Abstract
Background
Rapid sequence induction and intubation (RSII) is a technique commonly used to resist regurgitation of gastric contents and protect the airway. A modification of this technique is implemented in certain clinical circumstances. However, there is currently no standard definition for a “modified RSII.” Therefore, we surveyed clinicians at academic centers across the United States to establish a working definition of a “modified RSII” as well as the clinical scenarios in which it is being used.
Methods
A survey was created that queried the use and definition of modified RSII, and validated with test respondents. We then mailed the survey to all 131 anesthesia residency training programs across the United States. Logistic regression models were created to estimate the percentage of affirmative responses among respondents that performed modified RSII procedures and answered survey items in a consistent manner. Similar quantities were calculated by physician status (resident and attending).
Results
Four hundred ninety surveys were received from 58 institutions (44% institution response rate), 93% of respondents reported using a modified RSII, and of those 85% consistently completed the survey instrument. A majority of respondents (71%, CI: 63–77%) reported administering oxygen before anesthesia induction, applying cricoid pressure, and attempting to ventilate the lungs via a facemask before securing the airway. Respondents noted that they would use a modified RSII procedure if the patient were either moderately or morbidly obese (each ~59%, 53–64%), had a history but no current symptoms of gastroesophageal reflux disease (52%, 46–57%), had a hiatal hernia (42%, 36–48%) or were a trauma patient who had been NPO for at least 8 hours (39%, 33–45%). Similar RSII results were obtained when repeating the analysis on the subset that did not enforce the consistency requirements.
Conclusions
Based on our survey we have established three defining features of a modified RSII: (1) oxygen administration before induction; (2) the use of cricoid pressure; and (3) an attempt to ventilate the patient’s lungs before securing the airway. Although this definition seems intuitively obvious, no previous work has tested whether it is commonly accepted.
Introduction
Rapid sequence induction and intubation (RSII) is a technique commonly used to protect the airway from aspiration of gastric contents by minimizing the likelihood of regurgitation during induction of anesthesia. Components of a classic RSII consist of oxygen administration, application of cricoid pressure, and the avoidance of mask ventilation before inserting an endotracheal tube to secure the airway.1–4
In certain clinical circumstances, a modified technique is implemented in an attempt to optimize patient outcomes and reduce excess risk exposure. Potential variations between a classic RSII and modified RSII may include: (1) selection of a different neuromuscular blocking drug (NMBD); (2) timing of NMBD administration in relation to induction; (3) use of positive pressure ventilation before securing the airway; (4) timing of cricoid pressure application; and (5) use of opioids and anxiolytics as premedication. In our experience, the most common deviation from a classic RSII has typically included the establishment of mask ventilation after induction, but before administration of a NMBD.
The only previous study regarding the use of modified RSII surveyed only a limited group of Certified Registered Nurse Anesthetists (CRNAs) from one hospital and therefore cannot be easily generalized.3 Therefore, because of the lack of a standard and widely accepted definition for an RSII in the literature1–4 or in any major anesthesiology textbook 5–7, we surveyed both attending physicians and residents from across the United States to establish the characteristic features of a modified RSII and to find the important distinctions in actual practice between a classic and modified RSII. We wanted to determine those characteristics of a modified RSII that a majority of participants report using, as well as the clinical scenarios in which it is being used. Having a standard definition for this term will help improve communication among providers about induction strategies.
Materials and Methods
This study was reviewed and approved by the Human Research Committee of the Massachusetts General Hospital (Boston, MA).
Instruments
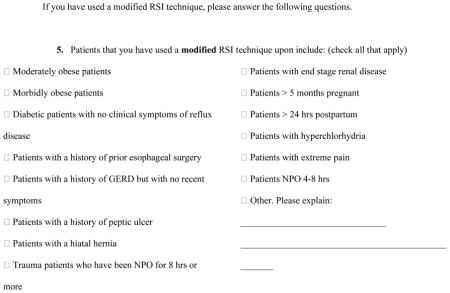
The survey we used contained four sections: (1) a demographic section, containing questions about the participant’s institution, the participant’s status in the institution (resident versus attending physician), and the participant’s length of practice (PGY status for residents and number of years in practice for attending physicians); (2) a section in which participants were asked to indicate how often they used RSII and how often they used modified RSII (choosing from never, rarely, occasionally, often or always); (3) a section in which participants were asked to select in which scenarios (of 15 options, including “other”) they have used a modified RSII technique; and (4) a section that included questions regarding how the participant would perform a modified RSII. The survey is reproduced in its entirety in the Appendix.
Procedures
We used FRIEDA online (the American Medical Association’s database of graduate medical education programs and combined specialty programs accredited by the Accreditation Council for Graduate Medical Education) to compile a list of all 131 anesthesia residency programs across the United States. We sent ten survey packets to the residency program director at each of these 131 programs. Each survey packet included a short cover letter describing the study and instructing the program director to distribute the survey to five residents and five attending physicians. The program director was given the option of completing one of the five attending surveys. We left it to the discretion of the program director to decide which residents and which attending physicians should complete the survey. Based on our survey validation, the survey required no more than 15 minutes to complete. The only identifying information on the survey was the specific hospital with which the participant was affiliated. Subjects were eligible to participate only if they were current anesthesiology residents or current attending anesthesiologists. Consent to participate was implied by return of the survey. Approximately 30 days after the initial mailing, we sent a follow-up email to the program director of each institution who had not yet returned a survey packet. The consistency of survey responses was assessed by comparing an item’s response patterns with its instructions and by comparing responses of gate questions with corresponding follow-up questions. Two categories of possible inconsistencies included: 1) a respondent selected more than one response for a question that asked for only one; and 2) a gate question was answered in such a way that the follow-up question should or should not have been completed. Six consistency checks were developed and responses were flagged for further analysis if any of these were violated.
Statistical Methods
Respondent characteristics, RSII usage and reasons for using the modified RSII procedure were tabulated across the entire data set and by status (resident versus attending). Categorical variables were represented as percentages and counts while continuous variables were summarized by the 10, 25, 50 (median), 75 and 90th percentiles. Separate intercept-only logistic regression models were fit to estimate these “overall” proportions while additional models that accounted for status were also constructed to directly estimate and test whether any RSII or modified RSII usage differed between residents and attending physicians. Model parameters were estimated using generalized estimating equations with an independence or diagonal working covariance structure along with robust standard errors to account for within institution correlations. Originally on the log-odds scale, these estimates and their confidence intervals were transformed to and reported on the probability scale.
In order to establish a working definition of a “modified RSII,” we examined which of the components of a classic RSII are also used during the modified technique. The components of interest included: (1) oxygen administration before induction; (2) application of cricoid pressure; (3) ventilation of the lungs using a facemask; and (4) administration of pharmacologic aspiration prophylaxis. Since these items were likely to be used in tandem, a series of new variables was derived that summarized all joint 2-, 3- and 4-way combinations. Proportions of respondents using each of these were calculated and summarized in a similar manner to that which was described previously. Status comparisons were assessed by computing the odds ratio (OR) and the Wald test comparing this odds ratio to the null (OR=1). All analyses were performed using R version 2.11.1.*
Results
We received 490 surveys from 58 institutions (44% institution response rate over a 3.5 month period); 231 from residents (47%) and 259 from attending physicians (53%). The average response rate within each institution was 84%. Table 1 describes the demographic information and the use of both the classic and modified RSII procedure of these respondents. Similar summaries were generated on additional subsets of interest including: 1) those respondents who at least rarely performed modified RSII procedures (n=454); 2) those who also consistently completed all of the items that pertained to modified definitions (Appendix, Items 8–11, n=430); and 3) those who also completed the follow-up items related to the modified definitions (Items 8–11 and all sub-items, n=387). To determine if the assumption that the data issues (either missing data or inconsistencies) occurred completely at random, all analyses were performed using subsets #2 and #3 which omitted roughly 5 and 15% of the sample, respectively. Similar results were obtained using both subsets and thus the following summaries correspond to the subset that applied the most stringent exclusion criteria.
Table 1.
Respondent demographics and rapid sequence induction (RSI) usage by analysis subset. Categorical variables are summarized as percentages and counts (if missing data were present, then denominators are also provided) while continuous variables are characterized by the 10, 25, 50 (median), 75 and 90th percentiles.
| All | Modified RSI | Modified RSI w/gate restrictions* | Modified RSI w/gate and follow-up restrictions* | |
|---|---|---|---|---|
| N = 490 | N = 454 | N = 430 | N = 387 | |
| Status | ||||
| Resident | 47.1% (231) | 46.3% (210) | 45.1% (194) | 45.2% (175) |
| Training (years) | 2, 3, 4, 12.75, 23 | 2, 3, 4, 13, 24 | 2, 3, 4, 13, 24 | 2, 3, 4, 13, 24 |
| Currently use RSI | ||||
| ≥ Occasionally | 90.4% (442/489) | 90.5% (411) | 90.5% (389) | 90.2% (349) |
| Currently use Modified RSI | ||||
| ≥ Occasionally | 77.0% (374/486) | 82.4% (374) | 82.1% (353) | 81.4% (315) |
Gate restrictions correspond to survey items 8–11 (Appendix), while gate and follow-up restrictions correspond to both survey items 8–11 along with the additional items related to each item. For example, the gate question for Item 9 is “In preparation for a modified RSI, do you usually preoxygenate prior to induction?” while the follow-up items investigate oxygen administration durations.
Table 2 summarizes RSII usage and patient characteristics that influence the use of a modified RSII procedure. Of this subset of respondents, 81% (77–85%) at least occasionally use a modified RSII procedure. Respondents noted that they would use a modified RSII procedure if the patient were either moderately or morbidly obese (each ~59%, 53–64%), had a history but no current symptoms of gastroesophageal reflux disease (52%, 46–57%), had a hiatal hernia (42%, 36–48%) or were a trauma patient who had been fasted (NPO) for at least 8 hours (39%, 33–45%). Succinylcholine (58%, 50–65%) and rocuronium (39%, 31–48%) were the preferred NMBDs and tended to be administered either at the same time as the anesthesia induction drug (45%, 40–51%) or after mask ventilation was attempted (32%, 27–38%).
Table 2.
Rapid sequence induction (RSI) usage and patient characteristics that warrant the modification of the RSI procedure by physician status. Estimates correspond to probabilities and their 95% confidence intervals.
| All | Status | ||
|---|---|---|---|
| N=387 | Resident N=175 |
Attending N= 212 |
|
| Currently use RSI | |||
| ≥ Occasionally | 0.90 (0.87, 0.93) | 0.94 (0.89, 0.97) | 0.87 (0.83, 0.91) |
| Currently use Modified RSI | |||
| ≥ Occasionally | 0.81 (0.77, 0.85) | 0.78 (0.71, 0.83) | 0.84 (0.79, 0.89) |
| Reasons for Modified RSI: | |||
| Moderately obsese | 0.59 (0.54, 0.64) | 0.57 (0.50, 0.63) | 0.61 (0.55, 0.68) |
| Morbidly obsese | 0.59 (0.53, 0.64) | 0.62 (0.53, 0.69) | 0.56 (0.49, 0.63) |
| Gastroesophageal reflux disease | 0.52 (0.46, 0.57) | 0.53 (0.44, 0.61) | 0.51 (0.44, 0.58) |
| Hiatal hernia | 0.42 (0.36, 0.48) | 0.39 (0.32, 0.46) | 0.44 (0.37, 0.51) |
| Trauma | 0.39 (0.33, 0.45) | 0.35 (0.27, 0.43) | 0.42 (0.35, 0.50) |
| NPO | 0.35 (0.30, 0.41) | 0.34 (0.25, 0.43) | 0.36 (0.30, 0.43) |
| Diabetic | 0.33 (0.28, 0.38) | 0.31 (0.25, 0.38) | 0.34 (0.28, 0.42) |
| Pregnant | 0.30 (0.26, 0.36) | 0.19 (0.14, 0.26) | 0.40 (0.33, 0.47) |
| Prior esophageal surgery | 0.23 (0.20, 0.27) | 0.19 (0.14, 0.25) | 0.27 (0.22, 0.32) |
| Renal disease | 0.21 (0.18, 0.25) | 0.16 (0.11, 0.22) | 0.25 (0.20, 0.31) |
| Postpartum | 0.21 (0.17, 0.25) | 0.10 (0.06, 0.15) | 0.30 (0.25, 0.36) |
| Peptic Ulcer | 0.19 (0.15, 0.23) | 0.19 (0.14, 0.25) | 0.19 (0.14, 0.25) |
| Pain | 0.16 (0.13, 0.20) | 0.10 (0.06, 0.15) | 0.22 (0.16, 0.28) |
| Other | 0.11 (0.08, 0.14) | 0.05 (0.03, 0.09) | 0.15 (0.11, 0.20) |
| Hyperchloryhydria | 0.06 (0.05, 0.09) | 0.03 (0.02, 0.08) | 0.09 (0.06, 0.13) |
| Type of muscle relaxant: | |||
| Vecuronium | 0.06 (0.03, 0.09) | 0.04 (0.02, 0.08) | 0.07 (0.04, 0.13) |
| Cisatracurium | 0.01 (0.00, 0.04) | 0.01 (0.00, 0.05) | 0.01 (0.00, 0.04) |
| Rocuronium | 0.39 (0.31, 0.48) | 0.45 (0.35, 0.54) | 0.35 (0.27, 0.44) |
| Succinylcholine | 0.58 (0.50, 0.65) | 0.54 (0.45, 0.64) | 0.61 (0.52, 0.69) |
| Administration time of muscle relaxant: | |||
| Before induction | 0.03 (0.02, 0.06) | 0.05 (0.02, 0.11) | 0.02 (0.01, 0.05) |
| At same time as induction | 0.45 (0.40, 0.51) | 0.48 (0.39, 0.57) | 0.43 (0.37, 0.51) |
| After lid reflex loss | 0.14 (0.10, 0.18) | 0.12 (0.07, 0.19) | 0.16 (0.11, 0.21) |
| After verbal contact loss | 0.06 (0.04, 0.08) | 0.07 (0.04, 0.11) | 0.05 (0.03, 0.08) |
Modifiable aspects of the RSII procedure are outlined in Tables 3† and 4. All respondents administered oxygen to their patients before anesthesia induction, while 91% (86–94%) applied cricoid pressure, 77% (70–82%) ventilated patients’ lungs with a facemask, and 71% (63–77%) applied both cricoid pressure and ventilated patients’ lungs. Attending physicians within this population had higher odds of using mask ventilation when compared to residents (OR=1.62, 1.09–2.41). Similar associations were observed for applying cricoid pressure and mask ventilation. Oxygen administration before induction generally lasted 3–5 minutes (55%, 51–60%) and differences were not detected by physician status. A majority of the respondents who applied cricoid pressure did so at the same time as the induction drug administration (57%, 52–62%). When compared to residents, attending physicians were less likely to apply cricoid pressure drug induction of anesthesia (OR=0.56, 0.34–0.91), but more likely to apply cricoid pressure after loss of verbal contact (OR=5.78, 1.76–19.0). Mask ventilation generally occurred either before or after an NMBD was administered [41% before (35–48%), 35% after (30–42%)]. The odds that attending physicians ventilated their patients’ lungs before the administration of the NMBD was 0.68 (0.46–1.01) times that of the residents, while it was 2.37 (1.22–4.61) when ventilation was started both before and after NMBD administration.
Table 3.
Modifiable aspects of the rapid sequence induction (RSI) procedure. Estimates correspond to either probabilities and their 95% confidence intervals or the odds ratio comparing attendings to residents (reference group).
| All: p (95% CI) | Status: p (95% CI) | Status Comparison | |||
|---|---|---|---|---|---|
| N=387 | Resident N=175 |
Attending N=212 |
OR (95% CI) | P-value | |
| Oxygenate before induction | 1.00 (---) | 1.00 (---) | 1.00 (---) | --- | --- |
| Apply cricoid pressure | 0.91 (0.86, 0.94) | 0.91 (0.85, 0.95) | 0.90 (0.83, 0.94) | 0.85 (0.41, 1.76) | 0.666 |
| Ventilate patient with a facemask | 0.77 (0.70, 0.82) | 0.72 (0.64, 0.79) | 0.81 (0.74, 0.86) | 1.62 (1.09, 2.41) | 0.017 |
| Apply cricoid pressure and ventilate patient with a facemask | 0.71 (0.63, 0.77) | 0.66 (0.57, 0.74) | 0.75 (0.66, 0.82) | 1.53 (1.02, 2.28) | 0.038 |
Discussion
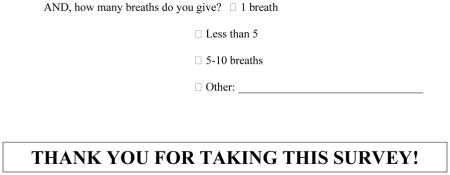
Despite the lack of a consistent definition in the literature or any major anesthesia textbook, 81% (95% CI: 77–85%) of anesthesiologists surveyed reported having used a “modified RSII.” Our results indicate that the three defining factors of a modified RSII include: (1) oxygen administration before anesthesia induction; (2) the use of cricoid pressure; and (3) an attempt to ventilate the patient’s lungs via positive pressure ventilation. A majority of respondents used all three elements. We can also conclude that less than 5 breaths are typically given when attempting to ventilate the lungs via positive-pressure ventilation, and that the majority of respondents who ventilated the lungs did so before administering an NMBD. Most practicing anesthesiologists in our survey reported using a modified RSII technique for at least some of their cases, with moderate or morbid obesity and gastroesophageal reflux disease being the most common medical reason. While our survey did not explicitly ask what differentiated the classic RSII from a modified RSII or how to choose between the two techniques, we constructed and validated the survey to discern this, because each question explicitly stated that it was asking about a modified RSII.
Our results allow us to highlight one important distinction between a classic RSII and a modified RSII; the attempt to ventilate the lungs using positive-pressure ventilation via a facemask. The avoidance of positive-pressure ventilation is one of the hallmarks of a classic RSII, and so this technique is specifically different from a modified RSII. Our results are consistent with the results of another study by Schleinger and Blanchfield regarding a modified RSII, in which 94 of percent of respondents who used a modified RSII technique reported that it was appropriate to attempt facemask ventilation.3 Additionally, about one-quarter of our respondents who reported attempting to ventilate the patients’ lungs via a facemask reported doing so both before and after administration of an NMBD. These results are slightly different than the other study regarding a modified RSII, in which half of the respondents who reported facemask ventilation did so both before and after administration of an NMBD.3 These differences could be accounted for by the fact that our survey includes almost 60 different institutions, whereas Schleinger and Blanchfield only surveyed CRNAs from a single hospital. Therefore, it is not surprising to find more variation in practice. Additionally, we surveyed residents and attending physicians, whereas Schlesinger and Blanchfield surveyed CRNAs. The results may therefore reflect training differences or exposure to different case types.
Although we did find a clear distinction between a modified RSII and a classic RSII, we were unable to detect differences between the two with respect to the use of oxygen administration, cricoid pressure, and selection of the NMBD. Consistent with previous literature regarding classic RSII,2,4 oxygen administration is almost always used. These results are also consistent with a study regarding modified RSII, in which 97% of respondents reported that oxygen administration was a requirement before induction.3
Although research remains inconclusive as to whether cricoid pressure is effective in preventing pulmonary aspiration of gastric contents,8,9 we found that a high percentage (91%, 86–94%) of participants reported applying cricoid pressure, which is consistent with previous literature regarding a classic RSII, and with previous literature regarding a modified RSII.2–4 Additionally, our results are consistent with other literature in terms of the timing of cricoid pressure application. We found that a majority of participants applied cricoid pressure during induction of anesthesia, which is consistent with other studies regarding classic RSII and modified RSII.2–4
The majority of respondents reported using succinylcholine most frequently. Succinylcholine remains widely used when performing a classic RSII,1–4 which is consistent with the results of a meta-analysis that reported that succinylcholine leads to better intubating conditions than rocuronium.10 With its properties of fast onset and fast recovery, it can expedite airway management during a rapid sequence tracheal intubation.11 However, succinylcholine can have dangerous side effects, such as malignant hyperthermia, hyperkalemic cardiac arrest, and phase II block.11 For this reason, other NMBDs are sometimes chosen. Our results demonstrated that 39% (31–48%) of participants most often administer rocuronium. These results are consistent with literature regarding a classic RSII, in which almost one-third of respondents reported having used rocuronium and succinylcholine during a classic RSII,2 while 40% of respondents used rocuronium for an asymptomatic hiatal hernia.1 We found that 35% of respondents in our survey administered the NMBD before starting mask ventilation, which is consistent with a recent paper that has called into question the necessity of establishing mask ventilation before administration of a NMBD during a standard anesthetic induction.12 There was little consistency regarding the use of aspiration prophylaxis, as only half of the respondents (57%, 48–65) reported using it.
One limitation of our survey is a possible voluntary response bias. Because we could not control who, specifically, completed the surveys, it is possible that the data are not representative of all anesthesiologists. For example, a very busy physician (who therefore opted out of the survey) may have a different perspective than a less busy physician (who did take the survey).
Additionally, we did not survey any private practice anesthesiologists, making it difficult to generalize our results to all practicing anesthesiologists. However, we received surveys from nearly half of all residency programs across the country and received almost 500 completed instruments. Therefore, our results should be generalizable among teaching hospitals and recent residency program graduates across the United States. An additional limitation was that we used a paper-based survey and thus respondents may not have followed the instructions as stated, leading to inconsistent responses. We attempted to rectify this by repeating the analysis on two increasingly restrictive subsets. Complete-case analyses may introduce biases, but we expect these biases to be minimal since the results were similar between the subset that excluded 5% and that, which excluded 15% of the sample. Finally, we did not examine the frequency with which patients were given premedications, such as opioids or anxiolytics. Although these types of medications were not included in the original description of a classic RSII, their use appears to be increasingly common today.
Our results indicate that an overwhelming majority (81%, 77–85%) of anesthesiologists at academic centers sometimes use a modified RSII technique. Based on our survey, we have been able to construct a meaningful definition of a modified RSII, which includes the use of oxygen administration, application of cricoid pressure, and an attempt to ventilate the lungs via positive pressure ventilation before securing the airway with a cuffed tracheal tube. While oxygen administration and cricoid pressure application are also key parts of a classic RSII, positive pressure ventilation is unique to a modified RSII. Having a standard definition and awareness of this key distinction between a classic and modified RSII will serve as a basis for future research to determine how to safely care for patients under circumstances where a modified RSII is indicated.
Supplementary Material
Table 4.
Specific modifications of the rapid sequence induction (RSI) procedure. Estimates correspond to either probabilities and their 95% confidence intervals or the odds ratio comparing attendings to residents (reference group). Counts (labeled “N”) are provided since these responses were provided if the gate question was answered affirmatively.
| All: p (95% CI) | Status: p (95% CI) | Status Comparison | |||
|---|---|---|---|---|---|
| Resident | Attending | OR (95% CI) | P-value | ||
| Oxygenate before induction | |||||
| N | 387 | 175 | 212 | --- | --- |
| For how long? | |||||
| > 5 minutes | 0.10 (0.07, 0.14) | 0.13 (0.08, 0.19) | 0.08 (0.05, 0.13) | 0.61 (0.30, 1.23) | 0.168 |
| 3–5 minutes | 0.55 (0.51, 0.60) | 0.54 (0.47, 0.61) | 0.56 (0.49, 0.63) | 1.08 (0.69, 1.68) | 0.742 |
| < 3 minutes | 0.11 (0.08, 0.15) | 0.13 (0.08, 0.19) | 0.10 (0.07, 0.14) | 0.76 (0.44, 1.32) | 0.337 |
| 4 vital capacity breaths | 0.12 (0.09, 0.16) | 0.13 (0.09, 0.18) | 0.12 (0.08, 0.18) | 0.97 (0.56, 1.70) | 0.921 |
| Until EtO2 >80% | 0.19 (0.14, 0.25) | 0.16 (0.11, 0.23) | 0.21 (0.16, 0.28) | 1.41 (0.87, 2.29) | 0.159 |
| Apply cricoid pressure | |||||
| N | 351 | 160 | 191 | --- | --- |
| When is it applied? | |||||
| Before the induction drug | 0.28 (0.25, 0.33) | 0.35 (0.28, 0.43) | 0.23 (0.18, 0.29) | 0.56 (0.34, 0.91) | 0.019 |
| At the same time as induction drug | 0.57 (0.52, 0.62) | 0.56 (0.48, 0.63) | 0.58 (0.52, 0.64) | 1.11 (0.73, 1.68) | 0.634 |
| After loss of lid reflex | 0.08 (0.06, 0.12) | 0.07 (0.04, 0.14) | 0.09 (0.06, 0.14) | 1.20 (0.53, 2.74) | 0.657 |
| After loss of verbal contact | 0.06 (0.04, 0.09) | 0.02 (0.01, 0.06) | 0.10 (0.07, 0.15) | 5.78 (1.76,19.00) | 0.004 |
| Ventilate patient with a facemask | |||||
| N | 297 | 126 | 171 | --- | --- |
| When do you attempt to ventilate? | |||||
| Before administration of muscle relaxant | 0.41 (0.35, 0.48) | 0.47 (0.38, 0.56) | 0.37 (0.30, 0.46) | 0.68 (0.46, 1.01) | 0.058 |
| After administration of muscle relaxant | 0.35 (0.30, 0.42) | 0.37 (0.28, 0.46) | 0.35 (0.27, 0.43) | 0.92 (0.55, 1.53) | 0.738 |
| Both before and after administration of muscle relaxant | 0.26 (0.20, 0.32) | 0.17 (0.10, 0.26) | 0.32 (0.25, 0.41) | 2.37 (1.22, 4.61) | 0.011 |
| How many breaths do you give? | |||||
| 1 breath | 0.28 (0.22, 0.36) | 0.31 (0.22, 0.42) | 0.26 (0.20, 0.34) | 0.80 (0.48, 1.31) | 0.373 |
| < 5 | 0.48 (0.42, 0.55) | 0.56 (0.45, 0.65) | 0.43 (0.36, 0.51) | 0.61 (0.38, 0.99) | 0.047 |
| 5–10 breaths | 0.20 (0.15, 0.25) | 0.15 (0.10, 0.22) | 0.23 (0.17, 0.30) | 1.66 (0.94, 2.94) | 0.080 |
Acknowledgments
Funding: Financial support for the preparation of this manuscript was provided from a Research Fellowship Grant from the Foundation for Anesthesia Education and Research, 5T32GM007592 from the National Institute of Health, as well as by department funds of the Department of Anesthesia, Critical Care, and Pain Medicine, Massachusetts General Hospital and the Department of Anesthesia, Vanderbilt University School of Medicine.
APPENDIX



Footnotes
Those participants who responded “never” to the question “how often do you use a modified RSI?” were instructed to discontinue the survey.
R Development Core Team (2010). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org.
See Online Supplement for a similar table generated using the less restrictive subset (e.g., only gate question consistency checks versus gate and follow-up checks).
DISCLOSURES: Name: Jesse M. Ehrenfeld, M.D., M.P.H.
Contribution: This author helped design the study, analyze the data, and write the manuscript.
Attestation: This author has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files.
Name: Eva A. Cassedy, B.A.
Contribution: This author helped analyze the data and write the manuscript.
Attestation: This author has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Name: Victoria E. Forbes, M.S.
Contribution: This author helped conduct the study.
Attestation: This author has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Name: Nathaniel D. Mercaldo, M.S.
Contribution: This author performed the statistical analysis
Attestation: This author has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Name: Warren S. Sandberg, M.D., Ph.D.
Contribution: This author helped design the study, analyze the data, and write the manuscript.
Attestation: This author has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Contributor Information
Jesse M. Ehrenfeld, Department of Anesthesiology, Vanderbilt University, Nashville, TN
Eva A. Cassedy, Massachusetts General Hospital, Boston, MA
Victoria E. Forbes, Massachusetts General Hospital, Boston, MA
Nathaniel D. Mercaldo, Vanderbilt University Medical Center
Warren S. Sandberg, Department of Anesthesiology, Vanderbilt University, Nashville, TN
References
- 1.Koerber JP, Roberts GE, Whitaker R, Thorpe CM. Variation in rapid sequence induction techniques: current practice in Wales. Anaesthesia. 2009;64:54–59. doi: 10.1111/j.1365-2044.2008.05681.x. [DOI] [PubMed] [Google Scholar]
- 2.Morris J, Cook TM. Rapid sequence induction: a national survey of practice. Anaesthesia. 2001;56:1090–1097. doi: 10.1046/j.1365-2044.2001.01962.x. [DOI] [PubMed] [Google Scholar]
- 3.Schlesinger S, Blanchfield D. Modified rapid-sequence induction of anesthesia: a survey of current clinical practice. Aana J. 2001;69:291–298. [PubMed] [Google Scholar]
- 4.Thwaites AJ, Rice CP, Smith I. Rapid sequence induction: a questionnaire survey of its routine conduct and continued management during a failed intubation. Anaesthesia. 1999;54:376–381. doi: 10.1046/j.1365-2044.1999.00738.x. [DOI] [PubMed] [Google Scholar]
- 5.Barash PG, Cullen BF, Stoelting RK, Cahalan M, Stock MC. Clinical Anesthesia. 6. Philadelphia: Lippincott Williams & Wilkins, a Wolters Kluwer Business; 2009. [Google Scholar]
- 6.Longnecker DE, Brown DL, Newman MF, Zapol WM. Anesthesiology. New York, NY: The McGraw Hill Companies; 2008. [Google Scholar]
- 7.Miller RD, Eriksson LI, Fleisher LA, Wiener-Kronish JP, Young WL. Miller’s Anesthesia. Philadelphia, PA: Churchill Livingstone Elsevier; 2010. [Google Scholar]
- 8.Ellis DY, Harris T, Zideman D. Cricoid pressure in emergency department rapid sequence tracheal intubations: a risk-benefit analysis. Ann Emerg Med. 2007;50:653–665. doi: 10.1016/j.annemergmed.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Lerman J. On cricoid pressure: “may the force be with you”. Anesth Analg. 2009;109:1363–1366. doi: 10.1213/ANE.0b013e3181bbc6cf. [DOI] [PubMed] [Google Scholar]
- 10.Perry JJ, Lee JS, Sillberg VAH, Wells GA. Rocuronium versus Succinylcholine for Rapid Sequence Induction Intubation (Review) The Cochrane Collaboration. 2009 doi: 10.1002/14651858.CD002788.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Lee C. Goodbye suxamethonium! Anaesthesia. 2009;64:73–81. doi: 10.1111/j.1365-2044.2008.05873.x. [DOI] [PubMed] [Google Scholar]
- 12.Broomhead RH, Marks RJ, Ayton P. Confirmation of the ability to ventilate by facemask before administration of neuromuscular blocker: a non-instrumental piece of information? Br J Anaesth. 2010;104:313–317. doi: 10.1093/bja/aep380. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


