Abstract
Background
Long-term survival of distal femoral endoprosthetic replacements is largely affected by aseptic loosening. It is unclear whether and to what degree surgical technique and component selection influence the risk of loosening.
Questions/purposes
We (1) established the overall failure and aseptic loosening rates in a tumor population and asked (2) whether stem diameter and specifically the diaphysis-to-stem ratio predicts loosening, and (3) whether resection percentage correlates with failure.
Methods
We retrospectively reviewed the charts of all 93 patients in whom 104 distal femoral replacements had been performed from 1985 to 2008. We extracted the following data: age, need for revision surgeries, tumor diagnosis, adjunct treatment, and implant characteristics. We reviewed radiographs and determined stem size, bone diaphyseal width, and resection percentage of the femur. Kaplan-Meier survivorship was calculated for all implant failures and failures resulting from aseptic loosening. We evaluated radiolucent lines in patients with radiographic followup over 5 years. We identified independent risk factors for loosening. The minimum followup for radiographic evaluation was 5 years (mean, 12.7 years; range, 5.4–23.5 years).
Results
Overall implant survival for 104 stems in 93 patients was 73.3% at 10 years, 62.8% at 15 years, and 46.1% at 20 years. Survival from aseptic loosening was 94.6% at 10 and 15 years and 86.5% at 20 years. Of the variables analyzed, only bone:stem ratio independently predicted aseptic failure. Patients with stable implants had larger stem sizes and lower bone:stem ratios than those with loose implants (14.5 mm versus 10.7 mm and 2.02 versus 2.81, respectively).
Conclusions
Our data suggest durability relates to selecting stems that fill the canal.
Level of Evidence
Level III, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Tumors of the distal femur continue to pose many challenges even as chemotherapy has improved patient survival [3, 10, 13, 15]. Obtaining a consistent, stable, long-term reconstruction is often an elusive goal for the tumor surgeon. Reconstruction options vary and include allograft reconstruction [4, 8], sterilized autograft replacement [6, 11, 20, 24], allograft with prosthetic resurfacing [9], and distal femoral endoprosthetic replacement (DFR) [2, 21, 23]. Cemented DFR offers the advantage of immediate stable fixation, early return to function, and durable implant survival [18].
We previously reported that mechanical failure and infection are the leading causes of implant revision [19]. Others, however, report aseptic loosening as the largest cause of failure [22, 25]. The cause of this discrepancy is unknown, although two groups have postulated that longer resection length (more than 40% of the femur) correlates with implant failure [12, 22]. This is likely the result of the longer lever arm, or increased offset, between the mechanical axis and stem tip in longer resections [5, 16, 22]. However, resection length alone cannot explain differences reported at major centers; one would reasonably expect that oncologic resection lengths would be similar across patient cohorts.
Observations by the senior author (RMH), spurred by revision of a DFR implanted elsewhere that loosened after less than 1 year, suggested loosening may be more common in implants surrounded by a large cement mantle. On the other hand, a large stem that fills the reamed femoral canal (resulting in a thin cement mantle) may protect the cement-stem interface by achieving three-point fixation of the stem within the bone canal independent of the cement mantle. In press-fit endoprosthetic reconstructions, using a stem smaller than 13.5 mm and having a diaphyseal bone:stem ratio over 2.5 predicted higher rates of aseptic loosening [7]. However, in cemented endoprosthetic reconstructions it is unclear whether stem size or diaphyseal bone:stem ratio influences durability.
In this study, we therefore (1) established the overall failure and aseptic loosening rates in a tumor population and asked (2) whether stem diameter, and specifically the diaphysis:stem ratio, predicts loosening, and (3) whether resection percentage correlates with failure.
Patients and Methods
Using an electronic registry we retrospectively identified all 93 patients with 104 distal femoral prostheses implanted at our institutions from 1985 to 2008. These patients are a subset of patients reported in a previous study [19]. Ninety-one patients had 91 primary arthroplasties, whereas two patients had only revision arthroplasties performed at our institution. Of the 91 patients initially treated at our institution, 11 received a revision arthroplasty for stem fractures, infections, or loosening. The indications for surgery were typical of an oncologic population and are documented in our previous report [19]. Specifically, the largest cause of oncologic resection was osteosarcoma (42 patients) and sarcomas in general accounted for 58% of our patient population (Table 1). Nineteen patients were treated for giant cell tumors and 10 of our patients were treated for metastatic disease. Three patients had hematologic malignancies and one patient was treated for a distal femoral fracture and another for osteoarthritis. The only contraindications for surgery were unresectable tumors or active infection. We included only skeletally mature patients including patients who presented to our institution from outside facilities for revision of their distal femoral replacement. Fifty-eight (56%) patients were men and 46 (43%) were women. The average age at treatment was 36.9 years. No patients were recalled specifically for this study; all data were obtained from medical records and radiographs. We had prior Institutional Review Board approval.
Table 1.
Diagnoses of patients treated with distal femoral endoprostheses
| Diagnostic group | Diagnosis | Number of patients |
|---|---|---|
| Sarcomas (58.1%) | Osteosarcoma | 42 |
| Chondrosarcoma | 5 | |
| Malignant fibrous histiocytoma | 3 | |
| Fibrosarcoma | 2 | |
| Pleomorphic sarcoma | 1 | |
| Spindle cell sarcoma | 1 | |
| Benign aggressive tumor of bone (20.4%) | Giant cell | 19 |
| Hematologic malignancies (3.2%) | Lymphoma | 2 |
| Plasmacytoma | 1 | |
| Metastatic cancers (10.8%) | Breast carcinoma | 2 |
| Lung carcinoma | 1 | |
| Poorly differentiated carcinoma | 3 | |
| Renal cell carcinoma | 1 | |
| Colona carcinoma | 1 | |
| Metastatic hemangiopericytoma | 1 | |
| Melanoma | 1 | |
| Nononcologic disease (7.5%) | Fracture | 1 |
| Osteoarthritis | 1 | |
| Revision/hardware failure | 5 |
All patients were treated with cemented Modular Replacement System/Global Modular Replacement System (MRS/GMRS) stems (Stryker/Howmedica, Mahwah, NJ, USA). Early stems were casted and some early custom stems were made without flutes. MRS/GMRS stems (since 1985–1986) were forged and fluted. Such changes appear to have reduced the incidence of mechanical failure of the stems (ie, stems bending or breaking). We choose our time period to exclude the early stems to have a more uniform implant design. The change from MRS to GMRS did not alter the length or composition of the stem itself to the best of our knowledge.
Canals were reamed with flexible reamers until we encountered cortical “chatter.” This permitted the largest stem size possible for the patient’s canal size. We attempted to ream only 1 to 1.5 mm over the stem diameter. If the stem would not seat, we would continue reaming only so far as to enable the stem to seat. A third-generation cement technique, using Simplex cement (Stryker Howmedica), was performed. Canals were pulse lavaged, cement restrictors were placed, canals were packed to minimize bleeding, cement was vacuum-mixed and injected under pressure, and stems seated and held in place until the cement had cured.
As per our protocol, tumor patients were seen every 3 months for 2 years after their resection, then every 6 months for a year, and then yearly. Patients received both a physical examination as well as radiographs of their affected extremity at each followup visit. Followup ranged from immediately postoperative to 23.5 years (mean, 5.6 years). For survivorship analysis, all but two patient charts were reviewed and included. Two patients did not have clinical or radiographic followup documented and were considered to have been lost to followup for survivorship analysis.
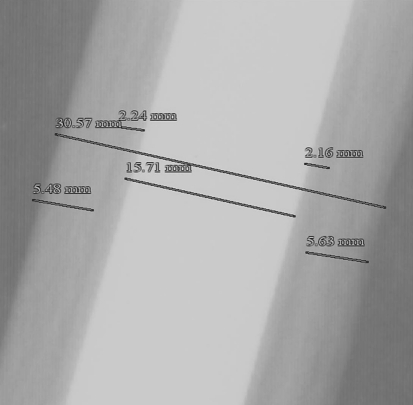
Relevant clinical information extracted from the charts included primary tumor diagnosis, age at the time of resection, chemotherapy use, radiation therapy, implant dimensions, and revisions or other surgical intervention. One of us (PFB) reviewed and analyzed all available films for resection length, average stem diameter, largest reaming diameter, bone diameter, cortical thickness, cement mantle thickness, and length of remaining femur after resection (Fig. 1). Known dimensions of implant components from the operative notes were used to verify image calibrations provided by the digital PACS system. From this, we calculated the resection percentage as used in prior studies [12, 22] as well as ratio of bone diameter to average stem diameter (Fig. 2A–B). As mentioned previously, two patients did not have adequate films for radiographic evaluation and were not included in the analysis.
Fig. 1.
This shows a radiograph with measurements of the entire diaphyseal width, the stem width, cortical thickness, and cement mantle. The bone:stem ratio is calculated by dividing the diaphyseal width from the stem width. In this case, that ratio is 30.57:15.71 = 1.95. All measurements are standardized to known quantities from the operative report to correct for magnification error.
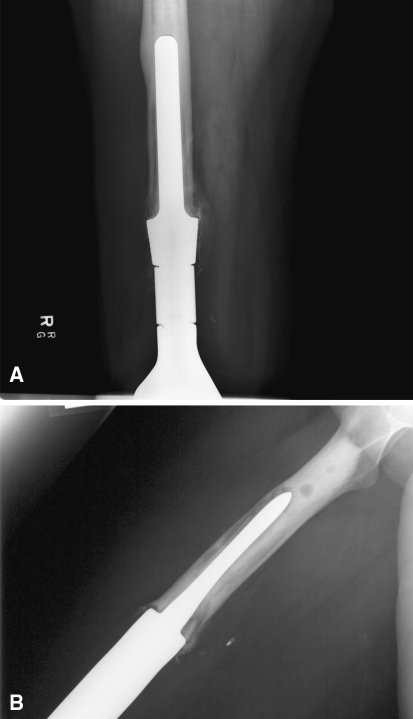
Fig. 2A–B.
(A) This radiographs shows an example of the large stem size relative to the diaphyseal width technique described in this article. It also shows cortical hypertrophy that can occur with transfer of the stress to the tip of the prosthesis. (B) The bottom radiograph is an example of the large bone:stem ratio technique with an undersized stem relative to the diaphyseal canal.
Implant failure was defined as the need for removal and/or revision of a cemented component. Minor procedures such as bushing exchanges or excision of pseudomenisci were not considered implant failures. We calculated 5-, 10-, 15-, and 20-year survivorship rates using Kaplan-Meier analysis for all causes of stem removal, including mechanical failures (stem fractures), biologic failures (removal or amputation because of infection), biomechanical failures (aseptic loosening or bony fracture), and oncologic failures (revision or amputation because of tumor recurrence) resulting in stem removal or amputation. Patients were censored in this analysis at last clinical followup or at death. We performed additional Kaplan-Meier analysis using aseptic loosening as the end point with patients censored for any other cause of stem removal, death, or at latest clinical followup.
To determine contributing factors that lead to aseptic loosening of distal femoral replacements, all 31 patients (34 stems) with a minimum of 5 years clinical and radiographic followup, no signs of infection, and no prior radiation therapy were included in a separate cohort for further analysis; 32 of the original stems had been implanted at our institution and two had been referred to us with aseptic loosening (Table 2). Serial radiographs were reviewed for evidence of aseptic loosening by a senior musculoskeletal radiologist (JSJ) in a blinded fashion. Aseptic loosening was defined as fracture of the cement mantle or progressive radiolucencies within an intact cement mantle, characterized according to the zones described by Shah et al. [16]. Each zone corresponds to 20% of the stem length, with Zone 1 being most distal and Zone 5 being most proximal. Patients with radiolucencies in Zones 1 to 3 (the most distal 60% of the stem) were noted. We recorded patients in the clinically stable cohort with radiographic signs of loosening along with their length of followup.
Table 2.
Explanation of patients analyzed for radiographic parameters that led to aseptic loosening
| Group | Exclusion criteria | Number of stems | Stems remaining |
|---|---|---|---|
| Full patient cohort | 104 stems | ||
| Death before 5-year followup | 18 | 86 | |
| Performed within last 5 years | 18 | 68 | |
| Infection | 12 | 56 | |
| Lost to followup | 11 | 45 | |
| Stem fracture | 6 | 39 | |
| Radiation therapy | 4 | 35 | |
| Amputation for local recurrence | 1 | 34 | |
| Analysis group | 34 stems |
We used a two-tailed Student’s t-test to assess for differences in stem size, bone:stem diameter ratio, resection percentage, and the difference between reaming and stem diameters between those patients revised for aseptic loosening and the clinically stable group. To determine which factor(s) independently predicted implant failure, we conducted bivariate analysis using aseptic loosening as the dependent variable, chi-square tests for nominal variables, and nominal logistic regression for continuous variables. Significant variables were modeled using multiple logistic regression analysis to identify independent risk factors for implant failure. We assessed age at the time of reconstruction, gender, resection length, oncologic diagnosis (sarcoma, benign aggressive tumor of bone, metastatic lesion, hematologic malignancy, or nonneoplastic causes), use of radiation therapy, chemotherapy administration, and the mentioned radiographic criteria as independent variables. We performed all statistical analysis using a commercially available software package (JMP 8; SAS Institute, Inc, Cary, NC, USA).
Results
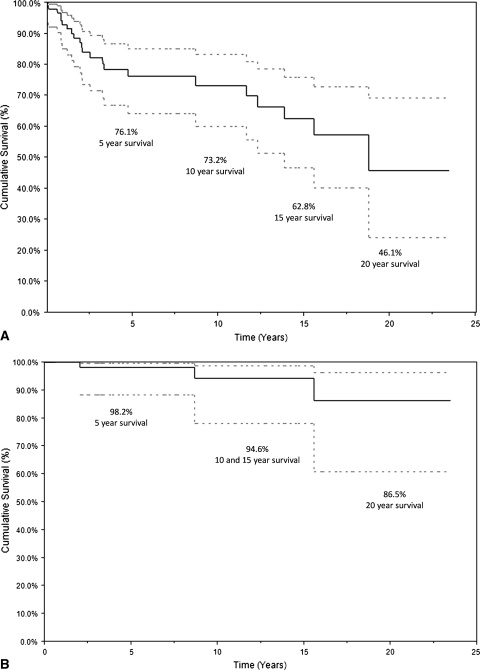
Survival using any cause of stem removal was 76.1% at 5 years, 73.3% at 10 years, 62.8% at 15 years, and 46.1% at 20 years (Fig. 3A). Survival from aseptic loosening was 98.2% at 5 years, 94.6% at 10 and 15 years, and 86.5% at 20 years (Fig. 3B). The largest cause of failure was infection (11.7%). Six (5.8%) implants were revised for stem fracture and one amputation was performed for recurrence (1.0%). Five (4.9%) implants were revised for aseptic loosening. Two of these had been inserted at outside institutions with a small stem technique. Therefore, our aseptic loosening rate was 2.9%.
Fig. 3A–B.
(A) Survival curve showing the rate of survival with any cause of stem removal counted as a failure. Patients were censored at death or last followup. This shows 5-, 10-, 15-, and 20-year survival rates of 76.1%, 73.5%, 62.8%, and 46.1%, respectively. (B) Survival curve where only aseptic loosening is the mode of failure. Patients were censored at death, last followup, or any other cause of stem removal or revision. This shows 20-year survival rates of 86.5%. Ninety-five percent confidence intervals are included on both curves.
Of the subset of 34 stems analyzed for loosening, three had been revised for aseptic loosening. None of the clinically stable implants had progressive loosening in Zones 1, 2, and 3. Four patients (14.8%) had an isolated lucency in the cement mantle of Zone 1. Two patients had a lucent line in Zone 2 (7.4%) and three patients had lucencies in Zone 3 (11.1%). None of these progressed throughout the distal cement mantle. The average radiographic followup was 11.5 years. Twenty-nine stems implanted at our institution (90.6%) were stable at an average followup of 12.8 years (range, 5.4–23.5 years). Stems requiring revision failed at an average of 7.8 years (range, 2.0–15.6 years).
In the initial analysis, only smaller stem sizes (p < 0.001) and higher bone:stem ratios (p < 0.001) predicted aseptic loosening. Although resection percentage did not predict (p = 0.07) aseptic loosening, it is a documented risk factor for loosening and was therefore included in our multivariate analysis. This revealed only bone:stem ratio as an independent risk factor for aseptic loosening (Table 3). Stems were 35% larger (p < 0.001) in stable patients compared with revisions for aseptic loosening, an average of 14.5 mm compared with 10.7 mm. The difference between the reaming and stem diameter was 1.27 mm (range, 0–2 mm) in the stable cohort. An average cement mantle of 2.54 mm was seen in patients with the large stem technique. The femoral diameters were similar (p = 0.70) between the groups (29.2 mm in the stable group versus 29.9 mm in the revisions). However, the average bone:stem ratio was larger (p < 0.001) in the revisions (2.81 versus 2.02) showing an undersized stem relative to similar bone stock.
Table 3.
Statistical analysis of factors that led to aseptic loosening
| Factor | 34-patient cohort | 104 stems in 93 patients | ||
|---|---|---|---|---|
| p values from Student’s t-test | p values from bivariate analysis | p values from multivariate analysis | Odds ratio | |
| Length of followup | 0.13 | 0.70 | ||
| Radiation treatment | 0.51 | |||
| Age | 0.51 | |||
| Gender | 0.86 | 0.47 | ||
| Diagnosis | 0.38 | |||
| Chemotherapy | 0.09 | |||
| Resection (%) | 0.08 | 0.07 | 1.00 | 7.2 (0.8–61.4) |
| Bone:stem ratio | < 0.001 | < 0.001 | 0.009 | 78.0 (5.1–1259.9) |
| Stem size | < 0.001 | < 0.001 | 1.00 | ∞ |
Resection percentage did not independently predict loosening during the followup time. When directly comparing revised and stable implants, the resection percentage in the stable group (41%) was statistically similar (p = 0.09) to that in the revision group (53%). However, there were no failures in patients with less than 40% resection and 29% of patients with more than 40% resection were revised.
Discussion
Aseptic loosening remains one of the largest causes of distal femoral endoprosthetic failure. The only identifiable risk factor in the literature is resection length, which is largely predetermined by oncologic requirements [12, 22]. Based on observations of failures with small stem components, we hypothesized that larger stems and specifically canal-filling stems would lower the rates of loosening in cemented distal femoral endoprostheses. We therefore asked three questions: (1) what is our rate of aseptic loosening and overall stem failure; (2) does stem size relative to the femoral canal (ie, the bone:stem ratio) predict aseptic loosening; and (3) does resection length affect endoprosthetic survival as has been previously postulated?
This observational study is limited by a number of factors. First, the study is largely a cohort study of patients who presented at our institution. The large majority of these patients were treated with a large stem technique. The revisions performed at other facilities were treated with a smaller stem technique. This introduces a selection bias that may affect our results. However, the patients who failed after primary surgeries at our institution have large bone:stem ratios (2.84) similar to the failures from other institutions. Second, we had a limited patient population. Given the potentially confounding variables, the study may have been underpowered to detect differences resulting from resection length. Other potentially independent risk factors besides bone:stem ratio might be seen with a larger patient population. However, we are limited as a result of the rarity of this condition; this patient cohort is in fact large with long followup compared with other published studies. Additionally, these patients were treated in a homogeneous fashion by a small group of highly trained surgeons using the same implant and surgical techniques.
Although the implant survival was less than 50% at 20 years, aseptic loosening was not the most important cause of failure. Infection was the single most common cause of failure with 11.7% of stems failing as a result of sepsis. This is a major problem that is recognized in other series as well with infections seen in 5% to 8% of patients with shorter followup [14, 17]. There was 94.6% survival at 10 and 15 years and 86.5% survival at 20 years from aseptic loosening in this study. The actual rate of loosening in our primary population (2.9%) was much lower compared with other series; loosening accounted for 44% of all failures in one study [25]. In the largest series of cemented distal femoral stems [22], implant survival was only 67% using aseptic loosening as the failure criteria with almost 10% of stems removed for aseptic loosening. Unfortunately, neither study reported on reaming techniques or on stem diameter.
When analyzing only those patients with greater than 5-year followup and no infection or radiation history, we found a higher bone:stem ratio in patients requiring revision than in those with stable implants. Stem size and reaming technique have not been explicitly stated in most studies. Springer et al. [21] suggest a 2-mm overream when using endoprostheses. Although they have not revised any stems at almost 5-year followup, four of the 26 stems showed radiographic signs of loosening. Another group attempts a 1- to 2-mm mantle and documents 2.4% loosening at 10 years [1]. Sharma et al. advocate line-to-line reaming with no loosening failures and 79% stem survival at 10 years [17]. The only other study that looked explicitly at bone:stem ratios was a review of uncemented, press-fit stems for distal femoral replacements [7]. They found a similar relationship between high bone:stem ratios (> 2.5) and implant failure from aseptic loosening. This suggests large stems filling the canal may have a mechanical advantage regardless of the type of fixation actually used. Large-stem cementation may prevent the early failures associated with poor osteointegration of uncemented stems while retaining the long-term benefits seen in this press-fit population.
In contrast to two previous studies [12, 22], we found resection percentage did not predict loosening, although there was a trend toward higher loosening rates in longer replacements. With a larger study population, the trend could be significant. However, it is clear from our data that this offers less of a correlation with aseptic failure than surgical technique.
A number of unsolved questions remain. Is there a tighter fit as a result of three-point fixation of a large straight stem placed into a curved canal and does this account for the results seen in our study? Would a more anatomic stem that matches the patient’s femoral bow be more or less likely to loosen? What is the optimal cement mantle in diaphyseal bone and do these results apply to other anatomic areas? Further study and large, multicenter trials would help answer these questions.
Distal femoral endoprosthetic reconstruction can be a highly successful option for limb salvage surgery. We present a technique that is associated with low rates of aseptic loosening in medium- to long-term followup. Radiographic analysis has not demonstrated radiolucencies associated with loosening, suggesting durable stable fixation may be expected with even longer-term followup. Although limited by the lack of a comparison cohort implanted with smaller stems, only a prospectively controlled cohort would provide better evidence of the efficacy of the technique. At 20-year analysis, we present loosening rates below 15% and few signs of radiographic failure. Cemented endoprostheses with large canal-filling stems provide excellent stability in limb salvage reconstructions.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the reporting of this case and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at Washington Cancer Institute, Washington Hospital Center, Washington, DC, USA.
References
- 1.Ahlmann ER, Menendez LR, Kermani C, Gotha H. Survivorship and clinical outcome of modular endoprosthetic reconstruction for neoplastic disease of the lower limb. J Bone Joint Surg Br. 2006;88:790–795. doi: 10.1302/0301-620X.88B6.17519. [DOI] [PubMed] [Google Scholar]
- 2.Bickels J, Wittig JC, Kollender Y, Henshaw RM, Kellar-Graney KL, Meller I, Malawer MM. Distal femur resection with endoprosthetic reconstruction: a long-term followup study. Clin Orthop Relat Res. 2002;400:225–235. doi: 10.1097/00003086-200207000-00028. [DOI] [PubMed] [Google Scholar]
- 3.Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M, Winkelmann W, Zoubek A, Jurgens H, Winkler K. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1, 702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–790. doi: 10.1200/JCO.20.3.776. [DOI] [PubMed] [Google Scholar]
- 4.Brigman BE, Hornicek FJ, Gebhardt MC, Mankin HJ. Allografts about the knee in young patients with high-grade sarcoma. Clin Orthop Relat Res. 2004;421:232–239. doi: 10.1097/01.blo.0000127132.12576.05. [DOI] [PubMed] [Google Scholar]
- 5.Cannon SR. Massive prostheses for malignant bone tumours of the limbs. J Bone Joint Surg Br. 1997;79:497–506. doi: 10.1302/0301-620X.79B3.14191. [DOI] [PubMed] [Google Scholar]
- 6.Chen WM, Chen TH, Huang CK, Chiang CC, Lo WH. Treatment of malignant bone tumours by extracorporeally irradiated autograft-prosthetic composite arthroplasty. J Bone Joint Surg Br. 2002;84:1156–1161. doi: 10.1302/0301-620X.84B8.13508. [DOI] [PubMed] [Google Scholar]
- 7.Farfalli GL, Boland PJ, Morris CD, Athanasian EA, Healey JH. Early equivalence of uncemented press-fit and Compress femoral fixation. Clin Orthop Relat Res. 2009;467:2792–2799. doi: 10.1007/s11999-009-0912-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gebhardt MC, Flugstad DI, Springfield DS, Mankin HJ. The use of bone allografts for limb salvage in high-grade extremity osteosarcoma. Clin Orthop Relat Res. 1991;270:181–196. [PubMed] [Google Scholar]
- 9.Gitelis S, Piasecki P. Allograft prosthetic composite arthroplasty for osteosarcoma and other aggressive bone tumors. Clin Orthop Relat Res. 1991;270:197–201. [PubMed] [Google Scholar]
- 10.Goorin AM, Schwartzentruber DJ, Devidas M, Gebhardt MC, Ayala AG, Harris MB, Helman LJ, Grier HE, Link MP. Presurgical chemotherapy compared with immediate surgery and adjuvant chemotherapy for nonmetastatic osteosarcoma: Pediatric Oncology Group Study POG-8651. J Clin Oncol. 2003;21:1574–1580. doi: 10.1200/JCO.2003.08.165. [DOI] [PubMed] [Google Scholar]
- 11.Harrington KD, Johnston JO, Kaufer HN, Luck JV Jr, Moore TM. Limb salvage and prosthetic joint reconstruction for low-grade and selected high-grade sarcomas of bone after wide resection and replacement by autoclaved [corrected] autogeneic grafts [erratum appears in Clin Orthop Relat Res. 1987;216:312]. Clin Orthop Relat Res. 1986;211:180–214. [PubMed]
- 12.Kawai A, Lin PP, Boland PJ, Athanasian EA, Healey JH. Relationship between magnitude of resection, complication, and prosthetic survival after prosthetic knee reconstructions for distal femoral tumors. J Surg Oncol. 1999;70:109–115. doi: 10.1002/(SICI)1096-9098(199902)70:2<109::AID-JSO9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 13.Link MP, Goorin AM, Miser AW, Green AA, Pratt CB, Belasco JB, Pritchard J, Malpas JS, Baker AR, Kirkpatrick JA. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med. 1986;314:1600–1606. doi: 10.1056/NEJM198606193142502. [DOI] [PubMed] [Google Scholar]
- 14.Myers GJ, Abudu AT, Carter SR, Tillman RM, Grimer RJ, Myers GJC. Endoprosthetic replacement of the distal femur for bone tumours: long-term results [erratum appears in J Bone Joint Surg Br. 2007;89:706] J Bone Joint Surg Br. 2007;89:521–526. doi: 10.1302/0301-620X.89B4.18631. [DOI] [PubMed] [Google Scholar]
- 15.Rosen G, Marcove RC, Huvos AG, Caparros BI, Lane JM, Nirenberg A, Cacavio A, Groshen S. Primary osteogenic sarcoma: eight-year experience with adjuvant chemotherapy. J Cancer Res Clin Oncol. 1983;106(Suppl):55–67. doi: 10.1007/BF00625054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah AD, Taylor SJ, Hua J, Taylor SJG. Correlation of radiographic and telemetric data from massive implant fixations. J Biomech. 2006;39:1304–1314. doi: 10.1016/j.jbiomech.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 17.Sharma S, Turcotte RE, Isler MH, Wong C. Cemented rotating hinge endoprosthesis for limb salvage of distal femur tumors. Clin Orthop Relat Res. 2006;450:28–32. doi: 10.1097/01.blo.0000229316.66501.fc. [DOI] [PubMed] [Google Scholar]
- 18.Sharma S, Turcotte RE, Isler MH, Wong C. Experience with cemented large segment endoprostheses for tumors. Clin Orthop Relat Res. 2007;459:54–59. doi: 10.1097/BLO.0b013e3180514c8e. [DOI] [PubMed] [Google Scholar]
- 19.Shehadeh A, Noveau J, Malawer M, Henshaw R. Late complications and survival of endoprosthetic reconstruction after resection of bone tumors. Clin Orthop Relat Res. 2010;468:2885–2895. doi: 10.1007/s11999-010-1454-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith WS, Struhl S. Replantation of an autoclaved autogenous segment of bone for treatment of chondrosarcoma. Long-term follow up. J Bone Joint Surg Am. 1988;70:70–75. [PubMed] [Google Scholar]
- 21.Springer BD, Sim FH, Hanssen AD, Lewallen DG. The modular segmental kinematic rotating hinge for nonneoplastic limb salvage. Clin Orthop Relat Res. 2004;421:181–187. doi: 10.1097/01.blo.0000126306.87452.59. [DOI] [PubMed] [Google Scholar]
- 22.Unwin PS, Cannon SR, Grimer RJ, Kemp HB, Sneath RS, Walker PS. Aseptic loosening in cemented custom-made prosthetic replacements for bone tumours of the lower limb. J Bone Joint Surg Br. 1996;78:5–13. [PubMed] [Google Scholar]
- 23.Unwin PS, Cobb JP, Walker PS. Distal femoral arthroplasty using custom-made prostheses. The first 218 cases. J Arthroplasty. 1993;8:259–268. doi: 10.1016/S0883-5403(06)80087-2. [DOI] [PubMed] [Google Scholar]
- 24.Uyttendaele D, Schryver A, Claessens H, Roels H, Berkvens P, Mondelaers W. Limb conservation in primary bone tumours by resection, extracorporeal irradiation and re-implantation. J Bone Joint Surg Br. 1988;70:348–353. doi: 10.1302/0301-620X.70B3.3163694. [DOI] [PubMed] [Google Scholar]
- 25.Wirganowicz PZ, Eckardt JJ, Dorey FJ, Eilber FR, Kabo JM. Etiology and results of tumor endoprosthesis revision surgery in 64 patients. Clin Orthop Relat Res. 1999;358:64–74. doi: 10.1097/00003086-199901000-00009. [DOI] [PubMed] [Google Scholar]