Abstract
Objective
The aim of our study was to investigate the frequency and compare the prevalence of IRS-1Gly972Arg and IRS-2 Gly1057Asp polymorphisms in PCOS patients and non-diabetic healthy women.
Material(s) and method(s)
Forty eight Iranian women diagnosed with PCOS were enrolled in this study. Fifty two non-diabetics, non-PCOS women were enrolled as the control group. HemoglobinA1c (HbA1c), fasting blood glucose (FBS), fasting insulin levels (FIL) and 2 h post-prandial blood glucose(2hpp BS) were evaluated from blood samples. Insulin resistance sample was estimated with Homeostasis Model Assessment index for insulin resistance (HOMA-IR). Genotyping of allelic variants Gly972Arg of IRS-1 and Gly1057Asp of IRS-2 was conducted using PCR.
Results
No statistically significant differences in the prevalence of IRS-1 Gly972Arg or IRS-2 Gly1057Asp polymorphisms or any combination of both were observed between controls and PCOS patients (P > 0.02). Control subjects with the IRS-1 polymorphism had higher levels of 2hpp BS compared with those with the Gly/Gly genotype (P = 0.037).
Conclusions
Considering that no association between the IRS-1 Gly972Arg and IRS-2 Gly1057Asp polymorphisms and PCOS were found, the results confirm that these polymorphisms should not be considered as major contributors to the pathogenesis of this disorder.
Keywords: PCOS, IRS-1, IRS-2, Polymorphisms, Insulin resistance
Introduction
Genetic studies contribute towards our understanding of disease pathogenesis and hold the promise of improving our ability to individualize treatment for patients [1].
Polycystic ovary syndrome (PCOS) is a disorder characterized by functional irregularities leading to anovulation and menstrual irregularity concomitant with hyperandrogenism [2]. Variable features of this syndrome lead to sub classifications, but insulin resistance is a common phenotype that is associated with it [3]. Insulin resistance in PCOS is a phenotype which is more frequent than healthy population adjusted by sex and age [4, 5]. Insulin resistance in type 2 diabetes and PCOS is likely to be due to post insulin receptor signaling pathway defects [6]. Insulin receptor substrates type 1 and 2 (IRS1 and IRS2) are shown to play important roles in insulin signaling. Thus, IRS-1 Gly972Arg and IRS-2 Gly1057Asp, which are the most common polymorphism of these two genes, may play a functional role on the insulin-resistant component of PCOS [7–9]. Many studies have found a relationship between IRS1 Gly972Arg polymorphism and PCOS metabolic features [10, 11]. However there are still controversies as to whether such relationships exists [12]. Association of IRS2 Gly1057Asp polymorphism with PCOS metabolic features have also been investigated but disagreements between results of the few existing studies of this polymorphism call for further investigations [9, 13].One study reported that the Gly972Arg in IRS-1 and Gly1057Asp in IRS-2 polymorphisms influence glucose homeostasis in premenopausal women, but are not associated with PCOS [14]. In addition, polymorphism studies are mostly dependent on ethnicity and variability within different geographic regions which could affect study results. Recently a study was done to find out the prevalence of different codons of IRS-1 in PCOS patients in different European ethnicities with different results [15, 16]. The present study was therefore undertaken to investigate the frequency of IRS1Gly972Arg and IRS2 Gly1057Asp polymorphisms and to compare the prevalence of these two polymorphisms in PCOS patients and non-diabetic healthy women in Iranian subjects.
Materials and methods
Subjects
Forty eight PCOS patients participated in our study following diagnostic criteria with reference to NIH consensus criteria. All patients had been on medication for PCOS and infertility. Fifty two non-diabetic, non-PCOS women referred to our clinic for non-PCOS related infertility were randomly collected as control group.
Metabolic assessment
All individuals had an oral glucose tolerance test (OGTT). After an overnight fast, HemoglobinA1c, fasting glucose and fasting insulin levels were obtained from blood samples. Dextrose (75 g) was then administered orally, and blood samples were obtained after 2 h for measurement of glucose concentrations. The degree of insulin resistance for each sample was estimated with Homeostasis Model Assessment (HOMA-IR) using the fallowing equation. [fasting insulin (micro units per milliliter) × fasting glucose (mill moles)]/22.5 [17]. The HOMA method has been recently validated to be a good index of insulin resistance in subjects with a broad range of insulin sensitivity and has a good correlation with the insulin-mediated glucose uptake calculated by the euglycemic hyperinsulinemic glucose clamp [14]
Molecular genetic studies
Genomic DNA was obtained from whole blood using a Qiagene DNA isolation kit. Genotyping of allelic variants Gly972Arg of IRS-1 and Gly1057Asp of IRS-2 was conducted using RFLP PCR technique. Primers were designed using Beacon Designer® software and provided by Meta Bion©. Forward and reverse primers sequences were 5′GCCTGGCAGGAGAGCACTG-3′ and 5′TCTGACGGGGACAACTCATCTG-3′ respectively for IRS-1 gene with 142 bp production length of amplicon and 5′GTCCCCGTCGTCGTCTCTG-3′ and 5′GCCACACCAAAAGCCATCTCG-3′ respectively for IRS-2 gene with 171 bp production length of amplicon. Digestions of amplified DNA sequences were conducted with SmaI restriction enzyme which could detect and cut CCCGGG sequences in two different sites of each amplicon in both IRS-1 and IRS-2 including Gly972 in IRS-1 and Gly1057 in IRS-2 gens. The digested segments were run on 5% agarose gel stained with ethidium bromide and images captured in UV photo duct.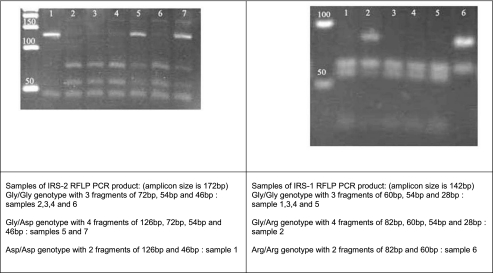
Statistical analysis
All statistical analyses were performed by using SPSS v.16 for Windows. Results are expressed as means ± SD unless otherwise stated. The Kolmogorov–Smirnov statistic was applied to continuous variables. Unpaired t-tests and one-way ANOVA followed by the least significant differences test for post hoc comparisons were used to compare the central tendencies of the different groups. Variables with no normal distribution or groups with small number (less than 6) of subjects were analyzed by non-parametric tests using Mann–Whitney or Kruskal–Wallis depending on number of groups compared. Cross tabulation was applied for evaluation of prevalence of polymorphisms and chi-square test was used for comparing the frequency of polymorphisms within groups.
Results
Clinical characteristics of the study population
The comparison of clinical and biochemical variables between PCOS patients and controls is shown in Table 1. The proportion of subjects with normal weight (BMI ≤25 kg/m2), overweight (BMI 25.0–29.9 kg/m2), obese (BMI 30–34.9 kg/m2) or morbid obesity (35≤ BMI kg/m2) was not statistically different (P > 0.200) between PCOS patients (normal weight 27.7%, overweight 44.7%, obesity 17% and morbid obesity 10.6%) and non-hyper androgenic controls (normal weight 40.4%, overweight 36.5%, obesity 19.2% and morbid obesity 3.8%). However compared with controls, PCOS patients had an increased prevalence of positive family history of PCOS (P < 0.001) and increased prevalence of hirsutism (P < 0.001).
Table 1.
Clinical characteristics of the study population
| Variable | Control mean ± SD | PCOS mean ± SD | Pvaluea |
| Age | 29.7 ± 6.4 | 26.5 ± 5.2 | .010 |
| BMI | 26.2 ± 4.0 | 27.4 ± 5.3 | 469 |
| FBS | 86.8 ± 9.4 | 87.9 ± 10.7 | .445 |
| 2hppBS | 93.1 ± 12.6 | 98.2 ± 15.1 | .154 |
| Fasting insulin | 12.7 ± 13.9 | 17.6 ± 25.9 | .167 |
| HOMA1-IR | 2.7 ± 3.0 | 3.7 ± 5.3 | .189 |
| Variable | Control (52) (%) | PCOS (48) (%) | Pvalueb |
| Positive family history | 1.9 | 27.1 | .000 |
| Presence of Hirsutism | 11.5 | 54.2 | .000 |
| Presence of insulin resistance | 25 | 27 | .81 |
| Presence of overweight and obesity | 59.6 | 70.8 | .24 |
aTested with Mann–Whitney
bTested with chi-square
IRS-1 and IRS-2 allele frequencies
No statistically significant differences in genotypes or allelic frequencies for both variants were observed between controls and PCOS patients. Moreover, no association was observed when considering the combination of both polymorphisms: 44% of controls and 42.6% of PCOS patients were homozygous for wild-type alleles of both genes, 52% of the controls and 51.1% of PCOS patients had mutated alleles of only IRS2 gene, and 4% of the controls and 6.4% of PCOS patients had mutated alleles for both IRS1 and IRS2 genes (P > 0.2).
IRS-1 Gly972Arg polymorphism effects on control subjects
Control subjects with the IRS-1 Gly/Arg genotype had higher levels of glucose at 2 h using the OGTT (107.0 ± 14 mg/dL) compared with those with the Gly/Gly (91.9 ± 11.9 mg/dL; P = 0.037) genotype (Table 2). Comparison of other variables including body mass index, fasting blood glucose, insulin and homeostasis model assessment of insulin resistance (HOMA-IR) with Gly/Gly and Gly/Arg genotype did not reveal any difference within the control group. Furthermore, other factors including family history, hirsutism, frequency of over weight status (BMI >25) and presence of insulin resistance (HOMA-IR >3.5) compared within allelic variables showed no significant difference (Table 3).
Table 2.
IRS-1 Gly972Arg polymorphism
| Control group | PCOS group | |||||
|---|---|---|---|---|---|---|
| Variable | Control Gly/Gly mean ± SD | Control Gly/Arg mean ± SD | Pvaluea | PCOS Gly/Gly (44) mean ± SD | PCOS X/Arg mean ± SD | Pvaluea |
| Age | 29.8 ± 6.5 | 28.7 ± 5.8 | .778a | 26.4 ± 5.1 | 27.7 ± 7.6 | .787a |
| BMI | 26.5 ± 4.1 | 23.5 ± 2.6 | .121a | 27.4 ± 5.2 | 28.2 ± 8.0 | .840a |
| FBS | 87.2 ± 9.0 | 82.5 ± 15.1 | .728a | 87.9 ± 11.0 | 89.2 ± 8.6 | .986a |
| 2hpp BS | 91.9 ± 11.9 | 107.0 ± 14.1 | .037a | 98.9 ± 15.6 | 92.0 ± 5.0 | .290a |
| Fasting insulin | 12.7 ± 14.5 | 13.0 ± 3.5 | .359a | 18.6 ± 26.9 | 7.1 ± 5.1 | .058a |
| HOMA1-IR | 2.7 ± 3.2 | 2.7 ± 1.0 | .455a | 4.0 ± 5.5 | 1.5 ± 1.1 | .076a |
| Family history | Positive | Negative | Pvalue | Positive | Negative | Pvalue |
| (Polymorphism frequency within group)%b | (0/1) 0 | (4/47) 7.8 | .77 | (0/13) 0 | (4/35) 11.4 | .20 |
| Hirsutism | Positive | Negative | Pvalue | Positive | Negative | Pvalue |
| (Polymorphism frequency within group)% | (0/6) 0 | (4/48) 8.7 | .45 | (3/12) 3.8 | (1/26) 13.6 | .22 |
| Insulin resistance | Positive | Negative | Pvalue | Positive | Negative | Pvalue |
| (Polymorphism frequency within group)% | (1/13) 7.7 | (3/39) 7.7 | 1.00 | (0/13) 0 | (4/35) 11.4 | .20 |
| Overweight and obesity | Positive | Negative | Pvalue | Positive | Negative | Pvalue |
| (Polymorphism frequency within group)% | (1/31) 3.2 | (3/21) 14.3 | .14 | (3/34) 8.8 | (1/14) 7.1 | .84 |
aTested with Mann–Whitney, p ≤ 0.05
bThe differences between total number of patients within groups are due to improper documentation
Table 3.
IRS-2 Gly1057Asp polymorphism
| Control group#52 | PCOS group#48 | |||||
|---|---|---|---|---|---|---|
| Variable | Control Gly/Gly mean ± SD | Control X/Asp mean ± SD | Pvalue | PCOS Gly/Gly mean ± SD | PCOS X/Asp mean ± SD | Pvalue |
| Age | 30.3 ± 6.7 | 29.3 ± 6.2 | .239a | 25.2 ± 4.1 | 27.6 ± 5.9 | .131 |
| BMI | 26.7 ± 4.1 | 25.9 ± 4.0 | .448a | 25.5 ± 2.7 | 28.2 ± 6.2 | .077 |
| FBS | 88.5 ± 9.6 | 85.4 ± 9.4 | .243 | 89.7 ± 9.5 | 86.6 ± 11.7 | .243 |
| Post prandial BS | 95.9 ± 12.9 | 90.6 ± 12.0 | .131 | 94.7 ± 11.1 | 99.0 ± 14.9 | .131 |
| Fasting insulin | 10.2 ± 5.3 | 11.6 ± 4.7 | .301 | 12.1 ± 4.8 | 11.8 ± 6.0 | .301 |
| HOMA1-IR | 2.2 ± 1.2 | 2.4 ± 1.1 | .481 | 3.0 ± 1.9 | 2.5 ± 1.3 | .481 |
| Control group | PCOS group | |||||
| Family history | Positive | Negative | Pvalue | Positive | Negative | Pvalue |
| (Polymorphism frequency within group)%b | (1/1) 100 | (27/54) 52.9 | .350 | (9/13) 69.2 | (18/35) 51.4 | .269 |
| Hirsutism | Positive | Negative | Pvalue | Positive | Negative | Pvalue |
| (Polymorphism frequency within group)% | (1/6) 16.7 | (27/46) 58.7 | .052 | (14/26) 53.8 | (13/22) 59.1 | .715 |
| Insulin resistance | Positive | Negative | Pvalue | Positive | Negative | Pvalue |
| (Polymorphism frequency within group)% | (7/13) 53.8 | (21/39) 53.8 | 1.000 | (7/13) 53.8 | (20/35) 57.1 | .838 |
| Overweight and obesity | Positive | Negative | Pvalue | Positive | Negative | Pvalue |
| (Polymorphism frequency within group)% | (17/31) 54.8 | (11/21) 52.4 | .862 | (18/34) 52.9 | (9/14) 64.3 | .471 |
aTested with Mann–Whitney
bThe differences between total number of patients within groups are due to improper documentation
IRS-1 Gly972Arg polymorphism effects on PCOS patients
Clinical and metabolic variables of wild type and polymorph alleles of IRS1 were compared (Table 2). Although fasting insulin and HOMA-IR were higher in polymorphic subjects compared with wild type subjects, statistical tests only showed a trend toward significance (P = 0.058 and 0.076, respectively).
IRS-2 Gly1057Asp polymorphism effects on control subjects
To compare the clinical and metabolic characteristics of IRS-2 alleles, we grouped Gly/Asp and Asp/Asp alleles as a single group and compared it with wild type Gly/Gly allele. Comparing variables within control subjects, no statistical significant difference was found. Subjects with wild type genotype had higher frequency of hirsutism compared with mutated genotypes (20.8% vs 3.6%) but this was not statistically significant (p = 0.054) (Table 3). After excluding non-overweight subjects (BMI ≤25), wild type overweight subjects were found to have higher post prandial blood sugar comparing with mutated over weight subjects (98.7 ± 12.1 vs 87 ± 13.5; P = 0.02) (Table 4).
Table 4.
IRS-2 Gly1057Asp polymorphism in overweight/obese samples
| Overweight/obese control group | Overweight/obese PCOS group | |||||
|---|---|---|---|---|---|---|
| Variable | Control Gly/Gly mean ± SD | Control X/Asp mean ± SD | Pvalue | PCOS Gly/Gly mean ± SD | PCOS X/Asp mean ± SD | Pvalue |
| Age | 30.6 ± 5.9 | 29.9 ± 6.6 | .765 | 25.5 ± 4.2 | 28.1 ± 6.4 | .166 |
| BMI | 29.5 ± 2.8 | 28.4 ± 2.8 | .200a | 27.9 ± 3.9 | 31.1 ± 5.1 | .008a |
| FBS | 86.0 ± 11.0 | 83.8 ± 10.9 | .527 | 88.9 ± 10.4 | 86.7 ± 11.8 | .568 |
| 2hpp BS | 98.7 ± 12.1 | 87.1 ± 13.5 | .020 | 93.2 ± 11.9 | 99.7 ± 12.8 | .153 |
| Fasting insulin | 11.1 ± 5.0 | 12.9 ± 3.9 | .291 | 12.1 ± 4.8 | 11.0 ± 6.1 | .579 |
| HOMA1-IR | 2.4 ± 1.2 | 2.7 ± 1.0 | .477 | 2.6 ± 1.0 | 2.3 ± 1.4 | .557 |
| Overweight/obese control group | Overweight/obese PCOS group | |||||
| Family history | Positive | Negative | Pvalue | Positive | Negative | Pvalue |
| (Polymorphism frequency within group)%b | (0/0) – | (17/31) 54.8 | – | (7/9) 77.8 | (11/25) 44 | .082 |
| Hirsutism | Positive | Negative | Pvalue | Positive | Negative | Pvalue |
| (Polymorphism frequency within group)% | (0/3) 0 | (17/28) 60.7 | .081 | (11/20) 55 | (7/14) 50 | .774 |
| Insulin Resistance | Positive | Negative | Pvalue | Positive | Negative | Pvalue |
| (Polymorphism frequency within group)% | (5/9) 55.6 | (12/22) 54.5 | .959 | (4/8) 50 | (14/26) 53.8 | .849 |
aTested with Mann–Whitney
bThe differences between total number of patients within groups are due to improper documentation
IRS-2 Gly1057Asp polymorphism effects on PCOS patients
As mentioned before, we compared IRS-2 Gly/Asp and Asp/Asp mutated alleles with Gly/Gly wiled type allele. No significant difference in variables between the two groups was observed (Table 3). Again, after excluding non-overweight subjects (BMI ≤25), this time differences were found in the BMI of wild type (27.8 ± 3.8) vs. mutated allelic groups (31.1 ± 5) (Table 4).
Discussion
Resistance to insulin is common in patients with PCOS [4, 5]. However genetic abnormalities that can fully account for this heritability have not been identified. Considering that IRS proteins play a critical role in insulin action based on both molecular and animal studies [8] polymorphism in their genes could affect their actions [18, 19]. IRS-1 Gly972Arg and IRS-2 Gly1057Asp polymorphisms have been studied in PCOS and other insulin resistant disorders like type 2 diabetes but the results are in major disagreement.
Therefore the role of these polymorphisms in etiology of insulin resistance in PCOS patients are not clearly understood and no practical conclusion is available. Furthermore different gene polymorphisms could participate in insulin signaling defects simultaneously and studying multiple polymorphism could reveal more interpretable results. Few studies have been conducted to date to investigate these combinational effects.
In this study we searched for differences between the distribution of both IRS-1 Gly972Arg and IRS-2 Gly1057Asp polymorphisms within PCOS patients and control subjects. Neither the IRS-2 Gly1057Asp polymorphisms, nor any combination of both, were associated with PCOS. This result is in agreement with other studies which have evaluated both IRS-1 and IRS-2 polymorphisms [9, 13] and others which have studied only IRS-1 polymorphism effects [12, 14]. For instance a study in Taiwanese population within 47 PCOS patients and 45 control subjects found no case of IRS-1 Gly972Arg polymorphism [20]. In contrast a study in Turkey reported different frequency of IRS-1 Gly972Arg, polymorphism in PCOS vs. control subjects 8.3% and 23.3% respectively (P = 0.04) [10]. Also other studies have shown more frequent IRS-1 polymorphism in PCOS patients [11]. These disagreements show that IRS-1 polymorphism alone may not play a major role in PCOS etiology. However its role cannot be ruled out since combination of other factors with IRS-1 polymorphism may change the extent of effects.
In addition, there is evidence implicating IRS-1 polymorphism in carbohydrate homeostasis and insulin sensitivity. In control subjects, polymorphism was associated with higher post prandial blood sugar levels comparing to wild type genotype (91.9 ± 11.9 vs.107 ± 14 mg/dl; P = 0.037). Although this difference is not clinically important since both of groups could be classified as normoglycemic, but metabolic effects of polymorphism could be evident by minimal changes. Other studies have found significant effects of IRS-1 polymorphism on fasting insulin and HOMA-IR but not on OGTT in both PCOS and control groups [10]. Another study of 103 PCOS patients and 48 control subjects in Spain did not find any metabolic differences within allelic genotypes of control group but PCOS subjects with mutated allele are found to have higher levels of fasting insulin and HOMA-IR [13]. In contrast, some studies found no effect of IRS-1Gly972Arg polymorphism on metabolic characteristics of PCOS or control groups [11, 12].
In evaluating IRS-2 Gly1057Asp polymorphism effects on metabolic characteristics of subjects, differences were revealed after restricting the analysis to over-weight/obese subjects (BMI >25). In over-weight PCOS patients, BMI found to be higher within mutated alleles compared to wild type allele (31.1 ± 5 vs. 27.8 ± 3.8; P = 0.008). Other studies evaluating IRS-2 polymorphism effects, did not find any differences between anthropometric measurement of allelic genotypes [9, 13, 14].
In addition, we found that IRS-2 mutated alleles within overweight/obese control subjects were associated with lower levels of post prandial glucose comparing with overweight/obese control wild types (87 ± 13.5 vs. 98.7 ± 12.1; P = 0.02). This result could be comparable with Ehrmann et al. who reported lower 2hpp BS levels in polymorphic PCOS subjects comparing with wild type, but no study reported this pattern of effects in control subjects [21].
In conclusion, considering that no association between the IRS-1 Gly972Arg and IRS-2 Gly1057Asp polymorphisms and PCOS were found, the results confirm that these polymorphisms should not be considered as major contributors to the pathogenesis of this disorder. Furthermore these polymorphisms influence glucose homeostasis indicated with 2hpp BS in control subjects in opposite manners so that IRS-1 Gly972Arg could be considered as a risk factor and IRS-2 Gly1057Asp could be mentioned as a supportive mutation in overweight/obese subjects against PPBS increase. Also body weight in overweight/obese PCOS patients was influenced by IRS-2 polymorphism; therefore it might influence the phenotypic characteristics of overweight/obese PCOS patients.
Acknowledgment
We would like to have a special thank to Mrs. Maryam Bagheri for managing the patients and to collect the data.
Footnotes
Capsule
Neither IRS-1 Gly972Arg nor IRS-2 Gly1057Asp polymorphisms should be considered as major contributors to the pathogenesis of PCOS.
References
- 1.Simoni M, Tempfer CB, Destenaves B, Fauser BCJM. Functional genetic polymorphisms and female reproductive disorders: part I: polycystic ovary syndrome and ovarian response. Hum Reprod Updat. 2008;14:459–484. doi: 10.1093/humupd/dmn024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franks S. Controversy in clinical endocrinology: diagnosis of polycystic ovarian syndrome: in defense of the Rotterdam criteria. J Clin Endocrinol Metab. 2006;91:786–789. doi: 10.1210/jc.2005-2501. [DOI] [PubMed] [Google Scholar]
- 3.Welt CK, Gudmundsson JA, Arason G, Adams J, Palsdottir H, Gudlaugsdottir G, et al. Characterizing discrete subsets of polycystic ovary syndrome as defined by the Rotterdam criteria: the impact of weight on phenotype and metabolic features. J Clin Endocrinol Metab. 2006;91:4842–4848. doi: 10.1210/jc.2006-1327. [DOI] [PubMed] [Google Scholar]
- 4.Carmina E, Napoli N, Longo RA, Rini GB, Lobo RA. Metabolic syndrome in polycystic ovary syndrome (PCOS): lower prevalence in southern Italy than in the USA and the influence of criteria for the diagnosis of PCOS. Eur J Endocrinol. 2006;154:141–145. doi: 10.1530/eje.1.02058. [DOI] [PubMed] [Google Scholar]
- 5.Wei HJ, Young R, Kuo IL, Liaw CM, Chiang HS, Yeh CY. Prevalence of insulin resistance and determination of risk factors for glucose intolerance in polycystic ovary syndrome: a cross-sectional study of Chinese infertility patients. Fertil Steril. 2009;91:1864–1868. doi: 10.1016/j.fertnstert.2008.02.168. [DOI] [PubMed] [Google Scholar]
- 6.Corbould A, Kim YB, Youngren JF, Pender C, Kahn BB, Lee A, et al. Insulin resistance in the skeletal muscle of women with PCOS involves intrinsic and acquired defects in insulin signaling. Am J Physiol Endocrinol Metab. 2005;288:E1047–E1054. doi: 10.1152/ajpendo.00361.2004. [DOI] [PubMed] [Google Scholar]
- 7.Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800. doi: 10.1210/er.18.6.774. [DOI] [PubMed] [Google Scholar]
- 8.Thirone AC, Huang C, Klip A. Tissue-specific roles of IRS proteins in insulin signaling and glucose transport. Trends Endocrinol Metab. 2006;17:72–78. doi: 10.1016/j.tem.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Mkadem SA, Lautier C, Macari F, Molinari N, Lefèbvre P, Renard E, et al. Role of allelic variants Gly972Arg of IRS-1 and Gly1057Asp of IRS-2 in moderate-to-severe insulin resistance of women with polycystic ovary syndrome. Diabetes. 2001;50:2164–2168. doi: 10.2337/diabetes.50.9.2164. [DOI] [PubMed] [Google Scholar]
- 10.Dilek S, Ertunc D, Tok EC, Erdal EM, Aktas A. Association of Gly972Arg variant of insulin receptor substrate-1 with metabolic features in women with polycystic ovary syndrome. Fertil Steril. 2005;84:407–412. doi: 10.1016/j.fertnstert.2005.01.133. [DOI] [PubMed] [Google Scholar]
- 11.Baba T, Endo T, Sata F, Honnma H, Kitajima Y, Hayashi T, et al. Polycystic ovary syndrome is associated with genetic polymorphism in the insulin signaling gene IRS-1 but not ENPP1 in a Japanese population. Life Sci. 2007;81:850–854. doi: 10.1016/j.lfs.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 12.Valdés P, Cerda A, Barrenechea C, Kehr M, Soto C, Salazar LA. No association between common Gly972Arg variant of the insulin receptor substrate-1 and polycystic ovary syndrome in Southern Chilean women. Clin Chim Acta. 2008;390:63–66. doi: 10.1016/j.cca.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 13.Ehrmann DA, Tang X, Yoshiuchi I, Cox NJ, Bell GI. Relationship of insulin receptor substrate-1 and -2 genotypes to phenotypic features of polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87:4297–4300. doi: 10.1210/jc.2002-020216. [DOI] [PubMed] [Google Scholar]
- 14.Villuendas G, Botella-Carretero JI, Roldán B, Sancho J, Escobar-Morreale HF. San Millán JL. Polymorphisms in the insulin receptor substrate-1 (IRS-1) gene and the insulin receptor substrate-2 (IRS-2) gene influence glucose homeostasis and body mass index in women with polycystic ovary syndrome and non-hyperandrogenic controls. Hum Reprod. 2005;20:3184–3191. doi: 10.1093/humrep/dei205. [DOI] [PubMed] [Google Scholar]
- 15.Pappalardo MA, Russo GT, Pedone A, Pizzo A, Borrielli I, Stabile G, Artenisio AC, Amato A, et al. Very high frequency of polymorphism for the insulin receptor substrate 1 (IRS-1) at codon 972 in Southern Italian women with polycystic ovary syndrome. Horm Metab Res. 2010;42:575–584. doi: 10.1055/s-0030-1249020. [DOI] [PubMed] [Google Scholar]
- 16.Christopoulos P, Mastorakos G, Gazouli M, Deligeoroglou E, Katsikis I, Diamanti-Kandarakis E, Panidis D, Creatsas G. Study of association of IRS-1 and IRS-2 genes polymorphisms with clinical and metabolic features in women with polycystic ovary syndrome. Is there an impact? J Gynecol Endocrinol. 2010;26:698–703. doi: 10.3109/09513591003649823. [DOI] [PubMed] [Google Scholar]
- 17.Chang AM, Smith MJ, Bloem CJ, Galecki AT, Halter JB, Supiano MA. Limitation of the homeostasis model assessment to predict insulin resistance and beta-cell dysfunction in older people. J Clin Endocrinol Metab. 2006;91:629–634. doi: 10.1210/jc.2005-1803. [DOI] [PubMed] [Google Scholar]
- 18.Marchetti P, Lupi R, Federici M, Marselli L, Masini M, Boggi U, et al. Insulin secretory function is impaired in isolated human islets carrying the Gly(972) Arg IRS-1 polymorphism. Diabetes. 2002;51:1419–1424. doi: 10.2337/diabetes.51.5.1419. [DOI] [PubMed] [Google Scholar]
- 19.Hribal ML, Federici M, Porzio O, Lauro D, Borboni P, Accili D, et al. The Gly972Arg amino acid polymorphism in insulin receptor substrate-1 affects glucose metabolism in skeletal muscle cells. J Clin Endocrinol Metab. 2000;85:2004–2013. doi: 10.1210/jc.85.5.2004. [DOI] [PubMed] [Google Scholar]
- 20.Lin TC, Yen JM, Gong KB, Kuo TC, Ku DC, Liang SF, et al. Abnormal glucose tolerance and insulin resistance in polycystic ovary syndrome amongst the Taiwanese population- not correlated with insulin receptor substrate-1 Gly972Arg/Ala513Pro polymorphism. BMC Med Genet. 2006;7:36. doi: 10.1186/1471-2350-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colilla S, Cox NJ, Ehrmann DA. Heritability of insulin secretion and insulin action in women with polycystic ovary syndrome and their first degree relatives. J Clin Endocrinol Metab. 2001;86:2027–2031. doi: 10.1210/jc.86.5.2027. [DOI] [PubMed] [Google Scholar]


