Abstract
Purpose
A symbiotic relationship between ovarian granulosa cells (GC) and the developing oocyte is critical. Genetic modulations in GC’s can lead to reproductive insufficiency, highlighting the role of GC’s in reproductive competence. Utilizing gene expression analyses in cumulus GC’s, we attempt to enhance our understanding of mechanisms that may contribute to poor reproductive capacity in young women with diminished ovarian reserve (DOR).
Methods
We measured gremlin 1 (GREM1) gene expression in GC’s from infertile women <38 years undergoing in vitro fertilization in the context of DOR.
Results
GREM1, a member of the differential screening-selected gene aberrative in neuroblastoma (DAN) family of genes known for its highly regulated expression pattern during folliculogenesis and a downstream effecter of oocyte-derived growth and differentiation factor 9, was down-regulated 3-fold (−3.08) in women with DOR versus control; down-regulation was confirmed by qRT-PCR (−4.02).
Conclusion
This is the first demonstration linking differential expression of Gremlin with etiology of infertility in women.
Keywords: Cumulus granulosa cell, Gremlin 1, Diminished ovarian reserve, GDF9, Gene expression
Introduction
Cumulus granulosa cells (GC’s) are in intimate contact with the enclosed oocyte, and cross-communication between the somatic GC’s and the developing germ cell are integral to the process of folliculogenesis [1, 2] and reproductive competence [3, 4]. Growth and differentiation factor 9 (GDF9) and its homologues bone morphogenic protein 15 (BMP15, also known as GDF9ß) and BMP6 are currently regarded as the best candidate molecules responsible for oocyte regulation of cumulus GC expansion during ovulation, which is imperative for normal oocyte development in vivo [5]. GDF9 induces cumulus GC gene expression of gremlin 1 (GREM1) [5], a highly-conserved member of the differential screening-selected gene aberrative in neuroblastoma (DAN) family of secreted factors involved in regulating the balance of GDF9 and BMP15 correlated with embryonic development [6].
A number of recent studies have evaluated cumulus cell GREM1 expression as a function of oocyte competence following ART. Increased GREM1 expression in human cumulus GC’s has been associated with better blastocyst formation [7, 8], good embryo quality and a non-significantly improved pregnancy rate [9], and increased female age and good embryo development but decreased serum progesterone on day of hCG in women who received FSH alone but not FSH and LH [10]. The literature reflects considerable between-patient variation for human cumulus GC GREM1 expression [6, 10], and given the complexity of the interactions observed and the currently limited knowledge about the function of the cumulus GC genes, it is still difficult to fully explain the biological/causal correlations seen [11].
We have previously reported on a differential pattern of GC gene expression in young women with diminished ovarian reserve (DOR) compared to those with normal ovarian reserve [12]; we herein report a significant down regulation in the expression of GREM1, as assessed by quantitative real time PCR (qRT-PCR) in cumulus GC’s of women with DOR (n = 4) versus controls (n = 4). Given the relevance of GREM1 for normal follicular development and oocyte competence, our observations suggest that down-regulation of GREM1 in GC’s of women with DOR may be contributory to the reproductive compromise that is well recognized in the context of DOR.
Materials and methods
Details of patient enrollment, inclusion criteria, IVF treatment protocol, cumulus GC isolation, RNA extraction, microarray analysis and PCR methodology have been previously described [12]. Briefly cumulus GC’s were collected from eligible infertile women undergoing in vitro fertilization (IVF) cycles at Montefiore’s Institute for Reproductive Medicine and Health who provided IRB-approved consent. As per our clinical practice guidelines, highest early follicular FSH levels (days 1–3) ≥ 10.0 mIU/mL identified those with DOR (n = 4), whereas levels ≤10 mIU/mL and E2 < 80 pg/mL were taken to reflect normal ovarian reserve (controls, n = 4). Secondary infertility diagnoses ovulatory dysfunction, endometriosis, and tubal disease with hydrosalpinx were exclusion criteria in an attempt to minimize confounding of data by conditions that have previously been related to distinct GC gene profiles. GREM1 gene expression in cumulus GC’s of the control and DOR patients was assessed by qRT-PCR. Results are expressed as relative fold-changes in genes, using the 2-ΔΔCT method.
Statistical analyses
More than 2-fold difference in GREM1 gene expression between the two groups was considered biologically relevant. Statistical analyses were performed using the nonparametric Mann–Whitney two-sample rank sum test to detect differences in patient characteristics (expressed as median + interquartile range IQR). Student’s t-test was used to assess differences in microarray data between control and DOR patients. STATA (Intercooled STATA version 9, Statacorp, College Station, TX) was utilized for microarray and qRT-PCR analyses and p < 0.05 was deemed as statistically significant.
Results
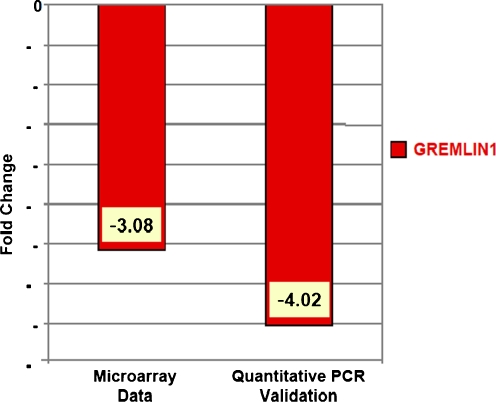
Patient characteristics for the two groups (control and DOR) were compared and previously published [12]. Microarray analysis revealed a number of genes down-regulated 2-fold or greater in the cumulus GC’s from women with DOR compared to controls [12]. Of interest, GREM1 expression was down-regulated as measured by microarray, and this was confirmed by qRT-PCR. Figure 1 compares the microarray and qRT-PCR validation old-changes between control and DOR groups. A 4-fold reduction in the expression of GREM1 was observed in cumulus GC’s of women with DOR compared to the controls.
Fig. 1.
GREMLIN1 is down-regulated in cumulus granulosa cells in women with diminished compared with normal ovarian reserve
Discussion
While GREM1 is recognized as relevant for folliculogenesis, we are the first to demonstrate an association of GREM1 gene expression in young women with DOR. The 4-fold down-regulation of GREM1 gene expression seen may indicate impaired oocyte function and cumulus GC expansion, associated with poor reproductive outcome. It has been previously shown that increased GREM1 expression in cumulus GC’s is a positive predictor of oocyte maturity, fertilization, embryo development and pregnancy rate in women undergoing IVF [6–11]. Our data suggests a corollary to these findings: that a decrease in GREM1 expression in cumulus GC’s is specific to women with diminished reproductive potential. It is possible that patients with markedly reduced cumulus GC GREM1 expression may have an underlying defect in GDF9 or other oocyte-secreted mediators of cumulus GC physiology, thus suggesting a mechanism for reproductive compromise. Genetic variants of GDF9 have been associated with polycystic ovarian syndrome, premature ovarian failure [13] and with DOR and poor IVF outcomes [14], perhaps through aberrant regulation of cumulus GC GREM1 expression, as is suggested by our data.
Further, the endocrine profiles of these women may be altered, in association with their infertility diagnosis [15], body mass index [16], or possible diminished ovarian reserve [17]. These altered endocrine profiles may in turn affect cumulus cell gene expression of folliculogenic factors such as GREM1, again suggesting a mechanism for impaired ovarian function.
We have previously demonstrated down-regulation of gene expression of members of the IGF family in luteinized cumulus GC’s and mural GC’s in young women with DOR versus control [12]. We hypothesized that this decrease in IGF gene expression in young women with DOR may explain the suboptimal serum estradiol levels during controlled ovarian hyperstimulation that are commonly noted in women with DOR. Here we confirm the association of GREM1 with etiology of infertility, specific to DOR and not related to aging.
The small sample size is an obvious limitation of our study that merits acknowledgement; the sample constraints can however be explained by the stringent exclusion and inclusion criteria that were employed in order to reduce a potential for confounding. Patients were allocated based on early follicular phase FSH levels, a single biomarker of ovarian reserve [12]. Though other biomarkers were not additionally assessed (e.g. anti-mullerian hormone), women evaluated to have DOR based on their FSH level required significantly more ampules of gonadotropin for controlled ovarian hyperstimulation, compared to the controls (data not shown), validating our clinical protocol. Further, the significant finding of decreased GREM1 expression was consistently seen in all DOR patients compared to the controls. In this study, cells for each patient were pooled for RNA isolation, meaning our findings could not be extrapolated to individual oocyte or embryo quality, or pregnancy rates. In a larger study of different design, the association of GREM1 expression in DOR patients with ART outcomes can be evaluated.
In summary, down-regulation of GREM1 was observed in the cumulus GC’s of young women with DOR compared to controls. While the causal relationship cannot be elucidated, these observations may reflect an underlying defect in GDF9 or other oocyte-secreted mediators of cumulus physiology, and merit further study.
Acknowledgements
The authors would like to acknowledge the invaluable contributions of Dr. Andrew Brooks in microarray and RT-PCR interpretation, and Joshua Hurwitz MD, Athena Zapantis BS, and Michael Nihsen MS for technical assistance.
Footnotes
All research was performed in the Division of REI, Department of Obstetrics, Gynecology & Women’s Health, Albert Einstein College of Medicine, Bronx, NY and Montefiore’s Institute for Reproductive Medicine and Health, Hartsdale, NY. A portion of this work was presented at the Annual Meeting of the Society for Gynecologic Investigation, March 2008.
Financial Support
This investigation was supported by grants from Ferring Pharmaceuticals, Inc (to SJ) and NIH grants 5K12 RR17672 (to LP) and K24 CD41978 (to NS).
Capsule Gremlin 1 expression is impaired in cumulus granulosa cells of young women with diminished ovarian reserve (DOR), suggesting a possible mechanism for the ovarian dysfunction associated with DOR.
References
- 1.Canipari R. Oocyte–granulosa cell interactions. Hum Reprod Update. 2000;6:279–289. doi: 10.1093/humupd/6.3.279. [DOI] [PubMed] [Google Scholar]
- 2.Gilchrist RB, Ritter LJ, Armstrong DT. Oocyte-somatic cell interactions during follicle development in mammals. Anim Reprod Sci. 2004;82–83:431–446. doi: 10.1016/j.anireprosci.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 3.Li R, Norman RJ, Armstrong DT, Gilchrist RB. Oocyte-secreted factor(s) determine functional differences between bovine mural granulosa cells and cumulus cells. Biol Reprod. 2000;63:839–845. doi: 10.1095/biolreprod63.3.839. [DOI] [PubMed] [Google Scholar]
- 4.Albertini DF, Combelles CM, Benecchi E, Carabatsos MJ. Cellular basis for paracrine regulation of ovarian follicle development. Reproduction. 2001;121:647–653. doi: 10.1530/rep.0.1210647. [DOI] [PubMed] [Google Scholar]
- 5.Pangas SA, Jorgez CJ, Matzuk MM. Growth differentiation factor 9 regulates expression of the bone morphogenetic protein antagonist gremlin. J Biol Chem. 2004;279:32281–32286. doi: 10.1074/jbc.M403212200. [DOI] [PubMed] [Google Scholar]
- 6.Wathlet S, Adriaenssens T, Segers I, Verheyen G, Velde H, Coucke W, et al. Cumulus cell gene expression predicts better cleavage-stage embryo or blastocyst development and pregnancy for ICSI patients. Hum Reprod. 2011;26:1035–1051. doi: 10.1093/humrep/der036. [DOI] [PubMed] [Google Scholar]
- 7.McKenzie LJ, Pangas SA, Carson SA, Kovanci E, Cisneros P, Buster JE, et al. Human cumulus granulosa cell gene expression: a predictor of fertilization and embryo selection in women undergoing IVF. Hum Reprod. 2004;19:2869–2874. doi: 10.1093/humrep/deh535. [DOI] [PubMed] [Google Scholar]
- 8.Cillo F, Brevini TA, Antonini S, Paffoni A, Ragni G, Gandolfi F. Association between human oocyte developmental competence and expression levels of some cumulus genes. Reproduction. 2007;134:645–650. doi: 10.1530/REP-07-0182. [DOI] [PubMed] [Google Scholar]
- 9.Anderson RA, Sciorio R, Kinnell H, Bayne RA, Thong KJ, Sousa PA, et al. Cumulus gene expression as a predictor of human oocyte fertilisation, embryo development and competence to establish a pregnancy. Reproduction. 2009;138:629–637. doi: 10.1530/REP-09-0144. [DOI] [PubMed] [Google Scholar]
- 10.Adriaenssens T, Segers I, Wathlet S, Smitz J. The cumulus cell gene expression profile of oocytes with different nuclear maturity and potential for blastocyst formation. J Assist Reprod Genet. 2011;28:31–40. doi: 10.1007/s10815-010-9481-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adriaenssens T, Wathlet S, Segers I, Verheyen G, Vos A, Elst J, et al. Cumulus cell gene expression is associated with oocyte developmental quality and influenced by patient and treatment characteristics. Hum Reprod. 2010;25:1259–1270. doi: 10.1093/humrep/deq049. [DOI] [PubMed] [Google Scholar]
- 12.Greenseid K, Jindal S, Hurwitz J, Santoro N, Pal L. Differential granulosa cell gene expression in young women with diminished ovarian reserve. Reprod Sci. 2011;18:892–899. doi: 10.1177/1933719111398502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilchrist RB, Lane M, Thompson JG. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum Reprod Update. 2008;14:159–177. doi: 10.1093/humupd/dmm040. [DOI] [PubMed] [Google Scholar]
- 14.Wang TT, Wu YT, Dong MY, Sheng JZ, Leung PC, Huang HF. G546A polymorphism of growth differentiation factor-9 contributes to the poor outcome of ovarian stimulation in women with diminished ovarian reserve. Fertil Steril. 2010;94:2490–2492. doi: 10.1016/j.fertnstert.2010.03.070. [DOI] [PubMed] [Google Scholar]
- 15.Wei HJ, Young R, Kuo IL, Liaw CM, Chiang HS, Yeh CY. Prevalence of insulin resistance and determination of risk factors for glucose intolerance in polycystic ovary syndrome: a cross-sectional study of Chinese infertility patients. Fertil Steril. 2009;91:1864–1868. doi: 10.1016/j.fertnstert.2008.02.168. [DOI] [PubMed] [Google Scholar]
- 16.Buyuk E, Seifer DB, Illions E, Grazi RV, Lieman H. Elevated body mass index is associated with lower serum anti-mullerian hormone levels in infertile women with diminished ovarian reserve but not with normal ovarian reserve. Fertil Steril. 2011;95:2364–2368. doi: 10.1016/j.fertnstert.2011.03.081. [DOI] [PubMed] [Google Scholar]
- 17.Gleicher N, Weghofer A, Barad DH. Anti-Mullerian hormone (AMH) defines, independent of age, low versus good live-birth chances in women with severely diminished ovarian reserve. Fertil Steril. 2010;94:2824–2827. doi: 10.1016/j.fertnstert.2010.04.067. [DOI] [PubMed] [Google Scholar]