Abstract
Subjects at risk for atherosclerosis may have dysfunctional high-density lipoprotein (HDL) despite normal cholesterol content in plasma. We considered whether efflux of excess cellular cholesterol to HDL from obese subjects is associated with impaired arterial endothelial function, a biomarker of cardiovascular risk. Fifty-four overweight (body mass index [BMI] 25 – 29.9 kg/m2) or obese women (BMI ≥ 30 kg/m2), age 46 ± 11 years, were enrolled in a worksite wellness program. HDL cholesterol averaged 57 ± 17 mg/dL and was inversely associated with BMI (r= −0.419, P= 0.002). Endothelial function was assessed by brachial artery flow-mediated dilation (FMD). Cholesterol efflux from 3H-cholesterol-labeled BHK cells transfected with the ATP-binding cassette transporter 1 (ABCA1) showed 8.2 to 22.5% cholesterol efflux over 18 hours when incubated with 1% serum and was positively correlated with FMD (P <0.05), especially in the 34 subjects with BMI ≥ 30 kg/m2 (r= 0.482, P= 0.004). This relation was independent of age, HDL or low-density lipoprotein cholesterol (LDL) concentrations in plasma, blood pressure or insulin resistance by stepwise multiple regression analysis (β= 0.31, R2= 0.21, P= 0.007). Nitration of apoA-I tyrosine residues (by sandwich ELISA) was significantly higher in women with BMI ≥ 30 kg/m2 and the lowest cholesterol efflux than in women with BMI 25 – 29.9 kg/m2 and the highest cholesterol efflux (P= 0.01). We conclude that decreased cholesterol efflux via the ABCA1 transporter is associated with increased nitration of apoA-I in HDL and is an independent predictor of impaired endothelial function in women with BMI ≥ 30 kg/m2. This finding suggests that functional measures of HDL may be better markers for cardiovascular risk than HDL cholesterol levels in this population.
It is widely accepted that plasma concentrations of high-density lipoprotein (HDL) are inversely related to the risk of developing atherosclerotic vascular disease.1,2 One mechanism for vasculoprotection by HDL may be through facilitation of nitric oxide bioactivity in arterial endothelium, resulting in an overall benefit to vascular homeostasis.3 HDL-mediated reverse cholesterol transport, the mechanism by which excess cholesterol is effluxed from cells and transported to the liver, may also play a role in endothelial function.4 Cells other than macrophages express cholesterol efflux transporters, including endothelial cells.5 Thus, variation in HDL-mediated cholesterol efflux from endothelial cells or other cells in the vasculature may contribute to overall endothelial function, with the possibility of adverse effects in populations suspected of having “dysfunctional HDL” associated with obesity and diabetes.6 Our objective was to measure HDL cholesterol efflux capacity in women with HDL cholesterol levels generally within the normal range, but at risk for atherosclerosis due to obesity. Because endothelial cells as well as other cells in the vasculature express the ABCA1 transporter, we hypothesized that this property of HDL may show an association with endothelial function and thus provide insight regarding the role of HDL quality, despite adequate quantity, in vasculoprotection.
Methods
This study was conducted at the Clinical Center of the National Institutes of Health with employees enrolled in a worksite wellness program initiated by the National Heart, Lung, and Blood Institute. The protocol, approved by the Institutional Review Board of the National Heart, Lung, and Blood Institute (NCT00666172), was open to women according to BMI (weight in kilograms divided by height in meters2) classification of overweight (25 – 29.9 kg/m2) or obese (≥ 30 kg/m2), who were without history of atherosclerotic vascular disease and were not participants in structured exercise or weight loss programs. All participants provided written consent to participate in the protocol. All subjects underwent focused cardiovascular physical examinations, and venous blood samples were drawn following overnight fast. Standard lipid profiles were measured, using enzymatic assay (Wako Chemical USA Inc, Richmond, VA). Insulin resistance was estimated from fasting glucose and insulin values using the Homeostasis Model Assessment (HOMA).7 For women of reproductive age reporting menses, testing was performed during the follicular phase (days 1–10) of the menstrual cycle.
Brachial artery flow-mediated dilation testing, as an index of endothelial nitric oxide bioactivity, was conducted by a single investigator (GZ) as follows: Imaging of the left brachial artery proximal to the antecubital fossa was performed using a high-resolution ultrasound (12.5-MHz linear-array transducer) after 10 minutes of rest. Arterial diameter was measured in millimeters from the leading edge of the intima–lumen interface of the near wall to the leading edge of the lumen–intima interface of the far wall, coincident with the R wave on the electrocardiogram (end-diastole), at ≥ 6 sites and averaged. The maximum increase in brachial artery diameter was then measured during reactive hyperemia following 5 minutes of forearm ischemia caused by inflation of a blood pressure cuff on the forearm to suprasystolic pressure (225 mm Hg). Brachial artery flow-mediated dilation (%) = (post-ischemia minus baseline diameter) divided by baseline diameter × 100.
HDL-associated proteins apoA-I and apoA-II were measured using turbidimetric immunoassay (Wako Chemicals USA, Richmond, VA). The HDL subparticle preβ-1 was measured by an enzyme-linked immunosorbent assay (Polymedco, Cortlandt Manor, NY).8,9 The subparticle HDL2b was measured electrophoretically as previously described10 with the 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA) and expressed as a percentage of the total HDL particle. The capacity of a serum specimen to accept cholesterol effluxed by ABCA1 was measured, using a stably transfected BHK cell line expressing the human ABCA1 transporter.11 BHK cells transfected with a hygromycin-resistant control plasmid were used as the control cell line. Cholesterol efflux was conducted at 37 °C in cells labeled with 3H-cholesterol for 24 hours, washed, and incubated for 18 hours with subject's whole serum at 1% concentration. The percent of efflux specific to the ABCA1 transporter was calculated by subtracting the radioactive counts in the blank medium (α-minimal essential medium with 0.1% bovine serum albumin) from the radioactive counts in the presence of HDL and then dividing the result by the sum of the radioactive counts in the medium plus the cell fraction.
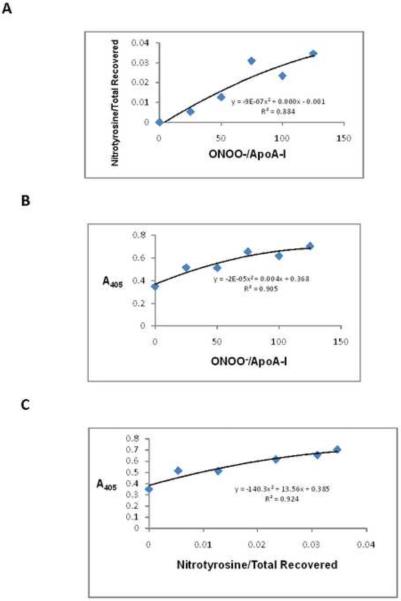
Nitrated apoA-I in serum of study participants with high and low efflux capacity was quantitated by a sandwich enzyme-linked immunoabsorbent assay.12 Briefly, sera from overweight and obese subjects with high and low cholesterol efflux were incubated in wells of a 96-well plate that was coated with anti-nitrotyrosine antibodies, enabling the capture of nitrated serum proteins, such as nitrated apoA-I. The plate wells were washed and an antibody to human apoA-I was added, specifically identifying nitrated apoA-I. Serum with higher levels of nitrated apoA-I will have increased nitrated apoA-I bound to the well, resulting in higher levels of anti-apoA-I antibody binding. HRP-conjugated secondary antibody followed by the HRP substrate OPD was used for detection. Absorbance at 405 nm was measured and corrected to absorbance of control wells in which no serum was added. The assay was validated experimentally through the range of values detected in subject samples by adding increasing concentrations of peroxynitrite to purified apoA-I and either performing ELISA on the nitrated apoA-I or digesting the nitrated apoA-I with trypsin and using LC-MS to quantify tyrosine nitration of apoA-I. The number of apoA-I tryptic peptides containing nitrotyrosine, normalized to the total number of apoA-I tryptic peptides recovered, increased with increasing peroxynitrite dosage (Figure 1A). Between absorbance (405) of 0.3 and 0.7 units, the fit between peroxynitrite dosage and absorbance (405) from the ELISA assay (y= −2E-05x2 + 0.004x + 0.368) was highly significant (Fig. 1B). Finally, the number of apoA-I tryptic peptides containing nitrotyrosine, normalized to the total number of apoA-I tryptic peptides recovered, was correlated with absorbance (405) in the ELISA assay (Figure 1C). Second-order polynomial equations and R2 values were calculated using Microsoft Excel.
Figure 1.
Validation of nitrated apoA-I ELISA assay. Purified human apoA-I was incubated with increasing doses of peroxynitrite (0, 25, 50, 75, 100, 125 mol peroxynitrite/mol apoA-I) at 37°C for 1 hour. Aliquots were removed for either trypsin digestion followed by LC/MS or for nitrated apoA-I determination by the ELISA assay. Panel A: Mass spectrometry analysis of purified apoA-I incubated with peroxynitrite. The number of apoA-I peptides containing nitrotyrosine (normalized to the number of peptides recovered) by mass spectrometry increased with peroxynitrite dosage. Panel B: ELISA analysis of purified apoA-I incubated with peroxynitrite. Absorbance (405) increased with peroxynitrite dosage. Panel C: Validation of nitrated apoA-I ELISA assay by mass spectrometry. The number of apoA-I peptides containing nitrotyrosine (normalized to the number of peptides recovered) by mass spectrometry strongly correlated with absorbance (405) in the nitrated apoA-I ELISA assay.
Data are reported as mean ± SD, unless otherwise indicated. All analyses were performed using the Instat3 or Prism statistical software (GraphPad Software Inc., San Diego, CA) or SAS (SAS Institute Inc, Cary, NC). Simple linear regression and the Pearson correlation coefficient were used to quantify associations between dependent and independent variables. Two-way analysis of variance (ANOVA) with interaction was performed to determine the effects of cholesterol efflux and HDL-C on brachial artery flow-mediated dilation. Multiple regression models for explaining brachial artery flow-mediated dilation were constructed, using stepwise model-building approaches by entering cholesterol efflux, age, HDL cholesterol, low-density lipoprotein cholesterol, triglycerides, blood pressure and HOMA as covariates using the SAS statistical analysis package and STEPWISE, SQUARE, GLM, and MEANS procedures (SAS User's Guide: Statistics, Version 9 Edition: SAS Institute Inc, Cary, NC). Statistical significance of differences in apoA-I tyrosine nitration were assessed by unpaired t-test. Both high and low efflux groups passed normality testing (Prism). P< 0.05 was considered to achieve statistical significance.
Results
Fifty-four consecutive women meeting eligibility criteria, ranging in age from 26 to 66 years, were enrolled during the initial year of the protocol and were evaluated in this study. Twenty subjects were identified as overweight (BMI 25 – 29.9 kg/m2) and 34 as obese (BMI≥ 30 kg/m2) (Table 1). Seven women (6 with BMI≥ 30 kg/m2 and 1 woman with 25 – 29.9 kg/m2) were adult-onset diabetics: 6 were on oral hypoglycemic medications and 1 took insulin. For the remainder with normal fasting blood glucose levels, insulin sensitivity was reduced (higher HOMA value) in obese compared with overweight women. Five women with BMI≥ 30 kg/m2 and 3 women with BMI 25 – 29.9 kg/m2 were on statin therapy for lipid management. Three subjects smoked cigarettes.
Table 1.
Clinical Characteristics
| Variable | Body Mass index (kg/m2) | ||
|---|---|---|---|
| 25–29.9 | ≥ 30 | P value | |
| n=20 | n=34 | ||
| Age (years) | 49 ± 11 | 45 ± 11 | 0.1866 |
| European American | 13 (65%) | 15 (44%) | 0.1670 |
| African American | 7 (35%) | 19 (56%) | |
| Waist circumference (cm) | 98 ± 8 | 116 ± 14 | <0.0001 |
| Homeostasis Model | 107 ± 1 | 301 ± 2.1 | 0.0057 |
| Assessment for insulin sensitivity | 203 ± 31 | 186 ± 32 | 0.0593 |
| Total cholesterol (mg/dL) | 118 ± 22 | 114 ± 33 | 0.05924 |
| Low-density lipoprotein cholesterol (mg/dL) | 66 ± 18 | 52 ± 15 | 0.0042 |
| High-density lipoprotein cholesterol (mg/dL) | 81 ± 41 | 96 ± 46 | 0.1279 |
| Triglycerides (mg/dL) | 150 ± 37 | 141 ± 28 | 0.3800 |
| Apolipoprotein A-I (mg/dL) | 34 ± 8 | 33 ± 628 | 0.8516 |
| Apolipoprotein A-II (mg/dL) | 53 ± 25 | 42 ± 15 | 0.0618 |
| Prebeta-1 (mg/dL) | 27.7 ± 6.3 | 23.1 ± 5.1 | 0.0053 |
| High-density lipoprotein 2b (%) | 7.4 ± 3.5 | 6.5 ± 3.5 | 0.3527 |
| Flow-mediated dilation (%) | |||
For the cohort, the mean HDL cholesterol was 57±17 mg/dL (range 32–111 mg/dL), and was inversely associated with BMI (r= −0.419, P= 0.002). ABCA1-specific cholesterol efflux averaged 13.7 ± 3.4% (range 8.2 to 22.5%) per 18 hours and was marginally higher in obese versus overweight women (14.4±3.6 versus 12.5±2.6%, P= 0.048). Cholesterol efflux values were independent of apoA-I or apoA-II levels, HDL cholesterol levels, HDL particle preβ-1 or 2b concentrations, or insulin sensitivity (all p>0.15). For all participants, there was a significant positive correlation between brachial artery flow-mediated dilation and ABCA1-specific cholesterol efflux (r= 0.271, P= 0.047), with the association particularly strong for the 34 obese women (r= 0.482, P= 0.004) (Figure 2). The interaction of cholesterol efflux and HDL cholesterol on brachial artery flow-mediated dilation in women with BMI≥ 30 kg/m2 is shown in Figure 3: Women with efflux values below the median had reduced brachial artery flow-mediated dilation (P= 0.011 versus efflux> median values) regardless of whether HDL cholesterol values were above or below the median (P= 0.755). By multiple regression analysis with cholesterol efflux, age, HDL cholesterol, low-density lipoprotein cholesterol, triglycerides, blood pressure and HOMA entered as covariates, only cholesterol efflux was determined to be an independent predictor of flow-mediated dilation (β= 0.31, R2= 0.21, P= 0.007).
Figure 2.
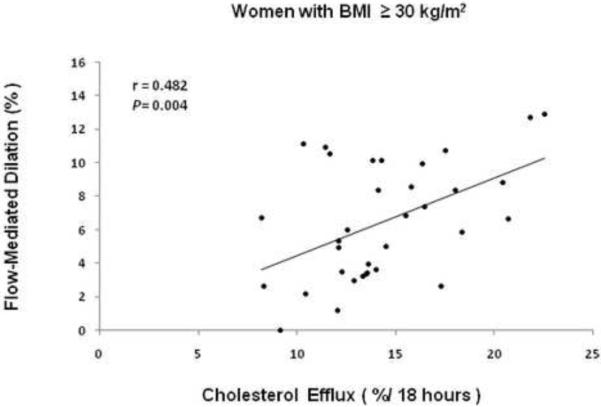
Association between brachial artery flow-mediated dilation as a measure of endothelial nitric oxide bioactivity and cholesterol efflux from BHK cell line expressing the human ABCA1 transporter incubated for 18 hours with subject's whole serum at 1% concentration in 34 women with BMI≥ 30 kg/m2.
Figure 3.
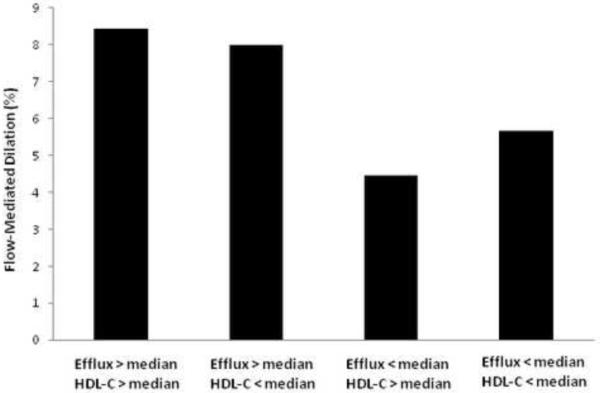
Interaction of cholesterol efflux and HDL cholesterol on brachial artery flow-mediated dilation in subjects with BMI≥ 30 kg/m2: Those with efflux values below the median had reduced brachial artery flow-mediated dilation (P= 0.011 versus efflux> median values) regardless of whether HDL cholesterol values were above or below the median.
Nitration of tyrosine residues in apoA-I was found to be significantly higher in the ten obese women with the lowest cholesterol efflux than in the ten overweight women with the highest cholesterol efflux (0.44 vs. 0.38 AU at 405 nm; Figure 4).
Figure 4.
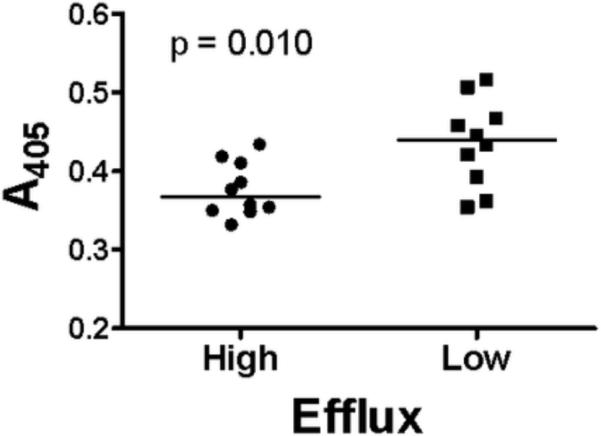
Nitrated apoA-I (by sandwich ELISA) in serum from the 10 women with BMI 25 – 29.9 kg/m2 and the highest ABCAI-dependent cholesterol efflux (left) and from 10 women with BMI≥ 30 kg/m2 and the lowest cholesterol efflux (right). Absorbance at 405 nm (A405) corresponds to the amount of nitrated apoA-I (in microliters) in serum.
Discussion
In our cohort of overweight (BMI 25 – 29.9 kg/m2) and obese (BMI≥ 30 kg/m2) women, we found that HDL cholesterol levels were inversely associated with obesity, consistent with previous reports from large cohorts of men and women.13–15 The range of HDL cholesterol levels was wide, and 17 women (including 6 of 34 subjects with BMI≥ 30 kg/m2) had values ≥ 60 mg/dL, which is well within the desirable range by current Adult Treatment Panel III guidelines.16 Using a cell-based assay to quantitate the capacity of serum to promote cholesterol efflux via the ABCA1 transporter, we demonstrated a facet of reverse cholesterol transport that was inversely associated with brachial artery flow-mediated dilation--a bioassay for nitric oxide bioactivity-- that was independent of HDL cholesterol levels. In this regard, women with BMI≥ 30 kg/m2 whose HDL cholesterol level was above the median for the group, but whose efflux was below the median, had an approximately 50% reduction in brachial artery flow-mediated dilation compared with women whose efflux levels were above the median. No associations among these covariates with flow-mediated dilation could be demonstrated in women with BMI 25 – 29.9 kg/m2. With age, lipid levels, blood pressure and insulin resistance added to cholesterol efflux in a multiple regression analysis, only cholesterol efflux emerged as an independent predictor of flow-mediated dilation in women with BMI≥ 30 kg/m2.
We considered whether nitration of apoA-I, the initial acceptor in reverse cholesterol transport, might account for variation in efflux by HDL from our subjects. Studies have shown that oxidative modification of apoA-I can reduce its ability to accept cholesterol from ABCA1 transporters of peripheral macrophages.17–19 In this regard, we found that nitration of tyrosine residues in apoA-I was significantly higher in the ten women with BMI≥ 30 kg/m2 and the lowest cholesterol efflux than in the ten women with BMI 25 – 29.9 kg/m2 and the highest cholesterol efflux. Increased apoA-I nitration may be a biomarker of increased oxidative stress in the obese state, even in the absence of diabetes or dyslipidemia, which has implications for oxidative inactivation of endothelial nitric oxide synthase and decreased nitric oxide production and bioavailability. Reactive oxygen species react with nitric oxide, forming peroxynitrite, among other highly reactive molecules. Conversion of nitric oxide to peroxynitrite not only decreases nitric oxide bioavailability but also results in oxidative modification and subsequent uncoupling of endothelial nitric oxide synthase, decreasing biogenesis of nitric oxide.20 Thus, increased oxidative stress in obesity may lead to increased peroxynitrite formation and increased apoA-I nitration that results not only in endothelial nitric oxide synthase oxidation and decreased nitric oxide formation, but also in decreased efflux and intracellular accumulation of cholesterol, further contributing to intravascular oxidant stress. Our findings may be relevant to the pathophysiology of endothelial dysfunction and preclinical atherosclerosis previously reported in obese subjects21,22, even when HDL cholesterol levels are within a desirable range by current guidelines.
Other groups, using non-macrophage or macrophage cells as the cholesterol source, have reported decreased efflux capacity of serum or plasma from patients with coronary artery disease or diabetes.23–25 The clinical relevance of HDL-mediated cholesterol efflux has been supported in a recent report by Khera et al25, in which cholesterol efflux from macrophages by serum from healthy subjects and patients undergoing coronary angiography was reported to be negatively correlated with carotid intimal thickness, independent of HDL cholesterol or apoA-I levels. Reduced cholesterol transport from cultured fibroblasts by plasma from obese subjects compared with plasma from lean subjects has been reported previously, with a positive correlation between efflux and levels of apoA-I.26 Although we did not detect a correlation between efflux and apoA-I levels, using BHK cells transfected with the ABCA1 transporter, in agreement with this report we found no correlation of efflux with HDL particle size.
Our findings are consistent with the notion of “dysfunctional HDL” in obese women who were commonly diabetic or pre-diabetic on the basis of reduced insulin sensitivity, including those with HDL cholesterol levels within the desirable range. Given the multiple roles of HDL, the conventional clinical practice of measuring HDL by its cholesterol content in plasma may not provide adequate insight into the functions of the particle. Thus, a post-hoc analysis of two large prospective studies showed that many patients with apparently high HDL cholesterol still develop major coronary events.27 These examples, among others, have led to a shift in focus from quantitative to qualitative HDL assessment. Significant associations between efflux and brachial artery flow-mediated dilation in our study suggest that interactions of HDL from obese subjects with the ABCA1 transporter may have relevance to endothelial function in humans, possibly directly through effects on lipid content of endothelium or indirectly through macrophages and other cells within the vessel wall—which may be abundant in obese subjects—that would otherwise diminish nitric oxide bioavailability as a consequence of increased intravascular oxidant stress.
Acknowledgements
This research was funded by the intramural research programs of the National Heart, Lung, and Blood Institute and the Clinical Center, National Institutes of Health. We gratefully acknowledge the technical contributions of Maureen Sampson and John Stonik to this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure No author has any conflict of interest related to this study.
References
- 1.Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977;62:707–714. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- 2.Gordon DJ, Rifkind BM. High-density lipoprotein- The clinical implications of recent studies. N Engl J Med. 1989;321:1311–1316. doi: 10.1056/NEJM198911093211907. [DOI] [PubMed] [Google Scholar]
- 3.Shaul PW, Mineo C. HDL action on the vascular wall: is the answer NO? J Clin Invest. 2004;113:509–513. doi: 10.1172/JCI21072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis GF, Rader DJ. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ.Res. 2005;96:1221–1232. doi: 10.1161/01.RES.0000170946.56981.5c. [DOI] [PubMed] [Google Scholar]
- 5.O'Connell BJ, Denis M, Genest J. Cellular physiology of cholesterol efflux in vascular endothelial cells. Circulation. 2004;110:2881–2888. doi: 10.1161/01.CIR.0000146333.20727.2B. [DOI] [PubMed] [Google Scholar]
- 6.Navab M, Ananthramaiah GM, Reddy ST, Van Lenten BJ, Ansell BJ, Harna S, Hough G, Bachini E, Grijalva VR, Wagner AC, Shaposhnik Z, Fogelman AM. The double jeopardy of HDL. Ann Med. 2005;37:173–178. doi: 10.1080/07853890510007322. [DOI] [PubMed] [Google Scholar]
- 7.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 8.Miyazaki O, Kobayashi J, Fukamachi I, Milda T, Bujo H, Saito Y. A new sandwich enzyme immunoassay for measurement of plasma pre-beta-1 HDL levels. J Lipid Res. 2000;41:2083–2088. [PubMed] [Google Scholar]
- 9.Milda T, Miyazaki O, Nakamura Y, Hirayama S, Hanyu O, Fukamachi I, Okada M. Analytical performance of a sandwich enzyme immunoassay for pre-beta-1 HDL in stabilized plasma. J Lipid Res. 2003;44:645–650. doi: 10.1194/jlr.D200025-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.Mueller O, Chang E, Deng D, Franz T, Jing D, Kincaid R, Konigshofer Y, Kratzmeier M, McNulty M, Qian H, Schneider J, Schulte H, Tian X, Van Cleve M, Yang D, Assmann G. PROCAM Study: risk prediction for myocardial infarction using microfluidic high-density lipoprotein (HDL) subfractionation is independent of HDL cholesterol. Clin Chem Lab Med. 2008;46:490–498. doi: 10.1515/CCLM.2008.117. [DOI] [PubMed] [Google Scholar]
- 11.Vaughan AM, Oram JF. ABCA1 and ABCG1 or ABCG4 act sequentially to remove cellular cholesterol and generate cholesterol-rich HDL. J Lipid Res. 2006 Nov;47:2433–2443. doi: 10.1194/jlr.M600218-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Bakillah A. Nitrated apolipoprotein A-I, a potential new cardiovascular marker, is markedly increased in low high-density lipoprotein cholesterol subjects. Chem Lab Med. 2009;47:60–69. doi: 10.1515/CCLM.2009.017. [DOI] [PubMed] [Google Scholar]
- 13.Glueck CJ, Taylor HL, Jacobs D, Morrison JA, Beaglehole R, Williams OD. Plasma high-density lipoprotein cholesterol: association with measurements of body mass. Circulation. 1980;62(Suppl IV):62–69. [PubMed] [Google Scholar]
- 14.Lamon-Fava S, Wilson PW, Schaefer EJ. Impact of body mass index on coronary heart disease risk factors in men and women. The Framingham Offspring Study. Arterioscler Thromb Vasc Biol. 1996;16:1509–1515. doi: 10.1161/01.atv.16.12.1509. [DOI] [PubMed] [Google Scholar]
- 15.Despres JP, Moorjani S, Ferland M, Tremblay A, Lupien PJ, Nadeau A, Pinault S, Theriault G, Bouchard C. Adipose tissue distribution and plasma lipoprotein levels in obese women. Importance of intra-abdominal fat. Arteriosclerosis. 1989;9:203–210. doi: 10.1161/01.atv.9.2.203. [DOI] [PubMed] [Google Scholar]
- 16.Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3121. [PubMed] [Google Scholar]
- 17.Nagano Y, Arai H, Kita T. High density lipoprotein loses its effect to stimulate efflux of cholesterol from foam cells after oxidative modification. Proc Natl Acad Sci USA. 1991;88:6457–6461. doi: 10.1073/pnas.88.15.6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shao B, Tang C, Heinecke JW, Oram JF. Oxidation of apolipoprotein A-I by myeloperoxidase impairs the initial interactions with ABCA1 required for signaling and cholesterol export. J Lipid Res. 2010;51:1849–1858. doi: 10.1194/jlr.M004085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng L, Nukuna B, Brennan ML, et al. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J Clin Invest. 2004;114:529–541. doi: 10.1172/JCI21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zou M-H, Shi C, Cohen RA. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest. 2002;109:817–826. doi: 10.1172/JCI14442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Juonala M, Viikari JS, Laitinen T, Marniemi J, Helenius H, Ronnemaa T, Raitakari OT. Interrelations between brachial artery endothelial function and carotid intima-media thickness in young adults: the Cardiovascular Risk in Young Finns Study. Circulation. 2004;110:2918–2923. doi: 10.1161/01.CIR.0000147540.88559.00. [DOI] [PubMed] [Google Scholar]
- 22.Burke GL, Bertoni AG, Shea S, Sun M, Goormastic M, Settle M, Schmitt D, Fu X, Thomson L, Fox PL, Ischiropoulos H, Smith JD, Kinter M, Hazen SL. The impact of obesity on cardiovascular risk factors and subclinical vascular disease: the Multi-Ethnic Study of Atherosclerosis. Arch Intern Med. 2008;168:928–935. doi: 10.1001/archinte.168.9.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sylvanne M, Castro G, Dengremont C, De Geitere C, Jauhiainen M, Enholm C, Michelagnoli S, Franceschini G, Kahri J, Taskinen MR. Cholesterol efflux from Fu5AH hepatoma cells induced by plasma of subjects with or without coronary artery disease and non-insulin dependent diabetes: importance of LpA-I:A-II particles and phospholipid transfer protein. Atherosclerosis. 1996;127:245–253. doi: 10.1016/s0021-9150(96)05962-x. [DOI] [PubMed] [Google Scholar]
- 24.Pujunen P, Sylvanne M, Castro G, Nieminen MS, Taskinen MR. Cholesterol efflux capacity in vitro predicts the severity and extent of coronary artery disease in patients with and without type 2 diabetes. Scand Cardiovasc J. 2001;35:96–100. doi: 10.1080/140174301750164736. [DOI] [PubMed] [Google Scholar]
- 25.Khera AV, Cuchel M, de la LLera-Moya M, Rodrigues A, Burke MF, Kashif J, French BC, Phillips JA, Wolfe ML, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sasahara T, Nestel P, Fidge N, Sviridov D. Cholesterol transport between cells and high density lipoprotein subfractions from obese and lean subjects. J Lipid Res. 1998;39:544–554. [PubMed] [Google Scholar]
- 27.van der Steeg WA, Holme I, Boekholdt SM, Larsen ML, Stroes ES, Tikkanen MJ, Wareham NJ, Faergeman O, Pedersen TR, Khaw KT, Kastelein JJ. High-density lipoprotein cholesterol, high-density lipoprotein particle size, and apolipoprotein A-I: significance for cardiovascular risk: the IDEAL and EPIC-Norfolk studies. J Am Coll Cardiol. 2008;51:634–642. doi: 10.1016/j.jacc.2007.09.060. [DOI] [PubMed] [Google Scholar]