 34–62 volunteering for bone density study, varying menopausal status | Energy expenditure in recreational, occupational, and housework activity, based on Minnesota Leisure Time Physical Activity survey (LTPA) | Kaufert and Syrotuik Symptom Index (VMS and general health symptoms) | Aerobic fitness | No relation between physical activity and vasomotor symptoms; inverse relation between recreational activity and somatic symptoms and other general health symptoms; direct relation between occupational activity and same outcomes |
| Aiello et al, 200410 | RCT of exercise vs stretching controls | 173 sedentary post-menopausal women, ages 50–75, 87 exercisers vs 86 in stretching control group | 45 mins moderate-intensity exercise, 5 days/wk for 12 months, 3 months facility-based training and 9 months home-based training | Occurrence and severity of VMS and other symptoms, assessed at baseline, 3, 6, 9 and 12 months | Body fat, sex hormones | No significant differences in occurrence of symptoms; non-significant decrease in occurrence of memory problems in exercise group, non-significant increase in risk of moderate-severe hot flashes (OR = 2.8, 95% CI, 0.8-9.3) |
| Elavsky and McAuley, 200727 | RCT of exercise vs yoga vs control | 164 inactive, symptomatic women, ages 42–58, 63 to exercise, 62 to yoga and 39 to control | 4 month walking program 3 times a week for an hour, intensity starting at 50% heart rate reserve (HRR), increased to 60–75% HRR; 4 month Iyengar yoga class 2 times a week for 90 mins/class | Greene Climacteric Scale | Fitness, body composition, affect, depression, Utian Quality of Life | Non-significant decreases in VMS in exercise and yoga groups relative to controls; significant increases in positive affect in exercise and yoga groups relative to controls; change in fitness was significant predictor of change in symptoms |
| Huang et al, 201025 | RCT of behavioral weight loss program | 338 overweight or obese women with urinary incontinence, 226 to intervention group and 112 to structured education control group | Based on Diabetes Prevention Program and Look AHEAD to achieve 7%–9% weight loss, physical activity goal was to increase to 200 minutes of moderate intensity exercise, mostly brisk walking | Bothersomeness of VMS and other symptoms, assessed at baseline and 56 months | Weight, waist circumference | Of those reporting any symptoms at baseline (n = 99 in intervention and n = 55 in control group), intervention resulted in more than a 2-fold likelihood of improvement in bother of flushing, relative to control group (OR = 2.3, 95% CI, 1.2-4.2), but this was attenuated to non-significance when adjusted for change in body size; decreases in weight and abdominal circumference significantly associated with improvement in reporting of hot flashes |
| Kemmler et al, 200432 | RCT to reduce menopause related bone loss | 137 women, ages 48–60 and 1–8 years post-menopause with low BMD; analysis based on 50 in exercise group, 31 in control group who completed study | 264 months of exercise 4/wk (2 facility-based group exercise and 2 home-based individual training), 25 minutes/session, including warm-up, endurance, jumping, strength and flexibility exercises | Frequency of hot flashes, other somatic and mood symptoms | Bone density, strength, endurance, blood lipids | Hot flashes improved in both groups with no significant between group difference; improvements in mood and insomnia in exercise group significantly different from no change in control group; significant improvements in fitness, BMD, and blood lipids |
| Lindh-Astrand et al, 200328 | RCT | 75 sedentary, naturally post-menopausal women, ages 48–63 with VMS | 12 weeks of exercise classes twice a week for 60 minutes, plus additional session on own | Daily frequency and severity of VMS for two weeks at beginning and at end of 12 weeks, plus baseline and follow-up for for one week monthly during extended 24 week follow-up | Kupperman Index, quality of life, mood and general psychological wellbeing | Non-significant decrease in number and severity of hot flashes in exercisers from baseline to 12 weeks; significant pre-post declines in symptom scale |
| McAndrew et al, 200931 | RCT designed to test three different approaches to promotion of physical activity | 280 inactive, healthy women, 113 with symptoms at follow-up | 12 month three arm design with one arm addressing stage and processes of change in physical activity behavior with tailored feedback (n = 95) vs, educational booklet based on social cognitive theory (n = 93) vs health-related print material on sleep and nutrition (control, n = 92) | MENQOL, administered at month 12 follow-up visit | Self-reported physical activity, depression, exercise self-efficacy, stress | Change in physical activity not associated with VMS; change in PA inversely associated with total symptoms, psychosocial and physical symptoms |
| Moriyama et al, 200829 | 2 by 2 RCT of exercise and estrogen therapy | 44 hysterectomized women | 6 months of moderate aerobic exercise for3 hrs/wk plus hormone therapy (n = 9), exercise and placebo (n = 11), HT and no activity (n = 14) and placebo and no activity (n = 11) | Kupperman index | Health-related quality of life (SF-12) | All groups had declines in symptoms; physical activity significantly associated with increases in physical functioning and decreases in bodily pain relative to no activity, regardless of drug or placebo assignment |
| Villaverde-Gutierrez et al, 200630 | Quasi-experimental (random group assignment, pre-post differences | 48 sedentary Spanish women, ages 55–72 | 12 month program of endurance (50–85% maximum HR), strengthening, flexibility and relaxation exercises, twice a week in supervised classes (n = 24) vs control group (n = 24) | Kupperman Index | Health-related quality of life | Significant decrease in severe symptoms from 50% to 37.5% in exercise group and significant increase in control group; significant improvement in HRQOL in exercise group but decrease in controls |
| Ueda, 200426 | Non-randomized intervention | 35 sedentary, symptomatic Japanese women, ages 40–60, 20 in intervention group vs 15 in control group | 12 week exercise and menopause education program of one 90 minute class/wk (30 minute lecture, 60 minutes of either aerobic or resistance exercise), plus aerobic exercise twice a week on own | Kupperman index | Quality of life, attitudes towards exercise | 22.5% decrease in overall Kupperman index in treatment group vs no change in controls, p<0.5; 32% decrease psychosomatic symptoms in treatment group vs 3% increase in controls, p<.05; non-significant decrease in VMS in intervention group vs control group |
| Wilbur et al, 200523 | RCT | 173 sedentary, healthy Caucasian and African American women, ages 45–65 | 24 week home-based moderate intensity walking program (50– 74% maximal HR), 4 times/week for 30 minutes at a time (n = 97) vs control (n = 66) | Frequency, severity and bother of VMS and other symptoms | BMI | Significant improvement in symptoms in both groups with no differences between groups; adherence to intervention led to significant improvement in sleep symptoms relative to controls |
Perhaps the strongest evidence for a protective association between physical activity and VMS in the observational data comes from one of the few prospective analyses, the longitudinal Melbourne Women’s Midlife Health Project, in which 438 women were followed over an 8-year interval.20 Although physical activity was not associated with VMS in this cohort at baseline,13 women who reported exercising every day at baseline were 49% less likely to report bothersome hot flashes during follow-up (odds ratio, 0.51; 95% confidence interval, 0.27–0.96). Furthermore, women whose exercise level decreased over the follow-up were more likely to experience bothersome hot flashes. In contrast, another longitudinal, observational study, also of Australian women (but not the same cohort), failed to find any association between change in physical activity over time and reporting of VMS.22
In recent years, a number of intervention studies have tested the effect of physical activity (generally aerobic exercise and most often walking) on VMS. Here again, findings, summarized in the second half of Table 1, are inconsistent, with several reporting no effect,23,25 one reporting a nonsignificant increase in hot flash severity in the exercisers compared with the controls,10 and several reporting reductions in frequency and severity of VMS.26-28 However, as with the observational studies, most of the trials also suffer from methodologic weaknesses, including very small sample sizes,26,29-31 nonrandomized designs,26,30,31 inadequately specified exercise dose,31 and large loss to follow-up.32 In one of the more carefully controlled trials, a 4-month intervention among 164 previously sedentary women randomized either to a walking group, yoga, or a control group27 led to a decrease in VMS in both the walking and yoga arms relative to the control group, but the differences were not significant. In this study, change in symptoms seemed to be mediated by increases in physical fitness, such that women who had the greatest increase in fitness were most likely to have the greatest decrease in symptoms.27
Despite the equivocal evidence for a protective effect of physical activity on VMS, the hypothesis remains compelling, partly because physical activity is a generally beneficial intervention with few risks or side effects, partly because the hypothesis has not yet been adequately tested, and partly because there are plausible biological mechanisms by which activity could alleviate VMS. Although the etiology of the hot flash is still not fully understood, neuroendocrine processes at the level of the hypothalamus are implicated.33 Physical activity, in turn, has a range of neuroendocrine responses that occur, both acutely, as a result of a single bout of exercise, and chronically, as a result of exercise training. Increases in brain norepinephrine and its metabolites occur in response to acute exercise,34,35 but 24-hour urinary norepinephrine seems to decrease with training,36 perhaps because of an increase in vagal tone. Decreases in resting heart rate and heart rate variability are a near universal response to aerobic exercise training37,38 and are typically ascribed to increased vagal output and a resulting shift in autonomic balance in favor of the parasympathetic nervous system.39
Because stress seems to be a precipitating factor in hot flashes40,41 and neuroendocrine substances, such as catecholamines and cortisol, are involved in the stress response and affect thermoregulation at the level of the hypothalamus,42 hot flashes may result from an imbalance in the autonomic nervous system, in which the “stress-buffering” role of the parasympathetic nervous system is not adequate to counter the increased activation of the sympathetic nervous system.43,44 If this is true, then the shift in that balance as a result of exercise training is a potential mechanism by which exercise could reduce the occurrence of VMS.
A second potential mechanism by which physical activity could have a favorable effect on the frequency or bother of VMS is through the release of endogenous opioids, particularly β-endorphins, that occurs in response to a single, sustained bout of vigorous exercise.45-47 The evidence for a role of β-endorphins in the pathogenesis of the hot flash comes primarily from animal studies, in which administration of naloxone, an opiate antagonist, in the morphine-dependent rat, causes symptoms similar to those of the hot flash, including a sudden increase in peripheral tail temperature and an luteinizing hormone (LH) surge.48,49 However, human experiments involving the infusion of naloxone in postmenopausal women have not consistently reduced the frequency of hot flashes or LH pulses,50,51 and studies of plasma β-endorphin levels before hot flashes have been contradictory,52,53 although plasma levels may not reflect the endorphin levels in the brain. It is not currently known whether β-endorphins are responsible for the so-called runner’s high,54 but the endogenous opioids are biochemically similar to exogenous opiates and have diverse physiologic effects, including temperature regulation (hypothermia), decreased sensitivity to pain, and decreased heart and respiratory rate, all of which could be responsible for a decrease in either frequency or bother of VMS.
Finally, physical activity could “distract” women from attention on their hot flashes by habituating them to the feelings of increased heat and heat dissipation through sweating that accompanies increases in physical effort and associating those feelings with behaviors that may make them feel good in other ways. This is similar to the “distraction” theory of how physical activity improves mental health and sense of well-being.55 On the other hand, given the acute rise in core temperature that occurs with exercise, exercise might actually induce hot flashes, particularly if symptomatic women have a narrowed thermoregulatory zone that lowers their threshold for sweating.56
Given these plausible biological mechanisms and the absence of methodologically rigorous studies, it is still not possible to draw firm conclusions about the efficacy of physical activity as a treatment for VMS. Many questions remain unanswered: Does participation in regular physical activity matter at all in terms of frequency, severity, or bother of hot flashes or even length of time for which VMS persist? If so, does exercise make VMS better or worse? Does the “dose” of physical activity matter? Is more vigorous intensity exercise more effective than moderate intensity exercise, and are there any differences by mode of exercise (aerobic vs resistance exercise vs mind–body disciplines)? And if physical activity is effective, how does it work? Well-designed and sufficiently powered randomized trials are required to answer these questions adequately. The Menopause Symptoms: Finding Lasting Answers for Symptoms and Health Research Network is currently conducting such a trial, in which 112 previously sedentary women with frequent and bothersome hot flashes randomized to moderate-to-vigorous aerobic exercise for 12 weeks will be compared with 150 women in a usual activity control group. The findings from this carefully designed and controlled trial, in which women participate in facility-based, individual exercise training at a prescribed target heart rate and constant caloric expenditure, relative to body weight, should provide unique and definitive evidence regarding the efficacy of physical activity for treating VMS that has been lacking up to this point. It represents an important step forward in closing the gap in our knowledge regarding this question.
PHYSICAL ACTIVITY AND CHANGES IN BODY SIZE AND COMPOSITION
On-going adult weight gain57,58 and the high prevalence of obesity59,60 are issues that loom large for the population as a whole. They are of particular concern for women during the menopausal transition, when many may not only be gaining weight, but are also experiencing changes in body composition and fat distribution. A number of studies of midlife women find an annual rate of weight gain of about 0.5 kg or more,61-65 but the evidence suggests that weight gain per se is more a function of aging than of the hormonal changes that define menopause.61,62,64,66 This conclusion comes both from cross-sectional comparisons of weight in women of similar chronological age but varying menopausal status (premenopausal, early perimenopausal, late perimenopausal, or postmenopausal, and surgically menopausal), as well as from longitudinal studies that examine rate of weight change over time by change in menopausal status.
A number of different analyses in SWAN support this conclusion. For instance, a comparison of BMI among the more than 16,000 women who participated in the cross-sectional screening survey found no difference between premenopausal and postmenopausal women after adjusting for chronological age and other covariates.67 Similarly, in a cross-sectional ancillary study of energy expenditure, body composition and menopausal status conducted at the Kaiser/UC Davis SWAN site at the 5-year follow-up visit for the SWAN longitudinal cohort, the median weight of the Chinese premenopausal and early perimenopausal women was not statistically different from that of the late perimenopausal and postmenopausal women [56.7 kg (interquartile range [IQR]), 50.3–61.5 vs 53.8 kg (IQR, 50.0–63.2), respectively; P = .51).68 The median weight among the white women, although significantly higher than the Chinese, also did not differ by menopausal status [71.7 kg (IQR, 60.5–82.0) for premenopause and early perimenopause vs 68.2 kg (IQR, 56.9–79.0); P = .21). In addition, in the SWAN cohort as a whole, mean weight gain over 3 years of follow-up was 2.1 kg (standard deviation. 4.8) overall, and was not associated with menopausal status.61
In contrast, changes in body composition (increased fat mass and decreased lean mass) and in fat distribution (from a more gynoid pattern to a more android pattern) do seem to be influenced by the menopausal transition, as well as by chronological aging.69-73 As discussed by Wildman and Sowers in this issue, a longitudinal analysis of 7-year changes in body composition, assessed by bioelectrical impedance at the Michigan SWAN site, showed substantial weight gain (3.4% or 2.9 kg over 6 years), significant increases in fat mass (10.1% or 3.4 kg), small but significant losses of skeletal muscle mass (1.06% or 0.23 kg), and a 6.2% (5.6 cm) increase in waist circumference.74 The change in weight was linear, suggesting only an age effect, whereas the changes in fat mass and skeletal muscle mass were more curvilinear over time, suggesting a menopause effect. Interestingly, although change in body composition did not vary by menopausal stage, as defined by bleeding criteria, increases in fat mass and in waist circumference and decreases in skeletal muscle mass were significantly associated with increasing follicle-stimulating hormone levels, independent of age, indicating an independent and significant menopause effect.
Despite the seeming inevitability of these changes in weight, body composition, and fat distribution with age and menopause, physical activity may attenuate the impact of both of these factors. To begin with, more active individuals tend to be leaner than sedentary individuals at any given point in time,75 which means that active midlife women have an advantage as they enter the menopausal transition in terms of starting out with a lower BMI, lower fat mass, greater lean mass, and less central adiposity. The site-specific Energy Expenditure SWAN study demonstrated this, particularly for the white women, in whom there was a strong, cross-sectional, inverse dose response relation between physical activity measured by accelerometry and both percent body fat and waist circumference.68
Second, physical activity may slow the rate of change of weight, both with menopause and over time. In a longitudinal analysis of the SWAN cohort, physical activity was inversely associated with changes in weight and waist circumference, independent of aging and change in menopausal status.61 Women whose activity decreased the most experienced the greatest increases in weight and waist circumference, whereas those whose physical activity was essentially stable experienced much smaller increases (Figs. 1 and 2). This pattern was true for both sports and exercise and lifestyle physical activity.
Fig. 1.
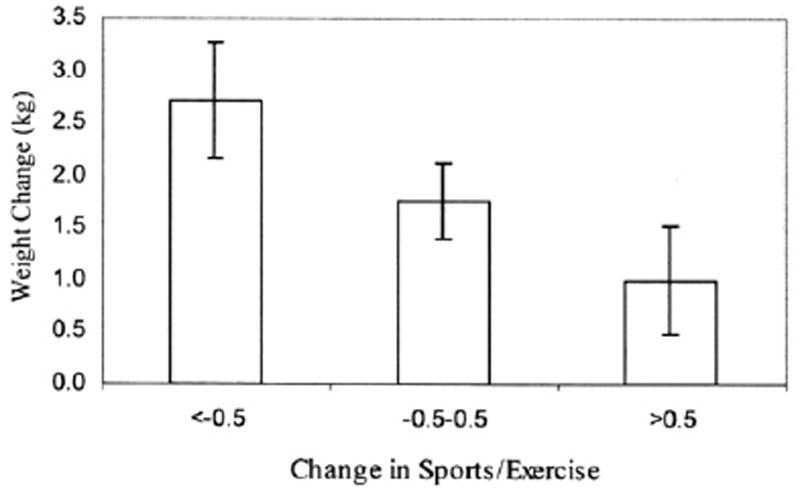
Change in weight associated with change in sports and exercise, adjusted for multiple confounding variables.
Fig. 2.
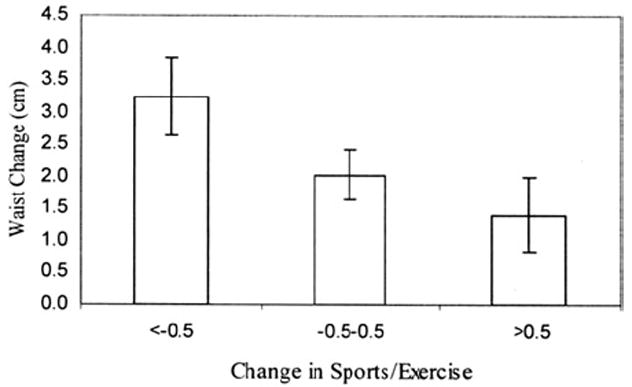
Change in waist associated with change in sports and exercise, adjusted for multiple confounding variables.
In addition, physical activity, although not entirely preventing weight gain with age, may protect against the development of obesity. This conclusion is suggested by the observation in SWAN of a 9% decrease in risk of obesity (odds ratio, 0.91; 95% confidence interval, 0.84–0.98) over a 9-year follow-up associated with a one unit higher level of baseline total physical activity, after adjustment for numerous confounding factors.76 Furthermore, a 1-unit increase in activity over time was independently associated with an even greater reduction in risk (odds ratio, 0.83; 95% confidence interval, 0.72–0.95). Finally, physical activity may protect against the accumulation not only of overall fat, but of intra-abdominal fat, which may be the most metabolically harmful type of fat. In a site-specific study conducted at the Chicago SWAN site, intra-abdominal fat, assessed by computed tomography, was significantly inversely associated with total physical activity (4.0 cm2 for every 1-unit increase in physical activity score), independent of total percent fat and other covariates.77 Compared with those women engaging in a level of physical activity approximating the current recommendations for general health (150 minutes a week of moderate intensity activity),1 less active women had significantly greater amounts of intra-abdominal fat, regardless of menopausal status, but did not differ in terms of the level of subcutaneous abdominal fat (Fig. 3).
Fig. 3.
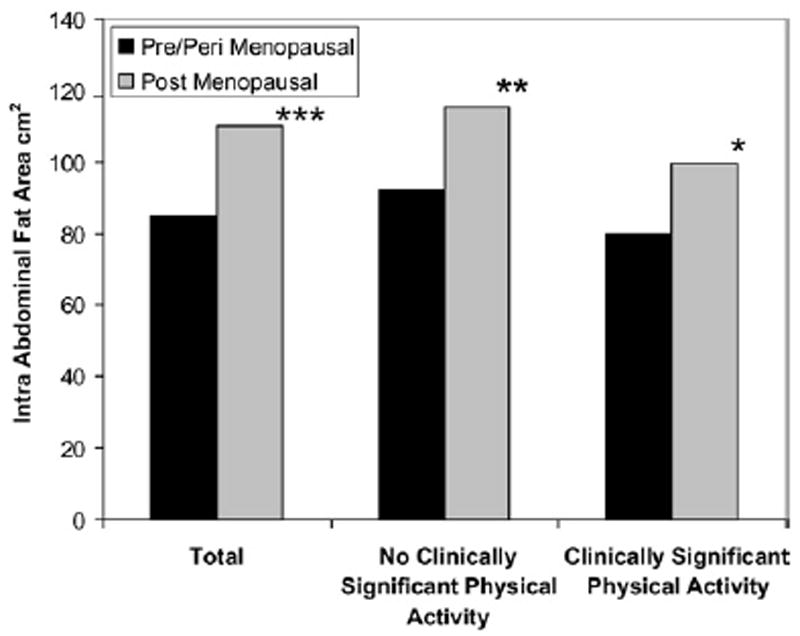
Mean IAF associated with physical activity by menopausal status and level of physical activity approximating current guidelines.
Other studies of midlife women confirm the protective role of physical activity against excess weight gain.62,78 Most notably, in a recent analysis of weight changes over 13 years of follow-up, different levels of activity were examined to clarify the amount of physical activity required for prevention of unhealthful weight gain.79 Although women expending 21 MET-hours or more a week (approximately equal to 60 minutes a day of moderate intensity activity) began at a lower weight than those expending between 7.5 and 21 and those expending fewer than 7.5 MET-hours per week (less than the recommended amount of 150 minutes a week of moderate intensity activity), the rate of weight gain in all 3 groups was essentially the same. However, in a fully adjusted model, the amount of weight gain was significantly greater in the 2 less active groups, relative to the most active group, particularly among women of normal weight to begin with. Finally, in the small group of women who maintained normal weight throughout the course of the study, the mean amount of physical activity was 21.5 MET-hours per week, which is equivalent to the recommendation for weight maintenance put forth by the Institute of Medicine in 2002 (60 minutes a day of moderate intensity activity).80 These findings suggest the importance of sustaining relatively high levels of physical activity starting in young adulthood to maintain normal weight through middle age, menopause, and beyond.
PHYSICAL ACTIVITY AND BONE DENSITY
The impact of the menopausal transition on bone mineral density (BMD) and risk of osteoporotic fractures, along with the insights into this concern that have come from SWAN, is discussed elsewhere in this volume by Lo et al. Regular physical activity is among the primary determinants of BMD and is a key contributor to overall musculoskeletal health, because of the responsiveness of bone to the mechanical forces that physical activities places on it.81 Both weight-bearing endurance activities, such as walking and running, and resistance exercises elicit this response, especially at the lumbar spine and femoral neck.82-86 Although the increase in BMD observed in exercise intervention studies in response to physical activity is modest (about 1%–2%), animal studies have shown that this is accompanied by a large increase in the resistance of the bone to fracture.1 This is in contrast with pharmacologic agents, in which the improvement in resistance of the bone is proportional to the improvement in BMD.87
The evidence for increased BMD in response to physical activity comes from a large number of cross-sectional studies,88 exercise training studies,89-91 and meta-analyses.83,86,86,92-95 In SWAN, a baseline, cross-sectional analysis of the relation between domain-specific physical activity and BMD at the lumbar spine, femoral hip, and total hip concluded that home and care-giving activity was positively associated with BMD, independent of other types of activity and other confounding variables.96 Spine and femoral BMD were both 1.7% greater in the highest tertile of home/care-giving activity than in the lowest tertile. Similarly, the highest tertile of sports and exercise was associated with 2.1% greater spine BMD and 2.6% greater femoral neck BMD than the lowest tertile. In contrast, neither lifestyle activity or occupational activity had a significant relation with BMD.
To date, the evidence is less clear about whether or not physical activity attenuates age-related loss of BMD.1 Several prospective, observational studies suggest that higher levels of physical activity at baseline are independently associated with higher BMD many years later97,98 and with less loss of BMD over time.99,100 They also suggest that maintenance of regular physical activity over time results in attenuated bone loss, compared with reduced physical activity or consistently sedentary behavior.97,98,101,102
Few, if any, studies have specifically examined the role of physical activity on the rate of bone loss during the menopausal transition, a time when bone loss accelerates, relative to the premenopausal or later postmenopausal period. With 10 years of follow-up, during which time most of the SWAN cohort has transitioned to postmenopause, there is now adequate statistical power to address this question and such an analysis is currently underway.
PHYSICAL ACTIVITY AND OTHER SYMPTOMS IN MIDLIFE WOMEN
In addition to VMS, other symptoms, such as joint pain and stiffness, fatigue, difficulty concentrating, poor sleep, irritability, and depression, are quite prevalent among midlife women, even though they are not directly associated with the menopause.103,104 In the SWAN cross-sectional survey, for instance, joint pain and stiffness were reported by more than 50% of the respondents, and difficulty sleeping was reported by just under 40%, although the prevalence of both of these complaints did not differ by menopausal status.2 Nevertheless, these symptoms may adversely impact the health of midlife women and decrease functioning and overall quality of life. The benefits of physical activity for ameliorating somatic and mood symptoms are well-documented and suggest another way in which regular physical activity may preserve and improve health in perimenopausal women.
Somatic Symptoms, Bodily Pain, Physical Functioning, and Quality of Life
Many of the studies cited that explored the influence of physical activity on VMS also considered a variety of somatic symptoms, ranging from headaches and joint pain to heart palpitations. In general, the findings suggest an inverse relation between greater levels of physical activity and lower rates of somatic complaints and fewer difficulties with sleep.11,23,105-108 In a relatively recent, cross-sectional analysis of menopause symptoms and lifestyle among Finnish women, for instance, women who were regularly active reported significantly fewer somatic symptoms and less pain, relative to women who were sedentary.14 Similarly, in a large cross-sectional survey of physical activity, BMI, and health-related quality of life in British women, the mean somatic symptom score was 28% lower among active women.15 In addition, in a longitudinal analysis based on 3300 Australian women, increases in physical activity over time were associated with decreases in somatic symptoms.22 Similarly, in a randomized trial designed to increase physical activity behavior, women who increased their activity reported fewer total menopausal symptoms (measured by the MENQOL) at follow-up, although symptoms were not measured at baseline,31 making it impossible to determine whether change in activity led to change in symptoms.
There is also considerable evidence that physical activity reduces feelings of bodily pain in general, and pain associated with osteoarthritis109 and low back problems.110 Several findings from SWAN reveal important relationships among bodily pain, menopausal status, and physical activity. At the baseline SWAN examination, the level of pain reported was higher among women in early perimenopause, relative to premenopause, but this difference did not remain after adjustment for multiple confounding variables.111 Furthermore, a longitudinal analysis of the SWAN cohort over 7 years of follow-up demonstrated that women who were more physically active at midlife experienced less bodily pain over time regardless of change in menopausal status, sociodemographic status, or medical conditions.112 In contrast, when pain was defined as a composite variable of aches and pains that included quantity of pain, interference from pain with work or sleep, stiffness and soreness, and low backaches or pain, postmenopausal women had significantly higher pain scores (more pain), relative to premenopausal women, even with adjustment for age, race/ethnicity, medical conditions, BMI, smoking, and depressive symptoms.113 This study, unfortunately, did not also examine the impact of physical activity. However, a subsequent longitudinal SWAN analysis by this same investigator showed that higher baseline physical activity score was independently associated with a 7% increased likelihood of high physical role functioning and a 10% greater likelihood of a low bodily pain score.114 Interestingly, the association between physical activity and physical role functioning seemed to be mediated by level of pain.
Evidence also suggests that physical activity improves physical function, not only in the elderly,115,116 but also in the middle aged.117 In SWAN, an analysis of the association between menopausal status and functional limitations based on the cross-sectional survey reported that almost 20% of the women reported some or substantial physical functioning limitations, and that the likelihood of physical limitations was significantly greater among postmenopausal women.118 Although that analysis did not consider physical activity, a longitudinal follow-up of the SWAN women at the Chicago site observed that, although the transition through menopause was associated with a decline in grip and pinch strength, greater physical activity was the strongest predictor of increased grip and pinch strength.119
Finally, there is increasing evidence that physical activity enhances overall quality of life in the population as a whole as well as in patient populations, such as breast cancer survivors.120 In the SWAN cohort, physical activity at baseline was significantly associated with reduced risk of impairment in health-related quality of life, as measured by the SF-12 for all 5 of the domains (role—physical, bodily pain, vitality, role—emotional, and social function),111 as well as with lower risk of impairment in these domains over time,112 independent of BMI, race/ethnicity, and other factors. Importantly, a recent randomized trial of several different doses of exercise conducted among sedentary postmenopausal women found a strong dose–response relation between volume of exercise and improvements in mental and physical aspects of quality of life, measured by the SF-36, and this finding was not accounted for by weight change.121
On the other hand, it is important to note that sports and exercise can cause acute activity-related musculoskeletal injuries, especially in deconditioned individuals or those with chronic injuries.122 Obese individuals with knee osteoarthritis, for example, may experience transient increases in pain immediately after exercise that decreases later in the day to below initial levels.123 These observations suggest a U-shaped dose–response curve for physical activity and pain, in which moderate activity decreases pain, relative to no or low activity, whereas excessive activity (for the specific individual) results in increased pain. In working with individual patients, it is important for clinicians to keep this possible U-shaped curve in mind.
Mood
Another significant benefit of regular physical activity is enhanced mental health, including protection against the onset of depressive and anxiety symptoms and disorders, reductions in existing symptoms of depression, anxiety and distress, and enhanced feelings of well-being.1 This evidence comes both from population-based, prospective cohort studies, and from randomized clinical trials (for a review of specific studies, see the Report from Physical Activity Guidelines Advisory Committee1). Both psychological and physiologic mechanisms, including increased levels of neurotransmitters (specifically, dopamine and serotonin), and enhanced brain aminergic synaptic transmission,124 increased endorphin secretion,125 distraction from stressful stimuli,55 and improved self-efficacy and self-esteem, may be responsible for these observed protective effects.126
Many of the studies of physical activity and symptoms in midlife women discussed previously also examined associations between physical activity and a range of mood states. Unlike the evidence regarding physical activity and VMS, the observational studies consistently show that physical activity is directly related to positive mood, vigor, and general well-being11,12,15,105,107,127,128 and inversely related to negative symptoms, such as depression, anxiety, and perceived stress.11,23,105-107 Evidence also suggests that increasing physical activity may improve mood and well-being, not only in midlife women with clinically meaningful symptoms of depression and anxiety, but in the general population as well. In the Melbourne Women’s Midlife Health Project, change in physical activity over a 3-year follow-up period was positively related to change in well-being, although this was only a marginally significant finding.129 Similarly, in an intervention study of sedentary midlife African-American women using a community-based walking program, adherence to walking was associated with lower depressive symptoms.130 These observations are supported by randomized trials as well, which show that women in the exercise groups report improvements in mood symptoms, relative to controls.26,27,30,31
Still unaddressed is the issue of whether physical activity can attenuate the risk of depressive symptomatology or other negative mood states associated with the menopausal transition itself, particularly in susceptible women. In SWAN, within woman changes in menopausal status from premenopause onwards were independently associated with increased risk of a high level of depressive symptoms, suggestive of clinical depression, although the impact of these changes was of a lesser magnitude than that of various health-related and psychosocial factors, such as having VMS or experiencing stressful life events.131 Although not undertaken to date, SWAN is in a position to examine whether physical activity is another factor independently associated with depressive symptoms and whether it may act as either a mediator or moderator of the effect of change in menopausal status. Hopefully, SWAN will, in the future, provide this type of insight into how lifestyle behavior might diminish the risk of mood changes during the menopause.
PHYSICAL ACTIVITY AND RISK OF BREAST CANCER
Cancer, particularly breast cancer, which has an increase in incidence rate after menopause, relative to premenopause, is another adverse outcome relevant to perimenopausal women that may be positively influenced by regular physical activity. A large body of observational studies suggests that women who are regularly active have a 25% to 30% lower risk of developing postmenopausal breast cancer than women who are inactive.132 There is also a reduction in risk for premenopausal breast cancer, although the magnitude is less (about 20%–25%).132 In addition, a growing body of literature suggests that physical activity after a breast cancer diagnosis and treatment is associated with lower rates of recurrence133 and lower risk of all-cause mortality.134,135 Potential mechanisms that may account for these observations include lower levels of circulating endogenous hormones, such as estrogen, sex hormone–binding globulin, and insulin-like growth factors, better maintenance of energy balance, and enhanced immune function.136,137
Although the SWAN cohort is too small to provide adequate numbers of breast cancer cases for any meaningful study of the disease, a SWAN ancillary study used measures of mammographic density, a powerful risk factor for breast cancer itself, and perhaps an intermediate marker of the disease, to examine the effect of hormonal status and sociodemographic, reproductive, and lifestyle factors on breast cancer risk among perimenopausal women. Both percent density and area of density were inversely associated with physical activity in all domains (sports/exercise, lifestyle activity, and home/care-giving activity), except for occupational activity, but these relationships were not significant after multiple adjustment for confounding factors.138 Physical activity was also not associated with change in dense area over time, although it was inversely related to change in non-dense area.139 Together, these findings do not provide any strong support for the hypothesis that the reduction in breast cancer risk associated with physical activity operates through the mechanism of mammographic density.
CLINICAL AND PUBLIC HEALTH IMPLICATIONS
Based on the SWAN findings described, and on many, many other studies,1 physical activity is a potent tool for health promotion and disease prevention in perimenopausal women as well as in the population as a whole. Unfortunately, less than half of the population regularly participates in physical activity at even the minimal level required for health benefits, and adherence to physical activity guidelines is even lower among women ages 40 to 60.140 This proportion may be even lower, depending on how physical activity is assessed.141 This presents a clear mandate to clinicians and public health professionals alike. All clinicians should prescribe regular physical activity to their patients and should be prepared to discuss and problem solve with their patients the barriers that exist to becoming more physically active (for information and resources for the Exercise is Medicine initiative, go to www.exerciseismedicine.org). Public health professionals need to continue and enhance their ongoing efforts to promote physical activity among midlife women, particularly in women of minority race/ethnicity and women with disabilities and chronic health conditions. Even if regular physical activity does not prevent or treat VMS, the other health benefits that it confers on midlife women will ensure both a healthy menopausal transition and healthy aging.
References
- 1.US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Physical Activity Guidelines Advisory Committee Report. Washington (DC): US Department of Health and Human Services, Office of Disease Prevention and Health Promotion; 2008. [Google Scholar]
- 2.Gold EB, Sternfeld B, Kelsey JL, et al. Relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40-55 years of age. Am J Epidemiol. 2000;152:463–73. doi: 10.1093/aje/152.5.463. [DOI] [PubMed] [Google Scholar]
- 3.Gold EB, Block G, Crawford S, et al. Lifestyle and demographic factors in relation to vasomotor symptoms: baseline results from the Study of Women’s Health Across the Nation. Am J Epidemiol. 2004;159:1189–99. doi: 10.1093/aje/kwh168. [DOI] [PubMed] [Google Scholar]
- 4.Gold EB, Colvin A, Avis N, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: study of women’s health across the nation. Am J Public Health. 2006;96:1226–35. doi: 10.2105/AJPH.2005.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gold EB, Lasley B, Crawford SL, et al. Relation of daily urinary hormone patterns to vasomotor symptoms in a racially/ethnically diverse sample of midlife women: study of women’s health across the nation. Reprod Sci. 2007;14:786–97. doi: 10.1177/1933719107308613. [DOI] [PubMed] [Google Scholar]
- 6.Sternfeld B, Cauley J, Harlow S, et al. Assessment of physical activity with a single global question in a large, multi-ethnic sample of midlife women. Am J Epidemiol. 2000;152:678–87. doi: 10.1093/aje/152.7.678. [DOI] [PubMed] [Google Scholar]
- 7.Sternfeld B, Ainsworth BE, Quesenberry CP. Physical activity patterns in a diverse population of women. Prev Med. 1999;28:313–23. doi: 10.1006/pmed.1998.0470. [DOI] [PubMed] [Google Scholar]
- 8.Romani WA, Gallicchio L, Flaws JA. The association between physical activity and hot flash severity, frequency, and duration in mid-life women. Am J Hum Biol. 2009;21:127–9. doi: 10.1002/ajhb.20834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitcomb BW, Whiteman MK, Langenberg P, et al. Physical activity and risk of hot flashes among women in midlife. J Womens Health (Larchmt) 2007;16:124–33. doi: 10.1089/jwh.2006.0046. [DOI] [PubMed] [Google Scholar]
- 10.Aiello EJ, Yasui Y, Tworoger SS, et al. Effect of a yearlong, moderate-intensity exercise intervention on the occurrence and severity of menopause symptoms in postmenopausal women. Menopause. 2004;11:382–8. doi: 10.1097/01.gme.0000113932.56832.27. [DOI] [PubMed] [Google Scholar]
- 11.Collins A, Landgren BM. Reproductive health, use of estrogen and experience of symptoms in perimenopausal women: a population-based study. Maturitas. 1995;20:101–11. doi: 10.1016/0378-5122(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 12.Elavsky S, McAuley E. Physical activity, symptoms, esteem, and life satisfaction during menopause. Maturitas. 2005;52:374–85. doi: 10.1016/j.maturitas.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 13.Guthrie JR, Smith AMA, Dennerstein L, et al. Physical activity and the menopause experience. Maturitas. 1995;20:71–80. doi: 10.1016/0378-5122(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 14.Moilanen J, Aalto AM, Hemminki E, et al. Prevalence of menopause symptoms and their association with lifestyle among Finnish middle-aged women. Maturitas. 2010;67:368–74. doi: 10.1016/j.maturitas.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Daley A, MacArthur C, Stokes-Lampard H, et al. Exercise participation, body mass index, and health-related quality of life in women of menopausal age. Br J Gen Pract. 2007;57:130–5. [PMC free article] [PubMed] [Google Scholar]
- 16.Sternfeld B, Quesenberry CP, Jr, Husson G. Habitual physical activity and menopausal symptoms: a case-control study. J Womens Health. 1999;8:115–23. doi: 10.1089/jwh.1999.8.115. [DOI] [PubMed] [Google Scholar]
- 17.Armstrong BK, White E, Saracci R. Principles of Exposure Measurement in Epidemiology. Oxford: Oxford University Press; 1994. [Google Scholar]
- 18.Gold EB, Sternfeld B, Kelsey JL, et al. Relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40-55 years of age. Am J Epidemiol. 2000;152:463–73. doi: 10.1093/aje/152.5.463. [DOI] [PubMed] [Google Scholar]
- 19.Hyde RE, Inui TS, Kleinman K, et al. Differential association of modifiable health behaviors with hot flashes in perimenopausal and postmenopausal women. J Gen Intern Med. 2004;19:740–6. doi: 10.1007/s11606-004-0002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guthrie JR, Dennerstein L, Taffe JR, et al. Hot flushes during the menopause transition: a longitudinal study in Australian-born women. Menopause. 2005;12:460–7. doi: 10.1097/01.GME.0000155200.80687.BE. [DOI] [PubMed] [Google Scholar]
- 21.Thurston RC, Joffe H, Soares CN, et al. Physical activity and risk of vasomotor symptoms in women with and without a history of depression: results from the Harvard Study of Moods and Cycles. Menopause. 2006;13:553–60. doi: 10.1097/01.gme.0000227332.43243.00. [DOI] [PubMed] [Google Scholar]
- 22.van Poppel MN, Brown WJ. “It’s my hormones, doctor” — does physical activity help with menopausal symptoms? Menopause. 2008;15:78–85. doi: 10.1097/gme.0b013e31804b418c. [DOI] [PubMed] [Google Scholar]
- 23.Wilbur J, Miller AM, McDevitt J, et al. Menopausal status, moderate-intensity walking, and symptoms in midlife women. Res Theory Nurs Pract. 2005;19:163–80. [PubMed] [Google Scholar]
- 24.Li S, Holm K. Physical activity alone and in combination with hormone replacement therapy on vasomotor symptoms in postmenopausal women. West J Nurs Res. 2003;25:274–88. doi: 10.1177/0193945902250413. [DOI] [PubMed] [Google Scholar]
- 25.Huang AJ, Subak LL, Wing R, et al. An intensive behavioral weight loss intervention and hot flushes in women. Arch Intern Med. 2010;170:1161–7. doi: 10.1001/archinternmed.2010.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ueda M. A 12-week structured education and exercise program improved climacteric symptoms in middle-aged women. J Physiol Anthropol Appl Human Sci. 2004;23:143–8. doi: 10.2114/jpa.23.143. [DOI] [PubMed] [Google Scholar]
- 27.Elavsky S, McAuley E. Physical activity and mental health outcomes during menopause: a randomized controlled trial. Ann Behav Med. 2007;33:132–42. doi: 10.1007/BF02879894. [DOI] [PubMed] [Google Scholar]
- 28.Lindh-Astrand L, Nedstrand E, Wyon Y, et al. Vasomotor symptoms and quality of life in previously sedentary postmenopausal women randomised to physical activity or estrogen therapy. Maturitas. 2004;48:97–105. doi: 10.1016/S0378-5122(03)00187-7. [DOI] [PubMed] [Google Scholar]
- 29.Moriyama CK, Oneda B, Bernardo FR, et al. A randomized, placebo-controlled trial of the effects of physical exercises and estrogen therapy on health-related quality of life in postmenopausal women. Menopause. 2008;15:613–8. doi: 10.1097/gme.0b013e3181605494. [DOI] [PubMed] [Google Scholar]
- 30.Villaverde-Gutierrez C, Araujo E, Cruz F, et al. Quality of life of rural menopausal women in response to a customized exercise programme. J Adv Nurs. 2006;54:11–9. doi: 10.1111/j.1365-2648.2006.03784.x. [DOI] [PubMed] [Google Scholar]
- 31.McAndrew LM, Napolitano MA, Albrecht A, et al. When, why and for whom there is a relationship between physical activity and menopause symptoms. Maturitas. 2009;64:119–25. doi: 10.1016/j.maturitas.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Kemmler W, Lauber D, Weineck J, et al. Benefits of 2 years of intense exercise on bone density, physical fitness, and blood lipids in early postmenopausal osteopenic women: results of the Erlangen Fitness Osteoporosis Prevention Study (EFOPS) Arch Intern Med. 2004;164:1084–91. doi: 10.1001/archinte.164.10.1084. [DOI] [PubMed] [Google Scholar]
- 33.Miller HG, Li RM. Measuring hot flashes: summary of a National Institutes of Health workshop. Mayo Clin Proc. 2004;79:777–81. doi: 10.4065/79.6.777. [DOI] [PubMed] [Google Scholar]
- 34.Dunn A, Reigle T, Youngstedt S, et al. Brain norepinephrine and metabolites after treadmill training and wheel running in rats. Med Sci Sports Exerc. 1996;28 doi: 10.1097/00005768-199602000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Dunn AL, Dishman RK. Exercise and the neurobiology of depression. Exerc Sports Sci Rev. 1991;19:41–98. [PubMed] [Google Scholar]
- 36.Rouveix M, Duclos M, Gouarne C, et al. The 24 h urinary cortisol/cortisone ratio and epinephrine/norepinephrine ratio for monitoring training in young female tennis players. Int J Sports Med. 2006;27:856–63. doi: 10.1055/s-2006-923778. [DOI] [PubMed] [Google Scholar]
- 37.Sandercock GR, Bromley PD, Brodie DA. Effects of exercise on heart rate variability: inferences from meta-analysis. Med Sci Sports Exerc. 2005;37:433–9. doi: 10.1249/01.mss.0000155388.39002.9d. [DOI] [PubMed] [Google Scholar]
- 38.Jurca R, Church TS, Morss GM, et al. Eight weeks of moderate-intensity exercise training increases heart rate variability in sedentary postmenopausal women. Am Heart J. 2004;147:e21. doi: 10.1016/j.ahj.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 39.Jackson DM, Reilly JJ, Kelly LA, et al. Objectively measured physical activity in a representative sample of 3- to 4-year-old children. Obes Res. 2003;11:420–5. doi: 10.1038/oby.2003.57. [DOI] [PubMed] [Google Scholar]
- 40.Swartzman LC, Edelberg R, Kemmann E. Impact of stress on objectively recorded menopausal hot flashes and on flush report bias. Health Psychol. 1990;9:529–45. doi: 10.1037//0278-6133.9.5.529. [DOI] [PubMed] [Google Scholar]
- 41.Thurston RC, Blumenthal JA, Babyak MA, et al. Emotional antecedents of hot flashes during daily life. Psychosom Med. 2005;67:137–46. doi: 10.1097/01.psy.0000149255.04806.07. [DOI] [PubMed] [Google Scholar]
- 42.Rebar RW, Spitzer IB. The physiology and measurement of hot flushes. Am J Obstet Gynecol. 1987;156:1284–8. doi: 10.1016/0002-9378(87)90165-7. [DOI] [PubMed] [Google Scholar]
- 43.Berntson GG, Bigger JT, Jr, Eckberg DL. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–48. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- 44.Watkins LL, Grossman P, Krishnan R, et al. Anxiety and vagal control of heart rate. Psychosom Med. 1998;60:498–502. doi: 10.1097/00006842-199807000-00018. [DOI] [PubMed] [Google Scholar]
- 45.Boecker H, Sprenger T, Spilker ME, et al. The runner’s high: opioidergic mechanisms in the human brain. Cereb Cortex. 2008;18:2523–31. doi: 10.1093/cercor/bhn013. [DOI] [PubMed] [Google Scholar]
- 46.Harber VJ, Sutton JR, MacDougall JD, et al. Plasma concentrations of beta-endorphin in trained eumenorrheic and amenorrheic women. Fertil Steril. 1997;67:648–53. doi: 10.1016/s0015-0282(97)81361-1. [DOI] [PubMed] [Google Scholar]
- 47.Heitkamp HC, Huber W, Scheib K. Beta-endorphin and adrenocorticotrophin after incremental exercise and marathon running—female responses. Eur J Appl Physiol Occup Physiol. 1996;72:417–24. doi: 10.1007/BF00242270. [DOI] [PubMed] [Google Scholar]
- 48.Simpkins JW, Katovich MJ, Song IC. Similarities between morphine withdrawal in the rat and the menopausal hot flush. Life Sci. 1983;32:1957–66. doi: 10.1016/0024-3205(83)90047-4. [DOI] [PubMed] [Google Scholar]
- 49.Simpkins JW, Katovich MJ. In: An animal model for pharmacologic evaluation of the menopausal hot flush. Notelovitz M, van Keep P, editors. Boston: MTP Press Ltd.; 1985. pp. 213–51. [Google Scholar]
- 50.Lightman SL, Jacobs HS, Maguire AK, et al. Climacteric flushing: clinical and endocrine response to infusion of naloxone. Br J Obstet Gynaecol. 1981;88:919–24. doi: 10.1111/j.1471-0528.1981.tb02229.x. [DOI] [PubMed] [Google Scholar]
- 51.DeFazio J, Verheugen C, Chetkowski R, et al. The effects of naloxone on hot flashes and gonadotropin secretion in postmenopausal women. J Clin Endocrinol Metab. 1984;58:578–81. doi: 10.1210/jcem-58-3-578. [DOI] [PubMed] [Google Scholar]
- 52.Tepper R, Neri A, Kaufman H, et al. Menopausal hot flushes and plasma B-endorphins. Obstet Gynecol. 1987;70:150–2. [PubMed] [Google Scholar]
- 53.Genazzani AR, Petraglia F, Facchinetti F, et al. Increase of proopiomelanocortin-related peptides during subjective menopausal flushes. Am J Obstet Gynecol. 1984;149:775–9. doi: 10.1016/0002-9378(84)90121-2. [DOI] [PubMed] [Google Scholar]
- 54.Dishman RK, O’Connor PJ. Lessons in exercise neurobiology: the case of endorphins. Ment Health Phys Act. 2009;2:4–9. [Google Scholar]
- 55.Leith LM. Foundations of exercise and mental health. Morgantown (WV): Fitness Information Technology, Inc; 1994. [Google Scholar]
- 56.Freedman RR, Krell W. Reduced thermoregulatory null zone in postmenopausal women with hot flashes. Am J Obstet Gynecol. 1999;181:66–70. doi: 10.1016/s0002-9378(99)70437-0. [DOI] [PubMed] [Google Scholar]
- 57.Lewis CE, Jacobs DR, Jr, McCreath H, et al. Weight gain continues in the 1990s: 10-year trends in weight and overweight from the CARDIA study. Coronary Artery Risk Development in Young Adults. Am J Epidemiol. 2000;151:1172–81. doi: 10.1093/oxfordjournals.aje.a010167. [DOI] [PubMed] [Google Scholar]
- 58.Lewis CE, Smith DE, Wallace DD, et al. Seven-year trends in body weight and associations with lifestyle and behavioral characteristics in black and white young adults: the CARDIA study. Am J Public Health. 1997;87:635–42. doi: 10.2105/ajph.87.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–7. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 60.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 61.Sternfeld B, Wang H, Quesenberry CP, Jr, et al. Physical activity and changes in weight and waist circumference in midlife women: findings from the Study of Women’s Health Across the Nation. Am J Epidemiol. 2004;160:912–22. doi: 10.1093/aje/kwh299. [DOI] [PubMed] [Google Scholar]
- 62.Owens JF, Matthews KA, Wing RR, et al. Can physical activity mitigate the effects of aging in middle-aged women? Circulation. 1992;85:1265–70. doi: 10.1161/01.cir.85.4.1265. [DOI] [PubMed] [Google Scholar]
- 63.Sammel MD, Grisso JA, Freeman EW, et al. Weight gain among women in the late reproductive years. Fam Pract. 2003;20:401–9. doi: 10.1093/fampra/cmg411. [DOI] [PubMed] [Google Scholar]
- 64.Guthrie JR, Dennerstein L, Dudley EC. Weight gain and the menopause: a 5-year prospective study. Climacteric. 1999;2:205–11. doi: 10.3109/13697139909038063. [DOI] [PubMed] [Google Scholar]
- 65.Macdonald HM, New SA, Campbell MK, et al. Longitudinal changes in weight in perimenopausal and early postmenopausal women: effects of dietary energy intake, energy expenditure, dietary calcium intake and hormone replacement therapy. Int J Obes Relat Metab Disord. 2003;27:669–76. doi: 10.1038/sj.ijo.0802283. [DOI] [PubMed] [Google Scholar]
- 66.Wing RR, Matthews KA, Kuller LH, et al. Weight gain at the time of menopause. Arch Intern Med. 1991;151:97–102. [PubMed] [Google Scholar]
- 67.Matthews KA, Abrams B, Crawford S, et al. Body mass index in mid-life women: relative influence of menopause, hormone use, and ethnicity. Int J Obes Relat Metab Disord. 2001;25:863–73. doi: 10.1038/sj.ijo.0801618. [DOI] [PubMed] [Google Scholar]
- 68.Sternfeld B, Bhat AK, Wang H, et al. Menopause, physical activity, and body composition/fat distribution in midlife women. Med Sci Sports Exerc. 2005;37:1195–202. doi: 10.1249/01.mss.0000170083.41186.b1. [DOI] [PubMed] [Google Scholar]
- 69.Wang Q, Hassanger C, Ravn P, et al. Total and regional body-composition changes in early postmenopausal women: age-related or menopause-related. Am J Clin Nutr. 1994;60:843–8. doi: 10.1093/ajcn/60.6.843. [DOI] [PubMed] [Google Scholar]
- 70.Pasquali R, Casimirri F, Labate AM, et al. Body weight, fat distribution, and the menopausal status in women. the VMH collaborative group. Int J Obes Relat Metab Disord. 1994;19:614–21. [PubMed] [Google Scholar]
- 71.Toth MJ, Tchernof A, Sites CK, et al. Effect of menopausal status on body composition and abdominal fat distribution. Int J Obes Relat Metab Disord. 2000;24:226–31. doi: 10.1038/sj.ijo.0801118. [DOI] [PubMed] [Google Scholar]
- 72.Ley CJ, Lees B, Stevenson JC. Sex- and menopause-associated changes in body-fat distribution. Am J Clin Nutr. 1992;55:950–4. doi: 10.1093/ajcn/55.5.950. [DOI] [PubMed] [Google Scholar]
- 73.Zamboni M, Armellini F, Milani MP, et al. Body fat distribution in pre- and post-menopausal women: metabolic and anthropometric variables and their inter-relationships. Int J Obes Relat Metab Disord. 1992;16:495–504. [PubMed] [Google Scholar]
- 74.Sowers M, Zheng H, Tomey K, et al. Changes in body composition in women over six years at midlife: ovarian and chronological aging. J Clin Endocrinol Metab. 2007;92:895–901. doi: 10.1210/jc.2006-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.DiPietro L. Physical Activity, Body Weight, and Adiposity: An Epidemiologic Perspective. Baltimore: Williams & Wilkins; 1995. [PubMed] [Google Scholar]
- 76.Sutton-Tyrrell K, Zhao X, Santoro N, et al. Reproductive hormones and obesity: 9 years of observation from the Study of Women’s Health Across the Nation. Am J Epidemiol. 2010;171:1203–13. doi: 10.1093/aje/kwq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dugan SA, Everson-Rose SA, Karavolos K, et al. Physical activity and reduced intra-abdominal fat in midlife African-American and white women. Obesity (Silver Spring) 2010;18:1260–5. doi: 10.1038/oby.2009.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brown WJ, Williams L, Ford JH, et al. Identifying the energy gap: magnitude and determinants of 5-year weight gain in midage women. Obes Res. 2005;13:1431–41. doi: 10.1038/oby.2005.173. [DOI] [PubMed] [Google Scholar]
- 79.Lee IM, Djousse L, Sesso HD, et al. Physical activity and weight gain prevention. JAMA. 2010;303:1173–9. doi: 10.1001/jama.2010.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Institute of Medicine. Dietary Reference Intake for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients) Washington DC: National Academies Press; 2002. [DOI] [PubMed] [Google Scholar]
- 81.American College of Sports Medicine position stand. Osteoporosis and exercise. Med Sci Sports Exerc. 1995;27:i–vii. [PubMed] [Google Scholar]
- 82.Kelley G. Aerobic exercise and lumbar spine bone mineral density in postmenopausal women: a meta-analysis. J Am Geriatr Soc. 1998;46:143–52. doi: 10.1111/j.1532-5415.1998.tb02530.x. [DOI] [PubMed] [Google Scholar]
- 83.Palombaro KM. Effects of walking-only interventions on bone mineral density at various skeletal sites: a meta-analysis. 2005;28:102–7. doi: 10.1519/00139143-200512000-00006. [DOI] [PubMed] [Google Scholar]
- 84.Kelley GA, Kelley KS, Tran ZV. Exercise and bone mineral density in men: a meta-analysis. J Appl Physiol. 2000;88:1730–6. doi: 10.1152/jappl.2000.88.5.1730. [DOI] [PubMed] [Google Scholar]
- 85.Martyn-St James M, Carroll S. Progressive high-intensity resistance training and bone mineral density changes among premenopausal women: evidence of discordant site-specific skeletal effects. Sports Med. 2006;36:683–704. doi: 10.2165/00007256-200636080-00005. [DOI] [PubMed] [Google Scholar]
- 86.Martyn-St James M, Carroll S. High-intensity resistance training and postmenopausal bone loss: a meta-analysis. Osteoporos Int. 2006;17:1225–40. doi: 10.1007/s00198-006-0083-4. [DOI] [PubMed] [Google Scholar]
- 87.McAteer ME, Niziolek PJ, Ellis SN, et al. Mechanical stimulation and intermittent parathyroid hormone treatment induce disproportional osteogenic, geometric, and biomechanical effects in growing mouse bone. Calcif Tissue Int. 2010;86:389–96. doi: 10.1007/s00223-010-9348-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Beck BR, Shaw J, Snow CM. Menopause. 2. San Diego: Academic Press; 2001. Physical activity and osteoporosis. [Google Scholar]
- 89.Snow-Harter C, Bouxsein ML, Lewis BT, et al. Effects of resistance and endurance exercise on bone mineral status of young women: a randomized exercise intervention trial. J Bone Miner Res. 1992;7:761–9. doi: 10.1002/jbmr.5650070706. [DOI] [PubMed] [Google Scholar]
- 90.Lohman T, Going S, Pamenter R, et al. Effects of resistance training on regional and total bone mineral density in premenopausal women: a randomized prospective study. J Bone Miner Res. 1995;10:1015–24. doi: 10.1002/jbmr.5650100705. [DOI] [PubMed] [Google Scholar]
- 91.Friedlander AL, Genant HK, Sadowsky S, et al. A two-year program of aerobics and weight training enhances bone mineral density of young women. J Bone Miner Res. 1995;10:574–85. doi: 10.1002/jbmr.5650100410. [DOI] [PubMed] [Google Scholar]
- 92.Kelley GA, Kelley KS, Tran ZV. Exercise and lumbar spine bone mineral density in postmenopausal women: a meta-analysis of individual patient data. J Gerontol A Biol Sci Med Sci. 2002;57:M599–M604. doi: 10.1093/gerona/57.9.m599. [DOI] [PubMed] [Google Scholar]
- 93.Kelley GA. Exercise and regional bone mineral density in postmenopausal women: a meta-analytic review of randomized trials. Am J Phys Med Rehabil. 1998;77:76–87. [PubMed] [Google Scholar]
- 94.Wolff I, van Croonenborg JJ, Kemper HC, et al. The effect of exercise training programs on bone mass: a meta-analysis of published controlled trials in pre- and postmenopausal women. Osteoporos Int. 1999;9:1–12. doi: 10.1007/s001980050109. [DOI] [PubMed] [Google Scholar]
- 95.Wallace BA, Cumming RG. Systematic review of randomized trials of the effect of exercise on bone mass in pre- and postmenopausal women. Calcif Tissue Int. 2000;67:10–8. doi: 10.1007/s00223001089. [DOI] [PubMed] [Google Scholar]
- 96.Greendale GA, Huang MH, Wang Y, et al. Sport and home physical activity are independently associated with bone density. Med Sci Sports Exerc. 2003;35:506–12. doi: 10.1249/01.MSS.0000056725.64347.C9. [DOI] [PubMed] [Google Scholar]
- 97.Morseth B, Emaus N, Wilsgaard T, et al. Leisure time physical activity in adulthood is positively associated with bone mineral density 22 years later. The Tromso study. Eur J Epidemiol. 2010;25:325–31. doi: 10.1007/s10654-010-9450-8. [DOI] [PubMed] [Google Scholar]
- 98.Rikkonen T, Salovaara K, Sirola J, et al. Physical activity slows femoral bone loss but promotes wrist fractures in postmenopausal women: a 15-year follow-up of the OSTPRE study. J Bone Miner Res. 2010;25:2332–40. doi: 10.1002/jbmr.143. [DOI] [PubMed] [Google Scholar]
- 99.Gudmundsdottir SL, Oskarsdottir D, Indridason OS, et al. Risk factors for bone loss in the hip of 75-year-old women: a 4-year follow-up study. Maturitas. 2010;67:256–61. doi: 10.1016/j.maturitas.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 100.Puntila E, Kroger H, Lakka T, et al. Leisure-time physical activity and rate of bone loss among peri- and postmenopausal women: a longitudinal study. Bone. 2001;29:442–6. doi: 10.1016/s8756-3282(01)00597-x. [DOI] [PubMed] [Google Scholar]
- 101.Tervo T, Nordstrom P, Neovius M, et al. Reduced physical activity corresponds with greater bone loss at the trabecular than the cortical bone sites in men. Bone. 2009;45:1073–8. doi: 10.1016/j.bone.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 102.Yasaku K, Ishikawa-Takata K, Koitaya N, et al. One-year change in the second metacarpal bone mass associated with menopause nutrition and physical activity. J Nutr Health Aging. 2009;13:545–9. doi: 10.1007/s12603-009-0105-y. [DOI] [PubMed] [Google Scholar]
- 103.Avis NE, Brockwell S, Colvin A. A universal menopausal syndrome? Am J Med. 2005;118(Suppl 12B):37–46. doi: 10.1016/j.amjmed.2005.09.057. [DOI] [PubMed] [Google Scholar]
- 104.Sherman S, Miller H, Nerurkar L, et al. Research opportunities for reducing the burden of menopause-related symptoms. Am J Med. 2005;118(Suppl 12B):166–71. doi: 10.1016/j.amjmed.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 105.Nelson DB, Sammel MD, Freeman EW, et al. Effect of physical activity on menopausal symptoms among urban women. Med Sci Sports Exerc. 2008;40:50–8. doi: 10.1249/mss.0b013e318159d1e4. [DOI] [PubMed] [Google Scholar]
- 106.Slaven L, Lee C. Mood and symptom reporting among middle-aged women: the relationship between menopausal status, hormone replacement therapy, and exercise participation. Health Psychol. 1997;16:203–8. doi: 10.1037//0278-6133.16.3.203. [DOI] [PubMed] [Google Scholar]
- 107.Dennerstein L, Smith AMA, Morse C. Psychological well-being, mid-life and the menopause. Maturitas. 1994;20:1–11. doi: 10.1016/0378-5122(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 108.Dunn AL, Trivedi MH, Kampert JB, et al. Exercise treatment for depression: efficacy and dose response. Am J Prev Med. 2005;28:1–8. doi: 10.1016/j.amepre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 109.Hughes SL, Seymour RB, Campbell R, et al. Impact of the fit and strong intervention on older adults with osteoarthritis. Gerontologist. 2004;44:217–28. doi: 10.1093/geront/44.2.217. [DOI] [PubMed] [Google Scholar]
- 110.Rainville J, Hartigan C, Martinez E, et al. Exercise as a treatment for chronic low back pain. Spine J. 2004;4:106–15. doi: 10.1016/s1529-9430(03)00174-8. [DOI] [PubMed] [Google Scholar]
- 111.Avis NE, Ory M, Matthews KA, et al. Health-related quality of life in a multiethnic sample of middle-aged women: Study of Women’s Health Across the Nation (SWAN) Med Care. 2003;41:1262–76. doi: 10.1097/01.MLR.0000093479.39115.AF. [DOI] [PubMed] [Google Scholar]
- 112.Avis NE, Colvin A, Bromberger JT, et al. Change in health-related quality of life over the menopausal transition in a multiethnic cohort of middle-aged women: Study of Women’s Health Across the Nation. Menopause. 2009;16:860–9. doi: 10.1097/gme.0b013e3181a3cdaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dugan SA, Powell LH, Kravitz HM, et al. Musculoskeletal pain and menopausal status. Clin J Pain. 2006;22:325–31. doi: 10.1097/01.ajp.0000208249.07949.d5. [DOI] [PubMed] [Google Scholar]
- 114.Dugan SA, Everson-Rose SA, Karavolos K, et al. The impact of physical activity level on SF-36 role-physical and bodily pain indices in midlife women. J Phys Act Health. 2009;6:33–42. doi: 10.1123/jpah.6.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kaplan GA, Strawbridge WJ, Camacho T, et al. Factors associated with change in physical functioning in the elderly: a six year prospective study. Journal of Aging and Health. 1993;5:140–53. [Google Scholar]
- 116.Sternfeld B, Ngo L, Satariano WA, et al. Associations of body composition with physical performance and self-reported functional limitation in elderly men and women. Am J Epidemiol. 2002;156:110–21. doi: 10.1093/aje/kwf023. [DOI] [PubMed] [Google Scholar]
- 117.Huang Y, Macera CA, Blair SN, et al. Physical fitness, physical activity, and functional limitation in adults aged 40 and older. Med Sci Sports Exerc. 1998;30:1430–5. doi: 10.1097/00005768-199809000-00013. [DOI] [PubMed] [Google Scholar]
- 118.Sowers M, Pope S, Welch G, et al. The association of menopause and physical functioning in women at midlife. J Am Geriatr Soc. 2001;49:1485–92. doi: 10.1046/j.1532-5415.2001.4911241.x. [DOI] [PubMed] [Google Scholar]
- 119.Kurina LM, Gulati M, Everson-Rose SA, et al. The effect of menopause on grip and pinch strength: results from the Chicago, Illinois, site of the Study of Women’s Health Across the Nation. Am J Epidemiol. 2004;160:484–91. doi: 10.1093/aje/kwh244. [DOI] [PubMed] [Google Scholar]
- 120.Mandelblatt JS, Luta G, Kwan ML, et al. Associations of physical activity with quality of life and functional ability in breast cancer patients during active adjuvant treatment: the Pathways Study. Breast Cancer Res Treat. 2011 Apr 8; doi: 10.1007/s10549-011-1483-5. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Martin CK, Church TS, Thompson AM, et al. Exercise dose and quality of life: a randomized controlled trial. Arch Intern Med. 2009;169:269–78. doi: 10.1001/archinternmed.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Garrick JG, Requa RK. Sports and fitness activities: the negative consequences. J Am Acad Orthop Surg. 2003;11:439–43. doi: 10.5435/00124635-200311000-00008. [DOI] [PubMed] [Google Scholar]
- 123.Focht BC, Ewing V, Gauvin L, et al. The unique and transient impact of acute exercise on pain perception in older, overweight, or obese adults with knee osteoarthritis. Ann Behav Med. 2002;24:201–10. doi: 10.1207/S15324796ABM2403_05. [DOI] [PubMed] [Google Scholar]
- 124.Weicker H, Struder HK. Influence of exercise on serotonergic neuromodulation in the brain. Amino Acids. 2001;20:35–47. doi: 10.1007/s007260170064. [DOI] [PubMed] [Google Scholar]
- 125.Janal MN, Colt EW, Clark WC, et al. Pain sensitivity, mood and plasma endocrine levels in man following long-distance running: effects of naloxone. Pain. 1984;19:13–25. doi: 10.1016/0304-3959(84)90061-7. [DOI] [PubMed] [Google Scholar]
- 126.Paluska SA, Schwenk TL. Physical activity and mental health: current concepts. Sports Med. 2000;29:167–80. doi: 10.2165/00007256-200029030-00003. [DOI] [PubMed] [Google Scholar]
- 127.Wilbur J, Holm K, Dan A. The relationship of energy expenditure to physical and psychologic symptoms in women at midlife. Nurs Outlook. 1992;40:269–76. [PubMed] [Google Scholar]
- 128.Wilbur J, Dan A, Hedricks C, et al. The relationship among menopausal status, menopausal symptoms, and physical activity in midlife women. Fam Community Health. 1990;13:67–78. [Google Scholar]
- 129.Guthrie JR, Dudley EC, Dennerstein L, et al. Changes in physical activity and health outcomes in a population-based cohort of mid-life Australian-born women. Aust N Z J Public Health. 1997;21:682–7. doi: 10.1111/j.1467-842x.1997.tb01780.x. [DOI] [PubMed] [Google Scholar]
- 130.Holm K, Dan A, Wilbur J, et al. A longitudinal study of bone density in midlife women. Health Care Women Int. 2002;23:678–91. doi: 10.1080/07399330290107421. [DOI] [PubMed] [Google Scholar]
- 131.Bromberger JT, Schott LL, Kravitz HM, et al. Longitudinal change in reproductive hormones and depressive symptoms across the menopausal transition: results from the Study of Women’s Health Across the Nation (SWAN) Arch Gen Psychiatry. 2010;67:598–607. doi: 10.1001/archgenpsychiatry.2010.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sternfeld B, Lee I-M. Physical activity and cancer: the evidence, the issues and the challenges. In: Lee I-M, editor. Physical Activity and Health Epidemiologic Methods and Studies. New York: Oxford University Press; 2009. [Google Scholar]
- 133.Holmes MD, Chen WY, Feskanich D, et al. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479–86. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 134.Sternfeld B, Weltzien E, Quesenberry CP, Jr, et al. Physical activity and risk of recurrence and mortality in breast cancer survivors: findings from the LACE study. Cancer Epidemiol Biomarkers Prev. 2009;18:87–95. doi: 10.1158/1055-9965.EPI-08-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Irwin ML, McTiernan A, Manson JE, et al. Physical activity and survival in postmenopausal women with breast cancer: results from the Women’s Health Initiative. Cancer Prev Res (Phila) 2011;4:522–9. doi: 10.1158/1940-6207.CAPR-10-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.McTiernan A, Ulrich C, Slate S, et al. Physical activity and cancer etiology: associations and mechanisms. Cancer Causes Control. 1998;9:487–509. doi: 10.1023/a:1008853601471. [DOI] [PubMed] [Google Scholar]
- 137.Lee I-M. Exercise and physical health: cancer and immune function. Res Q Exerc Sport. 1995;66:286–91. doi: 10.1080/02701367.1995.10607913. [DOI] [PubMed] [Google Scholar]
- 138.Oestreicher N, Capra A, Bromberger J, et al. Physical activity and mammographic density in a cohort of midlife women. Med Sci Sports Exerc. 2008;40:451–6. doi: 10.1249/MSS.0b013e31815f5b47. [DOI] [PubMed] [Google Scholar]
- 139.Conroy SM, Butler LM, Harvey D, et al. Physical activity and change in mammographic density: the Study of Women’s Health Across the Nation. Am J Epidemiol. 2010;171:960–8. doi: 10.1093/aje/kwq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Centers for Disease Control and Prevention (CDC). Prevalence of physical activity, including lifestyle activities among adults—United States, 2000-2001. MMWR Morb Mortal Wkly Rep. 2003;52:764–9. [PubMed] [Google Scholar]
- 141.Troiano RP, Berrigan D, Dodd KW, et al. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–8. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]


