Abstract
Background
Chondrosarcoma is treated primarily by surgery. The prognosis of patients after local recurrence is not well defined. Both the survival of patients and the risk of further local relapse after surgical treatment of local recurrence have yet to be established.
Questions/purposes
We determined survival after local recurrence of chondrosarcoma, the rate of further local recurrences, and prognostic factors predicting survival.
Patients and Methods
We retrospectively reviewed 52 patients treated for locally recurrent conventional chondrosarcoma between 1975 and 2008. All patients had nonmetastatic disease at the time of diagnosis. There were 36 males and 16 females with a median age of 39 years (range, 16–79 years). We analyzed variables affecting overall and disease-free survival. The minimum followup was 12 months unless patients died of disease before 12 months (median, 68 months; range, 4–387 months).
Results
Thirty patients developed their first local recurrence in axial locations, while 22 developed recurrence in the appendicular skeleton. After local recurrence, overall survival was 74% at 5 years and 60% at 10 years. The mean number of local recurrences was three (range, 1–14). Surgical margin correlated with further local recurrence but not survival. Tumor grade, axial location, metastases, and age independently predicted survival.
Conclusions
Prolonged survival of patients after local recurrence of conventional chondrosarcoma is possible, albeit with further recurrences in many patients.
Level of Evidence
Level IV, prognostic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Of the most common sarcomas that arise in bone, chondrosarcoma is distinguished by the fact that surgery is the primary form of treatment [8]. Unlike osteosarcoma, Ewing’s sarcoma, and malignant fibrous histiocytoma, conventional chondrosarcoma does not respond to systemic chemotherapy [2], and the patient’s best hope for cure is complete surgical excision.
Fifteen percent to 25% of patients develop local recurrence [2, 3, 6, 8, 13, 17]. The prognosis for patients after local relapse is not well defined, and few reports [15, 18] have studied the survival of patients after local recurrence. One study examined local recurrence of low-grade chondrosarcoma in the long bones and found local recurrence was associated with worse survival [15]. A study of local recurrence in the pelvis found a 71% rate of second local recurrence [18]. It should not be assumed local relapse has the same prognostic implication in chondrosarcoma as in osteosarcoma or Ewing’s sarcoma. Local recurrence in these diseases is associated with long-term survival rates of less than 20% [10, 11].
Previous studies report the development of local recurrence in chondrosarcoma is associated with an increased rate of metastasis ranging from 33% to 49% and worse overall patient survival (compared with patients without local recurrence) ranging from 17% to 76% at 10 to 20 years [8, 13, 15]. One study of locally recurrent pelvic chondrosarcoma found 71% of patients have at least a second local recurrence, but only 24% of patients develop metastases [18]. This suggests, for a substantial number of patients, local recurrence can be a poor prognostic sign and can contribute directly to diminished rates of survival [2]. However, in contrast to these studies, one study found no association between local recurrence and patient survival [14].
Despite improvements in diagnostic modalities and surgical techniques, there has not been an improvement in survival of patients with chondrosarcoma over the past 30 years, according to an analysis of US National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) data [7]. To improve the outcome of patients, it seems important to determine how to optimize the treatment of patients who develop recurrence of disease. We therefore determined (1) the survival rate of patients after local recurrence of chondrosarcoma; (2) the rate of repeat local recurrences; and (3) whether various factors, including amputation, surgical margins, and further recurrences, affected survival of patients after a first local recurrence.
Patients and Materials
We retrospectively identified 75 patients treated for locally recurrent chondrosarcoma at our institution between January 1975 and December 2008. We included patients with conventional skeletal chondrosarcoma of the extremities, pelvic girdle, sacrum, or shoulder girdle and who underwent surgical treatment at our institution. We excluded 20 patients due to dedifferentiated chondrosarcoma (six patients), clear-cell chondrosarcoma (three), unverified pathology (three), extraskeletal chondrosarcoma (three), and spine disease (two). Three patients were lost to followup between 1 and 8 months. This left 52 patients, forming the cohort for this study, which included 36 males and 16 females with a median age of 39 years (range, 16–79 years) (Table 1). The minimum followup was 12 months unless patients died of disease before 12 months (median, 68 months; mean, 104 months; range, 4–387 months). No patients were recalled specifically for this study; all data were obtained from medical records and imaging. The study was approved by the Institutional Review Board.
Table 1.
Patient demographics
| Demographic | Number of patients |
|---|---|
| Total | 52 |
| Median age (years) | 39 |
| Median followup (months) | 68 |
| Sex | |
| Male | 36 (69%) |
| Female | 16 (31%) |
| Location | |
| Axial | 30 (56%) |
| Peripheral | 22 (44%) |
| Grade | |
| 1 | 23 (44%) |
| 2 | 20 (39%) |
| 3 | 9 (17%) |
| Recurrence type | |
| Soft tissue | 23 (44%) |
| Bone | 29 (56%) |
| Time of recurrence | |
| ≤ 2 years | 27 (52%) |
| > 2 years | 25 (48%) |
| Number of recurrences | |
| 1 | 19 (37%) |
| 2 | 16 (31%) |
| 3 or more | 17 (32%) |
| Type of surgery (primary tumor) | |
| Curettage | 17 (33%) |
| Wide local incision | 35 (67%) |
The initial surgery for the primary tumor consisted of en bloc wide excision with negative margins in 27 cases, contaminated wide or marginal excision with positive margins in 8 cases, and intralesional excision in 17 cases. Eight of the 17 intralesional excisions were performed at an outside hospital, and the patients were referred after development of local recurrence. Positive margins were defined as the presence of tumor at the inked surface of the specimen submitted to surgical pathology.
After the initial surgery, we monitored patients for recurrence and metastasis every 3 months for the first 2 years and then gradually lengthened the interval between visits to yearly by the fifth year if no relapse of disease was observed. Patients underwent history, physical examination, chest radiography, and radiography of the affected region at each visit. CT, MRI, and whole-body bone scans were obtained selectively, depending on whether there was evidence from the history, physical examination, and radiographs to warrant further study. We reviewed patients’ charts, electronic medical records, and imaging studies. Data extracted included age at diagnosis, sex, site of the primary tumor, axial or appendicular location, time of the recurrence, final vital status, cause of death, followup period, number of recurrences, date of primary surgery, type of surgery, tumor grade, and location of the recurrence in soft tissue or bone. The first local recurrence developed in bone for 29 of the 52 patients and soft tissue for 23. The location of the recurrence was axial in 30 patients and peripheral in 22 patients. The median time to local recurrence was 24 months (range, 4–136 months) after initial surgery, demonstrating local recurrence could develop more than 10 years after the primary operation. The mean time to local recurrence was similar for all three grades: 40 months for Grade 1, 29 months for Grade 2, and 34 months for Grade 3 (p = 0.49). Fifteen of 52 patients (29%) developed metastases between 0 and 75 months after the local recurrence.
We calculated overall and disease-free survival using Kaplan-Meier analysis. We calculated overall survival after local recurrence from the date of recurrence until death or last followup. Patients who died of other unrelated causes were censored at the time of death. We defined disease-free survival as the period from the time of first local recurrence until another local recurrence or metastasis. The effect of various factors (age, sex, further local recurrences, tissue of local recurrence, time to first local recurrence, number of local recurrences, axial location, grade, subsequent development of metastasis, amputation, surgical margins) on Kaplan-Meier survival curves was determined with the log-rank test. We performed Cox regression analysis using forward conditional analysis to identify independent predictive factors for survival of patients. We used the SPSS® 15.0 program for Windows® (SPSS Inc, Chicago, IL, USA) to perform statistical calculations and calculated confidence intervals of 95% for statistical parameters.
Results
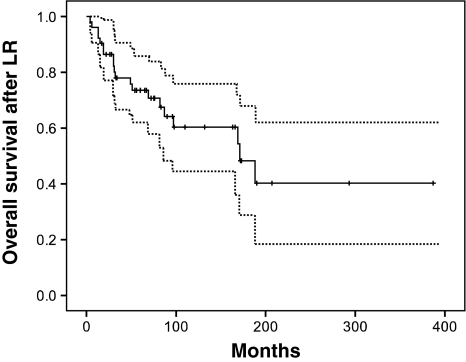
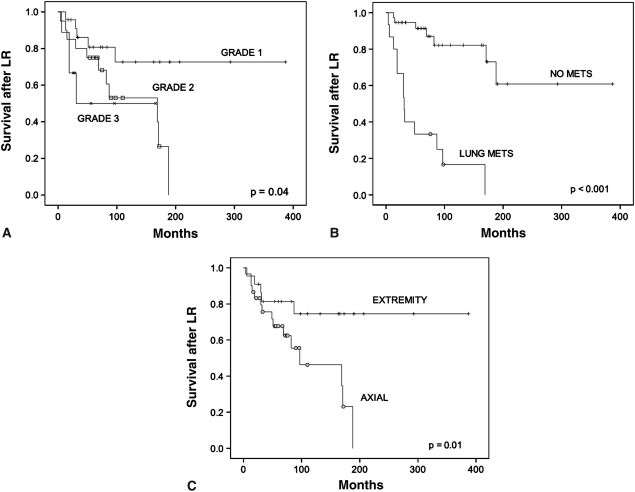
The overall survival of patients after local recurrence of chondrosarcoma was 74% at 5 years, 60% at 10 years, and 40% at 20 years (Fig. 1). Twenty of 52 patients were alive without disease, but only eight were continuously free of disease (Table 2). Fifteen patients developed metastases. In 14 of 15 patients, the grade of the local recurrence was Grade 2 or 3. In one patient, there was a massive local recurrence in the pelvis, which was predominantly Grade 1 with minute areas of Grade 2 tumor. The appearance of metastases at any time during the course of disease was associated with a poor prognosis, and 13 of 15 patients with metastases died of disease (Fig. 2). Five of 15 patients with pulmonary metastases underwent thoracotomy and resection of metastatic disease. One of these patients is still alive without evidence of disease at last followup. Of the six patients who received chemotherapy agents, one patient is still alive with disease. A variety of different systemic agents were employed, including doxorubicin, ifosfamide, gemcitabine, taxotere, and investigational agents. Not all patients who died had known metastases: six patients (12%) died of disease without distant metastases. All of these patients had axial disease in the pelvic-sacral area, which was associated with worse overall survival (Fig. 2). The survival curve for axial disease after local recurrence fell to zero by 200 months and never reached a plateau (Fig. 2). Adjuvant radiation was employed in two patients with locally advanced disease. Both patients had pelvic tumors. One patient has died of local disease progression without metastasis while the other patient is alive with stable disease 21 months after completion of radiation.
Fig. 1.
Kaplan-Meier analysis for overall survival shows long-term survival of 74% at 5 years, 60% at 10 years, and 40% at 20 years. Dotted lines indicate 95% confidence intervals. LR = local recurrence.
Table 2.
Vital status
| Status | Number of patients |
|---|---|
| Alive (n = 25) | |
| Without disease (NED) | 20 (38%) |
| With disease (AWD) | 5 (9%) |
| Dead (n = 27) | |
| Without disease (other) | 7 (13%) |
| With disease (DOD) | 20 (38%) |
NED = no evidence of disease; AWD = alive with disease; DOD = died of disease.
Fig. 2A–C.
Graphs show the effect of factors on survival after local recurrence (LR). (A) Grade 1 tumors had better survival compared to Grade 2 or 3 tumors after local recurrence (p = 0.04). The survival at 10 years by Kaplan-Meier analysis was 73% for Grade 1 tumors (95% confidence interval, 51%–94%), 53% for Grade 2 tumors (95% confidence interval, 28%–78%), and 50% for Grade 3 tumors (95% confidence interval, 14%–87%). (B) The development of lung metastasis predicted worse (p < 0.001) survival. The survival at 10 years by Kaplan-Meier analysis was 82% for patients who did not develop lung metastasis (NO METS) (95% confidence interval, 69%–97%) compared to 17% for patients who developed lung metastasis (LUNG METS) (95% confidence interval, 0%–37%). At last followup, only two patients with lung metastases were still alive. (C) Axial location of the tumor was associated with worse overall survival compared to peripheral location (p = 0.01). The survival at 10 years by Kaplan-Meier analysis was 75% for patients who had tumors in the extremities (95% confidence interval, 55%–95%) compared to 46% for patients who had tumors in the axial skeleton (95% confidence interval, 22%–70%). At 200 months, there were no survivors with axial disease.
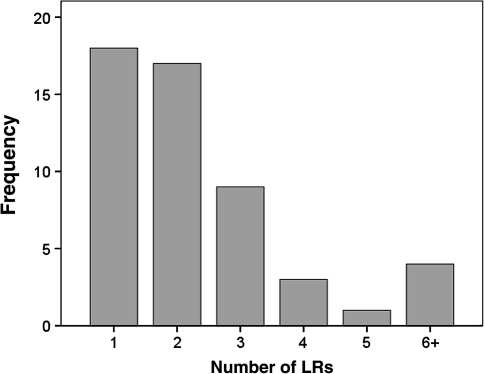
After the first local recurrence, six patients underwent amputation, and 46 patients underwent limb-sparing surgery. Six patients subsequently were treated with amputation for further recurrence of disease. Of the 12 patients who had undergone amputation, five patients died of disease; one patient was alive with disease; one patient died of other causes; and five patients were alive and free of disease. Thirty-four patients (65%) developed at least one additional local recurrence. The median number of local recurrences per patient was two, and the mean was 2.7 (range, 1–14) (Fig. 3). The mean number of local recurrences was similar (p = 0.29) for all three grades: 3.0 for Grade 1, 2.8 for Grade 2, and 1.8 for Grade 3.
Fig. 3.
A graph shows distribution of number of local recurrences (LRs). Most patients had more than one local recurrence, and the mean number of local recurrences was three. Four patients had six or more local recurrences.
Age, sex, tissue of local recurrence, time to first local recurrence, and number of local recurrences did not affect survival, whereas axial location, grade, and subsequent development of metastasis did impact survival (Table 3). Patients whose initial tumor grade was Grade 1 had better survival than patients with Grade 2 or 3 tumors, but not all patients with Grade 1 disease survived (Fig. 2). We observed an increase in tumor grade in 18 of 52 patients (35%), including 13 patients with Grade 1 disease and five with Grade 2 disease. Axial site of disease, high tumor grade (2 or 3), metastases, and age of 40 years or greater independently predicted disease-related overall survival (Table 4). The removal of the first local recurrence with positive margins resulted in a better chance (p = 0.02) that a second local recurrence would develop, but it was not associated with a difference in overall survival (p = 0.18). Thirty-six patients had excision of the local recurrence with negative margins, while 16 patients had positive margins. Of the patients with positive margins, 10 (63%) were in an axial location (p = 0.43). To address the possibility that Grade 1 tumors might skew the effect of surgical margins on survival, a separate Kaplan-Meier analysis was performed on the subset of patients with Grade 2 and 3 tumors. The 5-year survival was similar (p = 0.37) for patients with negative margins compared to those with positive margins: 72% versus 64%, respectively.
Table 3.
Kaplan-Meier analysis of individual factors affecting overall patient survival
| Factor | Number of patients | 10-year survival (%) | p Value |
|---|---|---|---|
| Sex | |||
| Male | 36 | 61 | 0.71 |
| Female | 16 | 63 | |
| Age at diagnosis | |||
| < 40 years | 27 | 71 | 0.20 |
| ≥ 40 years | 25 | 52 | |
| Number of recurrences | |||
| Single | 19 | 67 | 0.87 |
| Multiple | 33 | 59 | |
| Recurrence type | |||
| Soft tissue | 23 | 49 | 0.47 |
| Bone | 29 | 70 | |
| Time of recurrence | |||
| Within 2 years | 27 | 62 | 0.37 |
| After 2 years | 25 | 57 | |
| Grade | |||
| 1 | 23 | 73 | 0.04 |
| 2 | 20 | 53 | |
| 3 | 9 | 50 | |
| Location | |||
| Axial | 30 | 47 | 0.04 |
| Peripheral | 22 | 75 | |
| Metastasis | |||
| Yes | 15 | 17 | < 0.001 |
| No | 37 | 82 | |
Table 4.
Cox regression multivariate analysis for patient survival
| Covariate | B | Wald statistic | p Value | Exp(B) | 95% CI for Exp(B) | |
|---|---|---|---|---|---|---|
| Metastasis | 2.50 | 17.3 | < 0.0001 | 12.1 | 3.7 | 39 |
| Axial location | 1.70 | 7.5 | 0.006 | 5.4 | 1.6 | 18 |
| Grade (2 or 3) | 1.50 | 7.5 | 0.006 | 4.5 | 1.5 | 13 |
| Age < 40 years | −1.21 | 4.5 | 0.034 | .30 | 0.1 | 0.9 |
B = coefficient for each covariate; Exp(B) = eB represents the relative risk of each covariate; CI = confidence interval.
Discussion
The prognosis of patients with local recurrence of chondrosarcoma is not well established. Previous studies have been limited in scope and were focused on patients with low-grade chondrosarcoma [15] or pelvic disease [18]. We therefore (1) determined the survival of patients after local recurrence of chondrosarcoma in a broader group of patients; (2) determined the rate of further local recurrence; and (3) identified factors that might affect survival.
Readers should be aware of limitations to our study. First, this is a retrospective analysis, and many variables could not be controlled, including the treatment of the primary tumor, the decision whether to perform limb preservation versus amputation after local recurrence, and the exact surgical technique for treating the local recurrence. Second, the number of patients is small, and the conclusions of this report should be considered preliminary. The results ideally would be validated with larger populations of patients. Third, the effect of chemotherapy and radiation could not be adequately assessed. These modalities were applied to only a few patients for palliative purposes, and no conclusions could be drawn regarding their efficacy for treating either the local recurrence or metastasis.
We found prolonged survival was possible for the majority of patients: 60% at 10 years. The survival however did not reach a plateau at 10 years and continued to decline to 40% at 20 years. Part of the reason for this is that patients may survive a long time with disease and have multiple recurrences. Most patients (65%) had at least one additional local recurrence, which is consistent with the findings of Weber et al. [18]. In our study, multiple local recurrences did not correlate with survival, and some patients lived many years with numerous local recurrences, but without metastasis. As there are yet to be proven adjuvant treatments that reduce the rate of local recurrence, it seems especially important for surgeons to deliver meticulous surgical care for locally recurrent disease. Even with one or two recurrences, it is still possible to achieve cure with surgery. It is also important to emphasize the fact that a considerable number of patients die of advanced, uncontrolled, locally recurrent disease without distant metastasis. In this study, six of 20 patients (30%) without metastasis eventually died of locally recurrent disease.
The factors that appeared to affect survival adversely included the development of metastasis at any time during the course of their disease, high grade, axial location of disease, and age greater than 40 years. This finding is not surprising since the predictors recall the factors previously reported for primary chondrosarcoma [4–6, 8, 12, 13, 16]. The development of metastasis is a poor prognostic sign. The survival curve for these patients suggests all patients would eventually die of disease, some albeit with a protracted course of disease. The reason why a certain subset of tumors develop the capacity to metastasize is currently unknown but likely relates to molecular and genetic changes. Understanding this process will undoubtedly be critical to treating patients with recurrent chondrosarcoma in the future. While this study verifies the initial grade of the tumor has predictive value, it also demonstrates the tumor is not a static entity. Many of the recurrent tumors increased in grade compared to the initial tumor, and this phenomenon is reported by others [2, 6, 8, 13, 18]. The finding is likely a manifestation in part of continued mutation and change in the tumor cells.
Contrary to our initial expectations, we found no association between surgical margins and overall patient survival. This finding must be interpreted carefully. It could mean either surgical technique is not important to patient survival or the current techniques for analyzing the margins of pathologic specimens by ink are not accurate enough to identify residual disease. Furthermore, the determination of whether a specimen has a negative margin may depend in part on how closely the surgical pathologist examines each specimen. It is possible inadequate numbers of samples submitted for processing and too few sections per sample could result in the pathologist missing an area of positive margin. Unfortunately, in our retrospective analysis, we could not determine how accurately each pathologic specimen was processed. Although our observations suggest surgical margin is not a primary determinant of outcome, one should not interpret this as meaning the quality of surgery does not have an important bearing on patient outcome. Our data indirectly reflect the importance of surgery in several ways. Negative surgical margins in the treatment of the first local recurrence were associated with a reduced risk of a second local recurrence. For some patients who might die of local recurrence as opposed to distant metastasis, this could be quite important in terms of survival. Another observation is that patients with extremity disease fare better than those with axial disease. It is possible, though difficult to prove, that the reason for this is because wide margins are more readily attainable in the extremities compared to central locations. While the definition of “wide” is difficult to state quantitatively, most surgeons accept the viewpoint that negative margins and wide margins are not necessarily the same thing. Amputation with wide negative margins can usually be accomplished in the extremities, but this is not always possible, even as a salvage operation, for patients with sacral-pelvic or other centrally located disease.
Seventeen cases of local recurrence developed after curettage of Grade 1 tumors. This raises a question as to the appropriateness of treating Grade 1 chondrosarcoma with curettage [1, 9]. Since some of the cases were initially treated elsewhere, the denominator in terms of total number of cases treated by curettage is unknown. The thoroughness or consistency of the surgical technique is also not known. It is therefore impossible for us to calculate the rate of recurrence or survival of Grade 1 chondrosarcoma in this cohort. While survival of patients who initially had Grade 1 disease is better than that of patients who had higher-grade disease, it is notable some eventually died of disease. This is consistent with the study of Schwab et al. [15], who found 13% of Grade 1 chondrosarcomas developed local recurrence, and 1/3 of the patients with local recurrence developed metastasis. The results seem to validate the concept of Grade 1 chondrosarcoma being distinct from benign enchondroma. Separation of these two entities diagnostically, however, remains difficult, challenging, and controversial. We will need more study and research to address this issue.
In summary, survival after local recurrence of conventional chondrosarcoma may be prolonged, but it is not necessarily continuously disease-free, and patients often develop subsequent recurrence of disease. Most patients have at least two recurrences. Tumor location in the axial skeleton, tumor grade (2 or 3), and metastases are independent predictors of worse survival. Surgical margins of the resected local recurrence were statistically associated with the risk subsequent local recurrence but did not predict survival.
Acknowledgment
The authors thank Robert L. Satcher, MD, PhD, for his advice and critical reading of the manuscript.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at MD Anderson Cancer Center.
References
- 1.Bauer HC, Brosjo O, Kreicbergs A, Lindholm J. Low risk of recurrence of enchondroma and low-grade chondrosarcoma in extremities: 80 patients followed for 2–25 years. Acta Orthop Scand. 1995;66:283–288. doi: 10.3109/17453679508995543. [DOI] [PubMed] [Google Scholar]
- 2.Bjornsson J, McLeod RA, Unni KK, Ilstrup DM, Pritchard DJ. Primary chondrosarcoma of long bones and limb girdles. Cancer. 1998;83:2105–2119. doi: 10.1002/(SICI)1097-0142(19981115)83:10<2105::AID-CNCR9>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 3.Bruns J, Elbracht M, Niggemeyer O. Chondrosarcoma of bone: an oncological and functional follow-up study. Ann Oncol. 2001;12:859–864. doi: 10.1023/A:1011162118869. [DOI] [PubMed] [Google Scholar]
- 4.Donati D, El Ghoneimy A, Bertoni F, Di Bella C, Mercuri M. Surgical treatment and outcome of conventional pelvic chondrosarcoma. J Bone Joint Surg Br. 2005;87:1527–1530. doi: 10.1302/0301-620X.87B11.16621. [DOI] [PubMed] [Google Scholar]
- 5.Evans HL, Ayala AG, Romsdahl MM. Prognostic factors in chondrosarcoma of bone: a clinicopathologic analysis with emphasis on histologic grading. Cancer. 1977;40:818–831. doi: 10.1002/1097-0142(197708)40:2<818::AID-CNCR2820400234>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 6.Gitelis S, Bertoni F, Picci P, Campanacci M. Chondrosarcoma of bone: the experience at the Istituto Ortopedico Rizzoli. J Bone Joint Surg Am. 1981;63:1248–1257. [PubMed] [Google Scholar]
- 7.Giuffrida AY, Burgueno JE, Koniaris LG, Gutierrez JC, Duncan R, Scully SP. Chondrosarcoma in the United States (1973 to 2003): an analysis of 2890 cases from the SEER database. J Bone Joint Surg Am. 2009;91:1063–1072. doi: 10.2106/JBJS.H.00416. [DOI] [PubMed] [Google Scholar]
- 8.Lee FY, Mankin HJ, Fondren G, Gebhardt MC, Springfield DS, Rosenberg AE, Jennings LC. Chondrosarcoma of bone: an assessment of outcome. J Bone Joint Surg Am. 1999;81:326–338. doi: 10.1302/0301-620X.81B5.9588. [DOI] [PubMed] [Google Scholar]
- 9.Leerapun T, Hugate RR, Inwards CY, Scully SP, Sim FH. Surgical management of conventional Grade I chondrosarcoma of long bones. Clin Orthop Relat Res. 2007;463:166–172. doi: 10.1097/BLO.0b013e318146830f. [DOI] [PubMed] [Google Scholar]
- 10.Lin PP, Jaffe N, Herzog CE, Costelloe CM, Deavers MT, Kelly JS, Patel SR, Madewell JE, Lewis VO, Cannon CP, Benjamin RS, Yasko AW. Chemotherapy response is an important predictor of local recurrence in Ewing sarcoma. Cancer. 2007;109:603–611. doi: 10.1002/cncr.22412. [DOI] [PubMed] [Google Scholar]
- 11.Picci P, Sangiorgi L, Rougraff BT, Neff JR, Casadei R, Campanacci M. Relationship of chemotherapy-induced necrosis and surgical margins to local recurrence in osteosarcoma. J Clin Oncol. 1994;12:2699–2705. doi: 10.1200/JCO.1994.12.12.2699. [DOI] [PubMed] [Google Scholar]
- 12.Pring ME, Weber KL, Unni KK, Sim FH. Chondrosarcoma of the pelvis: a review of sixty-four cases. J Bone Joint Surg Am. 2001;83:1630–1642. [PubMed] [Google Scholar]
- 13.Pritchard DJ, Lunke RJ, Taylor WF, Dahlin DC, Medley BE. Chondrosarcoma: a clinicopathologic and statistical analysis. Cancer. 1980;45:149–157. doi: 10.1002/1097-0142(19800101)45:1<149::AID-CNCR2820450125>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 14.Rizzo M, Ghert MA, Harrelson JM, Scully SP. Chondrosarcoma of bone: analysis of 108 cases and evaluation for predictors of outcome. Clin Orthop Relat Res. 2001;391:224–233. doi: 10.1097/00003086-200110000-00025. [DOI] [PubMed] [Google Scholar]
- 15.Schwab JH, Wenger D, Unni K, Sim FH. Does local recurrence impact survival in low-grade chondrosarcoma of the long bones? Clin Orthop Relat Res. 2007;462:175–180. doi: 10.1097/BLO.0b013e3180caac2c. [DOI] [PubMed] [Google Scholar]
- 16.Sheth DS, Yasko AW, Johnson ME, Ayala AG, Murray JA, Romsdahl MM. Chondrosarcoma of the pelvis: prognostic factors for 67 patients treated with definitive surgery. Cancer. 1996;78:745–750. doi: 10.1002/(SICI)1097-0142(19960815)78:4<745::AID-CNCR9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 17.Loon CJ, Veth RP, Pruszczynski M, Wobbes T, Lemmens JA, Horn J. Chondrosarcoma of bone: oncologic and functional results. J Surg Oncol. 1994;57:214–221. doi: 10.1002/jso.2930570403. [DOI] [PubMed] [Google Scholar]
- 18.Weber KL, Pring ME, Sim FH. Treatment and outcome of recurrent pelvic chondrosarcoma. Clin Orthop Relat Res. 2002;397:19–28. doi: 10.1097/00003086-200204000-00004. [DOI] [PubMed] [Google Scholar]