Abstract
Background
Using patient-specific cutting blocks for TKA increases the cost to the hospital for these procedures, but it has been proposed they may reduce operative times and improve implant alignment, which could reduce the need for revision surgery.
Questions/purposes
We compared TKAs performed with patient-specific cutting blocks with those performed with traditional instrumentation to determine whether there was improved operating room time management and component coronal alignment to support use of this technology.
Methods
We retrospectively reviewed 57 patients undergoing primary TKAs using patient-specific custom cutting blocks for osteoarthritis and compared them with 57 matched patients undergoing TKAs with traditional instrumentation during the same period (January 2009 to September 2010). At baseline, the groups were comparable with respect to age, sex, and BMI. We collected data on operative time (total in-room time and tourniquet time) and measured component alignment on plain radiographs.
Results
On average, TKAs performed with patient-specific instrumentation had similar tourniquet times (61.0 versus 56.2 minutes) but patients were in the operating room 12.1 minutes less (137.2 versus 125.1 minutes) than those in the standard instrumentation group. We observed no difference in the femorotibial angle in the coronal plane between the two groups.
Conclusions
Patient-specific instrumentation for TKA shows slight improvement in operating room time management but none in component alignment postoperatively. Therefore, routine use of this new technology may not be cost-effective in its current form.
Level of Evidence
Level III, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
TKA is one of the most successful and commonly performed surgical procedures in the world. Improvements in surgical technique and implant durability, and the aging population, have resulted in an increased demand for these procedures, which is expected to double by 2017 [14]. Currently, Medicare pays for approximately 60% of the total joint arthroplasties performed in the United States, and the current payment formula for federal-sponsored healthcare in the United States is unsustainable. The economic burden of providing the highest standard of care to every patient, while integrating the latest technologic innovations, will prove to be challenging [14]. Critical evaluation of new technology for TKA will be important to ensure substantial improvement in clinical efficiency or patient outcomes is achieved to justify any additional costs to the healthcare system.
Computer-aided navigation for TKA was developed to improve component alignment and avoid outliers [7]. However, it has not become widely used in the orthopaedic community because of the high initial capital costs [3, 5, 10], the steep learning curve [8], and the increased surgical time to perform these procedures [3, 5, 10]. During the past decade, TKA technology evolved to include patient-specific cutting guides [12, 13, 18, 22, 25, 30], which have the proposed benefits of improved accuracy and more efficient intraoperative resource and time management, without the need for costly investments in capital equipment. The patient-specific guides also have the additional benefit of incorporating preoperative three-dimensional imaging to allow greater adjustments in the proposed component position before taking the patient to surgery. To date, studies in the literature on patient-specific instrumentation have been limited to small sample sizes without comparative control groups, but they have shown some decrease in operative time and acceptable component alignment [22, 25]. Theoretically, advantages include improving operating room time management, reducing costs in the perioperative period, and improving component alignment compared with conventional instrumentation, but these have not been confirmed in the literature to date.
We therefore compared TKAs performed with patient-specific cutting blocks with TKAs performed with traditional instrumentation to determine whether there was improved (1) operating room time management, (2) component coronal alignment to support the use of this technology, and (3) whether there were differences in soft tissue balancing.
Patients and Methods
We retrospectively reviewed all patients undergoing primary TKA for osteoarthritis by one surgeon (RLB) at a high-volume teaching university from January 2009 to September 2010. All patients received the same cemented, cruciate-retaining, Vanguard® total knee system (Biomet Inc, Warsaw, IN, USA). We identified 57 patients who underwent TKAs with the SignatureTM system (Biomet Inc), which uses the patient-specific cutting block technology (patient-specific cohort) and compared them with a cohort of 57 patients who had TKAs using standard instrumentation (standard cohort). Of the 57 patients in each cohort, there were 23 men and 34 women; the average age in both groups was 65 years at the time of surgery, and the average BMI was 32.0 in the patient-specific cohort and 33.0 in the standard cohort (Table 1).
Table 1.
Patient demographics
| Variable | Standard cohort (n = 57) | Patient-specific cohort (n = 57) | p value |
|---|---|---|---|
| Mean age at surgery (years) | 64.9 | 64.6 | 0.9 |
| Mean BMI | 33.0 | 32.0 | 0.3 |
| Sex (male/female) | 23/34 | 23/34 | NA |
| Operative side (left/right) | 27/30 | 31/26 | NA |
NA = not applicable.
All patients were evaluated in the outpatient clinic of the senior author (RLB) and judged good candidates for elective TKA for osteoarthritis. In addition, nonoperative treatment had failed for these patients, and they did not have any major flexion contractures (< 7°) or coronal deformity (< 10° varus/valgus). During the discussion regarding operative options, each patient was offered the choice between a TKA using the patient-specific cutting block technology and a conventional TKA with the standard instrumentation. Each patient made his or her decision independent of the treating physician, and the treating physician did not recommend one procedure over the other. Patients who wanted the patient-specific cutting blocks underwent MRI using the SignatureTM system manufacturing protocol, which includes 1-mm high-resolution slices at the knee and selected spot images at the hip and ankle. The MR images then were uploaded and sent to the manufacturer for processing using the predetermined default settings specific to this surgeon. After the MR images were processed by the manufacturer, a preliminary surgical plan with the proposed resection levels was uploaded and reviewed electronically by the surgical team for final approval before manufacturing of the custom blocks.
All patients in the standard group had the same operative setup and wound closure as the patients in the custom-guide group. The only difference was the intraoperative alignment using the standard instrumentation. On the femoral side, an intramedullary femoral guide set to 5° valgus was used to make the distal femoral cut and the proximal tibial cut was made perpendicular to the mechanical axis of the tibia using an extramedullary guide.
Operative times were recorded in real time by the surgical staff on the day of surgery as part of routine nursing documentation at our hospital and were part of the patient’s permanent medical record. We collected three times for all patients: (1) total time in the operating room, which was calculated from the time the patient was rolled into the operating room to the time the patient was rolled out of the room; (2) surgical time, which was calculated from skin incision to skin closure; and (3) total tourniquet time, which was calculated from the time the tourniquet was inflated to the time the tourniquet was released. Operative data were available for all patients. We subjectively assessed all patients for soft tissue balancing intraoperatively and at their first postoperative visit.
Postoperatively, all patients received an AP CT scanogram with a field of view from the hip to the ankle as part of standard clinical care (Fig. 1). To minimize projection errors and to standardize analysis, the limb was rotated until the two augment holes on the femoral condyles were partially visible on either side of the anterior flange of the femoral component [23] (Fig. 2). All radiographic measurements were performed on this CT scanogram by the same blinded reviewer (BMW) who was not involved with the clinical management of these patients. The measurements used to determine component coronal alignment included (1) the femorotibial angle (FTA), (2) hip-knee-ankle axis (HKA), (3) zone of mechanical axis (ZMA), and (4) mechanical axis deviation (MAD). Accepted values for the normal range of FTA were between 2.4° and 7.2° valgus [1, 2, 11]. The accepted normal value for HKA was 0° ± 3° varus or valgus [2, 4, 15, 16]. The accepted normal value for ZMA was the central zone, and the accepted normal value for MAD was 0° ± 10° deviation. Full radiographic data were available for all patients.
Fig. 1.

A CT scout image shows the lower-extremity alignment with a field of view from the hip to ankle used to make measurements for this study.
Fig. 2.
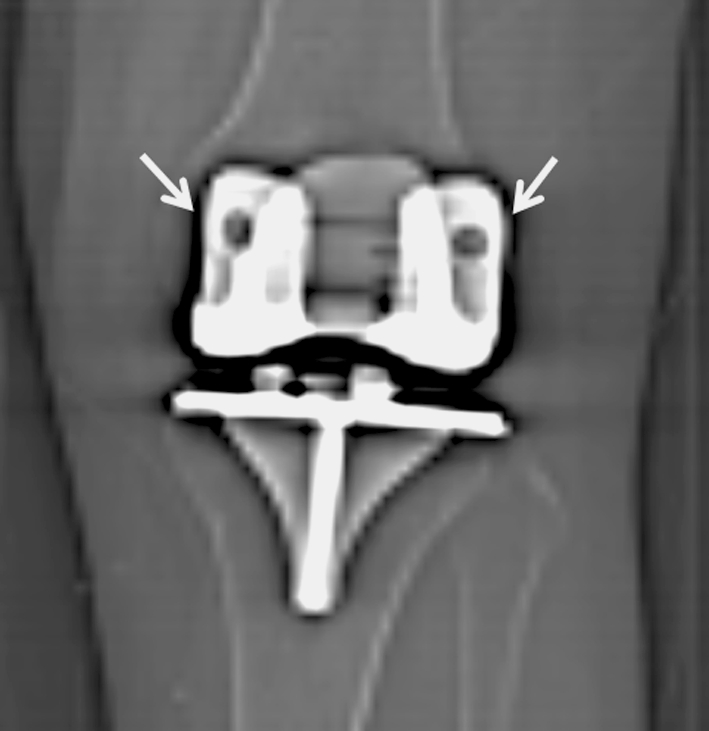
A CT scout image magnified at the knee shows the two augment holes (arrows) on the posterior condyles of the femoral component used to help standardize the extremity rotation before making measurements by ensuring the holes are visible on either side of the anterior flange of the femoral component.
The two-sample t-test was used to compare the two cohorts for age, BMI, and operative times. The chi-square test was used for categorical variables and analysis of the radiographic measurements. The analysis was performed with SPSS® software (SPSS Inc, Chicago, IL, USA).
Results
We found no improvement in the patient-specific cohort compared with the standard cohort for tourniquet time (a mean of 4.8 minutes saved; p = 0.099) and surgical time from skin incision to closure (3.8 minutes saved; p = 0.262), but we did find an improvement in total time in the operating room (12.1 minutes less; p = 0.028) (Table 2).
Table 2.
Operative times in the two cohorts
| Time | Standard cohort (n = 57) | Patient-specific cohort (n = 57) | p value |
|---|---|---|---|
| Tourniquet time (minutes) | 61.0 ± 15.0 | 56.2 ± 15.1 | 0.099 |
| Time in operating room (minutes) | 137.2 ± 33.6 | 125.1 ± 22.7 | 0.028 |
| Incision to closure time (minutes) | 93.4 ± 15.9 | 89.6 ± 18.2 | 0.262 |
Values are expressed as mean ± SD.
We found no improvement in the patient-specific cohort compared with the standard cohort regarding the proportion of patients with FTA between 2.4° and 7.2° (p = 0.85), HKA 0° ± 3° varus or valgus (p = 0.37), ZMA through the central zone (p = 0.85), or MAD of 0° ± 10° (p = 0.56) (Table 3).
Table 3.
Radiographic measurements in the two cohorts
| Radiographic measurement | Standard cohort (n = 57) | Patient-specific cohort (n = 57) | p value |
|---|---|---|---|
| Femorotibial angle | 0.85 | ||
| Between 2.4° and 7.2° valgus (number of patients) | 35 | 33 | |
| Outliers (number of patients) | 22 | 24 | |
| Hip-knee-ankle axis | 0.37 | ||
| Within ± 3° (number of patients) | 47 | 42 | |
| Outliers (number of patients) | 10 | 15 | |
| Zone of mechanical axis | 0.85 | ||
| Within the central zone (number of patients) | 34 | 36 | |
| Outliers (number of patients) | 23 | 21 | |
| Mechanical axis deviation | |||
| Within ± 10% (number of patients) | 34 | 38 | 0.56 |
| Outliers (number of patients) | 23 | 19 |
We noted no differences in soft tissue balancing intraoperatively and at the first postoperative visit. No patient has required revision surgery at this time.
Discussion
The demand for TKA will continue to increase owing to the success of the operation and the aging patient population in the United States [20, 21]. Hospital reimbursements for this procedure have not kept pace with inflation and it is likely Medicare will continue to decrease reimbursements in the future [6]. At the same time, hospital expenses for TKAs have increased with time owing to labor costs and the increasing cost of implants and new technology [6]. It has been well documented that correct component alignment is important in TKA to avoid early failures and the need for revision surgery [11, 19, 26, 29]. Computer-aided surgery has been used for more than a decade but continues to have high initial capital costs, and although it has shown some improvement in component positioning, it has not completely eliminated component outliers [5, 7, 9, 17, 24, 27, 28]. Patient-specific cutting blocks have many proposed advantages, including enhanced operative efficiency and improved component alignment, but they impose a considerable cost to the healthcare system with expensive imaging in the outpatient setting and additional implant charges to the hospital. We evaluated and compared the clinical efficiency and coronal alignment of primary TKAs performed with either patient-specific cutting blocks or standard instrumentation to determine the cost-utility of this new technology.
Our study was subject to certain limitations. First, this was a retrospective review and the patients were not randomized into the two cohorts. This introduced selection bias as the patients were allowed to choose between the two different cohorts and some patients were not interested in this new technology since it was not yet proven and others wanted to have the new technology because they likely believed new technology was usually superior. Other patients were claustrophobic, had bad experiences with MRI, had a copayment for the MRI, or did not want to wait 6 weeks for the cutting blocks to be manufactured and therefore did not want to be in the patient-specific cohort and this might have skewed our results. However, the patient demographics are so similar between the groups that it almost appears we attempted to match the two groups; therefore, we do not think it is likely selection bias substantially influenced our results. Second, although all the surgical procedures were performed by the same experienced surgeon, they were performed in an academic teaching environment in which different residents and fellows were present for these cases and may have varied the surgical times. The senior surgeon performed the majority of the bone cuts and was the sole decision maker when assessing accuracy of the bone cuts and the soft tissue balancing. Since this was a nonrandomized study, it is likely any additional time added to the cases for teaching would be distributed randomly over both cohorts. We also eliminated the first 25 cases performed using this new technology to allow time for the surgeon and operative staff to become comfortable with these cutting blocks to avoid any confounding variables during the learning curve period. These cases were performed by a high-volume surgeon who has performed several thousand TKAs with conventional instrumentation before using patient-specific instrumentation and therefore the results in this study may not be applicable to a less experienced surgeon or lower-volume surgeon. Third, we limited our assessment of the component alignment to the coronal plane and did not include any lateral measurements or assessments of rotational alignment. We use a scout CT image as part of our routine postoperative evaluation. The CT scout image is nonweightbearing compared with a full-leg standing radiograph but has the added advantage of being able to control the extremity for rotation, which is helpful when making these radiographic measurements. Finally, we included only comparisons of the operative times and radiographic measurements to determine cost-utility for this study and we did not factor in clinical results or patient satisfaction scores.
To date, there has been little published looking at the use of patient-specific cutting guides for TKA [12, 13, 18, 22, 25, 30] and there have been no reports on the cost-utility of this new technology. Two of the published articles [13, 18] focused on the kinematic alignment for their patient-specific cutting guides, which varies considerably from the mechanical alignment techniques used in our study. Two additional studies were reviewed and neither included human subjects as they looked at (1) finite analysis of stress distribution using patient-specific technology [12] and (2) the accuracy of CT versus MRI to determine which imaging modality is more accurate when manufacturing patient-specific guides [30]. In one study, the authors reported on their initial experience with 21 patient-specific cutting blocks and reported a mean reduction in tourniquet time of 13 minutes and subjectively they reported experiencing a decrease in operative setup time and turnover time; however, they stated they did not study these data [25].
Although several articles have described component alignment [13, 18, 22, 25] when using patient-specific guides, none to date has compared coronal alignment between TKAs using patient-specific cutting blocks using the mechanical alignment design and those using conventional techniques.
We found a trend (nonsignificant) for slight improvement in tourniquet time and skin-to-skin time. This study was conducted during the early learning curve for this high-volume surgeon who has performed several thousand TKAs with conventional instrumentation and it is possible the patient-specific guides might have been able to achieve greater improvement in tourniquet time and skin-to-skin time with more experience. In certain high-volume centers, it may be possible to group together all patient-specific TKAs on the same day and achieve greater time savings to allow the surgeon to either perform more procedures that day or go home earlier. In our study, we did have less total operating room time for the patient-specific group and we relate this to the fact that we were able to eliminate opening as many trays at the beginning of the surgery and then removing them at the end of the surgery. With fewer trays, this allowed the room circulator more time to help with getting the patient on the table, placing the Foley catheter, and preparing the extremity at the beginning and they also were more available to help get the dressing on, drapes off, and patient off the operating table at the end of the procedure.
Additional time commitment was required by patients and physicians for the patient-specific guides. On the patient side, this included the time for the MRI, which at our institution usually was not available the same day as the patient’s office visit. Therefore, most patients had to make an additional trip to the hospital on a different day to get the MRI, which takes a minimum of 2 hours at our institution (parking, waiting room, actual MRI). This also does not take into account the amount of time it takes for the patient, and possibly their family members, to travel to the hospital and any time lost from work to make this additional visit. On the surgeon side, there was the increased time needed for ancillary staff to schedule the MRI and ensure the scans were performed accurately to the specifics of the manufacturer and properly uploaded onto the manufacturer’s system. After the engineers created the surgical plan for the bone cuts, the surgeon had to log into the system and approve this plan, which took approximately 5 to 15 minutes per case depending on the complexity of the knee, surgeon’s computer skills, and tendency to want to make changes to the cut plan. If the surgeon made changes or had any questions about the surgical plan, this generally involved additional time spent communicating with the engineer. This time commitment by the patient and surgeon was in addition to the actual cost of the MRI, which ranged from several hundred dollars to more than a thousand dollars, and the up-charge to the hospital for actual customized cutting guides by the implant vendors, which also ranged from several hundred dollars to a thousand dollars.
Critically evaluating new technology for TKA is important to ensure it improves either clinical efficiency or patient outcomes to justify any additional costs to the healthcare system. Patient-specific cutting blocks have many proposed advantages over conventional instrumentation for TKA, but in the literature and in this study, we were unable to show any substantial improvement in clinical efficiency or component alignment to justify the increased costs associated with this new technology. At this time, routine use of this new technology does not appear to be cost-effective for a high-volume subspecialty-trained surgeon, but future studies are needed to determine whether there are any clinically important improvements in outcomes or patient satisfaction when using patient-specific cutting blocks for TKA.
Footnotes
One author (RMN) is a paid consultant for Smith & Nephew Orthopaedics (Memphis, TN, USA), Wright Medical Technology Inc (Arlington, TN, USA), Salient Surgical Technologies Inc (Portsmouth, NH, USA), and CardioMEMS Inc (Atlanta, GA, USA). One author (RLB) has received royalties from Smith & Nephew Orthopaedics. Each of the remaining authors certifies that he or she, or a member of his or her immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at Washington University.
References
- 1.Bargren JH, Blaha JD, Freeman MA. Alignment in total knee arthroplasty: correlated biomechanical and clinical observations. Clin Orthop Relat Res. 1983;173:178–183. [PubMed] [Google Scholar]
- 2.Bathis H, Perlick L, Tingart M, Luring C, Zurakowski D, Grifka J. Alignment in total knee arthroplasty: a comparison of computer-assisted surgery with the conventional technique. J Bone Joint Surg Br. 2004;86:682–687. doi: 10.1302/0301-620X.86B5.14927. [DOI] [PubMed] [Google Scholar]
- 3.Bauwens K, Matthes G, Wich M, Gebhard F, Hanson B, Ekkernkamp A, Stengel D. Navigated total knee replacement: a meta-analysis. J Bone Joint Surg Am. 2007;89:261–269. doi: 10.2106/JBJS.F.00601. [DOI] [PubMed] [Google Scholar]
- 4.Berend ME, Ritter MA, Meding JB, Faris PM, Keating EM, Redelman R, Faris GW, Davis KE. Tibial component failure mechanisms in total knee arthroplasty. Clin Orthop Relat Res. 2004;428:26–34. doi: 10.1097/01.blo.0000148578.22729.0e. [DOI] [PubMed] [Google Scholar]
- 5.Blakeney WG, Khan RJ, Wall SJ. Computer-assisted techniques versus conventional guides for component alignment in total knee arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2011;93:1377–1384. doi: 10.1302/0301-620X.93B10.27224. [DOI] [PubMed] [Google Scholar]
- 6.Bozic KJ, Morshed S, Silverstein MD, Rubash HE, Kahn JG. Use of cost-effectiveness analysis to evaluate new technologies in orthopaedics: the case of alternative bearing surfaces in total hip arthroplasty. J Bone Joint Surg Am. 2006;88:706–714. doi: 10.2106/JBJS.E.00614. [DOI] [PubMed] [Google Scholar]
- 7.Brin YS, Nikolaou VS, Joseph L, Zukor DJ, Antoniou J. Imageless computer assisted versus conventional total knee replacement: a Bayesian meta-analysis of 23 comparative studies. Int Orthop. 2011;35:331–339. doi: 10.1007/s00264-010-1008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandrasekaran S, Molnar RB. Minimally invasive imageless computer-navigated knee surgery: initial results. J Arthroplasty. 2008;23:441–445. doi: 10.1016/j.arth.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 9.Chauhan SK, Scott RG, Breidahl W, Beaver RJ. Computer-assisted knee arthroplasty versus a conventional jig-based technique: a randomised, prospective trial. J Bone Joint Surg Br. 2004;86:372–377. doi: 10.1302/0301-620X.86B3.14643. [DOI] [PubMed] [Google Scholar]
- 10.Desai AS, Dramis A, Kendoff D, Board TN. Critical review of the current practice for computer-assisted navigation in total knee replacement surgery: cost-effectiveness and clinical outcome. Curr Rev Musculoskelet Med. 2011;4:11–15. doi: 10.1007/s12178-011-9071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang DM, Ritter MA, Davis KE. Coronal alignment in total knee arthroplasty: just how important is it? J Arthroplasty. 2009;24(6 suppl):39–43. doi: 10.1016/j.arth.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 12.Harrysson OL, Hosni YA, Nayfeh JF. Custom-designed orthopedic implants evaluated using finite element analysis of patient-specific computed tomography data: femoral-component case study. BMC Musculoskelet Disord. 2007;8:91. doi: 10.1186/1471-2474-8-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howell SM, Hodapp EE, Kuznik K, Hull ML. In vivo adduction and reverse axial rotation (external) of the tibial component can be minimized. Orthopedics. 2009;32:319. doi: 10.3928/01477447-20090501-04. [DOI] [PubMed] [Google Scholar]
- 14.Iorio R, Robb WJ, Healy WL, Berry DJ, Hozack WJ, Kyle RF, Lewallen DG, Trousdale RT, Jiranek WA, Stamos VP, Parsley BS. Orthopaedic surgeon workforce and volume assessment for total hip and knee replacement in the United States: preparing for an epidemic. J Bone Joint Surg Am. 2008;90:1598–1605. doi: 10.2106/JBJS.H.00067. [DOI] [PubMed] [Google Scholar]
- 15.Jeffery RS, Morris RW, Denham RA. Coronal alignment after total knee replacement. J Bone Joint Surg Br. 1991;73:709–714. doi: 10.1302/0301-620X.73B5.1894655. [DOI] [PubMed] [Google Scholar]
- 16.Kamath AF, Israelite C, Horneff J, Lotke PA. Editorial: What is varus or valgus knee alignment? A call for a uniform radiographic classification. Clin Orthop Relat Res. 2010;468:1702–1704. [DOI] [PMC free article] [PubMed]
- 17.Kim YH, Kim JS, Choi Y, Kwon OR. Computer-assisted surgical navigation does not improve the alignment and orientation of the components in total knee arthroplasty. J Bone Joint Surg Am. 2009;91:14–19. doi: 10.2106/JBJS.G.01700. [DOI] [PubMed] [Google Scholar]
- 18.Klatt BA, Goyal N, Austin MS, Hozack WJ. Custom-fit total knee arthroplasty (OtisKnee) results in malalignment. J Arthroplasty. 2008;23:26–29. doi: 10.1016/j.arth.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Kumar PJ, Dorr LD. Severe malalignment and soft-tissue imbalance in total knee arthroplasty. Am J Knee Surg. 1997;10:36–41. [PubMed] [Google Scholar]
- 20.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 21.Kurtz SM, Ong KL, Schmier J, Mowat F, Saleh K, Dybvik E, Karrholm J, Garellick G, Havelin LI, Furnes O, Malchau H, Lau E. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(suppl 3):144–151. doi: 10.2106/JBJS.G.00587. [DOI] [PubMed] [Google Scholar]
- 22.Lombardi AV, Jr, Berend KR, Adams JB. Patient-specific approach in total knee arthroplasty. Orthopedics. 2008;31:927–930. doi: 10.3928/01477447-20080901-21. [DOI] [PubMed] [Google Scholar]
- 23.Nunley RM, Ellison BS, Zhu J, Ruh EL, Howell SM, Barrack RL. Do patient-specific guides improve coronal alignment in total knee arthroplasty? Clin Orthop Relat Res. 2011. doi:10.1007/s11999-011-2222-2. [DOI] [PMC free article] [PubMed]
- 24.Sparmann M, Wolke B, Czupalla H, Banzer D, Zink A. Positioning of total knee arthroplasty with and without navigation support: a prospective, randomised study. J Bone Joint Surg Br. 2003;85:830–835. [PubMed] [Google Scholar]
- 25.Spencer BA, Mont MA, McGrath MS, Boyd B, Mitrick MF. Initial experience with custom-fit total knee replacement: intra-operative events and long-leg coronal alignment. Int Orthop. 2009;33:1571–1575. doi: 10.1007/s00264-008-0693-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stulberg SD, Loan P, Sarin V. Computer-assisted navigation in total knee replacement: results of an initial experience in thirty-five patients. J Bone Joint Surg Am. 2002;84(suppl 2):90–98. [PubMed] [Google Scholar]
- 27.Tingart M, Luring C, Bathis H, Beckmann J, Grifka J, Perlick L. Computer-assisted total knee arthroplasty versus the conventional technique: how precise is navigation in clinical routine? Knee Surg Sports Traumatol Arthrosc. 2008;16:44–50. doi: 10.1007/s00167-007-0399-4. [DOI] [PubMed] [Google Scholar]
- 28.Victor J, Hoste D. Image-based computer-assisted total knee arthroplasty leads to lower variability in coronal alignment. Clin Orthop Relat Res. 2004;428:131–139. doi: 10.1097/01.blo.0000147710.69612.76. [DOI] [PubMed] [Google Scholar]
- 29.Wasielewski RC, Galante JO, Leighty RM, Natarajan RN, Rosenberg AG. Wear patterns on retrieved polyethylene tibial inserts and their relationship to technical considerations during total knee arthroplasty. Clin Orthop Relat Res. 1994;299:31–43. [PubMed] [Google Scholar]
- 30.White D, Chelule KL, Seedhom BB. Accuracy of MRI vs CT imaging with particular reference to patient specific templates for total knee replacement surgery. Int J Med Robot. 2008;4:224–231. doi: 10.1002/rcs.201. [DOI] [PubMed] [Google Scholar]


