Abstract
Purpose
The in-vitro environment influences oocyte competence and gene expression in cumulus cells and oocytes. Effects of culturing under non-attachment conditions and varying follicle exposure to FSH were investigated at the mRNA level and on oocyte developmental capacity.
Methods
Quantitative PCR analysis of Gdf9, Mater, Nmp2 (in oocytes), Lhcgr and Amh (in cumulus cells), and oocyte developmental competence after in vitro follicle culture were evaluated.
Results
Follicle survival (98.7%) and polar body rate (94%) were similar for all conditions. Estradiol and progesterone production were significantly lower in non-attachment follicles (10-fold and 3-fold, respectively). Under non-attachment conditions, a higher two-cell rate (69.9%) and total blastocyst yield (48.5%) were obtained and, by decreasing FSH levels during culture, Lhcgr transcripts were significantly reduced to levels similar to in-vivo. Levels of oocyte-specific transcripts were not significantly influenced by in-vitro conditions.
Conclusion
Non-attachment conditions influence follicle steroid secretory capacity and, together with dynamic FSH doses, positively influence cumulus cell gene expression and oocyte developmental competence.
Keywords: Follicle culture, Gene expression, Oocyte competence, Follicle-stimulating hormone, Luteinizing hormone receptor
Introduction
Follicle culture and oocyte in vitro maturation (IVM) are techniques with future clinical applications in fertility preservation for patients undergoing chemotherapy for cancer and other severe diseases. Mouse in vitro follicle culture systems have been successfully developed, supporting growth of primordial and early preantral follicles up to developmentally competent oocytes giving healthy offspring [1–3]. Similarly, in large mammalian models and in human, the growth of primordial follicles up to the secondary and antral stage has been accomplished with some success (For review see [4]).
Given the fact that competence of oocytes obtained with in vitro techniques is as yet suboptimal when compared to oocytes grown and matured in vivo [3, 5–9], the development of more appropriate in vitro conditions is a prerequisite for obtaining large numbers of competent oocytes in a reproducible manner.
The influence of the presence of insulin and follicle-stimulating hormone (FSH) in the in vitro follicle culture media has been previously studied and it is currently known that high doses of both hormones (alone or in combination) induce an inappropriate differentiation of granulosa cells and have a negative influence on oocyte competence [10–14].
Although gonadotrophins are essential for follicle survival in vivo beyond the antral stages, FSH is also needed in vitro at an earlier stage for antrum formation to occur [2, 15, 16]. A minimally effective FSH dose during the late preantral stages of in vitro growth is crucial for follicle survival in vitro [17], however, decreased FSH concentrations during the last part of the antral stage of in vitro culture demonstrated to be beneficial for maintaining the cumulus cells phenotype more similar to in vivo conditions [13]; whereas insulin in low concentrations (close to physiology) allows the expression levels of oocyte and cumulus cells genes to be more comparable to in vivo [14]. As suggested by Latham et al. [11], improvement in the culture system for obtaining good quality oocytes should also involve conditions that promote the cumulus cells phenotype.
Besides the culture media components, other factors such as the spatial distribution of the follicle cells in combination with the concentration of the major hormones FSH and insulin may play a role in the embryo developmental potential of in vitro grown oocytes. Since the first report on in vitro spherical follicle culture [15], many culture systems in which the attachment of the follicles is inhibited by different strategies have been developed along the years [18]. These techniques have allowed the growth of preantral follicles up to the preovulatory stage providing oocytes capable of fertilizing and developing to the blastocyst stage [19–22]. Latter studies made use of late preantral follicles with a follicle diameter ranging from 150 to 200 μm. Considering that follicles of different sizes at the beginning of culture have an influence on the final blastocyst rate [22], this study aimed to develop a system allowing early preantral follicles 100–130 to grow in conditions ensuring a differentiation closer to in vivo.
Over the last years, a new approach has been taken to develop a spherical culture system for follicles using an alginate matrix [23], which allowed obtaining developmentally competent oocytes from early secondary mouse follicles [24, 25].
Given that three dimensional relationships (spatial positioning) between the different cell types in culture may also influence cumulus differentiation and impact oocyte developmental competence, our study proposed to use round-bottomed ultra low attachment plates to prohibit attachment and flattening out of the early preantral follicles. The intention was: 1) to describe follicle and oocyte development when follicle attachment was inhibited by hydrogel coating; 2) to assess the influence of the spatial cellular relationship and different FSH concentrations on gene expression in oocytes and cumulus cells; and 3) assessment of oocyte developmental capacity. In this study we compared non-attachment (NA) cultures with the routine attachment (AT) follicle culture procedure [26]. It was hypothesized that the combination of the mentioned modifications would allow a better development of the oocyte and their surrounding cumulus cells positively influencing oocytes competence.
Material and methods
Animal model
Mice (F1 hybrids: C57Bl/6j × CBA/ca) used for these experiments were housed and bred following the national legislation and with the consent of the local ethical committee of the Vrije Universiteit Brussel (Project number: 09-216-1).
Follicle culture
Early secondary follicles from 13-day-old female mice ovaries were mechanically isolated as described by Cortvrindt & Smitz [26]. Ovaries were placed in Leibovitz L-15, 10% heat-inactivated Foetal Bovine Serum (FBS), containing 100 IU/ml penicillin, 100 μg/ml streptomycin (Penicillin/Streptomycin-Mix) (all from Invitrogen, Merelbeke, Belgium) and preantral follicles were dissected out.
Follicle culture was carried out as previously reported [14]. On average, 12–18 follicles (diameters between 110 and 130 μm) were individually transferred to either 96-well tissue-culture treated microplates (Costar, Elscolab, Kruibeke, Belgium), which are referred to as ‘Attachment culture’ (AT); or to a 96-well ‘ultra low attachment’ microplates (Costar, Elscolab, Kruibeke, Belgium), which are referred to as ‘Non-attachment culture’ (NA). These latter hydrogel coated-round bottomed plates were used in order to avoid the attachment of the ovarian follicles to their surface. In both cases, the set-up medium contained: 75 μl α-MEM glutamax (Invitrogen), 5% FBS (Invitrogen), 5 ng/ml Insulin, 5μg/ml human apo-Transferrin, 5 ng/ml Sodium selenite (All from Sigma), 10 mIU/ml r-FSH (Gonal-F) and 10mIU/ml r-LH (Both from Ares-Serono, Geneva, Switzerland).
The follicle culture period was of 9 days at 37°C, 20% O2, 5% CO2 and 100% humidity. Medium refreshment was done every 3 days by replacing 30 μl of spent medium with a fresh medium.
On day 9 of culture, all follicles from the different conditions were stimulated to allow ovulation. Ovulation was triggered with a combination of 1.2 IU human Chorionic Gonadotrophin (hCG; Ares-Serono, Geneva Switzerland) and 4 ng/ml recombinant human Epidermal Growth Factor (EGF; Roche Diagnostics, Mannheim Germany) [27]. Regardless of the composition of the media where follicles were grown in, the stimulation media contained 10mIU/ml FSH. Oocyte meiotic resumption, given by the extrusion of the polar body (PB) was assessed 16 hours after stimulation under an inverted microscope equipped with a Hoffman modulation contrast system (Nikon, Tokyo, Japan). From 20 to 40 oocytes in each group were assessed for oocyte meiotic resumption.
Follicle survival during culture was assessed by evaluating oocyte degeneration and/or oocyte release from the follicles. Follicles without oocyte quickly degenerate (and die), and are therefore deducted from the total number of follicles put in culture.
Sample selection procedure from the two follicle culture systems
For the sake of clarity a brief explanation of the follicular development under the AT and NA cultures as well as the selection of samples studied follows.
In the AT culture, the onset of antral cavity formation occurs on day 6 of culture. By then, two morphologically different follicle types can be distinguished: follicles at the ‘late preantral’ stage (no antral-like cavities yet present); and follicles at an ‘early antral’ stage, in which the antral-like cavity and a cumulus-oocyte complex (COC) start to form and can be clearly distinguished under the stereo microscope (60x).
In the NA cultures, follicle morphology was different compared to the AT cultures in that onset of antral cavity was not apparent by culture day 6. By day 9, the last day of culture, follicles can be morphologically differentiated in two types: 1) follicles having reached the large antral stage (diameter >350 μm) with a multilayered compact COC, as assessed under the stereo-microscope; 2) follicles in which an antral cavity was not visible and having a smaller size (ranging from 220 to 350 μm). The antral cavity formation on in vitro follicles reaching approximately 300 μm has also been reported by other researchers using spherical culture systems [22, 28]. Therefore, it was decided to divide cultured follicles in two groups (based on their size and presence of antral cavities) for a preliminary analysis of nuclear chromatin configuration.
Assessment of oocyte chromatin configuration in the AT and NA systems
Oocyte chromatin configuration was assessed on GV oocytes from day 8 of culture (based on previous data [29]). Oocytes were stained with 10 μg/ml Hoechst 33258 (Sigma) for 15 minutes. Nucleolar chromatin conformation was analyzed under a fluorescence microscope (IX70; Olympus). Chromatin conformation was classified as non-surrounded nucleolus (NSN), surrounded nucleolus (SN), or transitional (NSN/SN) stage. Oocytes for this purpose were collected from 3 independent experiments (on average 26 oocytes per condition were analyzed).
Effect of decreasing FSH concentration on follicle development in the AT and NA systems
In order to test the effects of decreasing the FSH concentration, FSH was omitted from the refreshment medium on day 3 and/or 6 for both AT and NA cultures. A first group of follicles were cultured under 10mIU/ml FSH (referred to as ‘AT FSH 10 IU’ and ‘NA FSH 10 IU’) during the entire culture period (9 days); a second group of follicles were exposed to 10mIU/ml FSH only from day 0 to day 3 (referred to as ‘AT No FSH D3’ and ‘NA No FSH D3’) and subsequently FSH was omitted; a third group of follicles were exposed to 10mIU/ml FSH only from day 0 to day 6 and subsequently FSH was omitted (referred to as ‘AT No FSH D6’ and ‘NA No FSH D6’).
Steroidal profile of cultured follicles
On day 9 of culture, spent medium from follicles (from both AT and NA cultures) was pooled and frozen for later measurement of estradiol, progesterone and testosterone levels. Progesterone and testosterone were measured as previously described [30]. Briefly, progesterone concentration was determined by a direct radioimmunoassay from Cisbio (Cisbio international, Gif-sur-Yvette cedex, France). Testosterone concentration was determined by a direct immunoassay from Orion Diagnostica (RIA, ORION Diagnostica; Espoo, Finland). 17b-estradiol was measured with a direct immunoassay from DIAsource Immunoassays (DIAsource E2-RIA-CT, Nivelles, Belgium). All immunoassays have been validated for their use on conditioned culture medium [30]. For the estradiol assay, the functional sensitivity was 20 ng/L, the coefficient of variation (%CV) tested on 3 levels with control samples ranged from 5.6% to 7.4% (%CV) allowing for a measurement range from 20 to 750 ng/L. The functional sensitivity for the progesterone assay was 1.0 μg/L and its variability, as measured on 3 levels, ranges from 5.2% to 15% (%CV), allowing for a working range from 1 to 40 μg/L. All samples were analyzed with the same production batch of reagents to minimize intrinsic technical variability. Steroids levels in culture media were measured for 7–12 samples per condition (each being a pool of 4–6 follicles).
Theca cell identification
Presence of theca cells was investigated via detection of alkaline phosphatase activity, typical for theca interna cells, and previously standardized for the AT follicle culture used in the current study [31, 32].
Follicles cultured under the NA system (10mIU FSH for the entire culture) were fixed with ice-cold absolute ethanol (Merck, Germany) for 10 minutes at -20°C, and incubated for 45 min in a mixture of naphthol AS-BI phosphosil acid sodium salt (25%) with Fast Blue BB salt (75%) in a 0.2 M Tris–HCl buffer solution (pH 9.3) (all from Sigma) at room temperature (in the dark). Follicles were then washed for 30 min in tap water, and rinsed 3 times with distilled water.
Counterstaining of granulosa cells was done with 0.5% Nuclear Fast Red for 10 min followed by 3 rinsing steps with distilled water. Cells with active alkaline phosphatase are stained blue (theca cells).
Oocyte and cumulus cells gene expression
The first aim of the study was to analyze and compare the gene expression levels in oocyte and cumulus cells from antral follicles grown under regular conditions or decreasing FSH concentration in both AT and NA cultures.
On day 9, COCs in the AT and NA cultures and from the different FSH conditions were isolated from antral follicles. Oocytes were freed from cumulus cells as previously described [13]. Oocytes and their corresponding cumulus cells were collected per pools of 4 to 7, frozen in liquid nitrogen and stored at -80°C for later gene expression analysis.
In vivo oocyte and cumulus cells samples from age-matched female mice primed 46 h with equine chorionic gonadotrophin (eCG) were included in the analysis as controls.
RNA isolation, cDNA synthesis and real-time PCR analysis
RNA isolation and quantification was performed as described previously [13]. Briefly, total RNA was extracted from 5 to 9 pools of oocytes or cumulus cells samples using the RNeasy Micro Kit (Qiagen) according to the instructions of the manufacturer. Reverse transcription to cDNA was performed by using the iScript cDNA Synthesis Kit (Bio-Rad Laboratories) according to manufacturer’s instructions and using blend oligo(dT) and random hexamers.
Quantification by Real-time RT-PCR was performed on the LightCycler 480 device (Roche Diagnostics). The amplification reaction was carried out in 15 μl, containing 2 μl cDNA, 7.5 μl SYBR Green PCR Master Mix 2x (Roche Diagnostics), 1.5 μl forward and reverse specific primer mix (0.6 μM final concentration) and 4 μl. nuclease-free water. The amplification cycle was as follows: 95°C for 10 min followed by 55 cycles of 95°C for 10s and 60°C for 30s. This was followed by the acquisition of the melting curve (95°C for 5s and 60°C for 1min), and a continuous fluorescence measurement.
Quantification of gene specific transcripts was calculated on the basis of the standard curve generated by amplification of serial dilutions of the specific amplicon for every gene analyzed. To ensure the specificity of the PCR products the melting curves after amplification cycle were analyzed. Transcripts levels in the oocytes were normalized by the number of oocytes in every sample and corrected for the loss of RNA and the efficiency of the cDNA synthesis by means of the recovery rate of the luciferase mRNA spiked before RNA extraction (exogenous control) as described previously (Sánchez et al., 2010). Transcripts levels in cumulus cells were normalized to the content of 18 s RNA. Sequences of PCR primers for mouse Gdf9, Mater, Npm2, Amh, Lhcgr and 18S are described in Table 1.
Table 1.
Primer sequences for the analyzed genes
| Gene | Sequence (5′-3′) | Fragment size (bp) | Accession N° |
|---|---|---|---|
| Gdf9 | F: taccgtccggctcttcagt | 93 | NM_008110.1 |
| R: ttaaacagcaggtccaccatc | |||
| Mater | F: caatgccctgtctctaacctg | 71 | NM_011860.2 |
| R: tgtcttctcactcgggcata | |||
| Npm2 | F: aatcactattgctacgctgaagg | 95 | NM_181345.1 |
| R: cagtcctgagccgaaaagtt | |||
| Amh | F: tgctagtcctacatctggctga | 120 | NM_007445.2 |
| R: gtccagggtatagcactaacagg | |||
| Lhcgr | F: gaaatggatttgaagaagtacaaag | 100 | NM_013582.2 |
| R: ccattgtgcatcttctccag | |||
| 18S | F: tcaagaacgaaagtcggagg | 489 | NR_003278.1 |
| R: ggacatctaagggcatcaca |
Developmental competence from in vitro grown oocytes: effects of follicle morphology in the AT and NA cultures and decreased FSH levels
In vitro fertilization of cultured follicles
In order to evaluate oocyte developmental capacity, ‘NA FSH 10 IU’ and ‘NA No FSH D6’ were compared with a standard control condition in the AT culture (‘AT FSH 10 IU’or control).
Oocytes grown in-vivo, obtained from superovulated 25-days-old mice receiving 2.5 IU eCG for 46 hours (Folligon) and 2.5 IU hCG (Chorulon) for 14 hours (both from Intervet, The Netherlands), were included in each experiment as controls for in vitro fertilization and early embryo development.
All media used for IVF and embryo culture were kindly donated by Cook medical (Sydney IVF media suite; Australia).
Briefly, cumulus oocyte complexes were collected from antral follicles (both from AT and NA cultures) and washed for 5 minutes in Gamete Buffer. In vitro fertilization (IVF) was performed in Fertilization Medium using fresh sperm (final dilution of 2x106/ml) obtained from a CBAB6F1 male donor. After incubation with sperm for 4 hour at 37°C in 6% CO2, 5% O2, 89% N2, presumptive zygotes were washed once in Gamete Buffer, twice in Cleavage Medium and cultured in groups of 10–15 in 20 μl Cleavage Medium drops over-layered with mineral oil at 37°C in 6% CO2, 5% O2 and 89% N2. Cleavage rate was scored 24 hours after IVF. On day 3, dividing embryos were transferred to Blastocyst medium and on day 5, blastocyst development and hatching were recorded. Oocyte from five independent follicle cultures (each consisting of 3 plates per condition) were assessed.
Statistical analysis
Unless mentioned otherwise, the results are shown as the Mean ± SEM. Differences in the percentage of oocytes at the SN and NSN nucleolar conformation, follicle survival, oocyte polar body rate and steroid production among the different in vitro conditions were assessed by ANOVA followed by a Tukey’s Multiple Comparison Test, p < 0.05. Percentages data were transformed (arcsine) before performing statistical analysis.
Oocyte gene expression results an ANOVA model was constructed with the log-fold ratio as response variable and treatment as fixed factor. Residual analysis showed normal and identical distribution of the error terms for the different treatments. All-pair wise comparisons were set up and Tukey’s correction for simultaneous hypothesis testing was applied. Transcript values were normalized to the amount of the exogenous control luciferase and are represented as relative to the control condition in the conventional attachment system (AT FSH 10 IU).
Cumulus cells gene expression results a Mann-Whitney U test was performed to compare between different groups. A Bonferroni correction was applied by considering only P-values below an ‘m’ as significant, where m is the number of comparisons made, p < 0.0024.
In vitro fertilization results A generalized linear model was fit, using a binomial distribution for the binary outcome and a probit link. Comparisons between the three in vitro treatments (‘AT FSH 10 IU’, ‘NA FSH 10 IU’ and ‘NA No FSH D6’) were set up and Tukey’s correction for simultaneous hypothesis testing was applied.
Results
Preliminary study: assessment of oocyte nucleolar conformation under AT and NA culture conditions
Chromatin remodeling and transition from a non-surrounded (NSN) to a surrounded nucleolus (SN) conformation is required for acquisition of both meiotic and developmental competence and is associated with oocyte transcriptional silencing [33–37]. Oocytes from antral follicles after 8 days of culture were collected and analyzed separately as described in Material and methods. Under AT culture conditions, day 8 antral follicles had a high percentage of oocytes at the SN stage, but this was dependent on whether follicles start to form antral-like cavities on day 6. Under NA conditions, a high percentage of oocytes acquired a SN configuration by day 8, coincident with the presence of a clear antral-like cavity at this stage. This demonstrated a comparable group of antral follicles in both systems, both having a higher percentage of oocytes at the SN stage (and referred to as ‘Antral’ follicles, Table 2). Remaining follicles (those at the late preantral stage on day 6 or 8 in the AT and NA cultures, respectively), did not exactly corresponded to similar developmental stages, but were an heterogeneous and less developed population (and referred to as ‘preantral’, Table 2).
Table 2.
Analysis of nucleolar chromatin configuration in oocytes from the attachment and non-attachment cultures on day 8 of culture
| Condition | NSN (%) | NSN/SN (%) | SN (%) |
|---|---|---|---|
| AT - ‘Preantral’ | 3,3 ± 3,3 | 53,9 ± 6,1 | 42,8 ± 8,3 |
| AT - ‘Antral’ | 0 | 20,5 ± 10,3 | 79,5 ± 10,3 |
| NA - ‘Preantral’ | 25,1 ± 16,9 | 58,0 ± 21,6 | 16,9 ± 10,6 |
| NA - ‘Antral’ | 0 | 23,3 ± 14,5 | 76,7 ± 14,5 |
Values are represented as Mean ± SEM
Groups of follicles were divided in ‘Preantral’ and ‘Antral’ differently for the AT and NA systems (see Material and methods)
Oocytes were collected from 3 independent experiments (on average 26 oocytes per condition were analyzed)
Therefore, based on these results, and in order to analyze and compare homogenous follicle populations, only the ‘Antral’ follicles, containing the highest proportion of oocytes at the SN chromatin configuration, were further studied.
Follicle development in AT and NA cultures
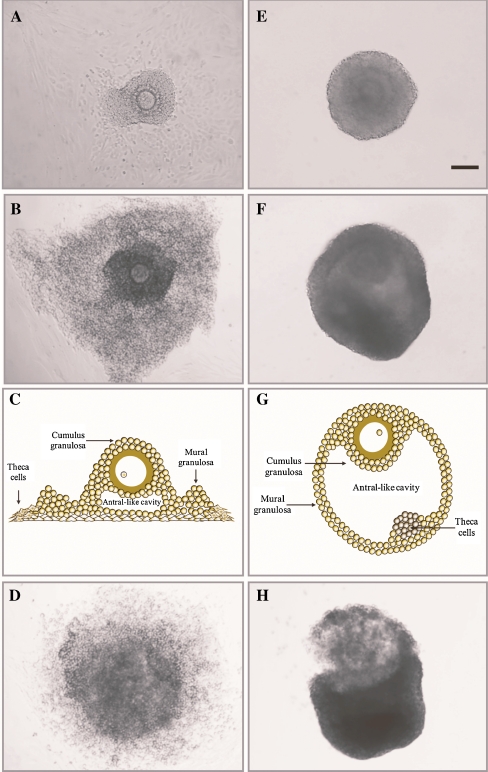
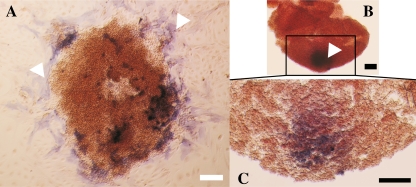
As reported earlier [26, 30, 32], follicles in AT cultures had a flattened-out morphology as a result of the rupture of the basement membrane and attachment of the theca and granulosa cells to the bottom of the culture well (Fig. 1a-c). In contrast, follicles under NA conditions were prevented from adhering to the bottom of the wells and developed into spherical-like structures (Fig. 1e-g). Thorough morphological analysis of NA follicles evidenced that the basement membrane was also breached during culture (~day 6 of culture), allowing the granulosa cells to out-grow. Staining for alkaline phosphatase activity demonstrated that theca cells were located within the layers of granulosa cells, differently positioned in comparison with those under attachment conditions (Fig. 2). At the end of culture, follicles under both culture conditions responded equally to the ovulatory stimulus by showing cumulus cells mucification and expansion (Fig. 1d and h).
Fig. 1.
Representative pictures of in vitro developed AT (a,b,d) and NA (e,f,h) follicles at day 6 of culture (a,e), and at the large antral stage before (b,f) and after (d,h) the ovulatory stimulus (day 9 and 10, respectively). c and g show a schematic view of how follicles develop in vitro under AT and NA systems, respectively. Scale bar = 100 μm
Fig. 2.
Staining for alkaline phosphatase activity. Theca cells in culture were evidenced by alkaline phosphatase staining giving purple color (arrow heads). In AT-cultured follicles, theca cells are distributed in the surroundings of the follicle and attached to the bottom of the culture dish (a). In the NA-cultured follicles, theca cells are kept entrapped into the follicle and visualized, under light microscopy, as a dark spot within the mass of cells (b, c). Black scale bars = 50 μm; white scale bar = 100 μm
Effects of AT and NA culture conditions, and decreasing FSH concentration on follicle survival, steroid secretion and oocyte meiotic resumption
Follicle survival rate was similar under the six different conditions studied: on average for all groups 98.7%. Induction of antrum formation was somehow reduced (ns) under the condition in which FSH was decreased from as early as day 3 (Table 3). There were no differences in oocyte meiotic resumption between any of the conditions after the ovulatory stimulus. The PB rate was, on average, 93.5% for all groups (Table 3).
Table 3.
Follicle survival rates, antral follicle development and oocyte polar body rate in the attachment and non-attachment culture systems
| Culture condition | Survival (%) | a Antral follicles analyzed/total (%) | PB rate (%) (Antral follicles) |
|---|---|---|---|
| AT FSH 10 IU | 100 | 44,6 ± 4,3 | 94.4 ± 5.6 |
| AT No FSH D6 | 100 | 98.2 ± 1.8 | |
| AT No FSH D3 | 97,3 ± 1,1 | 39,0 ± 4,3 | 88.8 ± 6.6 |
| NA FSH 10 IU | 98,9 ± 0,8 | 56,9 ± 3,9 | 93.9 ± 3.6 |
| NA No FSH D6 | 98,2 ± 1,0 | 49,3 ± 4,4 | 96.4 ± 3.6 |
| NA No FSH D3 | 97,8 ± 0,9 | 33,5 ± 4,0 | 90.0 ± 5.8 |
Values are represented as Mean ± SEM
No statistically differences were recorded (ANOVA P > 0.05)
Survival rate was calculated over total follicles put in culture at the end of culture (day 9)
PB rate was calculated only on ‘Antral’ follicles (meaning the all follicles included in the study) and assessed on day 10 (from 20 to 40 oocytes per condition)
a = Antral follicles analyzed over the total follicles put in culture. For the AT cultures this was based on day 6 and for the NA cultures on day 9 (see Material and methods)
Estradiol and progesterone levels produced by follicles from the AT cultures were significantly higher than in follicles from NA cultures (10-fold difference for estradiol and a more than 3-fold difference for progesterone) (Table 4). Moreover, Testosterone concentrations in the NA cultures were below the level of quantification. Duration of FSH exposure did not have an influence on any of the steroids measured.
Table 4.
Steroid measurements on day 9 antral follicles
| Condition | Progesterone (μg/L) | Testosterone (μg/L) | Estradiol (ng/L) |
|---|---|---|---|
| AT FSH 10 IU | 1,24 ± 0,17 a | 0,31 ± 0,05 | 7497 ± 1053 a |
| AT No FSH D6 | 0,86 ± 0,05 a | 0,60 ± 0,19 | 7760 ± 1203 a |
| AT No FSH D3 | 1,27 ± 0,25 a | 0,77 ± 0,17 | 8360 ± 1677 a |
| NA FSH 10 IU | 0,40 ± 0,06 b | ND | 621,9 ± 88,2 b |
| NA No FSH D6 | 0,37 ± 0,05 b | ND | 527 ± 56,6 b |
| NA No FSH D3 | 0,17 ± 0,03 b | ND | 560,4 ± 89,5 b |
Values are represented as Mean ± SEM
ab Different letters within the same column denote statistically significant differences among the conditions. (ANOVA P < 0.05)
ND not detectable values
Steroids levels were measured for 7–12 samples per condition (each being a pool of 4–6 follicles)
Table 3 shows the percentage of antral follicles at the different conditions and therefore represents the percentage of follicles included in the study.
Effect of decreasing FSH concentration on oocyte and cumulus cells gene expression in the AT and NA culture systems
Gene expression in oocytes
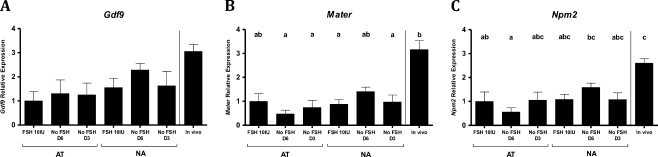
Overall, the expression of the oocyte transcripts analyzed on culture day 9 was not significantly different among the in vitro conditions (different FSH groups or the different follicular morphologies) (Fig. 3). The one exception was that levels of Npm2 mRNA were significantly lower in the ‘AT No FSH D6’ compared to the ‘NA No FSH D6’. Similarly, amounts of Mater and Npm2 expressed by oocytes from the ‘AT No FSH D6’ condition, tended to be lower than the other in vitro groups.
Fig. 3.
Expression of Gdf9 (a), Mater (b) and Npm2 (c) transcripts in in-vitro day 9 germinal vesicle oocytes from AT-follicles (left side) and NA-follicles (right side) exposed to the variable FSH conditions. Germinal vesicle oocytes from 46 h post eCG female mice ovarian antral follicles are included as in vivo control for gene expression and also considered in the statistical analysis. Five to nine pools of oocytes (each pool from 4 to 6 oocytes) were analyzed. Different letters indicate significant differences. ANOVA, p < 0.05
In vivo levels of the three transcripts tended to be higher compared to in vitro conditions (significantly different in some cases). Gdf9 expression levels did not differ significantly between in vivo and in vitro oocytes, however, it was noticeable that the ‘AT FSH 10 IU’ condition was ~3-fold lower than in vivo (Fig. 3a). Transcript levels of Mater, though, were significantly higher in in-vivo compared to most of the in vitro conditions, except when compared to ‘AT FSH 10 IU’ and ‘NA No FSH D6’ (Fig. 3b). Npm2 mRNA levels did not differ from in vivo, except for the control ‘AT FSH 10 IU’ and ‘AT No FSH D6’ (Fig. 3c). Notably, for the three genes analyzed, oocytes from ‘NA No FSH D6’ condition had consistently higher values (ns) than the other in vitro groups; and similar to in vivo levels.
Gene expression in cumulus cells
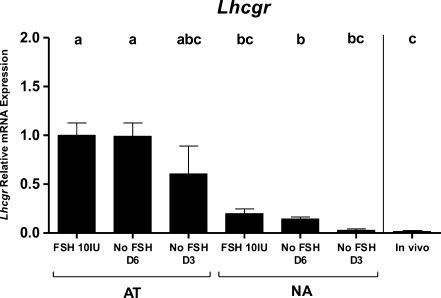
Amh and Lhcgr transcript levels were quantified in cumulus cells corresponding to the analyzed oocytes. Amh mRNA levels were below the limit of quantification in all samples from the AT cultures. Cumulus cells of follicles from the NA cultures expressed very low levels of Amh; it was however noticed that the lower was the FSH concentration, the higher the number of samples with a quantifiable Amh level, in both culture systems. Given the remarkably low levels of Amh RNA, effects of the different follicle morphologies (AT and NA cultures) on Amh gene expression was not assessed. In contrast, cumulus cells from the two types of follicle cultures differed in their amount of Lhcgr mRNA (Fig. 4). Cumulus cells grown in the NA cultures expressed significantly lower Lhcgr transcript levels compared to those grown in the AT cultures. Lhcgr mRNA levels were consistently lower in cumulus cells of NA cultures and decreasing FSH concentration, reaching levels very similar to in vivo (Fig. 4).
Fig. 4.
Messenger RNA expression of Lhcgr in in-vitro day 9 cumulus cells from AT-follicles and NA-follicles (right side) exposed to the variable FSH conditions. In vivo control are cumulus cells from eCG injected mice. Five to nine pools of cumulus cells (each pool of cumulus cells sample coming from 4 to 6 oocytes) were analyzed. Different letters indicate significant differences. Mann-Whitney U test followed by a Bonferroni correction, p < 0.0024
Developmental competence of fully grown oocytes from the AT and NA cultures under decreased FSH conditions
In order to evaluate the effects of the gene expression changes in oocyte and cumulus cells upon oocyte developmental competence, oocytes from the ‘NA FSH 10 IU’, ‘NA No FSH D6’ and ‘AT FSH 10 IU’ (this latter, the standard control condition in the attachment system) were fertilized and cultured up to the blastocyst stage.
The condition ‘NA No FSH D3’ was not selected for this purpose. Although it induced very low Lhcgr levels in the cumulus cells, the induction of antral cavity was reduced under this condition, providing less fertilizable oocytes. Moreover, Mater expression in these oocytes was significantly lower compared to in vivo.
In vitro fertilization of cultured follicles
Oocytes obtained from ‘NA No FSH D6’ condition had a significantly higher two-cell embryo rate (69.9%) compared to the other two conditions (Table 5). The rate of blastocysts over two-cell embryos was equal for both the ‘AT FSH 10 IU’ and ‘NA No FSH D6’ conditions. However, total blastocysts yield, considering the total number of oocytes used in the study, was significantly higher in the ‘NA NO FSH D6’ (48.5%) condition compared to ‘NA FSH 10 IU’ (24.4%) and tended (p = 0.059) to be higher than the ‘AT FSH 10 IU’ (30.9%) condition. The in vivo controls done in parallel demonstrated, as expected, a high developmental potential (90% blastocyst/total).
Table 5.
In vitro fertilization and embryo development
| Condition | Total N° of oocytes | Two-cell embryos N (%) | Blastocysts/two-cell N (%) | Total blastocysts 1 N (%) |
|---|---|---|---|---|
| AT FSH 10 IU | 68 | 35 (51.5) a | 21 (60) ab | 21 (30.9) ab |
| NA FSH 10 IU | 123 | 67 (54.5) a | 30 (44.8) a | 30 (24.4) a |
| NA No FSH D6 | 103 | 72 (69.9) b | 50 (69.4) b | 50 (48.5) b |
1 Total blastocyst = total oocytes that developed to blastocyst
ab = Different letters within a same column represent statistical significant differences among the conditions, p < 0.05
Oocytes from five independent follicle cultures were assessed
Discussion
Different spherical follicle culture systems have used strategies to prevent the attachment of follicles to the culture wells, with the aim to provide a more natural environment to the growing oocyte [15, 18, 20, 22, 24, 25, 28, 38–41]. The present study evaluates the impact of culturing follicles in a non-attachment system by comparing gene expression levels in oocytes and their surrounding cumulus cells to those found in a previously characterized attachment follicle culture system and to in-vivo conditions. The approach employed here was the use of hydrogel-coated well plates that avoids the need of transferring follicles daily to prevent their attachment, and at the same time, it provides a more in-vivo-like relationship among the follicular cells.
Moreover, our research group and others have shown that hormonal supplements in culture media play an important role. For instance, FSH and insulin modulate the differentiation of granulosa cells and oocyte developmental capacity [10, 11, 13, 14]. Therefore, in the current study, follicles were cultured under a physiological dose of insulin (5 ng/ml) and under a dynamic FSH dose (decreasing over time), which induces cumulus cells differentiation more similar to in-vivo [13, 14].
Follicle and oocyte development under the different in vitro conditions
A similar high follicle survival rate was obtained in both NA and AT conditions, and this was regardless of the FSH levels during culture. Both in-vivo and in-vitro, FSH is essential for follicle survival. Moreover, in-vitro grown follicles under both AT and NA conditions need FSH to grow and develop antral-like cavities [2, 15, 22, 28, 40, 42, 43]. Herein, we report that decreasing FSH concentration from the previously reported minimal essential dose of 10mIU/ml [17]; supports antrum formation, follicle differentiation and development through the antral phase. However, the proportion of follicles becoming antral by day 9 was, to some extent, diminished when FSH was decreased from day 3. These results confirm the important role of FSH, but also stress the fact that low FSH concentrations are able to support follicle survival, antral cavity development and granulosa cell differentiation, yet allowing the oocyte to resume meiosis (more than 93%) and being fertilizable. Furthermore, follicles in the NA cultures reached large antral stages (mean diameter: 424 ± 18 μm), with a recognizable antral-like cavity at diameters ≥350 μm; similar to those reported under other non-attachment conditions (~400 μm and ~300 μm, respectively) [15, 20, 22, 28, 38, 39]. This becomes significant since initial follicle diameters in previous reports differ from ours (150–200 μm; with a length of 5–6 days of culture versus 110–130 μm; with a length of 9 days of culture, respectively).
Non-attachment-follicles secreted significantly lower steroid levels than those measured for an equivalent developmental stage in AT-follicles. Follicle steroid production during growth is driven by theca-derived progesterone and androgens [44]. In the new present NA system, theca cells were localized in an internal follicle compartment, a different localization than under AT conditions (Fig. 3), which may influence follicular steroid production. In other non-attachment systems, low levels of progesterone and testosterone have also been reported, despite estradiol levels being normal under FSH stimulus [22, 28]. In contrast, in absence of FSH, estradiol production does not increase [28]. Overall, estradiol production seems enough to support follicle growth and differentiation, and normal cumulus expansion under NA conditions. The constantly low progesterone production throughout growth evidenced that premature luteinization did not occur during culture.
Gene expression in oocytes and their corresponding cumulus cells
To our knowledge, this is the first report showing gene expression in cultured oocytes and their corresponding cumulus cells obtained from large antral follicles grown under non-attachment conditions.
In oocytes In general, transcript levels of oocyte-specific Gdf9, Mater and Npm2 were higher in in-vivo compared to in-vitro. The condition ‘NA No FSH D6’, though, with consistently higher transcript values (ns) than other in vitro groups, did not differ from in-vivo. GDF9 is a key factor that regulates granulosa cells function during oocyte growth and maturation [45–48]; whereas Npm2 and Mater are two maternal-effect transcripts required for normal early embryo development [49, 50], and Npm2 also being crucial for the oocyte organization of nuclear and nucleolar domains and chromatin compaction [50, 51]. Having levels of these key transcripts more similar to in-vivo might indicate a more adequate oocyte expression pattern under the ‘NA No FHS D6’ condition, that also appears to be associated with an improvement of oocyte fertilization and developmental potential.
In cumulus cells As markers of cumulus cell function, levels of Amh and Lhcgr, two transcripts regulated by oocyte-secreted factors (induced and repressed in cumulus cells respectively), were quantified [46, 52–54]. Due to its high expression levels, Amh is considered a marker of cumulus cells phenotype [52–55]. However, under both culture systems studied herein, Amh levels were barely detected. Although this was not surprising under AT conditions [13], the promotion of a more enclosed follicular structure under NA conditions could not maintain Amh levels as high as in in-vivo; which may indicate that, under current in vitro conditions, Amh might be regulated by factors still unknown.High Lhcgr mRNA expression as quantified in total antral follicles, has been shown to positively correlate with oocyte fertilization and blastocyst rate [24, 25]. However, up-regulation of Lhcgr in differentiated cumulus cells is associated with abnormal cumulus cell phenotype and poor oocyte quality [10, 11, 13]. Based on our results on Lhcgr expression, NA conditions promote a more in vivo-like cumulus cell differentiation. In addition, the lowest exposure to FSH in the ‘NA No FSH D3’ and ‘NA No FSH D6’ conditions resulted in the lowest Lhcgr transcript levels, indicating a time/dose-dependent relationship.
Oocyte developmental competence
Given the previous results, effects on oocyte developmental capacity were evaluated and compared between the control routine group ‘AT FSH 10 IU’ and the most relevant non-attachment culture conditions: ‘NA FSH 10 IU’ and ‘NA No FSH D6’.
Fertilization and development to the blastocyst stage rates in oocytes from spherical cultured follicles range from 30 to 80% and 9–40%, respectively [19–22]. Nevertheless, initial diameters of those follicles were ≥150 μm, whereas those between 110 and 150 μm did not develop to blastocysts or developed at a lower rate (~10%, over total MII oocytes) [22, 24]. Oocytes under all the conditions analyzed in this study were able to fertilize and develop to the blastocyst stage; however, they differed on such capacities. Two-cell and blastocyst rate over the total oocyte number were highest in the ‘NA No FSH D6’ group (69.9% and 48.5%, respectively), meaning that combination of both non-attachment conditions and decreasing the FSH levels during antral stages leads to an improvement of oocyte developmental competence. This emphasizes the importance of previous findings in which FSH exposure during early in-vitro preantral stages maximizes oocyte developmental capacity [17], and strongly suggests that timely FSH exposure is a critical factor in in-vitro systems.
In summary, our results suggest that adjustments of oocyte-cumulus cell differentiation rather than structural follicle intactness are important for further oocyte competence. Further progress on follicle culture conditions, could address the altered compartmentalization of theca cells, as well as the low Amh levels, which might depend on factors lacking in the current set-up.
In conclusion, the current study demonstrates that mouse early secondary follicles (110–130 μm) can be grown under non-attachment conditions and produce mature MII oocytes. Moreover, this new non-attachment condition in combination with decreased FSH concentrations during the antral growth stages positively affected cumulus cells Lhcgr expression, and led to an improvement in oocyte developmental potential.
Acknowledgements
The authors wish to acknowledge Mrs. Katy Billooye for her valuable technical assistance with staining for alkaline phosphatase in theca cells and for performing the radioimmunoassays on the conditioned media. The authors are very grateful to Ms. Sandra De Schaepdryver for her editorial support.
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Financial support This work was supported by The Belgian Foundation Against Cancer (project no. 221.2008).
Footnotes
Flor Sánchez and Sergio Romero contributed equally to this work.
Capsule
Non-attached follicle culture and decreased FSH levels modulate follicle steroidogenesis and positively influence gene expression in cumulus cells and oocyte developmental capacity.
References
- 1.Eppig JJ, O’Brien MJ. Development in vitro of mouse oocytes from primordial follicles. Biol Reprod. 1996;54:197–207. doi: 10.1095/biolreprod54.1.197. [DOI] [PubMed] [Google Scholar]
- 2.Cortvrindt R, Smitz J, Steirteghem AC. In-vitro maturation, fertilization and embryo development of immature oocytes from early preantral follicles from prepuberal mice in a simplified culture system. Hum Reprod. 1996;11:2656–2666. doi: 10.1093/oxfordjournals.humrep.a019188. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien MJ, Pendola JK, Eppig JJ. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol Reprod. 2003;68:1682–1686. doi: 10.1095/biolreprod.102.013029. [DOI] [PubMed] [Google Scholar]
- 4.Smitz J, Dolmans MM, Donnez J, Fortune JE, Hovatta O, Jewgenow K, et al. Current achievements and future research directions in ovarian tissue culture, in vitro follicle development and transplantation: implications for fertility preservation. Hum Reprod Update. 2010;16:395–414. doi: 10.1093/humupd/dmp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trounson A, Anderiesz C, Jones G. Maturation of human oocytes in vitro and their developmental competence. Reproduction. 2001;121:51–75. doi: 10.1530/rep.0.1210051. [DOI] [PubMed] [Google Scholar]
- 6.Smitz JE, Cortvrindt RG. The earliest stages of folliculogenesis in vitro. Reproduction. 2002;123:185–202. doi: 10.1530/rep.0.1230185. [DOI] [PubMed] [Google Scholar]
- 7.Kim DH, Ko DS, Lee HC, Lee HJ, Park WI, Kim SS, et al. Comparison of maturation, fertilization, development, and gene expression of mouse oocytes grown in vitro and in vivo. J Assist Reprod Genet. 2004;21:233–240. doi: 10.1023/B:JARG.0000042008.83699.cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Combelles CMH, Fissore RA, Albertini DF, Racowsky C. In vitro maturation of human oocytes and cumulus cells using a co-culture three-dimensional collagen gel system. Hum Reprod. 2005;20:1349–1358. doi: 10.1093/humrep/deh750. [DOI] [PubMed] [Google Scholar]
- 9.Eppig JJ, O’Brien MJ, Wigglesworth K, Nicholson A, Zhang W, King BA. Effect of in vitro maturation oocytes on the health of adult offspring. Hum Reprod. 2009;24:922–928. doi: 10.1093/humrep/den466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eppig JJ, O’Brien MJ, Pendola FL, Watanabe S. Factors affecting the developmental competence of mouse oocytes grown in vitro: follicle-stimulating hormone and insulin. Biol Reprod. 1998;59:1445–1453. doi: 10.1095/biolreprod59.6.1445. [DOI] [PubMed] [Google Scholar]
- 11.Latham KE, Bautista FDM, Hirao Y, O’Brien MJ, Eppig JJ. Comparison of protein synthesis patterns in mouse cumulus cells and mural granulosa cells: effects of follicle-stimulating hormone and insulin on granulosa cell differentiation in vitro. Biol Reprod. 1999;61:482–492. doi: 10.1095/biolreprod61.2.482. [DOI] [PubMed] [Google Scholar]
- 12.Eppig JJ, Hosoe M, O’Brien MJ, Pendola FM, Requena A, Watanabe S. Conditions that affect acquisition of developmental competence by mouse oocytes in vitro: FSH, insulin, glucose and ascorbic acid. Mol Cell Endocrinol. 2000;163:109–116. doi: 10.1016/S0303-7207(99)00247-6. [DOI] [PubMed] [Google Scholar]
- 13.Sánchez F, Adriaenssens T, Romero S, Smitz J. Different follicle-stimulating hormone exposure regimens during antral follicle growth alter gene expression in the cumulus-oocyte complex in mice. Biol Reprod. 2010;83:514–524. doi: 10.1095/biolreprod.109.083311. [DOI] [PubMed] [Google Scholar]
- 14.Sánchez F, Romero S, Smitz J. Oocyte and cumulus cell transcripts from cultured mouse follicles are induced to deviate from normal in vivo condition by combinations of insulin, follicle-stimulating hormone, and human chorionic gonadotropin. Biol Reprod. 2011;85:565–574. doi: 10.1095/biolreprod.111.091744. [DOI] [PubMed] [Google Scholar]
- 15.Nayudu PL, Osborn SM. Factors influencing the rate of preantral and antral growth of mouse ovarian follicles in vitro. J Reprod Fertil. 1992;95:349–362. doi: 10.1530/jrf.0.0950349. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell LM, Kennedy CR, Hartshorne GM. Effects of varying gonadotropin dose and timing on antrum formation and ovulation efficiency of mouse follicles in vitro. Hum Reprod. 2002;17:1181–1188. doi: 10.1093/humrep/17.5.1181. [DOI] [PubMed] [Google Scholar]
- 17.Adriaens I, Cortvrindt R, Smitz J. Differential FSH exposure in preantral follicle culture has marked effects on folliculogenesis and oocyte developmental competence. Hum Reprod. 2004;19:398–408. doi: 10.1093/humrep/deh074. [DOI] [PubMed] [Google Scholar]
- 18.Nayudu PL, Fehrenbach A, Kiesel P, Vitt UA, Pancharatna K, Osborn S. Progress toward understanding follicle development in vitro: appearances are not deceiving. Arch Med Res. 2001;32:587–594. doi: 10.1016/S0188-4409(01)00339-3. [DOI] [PubMed] [Google Scholar]
- 19.Spears N, Boland NI, Murray AA, Gosden RG. Mouse oocytes derived from in vitro grown primary ovarian follicles are fertile. Hum Reprod. 1994;9:527–532. doi: 10.1093/oxfordjournals.humrep.a138539. [DOI] [PubMed] [Google Scholar]
- 20.Rose UM, Hanssen RG, Kloosterboer HJ. Development and characterization of an in vitro ovulation model using mouse ovarian follicles. Biol Reprod. 1999;61:503–511. doi: 10.1095/biolreprod61.2.503. [DOI] [PubMed] [Google Scholar]
- 21.Vitt UA, Nayudu PL, Rose UM, Kloosterboer HJ. Embryonic development after follicle culture is influenced by follicle-stimulating hormone isoelectric point range. Biol Reprod. 2001;65:1542–1547. doi: 10.1095/biolreprod65.5.1542. [DOI] [PubMed] [Google Scholar]
- 22.Bishonga C, Takahashi Y, Katagiri S, Nagano M, Ishikawa A. In vitro growth of mouse ovarian preantral follicles and the capacity of their oocytes to develop to the blastocyst stage. J Vet Med Sci. 2001;63:619–624. doi: 10.1292/jvms.63.619. [DOI] [PubMed] [Google Scholar]
- 23.Pangas SA, Saudye H, Shea L, Woodruff T. Novel approach for the three-dimensional culture of granulosa cell–oocyte complexes. Tissue Eng. 2003;9:1013–1021. doi: 10.1089/107632703322495655. [DOI] [PubMed] [Google Scholar]
- 24.Xu M, West E, Shea LD, Woodruff TK. Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biol Reprod. 2006;75:916–923. doi: 10.1095/biolreprod.106.054833. [DOI] [PubMed] [Google Scholar]
- 25.West-Farrell E, Xu M, Gomberg MA, Chow YH, Woodruff TK, Shea LD. The mouse follicle microenvironment regulates antrum formation and steroid production: alterations in gene expression profiles. Biol Reprod. 2009;80:432–439. doi: 10.1095/biolreprod.108.071142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cortvrindt R, Smitz J. Follicle culture in reproductive toxicology: a tool for in-vitro testing of ovarian function? Hum Reprod Update. 2002;8:243–254. doi: 10.1093/humupd/8.3.243. [DOI] [PubMed] [Google Scholar]
- 27.Romero S, Sánchez F, Adriaenssens T, Smitz J. Mouse cumulus-oocyte complexes from in vitro-cultured preantral follicles suggest an anti-luteinizing role for the EGF cascade in the cumulus cells. Biol Reprod. 2011;84:1164–1170. doi: 10.1095/biolreprod.110.087551. [DOI] [PubMed] [Google Scholar]
- 28.Boland NI, Humpherson PG, Leese HJ, Gosden RG. Pattern of lactate production and steroidogenesis during growth and maturation of mouse ovarian follicles in vitro. Biol Reprod. 1993;48:798–806. doi: 10.1095/biolreprod48.4.798. [DOI] [PubMed] [Google Scholar]
- 29.Segers I, Adriaenssens T, Ozturk E, Smitz J. Acquisition and loss of oocyte meiotic and developmental competence during in vitro antral follicle growth in mouse. Fertil Steril. 2010;93:2695–2700. doi: 10.1016/j.fertnstert.2009.11.035. [DOI] [PubMed] [Google Scholar]
- 30.Romero S, Smitz J. Improvement of in vitro culture of mouse cumulus–oocyte complexes using PDE3-inhibitor followed by meiosis induction with epiregulin. Fertil Steril. 2010;93:936–944. doi: 10.1016/j.fertnstert.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 31.Convery M, Brawer JR. Thecal and interstitial cells in polycystic ovaries (PCO) in the rat. Anat Rec. 1991;231:324–332. doi: 10.1002/ar.1092310305. [DOI] [PubMed] [Google Scholar]
- 32.Lenie S, Smitz J. Estrogen receptor subtypes localization shifts in cultured mouse ovarian follicles. Histochem Cell Biol. 2008;129:827–840. doi: 10.1007/s00418-008-0408-9. [DOI] [PubMed] [Google Scholar]
- 33.Wickramasinghe D, Ebert KM, Albertini DF. Meiotic competence acquisition is associated with the appearance of M-phase characteristics in growing mouse oocytes. Dev Biol. 1991;143:162–172. doi: 10.1016/0012-1606(91)90063-9. [DOI] [PubMed] [Google Scholar]
- 34.Zuccotti M, Piccinelli A, Giorgi Rossi P, Garagna S, Redi CA. Chromatin organization during mouse oocyte growth. Mol Reprod Dev. 1995;41:479–485. doi: 10.1002/mrd.1080410410. [DOI] [PubMed] [Google Scholar]
- 35.Zuccotti M, Giorgi Rossi P, Martinez A, Garagna S, Forabosco A, Redi CA. Meiotic and developmental competence of mouse antral oocytes. Biol Reprod. 1998;58:700–704. doi: 10.1095/biolreprod58.3.700. [DOI] [PubMed] [Google Scholar]
- 36.Bouniol-Baly C, Hamraoui L, Guibert J, Beaujean N, Szollosi MS, Debey P. Differential transcriptional activity associated with chromatin configuration in fully grown mouse germinal vesicle oocytes. Biol Reprod. 1999;60:580–587. doi: 10.1095/biolreprod60.3.580. [DOI] [PubMed] [Google Scholar]
- 37.Fuente R. Chromatin modifications in the germinal vesicle (GV) of mammalian oocytes. Dev Biol. 2006;296:1–12. doi: 10.1016/j.ydbio.2006.04.445. [DOI] [PubMed] [Google Scholar]
- 38.Fehrenbach A, Nüsse N, Nayudu PL. Patterns of growth, oestradiol and progesterone released by in vitro cultured mouse ovarian follicles indicate consecutive selective events during follicle development. J Reprod Fertil. 1998;113:287–297. doi: 10.1530/jrf.0.1130287. [DOI] [PubMed] [Google Scholar]
- 39.Vitt UA, Kloosterboer HJ, Rose UM, Mulders JW, Kiesel PS, Bete S, et al. Isoforms of human recombinant follicle-stimulating hormone: comparison of effects on murine follicle development in vitro. Biol Reprod. 1998;59:854–861. doi: 10.1095/biolreprod59.4.854. [DOI] [PubMed] [Google Scholar]
- 40.Rowghani NM, Heise MK, McKeel D, McGee EA, Koepsel RR, Russell AJ. Maintenance of morphology and growth of ovarian follicles in suspension culture. Tissue Eng. 2004;10:545–552. doi: 10.1089/107632704323061906. [DOI] [PubMed] [Google Scholar]
- 41.Kreeger PK, Deck JW, Woodruff TK, Shea LD. The in vitro regulation of ovarian follicle development using alginate-extracellular matrix gels. Biomaterials. 2006;27:714–723. doi: 10.1016/j.biomaterials.2005.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu J, Nayudu PL, Kiesel PS, Michelmann HW. Luteinizing hormone has a stage-limited effect on preantral follicle development in vitro. Biol Reprod. 2000;63:320–327. doi: 10.1095/biolreprod63.1.320. [DOI] [PubMed] [Google Scholar]
- 43.Gomes JE, Correia SC, Gouveia-Oliveira A, Cidadão AJ, Plancha CE. Three-dimensional environments preserve extracellular matrix compartments of ovarian follicles and increase FSH-dependent growth. Mol Reprod Dev. 1999;54:163–172. doi: 10.1002/(SICI)1098-2795(199910)54:2<163::AID-MRD8>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 44.Hillier SG, Whitelaw PF, Smyth CD. Follicular oestrogen synthesis: the ‘two-cell, two-gonadotrophin’ model revisited. Mol Cell Endocrinol. 1994;100:51–54. doi: 10.1016/0303-7207(94)90278-X. [DOI] [PubMed] [Google Scholar]
- 45.Carabatsos MJ, Elvin J, Matzuk MM, Albertini DF. Characterization of oocyte and follicle development in growth differentiation factor-9-deficient mice. Dev Biol. 1998;204:373–384. doi: 10.1006/dbio.1998.9087. [DOI] [PubMed] [Google Scholar]
- 46.Elvin JA, Clark AT, Wang P, Wolfman NM, Matzuk MM. Paracrine actions of growth differentiation factor-9 in the mammalian ovary. Mol Endocrinol. 1999;13:1035–1048. doi: 10.1210/me.13.6.1035. [DOI] [PubMed] [Google Scholar]
- 47.Vitt UA, Hayashi M, Klein C, Hsueh AJW. Growth differentiation factor-9 stimulates proliferation but suppresses the follicle-stimulating hormone-induced differentiation of cultured granulosa cells from small antral and preovulatory rat follicles. Biol Reprod. 2000;62:370–377. doi: 10.1095/biolreprod62.2.370. [DOI] [PubMed] [Google Scholar]
- 48.Gilchrist RB, Ritter LJ, Armstrong DT. Oocyte-somatic cell interactions during follicle development in mammals. Anim Reprod Sci. 2004;82–83:431–446. doi: 10.1016/j.anireprosci.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 49.Tong ZB, Gold L, Pfeifer KE, Dorward H, Lee E, Bondy CA, et al. Mater, a maternal effect gene required for early embryonic development in mice. Nature Genet. 2000;26:267–740. doi: 10.1038/81547. [DOI] [PubMed] [Google Scholar]
- 50.Burns KH, Viveiros MM, Ren Y, Wang P, DeMayo FJ, Frail DE, et al. Roles of NPM2 in chromatin and nucleolar organization in oocytes and embryos. Science. 2003;300:633–636. doi: 10.1126/science.1081813. [DOI] [PubMed] [Google Scholar]
- 51.Inoue A, Aoki F. Role of the nucleoplasmin 2 C-terminal domain in the formation of nucleolus-like bodies in mouse oocytes. FASEB J. 2010;24:485–494. doi: 10.1096/fj.09-143370. [DOI] [PubMed] [Google Scholar]
- 52.Baarends WM, Uilenbroek JTJ, Kramer P, Hoogerbrugge JW, Leeuwen ECM, Themmen APN, et al. Anti-Müllerian hormone and anti-Müllerian hormone type II receptor messenger ribonucleic acid expression in rat ovaries during postnatal development, the estrous cycle, and gonadotropin-induced follicle growth. Endocrinol. 1995;136:4951–4962. doi: 10.1210/en.136.11.4951. [DOI] [PubMed] [Google Scholar]
- 53.Salmon NA, Handyside AH, Joyce IM. Oocyte regulation of anti-Müllerian hormone expression in granulosa cells during ovarian follicle development in mice. Dev Biol. 2004;266:201–208. doi: 10.1016/j.ydbio.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 54.Diaz FJ, Wigglesworth K, Eppig JJ. Oocytes determine cumulus cell lineage in mouse ovarian follicles. J Cell Sci. 2007;120:1330–1340. doi: 10.1242/jcs.000968. [DOI] [PubMed] [Google Scholar]
- 55.Grøndahl ML, Nielsen ME, Canto MB, Fadini R, Rasmussen IA, Westergaard LG, et al. Anti-Müllerian hormone remains highly expressed in human cumulus cells during the final stages of folliculogenesis. Reprod Biomed Online. 2011;22:389–398. doi: 10.1016/j.rbmo.2010.12.005. [DOI] [PubMed] [Google Scholar]