Abstract
Background
Pathologic proximal femur fractures result in substantial morbidity for patients with skeletal metastases. Surgical treatment is widely regarded as effective; however, failure rates associated with the most commonly used operative treatments are not well defined.
Questions/purposes
We therefore compared surgical treatment failure rates among intramedullary nailing, endoprosthetic reconstruction, and open reduction-internal fixation when applied to impending or displaced pathologic proximal femur fractures.
Patients and Methods
We retrospectively compared the clinical course of 298 patients who underwent intramedullary nailing (n = 82), endoprosthetic reconstruction (n = 197), or open reduction-internal fixation (n = 19) from 1993 to 2008. Primary outcome was treatment failure, which was defined as reoperation for any reason. Treatment groups were compared for differences in demographic and clinical parameters.
Results
The number of treatment failures in the endoprosthetic reconstruction group (3.1%) was significantly lower than in the intramedullary nailing (6.1%) and open reduction-internal fixation (42.1%) groups. The number of revisions requiring implant exchange also was significantly lower for endoprosthetic reconstruction (0.5%), compared with intramedullary nailing (6.1%) and open reduction-internal fixation (42.1%).
Conclusions
Endoprosthetic reconstruction is associated with fewer treatment failures and greater implant durability. Prospective studies are needed to determine the impact of operative strategy on function and quality of life.
Level of Evidence
Level III, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Femoral metastasis is a leading cause of morbidity in patients with advanced-stage cancer [2]. Surgical treatment of pathologic fractures is widely regarded as an effective measure for managing the sequelae of femoral metastases [5, 7]. Given the increasing longevity of patients with skeletal metastases, surgical treatment now plays an even more important role in maintaining function and quality of life. Currently, surgical indications for pathologic femur fractures are heavily informed by retrospective series describing the performance of a single surgical technique with time. Moreover, few attempts have been made to compare the effectiveness of the most commonly used surgical techniques [13, 17]. Therefore, it is unclear whether there are actual differences in the frequency and type of treatment failures among common surgical techniques.
Two-thirds of all long-bone pathologic fractures occur in the femur, the majority of which involve the proximal half, specifically the intertrochanteric/subtrochanteric region and the femoral neck [4]. Surgical treatment of impending or displaced pathologic proximal femur fractures can be reasonably accomplished using one of several different approaches. Three operative strategies are commonly used, each of which features a different implant: (1) endoprosthetic reconstruction (EPR); (2) intramedullary nailing (IMN); or (3) extramedullary plate/screw fixation devices (open reduction-internal fixation [ORIF]). After the selection of an operative strategy, other treatment methods that are thought to enhance the effectiveness of surgical treatment are routinely incorporated into the overall treatment plan: (1) preoperative or postoperative radiation therapy [14, 15]; (2) simultaneous tumor debulking [4]; and (3) construct augmentation with methylmethacrylate cement [3]. Few published series provide a detailed rationale for incorporating radiation or surgical adjuvants into the overall treatment plan, nor do they analyze the effect these important covariates might have on treatment outcomes [7, 12–15, 17].
When treating pathologic proximal femur fractures, the surgeon must first select an operative strategy and then decide whether to perform tumor debulking, use construct augmentation, or recommend radiation therapy in combination with surgical treatment. Because suitable comparative analyses are lacking to guide these decisions, we compared the failure rates among the three operative strategies most commonly used to treat pathologic proximal femur fractures (ie, EPR, IMN, and ORIF). Additionally, we compared the rate of revision requiring implant exchange to determine whether there are any differences in implant durability among these operative strategies.
Patients and Methods
We performed a comparison of patients treated surgically for impending or displaced pathologic femur fractures in one institution to determine the frequency of treatment failure, which was defined as reoperation for any reason. We obtained approval from our institutional review board to perform this study. In a retrospective review of prospectively gathered data from our departmental surgical database, we identified 298 consecutive patients during a 15-year period (1993–2008) treated surgically for impending or displaced fractures above the femoral isthmus, excluding the femoral neck. Because reoperations often occurred in the immediate postoperative period, we did not assign a minimum followup criterion to capture this important clinical end point. Patients treated nonoperatively were not readily identifiable using available methods, so they were excluded from the analysis. Other exclusion criteria included simultaneous acetabular resurfacing (ie, THA) and primary tumors of the proximal femur.
Three treatment groups were created based on operative strategy: (1) EPR (n = 197); (2) IMN (n = 82); and (3) ORIF (n = 19). Among the treatment groups, we compared age, the presence or absence of fracture displacement, the number of major medical comorbidities, Eastern Cooperative Oncology Group (ECOG) status score, the use of preoperative or postoperative radiation therapy, and preoperative laboratory values (Table 1). Datasets were greater than 85% complete for each category analyzed. The treatment groups also were analyzed according to type of implant used (Fig. 1), distribution of oncologic diagnoses (Fig. 2), and site(s) of proximal femoral involvement (Table 2).
Table 1.
Patient characteristics by treatment group
| Characteristic | Endoprosthetic reconstruction (n = 197) | Intramedullary nailing (n = 82) | Open reduction-internal fixation (n = 19) | p Value |
|---|---|---|---|---|
| Median age (years) | 62.4 | 61.8 | 55.7 | 0.09, 0.67, 0.13 |
| Displaced fractures | 40% | 33% | 32% | 0.02 |
| Number of comorbidities* | 1 (0–5) | 1 (0–5) | 1 (0–3) | 0.87 |
| ECOG ≤ 2 | 61% | 88% | 47% | 0.00 |
| ECOG ≥ 3 | 39% | 12% | 53% | |
| Radiation therapy | 28% | 41% | 26% | 0.12 |
| Preoperative | 47% | 39% | 100% | |
| Postoperative | 53% | 61% | 0% | |
| Preoperative laboratory values | ||||
| Total calcium (mg/dL) | 8.0 | 8.2 | 9.4 | |
| Creatinine (mg/dL) | 0.8 | 0.8 | 1.0 | |
| Albumin (mg/dL) | 3.9 | 3.8 | 4.5 | |
| Hemoglobin (g/dL) | 10.8 | 10.0 | 11.7 | |
| Alkaline phosphatase (units/L) | 89 | 93 | 86 | |
* Values are expressed as median, with range in parentheses; ECOG = Eastern Cooperative Oncology Group.
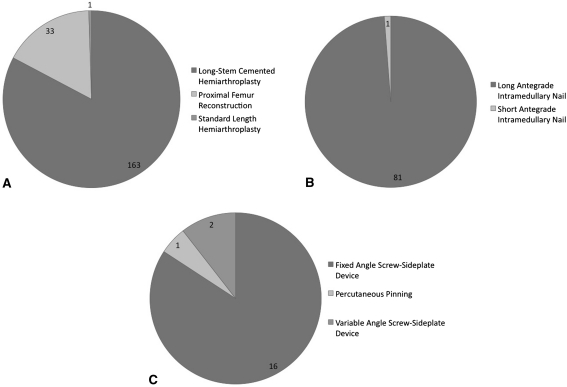
Fig. 1A–C.
The pie charts show the distribution of implants by surgical procedure type. (A) The majority if EPRs were long-stem cemented hemiarthroplasties. (B) For IMN, all implants were long antegrade nails, with the exception of one short antegrade nail. (C) For ORIF, most implants were hip screw-sideplate devices.
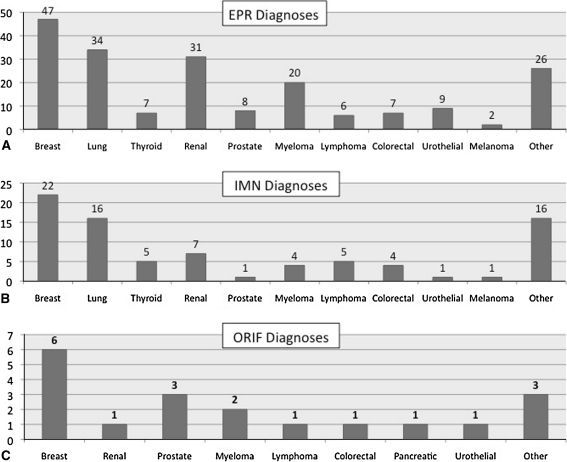
Fig. 2A–C.
The histograms show the distribution of oncologic diagnoses in the (A) EPR, (B) IMN, and (C) ORIF groups. The most common diagnoses in the EPR and IMN groups were breast, lung, and renal cell carcinoma. Breast carcinoma, prostate carcinoma, and myeloma, which are types of cancer typically considered to be radiosensitive, were the three most common diagnoses in the ORIF group.
Table 2.
Sites of proximal femur involvement by treatment group
| Femoral site | Endoprosthetic reconstruction (n = 197) | Intramedullary nailing (n = 82) | Open reduction-internal fixation (n = 19) |
|---|---|---|---|
| Intertrochanteric | 62 (32%) | 5 (6%) | 10 (53%) |
| Intertrochanteric/subtrochanteric | 53 (27%) | 2 (2%) | 6 (32%) |
| Subtrochanteric/proximal diaphysis | 82 (41%) | 75 (92%) | 3 (15%) |
All surgical procedures were performed by four fellowship-trained musculoskeletal oncologists (PJB, CMM, EA, JHH). Because of the retrospective study design and multiple surgeons contributing to the study, the selection of operative strategy was not based on uniform criteria but was strongly influenced by the treating surgeon’s clinical judgment. Surgical decision making was determined by (1) anatomic location of the lesion in the proximal femur; (2) degree of cortical destruction; (3) presence or absence of fracture displacement; (4) severity of pain; (5) degree of lesion mineralization; (6) surgeon’s estimate of survival; and (7) the patient’s treatment preference [8, 9].
In all EPRs, tumor debulking was performed using an intralesional technique followed by implant cementation. In general, intralesional resection was performed for IMN and ORIF for lesions of intermediate or low radiosensitivity. When intralesional resection was performed for IMN or ORIF, methylmethacrylate cement augmentation also was applied. All patients were allowed to bear full weight immediately after surgery. The rationale for assigning patients to ORIF was consistent among the treating surgeons insofar as ORIF was selected for patients with heavily mineralized lesions such as prostate cancer that would make instrumentation of the intramedullary canal exceedingly difficult and for patients with severely compromised pulmonary function that risked worsening after pressurization of the intramedullary canal with EPR or IMN. EPR, IMN, or ORIF were evenly distributed throughout the study period.
For all patients, we reviewed data regarding patient comorbidities, ECOG status score, timing and frequency of reoperations, indications for reoperation, number of revisions requiring implant exchange, patient survival, use of preoperative or postoperative radiation therapy, use of curettage and cementation in IMN, and surgery-related complications. We also sought to define the mechanisms of failure for each patient based on the most common mechanisms cited in the literature, and on observations from our clinical experience: (1) disease progression, defined by increasing lesion size on serial radiographs associated with pain and dysfunction; (2) nonunion, defined as failure to achieve painless radiographic union within 6 months of surgical treatment; (3) fracture displacement or conversion of an impending to displaced pathologic fracture; (4) hardware failure, defined as screw cutout or frank hardware fracture; (5) infection; and (6) dislocation. We also reviewed preoperative radiographs for each patient to confirm whether displacement was present before surgery and to verify the anatomic location of the metastatic lesion.
Univariate analysis of treatment group characteristics was conducted using the Mann-Whitney U test. Kaplan-Meier survival analysis was performed using the log-rank test to compare overall survival among the treatment groups. Differences were considered statistically significant when the p value was less than 0.05. All statistical analyses were performed using SPSS® 16.0 software (SPSS Inc, Chicago, IL, USA). Bonferroni correction was used for multiple comparisons.
Results
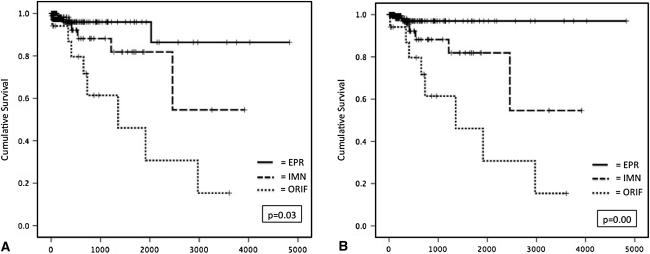
There was a significant difference in the treatment failure rates among the EPR (3.1%; six of 197), IMN (6.0%; five of 82), and ORIF (42.1%; eight of 19) groups (p < 0.05, log-rank test) (Fig. 3A). The mechanisms of treatment failure varied by surgical method (Table 3). The most common reason for reoperation in the EPR group was dislocation, most of which occurred within 90 days of surgery. In nearly all cases, one unsuccessful closed reduction was attempted followed by open reduction and reconstruction of soft tissue constraints about the hip. No subsequent dislocations occurred. The most common reasons for reoperation in the IMN and ORIF groups were nonunion and painful disease progression (Table 3). In most instances of treatment failure, both were present.
Fig. 3A–B.
Kaplan–Meier survival analysis was used to compare (A) the number of reoperations and (B) the number of reoperations requiring implant exchange revision among the treatment groups. There was a significant difference in the number of reoperations (p = 0.03) and revisions requiring implant exchange (p = 0.00) among the EPR, IMN, and ORIF groups.
Table 3.
Indications for reoperations by treatment group
| Treatment group | Indications for reoperation |
|---|---|
| Endoprosthetic reconstruction (6 of 197 patients) | Dislocation (n = 5) |
| Conventional hemiarthroplasty (n = 3) | |
| Proximal femur replacement (n = 2) | |
| Painful disease progression (n = 1) | |
| Intramedullary nailing (5 of 82 patients) | Nonunion/painful disease progression (n = 3) |
| Implant fracture secondary to nonunion (n = 1) | |
| Screw cutout (n = 1) | |
| Open reduction-internal fixation (8 of 19 patients) | Nonunion (n = 2) |
| Painful disease progression (n = 3) | |
| Screw cutout (n = 2) | |
| Implant fracture (n = 1) |
There was also a significant difference in the rate of revision requiring implant exchange among the treatment groups (EPR = 0.5%, one of 197; IMN = 6.0%, five of 82; ORIF = 42.1%, eight of 19) (p < 0.01, log-rank test) (Fig. 3B). For the IMN and ORIF groups, no difference in implant survival was observed between patients who were treated with curettage and cementation (IMN, n = 31 patients; ORIF, n = 8 patients) versus those who received an implant alone (IMN, n = 51; ORIF, n = 11) (IMN, p = 0.44; ORIF, p = 0.27; Mann-Whitney U test). All IMN and ORIF failures requiring an implant exchange procedure were converted to EPR, except for one IMN failure, which was treated with exchange nailing. No EPRs performed for salvage of internal fixation required reoperation.
Discussion
Pathologic proximal femur fractures cause substantial morbidity for patients with advanced-stage cancer. In this study, we compared the effectiveness of the most common operative strategies by assigning reoperation as the de facto definition of treatment failure. To our knowledge, this study represents the largest series of patients treated for impending or displaced pathologic proximal femur fractures.
We attempted to minimize the effects of random variability in our group comparisons by using large sample sizes. This was not possible for ORIF as they were performed at a substantially lower frequency during the study period than EPR and IMN. Four fellowship-trained musculoskeletal oncologists performed all of the surgical procedures; however, because nonuniform surgical indications were applied, selection bias could not be eliminated from the analysis. Furthermore, in the context of a 15-year study period, surgeon preference and experience with EPR, IMN, and ORIF likely evolved. Although data were not presented formally, the use of EPR, IMN, and ORIF was consistent and did not vary throughout the study period. Given that we selected a retrospective comparison study design, it is impossible to remove certain systematic biases, such as selection or ascertainment bias. Therefore, we presented data on baseline ECOG status score, use of preoperative or postoperative radiation therapy, use of methylmethacrylate augmentation, and broadly relevant laboratory parameters to better characterize the treatment groups.
In our cohort, EPR was associated with the fewest reoperations and implant exchange revisions and therefore should be strongly considered for treatment of pathologic proximal femur fractures, particularly for anatomic regions where surgical indications overlap. The most successful operative strategies offer the greatest implant durability for the patient’s remaining lifespan, minimizing the risk of reoperation-related morbidity. Reoperations are intrusive events that often parallel a patient’s declining health status. Frequently, complex procedures are necessary to remedy treatment failures, such as implant exchange revision, that can be disabling and require extensive rehabilitation. Certainly, the major acute risk associated with EPR and IMN is the potential for cardiopulmonary arrest with instrumentation of the intramedullary canal. Intraoperative deaths have been reported for EPR and IMN [1, 10, 11]; however, no difference in early mortality has been shown among EPR, IMN, and ORIF.
Although they did not calculate a specific failure rate, Jacofsky et al. [6] reported on the results and complications of hip arthroplasty performed as a salvage procedure for failed treatment of pathologic proximal femoral fractures secondary to malignancy. During a 20-year period, 42 patients required hip arthroplasties to salvage failed internal fixation. The most common indications for salvage were nonunion and painful disease progression. The mean time from internal fixation to the salvage procedure was 14.7 months; however, eight of the 42 patients (20%) required revision within 3 months. Salvage of internal fixation to hip arthroplasty was associated with a 12% incidence of major complications, including deep prosthetic infection, myocardial infarction, cerebrovascular accident, and exacerbation of congestive heart failure.
Our results are also consistent with those of Wedin and Bauer [17], who performed a retrospective comparison of the risk of reoperation among patients treated for proximal femoral metastases with internal fixation or EPR. Routine use of EPR was recommended based on a higher risk of reoperation at 2 years for patients treated with any type of osteosynthetic device, compared with patients treated with any type of endoprosthesis. As in our study, all internal fixation failures were converted to endoprostheses, except in one case in which exchange nailing was required owing to a technical error during the initial procedure. In the study by Wedin and Bauer [17], the local rate of failure for each treatment method was 16.2% (six of 37 procedures involving osteosynthetic devices) and 8.3% (nine of 109 procedures involving endoprostheses). Thus, EPR was associated with an approximately 50% reduction in failure rate.
Others have reported more favorable primary results after the use of internal fixation devices than ours. For example, Ward et al. [16] reported on 128 patients treated with IMN or other forms of internal fixation for impending or displaced pathologic femur fractures. Of these 128 patients, four (3.1%) underwent revision for nonunion, painful disease progression, or infection. Sarahrudi et al. [12] reviewed 142 patients who underwent surgical treatment of femoral metastases and found similar complication rates for IMN and EPR.
A significant difference in treatment failure rates exists among the three operative strategies most commonly used to treat impending or displaced pathologic proximal femur fractures. EPR was the most effective method because it had the lowest reoperation rate and the highest implant retention rate. Nearly all failed internal fixations were successfully converted to EPRs. In recognition of the study limitations, we cannot give firm recommendations for the treatment of impending or displaced pathologic fractures of the proximal femur. However, based on our findings, we believe EPR should be considered for the management of lesions involving the intertrochanteric, pertrochanteric, and subtrochanteric/proximal diaphyseal regions of the proximal femur. Prospective studies are needed to better define the relationship between surgical treatment method and other factors that influence the success or failure of treatment, such as pain management, functional restoration, and maintenance of quality of life.
Footnotes
One or more of the authors (MS) have received support from the Major Family Fellowship in Musculoskeletal Oncology.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Memorial Sloan-Kettering Cancer Center.
References
- 1.Barwood SA, Wilson JL, Molnar RR, Choong PF. The incidence of acute cardiorespiratory and vascular dysfunction following intramedullary nail fixation of femoral metastasis. Acta Orthop Scand. 2000;71:147–152. doi: 10.1080/000164700317413111. [DOI] [PubMed] [Google Scholar]
- 2.DeVita VT Jr, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. 7. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 3.Harrington KD. The use of methylmethacrylate as an adjunct in the internal fixation of unstable comminuted intertrochanteric fractures in osteoporotic patients. J Bone Joint Surg Am. 1975;57:744–750. [PubMed] [Google Scholar]
- 4.Harrington KD. The management of malignant pathologic fractures. Instr Course Lect. 1977;26:147–162. [Google Scholar]
- 5.Healey JH, Lane JM. Treatment of pathologic fractures of the distal femur with the Zickel supracondylar nail. Clin Orthop Relat Res. 1990;250:216–220. [PubMed] [Google Scholar]
- 6.Jacofsky DJ, Haidukewych GJ, Zhang H, Sim FH. Complications and results of arthroplasty for salvage of failed treatment of malignant pathologic fractures of the hip. Clin Orthop Relat Res. 2004;427:52–56. doi: 10.1097/01.blo.0000143572.96021.93. [DOI] [PubMed] [Google Scholar]
- 7.Lane JM, Sculco TP, Zolan S. Treatment of pathological fractures of the hip by endoprosthetic replacement. J Bone Joint Surg Am. 1980;62:954–959. [PubMed] [Google Scholar]
- 8.Mirels H. Metastatic disease in long bones: a proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res. 1989;249:256–264. [PubMed] [Google Scholar]
- 9.Nathan SS, Healey JH, Mellano D, Hoang B, Lewis I, Morris CD, Athanasian EA, Boland PJ. Survival in patients operated on for pathologic fracture: implications for end-of-life orthopedic care. J Clin Oncol. 2005;23:6072–6082. doi: 10.1200/JCO.2005.08.104. [DOI] [PubMed] [Google Scholar]
- 10.Patterson BM, Healey JH, Cornell CN, Sharrock NE. Cardiac arrest during hip arthroplasty with a cemented long-stem component: a report of seven cases. J Bone Joint Surg Am. 1991;73:271–277. [PubMed] [Google Scholar]
- 11.Peter RE, Schopfer A, Le Coultre B, Hoffmeyer P. Fat embolism and death during prophylactic osteosynthesis of a metastatic femur using an unreamed femoral nail. J Orthop Trauma. 1997;11:233–234. doi: 10.1097/00005131-199704000-00018. [DOI] [PubMed] [Google Scholar]
- 12.Sarahrudi K, Greitbauer M, Platzer P, Hausmann JT, Heinz T, Vécsei V. Surgical treatment of metastatic fractures of the femur: a retrospective analysis of 142 patients. J Trauma. 2009;66:1158–1163. doi: 10.1097/TA.0b013e3181622bca. [DOI] [PubMed] [Google Scholar]
- 13.Talbot M, Turcotte RE, Isler M, Normandin D, Iannuzzi D, Downer P. Function and health status in surgically treated bone metastases. Clin Orthop Relat Res. 2005;438:215–220. doi: 10.1097/01.blo.0000170721.07088.2e. [DOI] [PubMed] [Google Scholar]
- 14.Townsend PW, Rosenthal HG, Smalley SR, Cozad SC, Hassanein RE. Impact of postoperative radiation therapy and other perioperative factors on outcome after orthopedic stabilization of impending or pathologic fractures due to metastatic disease. J Clin Oncol. 1994;12:2345–2350. doi: 10.1200/JCO.1994.12.11.2345. [DOI] [PubMed] [Google Scholar]
- 15.Townsend PW, Smalley SR, Cozad SC, Rosenthal HG, Hassanein RE. Role of postoperative radiation therapy after stabilization of fractures caused by metastatic disease. Int J Radiat Oncol Biol Phys. 1995;31:43–49. doi: 10.1016/0360-3016(94)E0310-G. [DOI] [PubMed] [Google Scholar]
- 16.Ward WG, Holsenbeck S, Dorey FJ, Spang J, Howe D. Metastatic disease of the femur: surgical treatment. Clin Orthop Relat Res. 2003;415(suppl):S230–S244. doi: 10.1097/01.blo.0000093849.72468.82. [DOI] [PubMed] [Google Scholar]
- 17.Wedin R, Bauer HC. Surgical treatment of skeletal metastatic lesions of the proximal femur: endoprosthesis or reconstruction nail? J Bone Joint Surg Br. 2005;87:1653–1657. doi: 10.1302/0301-620X.87B12.16629. [DOI] [PubMed] [Google Scholar]