Abstract
Although inflammatory responses increase stroke severity, the role of immune cells specific for central nervous system (CNS) antigens remains controversial. Disruption of the blood-brain barrier (BBB) during stroke allows CNS antigens to leak into the peripheral circulation and enhances access of circulating leukocytes to the brain, including those specific for CNS antigens such as myelin oligodendrocyte glycoprotein (MOG) that can induce experimental autoimmune encephalomyelitis (EAE). We here demonstrate for the first time that myelin reactive splenocytes specific for MOG transferred into severe combined immunodeficiency (SCID) mice can migrate into the infarct hemisphere of recipients subjected to 60 minutes middle cerebral artery occlusion (MCAO) and 96 hours reperfusion; moreover these cells exacerbate infarct volume and worsen neurological deficits compared to animals transferred with naïve splenocytes. These findings indicate that autoimmunity in the CNS can exert detrimental injury on brain cells and worsen the damage from ischemic stroke.
Keywords: experimental stroke, myelin reactive splenocytes, inflammatory responses, neurologic deficit
INTRODUCTION
Central nervous system (CNS) immune privilege is construed as CNS isolation from the immune system by the blood brain barrier (BBB) and the lack of draining lymph nodes. Stroke is a devastating acute CNS condition marked by ischemic brain cell death and breakdown of BBB that likely promotes leakage of brain auto-antigens (e.g. myelin basic protein (MBP), proteolipid protein (PLP) and myelin oligodendrocyte glycoprotein (MOG)) to the periphery, activation of the immune system, migration of activated immunocytes into the CNS and inflammation leading to further CNS damage. The functional consequences of this autoimmune response are not well characterized, but could potentially contribute to neurological injury or protection after stroke.
There is strong evidence showing that acute inflammatory responses may contribute to ischemic injury in the brain of stroke patients and experimental cerebral ischemia (Allan and Rothwell 2001; Allan and Rothwell 2003; Hurn et al. 2007). It is well established that T helper type 1 (TH1) autoimmune responses to CNS antigens involving interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α), may result in an inflammatory demyelinating disease of the CNS (eg. multiple sclerosis (MS) in humans and experimental autoimmune encephalomyelitis (EAE) in mice (Fletcher et al. 2010)), and it is possible that Th1-responses to myelin antigens might also be induced after stroke that could exacerbate CNS damage. Indeed, a growing body of evidence indicates that active immunologic tolerance to CNS antigens may decrease delayed-type hypersensitivity reactions and infarct size (Becker et al. 2003; Becker et al. 1997). These effects may be important for CNS recovery after injury (Moalem et al. 1999), possibly through increased secretion of transforming growth factor β1 (TGF-β1) (Khoury et al. 1992; Miller et al. 1992) or interleukin-10 (IL-10)) (Frenkel et al. 2005) that may be neuroprotective either directly or indirectly by modulating the immune response.
Here we have evaluated the functional outcomes of an autoimmune response in experimental cerebral ischemia in mice. We have developed an adoptive transfer model for studying the role of MOG reactive cells in experimental stroke in mice. We demonstrate morphologically that MOG-reactive splenocytes can migrate into the ischemic hemisphere, increase infarct volume and worsen neurological deficits after ischemic stroke. Our study sheds new light on the long-held debate regarding the contribution of immune system to CNS injury, and has potentially far-reaching therapeutic implications.
MATERIALS AND METHODS
Animals
Green fluorescent protein (GFP+) mice on the C57BL/6 background were bred at the VA Animal Resource Facility. 8–12 week old male mice were used for the study. Age-matched severe combined immunodeficiency (SCID) mice on the C57BL/6 background (the Jackson Laboratory, USA) were used as recipient mice for MCAO induction. Animals were bred and cared for according to institutional guidelines in the Animal Resource Facility at the Veterans Affairs Medical Center, Portland, OR. All experiments were performed under approved institutional protocols from the VA and Oregon Health & Science University and conformed to the National Institutes of Health guidelines for the care and use of animals in research.
Immunization of Donors
GFP+ mice were immunized subcutaneously in four sites over the flanks with 200 μg MOG35–55 peptide (PolyPeptide Laboratories, San Diego, CA) in 400 μg complete Freund’s adjuvant (CFA, H37Ra, Difco).
In vitro Splenocyte Culture
Eight days after immunization with MOG35–55, spleens were removed and a single-cell suspension was prepared by pressing whole spleens through a sterile cell strainer (BD Falcon). Splenocyte suspensions were depleted of erythrocytes using sterile RBC lysis buffer (eBioscience). Cell number and viability were assessed by trypan blue exclusion and a single cell suspension of splenocytes was cultured with MOG35–55 peptide (25 μg/ml) at 4 × 106 cells/ml in stimulation medium (RPMI 1640 medium supplemented with nonessential amino acids, sodium pyruvate, 2-βME, and 10% fetal bovine serum) as published (Polanczyk et al. 2004).
Adoptive Transfer
After 48 hours re-stimulation with MOG35–55 in vitro, the cells were harvested and washed with RPMI 1640 3 times. SCID mice were injected i.p. one day prior to MCAO or sham treatment with a total of 1×108 cells for all cell transfer experiments. SCID recipients receiving GFP+ naive splenocytes served as a control group compared to GFP+ MOG-stimulated splenocytes.
MCAO Model
The mice were subjected to MCAO as previously published (Offner et al. 2006b) by reversible right MCA occlusion (60min) under isoflurane anesthesia, followed by 96h reperfusion. Body and head temperatures were controlled at 37 ± 0.5°C with a warming blanket and heat lamps during surgery and ischemia. A laser-Doppler probe (Moor Instruments, Oxford, UK) was affixed to the skull to monitor cortical perfusion and verify vascular occlusion and reperfusion. A silicone-coated 6-0 nylon monofilament was inserted into the right internal carotid artery via the external carotid artery until a drop in laser-Doppler signal was observed. Occlusion was confirmed by a decrease in laser-Doppler signal to less than 25% of baseline. After securing the filament in place, the surgical site was closed, and the animal was awakened. At 60 min of occlusion, the mouse was re-anesthetized, the laser-Doppler probe re-positioned over the same site on the skull, and the occluding filament withdrawn to allow for reperfusion. Mice were then allowed to recover for 96h after occlusion.
Quantification of Infarct Volume
As previously published (Dziennis et al. 2011), infarct size was measured at 96h after MCAO from a 1-mm thick coronal brain section using 2,3,5-triphenyltetrazolium chloride (TTC, Sigma Aldrich) staining and digital image analysis expressed as a percentage of the contralateral cortex and striatum structures. Each mouse was transcardially perfused with 30ml saline to exclude blood cells before tissues were removed. The 1-mm brain sections were incubated in 1.2% TTC for 15 min at 37°C, and then fixed in 10% formalin for 24h. Slices were photographed, and analyzed for infarct size with SigmaScan Pro 5.0 software (Jandel, San Real, Calif). The remaining ipsilateral and contralateral hemispheres were used for immunohistochemistry staining and for isolation of brain mononuclear cells, antibody staining and flow cytometry.
Neurological Deficit Score
Neurological function was evaluated using a 0–5 point-scale neurological score as previously published (Ren et al. 2011b): 0 = no neurological dysfunction; 1 = failure to extend left forelimb fully when lifted by tail: 2 = circling to the contralateral side; 3 = falling to the left; 4 = no spontaneous walk or in a comatose state; 5 = death. The scores were assessed in a blinded fashion.
Cell Isolation
Peripheral blood mononuclear cells were prepared by using red cell lysis buffer (eBioscience, San Diego, CA) following manufacturer’s Instructions. Single-cell suspensions from lymph nodes (superficial cervical, mandibular, axillary, lateral axillary, superficial inguinal and mesenteric) and spleens were prepared by mechanical disruption. For preparation of inflammatory cells in the brain, the perfused forebrain was dissected from the cerebellum and suspended in RPMI-1640 medium. The suspension was digested with type IV collagenase (1 mg/ml, Sigma-Aldrich, St. Louis, MO) and DNase I (50 μg/ml, Roche Diagnostics, Indianapolis, IN) at 37°C for 45 min in a shaker at 180 times per min. Inflammatory cells were isolated by 37–70% Percoll (GE Healthcare, Piscataway, NJ) density gradient centrifugation according to a method described elsewhere (Campanella et al. 2002). Inflammatory cells were removed from the interface for further analysis. The cells were then washed twice with RPMI 1640, counted, and resuspended in stimulation medium containing 10% FBS for phenotyping.
Analysis of Cell Populations by FACS
Anti-mouse antibodies used for this study are included in Table 1. Single-cell suspensions were washed with staining medium (PBS containing 0.1% NaN3 and 2% FCS). After incubation with appropriate mAb and washing, cells were acquired with LSRII (BD Biosciences). For each experiment, cells were stained with appropriate isotype control antibodies to establish background staining and to set quadrants before calculating the percentage of positive cells. Data were analyzed using Flowjo software (TreeStar, Ashland, OR).
Table 1.
Antibodies used in the study.
| Antibodies | Clones | Conjugates | Source |
|---|---|---|---|
| CD11b | M1/70 | PE-Cy7 | eBioscience |
| CD19 | 1D3 | APC | BD PharMingen, San Diego, CA |
| CD45 | 30-F11 | Pacific Orange™ | Invitrogen, Carlsbad, CA |
| Gr1 | IA8 | Pacific Blue™ | BD Horizon |
| CD3 | 17A2 | APC-eFluor 780 | eBioscience |
| CD4 | L3T4 | APC | eBioscience |
| CD8 | 53.6.7 | PE | eBioscience |
Calculations of Cell Subset Numbers from Cell Counts
The absolute number of GFP+ cell subsets within a sample is determined using the following equation:
For the example shown in Figure 5A, the total number of GFP+ cells harvested from the recipient mouse in the right hemisphere is:
For the example shown in Figure 5B, the total number of GFP+ monocytes harvested from the recipient mouse in the right hemisphere is:
Immunohistochemistry
Brains, lymph nodes and spleens were collected from perfused recipient SCID mice after adoptive transfer of GFP+ immunocytes after 60min MCAO and 96 hours reperfusion. Tissues were fixed with 4% buffered formalin, paraffin embedded, and sectioned. Anti-GFP staining: Sections were incubated with anti-GFP (Cell signaling), followed by incubation with secondary biotinylated antibody (goat anti-rabbit, Cell signaling) and staining with VECTASTAIN ABC Peroxidase Kit (Vector) and 3′, 3-diaminobenzidine (Sigma-Aldrich). Nuclear staining was carried out with hematoxylin (Sigma-Aldrich). Slides were analyzed by light microscopy (Olympus BX40, Japan).
Statistical Analysis
Data were reported as means ± SEM. Statistical analyses were performed using the appropriate test indicated in the Figure Legends as follows: Two tailed Student’s t test for infarct volume and cell counts; 2-Way ANOVA with Student-Newman-Keuls post-hoc analysis for neuroscores. P<0.05 were considered statistically significant.
RESULTS
In order to evaluate the effect of myelin reactive cells on outcome following MCAO, we developed an adoptive transfer assay to track immune cell subsets after transfer of cells from MOG-immunized GFP+ mice. The general steps for obtaining and transferring cells, time course for animal surgery and harvesting samples are depicted in Supplementary Fig. 1. The gating strategy for detecting passively transferred GFP+ cell subsets is described in Supplementary Fig. 2. These techniques are broadly used in many different laboratories and this report presents for the first time a merging of protocols with numerous variations.
Identification of GFP+ immune cell subsets and histological outcomes in brain and periphery in SCID recipients after transfer of GFP+ MOG-immunized splenocytes
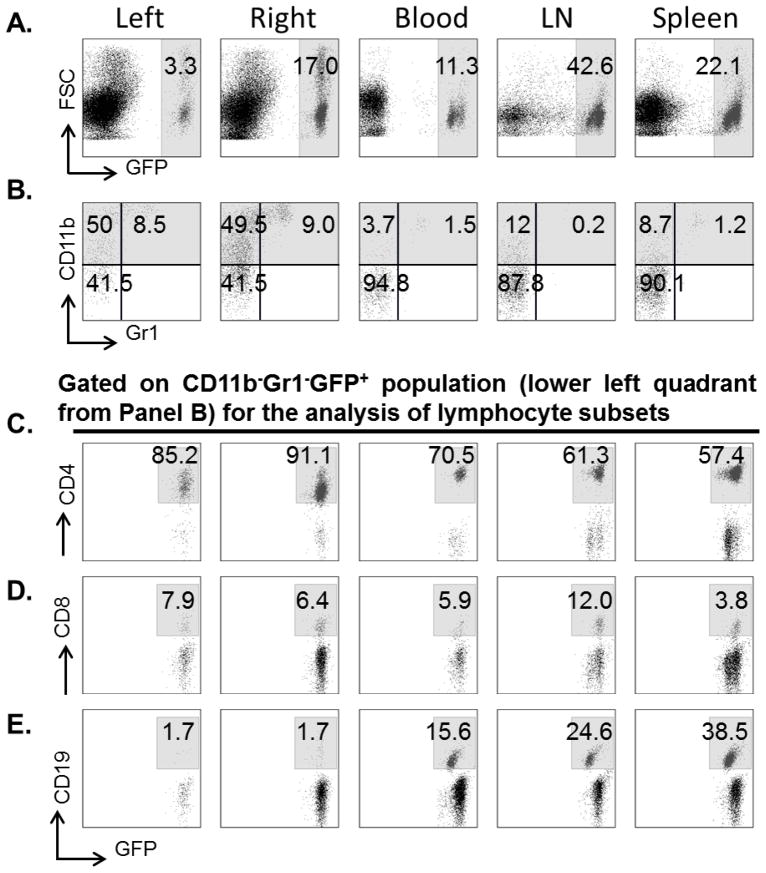
One day following adoptive transfer of 1×108 MOG-stimulated splenocytes, recipient mice underwent 60 min MCAO or Sham procedures and 4 days reperfusion. The results demonstrated increased total GFP+ cells in the ischemic right hemisphere vs. the non-ischemic left hemisphere (Fig. 1A) and more labeled cells in the LN vs. Spleen and Blood (Fig. 1A), but essentially no differences in the distribution of monocytes, granulocytes and lymphocytes between ischemic hemispheres or among peripheral immune organs (Fig. 1B). However, substantial differences were noted in the distribution of lymphocyte subtypes (Fig. 1C–E), with a predominance of CD4+ vs. CD8+ T-cells in all evaluated tissues and an increase in CD19+ B-cells in Spleen vs. LN and Blood, but their absence in brain.
Figure 1.
Representative flow cytometric plots of immunocyte subsets in ischemic (right) and non-ischemic (left) brain, peripheral blood, lymph nodes (LN) and spleens after adoptive transfer of MOG stimulated GFP+ splenocytes following 60min MCAO and 96h reperfusion. (A) GFP+ population (right panel in each image), (B) CD11b+Gr1− monocytes (upper left quadrant) and CD11b+Gr1+ granulocytes (upper right quadrant), (C) CD4+GFP+ T cells, (D) CD8+GFP+ T cells, and (E) CD19+GFP+ B cells. Results represent one of 5 independent experiments producing similar results.
Comparison of infiltrating GFP+ cells in SCID recipients of Naive vs. MOG-immunized splenocytes
Further analyses of the FACS data from SCID recipients of 1×108 MOG-immunized GFP+ splenocytes (n=5) or 1×108 naive GFP+ splenocytes (n=5) provided quantitative information (%) of gated cells (from Supplementary Fig. 2) and allowed statistical comparisons of the total number of GFP+ cell types in each tissue after 60 min MCAO and 4 days reperfusion (Fig. 2). At 4 days after stroke, the total number of GFP+ cells in the ischemic hemisphere of animals receiving MOG-immunized splenocytes was ~15-fold greater than that in the ischemic hemisphere of animals receiving naive splenocytes (Fig. 2A), and similar differences were noted for GFP+ monocytes, granulocytes and CD4+ and CD8+ T-cells (Fig. 2B–E), but not B-cells which were found only in the periphery (Fig. 2F). Although total numbers were variable and significantly smaller than in the ischemic hemisphere, a similar pattern showing increases in total numbers of GFP+ splenocytes and cell subtypes from MOG-immunized vs. Naïve splenocyte recipients were observed for the non-ischemic hemisphere, blood (number/ml), LN and spleen (Fig. 2), with the exception of CD8+ T-cells and B-cells (Fig. 2E–F).
Figure 2.
Analysis of infiltrating GFP+ immunocyte subsets into left (L=nonischemic hemisphere) and right (R=ischemic hemisphere), blood (B), lymph nodes (LN) and spleen (SP) after adoptive transfer of naive (gray bar) or MOG stimulated (black bar) GFP+ splenocytes following 60min MCAO and 96h reperfusion. Determinations of GFP+ cells (A), GFP+ monocytes (B), GFP+ granulocytes (C), GFP+CD4+ T cells (D), GFP+CD8+ T cells (E), GFP+CD19+ B cells (F). Values represent mean numbers ± SEM of indicated cell subsets from five mice of each group. Statistical analysis was performed with two tailed Student’s t test respectively. Significant differences between sample means are indicated. *P<0.05; **P<0.01; ***P<0.001..
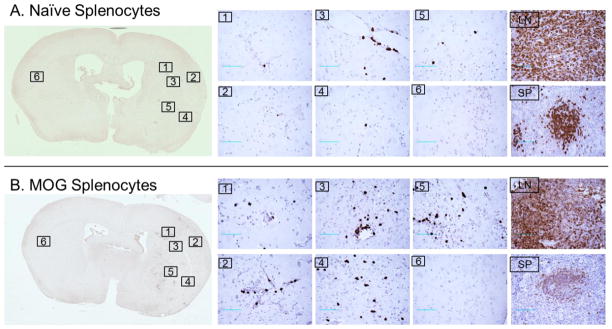
The GFP label allowed direct detection of transferred splenocytes in brain and peripheral tissues using anti-GFP immunohistochemical staining (Fig. 3). The results confirmed increased numbers of GFP+ cells in the ischemic cortex region and striatum region of animals receiving MOG-immunized splenocytes (Fig. 3B), but few in brain of animals receiving naïve splenocytes (Fig. 3A). Moreover, the GFP+ cells were found largely in the parenchyma, but not in the vessels, as would be expected after perfusion, and were easily detected in both spleen and lymph nodes of SCID recipients of transferred MOG-immunized and control GFP+ cells (Fig. 3).
Figure 3.
Detection of GFP+ cells in brain, lymph node and spleen by immunohistochemistry after adoptive transfer of naive (A) or MOG stimulated (B) GFP+ splenocytes following 60min MCAO and 96h reperfusion. Sections are shown from Brain (with higher 40X magnifications shown from six comparable locations), lymph nodes (LN) and spleen (SP) from recipient mice after transfer of GFP+ splenocytes (Scale bars: 200 μm). Results represent one of three independent experiments that included five animals per group.
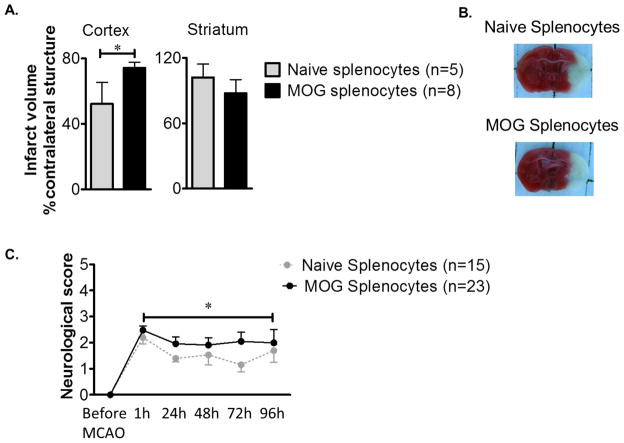
Transfer of MOG-immunized spleen cells exacerbates stroke severity in SCID recipient mice
SCID mice receiving MOG-immunized splenocytes (n=8) sustained significantly larger cortical infarcts (P<0.05) compared to animals receiving naive splenocytes (n=5) (Fig. 4A). However, there was not a significant effect on infarct size in the striatum (Fig. 4A). Representative histological staining of injured brain confirming increased infact size in recipients of cells from MOG-immunized donors is shown in Figure 4B. These data clearly implicate the role of MOG reactive splenocytes in exacerbating histological damage after MCAO. In a further cohort, there was significant recovery of neurological scores in animals receiving naive splenocytes (n=15) that did not occur in mice receiving MOG-immunized splenocytes (n=23) (Fig. 4C).
Figure 4.
Passively transferred MOG stimulated splenocytes exacerbate infarct size and worsen neurological outcomes after MCAO. (A) Infarct volume, corrected for the presence of edema, at 96 h of reperfusion after 60 min of MCAO given as bar graphs are visualized (mean ± SEM). Statistical analysis was performed with the Student’s t test. There was a significant difference in cortical infarct volumes between animals receiving MOG immunized splenocytes (n=8) and naive splenocytes (n=5). (B) Representative 2,3,5-triphenyltetrazolium chloride-stained cerebral sections of MCAO modeled to analyze infarct volume. (C) Neurological dysfunction scores (mean ± SEM) after reperfusion were significantly worse in animals receiving MOG splenocytes (n=23) (*p<0.05) vs. mice receiving naïve splenocytes (n=15). The statistics were performed with 2-Way ANOVA with Student-Newman-Keuls post-hoc analysis.
DISCUSSION
The CNS is a unique immune-privileged site in which local immune responses are restricted and immunological reactions are relatively limited (Streilein 1993; Streilein 1995; Streilein and Stein-Streilein 2000). However, ischemic stroke with the sudden occlusion of a cerebral blood vessel initiates microvascular injury and induces blood-brain barrier dysfunction, which allows infiltration into the brain of peripheral immune cells that promote further cerebral post-ischemic inflammation and neuronal degeneration (Lakhan et al. 2009). Moreover, inflammatory signals and antigenic products derived from brain (e.g. MOG, MBP & PLP) may leak across the damaged BBB and produce reciprocal systemic activation. Here, we demonstrate for the first time that myelin reactive cells, as compared with naïve splenocytes, selectively migrate into ischemic brain tissue of animals following MCAO, resulting in significantly increased infarct volume and exacerbated functional neurological deficits. Thus, regulation of the immune response to CNS components would seem to be a useful strategy for limiting CNS inflammation and damage after stroke.
Inflammatory immune responses directed against CNS antigens (eg. passive transfer of lymphocytes specific for MBP (Cross and Raine 1990), MOG (Stromnes and Goverman 2006), or PLP (Miller et al. 2010)) typically result in paralytic and demyelinating EAE. We now further demonstrate conclusively that MOG-reactive splenocytes, which secrete neurotoxic Th1 cytokines, IFN-γ and TNF-α (Downen et al. 1999; Murphy et al. 2010), can also exacerbate CNS injury due to stroke. The effects of the MOG-reactive splenocytes may be to directly induce death or damage to neurons or to indirectly alter cerebrovasculature endothelium to increase permeability of the BBB and enhance transendothelial transport resulting in the infiltration of inflammatory cells into the brain parenchyma (Engelhardt and Sorokin 2009). We chose MOG as the antigen since it is released into the peripheral circulation after stroke and thus could increase activation of MOG-reactive T cells (Dirnagl et al. 2007). Further studies are warranted to assess CNS antigen specificity, as the current study did not use a non-CNS antigen control, such as ovalbumin (OVA) immunized splenocytes or other CNS-specific antigens. Recombinant T cell receptor ligands (RTLs), a partial TCR agonist that inhibits myelin-reactive T-cell activation, limits CNS inflammation and reverses clinical signs of EAE (Offner et al. 2011; Offner et al. 2008) and has recently been demonstrated to reduce infarct volume, CNS inflammation and improve functional outcomes after experimental stroke (Akiyoshi et al. 2011; Dziennis et al. 2011; Subramanian et al. 2009). Moreover, adoptive transfer of MBP tolerized splenocytes reduces infarct size in experimental stroke (Becker et al. 2003). In this model, mucosal administration of MBP induces immunologic tolerance such that tolerized T-cells secrete Th2 cytokines (TGF-β1 and IL-10) that produce anti-inflammatory effects in the CNS and suppress Th1 immune responses (Fillatreau et al. 2002; Gee et al. 2008; Peron et al. 2010). Taken together, these studies lend broad support to the concept that Th1 immune responses are detrimental, whereas Th2 responses are beneficial for post-ischemic outcomes.
Chemokines and their G protein-coupled receptors are key regulators of leukocyte trafficking to sites of injury. Ischemia activates chemokine signals in brain tissue, including CCL2 (Kim et al. 1995), CCL5 (Offner et al. 2006a), CXCL2 (Yamasaki et al. 1995), CXCL8 (Montecucco et al. 2010) and CXCL10 (Wang et al. 1998) that promote expression of adhesion molecules by vascular endothelial cells. MOG-reactive splenocytes expressed striking differences in chemokine receptor levels that are essential for the infiltration, recruitment and localization of migrating leukocytes to the perivascular compartment (Horuk 2001; Prinz and Priller 2010). Consistent with the hypothesis that ischemic induction of chemokine signals enhances MOG-stimulated splenocyte trafficking into the injured brain, our data indicate that animals receiving MOG-reactive splenocytes had a higher percentage of infiltrating immune cells in the ischemic hemisphere (Fig. 1A & 2A). Similarly, we observed that mice receiving MOG-stimulated splenocytes sustained significantly larger cortical infarcts (P<0.05) (Fig. 4A), and worsened neurological deficits (Fig. 4C), relative to animals receiving naive splenocytes. Therefore, our data demonstrate for the first time that ischemia preferentially attracts CNS-antigen activated splenocytes to the ischemic brain, resulting in exacerbated injury.
In addition to the question of antigenic specificity, effects on post-ischemic injury by lymphocyte subsets need to be further explored. Infiltrating monocytes, granulocytes, CD4+ and CD8+ T-cells were greater in the ischemic hemisphere of animals receiving MOG-immunized splenocytes than in mice receiving naïve splenocytes (Fig. 2B–E). One of the major populations, granulocytes, was also detected in the ischemic hemisphere of animals receiving MOG-immunized splenocytes (Fig. 1B & 2C). After cerebral ischemia, granulocytes transmigrate into the tissues and release major proinflammatory molecules from granules and vesicles, including iNOS, NADPH oxidase, myeloperoxidase, MMP-8, MMP-9, elastase and cathepsins (Yilmaz and Granger 2010), The exact role of monocytes, which can produce the anti-inflammatory cytokine IL-10 or the proinflammatory cytokines, IL-1 and TNFα, is still not yet clear in stroke (Iadecola and Anrather 2011). However, monocytes are the most highly represented cell population detected in our study (Fig. 1B & 2B). Moreover, B-cells, which we have recently implicated as being highly cerebroprotective in stroke (Ren et al. 2011a), were essentially absent in brain (Fig. 1E & 2F). In the study by Kleinschnitz (Kleinschnitz et al. 2010), transfer of CD3+ T cells was also detrimental for cerebral ischemia. Given our current data, leukocytes including Treg cells and B-cells might exert neuroprotection in the periphery, but may act directly on neurons or glial cells or indirectly on cerebrovasculature endothelium in the CNS. The adoptive transfer of sorted cell subsets would give us more evidence of their effects on worsening or modulating stroke severity.
The findings presented here not only demonstrate that MOG-reactive cells migrate into brain and exacerbate stoke severity, but also further substantiate the idea that the type of immune response might affect stroke outcomes. These results open a new window to enhance our understanding of the effects of the immune system on stroke outcome.
Supplementary Material
Acknowledgments
We thank Dr. Heng Hu, Dr. Sushmita Sinha, Dr. Sheetal Bodhankar, Dr. Suzan Dziennis, Dr. Takeru Shimizu and Ms. Sandhya Subramanian for helpful discussions; Ms. Xiao Jing Nie for performing histological staining and Ms. Lisa Miller for help with experiments; and Ms. Eva Niehaus for assistance in preparing the manuscript. This work was supported by NIH Grants NR03521 (PDH), NS49210 (PDH) and the Collins Medical Trust (XR). This material is based upon work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development. The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors have no financial conflicts of interest.
References
- Akiyoshi K, Dziennis S, Palmateer J, Ren X, Vandenbark AA, Offner H, Herson PS, Hurn PD. Recombinant T Cell Receptor Ligands Improve Outcome After Experimental Cerebral Ischemia. Translational stroke research. 2011 doi: 10.1007/s12975-011-0085-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nature reviews Neuroscience. 2001;2(10):734–744. doi: 10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- Allan SM, Rothwell NJ. Inflammation in central nervous system injury. Philosophical transactions of the Royal Society of London Series B. Biological sciences. 2003;358(1438):1669–1677. doi: 10.1098/rstb.2003.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker K, Kindrick D, McCarron R, Hallenbeck J, Winn R. Adoptive transfer of myelin basic protein-tolerized splenocytes to naive animals reduces infarct size: a role for lymphocytes in ischemic brain injury? Stroke; a journal of cerebral circulation. 2003;34(7):1809–1815. doi: 10.1161/01.STR.0000078308.77727.EA. [DOI] [PubMed] [Google Scholar]
- Becker KJ, McCarron RM, Ruetzler C, Laban O, Sternberg E, Flanders KC, Hallenbeck JM. Immunologic tolerance to myelin basic protein decreases stroke size after transient focal cerebral ischemia. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(20):10873–10878. doi: 10.1073/pnas.94.20.10873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanella M, Sciorati C, Tarozzo G, Beltramo M. Flow cytometric analysis of inflammatory cells in ischemic rat brain. Stroke. 2002;22(2):586–592. doi: 10.1161/hs0202.103399. [DOI] [PubMed] [Google Scholar]
- Cross AH, Raine CS. Serial adoptive transfer of murine experimental allergic encephalomyelitis: successful transfer is dependent on active disease in the donor. Journal of neuroimmunology. 1990;28(1):27–37. doi: 10.1016/0165-5728(90)90038-o. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Klehmet J, Braun JS, Harms H, Meisel C, Ziemssen T, Prass K, Meisel A. Stroke-induced immunodepression: experimental evidence and clinical relevance. Stroke; a journal of cerebral circulation. 2007;38(2 Suppl):770–773. doi: 10.1161/01.STR.0000251441.89665.bc. [DOI] [PubMed] [Google Scholar]
- Downen M, Amaral TD, Hua LL, Zhao ML, Lee SC. Neuronal death in cytokine-activated primary human brain cell culture: role of tumor necrosis factor-alpha. Glia. 1999;28(2):114–127. [PubMed] [Google Scholar]
- Dziennis S, Mader S, Akiyoshi K, Ren X, Ayala P, Burrows GG, Vandenbark AA, Herson PS, Hurn PD, Offner HA. Therapy with recombinant T-cell receptor ligand reduces infarct size and infiltrating inflammatory cells in brain after middle cerebral artery occlusion in mice. Metabolic brain disease. 2011;26(2):123–133. doi: 10.1007/s11011-011-9241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt B, Sorokin L. The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction. Semin Immunopathol. 2009;31(4):497–511. doi: 10.1007/s00281-009-0177-0. [DOI] [PubMed] [Google Scholar]
- Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nature immunology. 2002;3(10):944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Lalor SJ, Sweeney CM, Tubridy N, Mills KH. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clinical and experimental immunology. 2010;162(1):1–11. doi: 10.1111/j.1365-2249.2010.04143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel D, Huang Z, Maron R, Koldzic DN, Moskowitz MA, Weiner HL. Neuroprotection by IL-10-producing MOG CD4+ T cells following ischemic stroke. Journal of the neurological sciences. 2005;233(1–2):125–132. doi: 10.1016/j.jns.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Gee JM, Kalil A, Thullbery M, Becker KJ. Induction of immunologic tolerance to myelin basic protein prevents central nervous system autoimmunity and improves outcome after stroke. Stroke; a journal of cerebral circulation. 2008;39(5):1575–1582. doi: 10.1161/STROKEAHA.107.501486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horuk R. Chemokine receptors. Cytokine & growth factor reviews. 2001;12(4):313–335. doi: 10.1016/s1359-6101(01)00014-4. [DOI] [PubMed] [Google Scholar]
- Hurn PD, Subramanian S, Parker SM, Afentoulis ME, Kaler LJ, Vandenbark AA, Offner H. T- and B-cell-deficient mice with experimental stroke have reduced lesion size and inflammation. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2007;27(11):1798–1805. doi: 10.1038/sj.jcbfm.9600482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nature medicine. 2011;17(7):796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury SJ, Hancock WW, Weiner HL. Oral tolerance to myelin basic protein and natural recovery from experimental autoimmune encephalomyelitis are associated with downregulation of inflammatory cytokines and differential upregulation of transforming growth factor beta, interleukin 4, and prostaglandin E expression in the brain. The Journal of experimental medicine. 1992;176(5):1355–1364. doi: 10.1084/jem.176.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Gautam SC, Chopp M, Zaloga C, Jones ML, Ward PA, Welch KM. Expression of monocyte chemoattractant protein-1 and macrophage inflammatory protein-1 after focal cerebral ischemia in the rat. Journal of neuroimmunology. 1995;56(2):127–134. doi: 10.1016/0165-5728(94)00138-e. [DOI] [PubMed] [Google Scholar]
- Kleinschnitz C, Schwab N, Kraft P, Hagedorn I, Dreykluft A, Schwarz T, Austinat M, Nieswandt B, Wiendl H, Stoll G. Early detrimental T-cell effects in experimental cerebral ischemia are neither related to adaptive immunity nor thrombus formation. Blood. 2010;115(18):3835–3842. doi: 10.1182/blood-2009-10-249078. [DOI] [PubMed] [Google Scholar]
- Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. Journal of translational medicine. 2009;7:97. doi: 10.1186/1479-5876-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A, Lider O, Roberts AB, Sporn MB, Weiner HL. Suppressor T cells generated by oral tolerization to myelin basic protein suppress both in vitro and in vivo immune responses by the release of transforming growth factor beta after antigen-specific triggering. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(1):421–425. doi: 10.1073/pnas.89.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SD, Karpus WJ, Davidson TS. Experimental autoimmune encephalomyelitis in the mouse. In: Coligan John E, et al., editors. Current protocols in immunology. Unit 15. Chapter 15. 2010. p. 11. [DOI] [PubMed] [Google Scholar]
- Moalem G, Leibowitz-Amit R, Yoles E, Mor F, Cohen IR, Schwartz M. Autoimmune T cells protect neurons from secondary degeneration after central nervous system axotomy. Nature medicine. 1999;5(1):49–55. doi: 10.1038/4734. [DOI] [PubMed] [Google Scholar]
- Montecucco F, Lenglet S, Gayet-Ageron A, Bertolotto M, Pelli G, Palombo D, Pane B, Spinella G, Steffens S, Raffaghello L, Pistoia V, Ottonello L, Pende A, Dallegri F, Mach F. Systemic and intraplaque mediators of inflammation are increased in patients symptomatic for ischemic stroke. Stroke; a journal of cerebral circulation. 2010;41(7):1394–1404. doi: 10.1161/STROKEAHA.110.578369. [DOI] [PubMed] [Google Scholar]
- Murphy AC, Lalor SJ, Lynch MA, Mills KH. Infiltration of Th1 and Th17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis. Brain, behavior, and immunity. 2010;24(4):641–651. doi: 10.1016/j.bbi.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Offner H, Sinha S, Burrows GG, Ferro AJ, Vandenbark AA. RTL therapy for multiple sclerosis: a Phase I clinical study. J Neuroimmunol. 2011;231(1–2):7–14. doi: 10.1016/j.jneuroim.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offner H, Sinha S, Wang C, Burrows GG, Vandenbark AA. Recombinant T cell receptor ligands: immunomodulatory, neuroprotective and neuroregenerative effects suggest application as therapy for multiple sclerosis. Reviews in the neurosciences. 2008;19(4–5):327–339. doi: 10.1515/revneuro.2008.19.4-5.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD. Experimental stroke induces massive, rapid activation of the peripheral immune system. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2006a;26(5):654–665. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- Offner H, Subramanian S, Parker SM, Wang C, Afentoulis ME, Lewis A, Vandenbark AA, Hurn PD. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. Journal of immunology. 2006b;176(11):6523–6531. doi: 10.4049/jimmunol.176.11.6523. [DOI] [PubMed] [Google Scholar]
- Peron JP, Yang K, Chen ML, Brandao WN, Basso AS, Commodaro AG, Weiner HL, Rizzo LV. Oral tolerance reduces Th17 cells as well as the overall inflammation in the central nervous system of EAE mice. Journal of neuroimmunology. 2010;227(1–2):10–17. doi: 10.1016/j.jneuroim.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Polanczyk MJ, Jones RE, Subramanian S, Afentoulis M, Rich C, Zakroczymski M, Cooke P, Vandenbark AA, Offner H. T lymphocytes do not directly mediate the protective effect of estrogen on experimental autoimmune encephalomyelitis. The American journal of pathology. 2004;165(6):2069–2077. doi: 10.1016/S0002-9440(10)63257-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz M, Priller J. Tickets to the brain: role of CCR2 and CX3CR1 in myeloid cell entry in the CNS. Journal of neuroimmunology. 2010;224(1–2):80–84. doi: 10.1016/j.jneuroim.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Ren X, Akiyoshi K, Dziennis S, Vandenbark AA, Herson PS, Hurn PD, Offner H. Regulatory B cells limit CNS inflammation and neurologic deficits in murine experimental stroke. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011a;31(23):8556–8563. doi: 10.1523/JNEUROSCI.1623-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Akiyoshi K, Vandenbark AA, Hurn PD, Offner H. Programmed Death-1 Pathway Limits Central Nervous System Inflammation and Neurologic Deficits in Murine Experimental Stroke. Stroke; a journal of cerebral circulation. 2011b doi: 10.1161/STROKEAHA.111.613182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streilein JW. Immune privilege as the result of local tissue barriers and immunosuppressive microenvironments. Current opinion in immunology. 1993;5(3):428–432. doi: 10.1016/0952-7915(93)90064-y. [DOI] [PubMed] [Google Scholar]
- Streilein JW. Unraveling immune privilege. Science. 1995;270(5239):1158–1159. doi: 10.1126/science.270.5239.1158. [DOI] [PubMed] [Google Scholar]
- Streilein JW, Stein-Streilein J. Does innate immune privilege exist? Journal of leukocyte biology. 2000;67(4):479–487. doi: 10.1002/jlb.67.4.479. [DOI] [PubMed] [Google Scholar]
- Stromnes IM, Goverman JM. Passive induction of experimental allergic encephalomyelitis. Nature protocols. 2006;1(4):1952–1960. doi: 10.1038/nprot.2006.284. [DOI] [PubMed] [Google Scholar]
- Subramanian S, Zhang B, Kosaka Y, Burrows GG, Grafe MR, Vandenbark AA, Hurn PD, Offner H. Recombinant T cell receptor ligand treats experimental stroke. Stroke; a journal of cerebral circulation. 2009;40(7):2539–2545. doi: 10.1161/STROKEAHA.108.543991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ellison JA, Siren AL, Lysko PG, Yue TL, Barone FC, Shatzman A, Feuerstein GZ. Prolonged expression of interferon-inducible protein-10 in ischemic cortex after permanent occlusion of the middle cerebral artery in rat. Journal of neurochemistry. 1998;71(3):1194–1204. doi: 10.1046/j.1471-4159.1998.71031194.x. [DOI] [PubMed] [Google Scholar]
- Yamasaki Y, Matsuo Y, Matsuura N, Onodera H, Itoyama Y, Kogure K. Transient increase of cytokine-induced neutrophil chemoattractant, a member of the interleukin-8 family, in ischemic brain areas after focal ischemia in rats. Stroke; a journal of cerebral circulation. 1995;26(2):318–322. doi: 10.1161/01.str.26.2.318. discussion 322–313. [DOI] [PubMed] [Google Scholar]
- Yilmaz G, Granger DN. Leukocyte recruitment and ischemic brain injury. Neuromolecular Med. 2010;12(2):193–204. doi: 10.1007/s12017-009-8074-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.