Abstract
Skin cancer incidence and mortality are higher in men compared to women, but the causes of this sex discrepancy remain largely unknown. Ultraviolet light exposure induces cutaneous inflammation and neutralizes cutaneous antioxidants. Gr-1+CD11b+ myeloid cells are heterogeneous bone marrow-derived cells that promote inflammation-associated carcinogenesis. Reduced activity of catalase, an antioxidant present within skin, has been associated with skin carcinogenesis. We utilized the outbred, immune competent Skh-1 hairless mouse model of ultraviolet light B (UVB)-induced inflammation and non-melanoma skin cancer to further define sex discrepancies in UVB-induced inflammation. Our results demonstrated that male skin had relatively lower baseline catalase activity, which was inhibited following acute UVB exposure in both sexes. Further analysis revealed that skin catalase activity inversely correlated with splenic Gr-1+CD11b+ myeloid cell percentage. Acute UVB exposure induced Gr-1+CD11b+ myeloid cell skin infiltration, which was inhibited to a greater extent in males by topical catalase treatment. In chronic UVB studies, we demonstrated that the percentage of splenic Gr-1+CD11b+ myeloid cells was 55% higher in male tumor-bearing mice compared to their female counterparts. Together, our findings indicate that lower skin catalase activity in male mice may at least in part contribute to increased UVB-induced Gr-1+CD11b+ myeloid cells and subsequent skin carcinogenesis.
Keywords: sex, ultraviolet light B, catalase, skin cancer, Gr-1+CD11b+ myeloid cells
Introduction
The incidence of non-melanoma skin cancer is higher in men compared to women (Armstrong and Kricker, 2001; Foote et al., 2001), with men facing a three-fold greater risk of developing cutaneous squamous cell carcinoma (SCC) and being twice as likely to develop cutaneous basal cell carcinoma (BCC) (Armstrong and Kricker, 2001). In accordance with this sex discrepancy in human skin carcinogenesis, similar findings have been reported by our group in the Skh-1 hairless mouse model of UVB-induced non-melanoma skin cancer (Thomas-Ahner et al., 2007). Likewise, women with malignant melanoma, another type of skin cancer, exhibit a survival advantage and concurrent decreased risk of metastasis compared to men with malignant melanoma (Joosse et al., 2011). We have previously demonstrated that female mice have higher cutaneous total antioxidant levels (Thomas-Ahner et al., 2007) and others have proposed that reactive oxygen species (ROS) may potentiate sex differences in melanoma incidence, metastasis, and survival (Joosse et al., 2010). However, specific cellular and molecular factors that may contribute to sex differences in skin cancer incidence and survival remain insufficiently characterized.
Catalase is an important antioxidant enzyme present within the skin that contributes to the maintenance of genomic integrity and preservation of ROS homeostasis by scavenging and detoxifying hydrogen peroxide into water and oxygen. Decreased catalase activity has been previously associated with skin carcinogenesis and progression (Gupta et al., 2001; Kwei et al., 2004; Liu et al., 2009; Pence and Naylor, 1990; Sander et al., 2002; Sander et al., 2003; Shindo et al., 1994). However, few studies have directly investigated the role of catalase in UVB-induced inflammation in vivo.
One contributing factor to inflammation-associated carcinogenesis is the expansion of bone marrow-derived Gr-1+CD11b+ myeloid cells. These heterogenous cell populations co-express Gr-1 (Ly-6C and Ly-6G) and CD11b (Mac-1) and are often referred to as myeloid derived suppressor cells (MDSC). Following an initial stimulus that promotes expansion, these cells can be activated by a variety of factors associated with inflammation, infection and cancer to suppress T cell and Natural Killer cell function. MDSC have been shown to accumulate within secondary lymphoid organs and at tumor sites in multiple mouse syngeneic and transgenic tumor models and in peripheral blood and tumor specimens from patients with various types of cancer (reviewed in (Gabrilovich and Nagaraj, 2009; Ostrand-Rosenberg and Sinha, 2009)). Interestingly, ex vivo catalase treatment has been shown to concurrently inhibit proliferation and promote differentiation of splenic Gr-1+CD11b+ myeloid cells isolated from tumor-bearing mice (Kusmartsev and Gabrilovich, 2003).
The current study set out to further define cellular and molecular mechanisms underlying sex differences in UVB-induced inflammation and skin carcinogenesis. The outbred, immune competent Skh-1 hairless mouse model allowed the opportunity to evaluate Gr-1+CD11b+ myeloid cells during acute inflammation in tumor-free mice and under chronic inflammatory conditions in tumor-bearing mice. Our current results demonstrated lower baseline skin catalase activity in male mice compared to females and showed that acute UVB exposure dramatically inhibits skin catalase activity in both sexes. A single UVB exposure was sufficient to increase the percentages of Gr-1+CD11b+ myeloid cells present in the spleen and skin, regardless of sex. Furthermore, skin catalase activity and splenic Gr-1+CD11b+ myeloid cell percentages were inversely correlated, and topical catalase supplementation significantly inhibited UVB-induced Gr-1+CD11b+ myeloid cell skin infiltration in male mice. Finally, we demonstrated that male sex was a predictor of increased splenic Gr-1+CD11b+ myeloid cells in tumor-bearing mice.
Results
Male mice demonstrate lower endogenous skin catalase activity compared to female mice
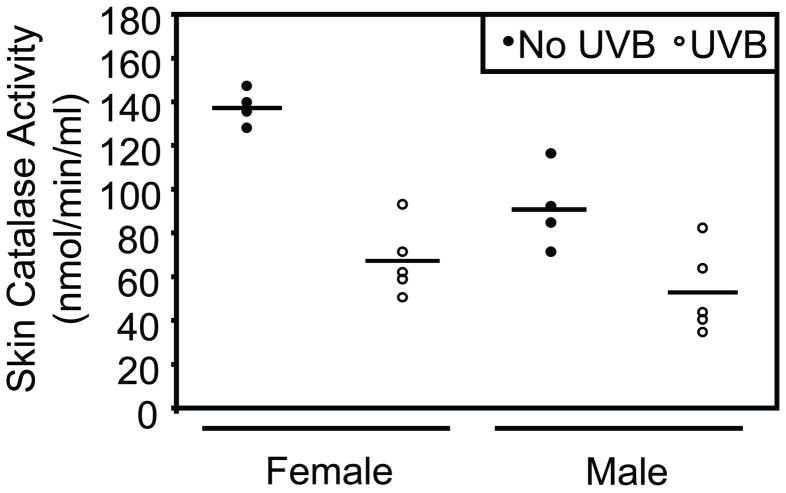
UVB exposure initiates an acute cellular inflammatory response that leads to the production of ROS, including hydrogen peroxide, within the injured skin tissue. Endogenous catalase is important for hydrogen peroxide detoxification and prevention of excessive oxidative stress in the skin and throughout the body (Schafer and Werner, 2008). Therefore, catalase activity was evaluated in male and female skin to determine the capacity for hydrogen peroxide detoxification at 48 hours following a single UVB exposure. In non-irradiated control mice, males demonstrated significantly less baseline catalase activity in the skin compared to female mice (p=0.0015). Consistent with previous reports in female Skh-1 mice (Pence and Naylor, 1990; Shindo et al., 1994), female mice showed a mean decrease in skin catalase activity of 70.25 nmol/min/ml at 48 hours post-UVB exposure (p<0.0001). Likewise, males showed a mean decrease in skin catalase activity of 37.97 nmol/min/ml at 48 hours post-UVB exposure (p=0.0043). Both male and female mice shared similar skin catalase activity at 48 hours following a single UVB exposure (Figure 1).
Figure 1. Male mice demonstrate lower skin catalase activity at baseline compared to females, and acute UVB exposure inhibits skin catalase activity in both sexes.
Dorsal skin catalase activity was quantified before and at 48 hours following a single UVB exposure (n=5 per sex). At baseline, non-irradiated male mice exhibited significantly lower skin catalase activity compared to female mice (p=0.0015). UVB significantly reduced skin catalase activity in male (p<0.0043) and female (p<0.0001) mice. Horizontal bars represent means.
A single UVB exposure increases splenic Gr-1+CD11b+ myeloid cells
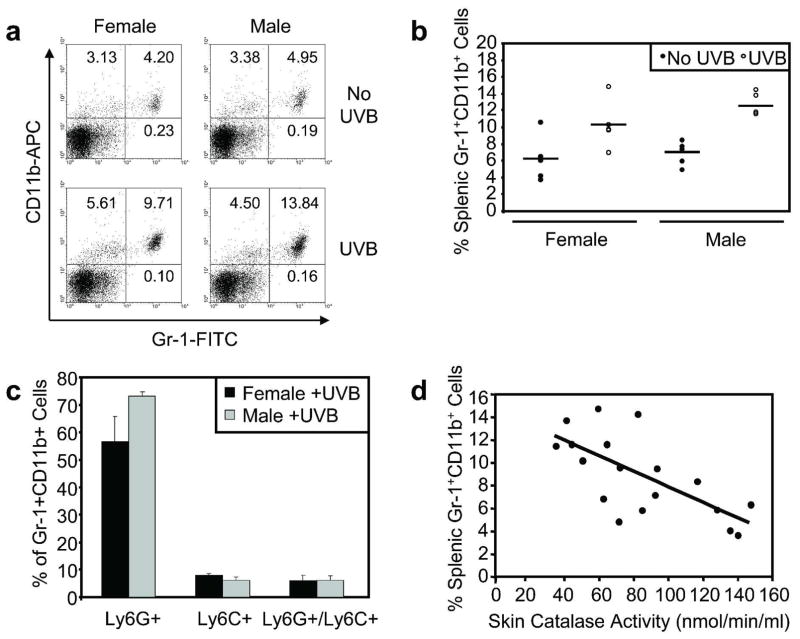
Catalase has been shown to concurrently inhibit proliferation and promote differentiation of splenic Gr-1+CD11b+ myeloid cells ex vivo (Kusmartsev and Gabrilovich, 2003). Our results demonstrating a sex discrepancy in baseline skin catalase activity along with confirmation that UVB inhibited skin catalase activity led us to consider how acute UVB affects systemic Gr-1+CD11b+ myeloid cells. Splenic Gr-1+CD11b+ myeloid cells were measured at 48 hours following a single UVB exposure, the peak of the cutaneous inflammatory response in the Skh-1 model. Representative flow cytometric analysis of splenocyte Gr-1 and CD11b surface staining is shown in Figure 2a. Relatively low percentages of splenic Gr-1+CD11b+ myeloid cells were detected in non-irradiated male and female control mice. However, a single UVB exposure significantly increased splenic Gr-1+CD11b+ myeloid cell percentages in both male (p=0.0022) and female (p=0.0057) mice (Figure 2b). Ly-6C and Ly-6G were separately analyzed by flow cytometry in Gr-1+CD11b+ myeloid cells to further characterize this splenic cell population. In both males and females, the vast majority of UVB-induced Gr-1+CD11b+ myeloid cells were Ly-6G-positive and Ly-6C-negative, indicating the predominance of the granulocytic subset of Gr-1+CD11b+ myeloid cells (Figure 2c). Furthermore, a significant inverse correlation was detected between skin catalase activity and the percentage of splenic Gr-1+CD11b+ myeloid cells when compared in all mice (r = −0.69; p=0.0015) (Figure 2d).
Figure 2. Acute UVB exposure increases the percentage of Gr-1+CD11b+ myeloid cells in the spleen.
Male and female mice were exposed to a single UVB dose (n=5 per sex). a, Splenic myeloid cells were assessed by flow cytometric analysis of Gr-1 and CD11b surface staining at 48 hours following UVB exposure and in non-irradiated mice. b, Relatively low percentages of splenic Gr-1+CD11b+ myeloid cells were detected in non-irradiated male and female control mice. UVB significantly increased splenic Gr-1+CD11b+ myeloid cell percentages in male (p=0.0022) and female (p=0.0057) mice. Horizontal bars represent means. c, Ly-6G and Ly-6C were analyzed separately to characterize Gr-1+CD11b+ myeloid cell subsets. The majority of UVB-induced splenic Gr-1+CD11b+ cells were Ly-6G-positive/Ly-6C-negative. d, Skin catalase activity and splenic Gr-1+CD11b+ myeloid cell percentage were compared in all mice. A significant inverse correlation was detected (r = −0.69; p=0.0015).
A single UVB exposure does not induce splenic CD4+CD25+FoxP3+ regulatory T-cells
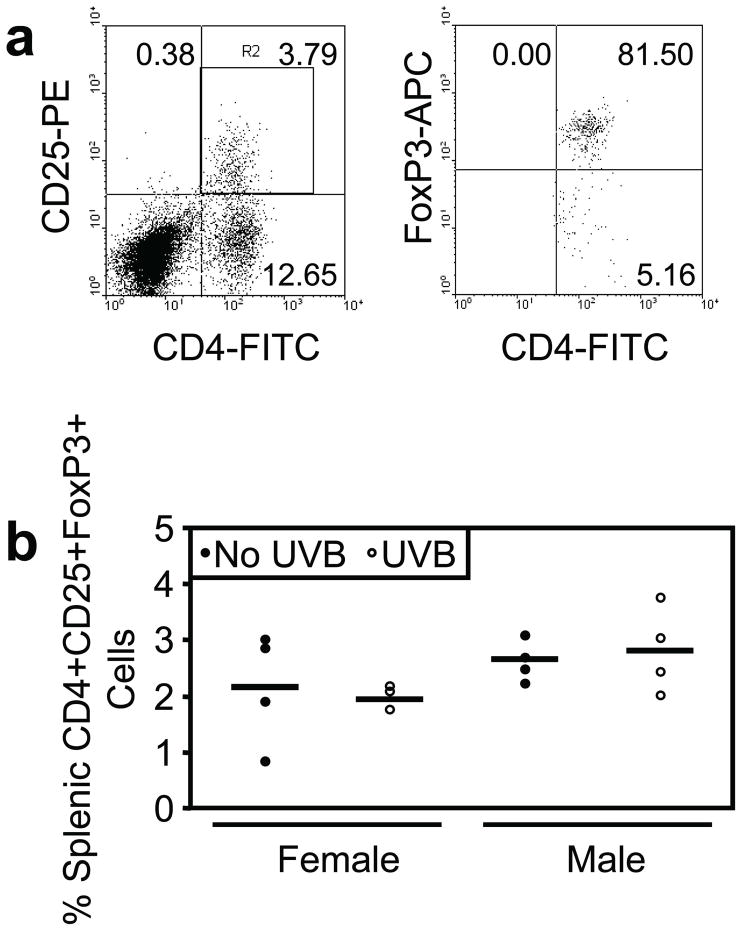
To characterize other inflammation-associated splenic immune cell populations that have been associated with UV-induced skin carcinogenesis (Daynes and Spellman, 1977; Elmets et al., 1983; Fisher and Kripke, 1982), we evaluated regulatory T-cells (T-regs) by flow cytometric analysis of cell surface CD4 and CD25 and intracellular FoxP3 in splenocytes following a single UVB exposure. Splenic T-regs were measured at 48 hours following a single UVB exposure in male and female mice. Representative flow cytometric analysis of splenocyte surface CD4 versus CD25 staining and subsequent intracellular FoxP3 staining is shown in Figure 3a. No change in CD4+CD25+FoxP3+ T-regs was detected in male or female mice following a single UVB exposure (Figure 3b).
Figure 3. Acute UVB exposure does not increase the percentage of splenic CD4+CD25+FoxP3+ regulatory T-cells.
Male and female mice were exposed to a single UVB dose (n=4 per sex). a, Splenic regulatory T-cells (T-regs) were assessed by flow cytometric analysis of cell surface CD4 and CD25 and intracellular FoxP3 at 48 hours following UVB exposure and in non-irradiated mice. b, UVB exposure did not enhance the percentage of T-regs in the spleen of male or female mice.
Catalase supplementation inhibits UVB-induced Gr-1+CD11b+ myeloid cell skin infiltration in male mice
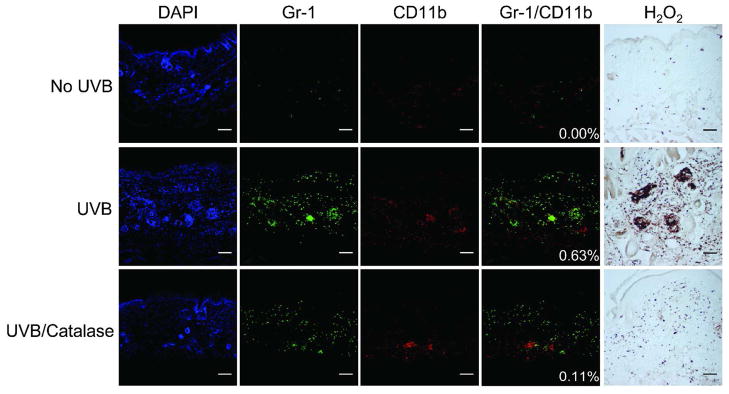
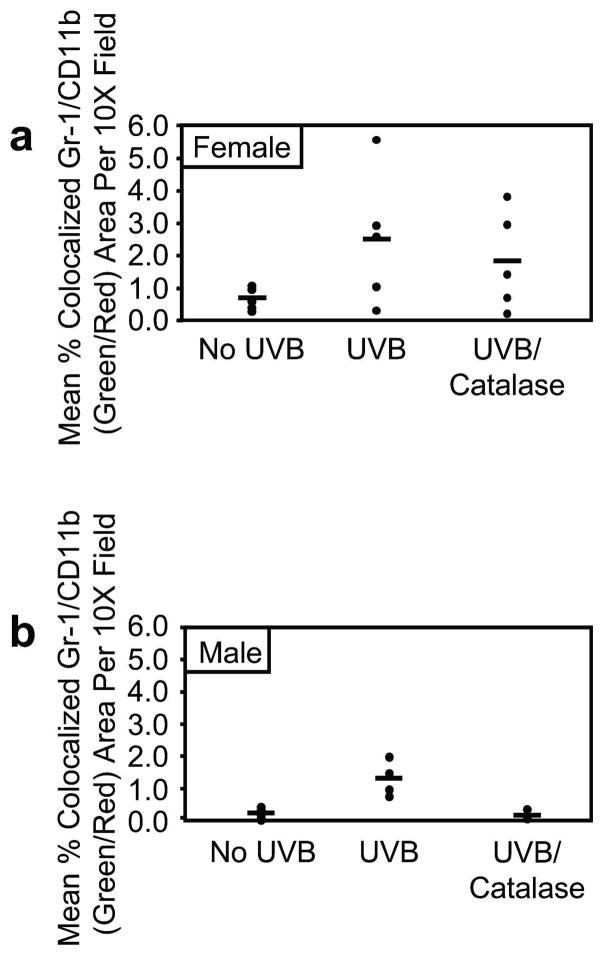
Our data demonstrated that UVB-mediated inhibition of skin catalase activity was associated with an increased percentage of Gr-1+CD11b+ myeloid cells in the spleen. In addition, previous studies have shown that catalase treatment concurrently inhibits proliferation while promoting differentiation of Gr-1+CD11b+ myeloid cells ex vivo (Kusmartsev and Gabrilovich, 2003). To directly test the effect of catalase on UVB-induced Gr-1+CD11b+ myeloid cell skin infiltration, male and female mice were treated topically with catalase immediately after and at 24 hours following a single UVB exposure. Gr-1+CD11b+ myeloid cell skin infiltration was evaluated by confocal immunofluorescence microscopy of Gr-1 (green) and CD11b (red) staining at 48 hours following a single UVB exposure. Representative skin images from male mice of green, red, and green/red merged (yellow) channels are shown in Figure 4. Histochemical staining of hydrogen peroxide was performed to validate the enzymatic neutralization of cutaneous hydrogen peroxide in catalase-treated mice. Representative images of male skin demonstrate elevated hydrogen peroxide at 48 hours following a single UVB exposure as compared to non-irradiated controls, and topical catalase treatment mitigated UVB-induced hydrogen peroxide levels (Figure 4). Whereas Gr-1 single-positive and CD11b single-positive cells were diffusely present in the skin of non-irradiated control mice, few to no Gr-1/CD11b double-positive cells were detected. Gr-1 single-positive and CD11b single-positive cells were detected at high levels following UVB exposure. In addition, Gr-1/CD11b double-positive cells in the skin were quantified as mean percentage of colocalized Gr-1/CD11b (yellow) area per 10X field. These cells were up-regulated upon UVB exposure in both male (p=0.0018) and female (p=0.0826) mice (Figure 5a-b). Although Gr-1 single-positive and CD11b single-positive cells were still detectable in the skin following UVB exposure and subsequent topical catalase treatment, catalase significantly reduced Gr-1/CD11b double-positive cells in the skin of male mice compared to UVB-exposed skin of vehicle-treated males (Figure 5b; p=0.0019). Interestingly, topical catalase led to a relatively modest reduction in Gr1/CD11b double-positive cells in the skin of female mice (Figure 5a).
Figure 4. Acute UVB exposure induces Gr-1+CD11b+ myeloid cell skin infiltration that is inhibited by topical catalase supplementation.
Mice (n=4 to 5 per treatment per sex) were treated topically with vehicle control or catalase immediately and at 24 hours following a single UVB exposure. Representative 10X images of Gr-1, CD11b, and Gr-1/CD11b merged channels are shown by confocal immunofluorescence microscopy in male skin. DAPI nuclear stain is shown in blue. Gr-1 and CD11b single-positive cells were present in the skin of non-irradiated control mice, but little to no Gr-1/CD11b colocalization was detected. At 48 hours post-UVB, skin Gr-1 and CD11b single-positive cell levels were higher, and Gr-1/CD11b colocalization was increased. Gr-1 and CD11b single-positive cells were still detected following UVB and subsequent topical catalase treatment. Catalase treatment inhibited Gr-1+CD11b+ myeloid cell skin infiltration. Histochemical staining of hydrogen peroxide showed UVB-induced cutaneous hydrogen peroxide, which was inhibited by topical catalase treatment. Scale bar = 5 mm.
Figure 5. Topical catalase supplementation inhibits acute UVB-induced Gr-1+CD11b+ myeloid cell skin infiltration in male mice.
Confocal immunofluorescence microscopy was used to quantify mean percentage of colocalized Gr-1/CD11b (green/red) area per 10X field in skin tissue sections. a, Compared to non-irradiated control mice, Gr-1/CD11b colocalization analysis demonstrated UVB-induced Gr-1+CD11b+ myeloid cell skin infiltration in female mice (p=0.0826). Topical catalase treatment had little effect in females. b, Male mice demonstrated UVB-induced Gr-1+CD11b+ myeloid cell skin infiltration (p=0.0018). Compared to UVB exposure and subsequent topical vehicle control treatment, catalase treatment significantly inhibited Gr-1+CD11b+ myeloid cell skin infiltration in male mice (p=0.0019). Horizontal bars represent means.
Male sex predicts elevated splenic Gr-1+CD11b+ myeloid cells in UVB-induced skin tumor-bearing mice
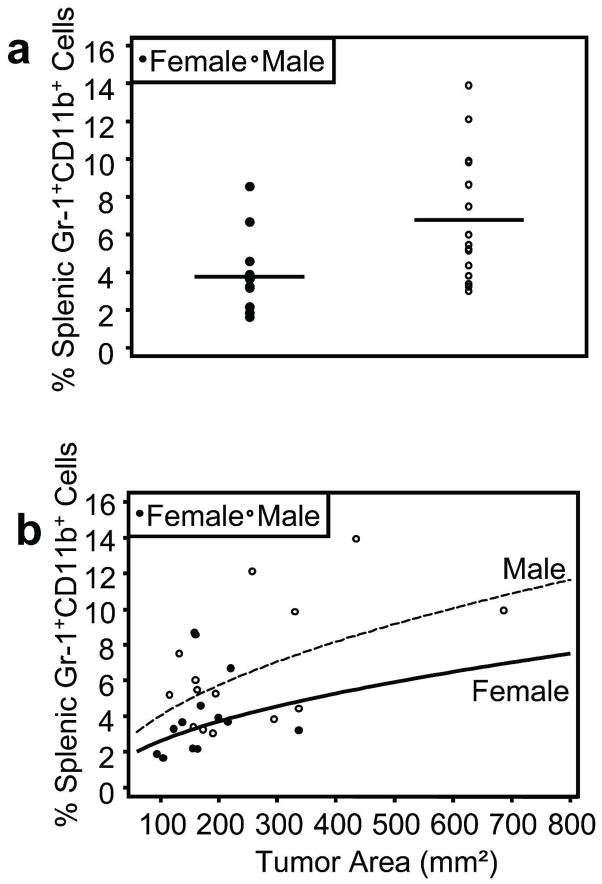
Because a single UVB exposure increased splenic percentages of Gr-1+CD11b+ myeloid cells, we evaluated this cell population in UVB-induced skin tumor-bearing mice. We have previously reported that male Skh-1 mice demonstrate an increased rate of UVB-induced skin carcinogenesis (Thomas-Ahner et al., 2007), which recapitulates that of human skin cancer. Therefore, we hypothesized that a greater number of systemic Gr-1+CD11b+ myeloid cells may be present in male tumor-bearing mice. Indeed, male tumor-bearing mice exhibited significantly higher percentages of splenic Gr-1+CD11b+ myeloid cells as compared to female tumor-bearing mice (p=0.005) (Figure 6a). To further evaluate the relationship between sex and splenic Gr-1+CD11b+ myeloid cell percentages, a multivariable linear regression model was constructed in which sex was evaluated as a predictor of splenic Gr-1+CD11b+ myeloid cell levels. After adjusting for tumor area, male sex was a significant predictor of elevated splenic Gr-1+CD11b+ myeloid cells (p=0.0280), and male tumor-bearing mice had an estimated 55% higher level of splenic Gr-1+CD11b+ myeloid cells for a given tumor area compared to female tumor-bearing mice (Figure 6b).
Figure 6. Male UVB-induced skin tumor-bearing mice exhibit higher percentages of splenic Gr-1+CD11b+ myeloid cells compared to females.
Male (n=15) and female mice (n=12) were exposed to UVB thrice weekly for 25 weeks to promote skin tumorigenesis. a, Flow cytometric analysis of Gr-1 and CD11b surface staining of splenocytes from skin tumor-bearing mice showed that males exhibited significantly higher percentages of splenic Gr-1+CD11b+ myeloid cells compared to females (p=0.005). Horizontal bars represent means. b, A multivariable linear regression model was constructed to determine if sex could predict splenic Gr-1+CD11b+ myeloid cell levels. After adjusting for total tumor area, male sex was a significant predictor of elevated splenic Gr-1+CD11b+ myeloid cells (p=0.0280) with an estimated 55% more splenic Gr-1+CD11b+ myeloid cells for a given tumor area.
Discussion
The incidence and mortality of skin cancer are higher in men compared to women. Although specific molecular and cellular mechanisms for this sex discrepancy remain poorly understood, imbalances in ROS have been proposed to underlie sex differences in skin cancer incidence, metastasis, and survival (Halliday, 2005; Joosse et al., 2010; Thomas-Ahner et al., 2007). Preceding data support our current findings to suggest that Gr-1+CD11b+ myeloid cells represent an additional cell population that contributes to UVB-induced inflammation and skin carcinogenesis. For example, previous reports have characterized myeloid cell skin infiltration during cutaneous inflammation and hypersensitivity reactions following acute UV exposure (Hammerberg et al., 1998; Katiyar et al., 1999; Sluyter and Halliday, 2001). Gr-1+ myeloid cells have been shown to support the growth of transplanted aggressive UV-induced tumors in athymic nude (Pekarek et al., 1995) and syngeneic euthymic mice (Seung et al., 1995). Likewise, Hammerberg, et al. showed that anti-CD11b treatment of immune competent mice inhibited UV-induced immune suppression (Hammerberg et al., 1996). In addition, MDSC were recently described in a chemically-induced cutaneous squamous cell carcinoma mouse model (Stumpfova et al., 2010). Our current results demonstrate a previously unreported link between UVB-mediated inhibition of skin catalase activity and the expansion of splenic and cutaneous Gr-1+CD11b+ myeloid cells. These findings highlight UVB-induced hydrogen peroxide as an important mediator of this initial expansion. Future work will be necessary to further investigate the molecular signaling pathways that promote the activation of UVB-induced Gr-1+CD11b+ myeloid cells in our model.
Catalase has been well-characterized as an antioxidant but has also been shown to regulate myeloid cell differentiation. For example, proliferation and differentiation of splenic Gr-1+CD11b+ myeloid cells purified from two independent orthotopic tumor models were inhibited and promoted, respectively, upon ex vivo catalase treatment (Kusmartsev and Gabrilovich, 2003). Our current results demonstrated lower baseline skin catalase activity in male mice as compared to females, and topical catalase supplementation inhibited Gr-1+CD11b+ myeloid cell skin infiltration in male mice. While female mice did show a reduction in Gr-1+CD11b+ myeloid cell skin infiltration following topical catalase treatment, this effect was less pronounced than that seen in males. This difference may be explained by our data demonstrating increased sensitivity to UVB-mediated inhibition of skin catalase activity in female mice compared to males. Collectively, these results indicate that the same UVB-induced factors that promote the inhibition of endogenous skin catalase activity, which was greater in females compared to males, could potentially neutralize the effects of any additional catalase supplementation. These data indicate that inherent sex differences could influence therapeutic intervention for controlling UVB-induced inflammation. Furthermore, we detected an inverse correlation between skin catalase activity and splenic Gr-1+CD11b+ myeloid cell percentages. In addition, male skin tumor-bearing mice exhibited higher percentages of splenic Gr-1+CD11b+ myeloid cells compared to their female counterparts. These data suggest that the level of skin catalase activity may influence inflammation and the expansion of Gr-1+CD11b+ myeloid cells following UVB exposure.
Increased systemic Gr-1+CD11b+ myeloid cells have been described in numerous murine tumor models, and we now show that this phenomenon extends to a model of UVB-induced non-melanoma skin cancer. Collectively, our findings demonstrate that endogenous skin catalase activity may regulate Gr-1+CD11b+ myeloid cell expansion to the spleen and skin of Skh-1 mice. Conversely, UVB exposure inhibited skin catalase activity, which correlated with increased splenic Gr-1+CD11b+ myeloid cell expansion and promoted Gr-1+CD11b+ myeloid cell skin infiltration. These data strongly suggest that excessive hydrogen peroxide contributes to Gr-1+CD11b+ myeloid cell expansion following UVB exposure. Furthermore, our results highlight inherent sex discrepancies in skin catalase activity and percentages of splenic Gr-1+CD11b+ myeloid cells in skin tumor-bearing mice. Together, these data emphasize the influence of sex in inflammation-related cancers.
Materials and Methods
Animal Treatments
Outbred, immune competent male and female Skh-1 hairless mice (6 to 8-week old; Charles River Laboratories, Wilmington, MA) were housed in the vivarium at The Ohio State University according to the requirements established by the American Association for Accreditation of Laboratory Animal Care. Prior to beginning all studies, procedures were approved by the appropriate Institutional Animal Care and Use Committee. All mice were dorsally exposed to 2,240 J/m2 UVB, previously determined to be one minimal erythemic dose. UVB dose was determined by a UVX meter (UVP Inc., Upland, CA) and emitted by Philips FS40 UV lamps (American Ultraviolet Company, Lebanon, IN). All acute studies utilized a single UVB dose, and all acute data are at 48 hours post-UVB. 100 IU per mouse of bovine catalase conjugated to polyethylene glycol (Sigma-Aldrich, St. Louis, MO) or Surgilube (Savage Laboratories, Melville, NY) vehicle control was administered topically to mouse dorsal skin immediately following UVB exposure and at 24 hours post-UVB. Non-irradiated control mice were treated topically with vehicle alone. For skin tumor studies, mice were exposed to UVB three times per week on non-consecutive days for twenty-five weeks. Age-matched, non-irradiated mice served as controls throughout chronic tumor studies.
Immediately following sacrifice, 0.5 cm2 dorsal skin was removed and incubated in 4% fresh paraformaldehyde prepared in sodium phosphate buffer (pH 7.2–7.4) for 4 hours at 4ºC. Remaining dorsal skin was removed and snap frozen in liquid nitrogen for catalase activity assays. Fixed skin was incubated in sodium phosphate buffer containing 20% sucrose at 4ºC overnight. Skin was then incubated in 5% glycerol in sodium phosphate buffer containing 20% sucrose for 2 hours at 4ºC, embedded in OCT freezing compound (Sakura, Torrance, CA), and stored at −80ºC for hydrogen peroxide and immunofluorescence staining.
Catalase activity
Snap frozen dorsal skin was crushed and catalase activity was determined using the Catalase Assay Kit (Cayman Chemical, Ann Arbor, MI) according to the manufacturer’s protocol.
Histochemical detection of hydrogen peroxide
Paraformaldehyde-fixed/OCT-embedded dorsal skin tissue sections were cut (5 μm) onto Superfrost Plus® microscope slides (Fisher Scientific, Pittsburgh, PA) and stored at −80ºC until needed. Histochemical methods are based on those described by Dannenberg and colleagues (Dannenberg et al., 1994). Briefly, slides were thawed for one hour at room temperature and incubated in 0.1M Tris-HCl buffer (pH 7.5) containing 1mg/ml glucose (Sigma-Aldrich) and 1mg/ml 3,3′-diaminobenzidine (DAB) tetrahydrochlorate hydrate (Sigma-Aldrich) for five hours at 37°C. Slides were washed in deionized water, dehydrated in the following order: 75% ethanol, 95% ethanol, 100% ethanol x 2, Clear Rite 3 (Richard-Allan Scientific, Kalamazoo, MI) x 2, and cover slipped with VectaMount mounting medium (Vector Laboratories, Burlingame, CA). Negative control slides were incubated with 150 μg/ml bovine catalase (Sigma-Aldrich) in phosphate-buffered saline (PBS) for 15 minutes at room temperature, and 150 μg/ml bovine catalase was included in the five-hour DAB incubation. Slides were evaluated with a Nikon Eclipse E400 microscope (Melville, NY, USA), and images were taken with a Nikon DXM1200 digital camera using Nikon ACT-1 version 2.63 software.
Direct immunofluorescence
Paraformaldehyde-fixed/OCT-embedded dorsal skin tissue or skin tumor sections were cut (10 μm) onto Superfrost Plus® microscope slides (Fisher Scientific) and stored at −80ºC until needed. Slides were thawed for one hour at room temperature, washed one time in PBS, and blocked in 1X casein (Vector Laboratories) for 30 minutes at room temperature. Slides were dual-labeled with rat anti-mouse Gr-1 (Ly-6C/Ly-6G)-Alexa Fluor® 488 (Invitrogen, Camarillo, CA) and rat anti-mouse CD11b-Alexa Fluor® 647 (BD Biosciences, San Jose, CA) antibodies diluted to 5 μg/ml and 2.5 μg/ml, respectively, in 1X casein and incubated for one hour at room temperature. Background fluorescence was determined using unstained control slides, and red-green channel bleed over was not detected using single-labeled slides. Following two PBS washes, slides were incubated with DAPI nuclear stain, and mounted with ImmuMount mounting medium (Thermo Fisher Scientific, Waltham, MA). All immunofluorescent images were acquired at 10X or 40X magnification with an Olympus FluoView™ 1000 spectral confocal microscope imaging system (Olympus, Center Valley, PA), and Image Processing and Analysis in Java (ImageJ) software (http://rsbweb.nih.gov/ij) was utilized for Gr-1/CD11b colocalization analysis of 10X images.
Splenocyte isolation
Spleens from Skh-1 mice were removed aseptically, disaggregated into single cell suspensions, and 0.2 μm filtered. Splenocytes were washed with PBS by centrifugation, underwent ammonium chloride red blood cell lysis, and were cryopreserved in 90% fetal bovine serum (FBS)/10% dimethyl sulfoxide (DMSO) until flow cytometric analysis.
Flow cytometric analyses
Splenocytes were thawed, washed with RPMI-1640 containing 10% FBS (hereafter referred to as splenocyte medium) by centrifugation, and resuspended in splenocyte medium. 5 x 105 cells per test were stained in 100 μl PBS containing 5% FBS (hereafter referred to as flow buffer). Antibodies used for myeloid cell surface staining were as follows. Rat anti-Gr-1-FITC or rat IgG2b-FITC (isotype control), rat anti-CD11b-APC or rat IgG2b-APC, rat anti-Ly-6G-V450 or rat IgG2b-V450, rat anti-Ly-6C-PeCy7 or rat IgM-PeCy7 (BD Biosciences). Ly-6G+ and Ly-6C+ events were sequentially gated from Gr-1+CD11b+ events for subset analyses. Cells were incubated on ice in the dark for one hour, washed with flow buffer by centrifugation, and fixed in PBS containing 1% formalin for flow cytometric analysis. The Mouse Regulatory T-cell Staining Kit #2 (eBioscience) was used for surface CD4 and CD25 staining and subsequent intracellular FoxP3 staining according to the manufacturer’s instructions. FoxP3+ events were sequentially gated from CD4+CD25+ events for CD4+CD25+FoxP3+ regulatory T-cell analysis. No fewer than 1 x 104 events per sample were collected on a BD FACSCalibur™ or LSRII flow cytometer (BD Biosciences) and subsequently analyzed with Windows Multiple Document Interface (WinMDI) Flow Cytometry Application version 2.9 (http://facs.scripps.edu/software.html).
Statistical analyses
Outcome data were analyzed using an ANOVA approach with linear contrasts used for testing the comparisons of interest. Residual plots were used to verify the model assumptions of normality and homoscedasticity and a logarithmic transformation was utilized, if necessary. Multivariable linear regression was used to model splenic Gr-1+CD11b+ myeloid cell levels by sex and tumor burden after log transforming both the outcome and tumor area. P-values ≤ 0.05 were considered statistically significant.
Acknowledgments
We thank the OSU CCC Analytical Cytometry and Biostatistics Shared Resources. Immunofluorescent confocal images used in this report were generated with the equipment and resources at The Campus Microscopy and Imaging Facility, The Ohio State University. We thank Dr. Pawel Kalinski for critical review of this manuscript.
Financial Support: This work was supported by NIH grants CA133629 (TMO), K22CA134551 (GBL), T32 60019501 (NJS), The Valvano Foundation for Cancer Research (GBL), and a seed grant from the OSUCCC Molecular Carcinogenesis and Chemoprevention Program (TMO).
Footnotes
Conflict of interest. The authors declare no conflicts of interest.
References
- Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8–18. doi: 10.1016/s1011-1344(01)00198-1. [DOI] [PubMed] [Google Scholar]
- Dannenberg AM, Jr, Schofield BH, Rao JB, Dinh TT, Lee K, Boulay M, et al. Histochemical demonstration of hydrogen peroxide production by leukocytes in fixed-frozen tissue sections of inflammatory lesions. J Leukoc Biol. 1994;56:436–443. doi: 10.1002/jlb.56.4.436. [DOI] [PubMed] [Google Scholar]
- Daynes RA, Spellman CW. Evidence for the generation of suppressor cells by ultraviolet radiation. Cell Immunol. 1977;31:182–187. doi: 10.1016/0008-8749(77)90018-1. [DOI] [PubMed] [Google Scholar]
- Elmets CA, Bergstresser PR, Tigelaar RE, Wood PJ, Streilein JW. Analysis of the mechanism of unresponsiveness produced by haptens painted on skin exposed to low dose ultraviolet radiation. J Exp Med. 1983;158:781–794. doi: 10.1084/jem.158.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher MS, Kripke ML. Suppressor T lymphocytes control the development of primary skin cancers in ultraviolet-irradiated mice. Science. 1982;216:1133–1134. doi: 10.1126/science.6210958. [DOI] [PubMed] [Google Scholar]
- Foote JA, Harris RB, Giuliano AR, Roe DJ, Moon TE, Cartmel B, et al. Predictors for cutaneous basal- and squamous-cell carcinoma among actinically damaged adults. Int J Cancer. 2001;95:7–11. doi: 10.1002/1097-0215(20010120)95:1<7::aid-ijc1001>3.0.co;2-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Butts B, Kwei KA, Dvorakova K, Stratton SP, Briehl MM, et al. Attenuation of catalase activity in the malignant phenotype plays a functional role in an in vitro model for tumor progression. Cancer Lett. 2001;173:115–125. doi: 10.1016/s0304-3835(01)00656-5. [DOI] [PubMed] [Google Scholar]
- Halliday GM. Inflammation, gene mutation and photoimmunosuppression in response to UVR-induced oxidative damage contributes to photocarcinogenesis. Mutat Res. 2005;571:107–120. doi: 10.1016/j.mrfmmm.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Hammerberg C, Duraiswamy N, Cooper KD. Reversal of immunosuppression inducible through ultraviolet-exposed skin by in vivo anti-CD11b treatment. J Immunol. 1996;157:5254–5261. [PubMed] [Google Scholar]
- Hammerberg C, Katiyar SK, Carroll MC, Cooper KD. Activated complement component 3 (C3) is required for ultraviolet induction of immunosuppression and antigenic tolerance. J Exp Med. 1998;187:1133–1138. doi: 10.1084/jem.187.7.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joosse A, de Vries E, Eckel R, Nijsten T, Eggermont AM, Holzel D, et al. Gender differences in melanoma survival: female patients have a decreased risk of metastasis. J Invest Dermatol. 2011;131:719–726. doi: 10.1038/jid.2010.354. [DOI] [PubMed] [Google Scholar]
- Joosse A, De Vries E, van Eijck CH, Eggermont AM, Nijsten T, Coebergh JW. Reactive oxygen species and melanoma: an explanation for gender differences in survival? Pigment Cell Melanoma Res. 2010;23:352–364. doi: 10.1111/j.1755-148X.2010.00694.x. [DOI] [PubMed] [Google Scholar]
- Katiyar SK, Challa A, McCormick TS, Cooper KD, Mukhtar H. Prevention of UVB-induced immunosuppression in mice by the green tea polyphenol (-)-epigallocatechin-3-gallate may be associated with alterations in IL-10 and IL-12 production. Carcinogenesis. 1999;20:2117–2124. doi: 10.1093/carcin/20.11.2117. [DOI] [PubMed] [Google Scholar]
- Kusmartsev S, Gabrilovich DI. Inhibition of myeloid cell differentiation in cancer: the role of reactive oxygen species. J Leukoc Biol. 2003;74:186–196. doi: 10.1189/jlb.0103010. [DOI] [PubMed] [Google Scholar]
- Kwei KA, Finch JS, Thompson EJ, Bowden GT. Transcriptional repression of catalase in mouse skin tumor progression. Neoplasia. 2004;6:440–448. doi: 10.1593/neo.04127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Gu X, Robbins D, Li G, Shi R, McCord JM, et al. Protandim, a fundamentally new antioxidant approach in chemoprevention using mouse two-stage skin carcinogenesis as a model. PLoS One. 2009;4:e5284. doi: 10.1371/journal.pone.0005284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekarek LA, Starr BA, Toledano AY, Schreiber H. Inhibition of tumor growth by elimination of granulocytes. J Exp Med. 1995;181:435–440. doi: 10.1084/jem.181.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pence BC, Naylor MF. Effects of single-dose ultraviolet radiation on skin superoxide dismutase, catalase, and xanthine oxidase in hairless mice. J Invest Dermatol. 1990;95:213–216. doi: 10.1111/1523-1747.ep12478037. [DOI] [PubMed] [Google Scholar]
- Sander CS, Chang H, Salzmann S, Muller CS, Ekanayake-Mudiyanselage S, Elsner P, et al. Photoaging is associated with protein oxidation in human skin in vivo. J Invest Dermatol. 2002;118:618–625. doi: 10.1046/j.1523-1747.2002.01708.x. [DOI] [PubMed] [Google Scholar]
- Sander CS, Hamm F, Elsner P, Thiele JJ. Oxidative stress in malignant melanoma and non-melanoma skin cancer. Br J Dermatol. 2003;148:913–922. doi: 10.1046/j.1365-2133.2003.05303.x. [DOI] [PubMed] [Google Scholar]
- Schafer M, Werner S. Oxidative stress in normal and impaired wound repair. Pharmacol Res. 2008;58:165–171. doi: 10.1016/j.phrs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Seung LP, Rowley DA, Dubey P, Schreiber H. Synergy between T-cell immunity and inhibition of paracrine stimulation causes tumor rejection. Proc Natl Acad Sci U S A. 1995;92:6254–6258. doi: 10.1073/pnas.92.14.6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindo Y, Witt E, Han D, Packer L. Dose-response effects of acute ultraviolet irradiation on antioxidants and molecular markers of oxidation in murine epidermis and dermis. J Invest Dermatol. 1994;102:470–475. doi: 10.1111/1523-1747.ep12373027. [DOI] [PubMed] [Google Scholar]
- Sluyter R, Halliday GM. Infiltration by inflammatory cells required for solar-simulated ultraviolet radiation enhancement of skin tumor growth. Cancer Immunol Immunother. 2001;50:151–156. doi: 10.1007/PL00006685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpfova M, Ratner D, Desciak EB, Eliezri YD, Owens DM. The immunosuppressive surface ligand CD200 augments the metastatic capacity of squamous cell carcinoma. Cancer Res. 2010;70:2962–2972. doi: 10.1158/0008-5472.CAN-09-4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas-Ahner JM, Wulff BC, Tober KL, Kusewitt DF, Riggenbach JA, Oberyszyn TM. Gender differences in UVB-induced skin carcinogenesis, inflammation, and DNA damage. Cancer Res. 2007;67:3468–3474. doi: 10.1158/0008-5472.CAN-06-3798. [DOI] [PubMed] [Google Scholar]