Abstract
Background
Coronal alignment may impact clinical outcome and survivorship in TKA. Patient-specific instrumentation has been developed to restore mechanical or kinematic axis and potentially reduce component malpositioning. Although it is clear these instruments add cost, it is unclear whether they improve alignment.
Questions/purposes
We determined whether the mean coronal alignment after TKA performed with conventional versus patient-specific instrumentation better restored the mechanical and kinematic axes and whether there were more outliers with one of the two methods.
Methods
We retrospectively evaluated 150 primary TKAs performed for osteoarthritis: Group 1 (n = 50) conventional instrumentation; Group 2 (n = 50) patient-specific instrumentation restoring the mechanical axis; Group 3 (n = 50) patient-specific instrumentation restoring the kinematic axis, and measured femorotibial angle, hip-knee-ankle angle, and the zone of the mechanical axis from scout CT images taken 0 to 6 weeks postoperatively.
Results
The mean femorotibial angle differed between the groups: Group 1 had the greatest varus mean alignment and most varus outliers. The mean hip-knee angle was similar between Groups 1 and 2, with Group 3 having greater valgus mean alignment and the most valgus outliers. For the zone of the mechanical axis, Groups 1 and 2 had similar percentages of outliers (40% versus 32%), whereas Group 3 had a greater number of outliers (64%) that were valgus.
Conclusions
TKAs with patient-specific instrumentation restoring the mechanical axis had a similar number of outliers as conventional instrumentation with both groups having more varus outliers than TKAs with patient-specific instrumentation restoring kinematic axis, which had more valgus outliers. Therefore, additional studies are needed to determine whether patient-specific instrumentation improves clinical function or patient satisfaction and whether their routine use can be justified in primary TKA.
Level of Evidence
Level III, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Variability in limb and knee alignment is an issue in TKA. Short term, limb and knee malalignment might be related to persistent pain, stiffness, instability, and dissatisfaction [8, 28]. Long term, malalignment might increase the risk of loosening, instability, wear, and osteolysis [1–3, 6, 9, 18, 20, 24, 30–32]. After a decade of clinical use, computer-assisted navigation has resulted in improved alignment but has not eliminated outliers and has not improved the clinical outcome [4, 7, 26, 35]. One recent alternative has been the development of patient-specific instrumentation using MRI or CT imaging to generate patient-specific guides that can be used intraoperatively to control all six degrees of freedom of component alignment, which also controls limb and knee alignment.
The goals of the patient-specific instrumentation technology are to customize disposable blocks unique to each patient’s anatomy in an attempt to increase accuracy of the bone preparation and eliminate outliers of component malpositioning. There are several commercially available patient-specific guides on the market using one of two basic alignment goals based on either (1) restoring the mechanical alignment [1, 18, 23, 24, 32, 34], or (2) restoring the kinematic alignment [11–13, 15, 16]. In the mechanical axis model, the goals of component placement are perpendicular to the mechanical axis of the tibia and parallel to the epicondylar axis of the femur (distal femoral resection in 5° to 8° valgus) [1, 18, 23, 24, 32, 34]. The historical precedent for coronal alignment after TKA originally was supported by John Insall [17], who believed restoration of alignment of the mechanical axis of the lower extremity should be through the center of the knee. In the kinematic model, the surgeon aligns components with the flexion-extension axis of the femur, which passes through the center of the medial and lateral condyles [16] and deviates from the epicondylar axis by an average of 5° and as much as 11° [11–13, 15]. Kinematic alignment was developed with the goal of improving patients’ clinical outcomes by restoring the prearthritic alignment of the limb and knee and not releasing the collateral or retinacular ligaments [5].
Several studies have compared limb and knee alignment of mechanically and kinematically aligned patient-specific instrumentation with conventional instrumentation [22, 33]. These studies are limited to small sample size and limited radiographic analysis and neither compared coronal alignment with patient-specific instrumentation based on the mechanical and kinematic axes.
We therefore determined whether the mean coronal alignment after TKA performed with conventional versus patient-specific instrumentation better restored the mechanical and kinematic axes and whether there were more outliers with one of the two methods.
Patients and Methods
We retrospectively reviewed 150 patients with osteoarthritis who had a primary TKA performed using the same cruciate-retaining cemented total knee system (VanguardTM; Biomet Inc, Warsaw, IN, USA) between January 2009 and September 2010. These 150 patients were selected from those who had a total of 285 primary TKAs performed during the study period meeting the inclusion and exclusion criteria and who had a full series of adequate radiographs. We included only patients with primary osteoarthritis with or without prior open or arthroscopic meniscectomy. We excluded patients with previous fractures of the femoral condyles, tibia plateau, or femoral/tibial shaft and patients with previous osteotomy around the knee. Patients with a pacemaker (owing to their inability to have MRI) and patients with metal around the knee (owing to potential metallic artifact) also were excluded. We established three cohorts of patients for comparison by choosing an unselected group of consecutive patients who had primary TKA and a scout CT scan on the day of discharge or within 6 weeks of surgery. Scans met criteria for being of sufficient quality for an accurate reading (had to include entire femoral head, entire ankle, and the femoral component flange had to be centered on the ovals of the posterior condyle).
In Group 1 (n = 50), we used conventional instrumentation and the distal femur was prepared with an intramedullary rod using a 5°-valgus resection cut. The proximal tibia was prepared with an extramedullary cutting jig perpendicular to the mechanical axis of the tibia. In Group 2 (n = 50) we used the patient-specific SignatureTM system (Biomet Inc) based on restoring the mechanical axis. In Group 3 (n = 50) we used the patient-specific OtisMedTM system (OtisMed Corp, Alameda, CA, USA) based on shape matching to restore the kinematic axis.
In Group 2, each patient had preoperative MRI using the SignatureTM system manufacturing protocol, which uses 1-mm high-resolution slices at the knee and selected 5-mm spot images at the hip and ankle to allow the engineers to derive the mechanical axis and correct for rotation of the extremity. In all of these cases, the surgeon’s predetermined default settings were used to construct the disposable patient-specific blocks. The default settings for the femoral preparation incorporated a 9-mm posterior resection measured from the cartilage, a 9-mm medial distal resection measured from the bone, and the femoral rotation was set at 0° parallel to the transepicondylar axis. The default settings for the tibial preparation were perpendicular to the tibial mechanical axis (0° varus/valgus), 12 mm below the lateral plateau high point, and 2° posterior slope.
In Group 3 each patient had preoperative MRI using the OtisMedTM system manufacturing protocol, which used 2-mm high resolution slices localized to the knee only and included a 16-cm field of view centered on the joint line, which is similar to using a short knee radiograph for preoperative surgical templating. The alignment of the coronal and axial plane was perpendicular to the corticocancellous junction of the distal femur and posterior femur, respectively. Using proprietary software, the arthritic knee was reconstructed by filling articular defects, removing osteophytes, and approximating the joint surface to its prearthritic natural alignment, and not the mechanical alignment. The proprietary software then shape matched the femoral and tibial components to the reconstructed natural knee model to align the flexion-extension rotation axis and then the corresponding patient-specific disposable cutting guides are manufactured to fit the arthritic knee.
One surgeon (RLB) performed all of the TKAs for Groups 1 and 2, whereas a different surgeon (SMH) performed all of the TKAs in Group 3. Both surgeons had experience with patient-specific instrumentation before the start of this study and we did not include any of their early cases during the learning curve for this new technology. In accordance with standard protocol for both surgeons, all patients had a postoperative coronal CT scanogram with a field of view from the hip to the ankle (Fig. 1). To minimize projection errors and standardize radiographic measurements, the leg was internally or externally rotated until the two augment holes on the posterior condyles of the femoral component were partially visible on either side of the anterior flange of the femoral component to control for rotation at the time of the scout CT scan (Fig. 2).
Fig. 1.
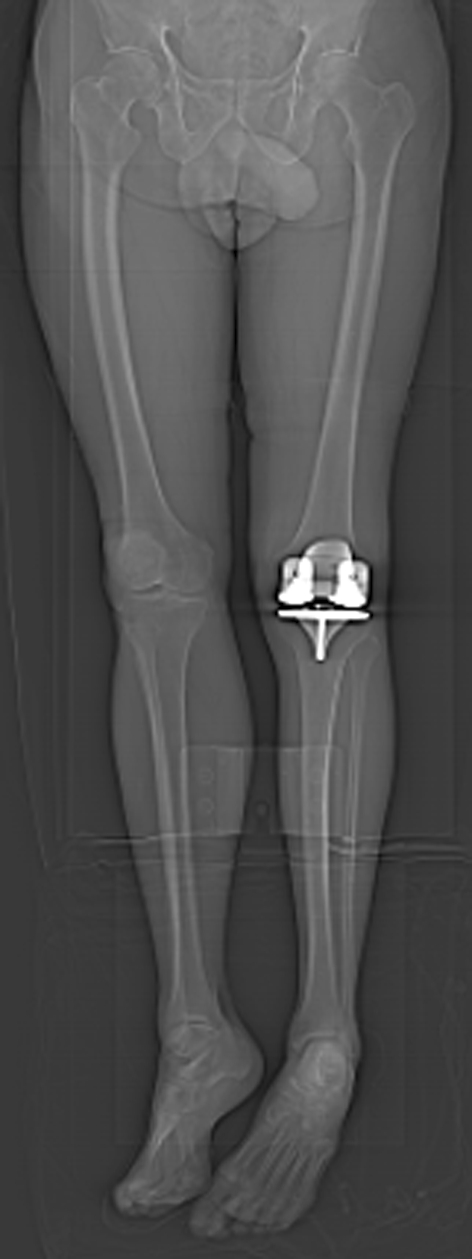
A CT scout image with a field of view from the hip to the ankle was used to make measurements for this study and was adjusted to correct for rotation.
Fig. 2.
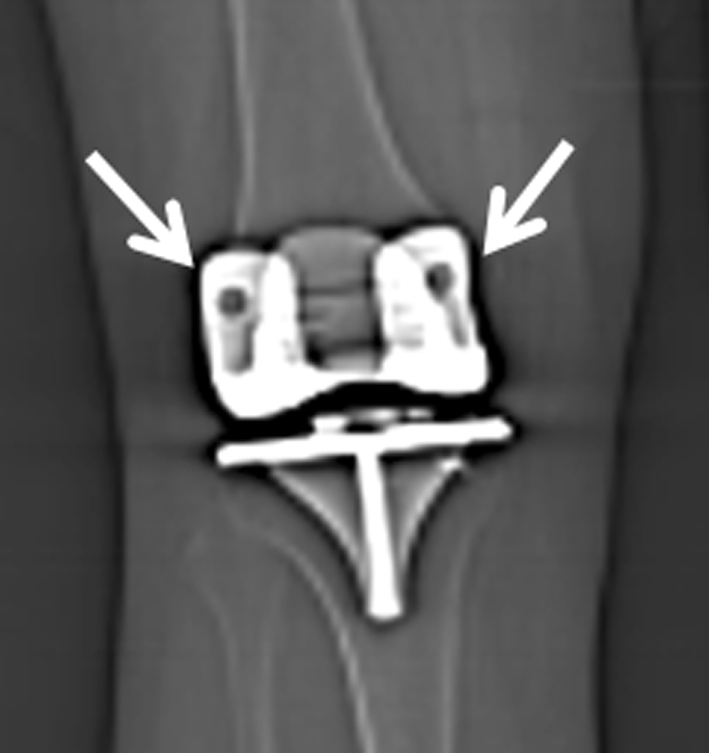
A CT scout image shows the two augment holes (arrows) on the posterior condyles of the femoral component, which are visible on either side of the anterior flange of the femoral component. These two augment holes are used to help the radiology technicians standardize the lower extremity rotational alignment to ensure reliability of coronal plane measurements.
Two reviewers (JZ and BMW) blinded to the surgical method performed the following three radiographic measurements on the AP projection of each CT scanogram [29]: (1) femorotibial angle (FTA), which was the angle formed by a line that bisected the distal fourth of the femur and a line that bisected the proximal fourth of the tibia (Fig. 3); (2) hip-knee-ankle angle (HKA), which was the angle formed by the mechanical axis of the femur (line between the center of the femoral head and the center of the knee) and the mechanical axis of the tibia (line between the center of the ankle and the center of the knee) (Fig. 4); and (3) zone of mechanical axis (ZMA), which was the zone of the tibial base plate (divided into five equal regions) where the mechanical axis of the limb (straight line from the center of the femoral head to the center of the ankle) intersected the tibial base plate (Fig. 5). Valgus angular values were ascribed a negative value (−), whereas varus angular values were ascribed a positive value (+). Measurements denoting lateral displacement from the center point of the tibial plateau were ascribed a positive value (+), whereas measurements indicating a medial distance from the center of the tibial plateau were considered negative (−). In this study, the accepted values for normal or in-range alignment were: (1) 2° to 8° valgus for FTA [1, 14, 25, 32, 34]; (2) 0° ± 3° varus/valgus for HKA [2, 3, 18, 19, 23]; and (3) the central zone for ZMA [25, 27, 30, 36]. We determined interrater reliability by calculating the intraclass correlation coefficient (ICC) using a two-way mixed effects model. The highest level of agreement among raters was seen for FTA (ICC = 0.87; 95% CI, 0.81–0.91; p < 0.001). HKA assessment had a good level of agreement among raters (ICC = 0.86; 95% CI, 0.81–0.89; p < 0.001), as did ZMA (ICC = 0.83; 95% CI, 0.79–0.87; p < 0.001).
Fig. 3.
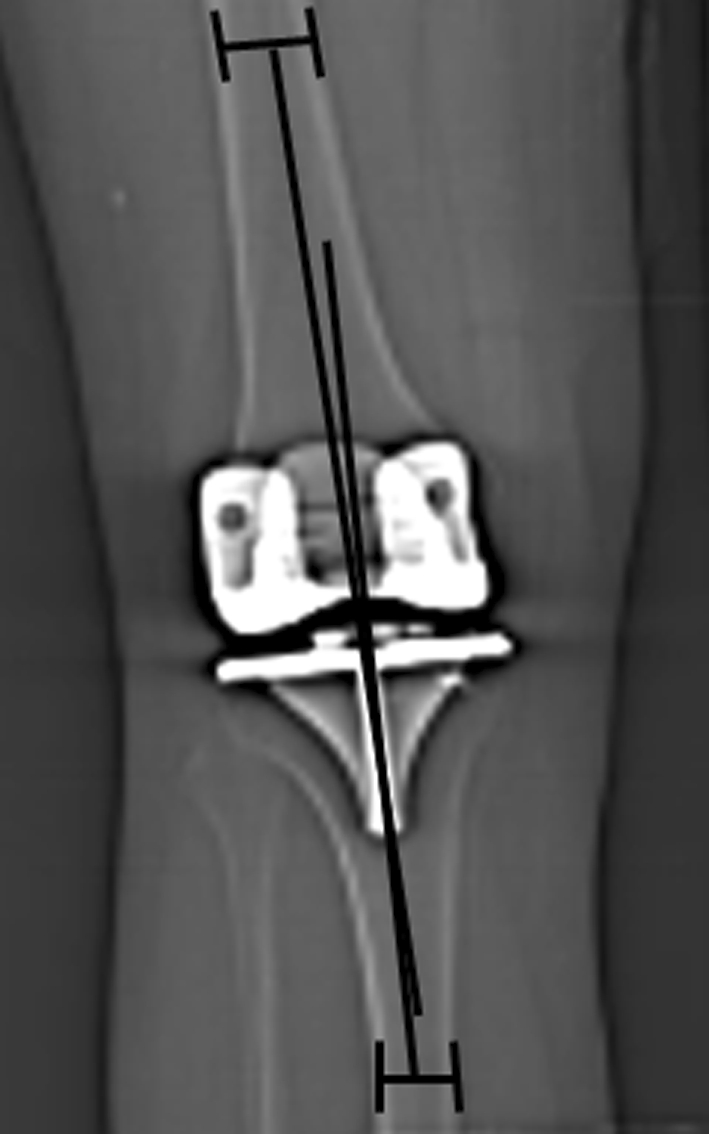
The femorotibial angle (FTA) angle measurement between the anatomic axis of the femur and the anatomic axis of the tibia was used to measure coronal alignment on a short image.
Fig. 4.
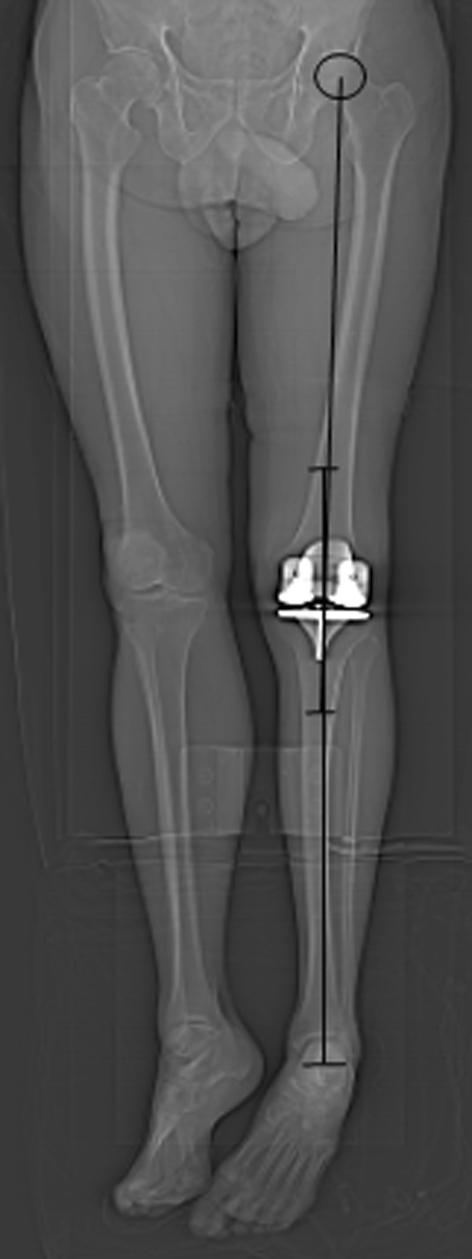
The hip-knee-ankle (HKA) angle measurement between the mechanical axis of the femur (line between the center of the femoral head and the center of knee) and mechanical axis of the tibia (line between the center of the ankle and the center of the knee) was used to measure coronal alignment on a full-length image.
Fig. 5.
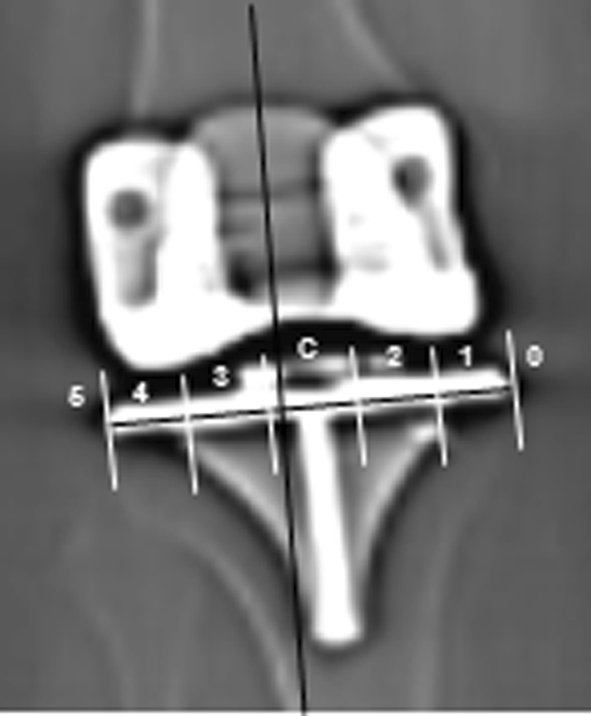
The zone of the mechanical axis (ZMA) was determined by dividing the width of the tibial base plate into five equal regions and then drawing a line through the mechanical axis of the lower extremity to determine through which zone it passes. In this image the mechanical axis of the lower extremity (black line) passes through the central zone labeled C.
We used a single-factor ANOVA and Tukey’s post hoc test to determine whether the FTA and HKA differed between the three surgical techniques. Fisher’s exact test was used to determine whether the percentage of in-range, varus, and valgus outliers of the FTA, HKA, and ZMA differed between the three surgical techniques. Statistical analyses were performed using SPSS for Windows, version 20 (SPSS Inc, Chicago, IL, USA).
Results
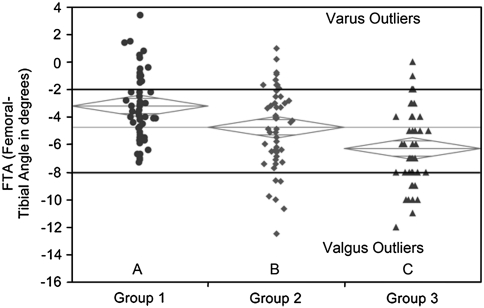
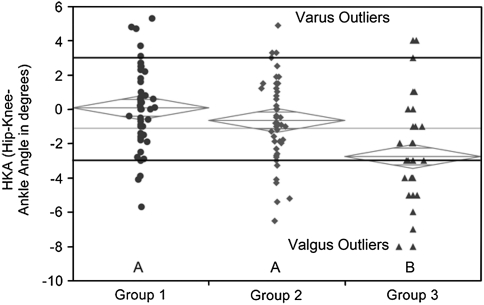
The mean coronal alignment measurements for all three groups assessed by the FTA and HKA angles fell within the accepted ranges for these measurements. The mean FTA in Group 1 (−3.21°; 95% CI, −2.42° to −3.99°) was more varus than Group 2 (−4.75°; 95% CI, −3.97° to −5.54°) (p = 0.03), and Groups 1 and 2 were less valgus than Group 3 (−6.30°; 95% CI, −5.51° to −7.09°) (p = 0.043) (Fig. 6). The mean HKA in Group 1 (0.08°; 95% CI, −0.61° to 0.78°) was similar to that of Group 2 (−0.65°; 95% CI, −1.35° to 0.04°) (p = 0.47), however Groups 1 and 2 were in less valgus than Group 3 (−2.76°; 95% CI, −3.46° to −2.06°) (p = 0.0002 and p = 0.0061, respectively) (Fig. 7). The ZMA intersected with the central zone of the tibial base plate in 60% of Group 1, 68% of Group 2, and 36% of Group 3 (Table 1). The ZMA was aligned through the second zone (mild varus) for 18% of Group 1, 12% of Group 2, and 6% of Group 3. The ZMA was through the third zone (mild valgus) for 22% of Group 1, 20% of Group 2, and 48% of Group 3. None of the patients in Groups 1 and 2 had alignment in Zone 4 (moderate valgus), but 10% of Group 3 had alignment through this zone (Table 2). The mechanical axis was never aligned in moderate or severe varus (Zones 0 or 1) or severe valgus (Zone 5) for any patients in all three groups.
Fig. 6.
The graph shows one-way analysis of the femorotibial angle (FTA) by group, depicting the variation in coronal alignment between the three groups and the number of varus and valgus outliers outside the accepted range of −2° to −8°. The diamonds indicate the 95% confidence interval for each group.
Fig. 7.
The graph shows one-way analysis of the hip-knee-ankle (HKA) angle by group, depicting the variation in coronal alignment between the three groups and the number of varus and valgus outliers outside the accepted range of 3° to −3°. The diamonds indicate the 95% confidence interval for each group.
Table 1.
Radiographic measurements comparing the three cohorts
| Radiographic measurement | Group 1 (n = 50) | Group 2 (n = 50) | Group 3 (n = 50) | |||
|---|---|---|---|---|---|---|
| FTA | FTA 2°–8° valgus | FTA outliers | FTA 2°–8° valgus | FTA outliers | FTA 2°–8° valgus | FTA outliers |
| Number of patients (%) | 36 (72%) | 14 (28%) | 34 (68%) | 16 (32%) | 36 (72%) | 14 (28%) |
| HKA | HKA 0° ± 3° varus/valgus | HKA outliers | HKA 0° ± 3° varus/valgus | HKA outliers | HKA 0° ± 3° varus/valgus | HKA outliers |
| Number of patients (%) | 42 (84%) | 8 (16%) | 41 (82%) | 9 (18%) | 28 (56%) | 22 (44%) |
| ZMA | ZMA through central zone | ZMA outliers | ZMA through central zone | ZMA outliers | ZMA through central zone | ZMA outliers |
| Number of patients (%) | 30 (60%) | 20 (40%) | 34 (68%) | 16 (32%) | 18 (36%) | 32 (64%) |
FTA = femorotibial angle; HKA = Hip-knee-ankle angle; ZMA = zone of mechanical axis.
Table 2.
Radiographic outliers of the three cohorts
| Radiographic measurement | FTA Outliers | HKA Outliers | ZMA Outliers | ||||
|---|---|---|---|---|---|---|---|
| Group 1 (n = 50) | Varus | Valgus | Varus | Valgus | Zone 2 | Zone 3 | Zone 4 |
| Number of patients | 14 | 0 | 5 | 3 | 9 | 11 | 0 |
| Group 2 (n = 50) | Varus | Valgus | Varus | Valgus | Zone 2 | Zone 3 | Zone 4 |
| Number of patients | 10 | 6 | 3 | 6 | 6 | 10 | 0 |
| Group 3 (n = 50) | Varus | Valgus | Varus | Valgus | Zone 2 | Zone 3 | Zone 4 |
| Number of patients | 3 | 11 | 2 | 20 | 3 | 24 | 5 |
FTA = femorotibial angle; HKA = hip-knee-ankle angle; ZMA = zone of mechanical axis.
We found coronal alignment outliers in all three groups and no reduction in the number of outliers using the patient-specific instrumentation when compared with conventional instrumentation. The total numbers of outliers for FTA were similar among the three groups (Table 1), but the prevalence of varus outliers was greatest in Group 1 (14 patients, 28%), and the prevalence of valgus outliers was greatest Group 3 (11 patients, 22%) (Table 2). The total numbers of outliers for HKA were similar between Group 1 (five varus and three valgus) and Group 2 (three varus and six valgus) (p = 1.0). Group 3 had 22 outliers (two varus and 20 valgus) which was more than Group 1 (p = 0.0041) and Group 2 (p = 0.0089) (Table 2). For the ZMA, we found no difference in the numbers of outliers between Groups 1 and 2 (p = 0.638), but there were differences between Groups 1 and 3 (p = 0.0012) and Groups 2 and 3 (p = 0.0008) in favor of Groups 1 and 2 having a higher percentage of patients with mechanical alignment through the central zone of the tibial base plate compared with Group 3. In Group 3 there were 32 outlier patients (64%) and of these, 29 had valgus alignment (Table 2).
Discussion
There is interest in the effect of coronal limb and knee alignment on clinical outcomes and survivorship after TKA. Despite improvements in conventional instrumentation and the use of intraoperative computer navigation technology, variability in component alignment still exists and a percentage of patients continue to experience pain, stiffness, and decreased ROM after TKA [28]. Patient-specific custom guides are relatively new and have several proposed advantages over conventional instrumentation, including improved component accuracy and elimination of outliers. Patient-specific instrumentation can be engineered to restore the knee alignment to either the mechanical axis or the kinematic alignment. Several studies [22, 33] have been published comparing kinematically aligned patient-specific instrumentation with conventional instrumentation, but these studies have been limited by small sample size, limited radiographic analysis, or lack of a comparable control group. There are no published studies comparing patient-specific instrumentation based on the mechanical axis versus conventional instrumentation. The objectives of our study were to compare postoperative coronal alignment after primary TKA performed using either conventional instrumentation or patient-specific instrumentation based on either the mechanical axis or kinematic axis.
Our study has several limitations. First, we had two separate, but experienced, total joint surgeons contributing patients for this study and we could not control for patient selection and referral patterns; therefore, there might have been some selection bias. These two surgeons are experienced and perform a high volume of primary TKAs per year and therefore their low number of outliers in this study might not be applicable to all surgeons performing primary TKAs using conventional instrumentation. Second, the patient-specific cutting guides used for Groups 2 and 3 were manufactured by different companies and used different MRI protocols. Although neither surgeon reported experiencing any difficulty seating the guides on the bone, the two companies might have different degrees of variability inherent in their software analysis programs, rapid prototyping technology, and the intraoperative tolerances of their blocks to lock securely onto the bone, which might have introduced some subtle variances into the final component alignment. It also is not unexpected that there were a greater number of outliers in Group 3 as this system uses only MRI of the knee and not the hip or ankle and attempts to restore the limb and knee to its prearthritic kinematic alignment and not a neutral mechanical axis, which exists in only 2% of the healthy population [13]. Third, this study assessed only two patient-specific instrumentation designs and our findings might not be applicable to all the other patient-specific designs that currently are commercially available. There are inherent advantages and disadvantages to using CT versus MRI when creating these custom guides. MRI is more accurate for assessing cartilage thickness but is more expensive and time consuming than using CT which is faster but exposes the patient to radiation. Fourth, we did not perform a power analysis before starting this study as it was designed as a pilot study to evaluate and provisionally compare these new types of patient-specific instrumentation, but we currently are enrolling patients in a randomized prospective study on a larger scale. Fifth, the results from this study are limited to the coronal plane and do not take into account component positioning on the lateral view. In addition, we could not assess for rotational alignment, which may play a major role in long-term survivorship of total knee implants. Last, we do not report on clinical outcomes such as pain, stiffness, ROM, patient satisfaction, or outcome scoring systems, which may limit the true clinical relevance of the findings in this study.
Despite the fact that patient-specific instrumentation has been commercially available for several years, little has been published on this new technology [16, 21, 22, 33]. Three of these articles focused on the results of patient-specific instrumentation based on the kinematic alignment model [16, 21, 33]. One study [21] reported on four TKAs performed using kinematic-based patient-specific instrumentation and found intraoperatively that the tibial components were between 3° valgus and 7° varus, but long radiographs were not taken, so overall alignment was not evaluated. In another study [33], the authors reported on 21 consecutive patients who had a TKA performed with patient-specific instrumentation based on the kinematic axis. They found the mean coronal alignment in this group was 1.2° varus ± 2.4° (range, 6° valgus to 4° varus) but they had no control group for comparison so they compared their results with results of previous studies reporting on coronal alignment between computer-assisted and conventional TKAs. No articles to date have compared coronal alignment measurements between patient-specific cutting blocks using the mechanical alignment design and the kinematic alignment design. We found patients who had TKAs and were treated with the guides based on the kinematic axis had a similar total number of outliers for the FTA measurement as the conventional and mechanical axes groups, but all of the these patients had valgus outliers, which is not unexpected as these guides are specifically created to restore the knee to its prearthritic alignment and not to neutral mechanical alignment. Fang et al. [14] measured the FTA angle on 6070 TKAs and found slightly better clinical survivorship and a lower revision rate in their patients with valgus outliers (97% survivorship, 1.5% revision rate) than their patients with varus outliers (95% survivorship, 1.8% revision rate). We also found that patients treated with guides based on the kinematic axis had an increased number of valgus outliers in the HKA measurement group compared with the other two groups. In a study of factors associated with polyethylene wear after TKA, Collier et al. [10] performed a regression analysis and found that only three factors were associated with increased wear in the medial compartment: shelf age of the liner, age of the patient, and postoperative varus alignment on the HKA measurement. Furthermore, another study looking at coronal alignment using the HKA angle between 0° ± 3° and assessing survivorship of outliers found that this measurement may be less predictable than previously believed in determining long-term survivorship of modern TKA prostheses.
We designed our study to compare postoperative coronal alignment measurements after primary TKA performed using either conventional instrumentation or patient-specific instrumentation by high-volume experienced knee surgeons. We also aimed to evaluate and compare coronal alignment achieved by the two different types of patient-specific instrument designs (mechanical axis or kinematic axis). Our data suggest there are no differences in coronal alignment between TKAs performed with patient-specific instrumentation based on restoring the mechanical axis over those performed with conventional instrumentation. TKAs performed with patient-specific instrumentation based on restoring the kinematic axis had a substantial number of valgus outliers. Future randomized controlled trials are needed to help critically evaluate clinical function and survivorship between these different types of surgical instrumentation to determine if there are important differences in patients’ clinical and functional outcomes not related to the coronal alignment to support this more expensive technology for use in routine TKAs.
Acknowledgment
We thank Brandon Williams for help with the radiographic images for this study.
Footnotes
One of the authors (RMN) is a paid consultant for Smith & Nephew Orthopaedics, Wright Medical Technology Inc, Salient Surgical, and CardioMEMS. One of the authors (RLB) has received royalties from Smith & Nephew Orthopaedics and is a paid consultant for Stryker. One of the authors (SMH) receives royalties from Biomet and is a paid consultant for Biomet and Stryker.
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at Washington University, St Louis, MO, USA.
An erratum to this article can be found at http://dx.doi.org/10.1007/s11999-012-2243-5.
References
- 1.Bargren JH, Blaha JD, Freeman MA. Alignment in total knee arthroplasty. Correlated biomechanical and clinical observations. Clin Orthop Relat Res. 1983;173:178–183. [PubMed] [Google Scholar]
- 2.Bathis H, Perlick L, Tingart M, Luring C, Zurakowski D, Grifka J. Alignment in total knee arthroplasty: a comparison of computer-assisted surgery with the conventional technique. J Bone Joint Surg Br. 2004;86:682–687. doi: 10.1302/0301-620X.86B5.14927. [DOI] [PubMed] [Google Scholar]
- 3.Berend ME, Ritter MA, Meding JB, Faris PM, Keating EM, Redelman R, Faris GW, Davis KE. Tibial component failure mechanisms in total knee arthroplasty. Clin Orthop Relat Res. 2004;428:26–34. doi: 10.1097/01.blo.0000148578.22729.0e. [DOI] [PubMed] [Google Scholar]
- 4.Blakeney WG, Khan RJ, Wall SJ. Computer-assisted techniques versus conventional guides for component alignment in total knee arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2011;93:1377–1384. doi: 10.1302/0301-620X.93B10.27224. [DOI] [PubMed] [Google Scholar]
- 5.Bolognesi MP, Pearle AD, Oryhon JM, Nunley RM. New technology for knee arthroplasty. In: Glassman AH, Lachiewicz PF, Tanzer M, editors. Orthopaedic Knowledge Update: Hip and Knee Reconstruction 4. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2011. pp. 119–137. [Google Scholar]
- 6.Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468:57–63. doi: 10.1007/s11999-009-1119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brin YS, Nikolaou VS, Joseph L, Zukor DJ, Antoniou J. Imageless computer assisted versus conventional total knee replacement: a Bayesian meta-analysis of 23 comparative studies. Int Orthop. 2011;35:331–339. doi: 10.1007/s00264-010-1008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choong PF, Dowsey MM, Stoney JD. Does accurate anatomical alignment result in better function and quality of life? Comparing conventional and computer-assisted total knee arthroplasty. J Arthroplasty. 2009;24:560–569. doi: 10.1016/j.arth.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 9.Churchill DL, Incavo SJ, Johnson CC, Beynnon BD. The transepicondylar axis approximates the optimal flexion axis of the knee. Clin Orthop Relat Res. 1998;356:111–118. doi: 10.1097/00003086-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Collier MB, Engh CA, Jr, McAuley JP, Engh GA. Factors associated with the loss of thickness of polyethylene tibial bearings after knee arthroplasty. J Bone Joint Surg Am. 2007;89:1306–1314. doi: 10.2106/JBJS.F.00667. [DOI] [PubMed] [Google Scholar]
- 11.Coughlin KM, Incavo SJ, Churchill DL, Beynnon BD. Tibial axis and patellar position relative to the femoral epicondylar axis during squatting. J Arthroplasty. 2003;18:1048–1055. doi: 10.1016/S0883-5403(03)00449-2. [DOI] [PubMed] [Google Scholar]
- 12.Eckhoff D, Hogan C, DiMatteo L, Robinson M, Bach J. Difference between the epicondylar and cylindrical axis of the knee. Clin Orthop Relat Res. 2007;461:238–244. doi: 10.1097/BLO.0b013e318112416b. [DOI] [PubMed] [Google Scholar]
- 13.Eckhoff DG, Bach JM, Spitzer VM, Reinig KD, Bagur MM, Baldini TH, Flannery NM. Three-dimensional mechanics, kinematics, and morphology of the knee viewed in virtual reality. J Bone Joint Surg Am. 2005;87(suppl 2):71–80. doi: 10.2106/JBJS.E.00440. [DOI] [PubMed] [Google Scholar]
- 14.Fang DM, Ritter MA, Davis KE. Coronal alignment in total knee arthroplasty: just how important is it? J Arthroplasty. 2009;24:39–43. doi: 10.1016/j.arth.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 15.Hollister AM, Jatana S, Singh AK, Sullivan WW, Lupichuk AG. The axes of rotation of the knee. Clin Orthop Relat Res. 1993;290:259–268. [PubMed] [Google Scholar]
- 16.Howell SM, Hodapp EE, Kuznik K, Hull ML. In vivo adduction and reverse axial rotation (external) of the tibial component can be minimized. Orthopedics. 2009;32:319. doi: 10.3928/01477447-20090501-04. [DOI] [PubMed] [Google Scholar]
- 17.Insall JN. The total knee replacement. In: Insall JN, editor. Surgery of the Knee. New York, NY: Churchill Livingstone; 1984. pp. 1447–1464. [Google Scholar]
- 18.Jeffery RS, Morris RW, Denham RA. Coronal alignment after total knee replacement. J Bone Joint Surg Br. 1991;73:709–714. doi: 10.1302/0301-620X.73B5.1894655. [DOI] [PubMed] [Google Scholar]
- 19.Kamath AF, Israelite C, Horneff J, Lotke PA. Editorial: What is varus or valgus knee alignment?: a call for a uniform radiographic classification. Clin Orthop Relat Res. 2010;468:1702–1704. doi: 10.1007/s11999-010-1334-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kennedy WR, White RP. Unicompartmental arthroplasty of the knee: postoperative alignment and its influence on overall results. Clin Orthop Relat Res. 1987;221:278–285. [PubMed] [Google Scholar]
- 21.Klatt BA, Goyal N, Austin MS, Hozack WJ. Custom-fit total knee arthroplasty (OtisKnee) results in malalignment. J Arthroplasty. 2008;23:26–29. doi: 10.1016/j.arth.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Lombardi AV, Jr, Berend KR, Adams JB, White D, Chelule KL, Seedhom BB. Patient-specific approach in total knee arthroplasty. Orthopedics. 2008;31:927–930. doi: 10.3928/01477447-20080901-21. [DOI] [PubMed] [Google Scholar]
- 23.Longstaff LM, Sloan K, Stamp N, Scaddan M, Beaver R. Good alignment after total knee arthroplasty leads to faster rehabilitation and better function. J Arthroplasty. 2009;24:570–578. doi: 10.1016/j.arth.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Lotke PA, Ecker ML. Influence of positioning of prosthesis in total knee replacement. J Bone Joint Surg Am. 1977;59:77–79. [PubMed] [Google Scholar]
- 25.Luo CF. Reference axes for reconstruction of the knee. Knee. 2004;11:251–257. doi: 10.1016/j.knee.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Mason JB, Fehring TK, Estok R, Banel D, Fahrbach K. Meta-analysis of alignment outcomes in computer-assisted total knee arthroplasty surgery. J Arthroplasty. 2007;22:1097–1106. doi: 10.1016/j.arth.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Mohanlal P, Jain S. Assessment and validation of CT scanogram to compare per-operative and post-operative mechanical axis after navigated total knee replacement. Int Orthop. 2009;33:437–439. doi: 10.1007/s00264-008-0639-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noble PC, Conditt MA, Cook KF, Mathis KB. The John Insall Award: Patient expectations affect satisfaction with total knee arthroplasty. Clin Orthop Relat Res. 2006;452:35–43. doi: 10.1097/01.blo.0000238825.63648.1e. [DOI] [PubMed] [Google Scholar]
- 29.Paley D. Principles of Deformity Correction. New York, NY: Springer-Verlag; 2002. [Google Scholar]
- 30.Parratte S, Pagnano MW, Trousdale RT, Berry DJ. Effect of postoperative mechanical axis alignment on the fifteen-year survival of modern, cemented total knee replacements. J Bone Joint Surg Am. 2010;92:2143–2149. doi: 10.2106/JBJS.I.01398. [DOI] [PubMed] [Google Scholar]
- 31.Petersen TL, Engh GA. Radiographic assessment of knee alignment after total knee arthroplasty. J Arthroplasty. 1988;3:67–72. doi: 10.1016/S0883-5403(88)80054-8. [DOI] [PubMed] [Google Scholar]
- 32.Ritter MA, Faris PM, Keating EM, Meding JB. Postoperative alignment of total knee replacement: its effect on survival. Clin Orthop Relat Res. 1994;299:153–156. [PubMed] [Google Scholar]
- 33.Spencer BA, Mont MA, McGrath MS, Boyd B, Mitrick MF. Initial experience with custom-fit total knee replacement: intra-operative events and long-leg coronal alignment. Int Orthop. 2009;33:1571–1575. doi: 10.1007/s00264-008-0693-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tew M, Waugh W. Tibiofemoral alignment and the results of knee replacement. J Bone Joint Surg Br. 1985;67:551–556. doi: 10.1302/0301-620X.67B4.4030849. [DOI] [PubMed] [Google Scholar]
- 35.Tingart M, Luring C, Bathis H, Beckmann J, Grifka J, Perlick L. Computer-assisted total knee arthroplasty versus the conventional technique: how precise is navigation in clinical routine? Knee Surg Sports Traumatol Arthrosc. 2008;16:44–50. doi: 10.1007/s00167-007-0399-4. [DOI] [PubMed] [Google Scholar]
- 36.Weng YJ, Hsu RW, Hsu WH. Comparison of computer-assisted navigation and conventional instrumentation for bilateral total knee arthroplasty. J Arthroplasty. 2009;24:668–673. doi: 10.1016/j.arth.2008.03.006. [DOI] [PubMed] [Google Scholar]