Abstract
Background
The proximal femur is the most common site of surgery for bone metastases, and stabilization may be achieved through intramedullary fixation (IMN) or endoprosthetic reconstruction (EPR). Intramedullary devices are less expensive, less invasive, and may yield improved function over endoprostheses. However, it is unclear which, if either, has any advantages.
Questions/purposes
We determined whether function, complications, and survivorship differed between the two approaches.
Methods
We retrospectively reviewed 158 patients with 159 proximal femur metastatic lesions treated with surgical stabilization. Forty-six were stabilized with IMN and 113 were treated with EPR. The minimum followup was 0.25 months (mean, 16 months; median, 17 months; range, 0.25–86 months).
Results
The mean Musculoskeletal Tumor Society score was 24 of 30 (80%) after IMN and 21 of 30 (70%) after EPR. There were 12 complications (26%) in the IMN group, including 10 nonunions, six of which went on to mechanical failure. There were complications in 20 of 113 (18%) of the EPR group, which consisted of 10 dislocations (9%) and 10 infections (9%). There were no mechanical failures with EPR. Both implants remained functional for the limited lifespan of these patients in each group at all time intervals. EPRs were associated with increased implant longevity compared with IMNs (100% versus 85% 5-year survival, respectively) and a decreased rate of mechanical failure (0% versus 11%, respectively) when compared with the intramedullary devices.
Conclusions
Patients with metastatic disease to the proximal femur may live for long periods of time, and these patients may undergo stabilization with either IMN or EPR with comparable functional scores and the implant survivorship exceeding patient survivorship at all time intervals. Endoprostheses demonstrate a lower mechanical failure rate and a higher rate of implant survivorship without mechanical failure than IMN devices.
Level of Evidence
Level III, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
One-third of metastatic lesions to the skeleton occurs in the proximal femur [11]. Approximately 10% of patients with metastatic disease will sustain pathologic fractures, and 65% of all fractures requiring surgery occurs in the femur [11, 12]. Impending and pathologic fractures of the proximal femur can have a disastrous effect on the patient’s quality of life, resulting in severe pain and limitation in function and mobility. Current estimates state that 40% of patients with pathologic fractures will survive for at least 6 months after their fracture and 30% will survive for more than 1 year [16]. Patients with metastatic disease are living longer, increasing the risk of failure of conventional techniques.
The primary goals of surgical stabilization of proximal femur metastatic lesions are to relieve pain, allow immediate return to function, improve the patient’s quality of life, facilitate medical testing and treatments, and decrease the chance of subsequent surgical revision. Surgical options that have emerged to achieve these goals include plate fixation [13, 27], intramedullary fixation [6, 13, 24, 26, 27], and endoprosthetic reconstruction [4, 5, 19, 28–30]. Plate fixation has an unacceptably high mechanical failure rate of 44% to 70% [6, 13, 30]. Intramedullary devices have a lower rate of mechanical failure of 2% to 22% but death from cardiopulmonary complications ranges from 1% to 10% [6, 24, 26, 28]. Endoprostheses have the lowest reported mechanical failure rate (under 3.7%) with a complication rate of 6% to 35% [5, 21, 25]. One study directly comparing the use of intramedullary devices and endoprostheses for proximal femur metastatic lesions found an increased 2-year risk of reoperation when comparing internal fixation with endoprosthetic reconstruction [28]. However, it is unclear whether function, complications, or implant survivorship differs between the two treatments.
We therefore compared (1) function; (2) complications; and (3) survivorship in patients treated with intramedullary devices and endoprosthetic reconstruction.
Patients and Methods
From our institution’s Orthopaedic Oncology Database we identified all 167 patients with metastatic disease of the proximal femur who underwent surgery with either intramedullary nailing or endoprosthetic reconstruction from 1998 to 2009. We included all patients with lesions of the proximal one-third of the femur who underwent either reconstruction nailing or resection of the proximal femur with placement of an endoprosthesis. Lesions located only in the femoral head without peritrochanteric extension were excluded as were patients with a lesion not amenable to either treatment method or who underwent revision of a preexisting implant. Indications for surgery were (1) an impending (Mirels score of 9 or greater [18]) or existing pathologic fracture; and (2) intractable pain and loss of ambulatory ability. Contraindications to surgery included (1) projected survival of less than 6 weeks; and (2) lack of medical fitness to undergo the procedure. After nine patients were excluded, 158 patients with 159 cases were available for review. There were 72 males and 86 females with a mean age of 60 years (range, 17–91 years) (Table 1). Impending fractures accounted for 43% (68 of 159) and actual pathologic fractures made up 53% (91 of 159) of the total lesions treated (Table 1). Diagnoses were comparatively distributed with breast adenocarcinoma (26% [41 of 158]) as the most common overall (Table 2). Thirty-four of 158 (22%) patients were alive at the time of this study. Of the patients treated with intramedullary fixation, 54% (25 of 46) had impending fractures and 46% (21 of 46) had existing fractures of the proximal femur; of the patients treated with endoprosthetic reconstruction, 38% (43 of 113) had impending fractures and 62% (70 of 113) had pathologic fractures (Table 1). The minimum patient followup from the time of surgery was 0.25 months (average, 16 months; range, 0.25–86 months) (Table 1) with no patients lost to followup. Thirty-five patients were alive at the time of this review (15 in the intramedullary fixation [IMN] group and 20 in the endoprosthetic reconstruction [EPR] group) with the minimum followup of these patients being 24.4 months (median, 17 months; range, 9–78 months). No patients were recalled specifically for this study; all data were obtained from medical records and radiographs, which were complete in all cases. This study was approved by our institution’s investigational board review.
Table 1.
Patient demographics in regard to type of surgical treatment
| Surgical treatment | Total number (M/F) | Average age (years) | Average followup (months) | Fracture | |
|---|---|---|---|---|---|
| Impending (% of total) | Pathologic (% of total) | ||||
| Endoprosthesis | 113 (53 M/60 F) | 61 (range, 17–86) | 14 (range, 0.75–86) | 43/113 (38%) | 70/113 (63%) |
| Reconstruction nail | 46 (19 M/26 F) | 56 (range, 16–91) | 20 (range, 0.25–78) | 25/46 (54%) | 21/46 (46%) |
M = male; F = female.
Table 2.
Distribution of diagnoses
| Diagnosis | Total (%) | Reconstruction nail (%) | Endoprosthesis (%) |
|---|---|---|---|
| Breast | 41 (26) | 10 (22) | 31 (27) |
| Sarcomas | 20 (13) | 12 (26) | 8 (7) |
| Lung | 19 (12) | 2 (4.3) | 17 (15) |
| Renal | 19 (12) | 5 (11) | 14 (12) |
| Multiple myeloma | 14 (9) | 9 (20) | 5 (4.5) |
| Prostate | 10 (6) | 1 (2.2) | 9 (8) |
| Colorectal | 7 (4.4) | 4 (6.5) | 3 (2.7) |
| Melanoma | 6 (3.8) | 2 (4.3) | 4 (3.6) |
| Thyroid | 4 (2.5) | 0 | 4 (3.6) |
| Liver | 4 (2.5) | 1 (2.2) | 3 (2.7) |
| Cervical/uterine | 4 (2.5) | 2 (4.3) | 2 (1.8) |
| Bladder | 4 (2.5) | 1 (2.2) | 3 (2.7) |
| Unknown | 3 (1.9) | 0 | 3 (2.7) |
| Lymphoma | 2 (1.3) | 2 (4.3) | 0 |
| Neuroendocrine | 1 (0.6) | 1 (2.2) | 0 |
| Total | 158 (100) | 45 (28) | 113 (72) |
The published mechanical failure rate of intramedullary devices has been roughly 20%, whereas that of EPR approximates 0% [5, 6, 21, 24–26, 28, 29]. Therefore, we wanted to detect at least a 20% to 25% higher failure rate of the intramedullary devices. A higher failure rate would be clinically unacceptable. We performed a power analysis sample size calculation for 25% IMN failure and 1% endoprosthetic failure and concluded that a sample size of 52 in each group will have 80% power to detect a 24% difference using a two-group t-test with a 0.050 two-sided significance level.
We presented patients with both treatment options, counseled about the risks and benefits of each, and allowed them to choose their preferred method of treatment. Patients were encouraged to undergo resection and EPR in the presence of an existing fracture, more than 50% canal involvement on plain films, or isolated renal cell or thyroid carcinoma. We performed IMN with either the TriGen Femoral Antegrade Nail in reconstruction locking mode (Smith and Nephew Inc, Memphis, TN) or the long Titanium Trochanteric Fixation Nail System (Synthes Inc, West Chester, PA) using the manufacturers’ recommended technique. The goal was to insert the largest possible diameter nail without reaming the femur to limit further spread of disease both locally and systemically. For patients with lesions involving greater than one-third of the diameter of the bone or greater than 2.5 cm in cortical destruction, we performed curettage and placement of supplemental cement for additional structural support. Postoperatively, patients with pathologic fractures treated with IMN were kept partially weightbearing until there was radiographic evidence of healing of at least two cortices. All other patients were allowed immediate weightbearing as tolerated after the procedure.
All proximal femur EPRs were performed using a standardized protocol through a posterolateral approach to the proximal femur. We performed proximal femoral reconstruction with the largest possible cemented stem using Global Modular Replacement System implants (Stryker Orthopaedics, Rutherford, NJ). Bipolar head components were used in all patients, and no acetabular resurfacing was performed in this series. The hip capsule was closed and the hip abductors repaired to holes in the implant both using #5 braided nonabsorbable suture. These patients were allowed full weightbearing as tolerated immediately after surgery, and as a result of the achievement of intraoperative stability throughout hip ROM, patients were not braced postoperatively.
Patients from the intramedullary fixation group received a standardized protocol of 30 Gray of external beam radiation starting 3 weeks after surgery. Both groups resumed their chemotherapy as indicated by their disease.
Routine followup examinations were performed at 1 week, 4 weeks, 2 months, 6 months, 1 year, and yearly thereafter. Charts and radiographs were available for review for all patients in this study, and recorded outcomes were based on the most recent followup evaluation. The Musculoskeletal Tumor Society (MSTS) functional scale for the lower extremity (which specifically evaluates supports, walking ability, and gait) [7] was administered by an investigator (LW) not involved in patient care to avoid potential bias. Complications were defined as adverse events that required additional surgical intervention; we did not consider other complications in this analysis. Charts were reviewed for the onset of complications, including infection, recurrence, aseptic loosening, fatigue failure, periprosthetic fracture, and dislocation.
Radiographs taken at the latest followup were evaluated for evidence of hardware failure or loosening, periprosthetic fracture, and nonunion. One investigator (ERA) evaluated preoperative radiographs for location of the lesion and percent of canal involvement as well as most recent followup radiographs for complications. Loosening was determined radiographically by the presence of circumferential radiolucency of greater than 2 mm around the implant in any zone and implant subsidence/migration of any distance in regard to the position of the implant stem to the surrounding cortical bone [9, 20]. Radiographic nonunion was defined as failure of a fracture to unite radiographically (bridging of four cortices on two orthogonal radiographs) with associated pain by 6 months from the date of fixation. Complication-free survival was defined as survivorship of the patient without any complications. Implant survivorship was defined as percentage of functional implants remaining that did not undergo revision of any or all components or removal of implants without revision. Survivorship of the implant without mechanical failure describes implant survival without experiencing a structural mechanical failure of the actual implant itself, ie, breakage of the nail, breakage of the stem, disassembly of the bipolar components, breakage of the nail’s interlocking bolts, etc.
We determined differences in MSTS scores between the two groups using a t-test. Differences in complications were determined using Fisher’s exact test and Kaplan–Meier survivorship curves using patient death and failure of the reconstruction as end points and corresponding 95% confidence intervals were generated using GraphPad Prism® software (GraphPad Software, Inc, San Diego, CA). We used log-rank analysis to determine differences in survival curves. Survivorship data met assumptions associated with the statistical analysis. The t-test comparison of MSTS score means agreed with our assumptions of continuous data, which followed a normal distribution, reflecting a random sample from the population. We used SAS/STAT® software (SAS® Institute, Inc, Cary, NC) for all analyses for all comparisons.
Results
The MSTS functional scores were similar (p = 0.28) for living patients treated with an intramedullary device and those treated with EPR: 80% (24 of 30; range, 8–30) versus 70% (21 of 30; range, 12–27), respectively.
We found no difference in the rate of adverse events requiring surgery (p = 0.097) or the infection rate (p = 0.283) between patients treated with an endoprosthesis and those treated with an intramedullary nail. We observed more complications (unrelated to recurrence or local disease) 1 year after surgery (p < 0.001) in patients treated with intramedullary fixation compared with endoprostheses: 67% versus 6%, respectively. There were 12 (26%) complications requiring further surgery in patients having intramedullary fixation. These included 10 (22%) symptomatic nonunions, of which six (13%) went on to have mechanical failure of the implant, one experienced deep infection, and one painful hardware (Table 3). We found no difference in the failure rate of the intramedullary devices based on location of the lesion (p = 0.723) or percent of canal involvement (p = 0.737). There were no failures in patients with greater than two-thirds canal involvement. All mechanical failures occurred in patients with one-third to two-thirds canal involvement. IMN cases with actual fractures had a higher rate of both mechanical failure (p = 0.028) and reoperation (p = 0.011) than those with impending fractures. Complications in patients treated with endoprostheses included 10 dislocations (9%) and 10 deep infections (9% [10 of 113]) (Table 4). We were unable to find a difference in the overall complication rate based on pathologic diagnosis. There was a lesser rate of mechanical failure in the endoprosthesis group compared with the intramedullary nail group (p = 0.003). There were no intraoperative deaths in this series and no patients treated with EPR died within 14 days of surgery, whereas only one patient who underwent intramedullary stabilization died from pneumonia and sepsis during this time.
Table 3.
Intramedullary nail complications
| Age (years), gender | Primary tumor | Complication | Time to complication | Treatment | Outcome | Followup |
|---|---|---|---|---|---|---|
| 46, M | Liposarcoma | Nonunion and nail breakage | 78 months | Removal of nail; placement of bipolar endoprosthesis | No further complications | 4 months |
| 16, F | Glomangiosarcoma | Nonunion and nail breakage | 14 months | Removal of nail; placement of bipolar endoprosthesis | No further complications | 45 months |
| 60, F | Breast | Nonunion and nail breakage | 18 months | Removal of nail; placement of bipolar endoprosthesis | No further complications | 52 months |
| 60, M | Renal | Nonunion and nail breakage | 11 months | Removal of nail; placement of bipolar endoprosthesis | No further complications | 19 months |
| 80, M | Renal | Nonunion and nail breakage | 19 months | Removal of nail; placement of bipolar endoprosthesis | No further complications | 27 months |
| 50, F | Breast | Nonunion and breakage of both distal interlocking screws | 6 months | Removal of nail; placement of bipolar endoprosthesis | No further complications | 8 months |
| 50, F | Sarcoma | Nonunion | 61 months | Revision to larger diameter nail; bone graft | Persistent nonunion | 3 months |
| 68, F | Sarcoma | Nonunion | 14 months | Revision to larger diameter nail; bone graft | Developed deep infection 4 months postoperatively; hip disarticulation | 36 months |
| 51, F | Sarcoma | Nonunion | 5 months | Revision to larger diameter nail | Union of fracture at 6 months | 19 months |
| 40, F | Sarcoma | Nonunion | 16 months | Revision to larger diameter nail | Union of fracture at 6 months | 22 months |
| 54, M | Sarcoma | Infection | 4 months | Hip disarticulation | No infection | 23 months |
| 31, F | Lymphoma | Painful hardware (distal interlocks) | 22 months | Removal of distal interlocks | No further complications | 46 months |
M = male; F = female.
Table 4.
Endoprosthetic reconstruction complications
| Age (years), gender | Primary tumor | Complication | Time to complication | Treatment | Outcome | Followup |
|---|---|---|---|---|---|---|
| 53, F | Breast | Dislocation | 1.5 months | Open reduction | Stable | 2 months |
| Infection | 2 months | I&D, IV Abx | No infection | |||
| 68, M | Prostate | Dislocation and infection | 1 month | I&D, resection arthroplasty, IV Abx | No infection, no reimplantation | 21 months |
| 52, F | Breast | Dislocation | 3 months | Open reduction | Stable | 9 months |
| Infection | 4 months | I&D, IV Abx | No infection | |||
| 67, F | Liposarcoma | Dislocation | 3 months | Open reduction | Stable | 6 months |
| Infection | 5 months | Hip disarticulation | No infection | |||
| 55, F | Sarcoma | Infection | 3 months | I&D, IV Abx | No infection | 29 months |
| 62, M | Lung | Infection | 3 months | I&D, IV Abx | No infection | 6 months |
| 61, F | Breast | Infection | 1 month | I&D, IV Abx | No infection | 3 months |
| 73, M | Lymphoma | Infection | 17 months | I&D, IV Abx | No infection | 19 months |
| 74, M | Thyroid | Infection | 2 months | I&D, IV Abx | No infection | 9 months |
| 28, M | Hepatic | Infection | 1 month | I&D, IV Abx | No infection | 6 months |
| 44, F | Melanoma | Dislocation | 1 month | Open reduction | Stable | 4 months |
| 68, M | Lung | Dislocation | 1 month | Closed reduction | Stable | 2 months |
| 82, M | Renal | Dislocation | 2 months | Closed reduced | Stable | 81 months |
| 68, F | Breast | Dislocation | 1 month | Open reduction, lengthening of intercalary parts for more stability | Stable | 29 months |
| 67, M | Melanoma | Dislocation | 1 month | Open reduction | Stable | 2 months |
| 59, F | Breast | Dislocation | 2 months | Open reduction | Stable | 10 months |
F = female; M = male; I&D = irrigation and débridement; IV Abx = intravenous antibiotics.
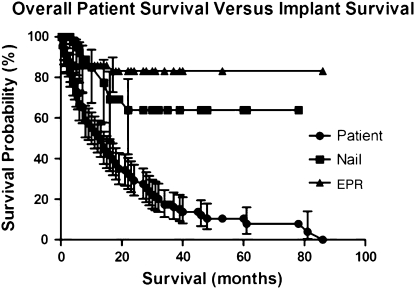
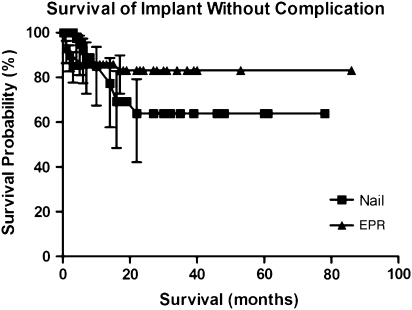
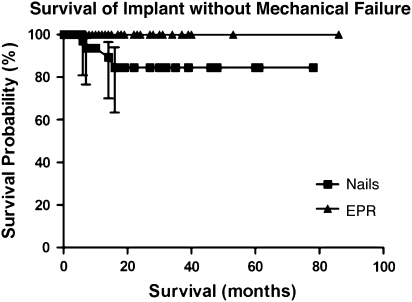
The overall patient survival in this series was 51%, 29%, and 11% at 1 year, 2 years, and 5 years, respectively. According to Kaplan-Meier analysis, the survival of both the intramedullary implants (p = 0.008, log-rank test) and the endoprostheses (p = 0.001) at 5 years exceeded that of the patients, a finding that was also unrelated to recurrence or local disease (Fig. 1). We found no difference (p = 0.306) in complication-free survival of the patients in either group (Fig. 2). However, the endoprosthesis group demonstrated increased survival of the implant without mechanical failure with 100% 2- and 5-year survival compared with 85% 2- and 5-year survival of the intramedullary fixation group (p = 0.003) (Fig. 3).
Fig. 1.
This Kaplan–Meier survivorship curve with 95% confidence intervals shows that both nails (p = 0.008, log-rank test) and endoprostheses (EPR) (p = 0.001, log-rank test) outlived the patients in whom they had been implanted.
Fig. 2.
This Kaplan–Meier survivorship curve with 95% confidence intervals shows that there was no difference (p = 0.31) in complication-free survival between nails and endoprostheses (EPR).
Fig. 3.
This Kaplan–Meier survivorship curve with 95% confidence intervals shows improved survival of the implant without mechanical failure in the endoprosthesis (EPR) group (p = 0.003).
Discussion
Surgical management of patients with metastatic disease of the proximal femur has evolved over the last 30 years with a move away from plate fixation to the use of intramedullary and endoprosthetic devices. However, the ideal method of treatment still remains unclear. We therefore compared (1) function; (2) complications; and (3) survivorship in patients treated with intramedullary devices and EPR.
We acknowledge several limitations of this study. First, owing to the nonrandomized retrospective design, we did not control for potentially confounding variables such as type or extent of disease, the degree of intraoperative resection, preoperative level of function, age of the patient, medical problems (osteoporosis), and associated adjuvant treatments (chemotherapy, bisphosphonates, etc). Metastatic lesions to the proximal femur occur in a very diverse group with a variety of diagnoses at different stages of disease and with differing lesion characteristics. Each patient’s treatment must be individualized to their current situation, making it difficult to conduct a truly randomized study comparing one surgical technique with another with sufficient numbers. However, all patients were treated at one institution using the same rationale, selection criteria, and surgical technique. Second, there is selection bias in the choice of surgical implant. When discussing surgical options with our patients, we presented risks and benefits of both IMN and EPR, allowing the patient to make an informed decision regarding implant choice based on their personal needs and expectations. For patients with extensive lesions (involving greater than 50% of the bone diameter) and fractures, we generally encouraged resection and EPR; however, not all patients chose this option. Even so, this bias would skew the endoprosthetic group toward more aggressive tumors and extensive lesions. Nonetheless, we observed no difference in overall complication rates but rather a decreased rate of mechanical failure (0% versus 11%) in the endoprosthetic group. Selection bias will be difficult to eliminate from future studies, because each patient will continue to be treated on an individual basis. Third, our complication rate only measured those complications requiring surgical intervention, which may exclude nonsurgical events (excessive bleeding, nerve palsy, chronic pain, etc) of clinical significance and should be noted. However, the objective nature of this measure lends itself to more accurate reporting of complications as opposed to the subjective nature of the measures “minor” or “major.” Next, we treated all patients with failed implants with surgical revision, although not all patients with metastatic disease will have revision surgery. This difference may skew functional results when compared with patients outside of this study. Patients with metastatic disease to bone have a decreased life expectancy; therefore, survivorship analysis may not accurately reflect the true longevity of the implant in all patients. However, the study specifically evaluates patients with metastatic disease and therefore should accurately reflect survivorship in the cohort being treated.
Our MSTS scores of 80% of normal in the intramedullary nail group and 70% of normal in the endoprosthesis group compare with scores reported by Potter et al. in patients with metastatic disease to the proximal femur treated with EPR [22]. Our MSTS score percentages of normal also compare with a Toronto Extremity Salvage Score of 61% of normal reported by Chandrasekar et al. in their series of proximal femoral replacement [5].
We found no difference in complications between our two groups. In our intramedullary fixation group, 22% (10 of 46) experienced painful nonunion, of which 60% (six of 10) of cases eventually went on to hardware failure. The presence of fracture resulted in an increased rate of reoperation and subsequent mechanical failure in the intramedullary nail group. Implant failure has been reported in other series evaluating IMN for metastatic lesions with a failure rate ranging between 2% and 22% [13, 26–28]. This probability of implant failure increases with longer patient survival and the presence of nonunion [6]. Intramedullary nails are designed to act as internal splints with load-sharing properties and are assumed to bear most of the load initially and then gradually transfer it to the bone as the fracture heals [3]. These devices were not designed to bear the entire load of the patient for the remainder of the patient’s lifetime and are thus at a higher risk for failure in patients with large bone defects or fractures may never heal. Furthermore, intralesional treatment of metastatic disease is treated with adjuvant external beam radiation therapy, which also may contribute to delayed union and resultant implant failure. In contrast, the load-bearing characteristics of endoprostheses allow immediate postoperative stability and weightbearing with a low reported mechanical failure rate [4, 5, 25, 28]. We found endoprostheses had a lower rate of mechanical failure than intramedullary devices (0% versus 11%). Another study that directly compared these two groups found a statistically insignificant trend toward decreased mechanical failure in the endoprosthesis group [28]. A majority of the intramedullary nail complications occurred late (after 1 year) as compared with a small minority of the endoprosthesis complications, a comparison not yet noted in previous literature but which should play a role in decision-making. Our relatively low dislocation rate of 9% (10 of 113) is comparable to previous series of endoprostheses using bipolar heads, which demonstrate a 1% to 12% dislocation rate [4, 5, 10, 21, 23, 25]. Other series in which acetabular resurfacing was also performed report a higher rate of instability of up to 30% when compared with bipolar implants [10, 17]. Dislocation may be further reduced by performing capsular repair [17]. It is our recommendation to use bipolar head components and repair the hip capsule whenever possible. Previous studies cite perioperative cardiopulmonary complications attributed to the use of long intramedullary implants with a rate of perioperative death as high as 10% [2, 14, 19, 25]. In our study, one patient died within 2 weeks of IMN and none within 14 days of EPR. We attribute our lack of mortality to our preoperative medical optimization, use of unreamed femoral nails, use of sharp reamers for long-stem endoprostheses, and careful cementing technique, allowing the anesthesiologist to optimize the patient’s vital parameters and increase intravenous fluids before cementing.
In a previous series of 228 pathologic long bone fractures, the most important risk factor for treatment failure was the length of survival after surgery [6]. Because patients are living longer with improved adjuvant therapies, this treatment failure is expected to further increase. Some authors argue that, for patients with a long projected survival, an endoprosthesis is the more durable surgical option [5, 21]. Some have even suggested that EPR should be the treatment of choice in isolated lesions from renal cell metastasis because these patients may have prolonged survival [1, 15]. In our series, 51% of patients survived 1 year, 29% survived 2 years, and 11% survived 5 years. Our endoprosthesis survivorship compares well with that reported in of the literature, which has demonstrated 86% to 93% revision-free implant survival [5, 8, 22]. Although we found no difference in complication-free survival between our two patient groups, we found higher implant survival without mechanical failure in the endoprosthesis group, which supports data showing increased mechanical failure among intramedullary devices.
When treating metastatic lesions to the proximal femur, both IMN and EPR may be used for stabilization with similar functional scores and overall complication rates, and both devices generally outlasted the patient. Patients with metastatic disease to the bone are living longer than previously considered, and surgery should be performed with this extended survival in mind. Intramedullary fixation in the presence of fracture leads to increased mechanical failure and reoperation rates, and most of the intramedullary complications occur after 1 year as opposed to EPR complications, which occur early. Advantages of EPR over IMN include the immediate ability to fully weightbear, the avoidance of nonunion, and the decreased incidence of mechanical failure and subsequent revision surgery. Therefore, we believe EPR should be considered in patients with proximal femur metastatic lesions with associated pathologic fracture and prolonged life expectancy.
Acknowledgment
We thank Stacy R. Collen, PhD, for her statistical analysis of the data in this study.
Footnotes
One author certifies that he (LRM) has or may receive payments or benefits, in any 1 year, an amount in excess of $10,000 from a commercial entity (Stryker Orthopaedics, Rutherford, NJ) related to this work.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and informed consent for participation in the study was obtained.
References
- 1.Baloch KG, Grimer RJ, Carter SR, Tillman RM. Radical surgery for the solitary bone metastasis from renal cell carcinoma. J Bone Joint Surg Br. 2000;82:62–67. doi: 10.1302/0301-620X.82B1.9995. [DOI] [PubMed] [Google Scholar]
- 2.Barwood SA, Wilson JL, Molnar RR, Choong PFM. The incidence of acute cardiopulmonary and vascular dysfunction following intramedullary nail fixation of femoral metastases. Acta Orthop Scand. 2000;71:147–152. doi: 10.1080/000164700317413111. [DOI] [PubMed] [Google Scholar]
- 3.Bong MR, Kummer FJ, Koval KJ, Egol KA. Intramedullary nailing of the lower extremity. J Am Acad Orthop Surg. 2007;15:97–106. doi: 10.5435/00124635-200702000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Cannon CP, Mirza AN, Lin PP, Lewis VO, Yasko AW. Proximal femoral endoprosthesis for the treatment of metastatic. Orthopedics. 2008;31:361. doi: 10.3928/01477447-20080401-03. [DOI] [PubMed] [Google Scholar]
- 5.Chandrasekar CR, Grimer RJ, Carter SR, Tillman RM, Abudu A, Buckley L. Modular endoprosthetic replacement for tumours of the proximal femur. J Bone Joint Surg Br. 2009;91:108–112. doi: 10.1302/0301-620X.91B1.20448. [DOI] [PubMed] [Google Scholar]
- 6.Dijstra S, Wiggers T, Geel BTV, Boxma H. Impending and actual pathological fractures in patients with bone metastases of long bones: a retrospective study of 233 surgically treated patients. Eur J Surg. 1994;160:535–542. [PubMed] [Google Scholar]
- 7.Enneking WF, Dunham W. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–246. [PubMed] [Google Scholar]
- 8.Finstein JL, King JJ, Fox FJ, Ogilvie CM, Lackman RD. Bipolar proximal femoral replacement prostheses for musculoskeletal neoplasms. Clin Orthop Relat Res. 2007;459:66–75. doi: 10.1097/BLO.0b013e31804f5474. [DOI] [PubMed] [Google Scholar]
- 9.Gruen TA, McNeice GM, Amstutz HC. ‘Modes of failure’ of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res. 1979;141:17–27. [PubMed] [Google Scholar]
- 10.Haentjens P, Neve W, Casteleyn PP, Opdecam P. Massive resection and prosthetic replacement for the treatment of metastases of the trochanteric and subtrochanteric femoral region bipolar arthroplasty versus total hip arthroplasty. Acta Orthop Belg. 1993;59(Suppl 1):367–371. [PubMed] [Google Scholar]
- 11.Hage WD, Aboulafia AJ, Aboulafia DM. Incidence, location, and diagnostic evaluation of metastatic bone disease. Orthop Clin North Am. 2000;31:515–528. doi: 10.1016/S0030-5898(05)70171-1. [DOI] [PubMed] [Google Scholar]
- 12.Harrington KD. Orthopaedic surgical management of skeletal complications of malignancy. Cancer. 1997;80:1614–1627. doi: 10.1002/(SICI)1097-0142(19971015)80:8+<1614::AID-CNCR12>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 13.Hunt KJ, Gollogy S, Randall RL. Surgical fixation of pathologic fractures. Bull Hosp Joint Dis. 2006;63:77–82. [PubMed] [Google Scholar]
- 14.Kerr PS, Jackson M, Atkins RM. Cardiac arrest during intramedullary nailing for femoral metastases. J Bone Joint Surg Br. 1993;75:972–973. doi: 10.1302/0301-620X.75B6.8245094. [DOI] [PubMed] [Google Scholar]
- 15.Lin PP, Mirza AN, Lewis VO, Cannon CP, Tu SM, Tannir NM, Yasko AW. Patient survival after osseous metastases from renal cell carcinoma. J Bone Joint Surg Am. 2007;89:1794–1801. doi: 10.2106/JBJS.F.00603. [DOI] [PubMed] [Google Scholar]
- 16.Marcove RC, Yang DJ.Survival times after treatment of pathologic fractures Clin Orthop Relat Res. 1982169109–114.7105565 [Google Scholar]
- 17.Menendez LR, Ahlmann ER, Kermani C, Gotha H. Endoprosthetic replacement for neoplasms of the proximal femur. Clin Orthop Relat Res. 2006;450:46–51. doi: 10.1097/01.blo.0000229332.91158.05. [DOI] [PubMed] [Google Scholar]
- 18.Mirels H. Metastatic disease in long bones. Clin Orthop Relat Res. 1989;249:256–264. [PubMed] [Google Scholar]
- 19.Nilsson J, Gustafson P. Surgery for metastatic lesions of the femur: good outcome after 245 operations in 216 patients. Injury. 2008;39:404–410. doi: 10.1016/j.injury.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 20.O’Neill DA, Harris WH. Failed total hip replacement: assessment by plain radiographs, arthrograms, and aspiration of the hip joint. J Bone Joint Surg Am. 1984;66:540–546. [PubMed]
- 21.Park DH, Jaiswal PK, Al-Hakim W, Aston WJS, Pollock RC, Skinner JA, Cannon SR, Briggs TWR. The use of massive endoprostheses for the treatment of bone metastases. Sarcoma. 2007;62:1–5. doi: 10.1155/2007/62151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Potter BK, Chow VE, Adams SC, Letson GD, Temple HT. Endoprosthetic proximal femur replacement: metastatic versus primary tumors. Surg Oncol. 2009;18:343–349. doi: 10.1016/j.suronc.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Rompe JD, Eysel P, Hopf C, Heine J. Metastatic instability at the proximal end of the femur. Comparison of endoprosthetic replacement and plate osteosynthesis. Arch Orthop Trauma Surg. 1994;113:260–264. doi: 10.1007/BF00443814. [DOI] [PubMed] [Google Scholar]
- 24.Samsani SR, Panikkar V, Venu KM, Georgiannos D, Calthorpe D. Breast cancer bone metastasis in femur: surgical considerations and reconstruction with long gamma nail. Eur J Surg Oncol. 2004;30:993–997. doi: 10.1016/S0748-7983(04)00200-8. [DOI] [PubMed] [Google Scholar]
- 25.Selek H, Basarir K, Yildiz Y, Saglik Y. Cemented endoprosthetic replacement for metastatic bone disease in the proximal femur. J Arthroplasty. 2008;23:112–117. doi: 10.1016/j.arth.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 26.Doorn R, Stapert JWJL. Treatment of impending and actual pathological femoral fractures with the long gamma nail in the Netherlands. Eur J Surg. 2000;166:247–254. doi: 10.1080/110241500750009366. [DOI] [PubMed] [Google Scholar]
- 27.Ward WG, Holsenbeck S, Dorey FJ, Spang J, Howe D. Metastatic disease of the femur: surgical treatment. Clin Orthop Relat Res. 2003;415(Suppl):S230–S244. doi: 10.1097/01.blo.0000093849.72468.82. [DOI] [PubMed] [Google Scholar]
- 28.Wedin R, Bauer HC. Surgical treatment of skeletal metastatic lesions of the proximal femur: endoprosthesis or reconstruction nail? J Bone Joint Surg Br. 2005;87:1653–1657. doi: 10.1302/0301-620X.87B12.16629. [DOI] [PubMed] [Google Scholar]
- 29.Wedin R, Bauer HCF, Wersall P. Failures after operation for skeletal metastatic lesions of long bones. Clin Orthop Relat Res. 1999;358:128–139. doi: 10.1097/00003086-199901000-00016. [DOI] [PubMed] [Google Scholar]
- 30.Yazawa Y, Frassica FJ, Chao EY, Pritchard DJ, Sim FH, Shives TC. Metastatic bone disease. A study of the surgical treatment of 166 pathologic humeral and femoral fractures. Clin Orthop Relat Res. 1990;251:213–219. [PubMed] [Google Scholar]