Abstract
Background
A limited number of studies have shown that modulation of cortical excitability using transcranial direct current stimulation (tDCS) is safe and tolerable. Few have directly evaluated whether sham and active stimulation are indistinguishable.
Objective
We aimed to demonstrate tDCS safety and tolerability in a large cohort, and to compare the occurrence and severity of side effects between sham and active stimulation sessions.
Methods
131 healthy subjects undergoing 277 tDCS sessions rated on a 1 to 5 scale the perception of side effects during and after stimulation. Proportions of active and sham sessions associated with side effects were compared using Fisher’s exact test, and distributions of severity ratings were compared using the Kruskal-Wallis test.
Results
No serious adverse effects occurred. Side effects most commonly reported were tingling (76%), itching (68%), burning (54%), and pain (25%). Side effect severity was mild, with fewer than two percent of responses indicating a severity >3 on all questions except tingling (15%), itching (20%), burning (7%), pain (5%) and fatigue (3%) during stimulation. Rates of sensory side effects were statistically significantly higher in active stimulation sessions compared to sham sessions. No other stimulation parameters had a statistically significant impact on side effect occurrence.
Conclusions
TDCS is a safe well-tolerated technique with no evidence of risk for serious adverse effects. Sensory side effects are common, but the severity is typically low. Because sensory side effects are more frequent and more severe in active compared to sham tDCS, the current method of sham stimulation may not be an adequate control condition for some studies.
Keywords: tDCS, safety, tolerability, side effects, sham
Introduction
Transcranial direct current stimulation (tDCS) is a noninvasive tool for modulation of cortical excitability which uses weak electrical currents applied to the scalp to alter resting membrane potentials of underlying neurons (1, 2). The effects of tDCS – increased cortical excitability with anodal stimulation and decreased cortical excitability with cathodal stimulation – have been validated using neurophysiologic methods, and appear to persist for up to 90 minutes beyond the period of stimulation (3–5). Modulation of cortical activity can be used as an investigative tool for understanding structure-function relationships of cognitive processes, and also has potential therapeutic applications for neurologic and psychiatric disorders (6–9). Compared to transcranial magnetic stimulation, another noninvasive technique for influencing neuronal activity, tDCS is inexpensive, easy to administer, and safe in healthy volunteers and in patients with central nervous system disease. In over 200 published studies with hundreds of subjects cumulatively undergoing thousands of tDCS sessions, no serious adverse events have been reported with current strength up to 2 mA and continuous application of current up to 30 minutes (1, 3, 6, 10, 11). In addition, stimulation is generally well tolerated, particularly at low current intensities using large stimulating electrodes (for lower charge density). Subjects frequently report an itching or tingling sensation at electrode sites at the beginning of stimulation, but discomfort fades after 30 seconds to 1 minute of stimulation at full current strength (12, 13). Because sudden initiation or interruption of the stimulation current can cause AC currents that result in neuronal firing and phenomena such as retinal phosphenes, gradually ramping up and tapering down current is recommended (14). Another putative advantage of tDCS over TMS is that sham (placebo) stimulation can be applied in a way that is indistinguishable from active stimulation(15). Sham stimulation is carried out by increasing current over several seconds to the target strength, and then tapering off over several seconds. Using this approach, subjects theoretically have the same experience of itching and tingling during sham stimulation as they would during active stimulation. In active stimulation, sensations are transient because the subject accommodates to the current, while in sham stimulation, sensations fade because the current is tapered off.
The adequacy of sham stimulation as a control condition depends on the assumption that the transient sensations produced by active stimulation are the same as those produced when current is tapered off. If active stimulation produces more intense or more prolonged discomfort than sham tDCS, performance of tasks during stimulation may be adversely impacted by these distracting sensations. Conversely, discomfort might heighten arousal, improving performance on some tasks. Differences in sensation during stimulation may also prevent true masking of subjects to the condition, potentially impacting performance on tasks during and after stimulation. Inadequate blinding may bias studies of the therapeutic effects of tDCS.
Prior studies have suggested that there is no difference in side effect occurrence during active and sham stimulation, but none have made direct comparisons between these two conditions in a large number of subjects (12, 16–18). In the present study, we aimed to compare the intensity of perceived sensations and other adverse effects between sham and active stimulation in subjects undergoing tDCS during studies of cognition.
Methods
Subjects
All subjects participating in tDCS studies at the Hospital of the University of Pennsylvania between April 2009 and May 2010 were asked to complete a questionnaire at the end of each stimulation session. Studies were approved by the University of Pennsylvania Institutional Review Board and all subjects consented to participation. All subjects were healthy adults who denied active exposure to CNS-acting pharmacologic agents. Subjects were seated comfortably and were asked to remain awake during experiments. Subjects were not told what type of stimulation was being given.
tDCS
All studies were conducted using a Magstim Eldith DC-stimulator (Magstim Company, Ltd, Wales, UK). Current was applied to the scalp with 5×5 cm or 5×7 cm saline-soaked sponge electrodes (25 cm2 or 35 cm2) with a current strength of 1.5 milliamperes (mA) in all sessions. Location of electrodes varied according to experimental paradigm. Some studies included anodal and cathodal stimulation conditions, and some compared either anodal or cathodal stimulation to sham only. In some studies, both the active electrode and reference electrode were placed directly on the scalp (thus, both electrodes potentially affected brain regions), and in some studies, the reference electrode was on the cheek or mastoid process. In all active tDCS sessions, current was ramped up over 10 seconds until 1.5 mA was reached, and was ramped down over an equal amount of time at the end of stimulation. The duration of stimulation for active stimulation sessions was between 600 and 1200 seconds, depending on the experimental paradigm. For sham stimulation, sponge electrodes were applied in the same manner and current was ramped up either over 10 seconds or 15 seconds, with an equal amount of time for tapering off. The investigator applying tDCS was not always blinded to the stimulation condition. Table 1 summarizes the experimental paradigms.
Table 1.
Summary of experimental conditions.
| Study | Subjects (n) | Sessions (n) | Task during or after stimulation | Active electrode location (number of sessions) | Reference electrode location | Active/Sham Duration (seconds) | Active/Sham Ramp time (seconds) | Electrode Size |
|---|---|---|---|---|---|---|---|---|
| Effects of stimulation on memory encoding and retrieval | 58 | 142 | During and after | Left or right frontal (42); Left or right parietal (100) | Right supraorbital; cheek | 600/30 | 10/15 | 35 cm2 |
| Disrupting temporal versus spatial dynamics of causality | 15 | 39 | After | Left or right parietal | Left supraorbital | 600/30 | 10/15 | 35 cm2 |
| Effect of anterior temporal lobe stimulation on proper name recall | 9 | 9 | Training task during; experimental task after | Left or right temporal | Cheek | 600/30 | 10/15 | 35 cm2 |
| Modulation of internal clock by stimulation of SMA | 29 | 29 | After | Frontocentral (25); Left parietooccipital (4) | Cheek | 600/-- | 10/-- | 35 cm2 |
| Effect of parietal stimulation on length of prism adaptation after effects. | 12 | 15 | During and after | Left parietal parasagittal (5); Right parietal parasagittal (10) | Central vertex (Cz) | 600/30 | 10/15 | 35 cm2 |
| Role of superior temporal gyrus in perception of brief (hundreds of milliseconds) time intervals | 13 | 13 | After | Right midtemporal | Cheek | 600/20 | 10/10 | 35 cm2 |
| Role of prefrontal cortex in delay discounting | 10 | 24 | After | Left frontal parasagittal | Mastoid | 900/20 | 10/10 | 25 cm2 |
| Hemispheric dominance in temporal lobe processing of phoneme perception | 5 | 6 | During and after | Posterior temporal (3); Central parietal (2) | Contralateral posterior temporal (3); Cheek (2) | 900/20 1200/20 |
10/10 | 35 cm2 |
Outcome measures
The questionnaire contained categorical rating scales (from 1 -- very mildly or not at all, to 5 -- a significant degree) for occurrence of 10 symptoms during stimulation (headache, difficulty concentrating, acute mood changes, changes in visual perception, tingling, itching, burning, pain, fatigue, and nervousness), the same 10 symptoms plus unpleasant sensation and nausea after stimulation, and visual sensation at the start or end of stimulation. Subjects were not asked to guess whether they received active or sham stimulation to avoid drawing attention to the difference and influencing future sessions.
Statistical analysis
The proportion of sessions during which side effects were experienced was determined by dichotomizing responses to each question into one of two categories: side effect absent (a rating of 1) or side effect present (ratings of 2 through 5). For each question, the proportion of sessions in which a side effect was present was compared between active stimulation and sham stimulation using Fisher’s exact test. Then, the severity of side effects (the median rating and distribution of answers) was compared between active and sham stimulation groups using the Wilcoxon rank sum (Mann-Whitney) test. To further evaluate whether there were differences in side effect severity between sham stimulation, stimulation with only the anode on the head (reference on cheek or mastoid), stimulation with only the cathode on the head, and stimulation with both anode and cathode on the head, comparisons were carried out using the Kruskal-Wallis test for equality of populations. Multivariate analyses using logistic regression were conducted to evaluate the influence of demographic variables, electrode location, or duration of stimulation on the association between active stimulation and the occurrence of sensory side effects. The outcome variable was a dichotomized combined sensory side effect score, where 0 was a response of 1 (absent/minimal) on the questions regarding tingling, itching, burning, or pain during stimulation, and 1 was any answer of 2 or higher on these questions.
Statistical significance was defined as a two-tailed p-value of less than 0.05, and no correction for multiple comparisons was made for this exploratory study.
Results
One hundred thirty-one healthy subjects (77 female), aged 18 to 73 years (mean 24.3, SD 7.64) underwent a total of 277 tDCS sessions (183 active, 94 sham) in eight studies. The main unit of analysis was session. Forty-one subjects (31%) underwent one session only, 34 (26%) underwent two sessions, and 56 (43%) underwent 3 sessions. No subjects asked to suspend a session or leave a study because of side effects. No subjects experienced seizures or other adverse effects requiring medical intervention. At the discretion of the investigator, one subject’s participation in a stimulation session was terminated early. The subject reported a burning sensation and saliva pooling under the cathode on the cheek while the stimulator was off, presumably due to device malfunction. The subject subsequently felt a transient shock sensation when the leads were detached and reinserted with the electrodes attached to her head. The subject reported no enduring adverse effects either shortly after the event, or the following day, and continued to volunteer for additional tDCS studies.
The occurrence of side effects is summarized in Table 2. The side effects most commonly reported during any type of stimulation session were tingling (76%), itching (68%), burning (54%), and pain (25%). Overall, 82.7% of sessions were associated with one 1 or more of the above side effects. Of the side effects not involving sensation, difficulty concentrating and fatigue were the two most commonly reported. The occurrence of difficulty concentrating was closely related to the occurrence of sensory symptoms – of the 99 sessions where subjects reported difficulty concentrating during stimulation, only 11 lacked either tingling, itching, burning or pain. The rates of tingling, itching, and burning during and after stimulation, and the rate of pain during stimulation were statistically significantly higher in active stimulation sessions compared to sham sessions, with differences ranging from 5 to 13%.
Table 2.
Side Effects During and After TDCS – proportion of sessions associated with side effects.
| Side Effect | Total (n= 277) %(n) | Active (n=183) %(n) | Sham (n=94) %(n) | p value* |
|---|---|---|---|---|
| During | ||||
| Headache | 13.4 (37) | 15.3 (28) | 9.6 (9) | 0.20 |
| Difficulties Concentrating | 35.7 (99) | 41.5 (76) | 24.5 (23) | <0.01 |
| Acute Mood Changes | 6.9 (19) | 8.2 (15) | 4.3 (4) | 0.32 |
| Changes in Visual Perception | 15.5 (43) | 18.6 (34) | 9.6 (9) | 0.10 |
| Tingling | 76.9 (213) | 89.1 (163) | 53.2 (50) | <0.001 |
| Itching Sensation | 68.2 (189) | 81.4 (149) | 42.6 (40) | <0.001 |
| Burning Sensation | 54.2 (150) | 65.0 (119) | 33.0 (31) | <0.001 |
| Pain | 24.9 (69) | 31.7 (58) | 11.7 (11) | <0.001 |
| Fatigue | 20.9 (58) | 19.7 (36) | 23.4 (22) | 0.53 |
| Nervousness | 10.1 (28) | 12.6 (23) | 5.3 (5) | 0.06 |
| Visual Sensation associated with start/end of stimulation | 12.3 (34) | 13.7 (25) | 9.6 (9) | 0.44 |
| After | ||||
| Headache | 13.4 (37) | 15.3 (28) | 9.6 (9) | 0.20 |
| Difficulties Concentrating | 20.2 (56) | 23.0 (42) | 14.9 (14) | 0.15 |
| Acute Mood Changes | 11.9 (33) | 14.8 (27) | 6.4 (6) | 0.05 |
| Changes in Visual Perception | 0 (0) | 0 (0) | 0 (0) | --- |
| Tingling | 24.9 (69) | 30.6 (56) | 13.8 (13) | <0.01 |
| Itching Sensation | 25.6 (71) | 33.3 (61) | 10.6 (10) | <0.001 |
| Burning Sensation | 12.3 (34) | 16.9 (31) | 3.2 (3) | <0.01 |
| Pain | 5.1 (14) | 6.0 (11) | 3.2 (3) | 0.40 |
| Fatigue | 17.7 (49) | 17.5 (32) | 18.1 (17) | 1.00 |
| Nervousness | 2.9 (8) | 2.2 (4) | 4.3 (4) | 0.45 |
| Unpleasant Sensation | 9.8 (27) | 10.9 (20) | 7.5 (7) | 0.40 |
| Nausea | 1.8 (5) | 1.1 (2) | 3.2 (3) | 0.34 |
2-sided Fisher’s exact
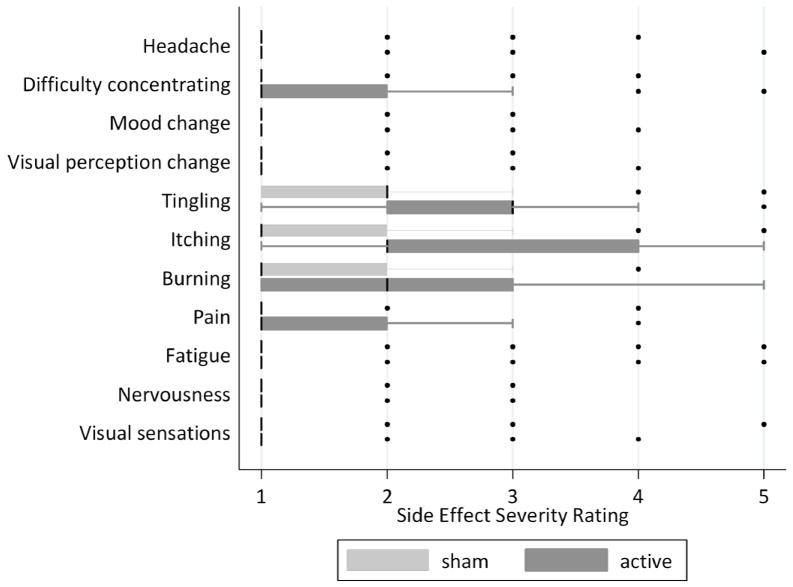
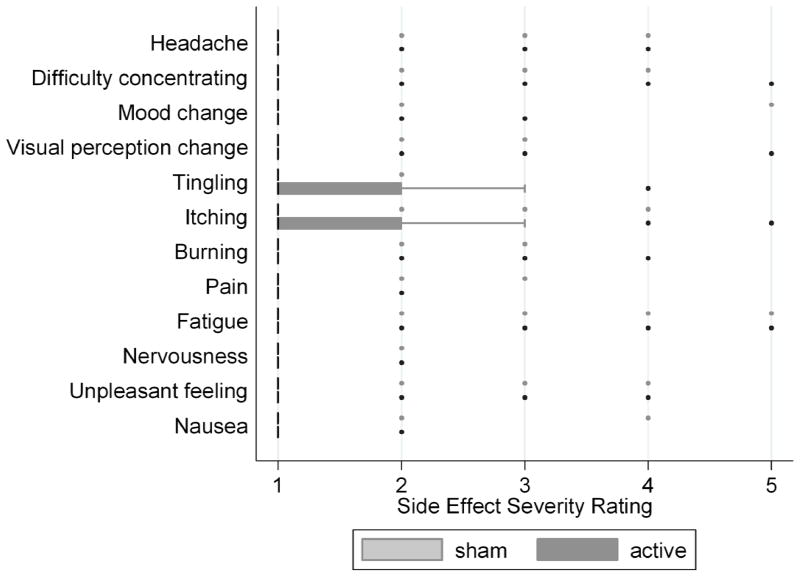
The severity of side effects was generally low, as summarized in Table 3. The median response was 1 (mild or none at all) for all side effect questions except for tingling, itching, and burning during stimulation. Fewer than two percent of responses were marked as a severity of 4 or 5 on all questions except tingling (15%), itching (20%), burning (7%), pain (5%) and fatigue (3%) during stimulation. Statistically significant differences in the distribution of side effect severity ratings between active and sham stimulation were seen for the sensory questions during and after stimulation, and difficulty concentrating during stimulation, summarized in Table 3 and illustrated in Figures 1 and 2. To understand whether certain individuals who were particularly sensitive to stimulation effects influenced the severity ratings, we evaluated the distribution of responses after excluding individuals who answered 3 or greater on the tingling, itching, burning, or pain questions during sham stimulation. The medians and distributions of severity responses during active stimulation did not change when these subject were excluded. For example, the median response for tingling during stimulation remained a three, with a range of 1 to 5, and tingling was reported in 75% of sessions.
Table 3.
Side Effects during and after tDCS –comparison of severity ratings
| Side Effect | Active Median (Range) | Sham Median (Range) | p value* |
|---|---|---|---|
| During | |||
| Headache | 1 (1–3) | 1 (1–4) | 0.19 |
| Difficulties Concentrating | 1 (1–5) | 1 (1–4) | <0.01 |
| Acute Mood Changes | 1 (1–3) | 1 (1–3) | 0.22 |
| Changes in Visual Perception | 1 (1–3) | 1 (1–3) | 0.05 |
| Tingling | 3 (1–5) | 2 (1–5) | <0.001 |
| Itching Sensation | 2 (1–5) | 1 (1–5) | <0.001 |
| Burning Sensation | 2 (1–4) | 1 (1–4) | <0.001 |
| Pain | 1 (1–3) | 1 (1–4) | <0.001 |
| Fatigue | 1 (1–4) | 1 (1–5) | 0.36 |
| Nervousness | 1 (1–3) | 1 (1–3) | 0.09 |
| Visual Sensation associated with start/end of stimulation | 1 (1–4) | 1 (1–5) | 0.35 |
| After | |||
| Headache | 1 (1–3) | 1 (1–4) | 0.21 |
| Difficulties Concentrating | 1 (1–5) | 1 (1–4) | 0.11 |
| Acute Mood Changes | 1 (1–3) | 1 (1–5) | 0.35 |
| Changes in Visual Perception | 1 (1–3) | 1 (1–3) | 0.09 |
| Tingling | 1 (1–4) | 1 (1–2) | <0.01 |
| Itching Sensation | 1 (1–5) | 1 (1–4) | <0.001 |
| Burning Sensation | 1 (1–4) | 1 (1–3) | <0.01 |
| Pain | 1 (1–2) | 1 (1–3) | 0.51 |
| Fatigue | 1 (1–5) | 1 (1–5) | 0.85 |
| Nervousness | 1 (1–2) | 1 (1–2) | 0.33 |
| Unpleasant Sensation | 1 (1–4) | 1 (1–4) | 0.37 |
| Nausea | 1 (1–2) | 1 (1–4) | 0.21 |
Wilcoxon rank-sum
Figure 1.
Box plots for severity of side effects during stimulation. Boxes represent the interquartile range, with median represented by a thick black line. Whiskers represent inner and outer adjacent values and circles represent outliers.
Figure 2.
Box plots for severity of side effects experienced after stimulation. Boxes represent the interquartile range, with median represented by a thick black line. Whiskers represent inner and outer adjacent values and circles represent outliers.
Controlling for active versus sham stimulation, there were no independent effects of sex, years of education, handedness, location of electrodes, electrode size/current density, ramp time, or duration of stimulation on the occurrence of sensory side effects. Age had a small but statistically significant protective effect against the occurrence of sensory side effects -- for each added year of age, the likelihood of a sensory side effect occurring dropped by 7% (adjusted OR 0.93; p = 0.001). In examining the active stimulation group in more detail, no differences were found when the distributions of responses were compared between sessions with the anode and cathode on the head, sessions with only the cathode on the head (reference electrode on the cheek or mastoid), and sessions with only the anode on the head. Data were not available regarding differences in the occurrence or intensity of sensation underlying the cathode compared to the anode.
Discussion
The results of this study add to the limited but growing body of literature indicating that tDCS is a safe and tolerable method of non-invasive brain stimulation. Our findings demonstrate that 1) no serious adverse effects occurred in a large sample of subjects and sessions 2) the occurrence of sensory side effects with tDCS is common 3) the severity of these side effects is low 4) there is a difference in the experience of active and sham stimulation conditions.
No subjects experienced adverse events requiring any medical intervention, and none were deterred from continuing to participate in tDCS studies. No lasting skin reactions in areas underlying the electrodes occurred. A single subject experienced a shock sensation, a side effect occasionally reported in other series (17, 18), which is presumably related to transient AC currents that occur with the abrupt making or breaking of the stimulating circuit. The shock sensation has been reported as unpleasant, but there appears to be no specific danger associated with it.
The findings of our study regarding the frequency and severity of side effects are consistent with the findings of prior studies, but we present new information regarding differences in the experience of active and sham stimulation. Poreisz and colleagues evaluated the occurrence of side effects in 102 subjects (77 healthy volunteers and 25 patients) undergoing 567 tDCS sessions (35 cm2 electrodes delivering 1mA of current lasting 20 minutes for active and 30 seconds for sham) using a questionnaire with rating scales for headache, difficulties concentrating, acute mood changes, visual perceptual changes, fatigue, pain, tingling, itching, and burning – both during and after tDCS. Side effects were reported by 71% of subjects, but were mild – a combined mean of 1.74 ±0.84 on a continuous analog scale of 1 to 5. Another prior study of discomfort during application of tDCS found that while the majority of subjects experienced some sensation with stimulation (namely, itching), most of them did not rate the sensation as uncomfortable (13). Similarly, in our study the occurrence of reported side effects was common but the intensity of the side effects was low: a sensory side effect (tingling, itching, burning, or pain) occurred in more than 80% of sessions, but few questions elicited median responses above the lowest response (minimal or none). In the Poreisz study, no comparison of side effect occurrence in the sham and active stimulation groups was reported, but only 17% of subjects claimed to feel a difference between anodal, cathodal, or sham stimulation when directly asked.
Gandiga and others evaluated the occurrence of perceived sensations and discomfort, as well as ratings of attentions and fatigue among an unspecified number of subjects undergoing 170 tDCS sessions. Again, sensations related to tDCS application were common. Tingling, for example, occurred in as many 74% of sessions but was mild – mean severity of side effects measured on a 1 to 10 visual analog scale ranged from 1.4 to 2.6 in healthy volunteers. The difference in side effect occurrence during active sessions versus sham stimulation sessions was not the primary comparison, making interpretation of results in relation to this question challenging. For some subpopulations, a difference between active and sham sessions in the rating of the duration of sensations was apparent. For example, chronic stroke patients reported feeling a mean of 40.20 (±7.99) seconds of sensations during active stimulation (applied for 20 minutes) compared to 24.63 (±4.56) seconds during sham stimulation (applied for 30 seconds). The authors report that none of the subjects were able to distinguish the type of stimulation given when directly asked. These data, in conjunction with the findings from our study, suggest that while subjects are unable to explicitly discriminate between active and sham stimulation, the implicit experience of the two conditions is different. The findings of the Gandiga study also suggest that the difference in duration of sensation might have been balanced between active and sham conditions by slightly increasing the duration of sham stimulation to the mean duration of sensations felt during active stimulation.
In both the Gandiga and Poreisz studies, current strength was 1 mA, lower than the 1.5 mA current strength used in this study. Higher current strength may exaggerate the difference in sensory side effect occurrence between the active and sham conditions. However, stimulation at 1.5 mA did not increase the overall proportion of subjects experiencing sensory side effects – as discussed above, the proportion perceiving sensations in all three studies was around 70%. Because all subjects in our study were stimulated at 1.5 mA, further delineation of the influence of current strength on differential rates of sensory side effects was not possible with the present data.
We did not directly ask our subjects whether they were able to distinguish between active and sham stimulation, or between anodal and cathodal stimulation, because we did not want to draw attention to these differences lest this awareness interfere with performance during the experiment. Thus, we cannot discern with the current data whether masking of the stimulation condition was successful among individual subjects. It is difficult to know whether differences in the proportion of subjects experiencing side effects as modest as 5 to 13% can influence the outcome of experiments of cognition or therapeutic trials. However, our data raises the possibility that current methods of sham stimulation may not be an adequate control condition, particularly in studies where sensory side effects may interfere with task performance.
Another comparable study was conducted by Tadini and colleagues, who studied cognitive, mood, and electroencephalographic effects of tDCS in 42 subjects exposed to continuous tDCS (and others exposed to intermittent tDCS or AC stimulation). Side effects were assessed using a questionnaire with yes/no answer choices. Of these 42 subjects, only three reported tingling, two during active stimulation only and 1 during sham only. There are several possible explanations for the unusually low rate of sensory side effects in this study. The current intensity (1 to 2 mA) was similar to our study and the studies previously discussed, but stimulation duration was shorter (10 minutes). Ramp times were not reported, but possibly contributed to the low rate of tingling. Scalp itching, which was among the most common side effects endorsed by our subjects, and has previously been reported as the most frequently used descriptor of the sensation induced by tDCS (13)was not specifically investigated in the Tadini study. Subjective adverse effects of any type were reported in 24 subjects (57%) during active stimulation and 20 subjects (48%) during sham stimulation, with headache and sleepiness being the most commonly endorsed. The lack of a difference in the occurrence of these side effects during active and sham stimulation suggests that these effects were related either to the onset of stimulation (which is the same for active and sham), or to the overall experience of the testing session, not to the effects of stimulation itself. In our study, more global symptoms such as headache and fatigue were far less common than sensory effects (occurring in less than a quarter of sessions), and rates were similar in sham and active sessions, suggesting that these effects may be more tied to the experimental tasks than stimulation itself.
Another interesting finding in our study is that increasing age has a protective effect on the experience of sensory side effects. We clearly found a reduction in the occurrence and severity of tingling, itching, burning, and pain with each increasing year of age. In the Gandiga study, young healthy volunteers (ages 20 to 35 years) reported higher rates of tingling sensations with active (73.7%) and sham (65%) than chronic stroke patients (mean ages 57.6 to 62.3 years) and older healthy volunteers (ages 56 to 85 years), where tingling was reported in 46.4% of active and 51.9% of sham stimulation sessions. Similarly, the authors report a statistically significant difference in the influence of age on severity of discomfort when young healthy volunteers were compared to older healthy volunteers. The reason for the effect of age is unclear. Possibilities include age-related differences in properties of skin conductance, density or effectiveness of small fiber nerve endings, and threshold for reporting a felt sensation.
Our study had several limitations. The rating scale we used grouped “absent” and “minimal” together for each side effect, making it impossible to distinguish whether any given symptom was experienced only mildly, or not at all. Though the number of sessions we evaluated was larger than in previous series, the study was not powered to investigate small differences in the effects of specific factors such as electrode location on the occurrence of side effects. In particular, all of the studies included in this analysis used a short ramp time (10 or 15 seconds), and it remains possible that sensations associated with active and sham stimulation would be more similar with more gradual ramping of current. Not all subjects in this study received both active and sham stimulation (incomplete crossover), so it is possible that those receiving active stimulation and not sham were more sensitive to sensory side effects than those receiving sham and not active. However, because subjects were assigned to study groups without knowledge of their sensitivity to side effects, there is no reason to suspect that systematic bias unbalanced these groups. Finally, investigators applying tDCS were not always blinded to the stimulation condition.
In summary, this study adds to the evidence that tDCS is a safe and well-tolerated method of noninvasive brain stimulation. However, with the stimulation parameters used here, the experience of active and sham stimulation is not the same among some individuals. While disparities in the occurrence or severity of sensory side effects may or may not affect blinding of subjects to the stimulation condition, they may differentially affect task performance, raising the possibility that the current method of sham stimulation is not an adequate control condition. Further investigation is warranted to understand how changes to the method of sham stimulation may mitigate this issue.
Acknowledgments
Dr. Kessler is supported by NIH NINDS K12 NS049453 (NSADA). Dr. Turkeltaub is supported by The American Academy of Neurology Foundation (Clinical Research Training Fellowship). Dr. Hamilton is supported by NIH NINDS 1K01 NS060995 and by the Harold Amos Medical Faculty Development Program of the Robert Wood Johnson Foundation.
The authors wish to thank the following investigators -- H. Branch Coslett, David A. Wolk, Ingrid R. Olsen, Laurel J. Buxbaum, Emily Hurwitz, Katharine Manning, Lauren Mancuso, Benjamin Straube, Bianca Bromberger, David McCoy, Cynthia M. Gooch, Steven A. Jax, and Joseph W. Kable -- for their cooperation with this study, and Sarah Zelonis for assistance in preparing this manuscript.
Footnotes
The authors have no actual or potential financial conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000 Sep 15;527(Pt 3):633–9. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Priori A, Berardelli A, Rona S, Accornero N, Manfredi M. Polarization of the human motor cortex through the scalp. Neuroreport. 1998 Jul 13;9(10):2257–60. doi: 10.1097/00001756-199807130-00020. [DOI] [PubMed] [Google Scholar]
- 3.Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001 Nov 27;57(10):1899–901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- 4.Ardolino G, Bossi B, Barbieri S, Priori A. Non-synaptic mechanisms underlie the after-effects of cathodal transcutaneous direct current stimulation of the human brain. J Physiol. 2005 Oct 15;568(Pt 2):653–63. doi: 10.1113/jphysiol.2005.088310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rango M, Cogiamanian F, Marceglia S, Barberis B, Arighi A, Biondetti P, et al. Myoinositol content in the human brain is modified by transcranial direct current stimulation in a matter of minutes: a 1H-MRS study. Magn Reson Med. 2008 Oct;60(4):782–9. doi: 10.1002/mrm.21709. [DOI] [PubMed] [Google Scholar]
- 6.Priori A. Brain polarization in humans: a reappraisal of an old tool for prolonged non-invasive modulation of brain excitability. Clin Neurophysiol. 2003 Apr;114(4):589–95. doi: 10.1016/s1388-2457(02)00437-6. [DOI] [PubMed] [Google Scholar]
- 7.Ferrucci R, Mameli F, Guidi I, Mrakic-Sposta S, Vergari M, Marceglia S, et al. Transcranial direct current stimulation improves recognition memory in Alzheimer disease. Neurology. 2008 Aug 12;71(7):493–8. doi: 10.1212/01.wnl.0000317060.43722.a3. [DOI] [PubMed] [Google Scholar]
- 8.Schlaug G, Renga V, Nair D. Transcranial direct current stimulation in stroke recovery. Arch Neurol. 2008 Dec;65(12):1571–6. doi: 10.1001/archneur.65.12.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrucci R, Bortolomasi M, Vergari M, Tadini L, Salvoro B, Giacopuzzi M, et al. Transcranial direct current stimulation in severe, drug-resistant major depression. J Affect Disord. 2009 Nov;118(1–3):215–9. doi: 10.1016/j.jad.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 10.Iyer MB, Mattu U, Grafman J, Lomarev M, Sato S, Wassermann EM. Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology. 2005 Mar 8;64(5):872–5. doi: 10.1212/01.WNL.0000152986.07469.E9. [DOI] [PubMed] [Google Scholar]
- 11.Nitsche MA, Liebetanz D, Lang N, Antal A, Tergau F, Paulus W. Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clin Neurophysiol. 2003 Nov;114(11):2220–2. doi: 10.1016/s1388-2457(03)00235-9. author reply 2–3. [DOI] [PubMed] [Google Scholar]
- 12.Poreisz C, Boros K, Antal A, Paulus W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res Bull. 2007 May 30;72(4–6):208–14. doi: 10.1016/j.brainresbull.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Dundas JE, Thickbroom GW, Mastaglia FL. Perception of comfort during transcranial DC stimulation: effect of NaCl solution concentration applied to sponge electrodes. Clin Neurophysiol. 2007 May;118(5):1166–70. doi: 10.1016/j.clinph.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Nitsche MA, Liebetanz D, Antal A, Lang N, Tergau F, Paulus W. Modulation of cortical excitability by weak direct current stimulation--technical, safety and functional aspects. Suppl Clin Neurophysiol. 2003;56:255–76. doi: 10.1016/s1567-424x(09)70230-2. [DOI] [PubMed] [Google Scholar]
- 15.Priori A, Hallett M, Rothwell JC. Repetitive transcranial magnetic stimulation or transcranial direct current stimulation? Brain Stimul. 2009 Oct;2(4):241–5. doi: 10.1016/j.brs.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006 Apr;117(4):845–50. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Tadini L, El-Nazer R, Brunoni AR, Williams J, Carvas M, Boggio P, et al. Cognitive, Mood, and Electroencephalographic Effects of Noninvasive Cortical Stimulation With Weak Electrical Currents. J ECT. 2010 Oct 5; doi: 10.1097/YCT.0b013e3181e631a8. [DOI] [PubMed] [Google Scholar]
- 18.Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008 Jul;1(3):206–23. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]