Abstract
High levels of prostaglandin E2 (PGE2) synthesis resulting from the upregulation of COX-2 has been shown to be critical for the development of non-melanoma skin tumors. This effect of PGE2 is likely mediated by one or more of its 4 G-protein coupled membrane receptors, EP1–4. A previous study showed that BK5.EP1 transgenic mice produced more carcinomas than wild type (WT) mice using initiation/promotion protocols, although the tumor response was dependent on the type of tumor promoter used. In this study, a single topical application of either 7,12-dimethylbenz[a]anthracene (DMBA) or benzo[a]pyrene (B[a]P), alone, was found to elicit squamous cell carcinomas (SCC) in the BK5.EP1 transgenic mice, but not in WT mice. While the epidermis of both WT and transgenic mice was hyperplastic several days after DMBA, this effect regressed in the WT mice while proliferation continued in the transgenic mice. Several parameters associated with carcinogen initiation were measured and were found to be similar between genotypes, including CYP1B1 and aromatase expression, B[a]P adduct formation, Ras activity and keratinocyte stem cell numbers. However, EP1 transgene expression elevated COX-2 levels in the epidermis and SCC could be completely prevented in DMBA-treated BK5.EP1 mice either by feeding the selective COX-2 inhibitor celecoxib in their diet or by crossing them onto a COX-2 null background. These data suggest that the tumor promoting/progressing effects of EP1 require the PGE2 synthesized by COX-2.
Keywords: Prostaglandin E2, EP1 receptor, skin carcinogensis, COX-2
INTRODUCTION
Extensive epidemiological studies show non-steroidal anti-inflammatory drugs (NSAIDs), which are cyclooxygenase (COX) inhibitors, reduce the risk of several types of cancer including colorectal, esophageal, lung, breast, bladder and prostate cancer [1–3]. Two isoforms of COX have been identified, i.e., COX-1 and COX-2. COX-1 is constitutively expressed in most cells and tissues and maintains normal physiological functions such as mucosal integrity. COX-2, the inducible isoform, is an immediate-early response gene that is not normally expressed in most tissues but is induced by mitogens, hypoxia, growth factors such as epidermal growth factor and platelet derived growth factor, hormones, tumor promoters, polysaccharides, and inflammatory stimuli [4–6]. In many epithelial tumors, including colon, gastric, cervix, liver, pancreas, esophagus, breast, bladder, lung and skin, COX-2 is constitutively upregulated [7–10].
The importance of COX-2 to cancer development has been shown in experimental animal models as well as in clinical studies [7,11–20]. Following ultraviolet light (UV) exposure, COX-2 is transiently upregulated in murine and human keratinocytes in vitro and in vivo and is consitutively upregulated in nonmelanoma skin tumors resulting from either UV exposure or chemical carcinogens [21–24]. In a 7,12-dimethylbenz[a]anthracene (DMBA)/12-O-tetradecanoylphorbol 13-acetate (TPA) skin carcinogenesis model, both COX-1 null mice and COX-2 null mice had significantly reduced tumor development [25]. Administration of COX inhibitors reduced DMBA/TPA as well as UV-induced tumor formation [24,26,27]. Consistent with these findings, transgenic mice that overexpress COX-2 in the epidermis develop more skin tumors than wild type mice [28,29].
PGE2 is the major COX-derived arachidonic acid metabolite in cultured mouse skin epidermal cells [30], human normal colorectal mucosa and human colorectal adenoma from familial adenomatous polyposis patients [31] and mouse mammary tissue [32]. In human and murine keratinocytes, the PGE2 level is increased after UVB exposure [23,26]. There are several studies that show that PGE2 is important in epithelial tumorigenesis. Celecoxib, a COX-2 selective inhibitor, inhibited human head and neck xenograft tumor growth with concomitant inhibition of PGE2 production by the tumor [33]. Administration of PGE2 reversed NSAID-mediated suppression of intestinal tumor formation in APCMin mice [34] and enhanced azoxymethane-induced colon cancer formation in rats [35]. Injection of an antibody against PGE2 decreased intestinal tumor formation in APCMin mice [34] and tumor growth in a head and neck squamous cell carcinoma (SCC) xenograft model [33]. PGE2 was also shown to abolish indomethacin– mediated growth inhibition of malignant keratinocytes [36].
PGE2 binds four subtypes of membrane receptors (EP1, EP2, EP3 and EP4) that are members of the family of seven-transmembrane G protein-coupled receptors. EP2 and EP4 are coupled to Gs and increase cAMP while EP3, which is coupled to Gi, reduces cAMP levels. PGE2 activation of the EP1 receptor raises the level of inositol-1,4,5-trisphosphate and cytosolic calcium, leading to the activation of protein kinase C (PKC) [37,38]. The EP2 and EP4 receptors have been shown to contribute significantly to the development of tumors of the skin and colon [39–41] while the EP3 receptor does not appear to be involved in skin carcinogenesis [42]. Several studies have suggested that the EP1 receptor, which is the most highly upregulated PGE2 receptor in nonmelanoma murine and human skin cancer, contributes to skin cancer development. After UV treatment of mice, the number of EP1-positive cells was reported to be increased in skin [43]. We recently showed that the mRNA level for EP1 increased significantly after either a single or multiple treatments with TPA or UV [44]. We and others have also shown that in DMBA/TPA and UVB-induced mouse papillomas and SCCs, and human SCCs, EP1 mRNA expression and protein levels were increased ~2- to 10-fold relative to untreated normal mouse skin and adjacent non-tumor bearing human skin [44–46]. Additionally, topical application of ONO-8713, an EP1 antagonist, reduced UV-induced skin tumor formation and UV-induced inflammatory markers, such as skin thickness, myeloperoxidase activity, PGE2 content and the number of p53-positive cells [43]. Finally, the EP1/EP3 agonist 17-phenyl trinor PGE2 was reported to abolish indomethacin–mediated growth inhibition in malignant keratinocytes that do not express EP3 [36].
To more definitively characterize the contribution of EP1 to skin carcinogenesis, we generated BK5.EP1 transgenic mice, which overexpress EP1 in the basal layer of the epidermis. Using either DMBA/TPA or DMBA/anthralin initiation/promotion protocols we found that EP1 transgenic mice produced significantly more carcinomas than wild type mice [44]. Because we observed the development of a few tumors following initiation but before promotion was started, we were interested in determining whether EP1 overexpression confers endogenous tumor promoting activity. Here we describe the development of aggressive skin tumors following treatment with DMBA, in the absence of treatment with tumor promoters. We also show that development of these tumors is entirely COX-2 dependent.
MATERIALS AND METHODS
Animals
Wild type female FVB/N mice at the age of 3–4 weeks were purchased from Harlan (Indianapolis, IN). BK5.EP1 transgenic mice, on a FVB background, were generated as previously described and bred at Science Park [44]. COX-2 knockout mice, on an FVB background, were obtained from Louise Howe [47] and crossed with BK5.EP1 mice. For all experiments, female mice at 6–9 weeks of age were used. All mice were maintained at the University of Texas MD Anderson Cancer Center – Science Park Research Division and housed in an air-conditioned (22 ± 1°C at 50% humidity) facility that is accredited by the Association for Assessment and Accreditation of Lab Animal Care; all experiments were carried out in accordance with IACUC approved procedures.
Carcinogenesis protocols
For experiment #1, 32 female BK5.EP1 transgenic mice on the FVB background and 32 female wild type FVB mice at 6–9 weeks of age were used. Dorsal hair of the mice was shaved 2 days prior to a single topical treament with DMBA (Sigma Aldrich, St. Louis, MO; 400μg/200μl acetone). Tumors were counted weekly to calculate tumor incidence (percentage of mice bearing tumors) and multiplicity (average number of tumors per mouse). At the end of the experiment, all skin lesions were removed and processed for histological analysis of pathological type. For experiment #2, groups of 7 female BK5.EP1 transgenic and wild type mice were treated with a single topical dose of 2,500 μg/200 μl benzo[a]pyrene (B[a]P). Lesions were assessed weekly for 10 weeks. For experiment #3, groups of 6 female BK5.EP1 transgenic mice were fed AIN-control diet or AIN diet containing 1000 ppm celecoxib starting one week before a single topical treatment with DMBA (400μg/200μl acetone). Two weeks after DMBA, one mouse from each group was sacrificed and the skin processed for histological evaluation. For experiment #4, groups of 4 female mice each of wild type, BK5.EP1, BK5.EP1/COX-2+/− and BK5.EP1/COX-2−/− were shaved 2 days prior to single topical treament with DMBA (400μg/200μl acetone). Lesions were assessed weekly for 10 weeks.
Histological evaluation and immunohistochemistry
Dorsal skins were fixed in 10% formalin and processed for paraffin embedding by the Histology and Tissue Processing Core at Science Park. Deparaffinized sections were stained with hematoxylin and eosin (H&E) or processed for Ki67 immunostaining using an overnight incubation of a 1:200 dilution of anti-Ki67 (DAKO, Carpinteria, CA). Following washes and incubation with a rabbit anti-rat antibody (Vector Laboratories, Inc., Burlingame, CA), 3,3′-diaminobenzidine tetrahydrochloride was used as a chromogen and sections were counterstained with hematoxylin. Ki67 positive and negative basal cells in the interfollicular region of the epidermis were counted under a microscope (200 X). At least 1,500 total basal cells were counted for each sample. An imaging system (Nikon ACT-1, Nikon, Melville, NY) was used to measure epidermal thickness. The on-screen thicknesses of the epidermis were obtained for at least 50 points of the interfollicular region per sample. The actual thickness of the epidermis was calculated by dividing the screen measurements by the magnification factor (×650). A veterinary pathologist, Dr. Donna Kusewitt, assessed the pathology of the lesions and tumors.
Western blot analysis
At specified times after treatment, total protein was extracted from epidermis with modified RIPA buffer (150 mM NaCl, 0.5% Triton X-100, 0.2 mM EDTA (pH 8.0), 50 mM Tris, pH 7.5). Fifty μg of protein was separated on 7 or 10% SDS-polyacrylamide gels by electrophoresis and transferred onto polyvinylidene difluoride membranes. The blots were incubated for 1 hr in 5% non-fat milk solution in Tris-buffered saline to block nonspecific binding. Blots were incubated with a 1:500 dilution of primary antibodies against CYP1B1 or CYP19a1 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) or COX-2 (Cayman Chemical Co., Ann Arbor, MI), for 1 hr at room temperature or overnight at 4°C. The blots were washed 3X with 0.1% Tween 20 in Tris-buffered saline for 5 min each. Horseradish peroxidase-conjugated secondary antibody (GE Healthcare, Piscataway, NJ) at 1:10,000 was applied for 1 hr at room temperature. The blots were washed, incubated with chemiluminescence substrate (Perkin-Elmer Life Sciences, Boston, MA), and exposed to autoradiography film.
3H-benzo[a]pyrene-DNA adduct formation
Four female wild type and transgenic mice were treated topically with 200 nmol 3H-B[a]P (American Radiolabled Chemicals, Inc., St. Louis, MO; specific activity 50 Ci/mmol). Fifteen hr later mice were sacrificed, and DNA was isolated from the dorsal epidermis as described by Cai et al., [48]. Briefly, the epidermis was scraped into guanidine isothiocyanate buffer, homogenized by trituration with a syringe, and dialyzed against 0.15 M NaCl. The samples were then digested with proteinase K and RNase A, extracted with isoamyl alcohol and chloroform, and DNA was precipitated with ammonium acetate and ethanol. Resuspended DNA was then quantified by absorbance and aliquots counted in a scintillation counter. Adduct formation was calculated as pmol adduct/mg DNA.
Ras activation assay
Ras activity was measured by a Ras activation assay kit according to the supplied instructions (Millipore Inc., Beillerica, MA). Briefly, the epidermis of 3 wild type and 3 transgenic mice was harvested and protein extracted with magnesium lysis buffer. Ten μg of the Ras assay reagent (Raf-1 RBD, agarose) was incubated with 500 μg protein extract overnight at 4° C. After centrifugation, washed beads were boiled and loaded onto a 12% SDS-polyacrylamide gel. Following electrophoresis, the proteins were transferred to polyvinylidene difluoride membranes. The membrane blot was incubated for 2 hr in 3% non-fat milk solution in Tris-buffered saline to block non-specific binding and incubated overnight at 4° C with an anti-Ras antibody. Washed blots were then incubated for 1 hr at room temperature with horseradish peroxidase conjugated anti-mouse IgG antibody, and the Ras protein was visualized with chemiluminescent substrate (Perkin-Elmer Life Sciences, Boston, MA) and exposure to autoradiography film.
Keratinocyte stem cell analysis
Bulge region keratinocyte stem cells were isolated from 3 WT and 3 BK5.EP1 transgenic mice 7 weeks of age as previously described [49]. Briefly, the fat was removed from the subcutis of dorsal skins, which were then incubated overnight with dispase at 4°C. After removing the epidermis, the dermis was incubated in 1% collagenase for 2 hr at 37°C. The hair follicle fraction was isolated by centrifugation at 300 × g for 5 min at 4°C followed by centrifugation at 52 × g for 5 min at 4°C. The hair follicles were then trypsinized for 10 min at 37°C, filtered first with a 70 μm cell strainer, then a 40 μm strainer (BD Biosciences, San Jose, CA). Following resuspension in 1X phosphate-buffered saline, the isolated stem cells were labeled with a CD34-biotin antibody (eBioscience, San Diego, CA), a keratinocyte hair follicle bulge region stem cell marker [49], followed with streptavidin coupled to allophycocyanin as well as an α6 integrin-PE (CD49f) antibody (BD Pharmingen, San Diego, CA) that is a marker of basal cells [50]. Cells were analyzed using a BD FACSAria SORP flow cytometer by the Cell and Tissue Analysis Facility Core at Science Park.
Statistical analysis
Data are shown as the mean ± standard deviation. To determine statistical differences between means, Student’s t-test, independent or proportions test, Poisson regression or Fisher’s exact test were used where applicable.
RESULTS
Effects of EP1 overexpression on DMBA-only skin carcinogenesis
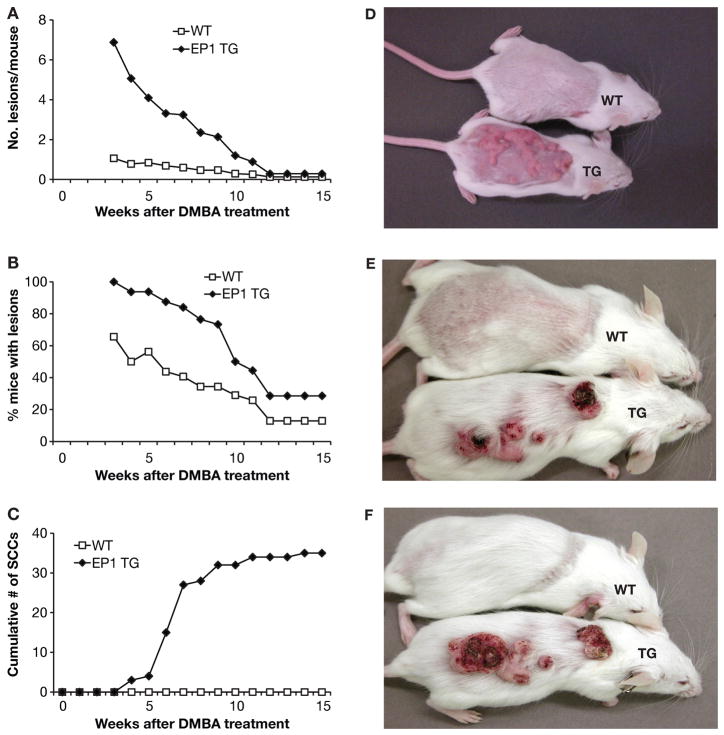
Tumor formation was observed in some EP1 transgenic mice prior to application of the tumor promoter TPA in a previously decribed DMBA/TPA study [44]; therefore, here a DMBA-only experimental protocol was used to determine whether expression of EP1 confered endogenous tumor promoting activity. However, a higher dose of DMBA (400μg) than in the DMBA/TPA protocol (100 μg) was used to ensure tumor formation in wild type mice. Raised hyperproliferative lesions, referred to as such because they were generalized and lacked discrete structure, appeared within one week of DMBA application in both wild type and transgenic mice, although the lesions were more prominent and covered a much larger area in the transgenic mice. The lesions often coalesced, making quantitation difficult. By two weeks the lesions were regressing in the WT mice, and did not reappear, but not BK5.EP1 mice, which had a 100% tumor incidence (Figure 1A,B,D). By 3–4 weeks the lesions in the transgenic mice were distinct tumors, defined as such because they were discrete structures, and could be counted (Figure 1A,B,E). The first carcinoma appeared at 4 weeks on a transgenic mouse and the number continued to increase up to ~9 weeks (Figure 1C,E,F). Mice with carcinomas larger than 1 cm in diameter were sacrificed in the middle of the experiment for humane reasons. No carcinomas appeared in wild type mice even after 49 weeks (Figure 1C,F and data not shown). By 6 weeks the majority of the wild type mice had no lesions while many of the lesions in transgenic mice were large and rapidly growing (Figure 1F). The closeness and aggressiveness of some of the tumors as they coalesced, reduced the apparent number of tumors (Figure 1A,D). Overall these data clearly show that overexpression of EP1 greatly enhanced malignant tumor formation in the DMBA-only skin carcinogenesis protocol suggesting that EP1 has intrinsic tumor promoting and progression activity.
Figure 1. The effect of EP1 overexpression in DMBA-only skin tumor development.
A DMBA-only skin carcinogenesis protocol was used with 32 BK5.EP1 transgenic mice (EP1 TG) and 32 wild type mice (WT). A: Tumor multiplicity; data are expressed as the average number of tumors per mouse. * p<0.001, Poisson regression. B: Tumor incidence; data are expressed as the percentage of mice bearing lesions/tumors. * p<0.001, Fisher exact test. C: Cumulative number of carcinomas; data are expressed as cumulative number of carcinomas. D–F: photographs of representative mice: D, Two weeks after DMBA treatment; E: Four weeks after DMBA treatment; F, Six weeks after DMBA treatment.
Effect of EP1 overexpression on DMBA–induced cell proliferation
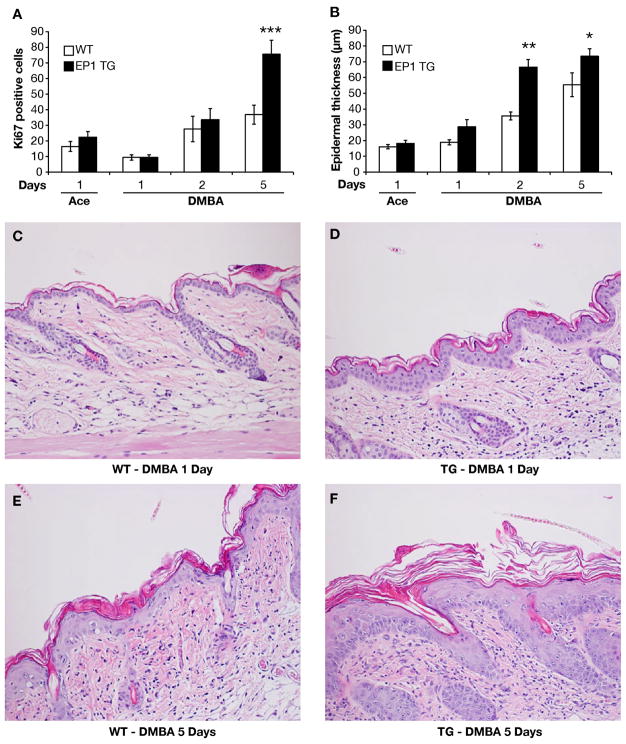
To begin to characterize the differences in response to DMBA between wild type and transgenic mice, the effect of EP1 overexpression on DMBA-induced epidermal cell proliferation was examined by assessing Ki-67 staining and epidermal thickness. With vehicle (acetone) treatment, the percent of Ki-67 positive cells and epidermal thickness were similar in wild type and BK5.EP1 transgenic mice, although there was a slight increase in the BK5.EP1 epidermis (Figures 2A and 2B). One day following DMBA treatment, proliferation of both WT and BK5.EP1 epidermis was reduced to the same extent, although both proliferation and epidermal thickness increased with time, up to day 5 (Figures 2A and 2B). The transgenic mice responded more robustly than the wild type such that at days 1 to 5 their epidermis was significantly more hyperplastic (Figures 2C–F). These data clearly show that overexpression of EP1 increases DMBA-induced epidermal cell proliferation which likely contributes to the more numerous and larger lesions seen in the transgenic mice.
Figure 2. Effect of DMBA on epidermal cell proliferation and hyperplasia in BK5.EP1 transgenic (EP1 TG) and wild type (WT) mice.
A: Ki-67 expression. Mice were topically treated with DMBA (400 μg/200 μl) or acetone (200 μl). At specified times after treatment, dorsal skin was removed, fixed in formalin, and immunostained for Ki-67. The data are shown as percentage of Ki-67 positive basal cells. B: Epidermal thickness. Dorsal skin was collected at specified times after acetone (200 μl) or DMBA (400 μg/200 μl acetone) treatment and stained with H&E. Epidermal thickness was measured under a microscope as described under ‘Methods and Materials’. * p <0.05, ** p<0.01, *** p <0.001, independent T or proportions test, BK5.EP1 compared to WT. C–F: Representative H&E stained sections were photographed at 200×.
Effect of EP1 overexpression on DMBA–induced histological changes
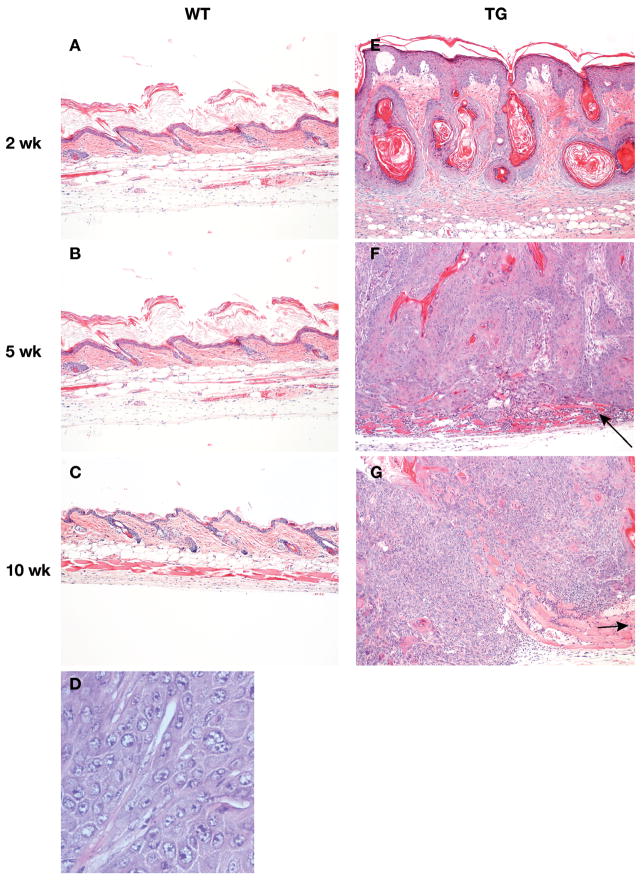
At 1 day after DMBA treatment, the epidermis of WT mice appeared unperturbed and there was a mild inflammatory cell infiltrate in the dermis (Figure 2C). By 5 days after exposure, mild epidermal hyperplasia and hyperkeratosis, as well as moderate dermal inflammation were evident (Figure 2E). However, by 2 weeks after DMBA treatment these changes had largely resolved, except for mild hyperpkeratosis (Figure 3A). At 5 and 10 weeks after exposure, previously treated WT skin was indistinguishable from untreated skin (Figure 3B,C). In contrast, BK5.EP1 mice developed widespread proliferative lesions that rapidly progressed to squamous cell carcinoma. Even at 1 day after DMBA treatment, moderate epidermal hyperplasia and dermal inflammation were apparent (Figure 2D), and by 5 days after treatment (Figure 2F), moderately severe hyperplasia affecting the interfollicular epithelium and infundibula of the hair follicles was seen. Two weeks after DMBA treatment (Figure 3E), there were large confluent areas characterized by marked hyperplasia of interfollicular and follicular epithelium and the formation of keratin-filled follicular cysts. By 5 weeks after exposure (Figure 3F), individual squamous cell carcinomas arose. These tumors were moderately well differentiated and many invaded the panniculus carnosus. By 10 weeks after exposure, the squamous cell carcinomas appeared much less differentiated and had penetrated well below the panniculus carnosus (Figure 3G).
Figure 3. Histological appearance of lesions and tumors after DMBA.
Mice were topically treated with DMBA (400 μg/200 μl). At specified times after treatment, dorsal skin was removed, fixed in formalin, and stained with H&E and photographed. A–C, wild type epidermis (100×). D: Squamous cell carcinoma from BK5.EP1 mice (400×). E–G, lesions and tumors from BK5.EP1 mice.
Effect of EP1 overexpression on tumor initiation
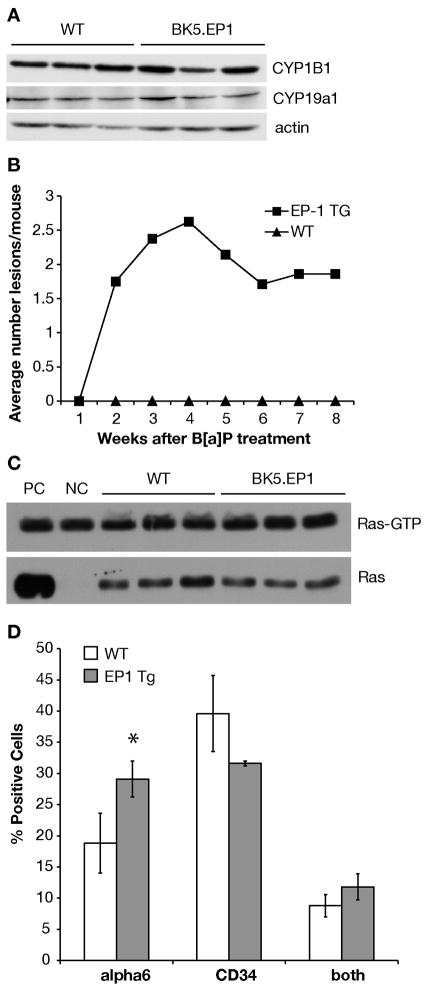
To detemine whether the tumorigenic effect of DMBA on EP1 transgenics was due to differences in events usually associated with initiation, several parameters associated wth carcinogen metabolism and DNA adduct formation were investigated. First, the effect of EP1 overexpression on the expression of CYP1B1, the enzyme that metabolizes DMBA to reactive intermediates, was examined by western blot. No differences in expression of CYP1B1 was discernable between BK5.EP1 and wild type mice (Figure 4A). The expression of CYP19a1 (aromatase) was also examined because it has been reported to be upregulated by EP1 in some tissues [51], although it is not known whether aromatase metabolizes DMBA. The level of expression was similar between EP1 transgenic and wild type mice (Figure 4A). Thus it appears that there are no differences in the expression of the metabolic enzymes.
Figure 4. Effect of EP1 overexpression on initiation.
A: Protein was extracted from epidermis at specified times from wild type (WT) and BK5.EP1 transgenic mice (EP1 TG). CYP1B1 and CYP19a1 protein levels were determined by western blot; actin levels were used as loading controls. B: Tumor response of BK5.EP1 and wild type FVB mice to a single B[a]P treatment. C: Protein was extracted from epidermis of untreated wild type (WT) and BK5.EP1 transgenic mice. Ras-GTP protein level was determined by pull down assay. PC, positive control, untreated wild type protein treated with GTPγS before pull down; NC, negative control, untreated wild type protein treated with GDP before pull down. Total Ras protein activity was determined by western blot. D: Quantification of bulge region keratinocyte stem cell markers by FACS analysis of keratinocytes from untreated BK5.EP1 (EP1 TG) and WT mice. Data presented as the mean (n=3) ± std. dev. of the percentage of total cells positive for α6 and/or CD34. *, p = 0.034.
Second, benzo[a]pyrene (B[a]P)-DNA adduct formation was measured. 3H-B[a]P was used because 3H-DMBA was not commercially available. B[a]P and DMBA are both polycyclic aromatic hydrocarbons and are metabolized by the same cytochrome P450 (CYP1B1) enzyme to their reactive forms [52]. Fifteen hours after 3H-B[a]P treatment, wild type mice had an average of 7.4 ± 1.5 pmol adduct/mg DNA while 6.5 ± 0.7 pmol adduct/mg DNA was detected in EP1 transgenic mice. To assure that the tumor response seen with DMBA was not unique to this carcinogen, another experiment was carried out using a single treatment of B[a]P. B[a]P caused the development of discrete tumors, 3 of which were carcinomas by week 8; the wild type mice on the other hand never developed visible lesions (Figure 4B).
Activation of EP1 has been reported to activate Ras through activation of phospholipase C [53]. To determine if this was occurring in the epidermis of BK5.EP1 mice, the level of Ras activity was measured by a pull-down assay. BK5.EP1 transgenic mice had a similar level of epidermal Ras activity as wild type mice (Figure 4C).
Because prostaglandins promote stem cell proliferation [54], the effect of EP1 overexpression on keratinocyte stem cell numbers was investigated. Keratinocyte stem cell from the bulge region express the hematopoietic stem cell marker CD34 on their cell surface [55], in addition to α6 integrin, a cell surface marker for basal keratinocytes that is associated with proliferation [56]. As shown in Figure 4D, there was an increase in the percentage of α6 integrin positive cells and a decrease in the percentage of CD34 positive cells in the BK5.EP1 mice, although these differences were not highly significant. The higher α6 integrin expression suggests increased proliferation in the BK5.EP1 mice. Overall, however, these data suggest that the increased number of carcinomas in the BK5.EP1 mice is not likely due to an increase in the number of stem cells or properties associated with carcinogen metabolism.
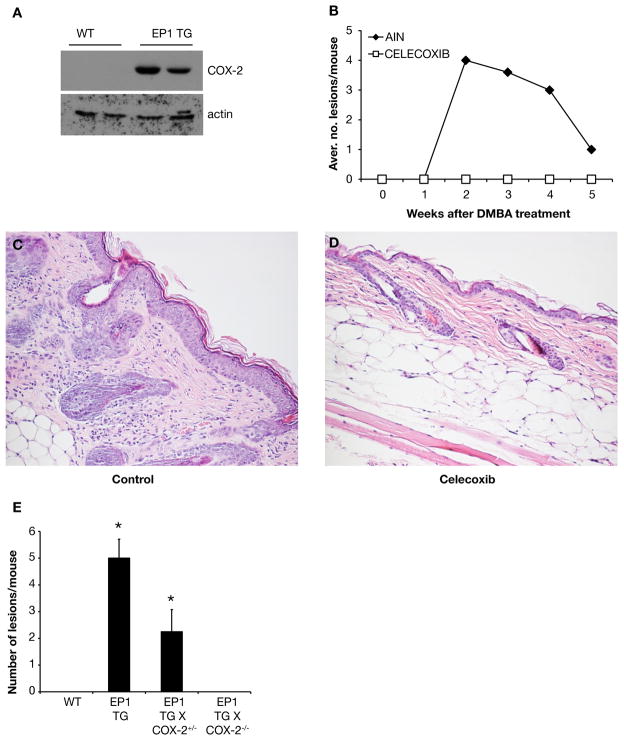
The effect of EP1 overexpression on skin carcinogenesis is COX-2 dependent
It was previously reported that the EP1 agonist 17-phenyl-2-trinor PGE2 induces COX-2 mRNA and increases PGE2 production in mouse osteoblastic cells [57]. In addition, application of the EP1 antagonist ONO-8713 on mouse skin effectively prevents UV-mediated induction of PGE2 [43]. In the present study, we found that there was no COX-2 protein expression in wild type mouse epidermis, as previously reported [22,58], whereas the COX-2 protein was clearly upregulated in the absence of treatment in BK5.EP1 transgenic mice (Figure 5A). To determine the significance of upregulated COX-2 in BK5.EP1 transgenic mouse, the selective COX-2 antagonist celecoxib (1000 ppm in the diet) was administered starting 1 week before DMBA treatment. The BK5.EP1 transgenic mice that were fed AIN control diet produced an average of 4 skin lesions/mouse 2 weeks after DMBA treatment. The BK5.EP1 transgenic mice that were fed celecoxib produced no lesions or tumors after DMBA treatment (Figure 5B). At the 2-week time point, hyperplasia of the epidermis and inflammatory cell infiltration in the dermis were observed in BK5.EP1 transgenic mice fed control diet (Figure 5C). However, celecoxib-fed BK5.EP1 transgenic mice showed relatively normal skin architecture and very few inflammatory cells (Figure 5D). As seen in the DMBA experiment shown in Figure 1A, there is an apparent drop in the number of hyperplastic lesions after week 2. This is largely due to the coalescence of many of the lesions (Figure 5B).
Figure 5. The effect of EP1 overexpression on DMBA-induced skin carcinogenesis is COX-2 dependent.
A: Protein was extracted from epidermis of untreated wild type (WT) and BK5.EP1 transgenic mice. COX-2 protein level was determined by western blot; actin levels were used as loading controls. B: Groups of six BK5.EP1 mice were fed AIN diet (control) or AIN diet containing celecoxib (1000 ppm) starting one week before a single DMBA (400 μg) application. Data are presented as the average number of lesions per mouse. C–D: Two weeks after DMBA treatment, a skin sample of one mouse from each group was collected, fixed in formalin and stained with H&E. C: Skin of AIN-control diet fed mice. D: Skin of celecoxib fed mice. E: BK5.EP1 mice were crossed with COX-2 null mice; groups of 4 mice of the indicated genotype were treated with 400 μg DMBA and the number of lesions assessed. Bars represent the mean number of lesions at 10 weeks ± std. dev.
To verify that this was not an off-target effect of celecoxib, which has been reported to inhibit Akt activation and mouse skin tumor promotion [59], BK5.EP1 mice were crossed with COX-2 null mice. As shown in Figure 5E, loss of one allele of COX-2 reduced tumor development by 50%, while loss of both alleles completely prevented tumor development. The above studies clearly show that expression of COX-2 in the BK5.EP1 transgenic mice is required for the increased DMBA-induced skin carcinogenesis in the EP1 transgenic mice.
DISCUSSION
In our earlier study using DMBA initiation and TPA promotion, we noticed some papilloma formation before TPA treatment was begun in the EP1 transgenics but not in WT mice. However, the number of papillomas arising after several months of repeated TPA promotion were considerably smaller in the transgenic mice, likely due to reduced PKC expression. When anthralin, which does not activate PKC, was used as a tumor promoter, more papillomas and carcinomas were observed in the transgenic than WT mice, suggesting that the transgenic animals have endogenous tumor promoting and progression activity [44]. In addition, Tober et al. [43] reported that topical application of a specific EP1 antagonist significantly reduced the number of UV-induced skin tumors and associated inflammation. Here we report that the BK5.EP1 transgenic mice are extremely sensitive to the carcinogenic actions of both DMBA and B[a]P compared to WT mice in a carcinogen-only protocol.
This observation is similar to that seen with PKCε transgenic mice, since PKCε transgenic mice show increased carcinoma development in a DMBA/acetone protocol [60,61]. COX-2 transgenic mice have also been reported to produce more tumors with a DMBA-only protocol [28,29]. The observation that both the COX-2 and EP1 transgenic mice produce considerably more skin tumors than their wild type counterparts suggests that the effects of prostaglandin E2 on tumor development are mediated in part via the EP1 receptor. We have also shown, however, that the EP2, but not EP3, receptor contributes significantly to the development of DMBA/TPA- and UV-induced skin cancer [42].
Interestingly, the BK5.EP1 transgenic mice differed from the previously described K14.COX-2 transgenic mice in the temporal aspects of tumor development. In the K14.COX-2 transgenic mice, more wild type mice initially developed skin tumors and it was only after 20 weeks that the transgenic mice developed more tumors/mouse and had a higher incidence than wild type mice [29]. In contrast, the BK5.EP1 mice developed more tumors than wild type mice from the beginning of the experiment. In addition, although K14.COX-2 transgenic mice had more total tumors than wild type, only 16% of these were SCC compared to 41% in wild type mice after 42 weeks [29]. In the experiments described here with BK5.EP1 mice, no SCC developed in wild type mice during the 49 week experiment, while 35 SCC developed in 32 BK5.EP1 mice. Thus, while elevated COX-2 levels are associated with increased promotion of skin tumors in both the K14.COX-2 and BK5.EP1 mice, PGE2 signaling through EP1 appears to also significantly enhance malignant progression.
The mechanism(s) by which EP1 contributes to skin carcinogenesis is not clear. Konger et al [37,62] found that in normal human epidermis EP1 is expressed in the differentiated compartment and is coupled to keratinocyte differentiation. However, Thompson et al [36] found that malignant murine keratinocytes depend in part on the EP1 receptor for their in vitro growth. We recently reported that proliferation of basal keratinocytes in vivo was essentially the same in untreated BK5.EP1 and wild type mice, although the epidermis was thicker in the transgenic animals [44]. The BK5.EP1 mice, however, showed a greater proliferative response and inflammatory cell recruitment after DMBA treatment than wild type mice. These features likely contribute to the tumor promoting activity of EP1.
To further elucidate the mechanism(s) by which EP1 overexpression increases tumor formation in the DMBA-only skin carcinogenesis model, processes associated with carcinogen initiation were measured, including B[a]P-DNA adduct formation, expression of CYP1B1 and CYP19a1, and Ras activation levels. The level of expression of CYP1B1, a cytochrome P450 enzyme that metabolizes DMBA to its active carcinogenic form, was not altered in the BK5.EP1 transgenic mice compared to wild type mice, suggesing that the metabolism of DMBA was the same in transgenic and wild type mice. This is further supported by the finding that B[a]P-DNA adduct formation was also similar in the BK5.EP1 transgenic mice compared to wild type mice. In addition, the level of the active form of Ras was essentially the same in the transgenic mice and wild type mice. Thus, EP1 overexpression does not appear to affect these aspects of the initiation stage of skin carcinogenesis.
The mouse skin carcinogenesis model has been the focus of investigations on the identification of initiated cells that give rise to skin tumors. Several studies have shown that keratinocyte stem cells, the self-renewing cells located in the bulge region of the hair follicle, are the target cells for skin carcinogenesis [63–65]. Because prostaglandin E2 has been shown to stimulate stem cell proliferation [54], presumably via one or more of the EP receptors, we sought to determine whether there were differences in stem cell number between BK5.EP1 and wild type mice that could explain their differential tumor response. Using the same stem cell markers and procedures used by others [49,50,65], we found only slight differences between the transgenic and wild type mice in the number ofα6 and/or CD34 cells. It is possible that the increased number of α6 expressing cells represents a more proliferative population of stem cells [55]; this may contribute to the increased carcinoma incidence since proliferation could prevent repair of DNA adducts.
The observation that COX-2 is constitutively expressed in the BK5.EP1 but not WT mice raised the question of whether this contributed to the high tumor burden in the transgenic mice. Administration of the selective COX-2 inhibitor celecoxib in the diet completely prevented DMBA-induced tumor development in the BK5.EP1 mice. To assure that this phenomenon was not due to off-target effects of celecoxib, the BK5.EP1 mice were crossed with COX-2 null mice. There was a clear COX-2 gene dose-dependent effect such that the absence of both alleles prevented tumor development while the presence of one allele of COX-2 gave a tumor response that was approximately half that of BK5.EP1 mice bearing both alleles. Thus, the up-regulated expression of COX-2 in the BK5.EP1 transgenic mice directly contributes to the enhanced susceptibility of these mice to skin carcinogenesis.
The requirement for COX-2 is likely that it supplies high levels of PGE2 that activates the EP1 receptor in the transgenic mouse. We previously showed that PKC is constitutively down-regulated in the epidermis of BK5.EP1 mice, which is likely the result of continuous activation followed by down regulation [44]. This may be responsible for the protumorigenic activity of EP1, which may involve a feedback cycle of PGE2 activating EP1, resulting in upregulated COX-2 and thus more PGE2. Further studies are needed to determine the significance of this feedback loop as well as determining the basis for the extreme sensitivity to the carcinogenic effects of DMBA and B[a]P.
Considering that the EP1 receptor is upregulated in papillomas and SCC of wild type mice following either a UV or initiation/promotion protocol, and the demonstration here that EP1 has tumor promoting/progressing activity, inhibitors of EP1 are likely to be effective skin cancer prevention agents. The extent to which the observations in skin are applicable to other epithelial tissues remains to be determined. However, if this generalization is correct, inhibition of EP1 (and EP2) should circumvent the cardiovascular problems associated with inhibition of COX-2 because the levels of thromboxane and prostacyclin should remain in balance [66].
Acknowledgments
This work was supported by the National Institutes of Health grants CA100140 and E007784 and the Center for Molecular and Cellular Toxicology, Division of Pharmacology and Toxicology, The University of Texas at Austin.
Abbreviations
- B[a]P
benzo[a]pyrene
- COX
cyclooxygenase
- DMBA
7,12-dimethylbenz[a]anthracene
- H&E
hematoxylin and eosin
- NSAID
nonsteroidal anti-inflammatory drug
- PG
prostaglandin
- PKC
protein kinase C
- SCC
squamous cell carcinoma
- TPA
12-O-tetradecanoylphorbol-13-acetate
- UV
ultraviolet light
- WT
wild type
References
- 1.Brown JR, DuBois RN. COX-2: a molecular target for colorectal cancer prevention. J Clin Oncol. 2005;23(12):2840–2855. doi: 10.1200/JCO.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 2.Pereg D, Lishner M. Non-steroidal anti-inflammatory drugs for the prevention and treatment of cancer. J Intern Med. 2005;258(2):115–123. doi: 10.1111/j.1365-2796.2005.01519.x. [DOI] [PubMed] [Google Scholar]
- 3.Rigas B, Kashfi K. Cancer prevention: a new era beyond cyclooxygenase-2. J Pharmacol Exp Ther. 2005;314(1):1–8. doi: 10.1124/jpet.104.080564. [DOI] [PubMed] [Google Scholar]
- 4.Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: subtypes and signaling. Annu Rev Pharmacol Toxicol. 2001;41:661–690. doi: 10.1146/annurev.pharmtox.41.1.661. [DOI] [PubMed] [Google Scholar]
- 5.Meric JB, Rottey S, Olaussen K, et al. Cyclooxygenase-2 as a target for anticancer drug development. Crit Rev Oncol Hematol. 2006;59(1):51–64. doi: 10.1016/j.critrevonc.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Wang D, Mann JR, DuBois RN. The role of prostaglandins and other eicosanoids in the gastrointestinal tract. Gastroenterology. 2005;128(5):1445–1461. doi: 10.1053/j.gastro.2004.09.080. [DOI] [PubMed] [Google Scholar]
- 7.Howe LR, Subbaramaiah K, Brown AM, Dannenberg AJ. Cyclooxygenase-2: a target for the prevention and treatment of breast cancer. Endocr Relat Cancer. 2001;8(2):97–114. doi: 10.1677/erc.0.0080097. [DOI] [PubMed] [Google Scholar]
- 8.Ristimaki A, Honkanen N, Jankala H, Sipponen P, Harkonen M. Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer Res. 1997;57(7):1276–1280. [PubMed] [Google Scholar]
- 9.Kagoura M, Toyoda M, Matsui C, Morohashi M. Immunohistochemical expression of cyclooxygenase-2 in skin cancers. J Cutan Pathol. 2001;28(6):298–302. doi: 10.1034/j.1600-0560.2001.028006298.x. [DOI] [PubMed] [Google Scholar]
- 10.Subbaramaiah K, Dannenberg AJ. Cyclooxygenase 2: a molecular target for cancer prevention and treatment. Trends Pharmacol Sci. 2003;24(2):96–102. doi: 10.1016/S0165-6147(02)00043-3. [DOI] [PubMed] [Google Scholar]
- 11.Arber N, Eagle CJ, Spicak J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355(9):885–895. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 12.Baron JA, Sandler RS, Bresalier RS, et al. A randomized trial of rofecoxib for the chemoprevention of colorectal adenomas. Gastroenterology. 2006;131(6):1674–1682. doi: 10.1053/j.gastro.2006.08.079. [DOI] [PubMed] [Google Scholar]
- 13.Bertagnolli MM, Eagle CJ, Zauber AG, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355(9):873–884. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 14.Boland GP, Butt IS, Prasad R, Knox WF, Bundred NJ. COX-2 expression is associated with an aggressive phenotype in ductal carcinoma in situ. Br J Cancer. 2004;90(2):423–429. doi: 10.1038/sj.bjc.6601534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chulada PC, Thompson MB, Mahler JF, et al. Genetic disruption of Ptgs-1, as well as Ptgs-2, reduces intestinal tumorigenesis in Min mice. Cancer Res. 2000;60(17):4705–4708. [PubMed] [Google Scholar]
- 16.Liu CH, Chang SH, Narko K, et al. Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem. 2001;276(21):18563–18569. doi: 10.1074/jbc.M010787200. [DOI] [PubMed] [Google Scholar]
- 17.Oshima M, Dinchuk JE, Kargman SL, et al. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2) Cell. 1996;87(5):803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- 18.Ristimaki A, Sivula A, Lundin J, et al. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 2002;62(3):632–635. [PubMed] [Google Scholar]
- 19.Steinbach G, Lynch PM, Phillips RK, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342(26):1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 20.Su JL, Shih JY, Yen ML, et al. Cyclooxygenase-2 induces EP1- and HER-2/Neu-dependent vascular endothelial growth factor-C up-regulation: a novel mechanism of lymphangiogenesis in lung adenocarcinoma. Cancer Res. 2004;64(2):554–564. doi: 10.1158/0008-5472.can-03-1301. [DOI] [PubMed] [Google Scholar]
- 21.An KP, Athar M, Tang X, et al. Cyclooxygenase-2 expression in murine and human nonmelanoma skin cancers: implications for therapeutic approaches. Photochem Photobiol. 2002;76(1):73–80. doi: 10.1562/0031-8655(2002)076<0073:ceimah>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 22.Athar M, An KP, Morel KD, et al. Ultraviolet B(UVB)-induced cox-2 expression in murine skin: an immunohistochemical study. Biochem Biophys Res Commun. 2001;280(4):1042–1047. doi: 10.1006/bbrc.2000.4201. [DOI] [PubMed] [Google Scholar]
- 23.Buckman SY, Gresham A, Hale P, et al. COX-2 expression is induced by UVB exposure in human skin: implications for the development of skin cancer. Carcinogenesis. 1998;19(5):723–729. doi: 10.1093/carcin/19.5.723. [DOI] [PubMed] [Google Scholar]
- 24.Muller-Decker K, Kopp-Schneider A, Marks F, Seibert K, Furstenberger G. Localization of prostaglandin H synthase isoenzymes in murine epidermal tumors: suppression of skin tumor promotion by inhibition of prostaglandin H synthase-2. Mol Carcinog. 1998;23(1):36–44. doi: 10.1002/(sici)1098-2744(199809)23:1<36::aid-mc5>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 25.Tiano HF, Loftin CD, Akunda J, et al. Deficiency of either cyclooxygenase (COX)-1 or COX-2 alters epidermal differentiation and reduces mouse skin tumorigenesis. Cancer Res. 2002;62(12):3395–3401. [PubMed] [Google Scholar]
- 26.Fischer SM, Lo HH, Gordon GB, et al. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, and indomethacin against ultraviolet light-induced skin carcinogenesis. Mol Carcinog. 1999;25(4):231–240. [PubMed] [Google Scholar]
- 27.Pentland AP, Schoggins JW, Scott GA, Khan KN, Han R. Reduction of UV-induced skin tumors in hairless mice by selective COX-2 inhibition. Carcinogenesis. 1999;20(10):1939–1944. doi: 10.1093/carcin/20.10.1939. [DOI] [PubMed] [Google Scholar]
- 28.Muller-Decker K, Neufang G, Berger I, Neumann M, Marks F, Furstenberger G. Transgenic cyclooxygenase-2 overexpression sensitizes mouse skin for carcinogenesis. Proc Natl Acad Sci U S A. 2002;99(19):12483–12488. doi: 10.1073/pnas.192323799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rundhaug JE, Pavone A, Kim E, Fischer SM. The effect of cyclooxygenase-2 overexpression on skin carcinogenesis is context dependent. Mol Carcinog. 2007;46(12):981–992. doi: 10.1002/mc.20340. [DOI] [PubMed] [Google Scholar]
- 30.Fischer SM, Baldwin JK, Jasheway DW, Patrick KE, Cameron GS. Phorbol ester induction of 8-lipoxygenase in inbred SENCAR (SSIN) but not C57BL/6J mice correlated with hyperplasia, edema, and oxidant generation but not ornithine decarboxylase induction. Cancer Res. 1988;48(3):658–664. [PubMed] [Google Scholar]
- 31.Yang VW, Shields JM, Hamilton SR, et al. Size-dependent increase in prostanoid levels in adenomas of patients with familial adenomatous polyposis. Cancer Res. 1998;58(8):1750–1753. [PubMed] [Google Scholar]
- 32.Chang SH, Liu CH, Conway R, et al. Role of prostaglandin E2-dependent angiogenic switch in cyclooxygenase 2-induced breast cancer progression. Proc Natl Acad Sci U S A. 2004;101(2):591–596. doi: 10.1073/pnas.2535911100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zweifel BS, Davis TW, Ornberg RL, Masferrer JL. Direct evidence for a role of cyclooxygenase 2-derived prostaglandin E2 in human head and neck xenograft tumors. Cancer Res. 2002;62(22):6706–6711. [PubMed] [Google Scholar]
- 34.Hansen-Petrik MB, McEntee MF, Jull B, Shi H, Zemel MB, Whelan J. Prostaglandin E(2) protects intestinal tumors from nonsteroidal anti-inflammatory drug-induced regression in Apc(Min/+) mice. Cancer Res. 2002;62(2):403–408. [PubMed] [Google Scholar]
- 35.Kawamori T, Uchiya N, Sugimura T, Wakabayashi K. Enhancement of colon carcinogenesis by prostaglandin E2 administration. Carcinogenesis. 2003;24(5):985–990. doi: 10.1093/carcin/bgg033. [DOI] [PubMed] [Google Scholar]
- 36.Thompson EJ, Gupta A, Vielhauer GA, Regan JW, Bowden GT. The growth of malignant keratinocytes depends on signaling through the PGE(2) receptor EP1. Neoplasia. 2001;3(5):402–410. doi: 10.1038/sj.neo.7900182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Konger RL, Billings SD, Prall NC, et al. The EP1 subtype of prostaglandin E2 receptor: role in keratinocyte differentiation and expression in non-melanoma skin cancer. Prostaglandins Leukot Essent Fatty Acids. 2009;81(4):279–290. doi: 10.1016/j.plefa.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Narumiya S. Prostanoids and inflammation: a new concept arising from receptor knockout mice. J Mol Med. 2009;87(10):1015–1022. doi: 10.1007/s00109-009-0500-1. [DOI] [PubMed] [Google Scholar]
- 39.Mutoh M, Watanabe K, Kitamura T, et al. Involvement of prostaglandin E receptor subtype EP(4) in colon carcinogenesis. Cancer Res. 2002;62(1):28–32. [PubMed] [Google Scholar]
- 40.Sonoshita M, Takaku K, Sasaki N, et al. Acceleration of intestinal polyposis through prostaglandin receptor EP2 in Apc(Delta 716) knockout mice. Nat Med. 2001;7(9):1048–1051. doi: 10.1038/nm0901-1048. [DOI] [PubMed] [Google Scholar]
- 41.Sung YM, He G, Hwang DH, Fischer SM. Overexpression of the prostaglandin E2 receptor EP2 results in enhanced skin tumor development. Oncogene. 2006;25(40):5507–5516. doi: 10.1038/sj.onc.1209538. [DOI] [PubMed] [Google Scholar]
- 42.Sung YM, He G, Fischer SM. Lack of expression of the EP2 but not EP3 receptor for prostaglandin E2 results in suppression of skin tumor development. Cancer Res. 2005;65(20):9304–9311. doi: 10.1158/0008-5472.CAN-05-1015. [DOI] [PubMed] [Google Scholar]
- 43.Tober KL, Wilgus TA, Kusewitt DF, Thomas-Ahner JM, Maruyama T, Oberyszyn TM. Importance of the EP(1) receptor in cutaneous UVB-induced inflammation and tumor development. J Invest Dermatol. 2006;126(1):205–211. doi: 10.1038/sj.jid.5700014. [DOI] [PubMed] [Google Scholar]
- 44.Surh I, Rundhaug J, Pavone A, Mikulec C, Abel E, Fischer SM. Upregulation of the EP1 receptor for prostaglandin E(2) promotes skin tumor progression. Mol Carcinog. 2011 doi: 10.1002/mc.20730. [DOI] [PubMed] [Google Scholar]
- 45.Lee JL, Kim A, Kopelovich L, Bickers DR, Athar M. Differential expression of E prostanoid receptors in murine and human non-melanoma skin cancer. J Invest Dermatol. 2005;125(4):818–825. doi: 10.1111/j.0022-202X.2005.23829.x. [DOI] [PubMed] [Google Scholar]
- 46.Tober KL, Thomas-Ahner JM, Kusewitt DF, Oberyszyn TM. Effects of UVB on E prostanoid receptor expression in murine skin. J Invest Dermatol. 2007;127(1):214–221. doi: 10.1038/sj.jid.5700502. [DOI] [PubMed] [Google Scholar]
- 47.Howe LR, Chang SH, Tolle KC, et al. HER2/neu-induced mammary tumorigenesis and angiogenesis are reduced in cyclooxygenase-2 knockout mice. Cancer Res. 2005;65(21):10113–10119. doi: 10.1158/0008-5472.CAN-05-1524. [DOI] [PubMed] [Google Scholar]
- 48.Cai Y, Kleiner H, Johnston D, et al. Effect of naturally occurring coumarins on the formation of epidermal DNA adducts and skin tumors induced by benzo[a]pyrene and 7,12-dimethylbenz[a]anthracene in SENCAR mice. Carcinogenesis. 1997;18(8):1521–1527. doi: 10.1093/carcin/18.8.1521. [DOI] [PubMed] [Google Scholar]
- 49.Trempus CS, Morris RJ, Bortner CD, et al. Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J Invest Dermatol. 2003;120(4):501–511. doi: 10.1046/j.1523-1747.2003.12088.x. [DOI] [PubMed] [Google Scholar]
- 50.Sonnenberg A, Calafat J, Janssen H, et al. Integrin alpha 6/beta 4 complex is located in hemidesmosomes, suggesting a major role in epidermal cell-basement membrane adhesion. J Cell Biol. 1991;113(4):907–917. doi: 10.1083/jcb.113.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Richards JA, Brueggemeier RW. Prostaglandin E2 regulates aromatase activity and expression in human adipose stromal cells via two distinct receptor subtypes. J Clin Endocrinol Metab. 2003;88(6):2810–2816. doi: 10.1210/jc.2002-021475. [DOI] [PubMed] [Google Scholar]
- 52.DiGiovanni J. Multistage carcinogenesis in mouse skin. Pharmacol Ther. 1992;54(1):63–128. doi: 10.1016/0163-7258(92)90051-z. [DOI] [PubMed] [Google Scholar]
- 53.Dhanasekaran N, Tsim ST, Dermott JM, Onesime D. Regulation of cell proliferation by G proteins. Oncogene. 1998;17(11):1383–1394. doi: 10.1038/sj.onc.1202242. Reviews. [DOI] [PubMed] [Google Scholar]
- 54.Li F, Huang Q, Chen J, et al. Apoptotic cells activate the “phoenix rising” pathway to promote wound healing and tissue regeneration. Sci Signal. 2010;3(110):ra13. doi: 10.1126/scisignal.2000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ohyama M. Hair follicle bulge: A fascinating reservoir of epithelial stem cells. J Dermatol Sci. 2007;46(2):81–89. doi: 10.1016/j.jdermsci.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 56.Webb A, Li A, Kaur P. Location and phenotype of human adult keratinocyte stem cells of the skin. Differentiation. 2004;72(8):387–395. doi: 10.1111/j.1432-0436.2004.07208005.x. [DOI] [PubMed] [Google Scholar]
- 57.Suda M, Tanaka K, Yasoda A, et al. Prostaglandin E2 (PGE2) autoamplifies its production through EP1 subtype of PGE receptor in mouse osteoblastic MC3T3-E1 cells. Calcif Tissue Int. 1998;62(4):327–331. doi: 10.1007/s002239900440. [DOI] [PubMed] [Google Scholar]
- 58.Bol DK, Rowley RB, Ho CP, et al. Cyclooxygenase-2 overexpression in the skin of transgenic mice results in suppression of tumor development. Cancer Res. 2002;62(9):2516–2521. [PubMed] [Google Scholar]
- 59.Kim N, Kim CH, Ahn DW, et al. Anti-gastric cancer effects of celecoxib, a selective COX-2 inhibitor, through inhibition of Akt signaling. J Gastroenterol Hepatol. 2009;24(3):480–487. doi: 10.1111/j.1440-1746.2008.05599.x. [DOI] [PubMed] [Google Scholar]
- 60.Jansen AP, Verwiebe EG, Dreckschmidt NE, Wheeler DL, Oberley TD, Verma AK. Protein kinase C-epsilon transgenic mice: a unique model for metastatic squamous cell carcinoma. Cancer Res. 2001;61(3):808–812. [PubMed] [Google Scholar]
- 61.Reddig PJ, Dreckschmidt NE, Zou J, Bourguignon SE, Oberley TD, Verma AK. Transgenic mice overexpressing protein kinase C epsilon in their epidermis exhibit reduced papilloma burden but enhanced carcinoma formation after tumor promotion. Cancer Res. 2000;60(3):595–602. [PubMed] [Google Scholar]
- 62.Konger RL, Billings SD, Thompson AB, et al. Immunolocalization of low-affinity prostaglandin E receptors, EP and EP, in adult human epidermis. J Invest Dermatol. 2005;124(5):965–970. doi: 10.1111/j.0022-202X.2005.23658.x. [DOI] [PubMed] [Google Scholar]
- 63.Kangsamaksin T, Park HJ, Trempus CS, Morris RJ. A perspective on murine keratinocyte stem cells as targets of chemically induced skin cancer. Mol Carcinog. 2007;46(8):579–584. doi: 10.1002/mc.20355. [DOI] [PubMed] [Google Scholar]
- 64.Morris RJ, Coulter K, Tryson K, Steinberg SR. Evidence that cutaneous carcinogen-initiated epithelial cells from mice are quiescent rather than actively cycling. Cancer Res. 1997;57(16):3436–3443. [PubMed] [Google Scholar]
- 65.Trempus CS, Morris RJ, Ehinger M, et al. CD34 expression by hair follicle stem cells is required for skin tumor development in mice. Cancer Res. 2007;67(9):4173–4181. doi: 10.1158/0008-5472.CAN-06-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ritter JM, Harding I, Warren JB. Precaution, cyclooxygenase inhibition, and cardiovascular risk. Trends Pharmacol Sci. 2009;30(10):503–508. doi: 10.1016/j.tips.2009.07.007. [DOI] [PubMed] [Google Scholar]