Abstract
Verapamil (VRP), a cardiovascular pharmaceutical widely distributed and persistent in the aquatic environment, has potential toxicity to fish and other aquatic organisms. However, the molecular mechanisms that lead to these toxic effects are not well known. In the present study, proteomic analysis has been performed to investigate the protein patterns that are differentially expressed in liver of rainbow trout exposed to sublethal concentrations of VRP (0.5, 27.0, and 270 μg/liter) for 42 days. Two-dimensional electrophoresis coupled with MALDI-TOF/TOF mass spectrometry was employed to detect and identify the protein profiles. The analysis revealed that the expression of six hepatic acidic proteins were markedly altered in the treatment groups compared with the control group; three proteins especially were significantly down-regulated in fish exposed to VRP at environmental related concentration (0.5 μg/liter). These results suggested that the VRP induce mechanisms against oxidative stress (glucose-regulated protein 78 and 94 and protein disulfide-isomerase A3) and adaptive changes in ion transference regulation (calreticulin, hyperosmotic glycine-rich protein). Furthermore, for the first time, protein Canopy-1 was found to be significantly down-regulated in fish by chronic exposure to VRP at environmental related levels. Overall, our work supports that fish hepatic proteomics analysis serves as an in vivo model for monitoring the residual pharmaceuticals in aquatic environment and can provide valuable insight into the molecular events in VRP-induced toxicity in fish and other organisms.
Over the past few decades, potential risks have been discovered with pharmaceutically derived chemicals in the aquatic environment. Certain pharmaceuticals, developed to promote human health and well being, are now attracting attention as a potentially new class of water pollutants (1). Such drugs as antibiotics, anti-depressants, and birth control pills have been detected in varied water sources (2). Verapamil (VRP),1 is an L-type calcium channel blocker of the phenylalkylamine class. It has been used in the treatment of hypertension, cardiac arrhythmia, and most recently, cluster headaches. During the last decade, the occurrence of VRP has also been reported in aquatic environments at concentrations ranged from 0.058 to 0.9 μg/liter (3, 4). Moreover, it has already been reported that VRP induced toxic effects on aquatic animals (e.g. Daphnia magna) after treatment at low doses (5). However, there is a lack of information about the toxic effects in fish. Fish have proven to be a better medium than flowing water to detect the impact and mechanisms of action of various contaminants on aquatic animals and human beings (6).
In our previous studies, the rainbow trout (Oncorhynchus mykiss) was used as a model system to study the toxic effects of VRP on biochemical and physiological responses. Our results showed that treatment with sublethal concentrations of VRP significantly changed the physiological and biochemical responses (including behavior changes, morphological indices, hematological parameters, and antioxidant responses) (7, 8). On the other hand, there was no significant change in all of the parameters measured in fish exposed to VRP at environmental related concentration (7).
Environmental stress has been shown to affect patterns of protein expression in fish (9); thus we hypothesize that protein expression profiles of VRP-exposed rainbow trout will differ from unexposed individuals. To measure this, we used proteomic approaches to get an insight on protein expression profiles in the liver of rainbow trout. The rainbow trout is one of the most extensively researched and characterized species of fish. In addition, its low tolerance to poor water quality has established it as one of the most useful sentinel species for aquatic toxicology. Moreover, the liver is the central organ in detoxifying of xenobiotics and processing metabolic products for degradation (10). Therefore, to increase our understanding of the biological response of rainbow trout liver, we studied the changes in hepatic proteome after exposure to VRP.
Proteomic methods have recently been employed to get deep knowledge about the toxicity and mechanisms of action of several toxicants, such as pentachlorophenol in rare minnow (Gobiocypris rarus) (11), microcystin in medaka (Oryzias latipes) (12), and Microcystin-LR in livers of zebrafish (Danio rerio) (13). In contrast to conventional biochemical methods, the proteomic approach offers great potential in identifying proteins involved in the response of organisms to contaminants through massive comparison of protein expression profiles and can help to identify novel and unbiased biomarkers related to toxicity (9).
The main objective of this study was to identify differentially expressed proteins in the livers of rainbow trout chronically exposed at different concentrations of VRP, which could be used as biomarkers for monitoring levels of exposure to VRP in contaminated water resources. We anticipate that this information will provide novel information about the chronic toxicity of VRP to rainbow trout and identify new protein biomarkers that can help us to increase our understanding of toxicity mechanisms in aquatic organisms and human beings.
EXPERIMENTAL PROCEDURES
Chemicals
Verapamil and other chemicals were obtained from Sigma-Aldrich. The VRP was dissolved in pure distilled water to make a stock solution at a concentration of 50 mg/ml.
Animals and Exposure
Juvenile rainbow trout weighing 40.43 ± 2.55 g (means ± S. D.) were obtained from a local commercial hatchery (Husinec, Czech Republic). They were held in aquariums containing 250 liters of freshwater continuously aerated to maintain dissolved oxygen values at 7.5–8.0 mg/liter. Temperature was 15 ± 1 °C, and pH was 7.4 ± 0.2. The photoperiod was a 12 h:12 h light-dark cycle. Fish were acclimatized for 14 days before the beginning of the experiment and were fed with commercial fish food (47% protein and 26% fat; BioMar Denmark). The fish were starved for 24 h prior to experimentation to avoid prandial effects during the assay.
Prior to the experiment, a semi-static system composed of eight aquariums (200 liters each) was used. The 40 fish were randomly distributed in the aquariums.
The nominal concentrations of VRP were used as follows:
E1 group, environmental related concentration, 0.5 μg/liter.
E2 group, 1% 96 h of LC50 of VRP in rainbow trout (8), 27.0 μg/liter.
E3 group, 10% 96 h of LC50 of VRP in rainbow trout, 270 μg/liter.
A control group exposed to clean freshwater.
Each experimental condition was duplicated. The fish were fed daily with commercial fish pellets at 1% of total body weight at a fixed time, and the extra food was removed. The exposure solution was renewed each day after 2 h of feeding to maintain the appropriate concentration of VRP and water quality. The treatment period in all experimental groups was 42 days. At the end of treatment, six fish from each aquarium were randomly sampled, and the livers were immediately frozen and stored at −80 °C.
To ensure agreement between nominal and actual compound concentrations in the aquariums, water samples were analyzed during the experimental period by LC-MS/MS. Water samples were collected from the test aquariums after 1 and 24 h of renewing the test solutions. The mean concentration of VRP in the water samples was always within 20% of the intended concentration (the measured concentrations of VRP in the water samples were 0.47 ± 0.05, 26.18 ± 1.36, and 251.33 ± 19.81 μg/liter corresponding to the nominal concentrations 0.5, 27.0, and 270 μg/liter).
Protein Sample Preparation
The 0.05–0.1 g of frozen tissue was immersed in lysis buffer (40 mm Tris, 8 m urea, 2 m thiourea, 2% Chaps, 3 mm EDTA, 50 mm DTT, 1 mm PMSF, and 5 μg/ml leupeptin) and treated by ultrasonication using (BANDELIN Sonopuls; HD2070). Then the suspension was centrifuged at 16,000 × g for 25 min to remove the cellular debris. After that, the protein supernatants were collected and stored at −20 °C for proteomics analysis. Protein levels were estimated spectrophotometrically by the method of Bradford (14) using bovine serum albumin as a standard.
Two-dimensional Polyacrylamide Gel Electrophoresis
Isoelectric focusing was performed using PROTEAN® IEF (Bio-Rad). For liver samples, 100 μg of protein in a total volume of 125 μl of rehydration buffer (7 m urea, 2 m thiourea, 2% Chaps, 50 mm DTT, 0.4% IPG buffer) was applied to 7-cm IPG strips (pH range was 4–7 and 7–10, respectively). Electrical current conditions for the separation were set up as follows: passive rehydration for 14 h; isoelectric focusing at 200 V for 1 h (gradient), 500 V for 1 h (gradient), 1000 V for 1 h (gradient), 4000 V for 1 h (gradient), and 4000 V for 2 h (rapid). After isoelectric focusing, the IPG strips were equilibrated in the first step with a solution containing 6 m urea, 29.3% glycerol, 2% SDS, 75 mm Tris-HCl, pH 8.8, and 2% (w/v) DTT for 15 min, and in the second step with a solution containing 2.5% (w/v) iodacetamide instead of DTT for another 15 min. Each IPG strip was laid onto a 12% SDS-PAGE gel for second dimension electrophoresis. Protein spots were visualized by Coomassie Brilliant Blue R-250 (AppliChem, Darmstadt, Germany) staining.
The stained gels were scanned and analyzed by nonlinear two-dimensional software. Average gels for each group were derived from three replicates. Protein spots were detected using automated routines from the software combined with manual editing to remove the artifacts. Spot abundance was determined by the area of the spot multiplied by the density and referred to as the spot volume. For each gel, normalized spot volumes were calculated as the ratio of each spot volume to total spot volume in the gel. The criteria for determination of differential expression of proteins in each group were as follows: 1) the protein spots were up- or down-regulated significantly (p < 0.05, ANOVA) with the statistic analysis, and the changes were consistent in the three replicate analyses; and 2) protein spots appeared or disappeared in experimental groups compared with control. In addition, spots were analyzed with adjusted spot filtration setting, by which spots with normalized volumes smaller than 0.05 were excluded.
In-gel Digestion and Mass Spectrometry
Spots of interest were excised from gels stained by Coomassie Brilliant Blue R-250, cut into small cubes (approximately 1 mm3), and destained using 0.1 m 4-ethylmorpholine acetate (pH 8.1) in 50% acetonitrile. After complete destaining, the gel was washed with water, dehydrated in acetonitrile, and rehydrated in water. The gel was partially dried using a SpeedVac concentrator and subsequently reconstituted with cleavage buffer containing 25 mm 4-ethylmorpholine acetate, 10% acetonitrile, and sequencing grade trypsin (Promega; 50 ng/μl). Digestion was carried out overnight at 37 °C. The resulting peptides were extracted with 40% MeCN, 0.4% acetic acid. Following extraction, the peptides were subjected directly to mass spectrometric analysis.
The mass spectra were measured on a MALDI-TOF/TOF mass spectrometer ultraFLEX III (Bruker-Daltonics, Bremen, Germany) equipped with a nitrogen laser (337 nm). The spectra were calibrated externally using the monoisotopic [M+H]+ ion of peptide standards PepMix II (Bruker-Daltonics). A 5 mg/ml solution of α-cyano-4-hydroxy-cinnamic acid in 50% MeCN, 0.3% acetic acid was used as a MALDI matrix. A 0.5-μl droplet of the sample was loaded onto the target. The droplet was allowed to dry at ambient temperature and overlaid with 0.4 μl of matrix solution. The positive MALDI-TOF and MS/MS LIFT spectra of selected ions were collected, peak lists were generated manually (using SNAP peak detection algorithm, S/N threshold higher 10, TopHat baseline subtraction in Bruker Daltonics flexAnalysis 3.0), and all of the matrix peaks, human keratin, and trypsin autoproteolytic peptides were removed. The obtained mass lists were searched against a NCBInr 20100904 protein database subset of the Chordata taxonomy group (11,744,690 sequences; 4,010,973,687 residues) using peptide mass fingerprinting MascotTM software (http://www.matrixscience.com/) with the following settings: enzyme chemistry - trypsin, missed cleavages 1, fixed carbamidomethyl modification of cysteine, variable single oxidation of methionine, peptide charge state 1+, and peptide mass tolerance ± 40 ppm. Mascot probability-based score is −10*Log(P), where P is the probability that the observed match is a random event, and a score greater than the recorded significant hit value was significant (p < 0.05). The MS/MS peak lists were searched against a NCBInr 20100910 protein database subset of the Chordata taxonomy group (11,759,209 sequences; 4,015,568,644 residues) using MS/MS ion search MascotTM software with the following settings: enzyme chemistry - trypsin, missed cleavages 2, fixed carbamidomethyl modification of cysteine, variable single oxidation of methionine, precursor charge state 1+, its mass tolerance ± 30 ppm, and fragment mass tolerance ± 0.6 Da. All of the obtained results of protein identification were interpreted in conjunction with the molecular weight and pI of proteins in the gels.
Statistical Tests
All of the measurements were replicated at least three times, and the data were expressed as mean values ± standard deviation. Statistical analysis was carried out using one-way ANOVA to evaluate whether the means were significantly different among the groups. Significant differences were indicated at p < 0.05. Prior to one-way ANOVA, the data were log-transformed to meet ANOVA assumptions of normality and homoscedasticity of variance.
RESULTS
Protein Expression
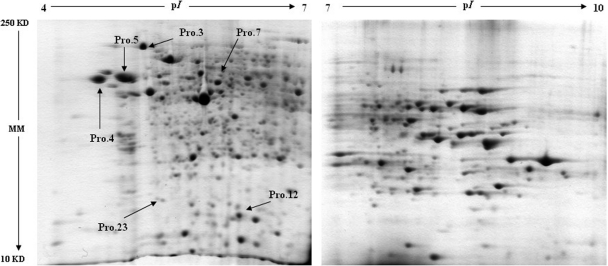
The two-dimensional PAGE gels of VRP-exposed and nonexposed rainbow trout livers are shown in Fig. 1, and quantitative spot comparisons were made with nonlinear two-dimensional software. On average, over 800 acidic protein spots (pI 4–7) and over 300 basic protein spots (pI 7–10) were detected in gels. Compared with the control, a total of six acidic protein spots from the VRP-exposed fish were observed to significantly alter in abundance. Moreover, none of the basic protein spots were significantly changed in accordance with the criterion.
Fig. 1.
Representative two-dimensional gels of rainbow trout liver protein. Protein spots marked by arrows were found to be differentially expressed as a result of VRP treatment. The molecular masses (MM) and pI scales are indicated. The corresponding number is the spot reference number.
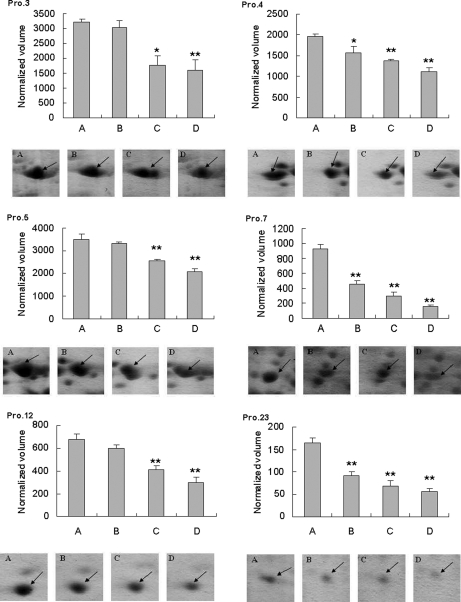
The all altered acidic proteins (Pro.3, 4, 5, 7, 12, and 23) were significantly down-regulated (p < 0.01) in fish exposed to VRP with high concentrations (27.0 and 270 μg/liter). Interestingly, the environmental related concentration (0.5 μg/liter) significantly altered expression pattern of three protein spots (Pro.4, 7, and 23). The quantitative comparisons of detected spots and the summary of significant differences observed between experimental groups are shown in Fig. 2. Protein spots that showed significant differences (p < 0.05) between treatment groups and control were selected for identification.
Fig. 2.
Changes in abundance of hepatic protein spots in fish among test groups. Protein identification is shown in Tables I and II. A, control group; B, VRP treatment group at 0.5 μg/liter; C, VRP treatment group at 27.0 μg/liter; D, VRP treatment group at 270 μg/liter. *, significant difference was p < 0.01 with one-way ANOVA; **, significant differences was p < 0.001 with one-way ANOVA.
Protein Identification
The selected hepatic acidic protein spots were cut from the gels and digested using trypsin protease, and the resulting mixtures of peptides were directly subjected to MALDI-TOF MS. Four of the six proteins were identified by the mass fingerprinting approach (Table I). These proteins were identified by their sequence similarities to the following proteins: glucose-regulated protein 78 (Pro.3), Calreticulin (Pro.4), Glucose-regulated protein 94 (Pro.5), and protein disulfide-isomerase A3 precursor (Pro.7).
Table I. Protein identification by mass fingerprinting approach.
| Spot no. | Protein name (organisms) | Accession no. | Molecular mass (kDa) | pI | Mascot scorea | Significant hit | Sequence coverage (%) | Matched/searched peptides |
|---|---|---|---|---|---|---|---|---|
| 3 | Glucose-regulated protein 78 (Oncorhynchus mykiss) | gi 60223019 | 70 | 5.02 | 234 | 73< | 36 | 21/29 |
| 4 | Calreticulin precursor (Salmo salar) | gi 209148412 | 48 | 4.33 | 205 | 73< | 49 | 16/23 |
| Calreticulin (Oncorhynchus mykiss) | gi 185134556 | 48 | 4.39 | 89 | 73< | 33 | 9/23 | |
| 5 | Glucose-regulated protein 94 (Oncorhynchus mykiss) | gi 303324549 | 91 | 4.69 | 206 | 73< | 28 | 20/21 |
| 7 | Protein disulfide-isomerase A3 precursor (Salmo salar) | gi 209153384 | 55 | 5.46 | 98 | 73< | 23 | 12/19 |
a Mascot score (probability-based score) is −10*Log(P), where P is the probability that the observed match is a random event. Scores greater than recorded significant hit were significant (p < 0.05). MALDI-TOF peak lists were searched against a NCBInr 20100904 protein database subset of the Chordata taxonomy group using MascotTM software with the following settings: enzyme chemistry - trypsin, missed cleavages 1, carbamidomethyl modification of cysteine, variable single oxidation of methionine, and peptide mass tolerance ± 40 ppm.
Moreover, the MS/MS ion search approach has been used in cases of Pro.3, 4, and 7 to assign unmatched m/z base peak signals to corroborate previous identification or to find another protein masked under the first significant protein identification by the MS fingerprinting method. The positive results, corroborating previous identification, are shown in Table II. A further two proteins not identified by mass fingerprinting were examined with the MS/MS ion search MascotTM tool from selected m/z signals (Table II). Pro.12 and 23 were identified as hyperosmotic glycine-rich protein (Pro.12) and Canopy-1 precursor (Pro.23), respectively. Among all of the indentified protein spots, Pro.3, 4, and 5 are present in O. mykiss, Pro.7 is present in Salmo salar or Gallus gallus, and the other two proteins are found in Salmo salar.
Table II. Protein identification by MS/MS ion search approach.
| Spot no. | Protein name (organisms) | Accession no. | Molecular mass (kDa) | pI | Mascot scorea | Significant het | Sequence | MS/MS (m/z) |
|---|---|---|---|---|---|---|---|---|
| MS fingerprinting | ||||||||
| 3 | Glucose-regulated protein 78 (Oncorhynchus mykiss) | gi 60223019 | 69 | 5.02 | 210 | 41< | K.VMEDSDLKKTDIDEI | 2520.290 |
| VLVGGSTR.I | ||||||||
| 4 | Calreticulin (Oncorhynchus mykiss) | gi 185134556 | 48 | 4.39 | 75 | 40< | K.KPEDWDDRPK.I | 1285.619 |
| 139 | 52< | A.TVYFKEQFQDGDAWK.S | 1861.875 | |||||
| 7 | Protein disulfide-isomerase A3 precursor (Gallus gallus) | gi 45383890 | 56 | 5076 | 117 | 52< | R.GFPTIYFAPAGKK.Q | 1396.721 |
| MS/MS ion search Mascot tool | ||||||||
| 12 | Hyperosmotic glycine rich protein (Salmo salar) | gi 185133178 | 21 | 8.88 | 65 | 36< | R.SYGGGGGGR.S | 767.343 |
| 160 | 33< | K.YDNPEDAKDAMDAMNGQSLDGR.T | 2413.011 | |||||
| 142 | 39< | K.LFVGGLSFDTTEQSLAEAFSK.Y | 2247.117 | |||||
| 23 | Canopy-1 precursor (Salmo salar) | gi 209737392 | 21 | 4.93 | 62 | 44< | K.TIHVGGFR.L | 886.503 |
| 243 | 41< | R.SSDAGDFPDFNNFKFDGPEGSNALK.F | 2676.192 | |||||
a Mascot score (probability-based score) is −10*Log(P), where P is the probability that the observed match is a random event. Scores greater than the recorded significant hit were significant (p < 0.05). The MS/MS peak lists were searched against a NCBInr 20100910 protein database subset of the Chordata taxonomy group using MascotTM software with the following settings: enzyme chemistry - trypsin, missed cleavages 2, carbamidomethyl modification of cysteine, variable single oxidation of methionine, peptide mass tolerance ± 30 ppm, and fragment mass tolerance ± 0.6 Da.
DISCUSSION
The exposure of organisms to sublethal levels of environmental pollution has been shown to trigger the cellular accumulation of proteins that mainly act as molecular chaperones (15, 16). Recent proteomic studies in the environmental monitoring field have aimed to decipher changes in protein expression patterns and to identify proteins governing the mechanism of toxicity of environmental xenobiotics. To our knowledge, this is the first study to apply a proteomics approach for the identification of fish liver proteins that are regulated in response to the exposure of Ca2+ antagonist (VRP). The results of the present study suggest that the fish hepatic proteome was significantly altered after exposure to VRP at environmental related concentrations. Moreover, the proteins that have been significantly affected in VRP treatment groups (Tables I and II) are involved in various processes such as immunity, redox signaling, and ion transference regulation.
Exposing cells to environmental stress induces expression of stress proteins in various intracellular compartments including the cytoplasm and the endoplasmic reticulum (ER) (17, 18). Among them, the glucose-regulated proteins (GRPs), a family of molecular chaperones and Ca2+-binding stress proteins located in ER, are regulated by environmental stress (19). Regulation of GRPs by ER stress could be altered as a response to a wide variety of natural, experimental, or anthropogenic stress, such as heat shock, oxidative stress, heavy metals, and organic contaminants (20–22). Thus multiple stress proteins may be important in the cellular tolerance response. In this study, the abundance of GRP78 (glucose-regulated protein 78) and GRP94 (glucose-regulated protein 94) was significantly decreased in the livers of fish exposed to higher concentrations of VRP, which indicates the VRP-induced ER stress. Moreover, our previous results showed that long term exposure of VRP induces oxidative stress in fish liver and other tissues as well (7). Similarly, some previous studies reported that GRPs were involved in the tolerance response of oxidative stress, Ca2+ disturbances, and cell death (23). Therefore, the possible explanation for the depression of these two proteins is that VRP-induced reactive oxygen species causes alterations in ER homeostasis and serves as a stress signal activating ER stress genes such as GRP78/BiP (immunoglobulin chain-binding protein) and GRP94, which is consistent with other reports (24–26).
Proteins that traverse the secretory pathway typically depend on disulfide bonds for their maturation and function, and these bonds are often crucial for the stability of a final protein structure (27). ER stress is also induced by high levels of misfolded proteins accumulation, which generates the unfolded protein response. The unfolded protein response results in the regulation of chaperones such as GRP58 and PDI to prevent protein aggregation and cell death (28, 29), which has been proved by many previous studies (30–32). In the present study, the inhibition of PDIA3 expression in all VRP-treated groups shows that the ER stress is present in fish hepatic cells. In particularly, this protein was down-regulated in fish exposed to VRP at environmental related concentrations, which indicated that the hepatic PDIA3 plays a role in an early adaptive response in VRP-induced ER stress. Moreover, the observed dose-dependent alterations of PDIA3 expression suggest that PDIA3 may have a protective function in the cellular stress response to VRP, in addition to its isomerase activities (33, 34). According to other reports, the PDI and GRP78 are induced by ER stress caused by interference with Ca2+ homeostasis, inhibition of disulfide bond formation of protein glycosylation, and oxidative stress to rescue the accumulation of misfolded or unfolded proteins with the ER (35). These results support our finding that the expression of PDIA3 and GRP78 was significantly induced by VRP treatment, suggesting that ER is the target for oxidative stress.
Calreticulin, a resident protein of ER, is a multifunctional protein, binding Ca2+ ions (a second messenger in signal transduction) and participating in transcription regulation. Decreased expression of calreticulin should lead to altered levels of intracellular calcium (36). Some reports suggested that calreticulin has been involved in buffering cytotoxic calcium (37). Furthermore, Poli (38) found that the suppression of calreticulin expression is linked to increased expression of C/EBP proteins, which are critical transcription factors for the inflammatory response in organism to the external stress. Because VRP, the chemical used in this study, is a calcium channel blocker, it is not surprising to find that calreticulin expression was significantly inhibited in the livers of all VRP-treated fish, especially at the lowest concentration (the environmental related level). However, despite the conservation of critical promoter elements, Kales et al. (39) reported that calcium homeostasis antagonists (e.g. A23187 and thapsigargin) do not appear to enhance the expression of rainbow trout calreticulin in vitro as demonstrated in mammals. Therefore, further investigations are required to elucidate the different mechanisms of calcium-mediated ER stress response in fish and mammals, while providing further insight into the regulation of this important protein.
In the ecotoxicological field, very few studies have described a possible induction of the hyperosmotic glycine-rich protein by toxicants. To our knowledge, only Connon et al. (40) have reported that hyperosmotic glycine-rich protein was significantly down-regulated by exposure to esfenvalerate in smelt larvae. In the present study, hyperosmotic glycine-rich protein was identified as significantly down-regulated by 42 days of exposure to higher concentrations of VRP in fish liver. Osmoregulation is physiologically controlled by chemical messages from the endocrine system, along with cell signaling and nerve transmission (40). The Ca2+ antagonists have been suggested to induce osmotic imbalances in fish (41) that are linked to effects on ATPase activity responsible for maintaining the sodium transmembrane electrochemical gradient (42). In this study, the decreased expression of this hyperosmotic glycine-rich protein may be directly caused by conditions affecting endocrine regulation in fish after VRP exposure.
Canopy-1 is a novel regulator of fibroblast growth factor (43). In the present study, this protein was significantly depressed in fish liver under VRP-induced stress. To our knowledge, the present study is the first report of Canopy-1 being altered in aquatic organisms by an external toxicant. However, the reason is not clear and needs more a detailed investigation in the future.
In summary, the present study demonstrated that environmentally relevant exposure to verapamil significantly modified the liver proteome of rainbow trout. Alterations in protein expression in fish liver provide some novel information of VRP toxicity, but the exact mechanisms behind the cellular stress responses are still to be identified. Therefore, further investigation of gene expression profile or immunobiology using specific antibodies could bring more insight into the molecular mechanisms of stress responses after verapamil exposure. However, the changes observed in this study have to be taken into account when estimating the toxicological hazard of pharmaceutically derived chemicals in the aquatic environment. Our study also indicates that fish hepatic proteome can be used as a good model for monitoring residual pharmaceuticals in aquatic environment.
Footnotes
* This work was supported by the CENAQUA CZ.1.05/2.1.00/01.0024; Grant agency of the Czech Republic GACR P503/11/1130, Grant agency of University of South Bohemia GAJU 047/2010/Z and 007/2010/Z, and Grant agency of the Czech Academy of Science GAAV KJB608030901; Czech National Agency for Agricultural Research Grant QH92308; the institutional research concept AV0Z50200510 (Institute of Microbiology); and Special Fund for Agro-scientific Research in the Public Interest of China Grant 200903048. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
1 The abbreviations used are:
- VRP
- verapamil
- GRP
- glucose-regulated protein
- ER
- endoplasmic reticulum
- PDI
- protein disulfide isomerase
- Chaps
- 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid
- ANOVA
- analysis of variance
- Pro.
- protein
- MM
- molecular mass.
REFERENCES
- 1. Fent K., Weston A. A., Caminada D. (2006) Ecotoxicology of human pharmaceuticals. Aquat. Toxicol. 76, 122–159 [DOI] [PubMed] [Google Scholar]
- 2. Li Z. H., Randak T. (2009) Residual pharmaceutically active compounds (PhACs) in aquatic environment—status, toxicity and kinetics: A review. Vet. Med.(Praha) 52, 295–314 [Google Scholar]
- 3. Al-Rifai J. H., Gabelish C. L., Schäfer A. I. (2007) Occurrence of pharmaceutically active and non-steroidal estrogenic compounds in three different wastewater recycling schemes in Australia. Chemosphere 69, 803–815 [DOI] [PubMed] [Google Scholar]
- 4. Khan S. J., Ongerth J. E. (2004) Modelling of pharmaceutical residues in Australian sewage by quantities of use and fugacity calculations. Chemosphere 54, 355–367 [DOI] [PubMed] [Google Scholar]
- 5. Villegas-Navarro A., Rosas-L E., Reyes J. L. (2003) The heart of Daphnia magna: Effects of four cardioactive drugs. Comp. Biochem. Physiol. C 136, 127–134 [DOI] [PubMed] [Google Scholar]
- 6. Arai T., Ikemoto T., Hokura A., Terada Y., Kunito T., Tanabe S., Nakai I. (2004) Chemical forms of mercury and cadmium accumulated in marine mammals and seabirds as determined by XAFS analysis. Enviro. Sci. Technol. 38, 6468–6474 [DOI] [PubMed] [Google Scholar]
- 7. Li Z. H., Velisek J., Zlabek V., Grabic R., Machova J., Kolarova J., Li P., Randak T. (2011) Chronic toxicity of verapamil on juvenile rainbow trout (Oncorhynchus mykiss): Effects on morphological indices, hematological parameters and antioxidant responses. J. Hazard. Mater. 185, 870–880 [DOI] [PubMed] [Google Scholar]
- 8. Li Z. H., Li P., Randak T. (2010) Ecotoxocological effects of short-term exposure to a human pharmaceutical verapamil in juvenile rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. C 152, 385–391 [DOI] [PubMed] [Google Scholar]
- 9. Dowling V. A., Sheehan D. (2006) Proteomics as a route to identification of toxicity targets in environmental toxicology. Proteomics 6, 5597–5604 [DOI] [PubMed] [Google Scholar]
- 10. Li Z. H., Zlabek V., Li P., Grabic R., Velisek J., Machova J., Randak T. (2010) Biochemical and physiological responses in liver and muscle of rainbow trout after long-term exposure to propiconazole. Ecotoxicol. Environ. Saf. 73, 1391–1396 [DOI] [PubMed] [Google Scholar]
- 11. Fang Y., Gao X., Zha J., Ning B., Li X., Gao Z., Chao F. (2010) Identification of differential hepatic proteins in rare minnow (Gobiocypris rarus) exposed to pentachlorophenol (PCP) by proteomic analysis. Toxicol. Lett. 199, 69–79 [DOI] [PubMed] [Google Scholar]
- 12. Mezhoud K., Praseuth D., Puiseux-Dao S., François J. C., Bernard C., Edery M. (2008) Global quantitative analysis of protein expression and phosphorylation status in the liver of the medaka fish (Oryzias latipes) exposed to microcystin-LR: I. Balneation study. Aquat. Toxicol. 86, 166–175 [DOI] [PubMed] [Google Scholar]
- 13. Wang M., Chan L. L., Si M., Hong H., Wang D. (2010) Proteomic analysis of hepatic tissue of zebrafish (Danio rerio) experimentally exposed to chronic Microcystin-LR. Toxicol. Sci. 113, 60–69 [DOI] [PubMed] [Google Scholar]
- 14. Bradford M. M. (1976) Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein dye binding. Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 15. Ellis R. J., van der Vies S. M. (1991) Molecular chaperones. Annu. Rev. Biochem. 60, 321–347 [DOI] [PubMed] [Google Scholar]
- 16. Feder M. E., Hofmann G. E. (1999) Heat-shock proteins, molecular chaperones, and the stress response: Evolutionary and ecological physiology. Annu. Rev. Physiol. 61, 243–282 [DOI] [PubMed] [Google Scholar]
- 17. Lindquist S. (1986) The heat shock response. Annu. Rev. Biochem. 55, 1151–1191 [DOI] [PubMed] [Google Scholar]
- 18. Welch W. J. (1992) Mammalian stress response: Cell physiology, structure function of stress proteins, and implications for medicine and disease. Physiol. Rev. 72, 1063–1081 [DOI] [PubMed] [Google Scholar]
- 19. Lee A. S. (1992) Mammalian stress response: induction of the glucose-regulated protein family. Curr. Opin. Cell Biol. 4, 267–273 [DOI] [PubMed] [Google Scholar]
- 20. Fulladosa E., Delmas F., Jun L., Villaescusa I., Murat J. C. (2002) Cellular stress induced in cultured human cells by exposure to sludge extracts from water treatment plants. Ecotoxicol. Environ. Saf. 53, 134–140 [DOI] [PubMed] [Google Scholar]
- 21. Delaney M. A., Klesius P. H. (2004) Hypoxic conditions induce Hsp70 production in blood, brain and head kidney of juvenile Nile tilapia Oreochromis niloticus (L.). Aquaculture 236, 633–644 [Google Scholar]
- 22. Sanders B. M., Martin L. S. (1993) Stress proteins as biomarkers of contaminant exposure in archived environmental samples. Sci. Total Environ. 139–140, 459–470 [DOI] [PubMed] [Google Scholar]
- 23. Liu H., Bowes R. C., 3rd, van de Water B., Sillence C., Nagelkerke J. F., Stevens J. L. (1997) Endoplasmic reticulum chaperones GRP78 and calreticulin prevent oxidative stress, Ca2+ disturbances, and cell death in renal epithelial cells. J. Biol. Chem. 272, 21751–21759 [DOI] [PubMed] [Google Scholar]
- 24. Schroder H. C., Efremova S. M., Margulis B. A., Guzhova I. V., Itskovich V. B., Muller W. E. (2006) Stress response in Baikalian sponges exposed to pollutants. Hydrobiologia 568, 277–287 [Google Scholar]
- 25. Martin S. A., Vilhelmsson O., Médale F., Watt P., Kaushik S., Houlihan D. F. (2003) Proteomic sensitivity to dietary manipulations in rainbow trout. Biochim. Biophys. Acta 1651, 17–29 [DOI] [PubMed] [Google Scholar]
- 26. Cohen S. D., Pumford N. R., Khairallah E. A., Boekelheide K., Pohl L. R., Amouzadeh H. R., Hinson J. A. (1997) Selective protein covalent binding and target organ toxicity. Toxicol. Appl. Pharmacol. 143, 1–12 [DOI] [PubMed] [Google Scholar]
- 27. Tu B. P., Weissman J. S. (2004) Oxidative protein folding in eukaryotes: Mechanisms and consequences. J. Cell Biol. 164, 341–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yoshimura F. K., Luo X. X. (2007) Induction of endoplasmic reticulum stress in thymic lymphocytes by the envelope precursor polyprotein of a murine leukemia virus during the preleukemic period. J. Virol. 81, 4374–4377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gillardin V., Silvestre F., Dieu M., Delaive E., Raes M., Thomé J. P., Kestemont P. (2009) Protein expression profiling in the African clawed frog Xenopus laevis tadpoles exposed to the polychlorinated biphenyl mixture Aroclor 1254. Mol. Cell. Proteomics 8, 596–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pastorelli R., Carpi D., Campagna R., Airoldi L., Pohjanvirta R., Viluksela M., Hakansson H., Boutros P. C., Moffat I. D., Okey A. B., Fanelli R. (2006) Differential expression profiling of the hepatic proteome in a rat model of dioxin resistance. Mol. Cell. Proteomics 5, 882–894 [DOI] [PubMed] [Google Scholar]
- 31. Oberemm A., Meckert C., Brandenburger L., Herzig A., Lindner Y., Kalenberg K., Krause E., Ittrich C., Kopp-Schneider A., Stahlmann R., Richter-Reichhelm H. B., Gundert-Remy U. (2005) Differential signatures of protein expression in marmoset liver and thymus induced by single-dose TCDD treatment. Toxicology 206, 33–48 [DOI] [PubMed] [Google Scholar]
- 32. Chen C. Y., Jia J. H., Zhang M. X., Meng Y. S., Kong D. X., Pan X. L., Yu X. P. (2005) Proteomic analysis on multi-drug resistant cells HL-60/DOX of acute myeloblastic leukemia. Chin. J. Physiol. 48, 115–120 [PubMed] [Google Scholar]
- 33. Kim-Han J. S., O'Malley K. L. (2007) Cell stress induced by the parkinsonian mimetic, 6-hydroxydopamine, is concurrent with oxidation of the chaperone, ERp57, and aggresome formation. Antioxid. Redox Signal. 9, 2255–2264 [DOI] [PubMed] [Google Scholar]
- 34. Huang T. S., Olsvik P. A., Krøvel A., Tung H. S., Torstensen B. E. (2009) Stress-induced expression of protein disulfide isomerase associated 3 (PDIA3) in Atlantic salmon (Salmo salar L.). Comp. Biochem. Physiol. B 154, 435–442 [DOI] [PubMed] [Google Scholar]
- 35. Hendershot L. M. (2004) The ER chaperone BiP is a master regulator of ER function. Mt. Sinai J. Med. 71, 289–297 [PubMed] [Google Scholar]
- 36. Merchant M., Kinney C., Sanders P. (2009) Differential protein expression in alligator leukocytes in response to bacterial lipopolysaccharide injection. Comp Biochem. Phys D 4, 300–304 [DOI] [PubMed] [Google Scholar]
- 37. Ruddat V. C., Whitman S., Klein R. D., Fischer S. M., Holman T. R. (2005) Evidence for downregulation of calcium signaling proteins in advanced mouse adenocarcinoma. Prostate 64, 128–138 [DOI] [PubMed] [Google Scholar]
- 38. Poli V. (1998) The role of C/EBP isoforms in the control of inflammatory and native immunity functions. J. Biol. Chem. 273, 29279–29282 [DOI] [PubMed] [Google Scholar]
- 39. Kales S. C., Bols N. C., Dixon B. (2007) Calreticulin in rainbow trout: A limited response to endoplasmic reticulum (ER) stress. Comp. Biochem. Physiol. B 147, 607–615 [DOI] [PubMed] [Google Scholar]
- 40. Connon R. E., Geist J., Pfeiff J., Loguinov A. V., D'Abronzo L. S., Wintz H., Vulpe C. D., Werner I. (2009) Linking mechanistic and behavioral responses to sublethal esfenvalerate exposure in the endangered delta smelt; Hypomesus transpacificus (Fam. Osmeridae). BMC Genomics 10, 608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang L., Wang W. X. (2007) Waterborne cadmium and zinc uptake in a euryhaline teleost Acanthopagrus schlegeli acclimated to different salinities. Aquat. Toxicol. 84, 173–181 [DOI] [PubMed] [Google Scholar]
- 42. Pedersen T. V., Bjerregaard P. (1995) Calcium and cadmium fluxes across the gills of the shore crab, Carcinus maenas. Mar. Pollut. Bull. 31, 73–77 [Google Scholar]
- 43. Hirate Y., Okamoto H. (2006) Canopy1, a novel regulator of FGF signaling around the midbrain-hindbrain boundary in zebrafish. Curr. Biol. 16, 421–427 [DOI] [PubMed] [Google Scholar]