Abstract
Objectives
• To validate previously published nomograms for predicting insignificant prostate cancer (PCa) that incorporate clinical data, percentage of biopsy cores positive (%BC+) and magnetic resonance imaging (MRI) or MRI/MR spectroscopic imaging (MRSI) results.
• We also designed new nomogram models incorporating magnetic resonance results and clinical data without detailed biopsy data.
• Nomograms for predicting insignificant PCa can help physicians counsel patients with clinically low-risk disease who are choosing between active surveillance and definitive therapy.
Patients and methods
• In total, 181 low-risk PCa patients (clinical stage T1c–T2a, prostate-specific antigen level < 10 ng/mL, biopsy Gleason score of 6) had MRI/MRSI before surgery.
• For MRI and MRI/MRSI, the probability of insignificant PCa was recorded prospectively and independently by two radiologists on a scale from 0 (definitely insignificant) to 3 (definitely significant PCa).
• Insignificant PCa was defined on surgical pathology.
• There were four models incorporating MRI or MRI/MRSI and clinical data with and without %BC+ that were compared with a base clinical model without %BC and a more comprehensive clinical model with %BC+.
• Prediction accuracy was assessed using areas under receiver–operator characteristic curves.
Results
• At pathology, 27% of patients had insignificant PCa, and the Gleason score was upgraded in 56.4% of patients.
• For both readers, all magnetic resonance models performed significantly better than the base clinical model (P ≤ 0.05 for all) and similarly to the more comprehensive clinical model.
Conclusions
• Existing models incorporating magnetic resonance data, clinical data and %BC+ for predicting the probability of insignificant PCa were validated.
• All MR-inclusive models performed significantly better than the base clinical model.
Keywords: magnetic resonance imaging, magnetic resonance spectroscopic imaging, nomograms, prostate neoplasms
Introduction
One of the greatest dilemmas in prostate cancer (PCa) management is choosing between active surveillance and definitive therapy for patients with clinically low-risk disease. Most PCa diagnosed today meets the standard definition of clinically low-risk disease: clinical stage T1c or T2a cancer, with Gleason score ≤ 6 and a pretreatment PSA level < 10 ng/mL [1–3]. However, at surgical pathology, some of these cancers prove to be higher-grade and more extensive than anticipated, whereas others appear to be insignificant and probably do not cause harm within the patient’s lifetime [4,5]. A number of nomograms have been developed to predict the probability of pathologically insignificant cancer and thus help treating physicians counsel patients regarding management options [6–10]. Such nomograms, which are typically based on clinical and biopsy findings, have shown similar levels of accuracy, even when derived from different patient populations and geographical locations [11]. The most accurate nomogram models incorporate detailed biopsy data not included in all biopsy reports. For example, Kattan et al. found that a ‘full model’ incorporating millimetres of cancerous and benign tissue in biopsy cores was more accurate than both a ‘medium’ model including the percentage of biopsy cores positive (%BC+) and a simpler base model. However, the use of the more comprehensive models often requires not only the re-review of biopsy specimens by an uropathologist, but also repeat biopsy [10,12]. MRI and magnetic resonance spectroscopic imaging (MRSI) have been shown to add significant incremental value to clinical variables in predicting organ-confined and insignificant PCa [13,14]. Our group has previously published nomogram models for predicting insignificant PCa before surgery that incorporated MRI and MRSI data along with clinical data and %BC+. In a preliminary retrospective study, these nomograms were significantly more accurate than both the base and medium nomogram models designed by Shukla-Dave et al. [13]. However, because millimetres of cancerous and benign tissue in the biopsy cores were not available for more than 90% of the patients in our study whose biopsies were obtained outside our institution, we were unable to compare the full model with our MR-inclusive models.
The present study aimed to validate the previously published preoperative MR-inclusive nomogram models for predicting the probability of insignificant PCa. We also designed new nomograms incorporating MRI, MRSI and clinical data without detailed biopsy data.
Patients and methods
Between December 2005 and November 2009, 357 patients provided their informed consent to be enrolled in a prospective National Institutes of Health (NIH) study investigating the use of pretreatment MRI and MRSI for assessing clinically low-risk PCa (clinical stage T1c or T2a, primary and secondary biopsy Gleason grades 1–3 [score ≤ 6], pretreatment PSA level < 10 ng/mL). For our analysis, we selected patients from the NIH study who had radical prostatectomy; 198 patients met this criterion, of whom three withdrew their consent and 14 did not have a complete MRI/MRSI study. Hence, the final analysis included 181 patients.
Endorectal MRI/MRSI data acquisition and processing
MRI/MRSI data were acquired on 1.5-T scanner (General Electric, Milwaukee, WI, USA). MRI was performed using a pelvic phased-array coil and an expandable endorectal coil. A standard prostate MRI protocol was used [13]. MRSI data acquisition and processing software were provided by the same vendor. The commercial acquisition software PROSE (for ‘prostate spectroscopy’) was used, which acquires data with the point-resolved spatially localized spectroscopy technique by using spectral-spatial pulses to excite choline, polyamines, creatine and citrate within the point-resolved spatially localized spectroscopy excitation volume, whereas water and lipids are suppressed in a voxel array with an in-plane resolution of 6.9 mm (total acquisition time of 17 min). Data were processed as described previously [15]. Peak areas were calculated by numerical integration. Choline + polyamines + creatine/citrate ratios were calculated for all diagnostic voxels.
Endorectal MRI/MRSI data interpretation
MRI examinations were prospectively interpreted by two radiologists who had > 10 and > 5 years of experience, respectively, of reading prostate MRI. The readers were blinded to clinical data and surgical pathology findings and used established criteria for identifying PCa in the peripheral zone and the transition zone [16]. Tumour volumes were estimated using a picture archiving and communications system [13]. MRI is limited in predicting exact tumour volumes as a result of confounding factors [17,18]. MRI readings were scored using a scale of 0–3, as used for the previously published MRI nomogram model [13]. Scores are defined in Table 1.
TABLE 1.
Magnetic resonance scoring systems
| Score | MRI | MRI/MRSI |
|---|---|---|
| 0 – definitely insignificant PCa |
No regions with abnormal T2W signal |
No abnormality on MRI or no suspicious volume on MRSI |
| 1 – Probably insignificant PCa |
Non-nodular decreased T2W signal < 0.5 cm3 |
Total combined MRI and MRSI suspicious volume < 0.5 cm3 |
| 2 – Indeterminate | Non-nodular reduced T2W signal > 0.5 cm3 or nodular < 0.5 cm3 |
Total combined MRI and MRSI suspicious volume ≈0.5 cm3 |
| 3 – Definitely significant PCa |
Nodular reduced T2W signal > 0.5 cm3 |
Combined MRI and MRSI suspicious volume > 0.5 cm3 |
MRSI, magnetic resonance spectroscopic imaging; PCa, prostate cancer; T2W, T2-weighted.
MRSI data were interpreted with the consensus of two spectroscopists who had > 5 years of experience reading prostate MRSI, using previously established metabolic criteria [13] and without reference to the MRI findings or knowledge of the clinical and pathology results. MRSI tumour volumes were estimated by multiplying the voxel size by the number of suspicious voxels. Using the MRSI results, the two radiologists each assigned an overall score for the probability of insignificant PCa on MRI/MRSI using the previously published scale [13] of 0–3 (Table 1). For statistical analysis, MRI or MRI/MRSI scores were included as nominal numbers to reflect the increasing likelihood of having significant disease as the scores increase.
Pathology
Insignificant PCa was defined as tumour confined to the prostate, with a total tumour volume ≤ 0.5 cm3 and no elements of Gleason grade 4 or 5. The volumes (cm3) of individual tumour foci were calculated using computerized planimetry with image analysis and Image-Pro Plus measurement software (version 5.0.0.39; Media Cybernetics, Inc., Bethesda, MD, USA) as described previously [13,19]. Whole-mount transverse serial sections of the prostate were prepared as previously described [13,19].
Statistical analysis
Nomogram models validated
In total, four nomogram models for predicting the probability of insignificant PCa were validated. Of these models, two were created by Kattan et al. [10] containing only clinical variables: a ‘base’ model and a more comprehensive ‘medium’ model incorporating %BC+. The other two were MRI and MRI/MRSI models [13], which combined clinical variables and %BC+.with MRI and MRI/MRSI findings, respectively. Henceforth, the term ‘medium’ will be applied to all models that include %BC+. Thus, the previously published MRI and MRI/MRSI models will be referred to as the mediumMRI and mediumMRI/MRSI models, respectively. Table 2 lists the variables included in each model.
TABLE 2.
Variables used in nomogram models
| Variable | PSA level |
Clinic al stage |
GG 1 | GG 2 | %BC+ | MRI prost ate volu me |
MRI sco re |
MRI/MRSI score |
|---|---|---|---|---|---|---|---|---|
| Base† | X | X | X | X | – | – | – | – |
| Medium† | X | X | X | X | X | X | – | – |
| MediumMRI*† | X | X | – | – | X | X | X | – |
| MediumMRI/MRSI*† | X | X | – | – | X | X | – | X |
| BaseMRI* | X | X | – | – | – | X | X | – |
| BaseMRI/MRSI* | X | X | – | – | – | X | – | X |
GG 1, primary Gleason grade; GG 2, secondary Gleason grade; %BC+, percentage of biopsy cores positive; MRSI, magnetic resonance spectroscopic imaging.
Denotes models for which Gleason grades were not included because all patients used to create the models had Gleason score 6 (3+3) disease.
Denotes models that were validated in the present study.
New nomogram models
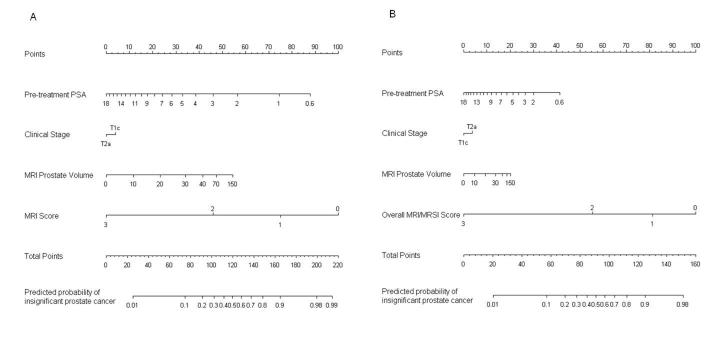
We designed a ‘baseMRI’ model (Fig. 1A) and a ‘baseMRI/MRSI’ model, neither of which included %BC+ (Fig. 1B and Table 2). These nomograms were created using data from the 181 patients who were part of the prospective NIH study described above. Biopsy Gleason grades were omitted because all of the patients had Gleason grade 3+3 cancer at biopsy [13].
FIG. 1.
BaseMRI (A) and baseMRI/magnetic resonance spectroscopic imaging (MRSI) (B) nomograms for predicting the probability of insignificant prostate cancer. Instructions: locate patient’s pre-treatment PSA level on ‘Pre-treatment PSA’ axis, then draw a perpendicular line to the ‘Points’ axis to determine associated points. Repeat for all remaining variables. Locate sum on ‘Total Points’ axis. Draw a perpendicular line down to find predicted probability.
Analyses
We calculated the predicted probabilities of insignificant PCa for each patient based on the six models; these probabilities were then utilized to quantify the discrimination ability of the nomogram models by calculating the concordance index, a measurement equivalent to the non-parametric area under the receiver–operator characteristic curve. The index shows the probability that, in a randomly selected pair of patients, the patient with insignificant disease will be assigned a higher risk of insignificant cancer than the patient without it. The scale ranges from 0.5 (no discrimination) to 1 (perfect discrimination). To visually inspect the calibration, we plotted the predictions on the x-axis and the observed outcomes on the y-axis in a calibration plot. In the plot, a 45° line indicates the ideal, where the predicted and observed outcomes correspond perfectly.
Differences between models were examined using bootstrapping with 1000 resamples. All calculations and tests were separately conducted for the two radiologists. P < 0.05 was considered statistically significant. All analyses were performed using the open source software R, version 2.10.0 (R Foundation for Statistical Computing, Vienna, Austria) with the Design, Hmisc, boot and ROCR libraries [20].
Results
Table 3 summarizes the patients’ clinical characteristics. At pathology, 27% of patients had insignificant PCa. For 56.4% of patients, the Gleason score was higher at surgical pathology than at biopsy (Table 4). The median number (range) of biopsy cores obtained per patient was 14 (4–27) (Table 5). There were 91 (50%) patients who had 12–14 biopsy cores (Table 5). At our institution, 31 (17%) patients had repeat biopsy. The medium clinical model performed significantly better than the base clinical model (Table 6) (P = 0.001) [13].
TABLE 3.
Predictive variables in the present study cohort stratified by the status of the cancer in the radical prostatectomy specimen.
| Status of cancer in RP specimen |
||
|---|---|---|
| Predictive variables | Insignificant | Significant |
| Number of patients (%) | 49 (27) | 132 (73) |
| Clinical (T) stage, n (%) | ||
| T1c | 43 (88) | 115 (85) |
| T2a | 6 (12) | 20 (15) |
| PSA level (ng/mL) | ||
| Mean (range) | 4.1 (0.5–8.0) | 4.6 (0.5–11.7) |
| Median (IQR) | 4.1 (2.8–5.4) | 4.4 (3.4–5.5) |
| MRI prostate volume (cm3) | ||
| Mean (range) | 45.1 (13.0–102.0) | 35.8 (11.0–186.9) |
| Median (IQR) | 39.3 (27.0–56.9) | 30 (23.4–42.0) |
| %BC+ | ||
| Mean (range) | 15.8 (4.2–50.0) | 23.0 (5.0–84.6) |
| Median (IQR) | 12.5 (8.3–21.6) | 17.7 (10.0–28.6) |
The biopsy Gleason score in all patients was 6. RP, radical prostatectomy; IQR, interquartile range; %BC+, percentage of biopsy cores positive.
TABLE 4.
Gleason score in the biopsy specimens and in the prostatectomy specimens
| Gleason score | Biopsy, n (%) | Prostatectomy, n (%)* |
|---|---|---|
| 3+3 | 181 (100) | 79 (43.6) |
| 3+4 | 0 | 92 (50.8) |
| 4+3 | 0 | 8 (4.4) |
| 4+4 | 0 | 1 (0.6) |
| 4+5 | 0 | 1 (0.6) |
| Total | 181 | 181 |
Compared to the Gleason score in the biopsy specimen, the score in the prostatectomy specimen was unchanged in 43.6% and higher in 56.4%.
TABLE 5.
Number of biopsy cores obtained
| Number of patients | Total number of biopsy cores |
|---|---|
| 1 | 4 |
| 2 | 5 |
| 4 | 6 |
| 8 | 7 |
| 16 | 8 |
| 6 | 9 |
| 15 | 10 |
| 9 | 11 |
| 54 | 12 |
| 20 | 13 |
| 17 | 14 |
| 7 | 15 |
| 4 | 16 |
| 4 | 17 |
| 5 | 18 |
| 2 | 19 |
| 2 | 20 |
| 1 | 21 |
| 1 | 23 |
| 1 | 24 |
| 1 | 25 |
| 1 | 27 |
TABLE 6.
Concordance indices indicating the accuracy levels of the models in predicting insignificant prostate cancer, listed in ascending order
| Models | Concordance index |
|---|---|
| Base model | 0.5582 |
| BaseMRI/MRSI model with reader 2 | 0.6619 |
| BaseMRI model with reader 2 | 0.6988 |
| MediumMRI/MRSI model with reader 2 | 0.7032 |
| Medium model | 0.7069 |
| MediumMRI model with reader 2 | 0.7329 |
| BaseMRI model with reader 1 | 0.7413 |
| BaseMRI/MRSI model with reader 1 | 0.7525 |
| MediumMRI model with reader 1 | 0.7618 |
| MediumMRI/MRSI model with reader 1 | 0.7727 |
MRSI, magnetic resonance spectroscopic imaging.
All magnetic resonance models showed good calibration for reader 1 and minor overfitting for reader 2 (Fig. 2). For reader 1, all four magnetic resonance models were more accurate than the base model (P ≤ 0.001) (Figs 3A and 4A and Table 6) but performed similarly to the medium model (P ≥ 0.065) (Figs 3A and 4A and Tables 6 and 7). For reader 2, all the magnetic resonance models were significantly more accurate than the base model (P < 0.05) but performed similarly to the medium model (P ≥ 0.342) (Figs 3B and 4B and Tables 6 and 7).
FIG. 2.
Calibration plots of models for (A) reader 1 and (B) reader 2. The y-axis represents the incidence rate for insignificant prostate cancer in the present study. The 45° solid line represents perfect prediction. Vertical bars above the x-axis indicate the relative frequency of model-predicted cancer probabilities in the validation cohort. MRSI, magnetic resonance spectroscopic imaging.
FIG. 3.
Receiver–operator characteristic (ROC) curves and areas under ROC curves (AUCs) for the base and medium clinical nomogram models and the baseMRI and mediumMRI nomogram models for (A) reader 1 and (B) reader 2.
FIG. 4.
A) Receiver–operator characteristic (ROC) curves and areas under ROC curves (AUCs) for the base and medium nomogram models and the baseMRI/MRSI and mediumMRI/magnetic resonance spectroscopic imaging (MRSI) models for (A) reader 1 and (B) reader 2.
TABLE 7.
P-values for the differences in the accuracy levels of the models in predicting the probability of insignificant prostate cancer
| Models compared | Reader 1 | Reader 2 |
|---|---|---|
| Base model vs medium model | 0.001 | 0.001 |
| Base model vs baseMRI model | < 0.001 | 0.014 |
| Base model vs baseMRI/MRSI model | 0.001 | 0.040 |
| Base model vs mediumMRI model | < 0.001 | 0.001 |
| Base model vs mediumMRI/MRSI model | < 0.001 | 0.007 |
| Medium model vs baseMRI model | 0.423 | 0.875 |
| Medium model vs baseMRI/MRSI model | 0.269 | 0.342 |
| Medium model vs mediumMRI model | 0.146 | 0.487 |
| Medium model vs mediumMRI/MRSI model | 0.065 | 0.905 |
MRSI, magnetic resonance spectroscopic imaging.
Magnetic resonance data for both readers was helpful in predicting significant PCa (Tables 8 and 9). For reader 1, 63 of 90 (70%) and, for reader 2, 69 of 106 (65%) patients with an MRI score of 3 (definitely significant) had a tumour volume > 0.5 cm3. MRI score 2 (indeterminate) was non-specific: Of 82 patients who received a score of 2 from reader 1, 52 (63%) had tumour volumes < 0.5 cm3 and 30 (37%) had tumour volumes > 0.5 cm3. Of 62 patients who received a score of 2 from reader 2, 37 (60%) had tumour volumes < 0.5 cm3 and 25 (40%) had tumour volumes > 0.5 cm3. For reader 1, six of nine (67%) patients with an MRI score of 0 (definitely insignificant) or 1 had a tumour volume < 0.5 cm3 and five of nine (56%) had insignificant cancer. For reader 2, 11 of 13 (85%) patients with an MRI score of 0 or 1 had a tumour volume < 0.5 cm3 and eight of 13 (61%) had insignificant cancer. Reader 1 misclassified one and reader 2 misclassified three pathologically significant cancers with a volume < 0.5 cm3 as being definitely or probably insignificant by MRI because the Gleason scores had been underestimated at biopsy (Tables 8 and 9).
TABLE 8.
Patients with insignificant or significant cancer in the prostatectomy specimen stratified by MRI and MRI/MRSI scores for reader 1
| MRI/MRSI score* |
|||||||
|---|---|---|---|---|---|---|---|
| 0–1 | 2 | 3 | Total | ||||
| Significance at pathology |
Tumour volume at pathology |
Surgical Gleason score |
MRI score |
||||
| Insignificant | < 0.5 | 6 | 0–1 | 5 | 0 | 0 | 5 |
| 2 | 1 | 30 | 2 | 33 | |||
| 3 | 0 | 0 | 11 | 11 | |||
| Total | 6 | 30 | 13 | 49 | |||
| significant | < 0.5 | > 6 | 0–1 | 1 | 0 | 0 | 1 |
| 2 | 0 | 17 | 2 | 19 | |||
| 3 | 0 | 0 | 16 | 16 | |||
| Total | 1 | 17 | 18 | 36 | |||
| significant | ≥ 0.5 | 6 | 0–1 | 0 | 1 | 0 | 1 |
| 2 | 0 | 10 | 3 | 13 | |||
| 3 | 0 | 0 | 16 | 16 | |||
| Total | 0 | 11 | 19 | 30 | |||
| significant | ≥ 0.5 | > 6 | 0–1 | 1 | 0 | 1 | 2 |
| 2 | 0 | 11 | 6 | 17 | |||
| 3 | 0 | 0 | 47 | 47 | |||
| Total | 1 | 11 | 54 | 66 | |||
Data in MRI/MRSI score column represent numbers of patients. MRSI, magnetic resonance spectroscopic imaging.
TABLE 9.
Patients with insignificant or significant cancer in the prostatectomy specimen stratified by MRI and MRI/MRSI scores for Reader 2
| MRI/MRSI score |
|||||||
|---|---|---|---|---|---|---|---|
| 0–1 | 2 | 3 | Total | ||||
| Significance at pathology |
Tumour volume at pathology |
Surgical Gleason score |
MRI score |
||||
| Insignificant | < 0.5 | 6 | 0–1 | 8 | 0 | 0 | 8 |
| 2 | 0 | 17 | 4 | 21 | |||
| 3 | 0 | 0 | 20 | 20 | |||
| Total | 8 | 17 | 24 | 49 | |||
| significant | < 0.5 | > 6 | 0–1 | 3 | 0 | 0 | 3 |
| 2 | 1 | 12 | 3 | 16 | |||
| 3 | 0 | 0 | 17 | 17 | |||
| Total | 4 | 12 | 20 | 36 | |||
| significant | ≥ 0.5 | 6 | 0–1 | 2 | 0 | 0 | 2 |
| 2 | 0 | 9 | 1 | 10 | |||
| 3 | 0 | 0 | 18 | 18 | |||
| Total | 2 | 9 | 19 | 30 | |||
| significant | ≥ 0.5 | > 6 | 0–1 | 0 | 0 | 0 | 0 |
| 2 | 0 | 15 | 0 | 15 | |||
| 3 | 0 | 0 | 51 | 51 | |||
| Total | 0 | 15 | 51 | 66 | |||
Data in MRI/MRSI score column represent numbers of patients. MRSI, magnetic resonance spectroscopic imaging.
After the addition of MRSI to MRI, reader 1 changed the imaging score for 13 patients and reader 2 changed the imaging score for eight patients from indeterminate to significant cancer; the change was correct in 11 of 13 patients for reader 1 and foru of eight patients for reader 2. MRSI increased uncertainty in only one patient for reader 1 and no patients for reader 2. Overall, the addition of MRSI improved predictive accuracy only marginally for reader 1 and not at all for reader 2 (Fig. 4 and Table 7.
Discussion
Nomograms validated
We successfully validated our previously published nomogram models incorporating magnetic resonance data, clinical data and %BC+ for the prediction of insignificant PCa (all patients had a biopsy Gleason score of 6) [13]. As in our previous study, these models performed significantly better than the clinical base model. However, they did not perform significantly better than the clinical medium model, indicating that %BC+ is an important predictive variable.
The area under the receiver–operator characteristic curve values for the above-mentioned MR-inclusive models (mediumMRI model, 0.7618; mediumMRI/MRSI model, 0.7727) were slightly lower than those reported in the previously published study (0.803 and 0.854, respectively) for reader 1, who was the only reader in the previous study. The discrepancy could be a result of the magnetic resonance readings being performed prospectively rather than retrospectively in the present study. In addition, the present study included patients with PSA levels < 10 ng/mL, whereas the previous study included patients with PSA levels < 20 ng/mL [13].
New nomograms without %BC+
The baseMRI and baseMRI/MRSI nomogram models without %BC+ performed significantly better than the clinical base model and similarly to all medium models. Therefore, these new MR-based models for predicting insignificant PCa could be used for patients considering active surveillance who do not have %BC+ in their biopsy reports, obviating the need for repeat biopsy for risk assessment, which was performed in 17% of patients in the present study.
TRUS-guided biopsy is a standard diagnostic procedure. Nevertheless, it is limited by random and systematic sampling errors and variations in the reporting of results [12]. The interpretation of biopsy results is also subject to interobserver variability [21–23]. In the present study, the biopsy Gleason score was upgraded in more than half of all patients at surgical pathology. The number of biopsy cores obtained varies considerably and is in the range 4–27 in the present study. Furthermore, fragmentation of biopsy cores may skew the interpretation of biopsy results, including the number and percentage of biopsy cores positive [24,25]. Biopsy can lead to complications and has been associated with mortality [26], although the causality of this association has not been confirmed.
The interpretation of MRI/MRSI is also subject to inter-reader variability and is affected by reader experience [27]. However, MRI is increasingly being used in clinical practice to evaluate PCa stage and aid in treatment planning. There is substantial evidence that MRI and MRSI can help with localization and assessment of the extent of tumour [13,16,28]. In one study, 33 of 158 patients with clinical stage T1c PCa (21%) who underwent MRI/MRSI before surgery [29] had a pathological stage of T3a or higher. It was shown that underestimation of tumour stage could be minimized by performing non-invasive MRI/MRSI [29].
In the present study, MRI and MRI/MRSI performed better with respect to identifying significant rather than insignificant disease. Hence, the nomogram models incorporating magnetic resonance findings may show that aggressive therapy is warranted in certain men whose disease would otherwise appear to be low risk. The MR-inclusive models could be used in medical centres where MRI of the prostate is used routinely and no added costs are involved.
The magnetic resonance nomograms, similar to the clinical nomograms, are limited by their inability to predict insignificant PCa with high probability. The next logical step for improving the prediction of insignificant PCa would be to add dynamic contrast-enhanced MRI and diffusion-weighted MRI to the MRI/MRSI examination. Both dynamic contrast-enhanced MRI and diffusion-weighted MRI have been shown to add significant incremental value to conventional MRI results with respect to PCa detection and staging [30–33].
In conclusion, we have successfully validated previously published nomogram models incorporating magnetic resonance data, clinical data and %BC+ for predicting the probability of insignificant PCa in patients with clinically low-risk disease. Those models, as well as magnetic resonance models without %BC+, performed significantly better than the base clinical nomogram model.
Acknowledgements
The authors thank Ada Muellner, MS, for editing the manuscript.
Conflict of interest This study was funded by NIH grant #R01 CA76423. The NIH did not take any part in the study design, data collection data analysis, manuscript preparation or publication decisions.
Abbreviations
- %BC+
percentage of biopsy cores positive
- MRSI
magnetic resonance spectroscopic imaging
- NIH
National Institutes of Health
- PCa
prostate cancer
References
- 1.Cooperberg MR, Lubeck DP, Meng MV, Mehta SS, Carroll PR. The changing face of low-risk prostate cancer: trends in clinical presentation and primary management. J Clin Oncol. 2004;22:2141–9. doi: 10.1200/JCO.2004.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Draisma G, Boer R, Otto SJ, et al. Lead times and overdetection due to prostate-specific antigen screening: estimates from the European Randomized Study of Screening for Prostate Cancer. J Natl Cancer Inst. 2003;95:868–78. doi: 10.1093/jnci/95.12.868. [DOI] [PubMed] [Google Scholar]
- 3.Etzioni R, Penson DF, Legler JM, et al. Overdiagnosis due to prostate-specific antigen screening: lessons from U.S. prostate cancer incidence trends. J Natl Cancer Inst. 2002;94:981–90. doi: 10.1093/jnci/94.13.981. [DOI] [PubMed] [Google Scholar]
- 4.Epstein JI, Walsh PC, Carmichael M, Brendler CB. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994;271:368–74. [PubMed] [Google Scholar]
- 5.Harnden P, Naylor B, Shelley MD, Clements H, Coles B, Mason MD. The clinical management of patients with a small volume of prostatic cancer on biopsy: what are the risks of progression? A systematic review and meta-analysis. Cancer. 2008;112:971–81. doi: 10.1002/cncr.23277. [DOI] [PubMed] [Google Scholar]
- 6.Di Blasio CJ, Rhee AC, Cho D, Scardino PT, Kattan MW. Predicting clinical end points: treatment nomograms in prostate cancer. Semin Oncol. 2003;30:567–86. doi: 10.1016/s0093-7754(03)00351-8. [DOI] [PubMed] [Google Scholar]
- 7.Kattan MW, Marasco J. What is a real nomogram? Semin Oncol. 2010;37:23–6. doi: 10.1053/j.seminoncol.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Carter HB, Sauvageot J, Walsh PC, Epstein JI. Prospective evaluation of men with stage T1C adenocarcinoma of the prostate. J Urol. 1997;157:2206–9. [PMC free article] [PubMed] [Google Scholar]
- 9.Epstein JI, Sanderson H, Carter HB, Scharfstein DO. Utility of saturation biopsy to predict insignificant cancer at radical prostatectomy. Urology. 2005;66:356–60. doi: 10.1016/j.urology.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Kattan MW, Eastham JA, Wheeler TM, et al. Counseling men with prostate cancer: a nomogram for predicting the presence of small, moderately differentiated, confined tumors. J Urol. 2003;170:1792–7. doi: 10.1097/01.ju.0000091806.70171.41. [DOI] [PubMed] [Google Scholar]
- 11.Dong F, Kattan MW, Steyerberg EW, et al. Validation of pretreatment nomograms for predicting indolent prostate cancer: efficacy in contemporary urological practice. J Urol. 2008;180:150–4. doi: 10.1016/j.juro.2008.03.053. [DOI] [PubMed] [Google Scholar]
- 12.Iczkowski KA, Bostwick DG. Sampling, submission, and report format for multiple prostate biopsies: a 1999 survey. Urology. 2000;55:568–71. doi: 10.1016/s0090-4295(99)00558-0. [DOI] [PubMed] [Google Scholar]
- 13.Shukla-Dave A, Hricak H, Kattan MW, et al. The utility of magnetic resonance imaging and spectroscopy for predicting insignificant prostate cancer: an initial analysis. BJU Int. 2007;99:786–93. doi: 10.1111/j.1464-410X.2007.06689.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Hricak H, Kattan MW, Chen HN, Scardino PT, Kuroiwa K. Prediction of organ-confined prostate cancer: incremental value of MR imaging and MR spectroscopic imaging to staging nomograms. Radiology. 2006;238:597–603. doi: 10.1148/radiol.2382041905. [DOI] [PubMed] [Google Scholar]
- 15.Mazaheri Y, Shukla-Dave A, Hricak H, et al. Prostate cancer: identification with combined diffusion-weighted MR imaging and 3D 1H MR spectroscopic imaging – correlation with pathologic findings. Radiology. 2008;246:480–8. doi: 10.1148/radiol.2462070368. [DOI] [PubMed] [Google Scholar]
- 16.Verma S, Rajesh A, Futterer JJ, et al. Prostate MRI and 3D MR spectroscopy: how we do it. AJR Am J Roentgenol. 2010;194:1414–26. doi: 10.2214/AJR.10.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qayyum A, Coakley FV, Lu Y, et al. Organ-confined prostate cancer: effect of prior transrectal biopsy on endorectal MRI and MR spectroscopic imaging. AJR Am J Roentgenol. 2004;183:1079–83. doi: 10.2214/ajr.183.4.1831079. [DOI] [PubMed] [Google Scholar]
- 18.Shukla-Dave A, Hricak H, Eberhardt SC, et al. Chronic prostatitis: MR imaging and 1H MR spectroscopic imaging findings – initial observations. Radiology. 2004;231:717–24. doi: 10.1148/radiol.2313031391. [DOI] [PubMed] [Google Scholar]
- 19.Ohori M, Wheeler TM, Dunn JK, Stamey TA, Scardino PT. The pathological features and prognosis of prostate cancer detectable with current diagnostic tests. J Urol. 1994;152:1714–20. doi: 10.1016/s0022-5347(17)32369-8. [DOI] [PubMed] [Google Scholar]
- 20.Harrell FE., Jr . Regression Modeling Strategies with Applications to Linear Models, Logistic Regression, and Survival Analysis. Springer Verlag; New York, NY: 2001. p. 568. [Google Scholar]
- 21.Carlson GD, Calvanese CB, Kahane H, Epstein JI. Accuracy of biopsy Gleason scores from a large uropathology laboratory: use of a diagnostic protocol to minimize observer variability. Urology. 1998;51:525–9. doi: 10.1016/s0090-4295(98)00002-8. [DOI] [PubMed] [Google Scholar]
- 22.di Loreto C, Fitzpatrick B, Underhill S, et al. Correlation between visual clues, objective architectural features, and interobserver agreement in prostate cancer. Am J Clin Pathol. 1991;96:70–5. doi: 10.1093/ajcp/96.1.70. [DOI] [PubMed] [Google Scholar]
- 23.Gleason DF. Histologic grading of prostate cancer: a perspective. Hum Pathol. 1992;23:273–9. doi: 10.1016/0046-8177(92)90108-f. [DOI] [PubMed] [Google Scholar]
- 24.Fajardo DA, Epstein JI. Fragmentation of prostatic needle biopsy cores containing adenocarcinoma: the role of specimen submission. BJU Int. 2010;105:172–5. doi: 10.1111/j.1464-410X.2009.08737.x. [DOI] [PubMed] [Google Scholar]
- 25.Reis LO, Reinato JA, Silva DC, Matheus WE, Denardi F, Ferreira U. The impact of core biopsy fragmentation in prostate cancer. Int Urol Nephrol. 2010;42:965–9. doi: 10.1007/s11255-010-9720-0. [DOI] [PubMed] [Google Scholar]
- 26.Gallina A, Suardi N, Montorsi F, et al. Mortality at 120 days after prostatic biopsy: a population-based study of 22,175 men. Int J Cancer. 2008;123:647–52. doi: 10.1002/ijc.23559. [DOI] [PubMed] [Google Scholar]
- 27.Mullerad M, Hricak H, Wang L, Chen HN, Kattan MW, Scardino PT. Prostate cancer: detection of extracapsular extension by genitourinary and general body radiologists at MR imaging. Radiology. 2004;232:140–6. doi: 10.1148/radiol.2321031254. [DOI] [PubMed] [Google Scholar]
- 28.Zakian KL, Sircar K, Hricak H, et al. Correlation of proton MR spectroscopic imaging with gleason score based on step-section pathologic analysis after radical prostatectomy. Radiology. 2005;234:804–14. doi: 10.1148/radiol.2343040363. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, Hricak H, Shukla-Dave A, et al. Clinical stage T1c prostate cancer: evaluation with endorectal MR imaging and MR spectroscopic imaging. Radiology. 2009;253:425–34. doi: 10.1148/radiol.2532081390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurhanewicz J, Vigneron D, Carroll P, Coakley F. Multiparametric magnetic resonance imaging in prostate cancer: present and future. Curr Opin Urol. 2008;18:71–7. doi: 10.1097/MOU.0b013e3282f19d01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mazaheri Y, Shukla-Dave A, Muellner A, Hricak H. MR imaging of the prostate in clinical practice. MAGMA. 2008;21:379–92. doi: 10.1007/s10334-008-0138-y. [DOI] [PubMed] [Google Scholar]
- 32.Turkbey B, Shah VP, Pang Y, et al. Is apparent diffusion coefficient associated with clinical risk scores for prostate cancers that are visible on 3-T MR images? Radiology. 2011;258:488–95. doi: 10.1148/radiol.10100667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vargas HA, Akin O, Franiel T, et al. Diffusion-weighted endorectal MR imaging at 3 T for prostate cancer: tumor detection and assessment of aggressiveness. Radiology. 2011;259:775–84. doi: 10.1148/radiol.11102066. [DOI] [PMC free article] [PubMed] [Google Scholar]