Abstract
Background
As measured via static stability tests, the PCL is the dominant restraint to posterior tibial translation while the posterolateral corner is the dominant restraint to external tibial rotation. However, these uniplanar static tests may not predict multiplanar instability. The reverse pivot shift is a dynamic examination maneuver that may identify complex knee instability.
Questions/purposes
In this cadaver study, we asked whether (1) isolated sectioning or (2) combined sectioning of the PCL and posterolateral corner increased the magnitude of the reverse pivot shift and (3) the magnitude of the reverse pivot shift correlated with static external rotation or posterior drawer testing.
Methods
In Group I, we sectioned the PCL followed by structures of the posterolateral corner. In Group II, we sectioned the posterolateral corner structures before sectioning the PCL. We performed posterior drawer, external rotation tests, and mechanized reverse pivot shift for each specimen under each condition and measured translations via navigation.
Results
Isolated sectioning of the PCL or posterolateral corner had no effect on the reverse pivot shift. Conversely, combined sectioning of the PCL and posterolateral corner structures increased the magnitude of the reverse pivot shift. The magnitude of the reverse pivot shift correlated with the posterior drawer and external rotation tests.
Conclusions
Combined sectioning of the PCL and posterolateral corner was required to cause an increase in the magnitude of the mechanized reverse pivot shift. The reverse pivot shift correlated with both static measures of stability.
Clinical Relevance
Combined injury to the PCL and posterolateral corner should be considered in the presence of a positive reverse pivot shift.
Introduction
Injuries to the PCL and the posterolateral corner (PLC) remain a challenge from the diagnostic and management perspective. The multiplanar instability patterns present in the ACL-deficient knee have been previously described [9, 33–35]. Additionally, there have been investigations related to the uniplanar kinematics of the PCL- and PLC-deficient knee [3, 8, 12, 13, 18, 24, 26, 27, 30]. These investigations document an intimate relationship between the PCL and PLC in controlling AP translation and axial rotation of the knee: the PCL is a primary restraint to posterior translation of the tibia and a secondary restraint to external rotation, while the PLC is a primary restraint to external and varus rotation and a secondary restraint to posterior translation [23]. The studies supporting this idea utilized conventional static uniplanar testing to assess stability in the ligament-deficient and reconstructed state. However, clinical studies evaluating PCL and PLC reconstruction [17, 20, 36, 39] have failed to demonstrate a correlation between the degree of knee laxity measured by uniplanar testing and subjective outcomes.
To our knowledge, dynamic stability tests evaluating the PCL- and PLC-deficient knee have not been extensively studied. Dynamic tests elicit instability in multiple planes by applying force over a ROM of the joint. Dynamic tests, such as the pivot shift, may more accurately recapitulate the clinical state and predict subjective symptoms [21]. Similar to the conventional pivot shift maneuver, the reverse pivot shift (RPS) is a dynamic test involving a pathologic, multiplanar motion path elicited by a combination of axial load and valgus force during knee extension from a flexed position [23]. The lateral tibial plateau shifts from a position of posterior subluxation to a position of reduction as the flexed knee is brought into extension, resulting in a characteristic clunk. Clinically, physicians commonly use this test to evaluate for the presence of posterolateral rotational instability of the knee. However, the assessment is qualitative and difficult to reproduce between observers. Furthermore, the relevance of a positive test is not clearly understood, as the relative contributions of the PCL and PLC to resisting this abnormal movement arc have not been quantified.
The refinement of computer-navigated surgery and associated data acquisition provide a unique opportunity to quantify dynamic tests of knee stability in various conditions of knee ligament deficiency. Our laboratory developed a mechanized device, which, in conjunction with computer navigation, quantifies translations in the medial, central, and lateral compartments of the knee during the pivot shift and RPS maneuvers [31]. By navigating the examination of the knee after selective sectioning of the PCL and structures of the PLC, we can gain a better understanding of how specific injury patterns increase the magnitude of this dynamic test of knee stability. Furthermore, we can delineate the relationship of this dynamic stability test to other established uniplanar tests, such as the posterior drawer and external rotation tests. Accordingly, practitioners can better interpret the importance of a positive RPS in the clinical setting and use this information to formulate a treatment plan.
We therefore asked whether (1) isolated sectioning of the PCL or the PLC increased the magnitude of the RPS, (2) combined sectioning of the PCL and PLC increased the magnitude of the RPS, and (3) the magnitude of the RPS correlated with static external rotation or posterior drawer testing.
Materials and Methods
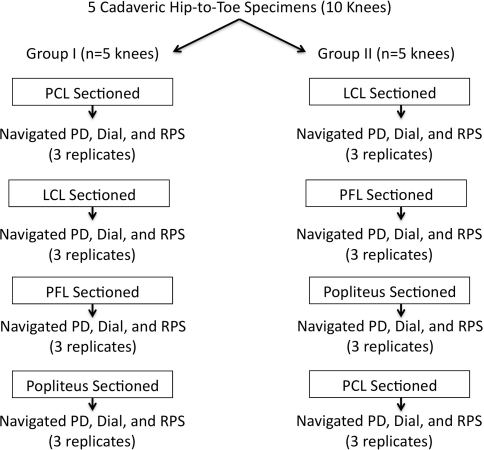
We obtained five fresh-frozen cadaveric hip-to-toe lower extremity specimens (10 knees; five knees allocated to each group) (average age of donor, 57 years; range, 42–58 years). For each cadaver specimen, we randomly assigned one knee to one of two experimental groups. In Group I, we sectioned the PCL and then sequentially sectioned the structures of the PLC, including the lateral collateral ligament (LCL), popliteal fibular ligament (PFL), and popliteus tendon. In Group II, we sequentially sectioned the structures of the PLC before sectioning of the PCL. We performed a navigated posterior drawer, external rotation dial test at 30° and 90° of knee flexion, and a mechanized RPS in the intact state and following section of each of the aforementioned structures (Fig. 1). Prior IRB approval was obtained for the use of human cadaveric tissue in this project.
Fig. 1.
A flowchart shows the distribution of the 10 cadaveric hip-to-toe specimens utilized in this protocol. Five knees were allocated to each group (I and II). In Group I, the PCL was sectioned, followed by sequential sectioning of the structures of the PLC. In Group II, the structures of the PLC were sectioned followed by the PCL. Mechanical testing consisting of a posterior drawer (PD) test, external rotation dial tests at 30° and 90°, and RPS were performed for each condition.
Based on previous data, we conducted a priori power analysis (power, 0.80; significance level, 0.05) to ensure we could detect differences of 3 mm in compartmental translation. We chose a difference of 3 mm as a clinically relevant value based on previous kinematic data obtained during examination of ACL-deficient knees studying similar parameters [6, 25, 31, 33, 34]. The power analysis indicated five specimens would be needed per group to discern a difference of 3 mm in compartmental translation per condition.
We used a surgical navigation system (PraximMedivision, Grenoble, France) to evaluate translations and rotations during the knee examination in the intact state and following serial sectioning of the PCL and structures of the PLC. The utilization of a surgical navigation system during clinical laxity examinations was reliable and repeatable; high intraclass correlation coefficients (0.998) were recorded for this surgical navigation system in comparison to a robotic manipulator [34].
For data acquisition, we used the PraximSurgetics navigation system (PraximMedivision) with customized software. We fixed rigid bodies to the distal femur and proximal tibia and traced reflective markers using an infrared camera, as previously described [7, 9]. We recorded surface landmarks, mapped intraarticular surface geometry, and created a three-dimensional model of the knee [37]. Using a proximally directed axial force to keep the tibial and femoral condyles in contact at all flexion positions, we manually cycled the knee from full extension to 90° of flexion. This represented the passive reference path from which we measured the deviation during each pivot shift examination. We performed the reference motion path 20 times, taking care to keep the leg in neutral rotation via navigation. The accuracy of this system was within 1 mm for translational measurements and 1° for measurements of rotation [14, 19].
To simulate the clinical examination, we performed a 65-N posterior drawer test [2–4] with a tensiometer attached to a 6.5-mm screw in the posterior tibia. This posterior load has been utilized by other authors to simulate a clinical examination of the knee and generate a posterior translation of the tibia in the absence of the PCL. However, this load does not necessarily recapitulate in vivo loading of the PCL that has been estimated to range from 65 to 765 N during athletic activities [10, 11]. Optical tracking of joint position allowed for consistent testing at 90° of knee flexion. The navigation system recorded posterior knee translation during the instrumented posterior drawer examination. We also performed a 5-Nm external rotation dial test [2–4] at 30° and 90° of knee flexion with a tensiometer attached to a 5-mm screw in the distal tibia. This torque has been utilized by other authors to simulate a clinical examination and generate external rotation of the tibia in the absence of PLC structures. Similarly, this load may not recapitulate the loads seen in vivo. However, in vivo torque and the resulting stress on the PLC have not been well described in contemporary biomechanical literature. The navigation system recorded medial and lateral compartment translations and tibial rotation during the external rotation examinations. We based the loads chosen for both the posterior drawer and external rotation tests on previous studies that attempted to recreate a clinical manual testing scenario, rather than a test of peak in vivo loading during activities of daily living [1–4, 38].
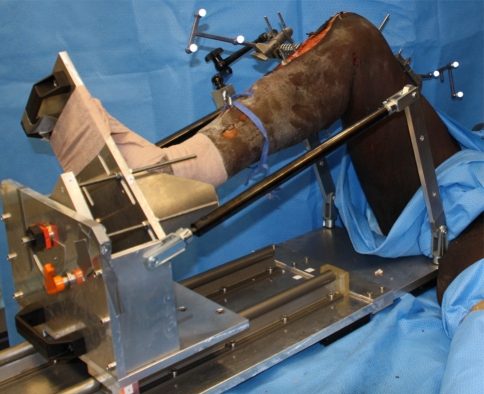
Using a previously described custom device [31], we performed a standardized mechanized RPS maneuver. The mechanized RPS utilized a customized machine that secured to the operating room table (Fig. 2). We achieved a 49-N valgus force by using a load cell positioned on the lateral aspect of the proximal tibia and secured to the mechanized pivot shifter. We maintained axial load on the limb throughout the RPS by securing the pelvis to the table during a flexion-extension cycle. The mechanized RPS device cycled the knee from 90° of knee flexion to maximal extension, while the navigation system simultaneously recorded knee kinematics.
Fig. 2.
A photograph shows how the right knee is secured in the mechanized RPS device. The leg holder suspends the proximal tibia. There is no thigh support allowing the femur to subluxate anteriorly during the reverse pivot. A load cell is mounted laterally to generate the valgus moment across the knee.
During the mechanized RPS, the navigation system recorded the three-dimensional path of a tracked point at the center of the tibia, center of the medial tibial plateau, and center of the lateral tibial plateau. Motion of these points was analyzed throughout a given motion path regarding a tracked central point in the notch of the femur. Customized software allowed us to compare the motion path during the pivot shift with the reference motion path of extension-flexion [9, 22]. We reported the maximum tibial translations and rotation during the RPS maneuver as the difference between reference motion path and RPS motion path in the medial and lateral compartments [5]. The utilization of a surgical navigation system during clinical laxity examinations was both reliable and repeatable [34].
A single surgeon (FAP) performed all surgical dissections. The surgeon transected the PCL by carefully dissecting the synovial sheath from the PCL at 90° of flexion and dissecting the tissue between the ACL and PCL. Using a Number 15 blade with the knee at 120° flexion, the femoral insertion of the PCL was dissected from the femoral wall. The stump was retracted posteriorly and the tibia was translated posteriorly to assure all soft tissue was from the medial wall of the femur. The arthrotomy was closed with Number 2 Ethibond® sutures (Ethicon, Inc, Somerville, NJ) and closed the skin with a running nylon suture.
The surgeon approached the PLC of the knee through a standard lateral hockey stick incision, divided the iliotibial band, and identified the LCL, popliteus tendon, and PFL. For each respective testing condition, the LCL ligament was divided sharply at its midsubstance, the PFL dissected off from its fibular attachment, and the popliteus dissected from its femoral insertion. Then, the iliotibial band was closed with Number 2 Ethibond® suture and the skin was closed with a running nylon suture after sequential sectioning of each structure.
To determine whether there was a difference in the magnitude of the RPS between the ligament intact state and after serial sectioning of the PCL and PLC structures, we used a repeated-measures ANOVA with four measures and a post hoc Tukey multiple-comparison test to compare the mean compartmental translations for each tracked point during the RPS examination within each group. Data were tested for normality using the Kolmogorov-Smirnoff test and for homogeneity of variance using Levene’s test (α = 0.05); the data were normally distributed. To determine whether the RPS correlated with external rotation or posterior drawer tests, we plotted the magnitude of the RPS against the magnitude of the posterior drawer and external rotation tests and established the slope of the best-fit line and r2 (coefficient of determination). We performed all statistical analysis using GraphPad Prism® (GraphPad Software, Inc, San Diego, CA).
Results
Isolated sectioning of the PCL in the presence of an intact PLC had no effect (p = 0.123) on the magnitude of the RPS as measured via lateral compartment translation when compared to the ligament-intact state (−7.4 mm versus −10.6 mm) (Fig. 3). Furthermore, isolated sectioning of the PCL had no effect (p = 0.212) on the magnitude of external tibial rotation during the RPS (−10.8° versus −7.0°). Isolated sectioning of each of the PLC structures in the presence of an intact PCL had no effect (p = 0.421) on the magnitude of the RPS as measured via lateral compartment translation as compared to the intact state (−8.3 mm versus −14.5 mm) (Fig. 4). Moreover, isolated sectioning of PLC structures in the presence of an intact PCL had no effect (p = 0.15) on external tibial rotation during the RPS (−6.5° versus −7.5°).
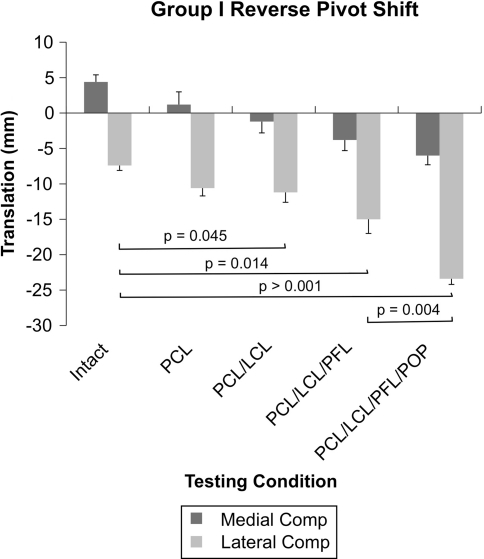
Fig. 3.
A graph shows medial and lateral compartment translations in response to a mechanized RPS. Sectioning of the PCL and LCL resulted in an increase in lateral compartment posterior translation. Sectioning of the PFL and popliteus resulted in further increases in lateral compartment posterior translations. Comp = compartment; POP = popliteus tendon.
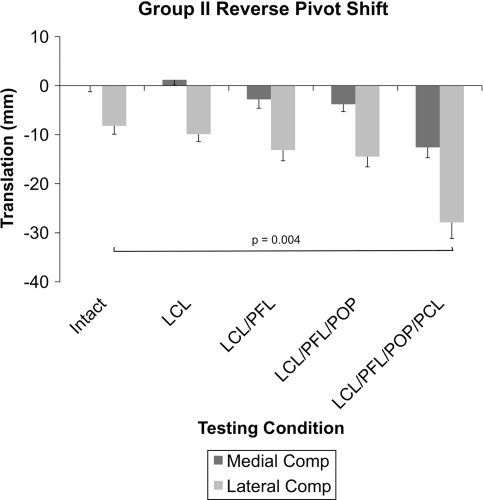
Fig. 4.
A graph shows medial and lateral compartment translations in response to a mechanized RPS. Sectioning of the PLC structures had no effect on lateral compartment posterior translation. Additional sectioning of the PCL resulted in further increases in lateral compartment posterior translations. Comp = compartment; POP = popliteus tendon.
Combined sectioning of the PCL and the LCL increased (p = 0.045) the magnitude of the RPS measured via lateral compartment translation as compared to the intact state (−7.4 mm versus −11.2 mm). Incremental increases in lateral compartment translation occurred after sectioning of the PFL (−7.4 mm versus −15 mm; p = 0.014) and popliteus tendon (−7.4 mm versus −23.4 mm; p > 0.001) versus the intact state. A 9-mm (15 mm versus 24 mm) increase (p = 0.004) in lateral compartment translation was noted after sectioning of the popliteus tendon.
Conversely, combined sectioning of the PCL and LCL had no effect (p = 0.081) on the magnitude of external tibial rotation during the RPS as compared to the intact state (−10.8° versus −9.0°). Furthermore, no change (p = 0.159) in external tibial rotation was noted after sectioning of the PFL (−10.8° versus −11.5°). However, further sectioning of the popliteus resulted in an increase (p < 0.001) in external tibial rotation (−10.8° versus −20.0°).
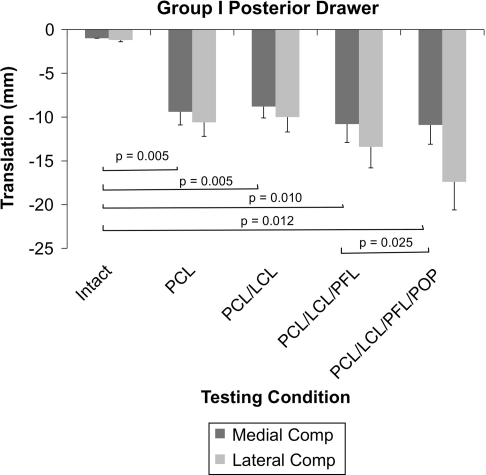
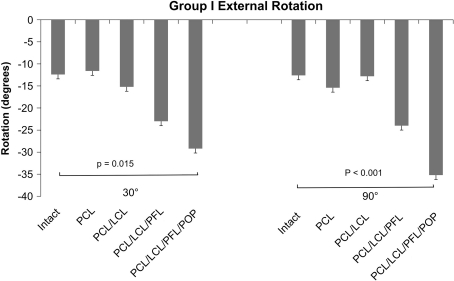
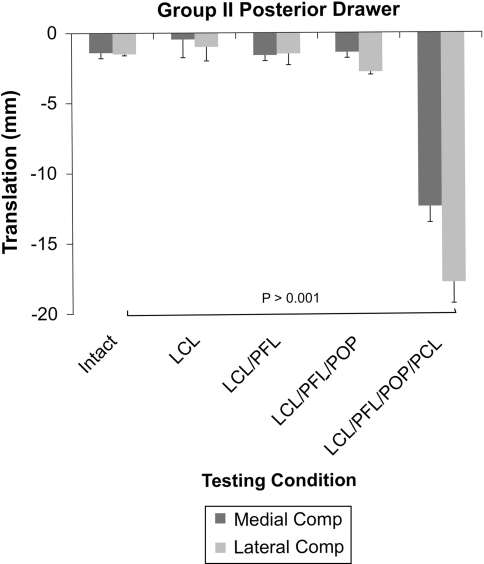
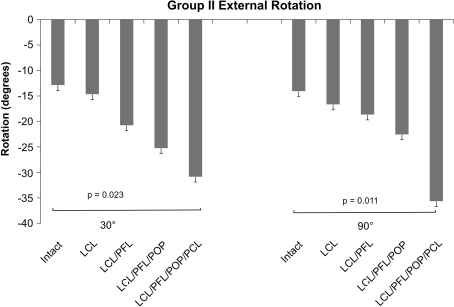
Static testing revealed sectioning of the PCL in the presence of an intact PLC led to an increase in medial and lateral compartment posterior translation in response to a 65-N posterior drawer test (Fig. 5) but had no effect on external rotation in response to a 5-Nm external rotation dial test at either 30° or 90° of knee flexion (Fig. 6). Sectioning of the PLC structures in the presence of an intact PCL had no effect on medial or lateral compartment posterior translation in response to a 65-N posterior drawer test (Fig. 7). Sectioning of the LCL and PFL in the presence of an intact PCL had no effect in response to a 5-Nm dial test, but additional sectioning of the popliteus tendon resulted in increases in external rotation at both 30º and 90º of knee flexion (Fig. 8). The magnitude of the RPS correlated with the magnitude of the lateral compartment translation during the posterior drawer test (Table 1) and tibial rotation during the external rotation test at 30° and 90° (Table 2). Collectively, we found the strongest correlations between the posterior drawer and lateral compartment translation during the mechanized RPS.
Fig. 5.
A graph shows medial and lateral compartment translations in response to a 65-N posterior drawer in Group I. Sectioning of the PCL resulted in an increase in posterior translation of the medial and lateral compartments. Further sectioning of the PLC structures did not result in an increase in medial compartment translation. Comp = compartment; POP = popliteus tendon.
Fig. 6.
A graph shows external tibial rotation in response to a 5-Nm external rotation force at 30° and 90° of knee flexion in Group I. Sectioning of the PCL, LCL, PFL, and popliteus were required to generate an increase in external tibial rotation at both 30° and 90° of knee flexion. Comp = compartment; POP = popliteus tendon.
Fig. 7.
A graph shows medial and lateral compartment translations in response to a 65-N posterior drawer in Group II. Sectioning of all PLC structures (LCL/PFL/popliteus) had no effect on posterior drawer. Further sectioning of the PCL resulted in an increase in medial compartment posterior translation. Comp = compartment; POP = popliteus tendon.
Fig. 8.
A graph shows external tibial rotation in response to a 5-Nm external rotation force at 30° and 90° of knee flexion in Group II. Sectioning of the LCL, PFL, popliteus, and PCL were required to generate an increase in external rotation at both 30° and 90° of knee flexion. Comp = compartment; POP = popliteus tendon.
Table 1.
Comparison between lateral compartment translation during the posterior drawer test and the mechanized RPS
| Comparison | Group I | Group II |
|---|---|---|
| RPS versus posterior drawer | ||
| R2 | 0.93 | 0.79 |
| P | 0.009 | 0.045 |
| Slope | 1.010 | 0.910 |
RPS = reverse pivot shift.
Table 2.
Comparison between tibial rotation during external rotation testing and lateral compartment translation during the mechanized RPS
| Comparison | Group I | Group II |
|---|---|---|
| RPS versus external rotation at 90° | ||
| R2 | 0.56 | 0.67 |
| P | 0.0035 | < 0.001 |
| Slope | 0.311 | 0.368 |
| RPS versus external rotation at 30° | ||
| R2 | 0.70 | 0.59 |
| P | 0.001 | 0.002 |
| Slope | 0.464 | 0.317 |
RPS = reverse pivot shift.
Discussion
Dynamic stability testing of the knee and the pivot shift phenomenon have drawn a great deal of attention from both clinicians and researchers as the proposed standard for assessing knee laxity in the ACL-deficient and ACL-reconstructed state. The impetus for the use of this test is predicated on studies suggesting subjective symptoms and patient satisfaction are more closely correlated with the presence of a pivot shift than abnormal laxity as measured by the Lachman examination [21]. The RPS may represent an important metric for measuring dynamic knee instability in both the biomechanical and clinical arenas; however, little is known about the relative contributions of PCL and PLC injuries to this pathologic movement arc. We therefore asked whether (1) isolated sectioning of the PCL or PLC increased the magnitude of the RPS, (2) combined sectioning of the PCL and PLC increased the magnitude of the RPS, and (3) the magnitude of the RPS correlated with static external rotation or posterior drawer testing.
We acknowledge limitations to our study. First, a limitation of many cadaveric studies utilizing serial sectioning is that not all permutations of sectioning protocols are examined owing to the limited number of specimens. Consequently, we only examined sectioning of the PLC structures in which the LCL was sectioned first, followed by the PFL, and finally the popliteus. Accordingly, we could not make definitive statements about the dominant role of any of these structures. Second, the magnitude of axial force applied to the knee during the mechanized RPS was unknown, complicating comparisons to other biomechanical studies on this topic. However, axial load was applied such that the knee moved from a flexed to extended position by our RPS simulator. While this closely recapitulated the clinical scenario and ensured a reproducible motion path [9, 33], the amount of axial load necessary to extend the knee was not recorded. Previous work demonstrated varying the axial load did not have an effect on the magnitude of the RPS [31] but simply changed the velocity of knee extension. While other studies evaluating a “simulated pivot shift” utilized axial loads of approximately 25 N to generate a positive test, the authors of these previous studies found this load differed among specimens [28, 29]. The utility of this study design was that we were able to precisely measure translation during both static and dynamic testing using a computer-assisted navigation system. Consequently, we were able to discern differences in the motion path between experimental states in the absence of known forces across the knee. Third, we did not evaluate the RPS as a predictive measure of subjective instability after PCL or PLC injury. Future studies addressing this deficiency in the literature would be useful. Finally, while our laboratory showed the mechanized pivot shifter produced reproducible findings and correlated well with the manual examination [31], that study did not take into consideration the potential for variability in the manner in which the examination is performed by different clinicians. Accordingly, we acknowledge the findings may not apply in all clinical examination scenarios.
Isolated sectioning of the PCL in the presence of an intact PLC had no effect on the magnitude of the RPS as measured via lateral compartment translation or external tibial rotation. These data were consistent with a cadaveric study in which Jakob et al. [16] graded the RPS after serial sectioning of the PLC and PCL and noted isolated sectioning of the PCL did not generate a RPS. We also found isolated sectioning of the PLC in the absence of PCL injury had no effect on the RPS. This finding contrasted with that of Jakob et al. [16], where the sequential sectioning of the PLC structures in the presence of an intact PCL led to an increase in the RPS, with sectioning of the popliteus resulting in the greatest increase in magnitude of the examination. It is important to note, in that study, the RPS was graded manually without any objective quantification.
When combined with sectioning of the PCL, sectioning of the LCL, PFL, and popliteus all increased the magnitude of the RPS as measured via lateral compartment translation. However, combined sectioning of the PCL and all of the structures of the PLC were necessary to generate a substantial increase in external tibial rotation during the RPS. Sectioning of the popliteus resulted in more than a 1.5-fold increase in lateral compartment translation while it doubled external tibial rotation. Nielsen and Helmig [32] demonstrated the LCL and popliteus could resist varus and external rotation forces, with the LCL providing greater restraint against varus rotation and the popliteus having a greater role against external rotation. We found both of these structures had a role in diminishing external rotation and the magnitude of the RPS. We have compared our findings to those documented in the literature (Table 3).
Table 3.
Comparative studies of PCL and PLC insufficiency
| Study | Study type | PCL insufficiency | PLC insufficiency | PCL and PLC insufficiency |
|---|---|---|---|---|
| Apsingi et al. [2] | Cadaveric biomechanical | Increased posterior laxity; no change in ER laxity | Isolated PLC sectioning not performed | Increased posterior laxity and ER laxity |
| Chun et al. [8] | Cadaveric biomechanical | Not tested | Sectioning of LCL had modest effect on ER; sectioning of the PFL and PT resulted in greater increases in ER laxity | Not tested |
| Jakob et al. [16] | Cadaveric subjective examination | No effect on the RPS | Increased subjective grade of RPS | Increased subjective grade of RPS |
| LaPrade and Terry [23] | Clinical subjective examination | Injury to the LCL, PT, or midthird lateral capsular ligament resulted in an abnormal RPS | Not tested | Not tested |
| Li et al. [24] | Clinical examination with in vivo imaging | Increase in posterior tibial translation beyond 30° of flexion | Not tested | Not tested |
| Nielsen and Helmig [32] | Cadaveric subjective examination | Not tested | Posterolateral instability noted only after sectioning PT | Not tested |
| Petrigliano et al. | Cadaveric biomechanical | Increased posterior laxity and no effect on RPS | Increased ER laxity and no effect on RPS | Increased posterior and ER laxity; increased RPS |
PLC = posterolateral corner; ER = external rotation; LCL = lateral collateral ligament; PT = popliteus tendon; RPS = reverse pivot shift; PFL = popliteofibular ligament.
Finally, we found the dynamic RPS examination correlated with the static posterior drawer and external rotation tests. The results via static testing were consistent with previous studies that described the PCL being the primary restraint to posterior translation and the PLC being the primary restraint to rotational laxity. Accordingly, we postulated the posterior drawer would not correlate to the RPS, which had a substantial rotational component. However, both static examinations correlated with the magnitude of the RPS. Interestingly, we found the posterior drawer had a stronger correlation with the magnitude of the RPS as measured via lateral compartment translation than did external rotation tests at either 30° or 90° of knee flexion. Similar findings were noted by Markolf et al. [29] when they compared the Lachman to the pivot shift in a cadaveric model. Those authors observed linear correlations between the pivot shift and Lachman examination when they initially tensioned the knees to match the laxities of the intact specimen and then loosened them between testing conditions.
The principal finding in our study was that combined sectioning of the PCL, in addition to components of the PLC, was necessary to generate an increase in the magnitude of the RPS and that static and dynamic stability of the knee were related. These findings suggest the PCL and PLC play complementary roles in resisting the multiplanar instability measured by this dynamic examination. Similar to the pivot shift, the RPS phenomenon appears to be composed of both AP and rotatory translation vectors. Typically, with isolated injuries to the PLC, external rotatory subluxation occurs as the tibia rotates around the axis of an intact PCL [15]. In the absence of the PCL, this rotation takes place through the medial compartment, resulting in greater AP and axial translation. We believe this combined ligament injury is necessary to generate the higher magnitude of dynamic instability noted during the RPS. Consequently, greater lateral compartment translation occurs during the RPS than during the external rotation or posterior drawer tests when performed independently. These findings underscore the interrelated function of the PLC and PCL. The mechanized RPS may prove a useful metric for dynamic testing of the knee with PCL and PLC injury, and the examiner should be alert of combined ligament injury in its presence.
Footnotes
Dr. Pearle received research funding from the Hospital for Special Surgery Institute for Sports Medicine Research. Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved or waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
The investigation was performed in the Computer Assisted Surgery Laboratory at Hospital for Special Surgery.
References
- 1.Apsingi S, Bull AMJ, Deehan DJ, Amis AA. Review: femoral tunnel placement for PCL reconstruction in relation to the PCL fibre bundle attachments. Knee Surg Sports Traumatol Arthrosc. 2009;17:652–659. doi: 10.1007/s00167-009-0747-7. [DOI] [PubMed] [Google Scholar]
- 2.Apsingi S, Nguyen T, Bull AM, Unwin A, Deehan DJ, Amis AA. Control of laxity in knees with combined posterior cruciate ligament and posterolateral corner deficiency: comparison of single-bundle versus double-bundle posterior cruciate ligament reconstruction combined with modified Larson posterolateral corner reconstruction. Am J Sports Med. 2008;36:487–494. doi: 10.1177/0363546508314415. [DOI] [PubMed] [Google Scholar]
- 3.Apsingi S, Nguyen T, Bull AM, Unwin A, Deehan DJ, Amis AA. The role of PCL reconstruction in knees with combined PCL and posterolateral corner deficiency. Knee Surg Sports Traumatol Arthrosc. 2008;16:104–111. doi: 10.1007/s00167-007-0444-3. [DOI] [PubMed] [Google Scholar]
- 4.Apsingi S, Nguyen T, Bull AMJ, Unwin A, Deehan DJ, Amis AA. A comparison of modified Larson and “anatomic” posterolateral corner reconstructions in knees with combined PCL and posterolateral corner deficiency. Knee Surg Sports Traumatol Arthrosc. 2009;17:305–312. doi: 10.1007/s00167-008-0696-6. [DOI] [PubMed] [Google Scholar]
- 5.Bedi A, Musahl V, Lane C, Citak M, Warren RF, Pearle AD. Lateral compartment translation predicts the grade of pivot shift: a cadaveric and clinical analysis. Knee Surg Sports Traumatol Arthrosc. 2010;18:1269–1276. doi: 10.1007/s00167-010-1160-y. [DOI] [PubMed] [Google Scholar]
- 6.Brophy RH, Pearle AD. Single-bundle anterior cruciate ligament reconstruction: a comparison of conventional, central, and horizontal single-bundle virtual graft positions. Am J Sports Med. 2009;37:1317–1323. doi: 10.1177/0363546509333007. [DOI] [PubMed] [Google Scholar]
- 7.Brophy RH, Voos JE, Shannon FJ, Granchi CC, Wickiewicz TL, Warren RF, Pearle AD. Changes in the length of virtual anterior cruciate ligament fibers during stability testing: a comparison of conventional single-bundle reconstruction and native anterior cruciate ligament. Am J Sports Med. 2008;36:2196–2203. doi: 10.1177/0363546508320764. [DOI] [PubMed] [Google Scholar]
- 8.Chun YM, Kim SJ, Kim HS. Evaluation of the mechanical properties of posterolateral structures and supporting posterolateral instability of the knee. J Orthop Res. 2008;26:1371–1376. doi: 10.1002/jor.20596. [DOI] [PubMed] [Google Scholar]
- 9.Colombet P, Robinson J, Christel P, Franceschi JP, Djian P. Using navigation to measure rotation kinematics during ACL reconstruction. Clin Orthop Relat Res. 2007;454:59–65. doi: 10.1097/BLO.0b013e31802baf56. [DOI] [PubMed] [Google Scholar]
- 10.Escamilla RF, Zheng N, Imamura R, Macleod TD, Edwards WB, Hreljac A, Fleisig GS, Wilk KE, Moorman CT, 3rd, Andrews JR. Cruciate ligament force during the wall squat and the one-leg squat. Med Sci Sports Exerc. 2009;41:408–417. doi: 10.1249/MSS.0b013e3181882c6d. [DOI] [PubMed] [Google Scholar]
- 11.Escamilla RF, Zheng N, Macleod TD, Imamura R, Edwards WB, Hreljac A, Fleisig GS, Wilk KE, Moorman CT, 3rd, Paulos L, Andrews JR. Cruciate ligament forces between short-step and long-step forward lunge. Med Sci Sports Exerc. 2010;42:1932–1942. doi: 10.1249/MSS.0b013e3181d966d4. [DOI] [PubMed] [Google Scholar]
- 12.Gill TJ, Velde SK, Wing DW, Oh LS, Hosseini A, Li G. Tibiofemoral and patellofemoral kinematics following reconstruction of an isolated posterior cruciate ligament injury: in vivo analysis during lunge. Am J Sports Med. 2009;37:2377–2385. doi: 10.1177/0363546509341829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gollehon DL, Torzilli PA, Warren RF. The role of the posterolateral and cruciate ligaments in the stability of the human knee: a biomechanical study. J Bone Joint Surg Am. 1987;69:233–242. [PubMed] [Google Scholar]
- 14.Hufner T, Kendoff D, Citak M, Geerling J, Krettek C. Precision in orthopaedic computer navigation] [in German. Orthopade. 2006;35:1043–1055. doi: 10.1007/s00132-006-0995-x. [DOI] [PubMed] [Google Scholar]
- 15.Hughston JC, Andrews JR, Cross MJ, Moschi A. Classification of knee ligament instabilities. Part II. The lateral compartment. J Bone Joint Surg Am. 1976;58:173–179. [PubMed] [Google Scholar]
- 16.Jakob RP, Hassler H, Staeubli HU. Observations on rotatory instability of the lateral compartment of the knee: experimental studies on the functional anatomy and the pathomechanism of the true and the reversed pivot shift sign. Acta Orthop Scand Suppl. 1981;191:1–32. doi: 10.3109/ort.1981.52.suppl-191.01. [DOI] [PubMed] [Google Scholar]
- 17.Jung TM, Lubowicki A, Wienand A, Wagner M, Weiler A. Knee stability after posterior cruciate ligament reconstruction in female versus male patients: a prospective matched-group analysis. Arthroscopy. 2011;27:399–403. doi: 10.1016/j.arthro.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Jung YB, Jung HJ, Kim SJ, Park SJ, Song KS, Lee YS, Lee SH. Posterolateral corner reconstruction for posterolateral rotatory instability combined with posterior cruciate ligament injuries: comparison between fibular tunnel and tibial tunnel techniques. Knee Surg Sports Traumatol Arthrosc. 2008;16:239–248. doi: 10.1007/s00167-007-0481-y. [DOI] [PubMed] [Google Scholar]
- 19.Khadem R, Yeh CC, Sadeghi-Tehrani M, Bax MR, Johnson JA, Welch JN, Wilkinson EP, Shahidi R. Comparative tracking error analysis of five different optical tracking systems. Comput Aided Surg. 2000;5:98–107. doi: 10.3109/10929080009148876. [DOI] [PubMed] [Google Scholar]
- 20.Kim SJ, Jung M, Moon HK, Kim SG, Chun YM. Anterolateral transtibial posterior cruciate ligament reconstruction combined with anatomical reconstruction of posterolateral corner insufficiency: comparison of single-bundle versus double-bundle posterior cruciate ligament reconstruction over a 2- to 6-year follow-up. Am J Sports Med. 2011;39:481–489. doi: 10.1177/0363546510385398. [DOI] [PubMed] [Google Scholar]
- 21.Kocher MS, Steadman JR, Briggs K, Zurakowski D, Sterett WI, Hawkins RJ. Determinants of patient satisfaction with outcome after anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2002;84:1560–1572. doi: 10.2106/00004623-200209000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Lane CG, Warren RF, Stanford FC, Kendoff D, Pearle AD. In vivo analysis of the pivot shift phenomenon during computer navigated ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2008;16:487–492. doi: 10.1007/s00167-008-0504-3. [DOI] [PubMed] [Google Scholar]
- 23.LaPrade RF, Terry GC. Injuries to the posterolateral aspect of the knee: association of anatomic injury patterns with clinical instability. Am J Sports Med. 1997;25:433–438. doi: 10.1177/036354659702500403. [DOI] [PubMed] [Google Scholar]
- 24.Li G, Papannagari R, Li M, Bingham J, Nha KW, Allred D, Gill T. Effect of posterior cruciate ligament deficiency on in vivo translation and rotation of the knee during weightbearing flexion. Am J Sports Med. 2008;36:474–479. doi: 10.1177/0363546507310075. [DOI] [PubMed] [Google Scholar]
- 25.Lopomo N, Zaffagnini S, Bignozzi S, Visani A, Marcacci M. Pivot-shift test: analysis and quantification of knee laxity parameters using a navigation system. J Orthop Res. 2010;28:164–169. doi: 10.1002/jor.20966. [DOI] [PubMed] [Google Scholar]
- 26.Markolf KL, Feeley BT, Jackson SR, McAllister DR. Biomechanical studies of double-bundle posterior cruciate ligament reconstructions. J Bone Joint Surg Am. 2006;88:1788–1794. doi: 10.2106/JBJS.E.00427. [DOI] [PubMed] [Google Scholar]
- 27.Markolf KL, Graves BR, Sigward SM, Jackson SR, McAllister DR. Effects of posterolateral reconstructions on external tibial rotation and forces in a posterior cruciate ligament graft. J Bone Joint Surg Am. 2007;89:2351–2358. doi: 10.2106/JBJS.F.01086. [DOI] [PubMed] [Google Scholar]
- 28.Markolf KL, Jackson SR, McAllister DR. A comparison of 11 o’clock versus oblique femoral tunnels in the anterior cruciate ligament-reconstructed knee: knee kinematics during a simulated pivot test. Am J Sports Med. 2010;38:912–917. doi: 10.1177/0363546509358321. [DOI] [PubMed] [Google Scholar]
- 29.Markolf KL, Jackson SR, McAllister DR. Relationship between the pivot shift and Lachman tests: a cadaver study. J Bone Joint Surg Am. 2010;92:2067–2075. doi: 10.2106/JBJS.I.00862. [DOI] [PubMed] [Google Scholar]
- 30.Mauro CS, Sekiya JK, Stabile KJ, Haemmerle MJ, Harner CD. Double-bundle PCL and posterolateral corner reconstruction components are codominant. Clin Orthop Relat Res. 2008;466:2247–2254. doi: 10.1007/s11999-008-0319-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Musahl V, Voos J, O’Loughlin PF, Stueber V, Kendoff D, Pearle AD. Mechanized pivot shift test achieves greater accuracy than manual pivot shift test. Knee Surg Sports Traumatol Arthrosc. 2010;18:1208–1213. doi: 10.1007/s00167-009-1004-9. [DOI] [PubMed] [Google Scholar]
- 32.Nielsen S, Helmig P. The static stabilizing function of the popliteal tendon in the knee: an experimental study. Arch Orthop Trauma Surg. 1986;104:357–362. doi: 10.1007/BF00454430. [DOI] [PubMed] [Google Scholar]
- 33.Pearle AD, Kendoff D, Musahl V, Warren RF. The pivot-shift phenomenon during computer-assisted anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2009;91(Suppl 1):115–118. doi: 10.2106/JBJS.H.01553. [DOI] [PubMed] [Google Scholar]
- 34.Pearle AD, Solomon DJ, Wanich T, Moreau-Gaudry A, Granchi CC, Wickiewicz TL, Warren RF. Reliability of navigated knee stability examination: a cadaveric evaluation. Am J Sports Med. 2007;35:1315–1320. doi: 10.1177/0363546507300821. [DOI] [PubMed] [Google Scholar]
- 35.Robinson J, Stanford FC, Kendoff D, Stüber V, Pearle AD. Replication of the range of native anterior cruciate ligament fiber length change behavior achieved by different grafts: measurement using computer-assisted navigation. Am J Sports Med. 2009;37:1406–1411. doi: 10.1177/0363546509331941. [DOI] [PubMed] [Google Scholar]
- 36.Sekiya JK, West RV, Ong BC, Irrgang JJ, Fu FH, Harner CD. Clinical outcomes after isolated arthroscopic single-bundle posterior cruciate ligament reconstruction. Arthroscopy. 2005;21:1042–1050. doi: 10.1016/j.arthro.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 37.Stindel E, Briard JL, Merloz P, Plaweski S, Dubrana F, Lefevre C, Troccaz J. Bone morphing: 3D morphological data for total knee arthroplasty. Comput Aided Surg. 2002;7:156–168. doi: 10.3109/10929080209146026. [DOI] [PubMed] [Google Scholar]
- 38.Damme G, Defoort K, Ducoulombier Y, Glabbeek F, Bellemans J, Victor J. What should the surgeon aim for when performing computer-assisted total knee arthroplasty? J Bone Joint Surg Am. 2005;87(Suppl 2):52–58. doi: 10.2106/JBJS.E.00447. [DOI] [PubMed] [Google Scholar]
- 39.Yoon KH, Lee JH, Bae DK, Song SJ, Chung KY, Park YW. Comparison of Clinical Results of Anatomic Posterolateral Corner Reconstruction for Posterolateral Rotatory Instability of the Knee With or Without Popliteal Tendon Reconstruction. Am J Sports Med. 2011 July 26 [Epub ahead of print]. [DOI] [PubMed]