Abstract
Purpose
This study was designed to investigate the effect of alpha-lipoic acid (ALA) on reactive oxygen species (ROS) production, total antioxidant capacity (TAC) and developmental competence of cultured pre-antral follicles derived from mouse ovarian tissue.
Methods
Pre-antral follicles were isolated from immature mouse ovaries and were cultured in α- minimal essential medium supplemented with different concentrations (0, 50, 100, 250 and 500 uM) of ALA. Follicular growth, oocyte maturation and embryo development were evaluated. Separately, ROS and TAC were measured after 0, 24, 48, 72 and 96 h of culture with spectrofluorometery and ferric reducing/antioxidant power (FRAP) assay, respectively.
Results
In the presence of 100 uM ALA, developmental rates of follicles, oocytes and embryos were significantly higher than other groups (p < 0.05). At 96 h after culture, a decrease in ROS and an increase in TAC were observed in ALA group compared to control group (p < 0.05).
Conclusion
ALA (100 uM) improves the in vitro development of follicles. This effect may be mediated by decreasing ROS concentration and increasing follicular TAC level during the culture period.
Keywords: Preantral follicles, Alpha-lipoic acid, Reactive oxygen species, Total antioxidant capacity
Introduction
Recent advances in the field of reproductive medicine were achieved utilizing assisted reproductive techniques (ART). Among these techniques, we can point to the in vitro culture of follicles. The culture of pre-antral follicles gives resources for the investigation of the physiology of follicular growth and ovulation and for producing a consistent population of competent oocytes for in vitro fertilization.
In vitro follicular development and maturation are affected by many factors. In this sense oxidative stress (OS) has been recently implicated as one of the most effective factors [3, 7, 44, 56]. Critical levels of reactive oxygen species (ROS) are necessary for many biochemical pathways involve in physiological functions. Oxidative stress has been known as excessive production of ROS or imbalance between the production of ROS and antioxidant defense system (i.e., oxidants exceed antioxidant [7, 22]). Under physiological conditions, generation of ROS occurs during various cellular metabolic reactions which is equilibrated by antioxidant defense systems of cells in order to neutralize the reactive intermediate [4, 6]. In the in vivo condition, enzymatic and non-enzymatic antioxidants provide adequate protection for OS-induced pathological changes and maintain an optimal level of ROS, whereas in the in vitro setup, higher oxygen levels and lack of physiological defense mechanisms against ROS result in OS (Sajal [34, 47]). Also, it has been shown that, OS can be induced during ART procedure by manipulation of gametes and embryos [50]. It has been indicated that OS may affect developmental competence of oocytes during in vitro maturation that may be obvious after fertilization [49]. It appears that extra ROS production in granulosa cells causes deleterious effect on oocyte fertilization and embryo development [12]. It has been reported that ROS may contribute in the oocyte meiotic arrest in the germinal vesicle (GV) stage [20] and induce embryonic developmental arrest and cell death [19].
Protection of embryos and oocytes against OS can be measured by total antioxidant capacity (TAC) which includes the enzymatic antioxidant system (e.g., glutathione peroxidase and superoxide dismutase) and nonenzymatic antioxidants system (e.g., vitamin C, glutathione, hypotaurine and taurine) that found in the oviductal and follicular fluids [33]. The levels of these antioxidants would be a demonstrator of the severity of oxidative stress. It has been shown that, TAC was significantly elevated in the follicullar fluids of those follicles which their oocytes were successfully fertilized [40]. On the other hand, absence of such a sophisticated defense system during in vitro culture and insufficient inherent antioxidant defenses in oocyte and embryos lead to greater oxidative stress. Hence, addition of antioxidants to culture media would be rational to control excessive OS during in vitro culture of gametes or embryos, [12, 22, 30, 40]. In this context, alpha-lipoic acid (ALA), (R)-5-(1,2-dithiolan-3-yl) pentanoic acid, is an important coenzyme of mitochondrial multienzyme complexes [14, 41, 42] and is well-known for its antioxidant rule in various biological processes [57]. ALA acts directly in scavenging of ROS, metal chelation and indirectly in recycling of other intracellular antioxidants [41, 42]. Since the role of ALA has not been examined in developmental competence of preantral follicle, the present study was designed to investigate the effects of different concentrations of ALA on the developmental competence of mouse preantral follicles through its supplementation in culture medium.
Materials and methods
Chemicals
All chemicals were purchased from Sigma-Aldrich (Hamburg, Germany), unless otherwise stated.
Animals
NMRI mice were cared for and used according to the Damghan University Animal Ethics Committee. Animals were housed and bred under controlled conditions (12-h light/12-h dark) and were provided with water and standard laboratory chow (Laboratory animals feed; Javane Khorasan Co, Tehran, Iran) ad libitum. As reported previously, 14 days old female mice were sacrificed by cervical dislocation and their ovaries were removed and separated from the connective tissue under aseptic condition and transferred to droplets of α- minimal essential medium (α- MEM; Gibco) supplemented with 2.2 g/L sodium bicarbonate, 25 mM HEPES, (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), 10% fetal bovine serum (FBS; Gibco), 100 mIU/ml penicillin and 75 mg/ml streptomycin [26]. Adult fertile males, 6–8 weeks old, were used for the collection of sperm.
Preantral follicle culture
Preantral follicles were isolated mechanically by using a 29 gauge needle under a stereomicroscope at × 10 magnification [2]. Isolated follicles were selected according to the following criteria: rounded follicular structure with 140–160 um diameters, containing visible centrally located healthy oocyte surrounded with intact several layers of granulosa cells and at least one layer of theca cells. During the operating procedures the culture medium was always kept at 37°C.
Experimental design
To evaluate the effects of ALA, isolated preantral follicles were divided in two main groups: one group was used and cultured for up to 12 days for assessment of developmental competence while other group was used and cultured for 96 h for analysis of ROS levels and TAC assessment.
Culture of preantral follicles
The isolated follicles were cultured individually in 20 uL droplets of α-MEM supplemented with, 0.23 mM sodium pyruvate, 100 mIU/ml of recombinant follicle-stimulating hormone (Gonal-f; Serono, Geneva, Switzerland), 1% ITS (Insulin, Transferring, Selenium; Gibco), 100 mIU/ml of penicillin, 75 mg/ml of streptomycin, 5% FBS and 2.2 g/L sodium bicarbonate, overlaid sterile mineral oil in falcon culture dishes at 37°C, 100% humidity and 5% CO2 for up to 12 days or 96 h according to the experimental design [26]. To evaluate the effect of ALA, stock solution was prepared in absolute ethanol and diluted appropriately, then 50, 100, 250 or 500 uM of ALA were added to culture medium. Media without ALA were considered as control group. At 48 h intervals, 10 uL of culture medium from each droplet was replaced by fresh medium.
Measurement of follicle diameter was assessed using precalibrated ocular micrometer at × 100 magnification on day 2 and 4 of culturing. Two cross sectional diameters of each follicle were measured and averaged. The survival rate of the follicles was checked by assessment of follicle morphology under inverted microscope [1, 26].
In vitro ovulation induction
In vitro ovulation was induced by replacement of culture media with fresh medium supplemented with 1.5 IU/ml human chronic gonadotropin (hCG) on 12th days of culture [2]. After 48 h, released oocytes were classified as germinal vesicle (GV), germinal vesicle breakdown (GVBD) when the GV was absent, and metaphase II oocytes (MII) when the first polar body was extruded. The proportions of GV, GVBD and MII were assessed in all groups.
In vitro fertilization
For in vitro fertilization, spermatozoa derived from caudae epididymis of adult male NMRI mice. Sperm suspensions were capacitated for 1.5 h in T6 medium supplemented with 5 mg/ml BSA. T6 medium composition was as described previously [11]. MII oocytes were collected from different groups and MII oocytes (n = 10) were inseminated into 50 uL droplets of T6 medium supplemented with 15 mg/ml BSA and a final motile sperm concentration of 1–2 × 106/ml then incubated for 4–6 h. At the end of this period, inseminated oocytes (n = 10) were cultured in 50 uL microdroplets of T6 medium supplemented with 5 mg/ml BSA for 120 h. The percentage of embryos developing to the blastocyte stages was determined. All fertilization steps and embryo culture were carried out under detoxified mineral oil at 37°C, 100% humidity and 5% CO2 in air [2].
Biochemical assays
Measurement of reactive oxygen species
The production of intracellular ROS of cultured preantral follicles was measured by 2′,7′ -dichlorodihydrofluorescin (DCHF) probe [2, 32]. Briefly, 10 follicles were collected from different times of culture (0, 24, 48, 72 and 97 h). The follicles were initially washed with phosphate buffer saline (PBS) and then incubated in 5 uM of 2′,7′-dichlorodihydrofluorescin diacetate (DCHFDA; Merck) at 37°C for 30 min. Then, follicles were washed three times with PBS and immediately transferred to 100 microliter of Tris-HCl buffer and sonicated at 50 W for 1 min. After sonication, solution was centrifuged at 10000 × g for 20 min at 4°C and supernatant were collected. The fluorescent intensity of supernatant was monitored by using a spectrofluorometer at 525 nm emission and at 488 nm excitation. Corrections for autofluorescence were made by including parallel blanks in each experiment. Values for ROS levels were expressed as uM H2O2 and the mean dichlorofluorescin (DCF) fluorescence intensity. All experiments were repeated at least four times.
Total antioxidant capacity assay
Assessment of TAC in cultured pre-antral was performed according to ferric reducing/antioxidant power (FRAP) method [2, 13]. For preparation of cellular supernatant, 10 follicles were collected from different times of culture period (0, 24, 48, 72 and 96 h) and homogenized in 100 ml Tris–HCl buffer and sonicated at 50 W for 1 min and then centrifuged at 10000 × g for 20 min at 4°C. Cellular supernatant (100 uL) was added to 2 ml of freshly FRAP reagent (Tripiridyltriazine; Merck) in a cuvette and incubated in 37°C for 10 min. Then, absorbance of blue-colored reagent was read against a blank reagent at 593 nm every 20 s for 10 min. Standard solutions of 100–1000 mM of Fe+2 (FeSO4 × 7 H2O) were used for calibration of assay. All experiments were repeated at least four times.
Statistical analysis
Data from replicate experiments were expressed as mean ± standard deviation (SD). Statistical analysis was performed using SPSS-ver.16 software package (SPSS Inc., Chicago, IL, USA). Differences in groups were evaluated by one-way analysis of variance (ANOVA) and Tukey’s HSD was used as post hoc tests. Differences were considered significant at a level of p < 0.05. Differences in ROS and TAC levels were assessed for each treatment group and each time point. All proportional data were subjected to an arc-sin transformation, and the transformed values were analyzed.
Results
In vitro follicle development
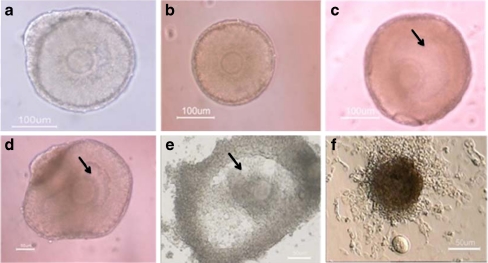
Every other day the development of in vitro cultured follicles was morphologically evaluated. By days 4 of culture, the follicles became attached to the bottom of the dish by the growing and adhesion of theca and granulosa cells. Granulosa cells proliferated and grew through basal membrane to form the irregular and diffuse appearance (Fig. 1).
Fig. 1.
Representative photographs of mouse preantral follicles (from 14-day old mouse) cultured in vitro for 12 days. The cultured isolated follicle on day 2 (a), day 4 (b), day 6 (c), day 8 (d), day 10 (e) and The oocyte ovulated of cultured preantral follicle after hCG administration (f). Antral-like cavities are indicated by black arrow
The diameter of cultured preantral follicles
The results of morphological development of the cultured isolated follicles in the presence of different concentrations of ALA were summarized in Tables 1 and 2. The diameter of preantral follicles was measured every other day during the first 4 days. The mean follicular size at day 0 was not significantly different among all groups (p > 0.05). The diameter of preantral follicles in all groups of study was increased during in vitro culture.
Table 1.
Diameter of preantral follicles cultured in the presence of different concentrations of ALA
| Dosage of ALA (uM) | Number of preantral follicle | Follicle diameter (um ± SD) | ||
|---|---|---|---|---|
| Initiate time | 2th day | 4th day | ||
| 0 (Control) | 83 | 156.0 ± 5.5 | 197.2 ± 25.0a | 297.7 ± 69.6a |
| 50 | 85 | 156.2 ± 5.5 | 187.3 ± 10.4b | 284.0 ± 38.6ac |
| 100 | 129 | 157.1 ± 4.4 | 220.6 ± 20.0c | 342.5 ± 33.8b |
| 250 | 133 | 157.4 ± 4.9 | 188.9 ± 10.0b | 273.0 ± 24.2c |
| 500 | 102 | 155.8 ± 4.9 | 171.3 ± 7.1d | 193.3 ± 12.9d |
In all cases 4 experimental replicates were performed
Groups with different superscripts in the same column are significantly different (p < 0.05)
ALA: Alpha lipoic acid;
Table 2.
Developmental rates of cultured preantral follicles in the presence of different concentration of ALA
| Dosage of ALA (uM) | Number of preantral follicle | Degenerated n (% ± SD) | Survived n (% ± SD) | Antrum formation n (% ± SD) | Developmental stages of oocyte | ||
|---|---|---|---|---|---|---|---|
| GV n (% ± SD) | MI n (% ± SD) | MII n (% ± SD) | |||||
| 0 (Control) | 83 | 14 a (17.3 ± 2.4) | 69a (82.7 ± 2.4) | 35a (41.0 ± 6.6) | 28 a (28.0 ± 14.6) | 14 ab (15.9 ± 3.0) | 27a (32.0 ± 4.5) |
| 50 | 85 | 12a (14.7 ± 6.1) | 73a (85.3 ± 6.1) | 34 a (40.7 ± 7.5) | 30a (34.3 ± 11.7) | 20b (23.7 ± 2.7) | 23a (27.3 ± 3.2) |
| 100 | 129 | 8b (6.2 ± 1.0) | 121b (93.8 ± 1.0) | 74b (57.6 ± 6.6) | 44a (34.0 ± 1.5) | 19a (14.6 ± 3.1) | 58b (45.1 ± 4.6) |
| 250 | 133 | 16a (12.0 ± 1.1) | 117 a (88.0 ± 1.1) | 34c (25.5 ± 2.4) | 77b (58.1 ± 4.7) | 17a (12.6 ± 5.1) | 23c (17.3 ± 2.2) |
| 500 | 102 | 102c (100 ± 0.0) | 0c (0 ± 0.0) | 0d (0 ± 0.0) | 0c (0 ± 0.0) | 0c (0 ± 0.0) | 0d (0 ± 0.0) |
In all cases 4 experimental replicates were performed
Groups with different superscripts in the same column are significantly different (p < 0.05)
ALA: Alpha lipoic acid; GV: germinal vesicle stage oocyte; MI: metaphase I stage oocyte; MII: metaphase II stage oocyte;
The diameter of preantal follicles on day 4 of culturing period in the presence of 100 uM concentration of ALA was significantly higher (p < 0.05) than other groups (Table 1).
The survival rate of cultured follicles
Every other day, by twelfth day of culture period, follicle survival was evaluated as those follicles that retained their oocyte wholly enclosed within the granulosa cell mass (Fig. 1). Folliclular degeneration was demonstrated by showing either spontaneous release of the oocytes or distress of further propagation of granulosa cells.
On day 12 of culture, the survival rate of cultured follicles in the presence of 0, 50, 100, 250 and 500 uM of ALA were 82%, 85%, 93%, 88% and 0%, respectively. The follicle survival rate was significantly higher in the presence of 100 uM concentration of ALA in comparison with other concentrations (p < 0.05; see Table 2). Significant differences were not observed among follicles which were cultured in the presence of 0, 50 and 250 uM concentrations of ALA (p > 0.05; see Table 2). In addition, until the 12th day, all follicles that were cultured in the presence of 500 uM concentration of ALA was degenerated.
Antrum formation
Lucent area in the granulosa cell mass around the oocyte that was formed from day 9 was considered as an antral-like cavity (Fig. 1). The rate of antrum formation in follicles cultured in media containing 0, 50, 100, 250 and 500 uM concentrations of ALA were 41%, 40%, 57%, 25% and 0%, respectively. In this sense, the antrum formation rate in the presence of 100 uM concentration of ALA was significantly higher compared to the other groups (p < 0.05; Table 2). There were no significant differences between antrum formation rates of follicles which were cultured in presence of 0 and 50 uM concentrations of ALA (p > 0.05). None of the follicles that were cultured in media containing 500 uM concentration of ALA formed antral-like cavity.
Maturation rate of in vitro grown oocytes
The maturation rates of oocytes derived from isolated pre-antral follicles that cultured in the presence of different concentrations of ALA are summarized in Table 2. The percentages of released MII oocyte in follicles after final oocyte maturation induced by hCG which were cultured in the presence of 0, 50, 100, 250 and 500 uM of ALA were 32%, 27%, 45%, 17% and 0%, respectively. These rates were significantly different. In addition, the MII rates were significantly higher in the presence of 100 uM concentration of ALA (p < 0.05). There were no significant differences between the percentage of MII oocytes in control group (0 uM ALA) and those that were cultured in the presence of 50 uM concentration of ALA (p > 0.05).
Fertilization rate and embryo development
The rates of oocyte fertilization and embryo development to blastocyte stage of MII oocytes derived from cultured isolated follicles in the presence of different concentrations of ALA are shown in Table 3. The percentages of fertilized oocytes and two-cell embyros derived from cultured preantral follicles in the presence of 50 uM (60% and 43%, respectively), and 250 uM (48% and 34%, respectively) of ALA were similar to the amount of control groups (0 uM; 61% and 45%, respectively; p > 0.05). Also, rate of oocyte fertilization and two cells embryo development derived from cultured follicles in medium supplemented with 100 uM of ALA (86.1% and 70.6% respectively) was significantly higher than other groups (p < 0.05).
Table 3.
Effects of different concentration of ALA on fertilization parameter of oocyte derived from cultured preantral follicles
| Dosage of LA(uM) | Number of MII oocytes | Fertization n (% ± SD) | Two cell n (% ± SD) | Morulas n (% ± SD) | Blastocytes n (% ± SD) |
|---|---|---|---|---|---|
| 0 (Control) | 27 | 17 (61.3 ± 4.9) | 13 (45.4 ± 7.0) | 7 (26 ± 3.4) | 5 (18.2 ± 4.0) |
| 50 | 23 | 14 (60.3 ± 15.3) | 10 (43.4 ± 14.3) | 7 (30.2 ± 2.7) | 5 (21.7 ± 7.2) |
| 100 | 58 | 50* (86.1 ± 3.5) | 41* (70.6 ± 4.2) | 29* (50.3 ± 9.3) | 27* (47.0 ± 7.6) |
| 250 | 23 | 11 (48.1 ± 7.8) | 8* (34.9 ± 7.3) | 3* (13.6 ± 3.4) | 2* (10.3 ± 9.0) |
| 500 | 0 | 0* (0.0 ± 0.0) | 0* (0.0 ± 0.0) | 0* (0.0 ± 0.0) | 0* (0.0 ± 0.0) |
In all cases 4 experimental replicates were performed. ALA: Alpha lipoic acid, MII: metaphase II oocyte
*: Significant differences compare to control group (0 uM ALA) in the same column (p < 0.05)
The rate of embryo reached to morula and blastocyst stages in the presence of 50 uM ALA (30.2% and 21.7%, respectively), were similar to control group (26% and 18.2% respectively; p > 0.05), however in the presence of 250 uM of ALA (13.6% and 10.3% respectively) were statistically lower than control group (p < 0.05), while in the presence of 100 uM of ALA (50.3% and 47% respectively) was statistically higher than control group (p < 0.05).
ROS production in cultured pre-antral follicles
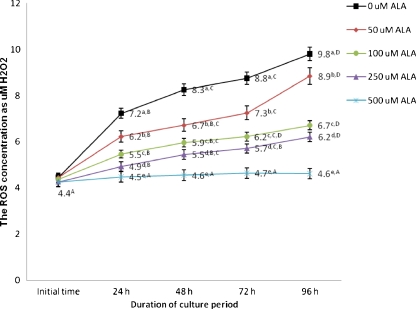
ROS intensity in isolated preantral follicles after 0, 24, 48, 72 and 96 h culture in the presence of different concentrations of ALA are summarized in Fig. 2. ROS levels were increased in control group with increasing length of culture period. The highest ROS levels were observed at 96 h of culture period, except for those follicles which were cultured in the presence of 500 uM ALA, which were similar at the beginning and the end of the culture period (p > 0.05). When concentrations of ALA in the culture medium were increased from 50 to 500 uM, ROS levels decreased in a dose dependent manner at different time intervals (i.e., 24, 48, 72 and 96 h).
Fig. 2.
ROS concentrations in cultured preantral follicles in the presence of different concentration of ALA. In all cases 4 experimental replicates were performed. Groups with different superscripts (a, b, c, d, e) are significantly different (p < 0.05) at same times of culture within the different groups. Different superscripts (A, B, C, D) describe significantly different (p < 0.05) at different times of culture within the same group. ROS: Reactive Oxygen Species; ALA: Alpha lipoic acid
Total antioxidant capacity levels in cultured pre-antral follicles
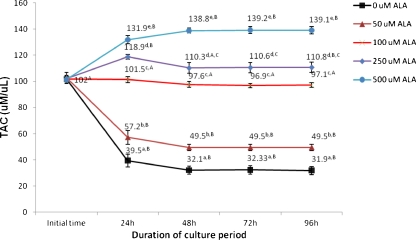
TAC levels in cultured follicles after 0, 24, 48, 72 and 96 h of culture in the presence of different concentrations of ALA were shown in Fig. 3. TAC levels were decreased in the control groups during the culture period up to 96 h (p < 0.05) In the control groups the highest level of TAC occurred at the beginning of the culture, and the TAC level was significantly decreased up to 96 h of culture (p < 0.05). However, TAC levels following treatment with 100 uM of ALA after 96 h of culture completely returned to the baseline and there were no significant differences between TAC level at the end and the beginning of the culture (p > 0.05). Maximum TAC levels were observed in the presence of 500 uM concentrations of ALA at 96 h (p < 0.05).
Fig. 3.
TAC levels in cultured preantral follicles in the presence of different concentration of ALA. In all cases 4 experimental replicates were performed. Groups with different superscripts (a, b, c, d, e) are significantly different (p < 0.05) at same times of culture within the different groups. Different superscripts (A, B, C, D) describe significantly different (p < 0.05) at different times of culture within the same group. TAC: Total Antioxidant capacity; ALA: Alpha lipoic acid
Discussion
The main purpose of the present study was to determine the effects of various concentrations of ALA on the developmental competence, generation of ROS and TAC of mouse cultured isolated pre-antral follicles.
The findings of this study showed that during culture period, diameter of follicles increased in all groups but diameters of follicles in medium containing 100 uM of ALA were statistically greater when compared with other groups. In addition, maturation and development of isolated follicles in the presence of 100 uM of ALA was statistically higher compared with other concentrations of ALA. Therefore, ALA (100 uM) would exerte an acceptable antioxidant activity here as shown previously [14, 17] since regulation of many intracellular signaling pathways is dependent to intracellular redox state [31].
There is considerable evidence showing that ROS are generated not only by cellular metabolism but also by external factors such as oxygen concentration, light, and manipulation during in vitro condition which may influence the overall metabolism of the cells [18, 25], which lead to OS [4, 5, 7]. It has been shown that in the in vivo condition, ROS production is a usual itinerary in the mitochondrial respiratory chain which is equilibrated by antioxidant defense systems of cells [4, 5]. Increased ROS production occurs in the in vitro milieu [18], which is in agreement with results of this study that showed an accrual of ROS formation in cultured preanral follicles up to 96 h. It has been founded that fortifying of the culture medium with antioxidants led to improved culture condition [25, 37–39]
It has been recommended that ALA beside its main role as a coenzyme of various enzymes like pyruvate dehydrogenase complex that catalyses the utilization of pyruvate in mouse oocytes and follicles [28, 29] also acts as a potent antioxidant and able to react directly with ROS [17, 36, 51]. These observations are in agreement with our data, which showed, ROS levels during IVM of follicles in the presence of ALA were significantly decreased in comparison to the control groups.
To our knowledge, there is no report about the effect of ALA on improving the IVM of cultured isolated prantral follicles; however ALA seems to play its role through several pathways. It seems that some deal of this activity may be due to its aptitude to chelation of metal ions and recycling of other cellular antioxidants, such as vitamin C and E, and glutathione [9, 23, 43].
Also it has been shown that ALA inhibits TNF-alpha-induced ROS generation [16] and potently prevents high glucose-induced OS and cell death [52, 53]. Additionally, [24] reported that ALA suppressed 6-hydroxydopamine-induced ROS generation and suggested that the ALA suppressed ROS generation and apoptosis depended on glutathione (GSH) synthesis. .
Our data indicated, reduction in TAC levels in control groups occurred in cultured preantral follicles up to 96 h, however in the presence of 100 uM ALA, stable as well as baseline levels of TAC were conserved after 96 h of culture. In agreement with this, [59] demonstrated that, ALA administration significantly elevated plasma total antioxidant status and could increase activities of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) and catalase (CAT) in the brain tissues of male rat exposed to restraint stress [8]. It appears that increase TAC levels in the presence of ALA is due to affect on nerve growth factor (NGF) which in turn induce expression of superoxide dismutase gene that result in increase of SOD [8]. In addition ALA enhances glucose uptake by cells, followed by increment of cellular nicotinamide adenine dinucleotide and nicotinamide adenine dinucleotide phosphate levels which is leading to increase the activity of CAT [10]. Also it has been demonstrated that administration of ALA could increase GPX-Px activity followed by increase intracellular GSH levels [8, 10]. Some evidences indicate that ALA might suppress cell death through GSH synthesis [54]. This is in agreement with our data that showed in the presence of 100 uM ALA, the degeneration rate of follicles was decreased significantly during IVM, when compared with control groups. Taken together, our study indicates that ALA has an excellent antioxidant activity, however we should consider its pivotal role in pyruvate oxidation during IVM and IVF to prepare an energy source for developing oocytes (see Culture of preantral follicles; [28, 29, 46]) On the other hand, the present study provides experimental data showing that increasing concentrations of ALA from 100 to 500 uM lead to increase degeneration rate of cultured preantral follicles. In support of our results, long time exposure to ALA results in increase of lipid peroxidation, mitochondrial damage and inhibition of glycogen synthesis [17, 36]. Also, it has been shown that high dose of ALA could arrest cell cycles in some cells [35, 55] or could provoke cell death through internucleosomal DNA fragmentation and caspase cleavage in numerous cancer cell lines [48, 55, 57]. Hence, it appears that high doses of ALA more than 100 uM are toxic for isolated preantral follicles.
On the other hand, it has been shown that ROS play important roles in somatic cell functions such as cell proliferation and differentiation [21, 45]. Consistent with the previous finding, our results showed that increase in concentration of ALA lead to decrease ROS concentration which in turn results in decrease follicles size, survival rate, and developmental competence. The results presented here showed that, ALA in 100 uM concentration promotes maturation and development of isolated follicles while higher concentrations more than 100 uM obliterate those events. Regarding to the effect of ALA on developmental competence of isolated follicles, there is no information available. Some reports have suggested that a relationship between redox-state of the follicular fluid and oocyte quality in which levels of TAC and ROS can be used as a predictive sign of successful IVF [44]. This is in consistent with our finding that showed presence of 100 uM of ALA in maturation medium results in decrease ROS production and increase TAC level which in turn lead to improve maturation and developmental competence of isolated follicles. Also it has been shown that reduced oxygen environment induce detrimental effects on maturation of squirrel Monkey’s oocytes [58].
On the other hand, it has been shown that less concentration of ROS may play a positive role in the induction of oocyte maturation and have beneficial effects on oocytes developmental competence [15, 27]. This observation may give explanation about our finding which why increasing of ALA concentrations results in decrease developmental competence of isolated follicles. In addition, as previous mentioned, high dose of ALA in numerous cancers cell lines could induce apoptosis [35, 48, 55, 57] which explicates, high degeneration rate of follicles in the presence of high concentrations of ALA.
In conclusion, culture of mouse isolated pre-antral follicles in the presence of 100 uM of ALA increased follicular TAC levels, decreased ROS levels, and finally improved the developmental competence of pre-antral follicles in vitro. Future studies are needed to explore metabolic role of ALA beside its antioxidative activity in ART.
Acknowledgements
This work was conducted and funded by the biology school of Damghan University, Damghan, Iran.
Footnotes
Capsule
Alpha lipoic acid improved the developmental competence of preantral follicles in mice
References
- 1.Abedelahi A, Salehnia M, Allameh AA. The effects of different concentrations of sodium selenite on the in vitro maturation of preantral follicles in serum-free and serum supplemented media. J Assist Reprod Genet. 2008;25(9–10):483–488. doi: 10.1007/s10815-008-9252-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abedelahi A, Salehnia M, Allameh AA, Davoodi D. Sodium selenite improves the in vitro follicular development by reducing the reactive oxygen species level and increasing the total antioxidant capacity and glutathione peroxide activity. Human Reprod (Oxford, England) 2010;25(4):977–985. doi: 10.1093/humrep/deq002. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal A, Allamaneni SS. Role of free radicals in female reproductive diseases and assisted reproduction. Reprod Biomed Online. 2004;9(3):338–347. doi: 10.1016/S1472-6483(10)62151-7. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal A, Gupta S, Sharma R. Oxidative stress and its implications in female infertility - a clinician’s perspective. Reprod Biomed Online. 2005;11(5):641–650. doi: 10.1016/S1472-6483(10)61174-1. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal A, Gupta S, Sikka S. The role of free radicals and antioxidants in reproduction. Curr Opin Obstet Gynecol. 2006;18(3):325–332. doi: 10.1097/01.gco.0000193003.58158.4e. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal A, Said TM, Bedaiwy MA, Banerjee J, Alvarez JG. Oxidative stress in an assisted reproductive techniques setting. Fertil Steril. 2006;86(3):503–512. doi: 10.1016/j.fertnstert.2006.02.088. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril. 2003;79(4):829–843. doi: 10.1016/S0015-0282(02)04948-8. [DOI] [PubMed] [Google Scholar]
- 8.Akpinar D, Yargicoglu P, Derin N, Aliciguzel Y, Agar A. The effect of lipoic acid on antioxidant status and lipid peroxidation in rats exposed to chronic restraint stress. Physiol Res Academ Sci Bohemoslov. 2008;57(6):893–901. doi: 10.33549/physiolres.931284. [DOI] [PubMed] [Google Scholar]
- 9.Amudha G, Josephine A, Varalakshmi P. Role of lipoic acid in reducing the oxidative stress induced by cyclosporine A. Clin Chim Acta; Int J Clin Chem. 2006;372(1–2):134–139. doi: 10.1016/j.cca.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 10.Arivazhagan P, Thilakavathy T, Panneerselvam C. Antioxidant lipoate and tissue antioxidants in aged rats. J Nutr Biochem. 2000;11(3):122–127. doi: 10.1016/S0955-2863(99)00079-0. [DOI] [PubMed] [Google Scholar]
- 11.Azadbakht M, Valojerdi MR. Development of vitrified-warmed mouse embryos co-cultured with polarized or non-polarized uterine epithelial cells using sequential culture media. J Assist Reprod Genet. 2008;25(6):251–261. doi: 10.1007/s10815-008-9231-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bedaiwy MA, Falcone T, Mohamed MS, Aleem AA, Sharma RK, Worley SE, et al. Differential growth of human embryos in vitro: role of reactive oxygen species. Fertil Steril. 2004;82(3):593–600. doi: 10.1016/j.fertnstert.2004.02.121. [DOI] [PubMed] [Google Scholar]
- 13.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 14.Bilska A, Wlodek L. Lipoic acid - the drug of the future? Pharmacol Rep. 2005;57(5):570–577. [PubMed] [Google Scholar]
- 15.Blondin P, Coenen K, Sirard MA. The impact of reactive oxygen species on bovine sperm fertilizing ability and oocyte maturation. J Androl. 1997;18(4):454–460. [PubMed] [Google Scholar]
- 16.Byun CH, Koh JM, Kim DK, Park SI, Lee KU, Kim GS. Alpha-lipoic acid inhibits TNF-alpha-induced apoptosis in human bone marrow stromal cells. J Bone Miner Res. 2005;20(7):1125–1135. doi: 10.1359/JBMR.050302. [DOI] [PubMed] [Google Scholar]
- 17.Cakatay U. Pro-oxidant actions of alpha-lipoic acid and dihydrolipoic acid. Med hypotheses. 2006;66(1):110–117. doi: 10.1016/j.mehy.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 18.Combelles CM, Gupta S, Agarwal A. Could oxidative stress influence the in-vitro maturation of oocytes? Reprod Biomed Online. 2009;18(6):864–880. doi: 10.1016/S1472-6483(10)60038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corso A, Cappiello M, Mura U. Thiol dependent oxidation of enzymes: the last chance against oxidative stress. Int J Biochem. 1994;26(6):745–750. doi: 10.1016/0020-711X(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 20.Downs SM, Mastropolo AM. The participation of energy substrates in the control of meiotic maturation in murine oocytes. Dev Biol. 1994;162(1):154–168. doi: 10.1006/dbio.1994.1075. [DOI] [PubMed] [Google Scholar]
- 21.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82(1):47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 22.Engel RH, Evens AM. Oxidative stress and apoptosis: a new treatment paradigm in cancer. Front Biosci. 2006;11:300–312. doi: 10.2741/1798. [DOI] [PubMed] [Google Scholar]
- 23.Freitas RM. The evaluation of effects of lipoic acid on the lipid peroxidation, nitrite formation and antioxidant enzymes in the hippocampus of rats after pilocarpine-induced seizures. Neurosci Lett. 2009;455(2):140–144. doi: 10.1016/j.neulet.2009.03.065. [DOI] [PubMed] [Google Scholar]
- 24.Fujita H, Shiosaka M, Ogino T, Okimura Y, Utsumi T, Sato EF, et al. Alpha-lipoic acid suppresses 6-hydroxydopamine-induced ROS generation and apoptosis through the stimulation of glutathione synthesis but not by the expression of heme oxygenase-1. Brain Res. 2008;1206:1–12. doi: 10.1016/j.brainres.2008.01.081. [DOI] [PubMed] [Google Scholar]
- 25.Goto Y, Noda Y, Narimoto K, Umaoka Y, Mori T. Oxidative stress on mouse embryo development in vitro. Free Radic Biol Med. 1992;13(1):47–53. doi: 10.1016/0891-5849(92)90165-D. [DOI] [PubMed] [Google Scholar]
- 26.Haidari K, Salehnia M, Rezazadeh Valojerdi M. The effect of leukemia inhibitory factor and coculture on the in vitro maturation and ultrastructure of vitrified and nonvitrified isolated mouse preantral follicles. Fertil Steril. 2008;90(6):2389–2397. doi: 10.1016/j.fertnstert.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 27.Halliwell B, Gutteridge JM. Free radicals and antioxidant protection: mechanisms and significance in toxicology and disease. Hum Toxicol. 1988;7(1):7–13. doi: 10.1177/096032718800700102. [DOI] [PubMed] [Google Scholar]
- 28.Harris S. Experimental and clinical investigation into mammalian oocyte metabolism, nutrition and fertility. Dissertation, University of Leeds; 2002.
- 29.Johnson M, Freeman E, Gardner D, Hunt P. Oxidative metabolism of pyruvate is required for meiotic maturation of murine oocytes in vivo. 77, 2–8. Biol Reprod. 2007;77:2–8. doi: 10.1095/biolreprod.106.059899. [DOI] [PubMed] [Google Scholar]
- 30.Kitagawa Y, Suzuki K, Yoneda A, Watanabe T. Effects of oxygen concentration and antioxidants on the in vitro developmental ability, production of reactive oxygen species (ROS), and DNA fragmentation in porcine embryos. Theriogenology. 2004;62(7):1186–1197. doi: 10.1016/j.theriogenology.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 31.Laurent A, Nicco C, Chereau C, Goulvestre C, Alexandre J, Alves A, et al. Controlling tumor growth by modulating endogenous production of reactive oxygen species. Cancer Res. 2005;65(3):948–956. [PubMed] [Google Scholar]
- 32.LeBel CP, Ischiropoulos H, Bondy SC. Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol. 1992;5(2):227–231. doi: 10.1021/tx00026a012. [DOI] [PubMed] [Google Scholar]
- 33.Li J, Foote RH, Simkin M. Development of rabbit zygotes cultured in protein-free medium with catalase, taurine, or superoxide dismutase. Biol Reprod. 1993;49(1):33–37. doi: 10.1095/biolreprod49.1.33. [DOI] [PubMed] [Google Scholar]
- 34.Luvoni GC, Keskintepe L, Brackett BG. Improvement in bovine embryo production in vitro by glutathione-containing culture media. Mol Reprod Dev. 1996;43(4):437–443. doi: 10.1002/(SICI)1098-2795(199604)43:4<437::AID-MRD5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 35.Marsh SA, Pat BK, Gobe GC, Coombes JS. Evidence for a non-antioxidant, dose-dependent role of alpha -lipoic acid in caspase-3 and ERK2 activation in endothelial cells. Apoptosis. 2005;10(3):657–665. doi: 10.1007/s10495-005-1901-4. [DOI] [PubMed] [Google Scholar]
- 36.Moini H, Packer L, Saris NE. Antioxidant and prooxidant activities of alpha-lipoic acid and dihydrolipoic acid. Toxicol Appl Pharmacol. 2002;182(1):84–90. doi: 10.1006/taap.2002.9437. [DOI] [PubMed] [Google Scholar]
- 37.Nasr-Esfahani MH, Johnson MH. How does transferrin overcome the in vitro block to development of the mouse preimplantation embryo? J Reprod Fertil. 1992;96(1):41–48. doi: 10.1530/jrf.0.0960041. [DOI] [PubMed] [Google Scholar]
- 38.Noda Y, Matsumoto H, Umaoka Y, Tatsumi K, Kishi J, Mori T. Involvement of superoxide radicals in the mouse two-cell block. Mol Reprod Dev. 1991;28(4):356–360. doi: 10.1002/mrd.1080280408. [DOI] [PubMed] [Google Scholar]
- 39.Nonogaki T, Noda Y, Narimoto K, Umaoka Y, Mori T. Protection from oxidative stress by thioredoxin and superoxide dismutase of mouse embryos fertilized in vitro. Hum Reprod (Oxford, England) 1991;6(9):1305–1310. doi: 10.1093/oxfordjournals.humrep.a137532. [DOI] [PubMed] [Google Scholar]
- 40.Oyawoye O, Abdel Gadir A, Garner A, Constantinovici N, Perrett C, Hardiman P. Antioxidants and reactive oxygen species in follicular fluid of women undergoing IVF: relationship to outcome. Hum Reprod (Oxford, England) 2003;18(11):2270–2274. doi: 10.1093/humrep/deg450. [DOI] [PubMed] [Google Scholar]
- 41.Packer L, Tritschler HJ, Wessel K. Neuroprotection by the metabolic antioxidant alpha-lipoic acid. Free Radic Biol Med. 1997;22(1–2):359–378. doi: 10.1016/S0891-5849(96)00269-9. [DOI] [PubMed] [Google Scholar]
- 42.Packer L, Witt EH, Tritschler HJ. Alpha-Lipoic acid as a biological antioxidant. Free Radic Biol Med. 1995;19(2):227–250. doi: 10.1016/0891-5849(95)00017-R. [DOI] [PubMed] [Google Scholar]
- 43.Papas AM. Antioxidant status, diet, nutrition and health. New York: CRC Press; 1998. pp. 133–210. [Google Scholar]
- 44.Pasqualotto EB, Agarwal A, Sharma RK, Izzo VM, Pinotti JA, Joshi NJ, et al. Effect of oxidative stress in follicular fluid on the outcome of assisted reproductive procedures. Fertil Steril. 2004;81(4):973–976. doi: 10.1016/j.fertnstert.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 45.Poli G, Leonarduzzi G, Biasi F, Chiarpotto E. Oxidative stress and cell signalling. Curr Med Chem. 2004;11:1163–1182. doi: 10.2174/0929867043365323. [DOI] [PubMed] [Google Scholar]
- 46.Roy S, Terada M. Activities of glucose metabolic enzymes in human preantral follicles: in vitro modulation by follicle-stimulating hormone, luteinizing hormone, epidermal growth factor, insulin-like growth factor i, and transforming growth factor β1. Biol Reprod. 1999;60:763–768. doi: 10.1095/biolreprod60.3.763. [DOI] [PubMed] [Google Scholar]
- 47.Gupta S, Sekhon L, Kim Y, Agarwal A. The role of oxidative stress and antioxidants in assisted reproduction. Women’s Health Rev. 2010;6:227–238. doi: 10.2174/157340410792007046. [DOI] [Google Scholar]
- 48.Simbula G, Columbano A, Ledda-Columbano GM, Sanna L, Deidda M, Diana A, et al. Increased ROS generation and p53 activation in alpha-lipoic acid-induced apoptosis of hepatoma cells. Apoptosis. 2007;12(1):113–123. doi: 10.1007/s10495-006-0487-9. [DOI] [PubMed] [Google Scholar]
- 49.Tarin JJ, Vendrell FJ, Ten J, Blanes R, Blerkom J, Cano A. The oxidizing agent tertiary butyl hydroperoxide induces disturbances in spindle organization, c-meiosis, and aneuploidy in mouse oocytes. Mol Hum Reprod. 1996;2(12):895–901. doi: 10.1093/molehr/2.12.895. [DOI] [PubMed] [Google Scholar]
- 50.Taylor C. Antioxidants and reactive oxygen species in infertility. Environ Toxical, Pharmaco. 2001;10:189–198. doi: 10.1016/S1382-6689(01)00099-0. [DOI] [Google Scholar]
- 51.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 52.Vincent AM, McLean LL, Backus C, Feldman EL. Short-term hyperglycemia produces oxidative damage and apoptosis in neurons. FASEB J. 2005;19(6):638–640. doi: 10.1096/fj.04-2513fje. [DOI] [PubMed] [Google Scholar]
- 53.Vincent AM, Stevens MJ, Backus C, McLean LL, Feldman EL. Cell culture modeling to test therapies against hyperglycemia-mediated oxidative stress and injury. Antioxid Redox Signal. 2005;7(11–12):1494–1506. doi: 10.1089/ars.2005.7.1494. [DOI] [PubMed] [Google Scholar]
- 54.Voloboueva LA, Liu J, Suh JH, Ames BN, Miller SS. (R)-alpha-lipoic acid protects retinal pigment epithelial cells from oxidative damage. Investig Ophthalmol Vis Sci. 2005;46(11):4302–4310. doi: 10.1167/iovs.04-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wenzel U, Nickel A, Daniel H. alpha-Lipoic acid induces apoptosis in human colon cancer cells by increasing mitochondrial respiration with a concomitant O2-*-generation. Apoptosis. 2005;10(2):359–368. doi: 10.1007/s10495-005-0810-x. [DOI] [PubMed] [Google Scholar]
- 56.Wiener-Megnazi Z, Vardi L, Lissak A, Shnizer S, Reznick A, Ishai D, et al. Oxidative stress indices in follicular fluid as measured by the thermochemiluminescence assay correlate with outcome parameters in in vitro fertilization. Fertil Steril. 2004;82(Suppl 3):1171–1176. doi: 10.1016/j.fertnstert.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 57.Yamasaki M, Kawabe A, Nishimoto K, Madhyastha H, Sakakibara Y, Suiko M, et al. Dihydro-alpha-lipoic acid has more potent cytotoxicity than alpha-lipoic acid. In Vitro Cell DevBiol-Animal. 2009;45:275–280. doi: 10.1007/s11626-008-9164-3. [DOI] [PubMed] [Google Scholar]
- 58.Yeoman RR, Williams LE, Abee CR. Low oxygen inhibits but complex high-glucose mediumfacilitates in vitro maturation of squirrel monkey oocyte-granulos a cell complexes. J Assist Reprod Genet. 1999;16:102–107. doi: 10.1023/A:1022525108241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zembron-Lacny A, Slowinska-Lisowska M, Szygula Z, Witkowski K, Szyszka K. The comparison of antioxidant and hematological properties of N-acetylcysteine and alpha-lipoic acid in physically active males. Physiol Res Academ Sci Bohemoslov. 2009;58(6):855–861. doi: 10.33549/physiolres.931590. [DOI] [PubMed] [Google Scholar]