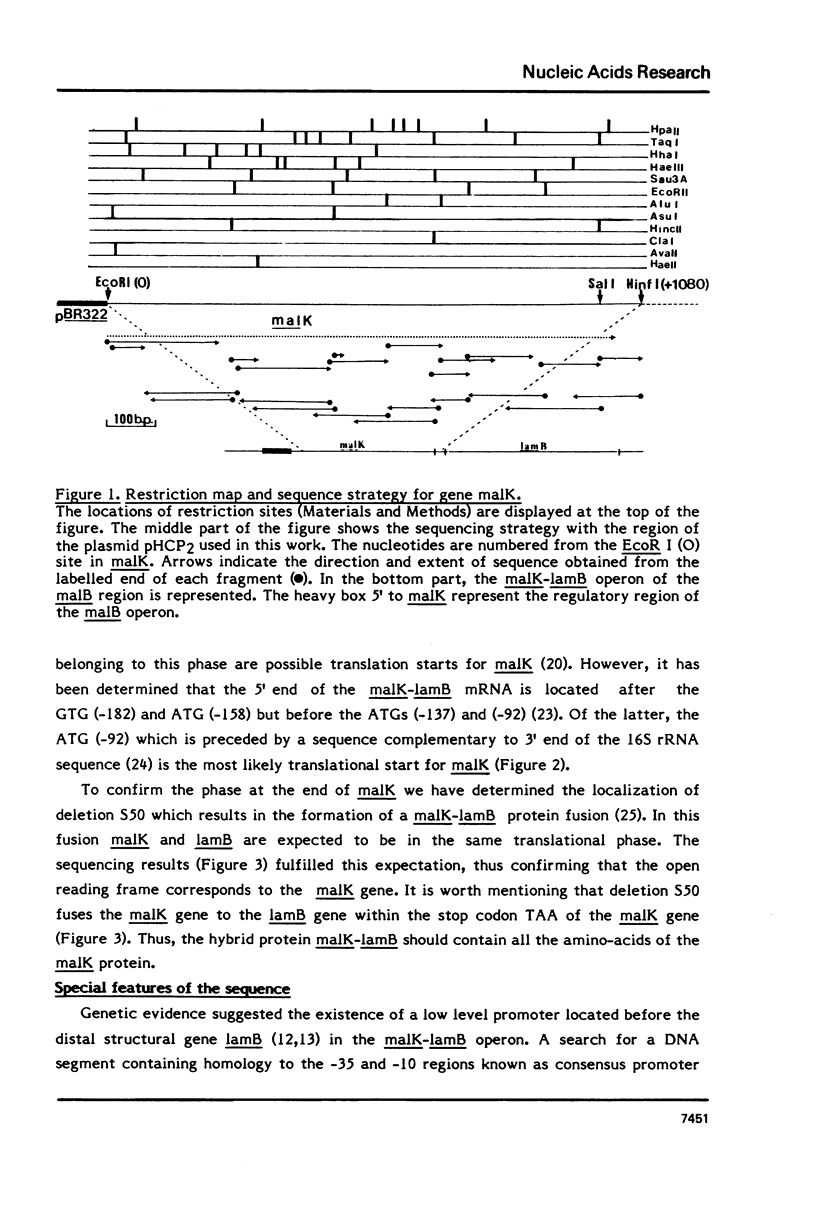
Abstract
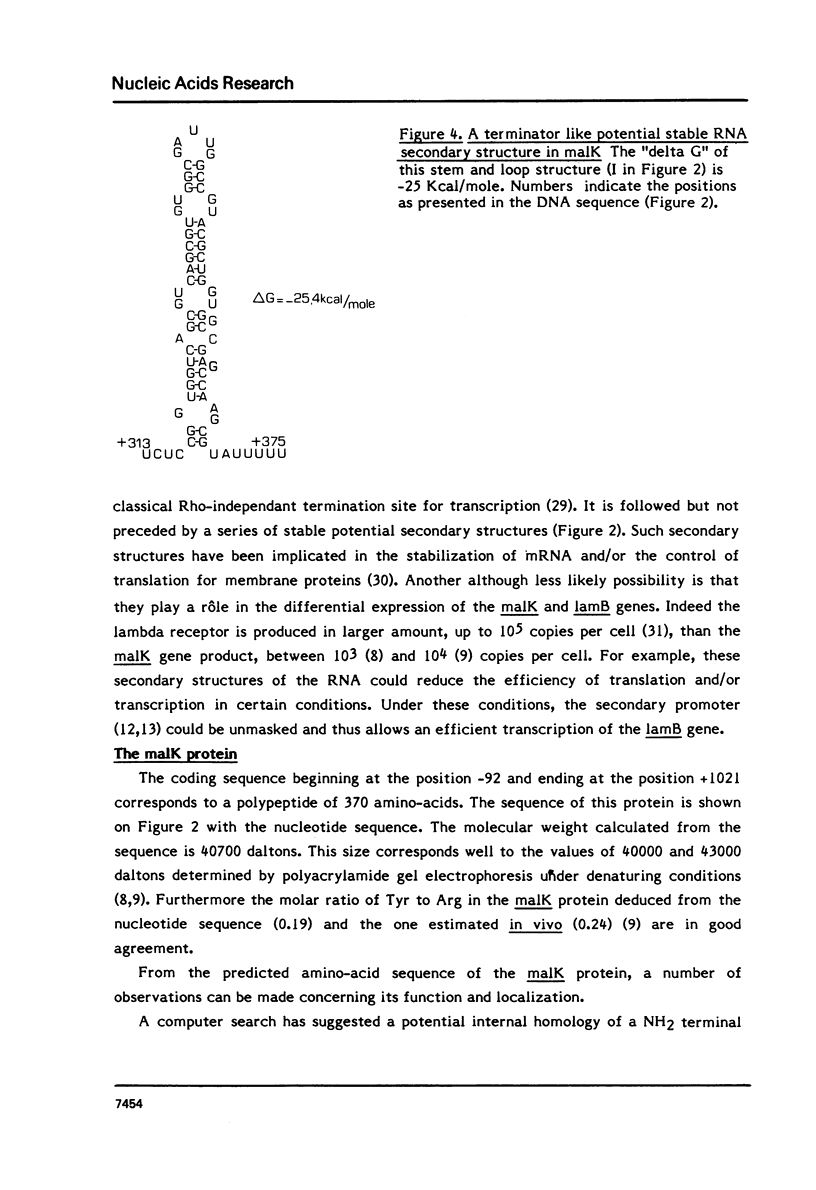
We present the sequence of gene malK which encodes a component of the system for maltose transport in E.coli K12. We also determined the position of deletion (S50) which fuses malK to the following gene lamB; the malK-lamB protein hybrid contains all of the malK protein. The mRNA corresponding to the last two thirds of gene malK could form stable stem and loop structures. The malK protein, as deduced from the gene sequence, would include 370 residues and correspond to a molecular weight of 40700. The sequence as well as sequence comparisons with the ndh protein of E.coli are discussed in terms of the location and function of the malK protein.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Nikaido K. Identification of a membrane protein as a histidine transport component in Salmonella typhimurium. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5447–5451. doi: 10.1073/pnas.75.11.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavoil P., Hofnung M., Nikaido H. Identification of a cytoplasmic membrane-associated component of the maltose transport system of Escherichia coli. J Biol Chem. 1980 Sep 25;255(18):8366–8369. [PubMed] [Google Scholar]
- Bedouelle H., Hofnung M. A DNA sequence containing the control regions of the malEFG and malK-lamB operons in Escherichia coli K12. Mol Gen Genet. 1982;185(1):82–87. doi: 10.1007/BF00333794. [DOI] [PubMed] [Google Scholar]
- Berkner K. L., Folk W. R. Polynucleotide kinase exchange reaction: quantitave assay for restriction endonuclease-generated 5'-phosphoroyl termini in DNA. J Biol Chem. 1977 May 25;252(10):3176–3184. [PubMed] [Google Scholar]
- Boos W. Aspects of maltose transport in Escherichia coli: established facts and educated guesses. Ann Microbiol (Paris) 1982 Jan;133A(1):145–151. [PubMed] [Google Scholar]
- Braun-Breton C., Hofnung M. Explanations accounting for transduction by bacteriophage lambda in maltose negative bacteriophage lambda resistant mutants of Escherichia coli K-12. Mol Gen Genet. 1978 Feb 16;159(2):143–149. doi: 10.1007/BF00270887. [DOI] [PubMed] [Google Scholar]
- Braun V., Krieger-Brauer H. J. Interrelationship of the phage lambda receptor protein and maltose transport in mutants of Escherichia coli K12. Biochim Biophys Acta. 1977 Aug 15;469(1):89–98. doi: 10.1016/0005-2736(77)90328-5. [DOI] [PubMed] [Google Scholar]
- Clement J. M., Perrin D., Hedgpeth J. Analysis of lambda receptor and beta-lactamase synthesis and export using cloned genes in a minicell system. Mol Gen Genet. 1982;185(2):302–310. doi: 10.1007/BF00330802. [DOI] [PubMed] [Google Scholar]
- Clément J. M., Hofnung M. Gene sequence of the lambda receptor, an outer membrane protein of E. coli K12. Cell. 1981 Dec;27(3 Pt 2):507–514. doi: 10.1016/0092-8674(81)90392-5. [DOI] [PubMed] [Google Scholar]
- Dills S. S., Apperson A., Schmidt M. R., Saier M. H., Jr Carbohydrate transport in bacteria. Microbiol Rev. 1980 Sep;44(3):385–418. doi: 10.1128/mr.44.3.385-418.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emr S. D., Silhavy T. J. Mutations affecting localization of an Escherichia coli outer membrane protein, the bacteriophage lambda receptor. J Mol Biol. 1980 Jul 25;141(1):63–90. doi: 10.1016/s0022-2836(80)80029-5. [DOI] [PubMed] [Google Scholar]
- Ferenci T., Boos W. The role of the Escherichia coli lambda receptor in the transport of maltose and maltodextrins. J Supramol Struct. 1980;13(1):101–116. doi: 10.1002/jss.400130110. [DOI] [PubMed] [Google Scholar]
- Gay N. J., Walker J. E. The atp operon: nucleotide sequence of the region encoding the alpha-subunit of Escherichia coli ATP-synthase. Nucleic Acids Res. 1981 May 11;9(9):2187–2194. doi: 10.1093/nar/9.9.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilson E., Higgins C. F., Hofnung M., Ferro-Luzzi Ames G., Nikaido H. Extensive homology between membrane-associated components of histidine and maltose transport systems of Salmonella typhimurium and Escherichia coli. J Biol Chem. 1982 Sep 10;257(17):9915–9918. [PubMed] [Google Scholar]
- Higgins C. F., Haag P. D., Nikaido K., Ardeshir F., Garcia G., Ames G. F. Complete nucleotide sequence and identification of membrane components of the histidine transport operon of S. typhimurium. Nature. 1982 Aug 19;298(5876):723–727. doi: 10.1038/298723a0. [DOI] [PubMed] [Google Scholar]
- Horowitz H., Christie G. E., Platt T. Nucleotide sequence of the trpD gene, encoding anthranilate synthetase component II of Escherichia coli. J Mol Biol. 1982 Apr 5;156(2):245–256. doi: 10.1016/0022-2836(82)90326-6. [DOI] [PubMed] [Google Scholar]
- Kellermann O., Szmelcman S. Active transport of maltose in Escherichia coli K12. Involvement of a "periplasmic" maltose binding protein. Eur J Biochem. 1974 Aug 15;47(1):139–149. doi: 10.1111/j.1432-1033.1974.tb03677.x. [DOI] [PubMed] [Google Scholar]
- Marchal C., Greenblatt J., Hofnung M. malB region in Escherichia coli K-12: specialized transducing bacteriophages and first restriction map. J Bacteriol. 1978 Dec;136(3):1109–1119. doi: 10.1128/jb.136.3.1109-1119.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Movva N. R., Nakamura K., Inouye M. Gene structure of the OmpA protein, a major surface protein of Escherichia coli required for cell-cell interaction. J Mol Biol. 1980 Nov 5;143(3):317–328. doi: 10.1016/0022-2836(80)90193-x. [DOI] [PubMed] [Google Scholar]
- Needleman S. B., Wunsch C. D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970 Mar;48(3):443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Luckey M., Rosenberg E. Y. Nonspecific and specific diffusion channels in the outer membrane of Escherichia coli. J Supramol Struct. 1980;13(3):305–313. doi: 10.1002/jss.400130304. [DOI] [PubMed] [Google Scholar]
- Raibaud O., Clément J. M., Hofnung M. Structure of the malB region in Escherichia coli K12. III. Correlation of the genetic map with the restriction map. Mol Gen Genet. 1979 Jul 24;174(3):261–267. doi: 10.1007/BF00267798. [DOI] [PubMed] [Google Scholar]
- Raibaud O., Roa M., Braun-Breton C., Schwartz M. Structure of the malB region in Escherichia coli K12. I. Genetic map of the malK-lamB operon. Mol Gen Genet. 1979 Jul 24;174(3):241–248. doi: 10.1007/BF00267796. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., Feldmann R. J. Membrane proteins: amino acid sequence and membrane penetration. J Mol Biol. 1974 Aug 25;87(4):853–858. doi: 10.1016/0022-2836(74)90090-4. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman H. A., Silhavy T. J., Beckwith J. R. Labeling of proteins with beta-galactosidase by gene fusion. Identification of a cytoplasmic membrane component of the Escherichia coli maltose transport system. J Biol Chem. 1980 Jan 10;255(1):168–174. [PubMed] [Google Scholar]
- Shuman H. A., Silhavy T. J. Identification of the malK gene product. A peripheral membrane component of the Escherichia coli maltose transport system. J Biol Chem. 1981 Jan 25;256(2):560–562. [PubMed] [Google Scholar]
- Silhavy T. J., Szmelcman S., Boos W., Schwartz M. On the significance of the retention of ligand by protein. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2120–2124. doi: 10.1073/pnas.72.6.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O., Birnstiel M. L. A simple method for DNA restriction site mapping. Nucleic Acids Res. 1976 Sep;3(9):2387–2398. doi: 10.1093/nar/3.9.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young I. G., Rogers B. L., Campbell H. D., Jaworowski A., Shaw D. C. Nucleotide sequence coding for the respiratory NADH dehydrogenase of Escherichia coli. UUG initiation codon. Eur J Biochem. 1981 May;116(1):165–170. doi: 10.1111/j.1432-1033.1981.tb05314.x. [DOI] [PubMed] [Google Scholar]



