Abstract
Background
Failure of endoprosthetic reconstruction with conventional stems due to aseptic loosening remains a challenge for maintenance of limb integrity and function. The Compress® implant (Biomet Inc, Warsaw, IN, USA) attempts to avoid aseptic failure by means of a unique technologic innovation. Though the existing literature suggests survivorship of Compress® and stemmed implants is similar in the short term, studies are limited by population size and followup duration.
Questions/purposes
We therefore compared (1) the rate of aseptic failure between Compress® and cemented intramedullary stems and (2) evaluated the overall intermediate-term implant survivorship.
Methods
We reviewed 26 patients with Compress® implants and 26 matched patients with cemented intramedullary stems. The patients were operated on over a 3-year period. Analysis focused on factors related to implant survival, including age, sex, diagnosis, infection, aseptic loosening, local recurrence, and fracture. Minimum followup was 0.32 years (average, 6.2 years; range, 0.32–9.2 years).
Results
Aseptic failure occurred in one (3.8%) patient with a Compress® implant and three (11.5%) patients with cemented intramedullary stems. The 5-year implant survival rate was 83.5% in the Compress® group and 66.6% in the cemented intramedullary stem group.
Conclusions
The Compress® implant continues to be a reliable option for distal femoral limb salvage surgery. Data regarding aseptic failure is encouraging, with equivalent survivorship against cemented endoprosthetic replacement at intermediate-term followup.
Level of Evidence
Level III, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
For primary tumors of the distal femur, endoprosthetic replacement (EPR) as a limb salvage option achieves functional and cosmetic results that make it a reasonable choice as compared to amputation [9, 16, 18, 23]. With advances in the medical management of musculoskeletal malignancies, patient survival has improved substantially [11]. Thus, specific means to address aseptic failure, the primary cause of EPR failure at intermediate to long term, are needed.
Compress® Compliant Pre-Stress (CPS) technology (Biomet Inc, Warsaw, IN, USA) utilizes fundamental principles of osseointegration to provide an innovative alternative to traditional cemented (CIS) or uncemented (UIS) intramedullary stems in EPR. Theoretical considerations of compressive osseointegration and practical applications of CPS technology have been previously described [2, 3, 5–7, 17, 21]. Briefly, the implant is secured to host cortical bone via a short intramedullary segment with transverse pins. This point of fixation provides anchorage for securing a spindle at the bone-prosthetic interface using high compressive force. Stress shielding is theoretically avoided, bone hypertrophy is induced, and the medullary canal is sealed from wear debris, with a resultant potential for more durable device survivorship.
Early reports of outcomes with CPS technology have been encouraging but are limited [2, 3, 5, 17, 21]. These studies suggest CPS implants induce viable local bone hypertrophy [17], have utility in various upper- and lower-extremity locations [6, 21], and minimize bone loss in revision settings [26]. The review with the longest followup of the CPS device is a single institution study demonstrating equivalent survivorship when compared to UIS EPR at an average of 45 months of followup (range, 3–85 months) [7]. The authors also found there were no failures of CPS implants after 1 year while the UIS devices continued to fail after a longer postoperative duration [7]. As a part of a study approved by the FDA, we previously compared a group of 26 patients who underwent distal femoral reconstruction with the CPS implant to a matched group of 26 patients who underwent CIS EPR at a separate institution [3]. Results from these two patient groups at 2-year average followup demonstrated equivalent implant survivorship when comparing implant revision or amputation for infection, local recurrence, fracture, or mechanical failure. To confirm the earlier findings, we now report data from the same patient groups at intermediate-term followup.
Based on this background, we presumed we would find (1) no difference in the number of patients requiring revision or amputation for aseptic failure and (2) no difference in overall implant survival between the CPS and CIS cohorts.
Patients and Methods
We retrospectively reviewed prospectively collected data of 26 patients who underwent distal femoral reconstruction with the CPS implant at one institution and 26 matched patients treated at another institution with a CIS implant. The CPS cohort consisted of 26 patients operated on by a single surgeon at University of California San Francisco as part of the original FDA approval process for the CPS implant. These patients were matched, according to age and reason for operation, over the operative time period of January 1, 2000, to December 31, 2002, to 26 CIS patients operated on by three surgeons at the Royal Orthopaedic Hospital. Data were reviewed from the orthopaedic databases at these institutions. Incomplete information was supplemented from the original patient records. The indication for surgery in both cohorts consisted of reconstruction of distal femoral defects after resection of a primary bone malignancy or failure of an oncologic distal femoral replacement. A relative contraindication for surgery was prior radiation therapy, and in the case of the CPS device, the presence of osteonecrotic bone. Osteosarcoma constituted the primary diagnosis in both groups (CPS: 65%; CIS: 69%) (Table 1). The average age for the 52 patients at time of surgery was 24.6 years (CPS: 24.9 years; range, 7–59; CIS: 24.3 years; range, 8–60). There were 31 males and 21 females in our study population. The minimum clinical followup was 0.32 years (average, 6.2 years; range, 0.32–9.8 years). Minimum clinical followup was 1.1 years (average, 6.3 years; range, 1.1–9.2 years) and 0.32 years (average, 6.1 years; range, 0.32–9.8 years) for the CPS and CIS cohorts, respectively. Followup specific to the CPS or CIS implant placed at the initial surgery was also calculated to account for those patients who may have had an amputation or implant revision but were still being seen at most recent review. The minimum implant followup was 0 years (average, 5.1 years; range, 0–9.2 years). For the individual CPS and CIS cohorts, minimum implant followup was 1.1 years (average, 5.6 years; range, 1.1–9.2 years) and 0.1 years (average, 4.5 years; range, 0.1–9.2 years), respectively. A total of nine (17.3%) patients in the study population were dead at most recent review, including two (7.7%) patients in the CPS group and seven (26.9%) patients in the CIS group (Table 2). One patient who received a CIS implant with metastatic synovial sarcoma died secondary to a perioperative myocardial infarction. The reason for death in all remaining patients in both cohorts was distant progression of osteosarcoma. Five patients (9.6 %), failing to comply with surveillance recommendations, were lost to followup, including four (15.4%) from the CPS cohort, at a postoperative average of 4.4 years (range, 2.2–6.2 years), and one (3.8%) from the CIS cohort, at 0.3 years. No patients were recalled specifically for this study; all data were obtained from medical records and radiographs. Approval to review the databases was obtained from ethical review boards of the institutions.
Table 1.
Patient demographics and characteristics
| Patient | Sex | Age (years) | Reason for surgery | Followup (years) |
|---|---|---|---|---|
| CPS | ||||
| 1 | Female | 11 | Osteosarcoma | 8.17 |
| 2 | Female | 13 | Osteosarcoma | 7.39 |
| 3 | Female | 26 | Osteosarcoma | 8.78 |
| 4 | Female | 48 | Revision TKA | 6.6 |
| 5 | Male | 8 | Osteosarcoma | 1.5 |
| 6 | Male | 18 | Osteosarcoma | 5.81 |
| 7 | Male | 18 | Giant cell tumor | 5.37 |
| 8 | Male | 58 | Osteosarcoma | 7.89 |
| 9 | Male | 59 | Osteosarcoma | 1.87 |
| 10 | Female | 12 | Osteosarcoma | 6.57 |
| 11 | Female | 15 | Osteosarcoma | 7.72 |
| 12 | Female | 15 | Osteosarcoma | 7.75 |
| 13 | Female | 15 | Osteosarcoma | 7.48 |
| 14 | Female | 15 | Osteosarcoma | 8.64 |
| 15 | Female | 18 | Osteosarcoma | 9.24 |
| 16 | Female | 18 | Osteosarcoma | 8.87 |
| 17 | Female | 33 | Osteosarcoma | 7.39 |
| 18 | Male | 10 | Osteosarcoma | 1.12 |
| 19 | Male | 10 | Osteosarcoma | 7.63 |
| 20 | Male | 12 | Osteosarcoma | 4.9 |
| 21 | Male | 16 | Osteosarcoma | 7.49 |
| 22 | Male | 20 | Osteosarcoma | 2.19 |
| 23 | Male | 20 | Burkitt’s lymphoma | 3.56 |
| 24 | Male | 22 | Osteosarcoma | 6.17 |
| 25 | Male | 51 | Leiomyosarcoma | 7.2 |
| 26 | Male | 52 | Chondrosarcoma | 6.91 |
| CIS | ||||
| 1 | Female | 10 | Osteosarcoma | 7.48 |
| 2 | Female | 13 | Osteosarcoma | 8.41 |
| 3 | Female | 15 | Osteosarcoma | 8.03 |
| 4 | Female | 15 | Osteosarcoma | 6.79 |
| 5 | Female | 17 | Osteosarcoma | 1.3 |
| 6 | Female | 18 | Osteosarcoma | 9.24 |
| 7 | Female | 19 | Osteosarcoma | 3.57 |
| 8 | Female | 48 | Synovial cell sarcoma | 0 |
| 9 | Female | 60 | Revision TKA | 7.83 |
| 10 | Male | 8 | Osteosarcoma | 8.16 |
| 11 | Male | 11 | Osteosarcoma | 8.38 |
| 12 | Male | 12 | Osteosarcoma | 5.13 |
| 13 | Male | 15 | Osteosarcoma | 2.13 |
| 14 | Male | 15 | Osteosarcoma | 5.45 |
| 15 | Male | 15 | Osteosarcoma | 2.39 |
| 16 | Male | 16 | Osteosarcoma | 6.9 |
| 17 | Male | 17 | Osteosarcoma | 8.59 |
| 18 | Male | 18 | Osteosarcoma | 8.21 |
| 19 | Male | 19 | Chondrosarcoma | 9.77 |
| 20 | Male | 21 | Osteosarcoma | 2.26 |
| 21 | Male | 22 | Osteosarcoma | 7.18 |
| 22 | Male | 27 | Malignant fibrous histiocytoma | 7.71 |
| 23 | Male | 51 | Spindle cell sarcoma | 7.88 |
| 24 | Male | 53 | Multiple myeloma | 0.32 |
| 25 | Male | 55 | Chondrosarcoma | 7.64 |
| 26 | Male | 58 | Leiomyosarcoma | 6.68 |
CPS = Compress® Compliant Pre-Stress Implant; CIS = cemented intramedullary stem.
Table 2.
Outcomes of patients with CPS and CIS implants
| Complication | Total (n = 52) | CPS (n = 26) | CIS (n = 26) | p value | |||
|---|---|---|---|---|---|---|---|
| Number | % | Number | % | Number | % | ||
| Alive and no revision/amputation | 31 | 59.6 | 19 | 73.1 | 12 | 46.1 | 0.61 |
| Aseptic failure | |||||||
| All cases | 4 | 7.7 | 1 | 3.8 | 3 | 11.5 | 1.00 |
| Requiring revision | 3 | 5.8 | 1 | 3.8 | 2 | 7.7 | 1.00 |
| Revision | |||||||
| All cases | 6 | 11.5 | 2 | 7.7 | 4 | 15.4 | 0.67 |
| Fracture | 1 | 1.9 | 1 | 3.8 | 0 | 0 | 1.00 |
| Infection | 2 | 3.8 | 0 | 0 | 2 | 7.7 | 0.49 |
| Amputation | |||||||
| All cases | 6 | 11.5 | 3 | 11.5 | 3 | 11.5 | 1.00 |
| Infection | 4 | 7.7 | 3 | 11.5 | 1 | 3.8 | 0.61 |
| Local recurrence | 2 | 3.8 | 0 | 0 | 2 | 7.7 | 0.49 |
| Patient death | 9 | 17.3 | 2 | 7.7 | 7 | 26.9 | 0.14 |
CPS = Compress® Compliant Pre-Stress Implant; CIS = cemented intramedullary stem.
Surgical details and implant specifics of the study groups were described in the earlier report comparing these two cohorts [3]. Briefly, the CPS anchor plug was secured proximally to the femoral diaphysis using transverse pins. The Compress® spindle was then attached to this proximal fixation through use of a helical spring compression device that applied up to 362.9 kg of force at the bone-prosthetic interface. The distal portion of the implant consisted of the standard Biomet Orthopaedic Salvage System™ rotating-hinge knee components. For the CIS group, the Stanmore long-stem rotating-hinge knee implant (Stanmore Implants Worldwide Ltd, Middlesex, UK) was used; in all patients, polymethylmethacrylate bone cement was used for stem fixation. The Stanmore prosthesis includes a hydroxyapatite collar that also promotes osseointegration at the bone-prosthesis interface.
The postoperative course for patients with CPS implants entailed 6 weeks of strict nonweightbearing, after which weightbearing was increased by 25% of body weight per week. Moreover, patients with CPS implants were instructed to avoid activities that may increase torque at the bone-implant interface for 3 months (such as kneeling or squatting). The postoperative course for patients with CIS implants entailed partial weightbearing for 48 hours, after which time activity and weightbearing were gradually increased as tolerated.
Office visits for clinical examination of lower-extremity function and plain radiographic review (AP and lateral views of the entire distal femoral endoprosthesis and knee) were undertaken at 6 and 12 weeks after surgery, with subsequent followup at 3- to 6-month intervals, depending on individualized surgical (infection), musculoskeletal (function), and oncologic (local recurrence) concerns.
From the database or medical records, we extracted the following information: sex, age, primary diagnosis, reasons and time for implant failure, additional surgeries, evidence of local recurrence or metastatic disease, and patient death. The only missing data were from those patients lost to followup.
The treating surgeons (RJO for CPS, RJG for CIS) evaluated all radiographs for evidence of aseptic failure; interobserver variability was not assessed. CPS aseptic failure was defined as the presence of (1) periprosthetic femoral fracture with implant failure; (2) implant fracture; (3) transverse pin migration; (4) progressive, gross decrease in the distance between the anchor plug base and the top of the spindle sleeve; and/or (5) progressive radiolucency at the bone-prosthetic interface, when compared to initial postoperative radiographs. CIS aseptic failure was defined as the presence of (1) periprosthetic femoral fracture with implant failure; (2) implant fracture; and/or (3) progressive circumferential lucency around the cement mantle when compared to initial postoperative radiographs. Aseptic clinical failure was defined as the need for revision due to complaints of pain or instability combined with the above-noted plain radiographic criteria and confirmed by clinical findings and the absence of infection at the time of revision. Criteria of presumed septic failure included: (1) leukocytosis and/or evidence of infectious organisms on microbiologic staining of a knee aspirate; (2) gross purulence confirmed histologically at the time of surgical exploration; and/or (3) positive microbiology cultures taken at the time of joint aspiration or revision surgery.
Our primary outcome was implant survival time with Kaplan-Meier analysis [14] using our definitions of failure as end points. Patients who died or underwent limb amputation during the course of the study period were censored at the time this event occurred if they had no evidence of implant failure. Patients who were lost to followup were censored as of the date of last recorded patient visit. We used the log-rank test to compare implant survival experienced between the two implants. All analyses were conducted using Stata® Version 10 (StataCorp LP, College Station, TX, USA).
Results
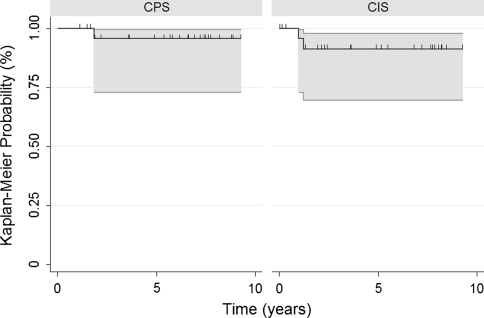
We found clinical or radiographic evidence of aseptic failure in four (7.7%) of the 52 patients, including one (3.8%) patient with a CPS implant versus three (11.5%) patients with CIS implants (Table 2). The single patient with a CPS implant underwent revision at 1.9 years postoperatively, and this revision to a new CPS implant remained stable at 5.6 years of followup (Fig. 1). Two of the three patients with CIS implants required revision at 1.0 and 1.2 years postoperatively while the third patient has not required revision. There was no difference (p = 0.22) in the 5-year survival rates using aseptic failure as the end point for the CPS implants (95.7%; 95% CI, 0.73–0.99) and the CIS implants (91.3%; 95% CI, 0.69–0.98) (Fig. 2).
Fig. 1.
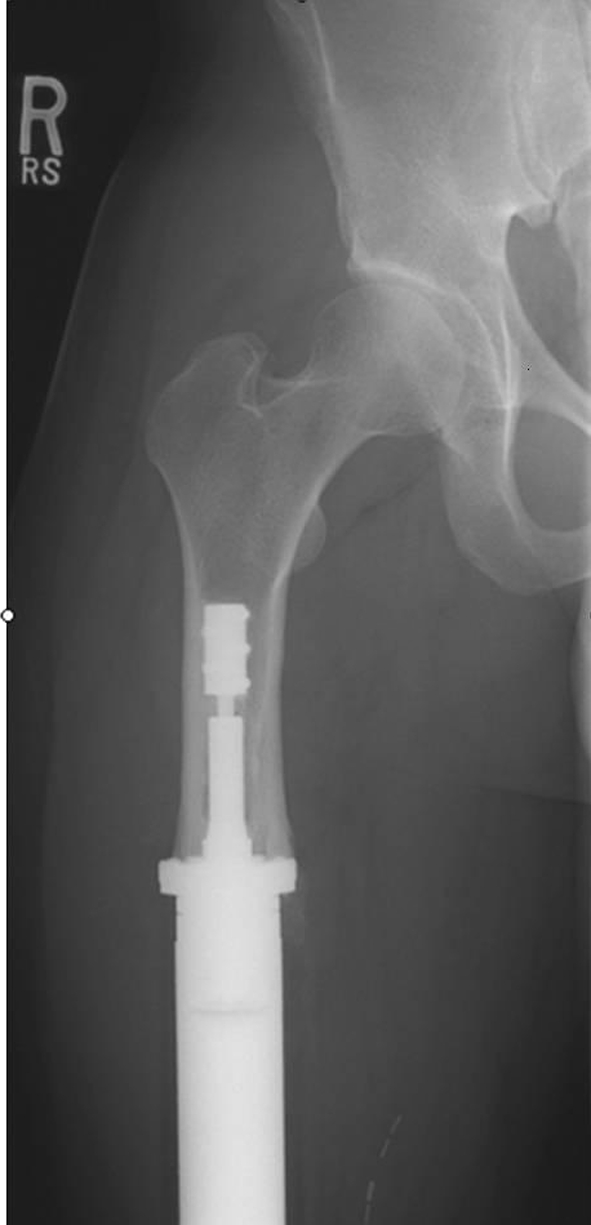
An AP proximal femoral radiograph of the only patient with CPS aseptic loosening demonstrates stable osseointegration at 5.6 years after revision to another CPS implant. Bone hypertrophy at the prosthetic interface has developed in response to compressive loading.
Fig. 2.
A Kaplan-Meier survival curve shows no difference (p = 0.22) in the 5-year implant survival rates using aseptic loosening as the end point between the CPS and CIS implants: 95.7% and 91.3%, respectively. Hash marks = censored data. Gray lines = 95% CIs.
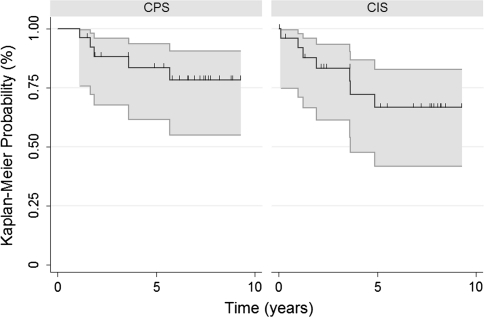
The overall 5-year implant survival rate using implant revision or amputation as the end point was longer (p < 0.0001) for patients with CPS implants (83.5%; 95% CI, 0.62–0.94) than for patients with CIS implants (66.6%; 95% CI, 0.42–0.83) (Fig. 3). With respect to revision alone, there were six (11.5%) revisions for any reason among the 52 patients (Table 2). Two (7.7%) CPS implants versus four (15.3%) CIS implants were revised (Table 2). For the patients with CPS implants requiring revision, one was for aseptic failure and one was for a proximal femoral periprosthetic fracture not associated with implant failure. For the patients with CIS implants requiring revision, two were for aseptic failure and two were for infection. The revisions for infection were at 3.6 and 4.9 years postoperatively. Six (11.5%) patients underwent an amputation, including three (11.5%) patients from each cohort (Table 2). Infection was the reason for amputation in all three (11.5%) patients with CPS implants. In the patients with CIS implants, infection accounted for one (3.8%) amputation and local recurrence accounted for two (7.7%) amputations.
Fig. 3.
A Kaplan-Meier survival curve shows a longer (p < 0.0001) overall 5-year implant survival rate using revision or amputation as the end point for the CPS implants than for the CIS implants: 83.5% and 66.6%, respectively. Hash marks = censored data. Gray lines = 95% CIs.
Discussion
The issue of EPR longevity remains a priority for orthopaedic oncologists as the overall survival and activity level of patients with musculoskeletal tumors continue to improve. The specific problem of aseptic failure deserves high attention, as it is the primary cause of late failure in traditional EPR. We believe the CPS implant presents an encouraging means to counter the problems of stress shielding and particulate-induced osteolytic loosening. We therefore compared patients with CPS implants to patients with CIS implants to determine whether there was a difference in (1) the number of patients requiring revision or amputation for aseptic failure and (2) overall implant survival.
We acknowledge limitations of our study. First, we had a small population. The sample size was a factor in being unable to find a difference between the CPS and CIS implant survivorship data. However, in the orthopaedic oncologic community, large sample sizes are nearly impossible to obtain due to the rarity of disease. Moreover, the 26 patients from the CPS cohort were the initial patients for the CPS FDA approval study and these data demonstrate results from these earliest cases. Second, we had a relatively short followup, although our study reports the longest followup duration for the CPS implant in the current literature. Third, conducting the study at separate institutions presents a challenge in terms of comparing surgical decision making and radiographic interpretation that may differ at respective locations. Nevertheless, similar indications and contraindications were used for surgical planning in both institutions, and criteria for failure assessed with similar intent, despite the differences inherent in implant design.
In a large study by Jeys et al. [13], including 228 distal femoral EPRs, aseptic loosening accounted for 13.6% of all failures with a median time to revision due to mechanical failure of 9.3 years. Moreover, Mittermayer et al. [20] and Unwin et al. [27] found, at 10 years postoperatively, 24% and 32.6%, respectively, of patients who had distal femoral EPR underwent revision for aseptic loosening. In our current study, the overall rate of aseptic failure was 7.7% at 6.2 years. For the CPS cohort, the rate of aseptic failure was 3.8% at 6.3 years (Table 2). The single patient with aseptic failure among the CPS group occurred within 2 years postoperatively and remains successfully revised at 5.6 years (Fig. 1). Furthermore, there have been no additional radiographic or clinical failures due to aseptic failure since the initial publication of the CPS cohort. This lack of aseptic failure beyond 2 years in CPS implants is in accordance with prior literature of compressive osseointegration technology, which postulates mechanical failure will be seen earlier rather than later since biomechanical integrity improves as osseointegration and bone hypertrophy develop over time [2, 3, 5, 7]. Although the rate of aseptic failure was 11.5% in patients with CIS implants at 6.1 years of followup, there was no statistical difference when the CPS and CIS implants were compared. The aseptic failures with the CIS can be explained by the presence of an expandable implant, a revised implant, and a modular implant. Two of these patients required revision, and one of these two implants was subsequently diagnosed as septic. Because an attempt was made to match patients who received CIS implants with the first 26 patients who received distal femoral CPS implants, the resultant patient selection prompted the inclusion of expandable and revision CIS cases. Were the study to be started today, it would be possible to more carefully match CIS primary oncologic nonexpandable implants with the same CPS indications, for which a more robust patient population now exists. It is also important to note the reliability of Stanmore CIS implants has been well-documented, and furthermore, technical design advancement in these cemented limb salvage prostheses have been associated with improved implant longevity [19]. Data from the Royal Orthopaedic Hospital published over a longer period of time with a greater number of patients demonstrate this point. The authors found an 83% implant survival at 5 years, and the risk of revision fell by 52% when rotating-hinge knee implants with hydroxyapatite collars were used in exchange for fixed-hinge knee implants with a smooth collar [19]. In that series, there were no patients with aseptic loosening in adult patients with cemented stems and hydroxyapatite collars at a mean followup of 12 years.
Although we observed no differences between the Compress® and Stanmore cohorts, literature on other CIS implants without hydroxyapatite collars indicates the aseptic loosening rate generally increases with time [10, 13, 18, 20, 23, 27]. Overall EPR survivability for distal femoral reconstruction varies greatly in the literature (Table 3). Published results show a survivorship of 75–90% at 5 years [10, 13, 15, 16, 20]. The 5-year survival of 83.5% in CPS implants is encouraging in light of these numbers. When compared to the 5-year survival of 66.6% in the CIS implants, the CPS implant demonstrated a longer survival rate. The two patients who did have revision of their CPS implant (for fracture and rotational failure) were successfully revised on a long-term basis with another CPS implant.
Table 3.
Representative literature review of distal femoral oncologic endoprosthetic survivorship
| Study | Number of patients available for review | Stated implant type | Survival (%) | Aseptic failure (%) | Septic failure (%) | Failure due to local recurrence (%) | ||
|---|---|---|---|---|---|---|---|---|
| 5-year | 10-year | 20-year | ||||||
| Unwin et al. [27] | 493 | CIS | 90.1 | 67.4 | 9.1 | 1.8 | 6.5 | |
| Meyers et al. [19] | 335 | CIS | 61.0 | 23.6 | 9.6 | 6.0 | ||
| Jeys et al. [13] | 228 | CIS | 52.7 | 30.5 | 13.6 | 12.7 | 5.3 | |
| Schwartz et al. [24] | 186 | CIS | 77.0 | 58.0 | 11.8 | 3.7 | ||
| Roberts et al. [22] | 135 | CIS | 75.0 | |||||
| Bickels et al. [4] | 110 | CIS | 93.0 | 88.0 | 5.4 | 4.5 | ||
| Gosherger et al. [8] | 103 | CIS/UIS | 65.9 | 14.6 | 11.7 | |||
| Ahlmann et al. [1] | 78 | CIS | 75.0 | 58.0 | 5.1 | 6.4 | 2.6 | |
| Torbert et al. [25] | 57 | CIS | 84.0 | 66.0 | ||||
| Farfalli et al. [7] | 50 | UIS | 85.0 | 71.0 | 24.0 | |||
| Farfalli et al. [7] | 41 | CPS | 88.0 | |||||
| Current study | 26 | CIS | 66.6 | 7.7 | 11.5 | 7.7 | ||
| Current study | 26 | CPS | 83.5 | 3.8 | 11.5 | |||
CPS = Compress® Compliant Pre-Stress Implant; CIS = cemented intramedullary stem; UIS = uncemented intramedullary stem.
In our study, 11.5% of the total 52 patients developed an infection that resulted in implant failure. Not only was the rate of infection the same in the CPS and CIS groups, but this rate is also similar to those of two studies in the literature reporting rates of 11.7% [8] and 10.3% [12]; septic failure remains a major unsolved problem for all types of massive EPR in young immunocompromised patients.
The CPS implant continues to be a reliable choice for limb salvage surgery. Equivalent survivorship when compared to CIS implants is demonstrated at intermediate-term followup. In the future, we expect it will be useful and appropriate to distinguish between mechanical, rotational CPS failure (which is early, uncommon, and included here under the rubric of aseptic failure) and mechanical/biomechanical/biologic CIS/UIS failure (which is late and historically more common in many implant types, being comprised of device fracture/stress shielding/particle-induced osteolysis) as a means of highlighting divergence in implant survivorship between CPS and conventional means of endoprosthetic fixation. We also believe CPS technology offers advantages in terms of ease of revision and the ability to be placed in short segments of bone. Further studies with more patients, longer followup, and anatomic locations or situations with greater mechanical stress are certainly warranted to correctly identify the best candidates for CPS EPR.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at the University of California San Francisco, the Royal Orthopaedic Hospital, and the Kaiser Permanente South San Francisco Medical Center.
References
- 1.Ahlmann ER, Menendez ER, Kermani C, Gotha H. Survivorship and clinical outcome for modular endoprosthetic reconstruction for neoplastic disease of the lower limb. J Bone Joint Surg Br. 2006;88:790–795. doi: 10.1302/0301-620X.88B6.17519. [DOI] [PubMed] [Google Scholar]
- 2.Avedian RS, Goldsby RE, Kramer MJ, O’Donnell RJ. Effect of chemotherapy on initial compressive osseointegration of tumor endoprostheses. Clin Orthop Relat Res. 2007;459:48–53. doi: 10.1097/BLO.0b013e3180514c66. [DOI] [PubMed] [Google Scholar]
- 3.Bhangu AA, Kramer MJ, Grimer RJ, O’Donnell RJ. Early distal femoral endoprosthetic survival: cemented stems versus the Compress implant. Int Orthop. 2006;30:465–472. doi: 10.1007/s00264-006-0186-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bickels J, Wittig JC, Kollender Y, Henshaw R, Kellar-Graney KL, Meller I, Malawer MM. Distal femoral resection with endoprosthetic reconstruction: a long-term followup study. Clin Orthop Relat Res. 2002;400:225–235. doi: 10.1097/00003086-200207000-00028. [DOI] [PubMed] [Google Scholar]
- 5.Bini SA, Johnston JO, Martin DL. Compliant prestress fixation in tumor prostheses: interface retrieval data. Orthopedics. 2000;23:707–711. doi: 10.3928/0147-7447-20000701-18. [DOI] [PubMed] [Google Scholar]
- 6.Davis JM, Robins RJ, Frink SJ, Rispoli DM. Use of a compression tumor implant with total elbow arthroplasty for traumatic distal humeral bone loss in a young woman. J Shoulder Elbow Surg. 2010;19:e24–e28. doi: 10.1016/j.jse.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Farfalli GL, Boland PJ, Morris CD, Athanasian EA, Healey JH. Early equivalence of uncemented press-fit and Compress femoral fixation. Clin Orthop Relat Res. 2009;467:2792–2799. doi: 10.1007/s11999-009-0912-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gosheger G, Gebert C, Ahrens H, Streitbuerger A, Winkelmann W, Hardes J. Endoprosthetic reconstruction in 250 patients with sarcoma. Clin Orthop Relat Res. 2006;450:164–171. doi: 10.1097/01.blo.0000223978.36831.39. [DOI] [PubMed] [Google Scholar]
- 9.Ham SJ, Schraffordt Koops H, Veth RP, Horn JR, Molenaar WM, Hoekstra HJ. Limb salvage surgery for primary bone sarcoma of the lower extremities: long-term consequences of endoprosthetic reconstructions. Ann Surg Oncol. 1998;5:423–436. doi: 10.1007/BF02303861. [DOI] [PubMed] [Google Scholar]
- 10.Horowitz SM, Glasser DB, Lane JM, Healey JH. Prosthetic and extremity survivorship after limb salvage for sarcoma: how long do the reconstructions last? Clin Orthop Relat Res. 1993;293:280–286. [PubMed] [Google Scholar]
- 11.Jawad MU, Cheung MC, Clarke J, Koniaris LG, Scully SP. Osteosarcoma: improvement in survival limited to high-grade patients only. J Cancer Res Clin Oncol. 2011;137:597–607. doi: 10.1007/s00432-010-0923-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeys LM, Grimer RJ, Carter SR, Tillman RM. Periprosthetic infection in patients treated for an orthopaedic oncological condition. J Bone Joint Surg Am. 2005;87:842–849. doi: 10.2106/JBJS.C.01222. [DOI] [PubMed] [Google Scholar]
- 13.Jeys LM, Kulkarni A, Grimer RJ, Carter SR, Tillman RM, Abudu A. Endoprosthetic reconstruction for the treatment of musculoskeletal tumors of the appendicular skeleton and pelvis. J Bone Joint Surg Am. 2008;90:1265–1271. doi: 10.2106/JBJS.F.01324. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan EL, Meier P. Nonparametric observations from incomplete observations. J Am Stat Assoc. 1958;53:457–481. doi: 10.2307/2281868. [DOI] [Google Scholar]
- 15.Kawai A, Lin PP, Boland PJ, Athanasian EA, Healey JH. Relationship between magnitude of resection, complication, and prosthetic survival after prosthetic knee reconstructions for distal femoral tumors. J Surg Oncol. 1999;70:109–115. doi: 10.1002/(SICI)1096-9098(199902)70:2<109::AID-JSO9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 16.Kawai A, Muschler GF, Lane JM, Otis JC, Healey JH. Prosthetic knee replacement after resection of a malignant tumor of the distal part of the femur: medium to long-term results. J Bone Joint Surg Am. 1998;80:636–647. doi: 10.1302/0301-620X.80B4.8216. [DOI] [PubMed] [Google Scholar]
- 17.Kramer MJ, Tanner BJ, Horvai AE, O’Donnell RJ. Compressive osseointegration promotes viable bone at the endoprosthetic interface: retrieval study of Compress implants. Int Orthop. 2008;32:567–571. doi: 10.1007/s00264-007-0392-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malawer MM, Chou LB. Prosthetic survival and clinical results with use of large-segment replacements in the treatment of high-grade bone sarcomas. J Bone Joint Surg Am. 1995;77:1154–1165. doi: 10.2106/00004623-199508000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Meyers GJ, Abudu AT, Carter SR, Tillman RM, Grimer RJ. Endoprosthetic replacement of the distal femur for bone tumors: long term results. J Bone Joint Surg Br. 2007;89:521–526. doi: 10.1302/0301-620X.89B4.18631. [DOI] [PubMed] [Google Scholar]
- 20.Mittermayer F, Krepler P, Dominkus M, Schwameis E, Sluga M, Heinzl H, Kotz R. Long-term followup of uncemented tumor endoprostheses for the lower extremity. Clin Orthop Relat Res. 2001;388:167–177. doi: 10.1097/00003086-200107000-00024. [DOI] [PubMed] [Google Scholar]
- 21.O’Donnell RJ. Compressive osseointegration of tibial implants in primary cancer reconstruction. Clin Orthop Relat Res. 2009;467:2807–2812. doi: 10.1007/s11999-009-0986-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts P, Chan D, Grimer RJ, Sneath RS, Scales JT. Prosthetic replacement of the distal femur for primary bone tumors. J Bone Joint Surg Br. 1991;73:762–769. doi: 10.1302/0301-620X.73B5.1894662. [DOI] [PubMed] [Google Scholar]
- 23.Safran MR, Kody MH, Namba RS, Larson KR, Kabo JM, Dorey FJ, Eilber FR, Eckardt JJ. 151 endoprosthetic reconstructions for patients with primary tumors involving bone. Contemp Orthop. 1994;29:15–25. [PubMed] [Google Scholar]
- 24.Schwartz AJ, Kasbo M, Eilber FC, Eilber FR, Eckardt JJ. Cemented distal femoral prostheses for musculoskeletal tumor: improved survival of modular vs. custom implants. Clin Orthop Relat Res. 2010;468:2198–2210. doi: 10.1007/s11999-009-1197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Torbert JT, Fox EJ, Hosalkar HS, Ogilvie CM, Lackman RD. Endoprosthetic reconstructions: results of long-term followup of 139 patients. Clin Orthop Relat Res. 2005;438:51–59. doi: 10.1097/01.blo.0000179735.37089.c2. [DOI] [PubMed] [Google Scholar]
- 26.Tyler WK, Healey JH, Morris CD, Boland PJ, O’Donnell RJ. Compress® periprosthetic fractures: interface stability and ease of revision. Clin Orthop Relat Res. 2009;467:2800–2806. doi: 10.1007/s11999-009-0946-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Unwin PS, Cannon SR, Grimer RJ, Kemp HB, Sneath RS, Walker PS. Aseptic loosening in cemented custom-made prosthetic replacements for bone tumours of the lower limb. J Bone Joint Surg Br. 1996;78:5–13. [PubMed] [Google Scholar]