Abstract
Background
Radiotherapy and surgery are routinely utilized to treat extremity soft tissue sarcoma. Multiple radiation modalities have been described, each with advantages and disadvantages, without one modality demonstrating clear superiority over the others.
Questions/purposes
We determined the overall initial complication rate in patients receiving surgery and radiotherapy, which specific complications were found when comparing different modalities, and whether combination therapy increased the overall rate of complications compared with surgery and single-modality radiotherapy.
Patients and Methods
We retrospectively reviewed the records of 190 patients who received external-beam radiotherapy (141 patients), high-dose-rate brachytherapy (37 patients), or both (12 patients). We evaluated 100 men and 90 women (mean age, 57 years; range, 18–94 years) for tumor size and subtype, comorbidities, stage, grade, margin of resection, type of adjuvant treatment, and complications. Minimum followup was 3 months (mean, 40 months; range, 3–155 months).
Results
The most frequent early complications in the high-dose-rate brachytherapy cohort were infection, cellulitis, and seroma and/or hematoma. In the external-beam radiotherapy cohort, chronic edema, fibrosis, and chronic radiation dermatitis were more frequently encountered. The total number of early complications and overall incidence of major complications requiring further surgery were similar among the three cohorts, but a larger number of patients in the high-dose-rate brachytherapy group required subsequent surgery for infection compared with the external-beam radiotherapy group.
Conclusions
High-dose-rate brachytherapy decreases radiation exposure and allows shorter duration of treatment compared with traditional external-beam radiotherapy but has a higher perioperative wound complication rate.
Level of Evidence
Level III, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Standard treatment of extremity soft tissue sarcoma (STS) consists of limb-sparing surgery and adjuvant radiation [2, 3, 5, 14, 15, 17, 21, 30, 42], although the mode and timing of radiotherapy continue to be debated [20–22, 27, 41]. Preoperative, intraoperative, and postoperative radiotherapy, administered by multiple delivery methods, all have described advantages and disadvantages [13, 38] without one adjunct clearly demonstrating superiority. Some patients are treated with mixed modalities to facilitate dose orientation or a postoperative dose boost. External-beam radiotherapy (EBRT) and brachytherapy are two established modalities used to deliver therapeutic radiation [4, 34, 35, 39], but current recommendations on the utilization of high-dose-rate brachytherapy (HDR-BT) alone or in conjunction with EBRT remain unclear due to a paucity of published data on the use of HDR-BT in the management of extremity STS [6, 8, 29].
Complications resulting from perioperative radiotherapy for STS are common, ranging from 6% to 48% [7, 11, 20, 32, 36], but prior studies reporting complications after HDR-BT have been generally limited by underreporting [8, 22] and small sample sizes [1, 9, 18, 23, 29], questioning the accuracy of the true incidence of perioperative complications after surgery and brachytherapy. Current brachytherapy guidelines are based on expert panel consensus of limited existing literature [29] without direct comparison between HDR-BT and EBRT.
Therefore, we asked whether (1) minor or major complication rates would be similar in patients treated with EBRT and HDR-BT; (2) the frequency of specific complications (eg, wound dehiscence, fibrosis) would differ; and (3) patients treated with combined HDR-BT and EBRT would experience complications more frequently than those treated with either modality alone.
Patients and Methods
After institutional review board approval, we retrospectively reviewed the medical records of all 320 adult patients with appendicular STS treated with surgery and radiotherapy between April 1990 and December 2009. Indications for surgery and radiotherapy and study inclusion were (1) a histologic diagnosis of intermediate- or high-grade STS, (2) appendicular location, and (3) ability to perform limb salvage surgery. Contraindications for surgery and radiotherapy included patients who were unable to tolerate surgery due to medical comorbidities or advanced disease state. Patients were eligible for inclusion in the study if complete histologic, surgical, clinical, and radiotherapy records were available for review. We excluded 220 patients with STS in axial locations, previous radiation to the affected extremity, incomplete records, patients receiving primary amputation, and pediatric patients. These exclusions left 100 (53%) men and 90 (47%) women with a mean age of 57 years (range, 18–94 years) at the time of presentation (Table 1). The lower extremity was the location of the primary tumor in 147 (78%) patients, and the upper extremity was the primary site in 43 (22%) patients. The most common histologic subtypes were high-grade pleomorphic undifferentiated sarcoma (36%) and liposarcoma (26%), followed by leiomyosarcoma (11%), synovial sarcoma (6%), malignant peripheral nerve sheath tumor (4%), and various other STSs [40]. Four (2%) patients had low-grade tumors, 21 (11%) patients had intermediate-grade lesions, and 165 (87%) patients had high-grade sarcomas [40]. The minimum followup was 3 months (mean, 10.6 months; range, 3–219 months). No patients were lost to followup during the time period of interest in this study. No patients were recalled specifically for this study; all data were obtained from medical records and imaging.
Table 1.
Summary data for all patients (n = 190) receiving HDR-BT, EBRT, or both
| Variable | HDR-BT | EBRT | Both | p Value* |
|---|---|---|---|---|
| Number of patients | 37 | 141 | 12 | |
| Mean age (years) | 57 | 56 | 69 | 0.64 |
| Sex (number of patients) | 0.71 | |||
| Male | 21 | 74 | 5 | |
| Female | 16 | 67 | 7 | |
| Mean duration of symptoms (months) | 6.87 | 9.9 | 27.2 | 0.09 |
| Mean tumor volume (cm3) | 644.6 | 640.5 | 1194.1 | 0.9 |
| Grade (number of patients) | 0.006 | |||
| Low | 2 | 1 | 1 | |
| Intermediate | 7 | 9 | 3 | |
| High | 28 | 131 | 8 | |
| Depth (number of patients) | 0.12 | |||
| Superficial to fascia | 9 | 18 | 1 | |
| Deep to fascia | 28 | 123 | 11 | |
| Stage (number of patients) | 0.80† | |||
| IIA | 2 | 9 | 0 | |
| IIB | 8 | 18 | 0 | |
| IIC | 0 | 4 | 1 | |
| III | 25 | 101 | 10 | |
| IV | 2 | 9 | 1 | |
| Primary versus recurrent (number of patients) | 0.6 | |||
| Primary | 37 | 138 | 11 | |
| Recurrent | 0 | 3 | 1 | |
| Mean total radiotherapy dose (Gy) | 33.4 | 62.3 | 54.8 | < 0.0001 |
| Mean duration of treatment (days) | 5.3 | 54.5 | 41.7 | < 0.0001 |
| Mean duration of followup (months) | 10.6 | 50.2 | 12.7 | < 0.0001 |
| Local recurrence (number of patients) | 3 (8%) | 15 (11%) | 2 (17%) |
* For HDR-BT versus EBRT; †results for pooled Stage II patients; HDR-BT = high-dose-rate brachytherapy; EBRT = external-beam radiotherapy.
There were no differences between EBRT and HDR-BT study cohorts with regard to patient age, sex, tumor size, duration of symptoms, or American Joint Committee on Cancer (AJCC) stage [16, 40] (Table 1). Preoperatively, the AJCC staging [16] on presentation was Stage IIA for 11 (6%) patients, Stage IIB for 26 (14%) patients, Stage IIC for five (3%) patients, Stage III for 136 (72%) patients, and Stage IV for 12 (6%) patients. Mean tumor volume was 670 cm3, defined by gross pathologic description or by preoperative imaging studies. The central portion of the tumor was deep to fascia in 162 (86%) patients and superficial to fascia in 28 (14%) patients. There were more high-grade tumors and fewer low-grade tumors in the EBRT cohort (p = 0.006). This would ostensibly lead to decreased survival and possibly an increased risk of local recurrence in this group, while decreasing the efficacy of radiotherapy; however, no impact on radiotherapy-related complications and sequelae would be expected.
All definitive surgeries were performed by three orthopaedic oncologists (HTT, SCA, JDP) at the study center, and one radiation oncologist (MEK) implanted afterloading HDR-BT catheters in the tumor bed in the brachytherapy group based on previously established brachytherapy protocols [6, 12, 29] (Fig. 1). One hundred forty-one (74%) patients had one planned definitive surgery, whereas 49 (26%) patients required reexcision after unplanned excision, defined as excisional biopsy without attempted wide resection or excision without preoperative imaging. All patients were treated with either wide local excision or focal marginal excision with preservation of vital neurovascular structures to maintain a functional limb. Limb salvage was performed in all but one patient, and primary closure was performed in 171 (91%) patients, with the remaining 18 (9%) requiring a muscle flap and/or split-thickness skin graft. R0 or R1 margins were obtained in all cases after definitive surgery. Microscopically positive margins were observed in 16 (8%) patients, six of which were referred after unplanned excision. Gross or microscopic residual tumor was noted in 35 of the 49 (71%) patients who underwent prior unplanned excision, and all of these patients received postoperative radiotherapy.
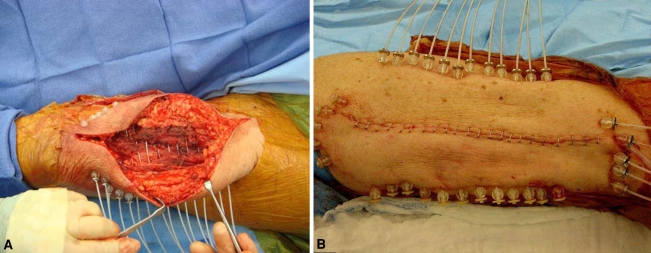
Fig. 1A–B.
(A) An intraoperative photograph demonstrates the placement of brachytherapy catheters in the tumor bed after resection of a thigh sarcoma. (B) An intraoperative photograph exhibits noncoplanar brachytherapy catheter placement, secured in place with stainless steel buttons and plastic beads.
One hundred forty-one (74%) patients received EBRT alone (11 preoperative EBRT and 130 postoperative EBRT), 37 (19%) patients received HDR-BT alone, and 12 (6%) received both EBRT and HDR-BT. The mean total dose in the EBRT group was 60.9 Gy administered over an average of 32 fractions with a median dose of 1.8 Gy per fraction over a mean of 54.5 elapsed days of treatment. EBRT was given with three-dimensional conformal radiotherapy and more recently intensity-modulated radiotherapy. The planning target volume was defined using standard guidelines, most commonly a 5-cm margin craniocaudally and a radial margin defined by the compartment and surgical bed. In the HDR-BT monotherapy cohort, the mean total dose was 33.6 Gy administered over an average of 10 fractions with a median dose of 3.4 Gy per fraction over a mean of 5.2 elapsed days of treatment. Catheters were loaded a mean of 4.5 days after placement. HDR-BT planning volume was determined by catheter placement, the majority of which were complex multiple noncoplanar implants. Simple volumes were treated using single-plane implants. A 2-cm proximal and distal margin was employed. Dose calculation was performed by placing source positions 5 mm outside the target volume. Target volume expansion was 5 mm in all directions. An inverse planning algorithm was used to cover planning target volume at the 95% dose level. Homogeneity was improved by setting the V150 and V200 (the percentage of volume receiving 150% and 200% of the prescribed dose) to 20% and 5%, respectively. Excessive high-dose regions outside the planning target volume were avoided by setting a source position volume constraint to V150 of 5%. In the combined group, the mean total dose was 69.6 Gy administered over an average of 44 elapsed days of treatment. Patients were seen daily during their initial hospitalization after surgery until discharge. HDR-BT patients were seen twice daily during their treatment until catheters were removed.
All patients were seen at 1 and/or 2 weeks postoperatively for wound evaluation and suture removal. Patients were subsequently evaluated at 1 and 3 months after surgery or more often as needed. Baseline MRI was obtained at 6 weeks after completion of radiotherapy and at 3-month intervals after baseline. Major complications were those requiring repeat operative intervention. All other complications were considered minor.
Descriptive statistics were performed for all groups. Potential differences among cohorts with regard to minor and major complication rates, rates of specific complications, and complication rates for combined versus monotherapy were assessed with use of chi square analysis (chronic pain/neuritis and pathologic fracture) or the Fisher exact test (other complications). Statistical analysis was performed with JMP® 7 statistical software (SAS Institute Inc, Cary, NC, USA).
Results
We noted 29 complications in 25 (68%) patients of the HDR-BT cohort and 166 complications in 106 (75%) patients in the EBRT cohort (Table 2). The most frequent complications in the HDR-BT cohort were deep infection requiring surgical débridement, cellulitis treated with antibiotics, and seroma/hematoma. In the EBRT group, the most frequent complications of radiotherapy treatment were chronic edema, fibrosis, and chronic radiation dermatitis. There was no difference between the HDR-BT and EBRT groups with regard to the total number of complications or the incidence of major (operative) complications, although there tended to be a greater (p = 0.08) proportion of reoperations in the HDR-BT cohort (eight, 22%) than in the EBRT group (21, 15%). There was no difference in local recurrence rates between the HDR-BT and EBRT groups (Table 1). There was also no difference in the rates of nonprimary closure (ie, flap and/or split-thickness skin grafts), which would ostensibly impact wound complication rates negatively.
Table 2.
Complications due to radiotherapy after HDR-BT, EBRT, or both after excision of extremity soft tissue sarcomas
| Variable | HDR-BT | EBRT | p Value (HDR-BT versus EBRT) | Both | p Value (HDR-BT versus combined) | p Value (EBRT versus combined) |
|---|---|---|---|---|---|---|
| Number of patients | 37 | 141 | 12 | |||
| Complications (number) | 29 | 164 | 17 | |||
| Cellulitis | 5 (14%) | 6 (4%) | 0.053 | 1 (8%) | 1 | 0.99 |
| Chronic pain/neuritis | 1 (3%) | 11 (8%) | 0.46 | 2 (17%) | 0.14 | 0.60 |
| Dehiscence/delayed wound healing | 2 (5%) | 13 (9%) | 0.53 | 1 (8%) | 1 | 1 |
| Chronic edema | 1 (3%) | 31 (22%) | 0.007 | 5 (42%) | 0.001 | 0.13 |
| Fibrosis | 4 (11%) | 30 (21%) | 0.17 | 1 (8%) | 1 | 0.46 |
| Pathologic fracture | 1 (3%) | 10 (7%) | 1 | 1 (8%) | 1 | 1 |
| Seroma/hematoma | 5 (14%) | 17 (12%) | 1 | 1 (8%) | 1 | 1 |
| Deep infection | 9 (24%) | 14 (10%) | 0.03 | 2 (17%) | 0.71 | 0.62 |
| Chronic radiation dermatitis | 1 (3%) | 32 (23%) | 0.008 | 1 (8%) | 0.43 | 0.31 |
| Other | 0 (0%) | 0 (0%) | 2 (17%) | 0.049 | 0.005 | |
| Total number of patients with any complication | 25 (68%) | 106 (75%) | 0.04 | 9 (75%) | 0.73 | 1 |
| Major (operative) complications (number) | 8 (22%) | 21 (15%) | 0.45 | 4 (33%) | 0.45 | 0.11 |
HDR-BT = high-dose-rate brachytherapy; EBRT = external-beam radiotherapy.
We noted differences between the HDR-BT and EBRT groups with regard to chronic edema (3% versus 22%; p = 0.007), infection (24% versus 10%; p = 0.028), and chronic radiation dermatitis (3% versus 23%; p = 0.008). The relative incidence of other complications was similar in the three cohorts.
We noted no differences between the EBRT and the combined-treatment groups for any of the specific complications. The rate of chronic edema was higher (p = 0.007) in the combined-treatment group than in the HDR-BT cohort (42% versus 3%, respectively).
Discussion
Complications related to perioperative radiotherapy in STS are common, with 6% to 48% incidence rates [5, 10, 31]. Variable definitions of complications and little emphasis on complications lead to large variability [2, 3, 5, 10]. Previous studies reported complications with low-dose-rate brachytherapy alone or in combination with EBRT or with HDR-BT in combination with EBRT in small numbers of patients [10, 29, 33], but no previous studies directly compare the complications of HDR-BT and EBRT as monotherapies [29]. We therefore addressed the following questions: (1) What is the overall complication rate in these two cohorts? (2) What is the frequency of specific complication subtypes? And (3) is the complication rate higher in patients treated with combined HDR-BT and EBRT compared with either modality alone?
Our study is associated with several limitations. First, patients were not randomized to the modality of adjuvant radiotherapy as it is necessary for local control in high-grade STS [35, 38, 39, 42]; therefore, a suitable control group with a similar spectrum of disease does not exist in our practice. Patients in the HDR-BT cohort were candidates for brachytherapy based on a multitude of factors, including tumor size, location, proximity to vital neurovascular structures, and anticipated patient compliance. Patient selection for HDR-BT implies a selection bias that must be considered when interpreting complications of treatment. Randomization was not possible given these considerations and the retrospective nature of this study. Second, because all patients received surgery and radiotherapy, it is not possible to attribute wound complications to surgery or radiation alone. The incidence of wound complications with surgery alone reportedly ranges from 33% to 50% [5, 10]. We were unable to compare wound complications to a group that received surgery alone as all patients received radiotherapy. Third, although our cohort of HDR-BT patients is larger than those in the majority of currently published reports [1, 6, 9, 19, 23, 33] (Table 3), an analysis of potentially confounding variables between HDR-BT and EBRT was limited due to small sample size. Further enrollment of patients into the HDR-BT group would enhance our understanding of the role of HDR-BT in the management of STS. Lastly, we had a relatively short duration of followup in the HDR-BT cohort and efficacy of local control cannot be determined; however, early complications were the primary end points of our study, and fewer than 3 months after surgery has previously been defined as a parameter for early complications, with very few reports documenting the time to complication occurrence from time of initial surgery [5, 10, 28, 29]. Additional followup of these patients for long-term complications is ongoing and will further define the role for adjuvant HDR-BT in STS, particularly with regard to local control, pathologic fracture, and secondary sarcoma rates. Despite these limitations, the findings in this direct comparative study build on limited existing literature for HDR-BT treatment for STS.
Table 3.
Summary of prior publications regarding complications of brachytherapy
| Study | Year | Number of patients | Followup (months) | Complications (%) |
|---|---|---|---|---|
| Alekhteyar et al. [1] | 1994 | 13 | 16 | NS |
| Crownover and Marks [9] | 1999 | 10 | 12 | 0 |
| Koizumi et al. [23] | 1999 | 16 | 30 | 6 |
| Petera et al. [33] | 2010 | 11 | 36 | NS |
| Itami et al. [19] | 2010 | 25 | 60 | NS |
| Emory et al. | 2011 | 37 | 13 | 68 |
NS = not specified.
Complications after EBRT are well documented, with preoperative treatment associated with higher rates of wound complications and postoperative radiotherapy associated with higher rates of subcutaneous fibrosis (Table 4) [7, 11, 12, 26, 29, 32, 33, 36, 37]. With no difference in overall survival or local recurrence between preoperative and postoperative EBRT [7, 20, 32, 36, 38], the timing of treatment is based on many factors, such as tumor size, surgeon preference, and previous unplanned excision. Although preoperative EBRT has advantages over postoperative EBRT, such as lower radiation dose and smaller field size, postoperative radiotherapy was most frequently used at our institution when EBRT was utilized due to previous reports of higher wound complication rates [6, 24, 29, 32, 36]. Complications associated with HDR-BT are also documented, but small sample sizes and lack of direct comparison between cohorts limit interpretation of this information [1, 8, 9, 18, 22, 23, 29]. The overall initial complication rates in EBRT and HDR-BT cohorts were similar, 75% and 68%, respectively, indicating surgery and radiotherapy for STS carry a high risk for perioperative morbidity.
Table 4.
Summary of prior publications regarding complications of external-beam radiotherapy for treatment of soft tissue sarcomas
| Study | Year | Number of patients | Followup (months) | Wound (%) | Fibrosis (%) | Edema (%) | Joint stiffness (%) |
|---|---|---|---|---|---|---|---|
| Stinson et al. [37] | 1991 | 145 | 36 | NS | 57 | 19 | 20 |
| Pollack et al. [36] | 1998 | ||||||
| Preoperative | 128 | 97 | 25 | NS | NS | NS | |
| Postoperative | 165 | 97 | 6 | NS | NS | NS | |
| O’Sullivan et al. [32] | 2002 | ||||||
| Preoperative | 88 | 39 | 35 | NS | NS | NS | |
| Postoperative | 94 | 39 | 17 | NS | NS | NS | |
| Davis et al. [11] | 2005 | 56 | 24 | NS | 48 | 23 | 23 |
| Emory et al. | 2011 | 141 | 51 | 28 | 36 | 22 | NS |
NS = not specified.
HDR-BT is an appealing radiotherapy option because of decreased radiation exposure, shorter duration of treatment compared with EBRT, targeted radiation to specific areas of concern with relative sparing of neurovascular structures (Fig. 2), and outpatient capabilities. Potential drawbacks of HDR-BT include the need for a radiation oncologist familiar with brachytherapy techniques, specialized facilities to perform brachytherapy, and initial wound complications as demonstrated in our study. Complications in our brachytherapy cohort primarily consisted of infection and required surgical intervention more often than the EBRT group; for this reason, brachytherapy may ostensibly be less appealing for resections in which vascular or skeletal reconstruction is required. The EBRT cohort demonstrated comparable rates of wound complications, fibrosis, and edema, as documented in other reports [6, 7, 11, 12, 24, 26, 29, 32, 33, 36, 37]. Our study highlights the differences in subtypes of complications, with edema and radiation dermatitis more common in the EBRT cohort and infection more common in the HDR-BT cohort.
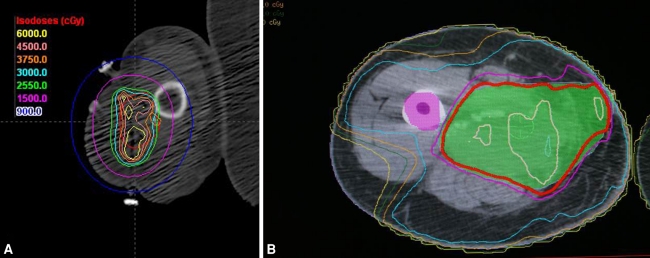
Fig. 2A–B.
(A) Three-dimensional reconstruction of catheter position enables focused radiotherapy dose distribution, demonstrated in this dose-volume histogram of the arm with relative sparing of the radial nerve. (B) Typical Intensity Modulate Radiation Therapy (IMRT) dose distribution is shown for postoperative treatment of a thigh sarcoma.
One study evaluating the complications of combined low-dose-rate brachytherapy and EBRT compared with brachytherapy alone reported the combined group had a higher rate of fibrosis, edema, and wound dehiscence [28]. The sample size of our combined-therapy cohort was inadequate to confirm this finding.
While followup in the HDR-BT cohort was inadequate to permit detailed assessment of long-term treatment efficacy, others [24–26, 30, 34] have demonstrated similar rates of local control and overall survival using HDR-BT monotherapy for STS compared with EBRT, without potential long-term complications of fibrosis and fracture. Further study is required to determine whether HDR-BT can be utilized as monotherapy in the management of STS; HDR-BT and EBRT both have high complication rates when scrutinized under stringent criteria. Early complication rates of HDR-BT are comparable to EBRT, with edema and radiation dermatitis more common in the EBRT cohort and infection more common in the HDR-BT cohort. Benefits of lower overall doses of radiation and shorter courses of treatment in HDR-BT must be weighed against increased postoperative infection rate when considering HDR-BT as a potential alternative to EBRT for select patients with intermediate- or high-grade extremity STS.
Acknowledgments
The authors thank Dr. H. Thomas Temple for his assistance with performing many of the surgeries, project development and data analysis. The authors thank Dr. J. David Pitcher for sharing his cases for which he was the primary surgeon. The authors also thank Dr. Jonathan Hilal and Dr. Jonathan Tresley for their assistance with data collection.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the University of Miami Miller School of Medicine.
References
- 1.Alekhteyar KM, Porter AT, Herskovic AM, Ryan J, Orton CG, Forman JD, Ahmad K. Preliminary results of hyperfractionated high dose rate brachytherapy in soft tissue sarcoma. Endocuriether Hypertherm Oncol. 1994;10:179–184. [Google Scholar]
- 2.Alektiar KM, Velasco J, Zelefsky MJ, Woodruff JM, Lewis JJ, Brennan MF. Adjuvant radiotherapy for margin-positive high-grade soft tissue sarcoma of the extremity. Int J Radiat Oncol Biol Phys. 2000;48:1051–1058. doi: 10.1016/S0360-3016(00)00753-7. [DOI] [PubMed] [Google Scholar]
- 3.Alektiar KM, Zelefsky MJ, Brennan MF. Morbidity of adjuvant brachytherapy in soft tissue sarcoma of the extremity and superficial trunk. Int J Radiat Oncol Biol Phys. 2000;47:1273–1279. doi: 10.1016/S0360-3016(00)00587-3. [DOI] [PubMed] [Google Scholar]
- 4.Andrews SF, Anderson PR, Eisenberg BL, Hanlon AL, Pollack A. Soft tissue sarcomas treated with postoperative external beam radiotherapy with and without low-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys. 2004;59:475–480. doi: 10.1016/j.ijrobp.2003.10.048. [DOI] [PubMed] [Google Scholar]
- 5.Arbeit JM, Hilaris BS, Brennan MF. Wound complications in the multimodality treatment of extremity and superficial sarcomas. J Clin Oncol. 1987;5:480–488. doi: 10.1200/JCO.1987.5.3.480. [DOI] [PubMed] [Google Scholar]
- 6.Aronowitz JN, Pohar SS, Liu L, Haq R, Damron TA. Adjuvant high dose rate brachytherapy in the management of soft tissue sarcoma: a dose-toxicity analysis. Am J Clin Oncol. 2006;29:508–513. doi: 10.1097/01.coc.0000231433.97407.c8. [DOI] [PubMed] [Google Scholar]
- 7.Cheng EY, Dusenbery KE, Winters MR, Thompson RC. Soft tissue sarcomas: preoperative versus postoperative radiotherapy. J Surg Oncol. 1996;61:90–99. doi: 10.1002/(SICI)1096-9098(199602)61:2<90::AID-JSO2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 8.Chun M, Kang S, Kim BS, Oh YT. High dose rate interstitial brachytherapy in soft tissue sarcoma: technical aspects and results. Jpn J Clin Oncol. 2001;31:279–283. doi: 10.1093/jjco/hye050. [DOI] [PubMed] [Google Scholar]
- 9.Crownover RL, Marks KE. Adjuvant brachytherapy in the treatment of soft-tissue sarcomas. Hematol Oncol Clin North Am. 1999;13:595–607. doi: 10.1016/S0889-8588(05)70078-2. [DOI] [PubMed] [Google Scholar]
- 10.Dalton RR, Lanciano RM, Hoffman JP, Eisenberg BL. Wound complications after resection and immediate postoperative brachytherapy in the management of soft-tissue sarcomas. Ann Surg Oncol. 1996;3:51–56. doi: 10.1007/BF02409051. [DOI] [PubMed] [Google Scholar]
- 11.Davis AM, O’Sullivan B, Turcotte R, Bell R, Catton C, Chabot P, Wunder J, Hammond A, Benk V, Kandel R, Goddard K, Freeman C, Sadura A, Zee B, Day A, Tu D, Pater J, Canadian Sarcoma Group; NCI Canada Clinical Trial Group Randomized Trial Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol. 2005;75:48–53. doi: 10.1016/j.radonc.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 12.Erickson B, Wilson JF. Clinical indications of brachytherapy. J Surg Oncol. 1997;65:218–227. doi: 10.1002/(SICI)1096-9098(199707)65:3<218::AID-JSO12>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 13.Fein DA, Lee WR, Lianciano RM, Corn BW, Herbert SH, Hanlon AL, Hoffman JP, Eisenberg BL, Coia LR. Management of extremity soft tissue sarcomas with limb-sparing surgery and postoperative irradiation: do total dose, overall treatment time, and the surgery-radiotherapy interval impact on local control? Int J Radiat Oncol Biol Phys. 1995;32:969–976. doi: 10.1016/0360-3016(95)00105-8. [DOI] [PubMed] [Google Scholar]
- 14.Gerrand CH, Bell RS, Wunder S, Kandel RA, O’Sullivan B, Catton CN, Griffin AM, Davis AM. The influence of anatomic location on outcome in patients with soft tissue sarcoma of the extremity. Cancer. 2003;97:485–492. doi: 10.1002/cncr.11076. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert NF, Cannon CP, Lin PP, Lewis VO. Soft-tissue sarcoma. J Am Acad Orthop Surg. 2009;17:40–47. doi: 10.5435/00124635-200901000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Green FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M. AJCC Cancer Staging Manual. 6. Philadelphia, PA: Lippincott-Raven; 2002. [Google Scholar]
- 17.Harrison LB, Franzese F, Gaynor JJ, Brennan MF. Long-term results of a prospective randomized trial of adjuvant brachytherapy in the management of completely resected soft tissue sarcomas of the extremity and superficial trunk. Int J Radiat Oncol Biol Phys. 1993;27:259–265. doi: 10.1016/0360-3016(93)90236-o. [DOI] [PubMed] [Google Scholar]
- 18.Hilaris BB, Bodner WR, Mastoras CA. Role of brachytherapy in adult soft tissue sarcomas. Semin Surg Oncol. 1997;13:196–203. doi: 10.1002/(SICI)1098-2388(199705/06)13:3<196::AID-SSU7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 19.Itami J, Sumi M, Beppu Y, Chuman H, Kawai A, Murakami N, Morota M, Mayahara H, Yoshimura R, Ito Y, Kagami Y. High-dose rate brachytherapy alone in postoperative soft tissue sarcomas with close or positive margins. Brachytherapy. 2010;9:349–353. doi: 10.1016/j.brachy.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 20.Jones JJ, Catton CN, O’Sullivan B, Couture J, Heisler RL, Kandel RA, Swallow CJ. Initial results of a trial of preoperative external-beam radiation therapy and postoperative brachytherapy for retroperitoneal sarcoma. Ann Surg Oncol. 2002;9:346–354. doi: 10.1007/BF02573869. [DOI] [PubMed] [Google Scholar]
- 21.Kaled MA, Brennan M, Healey J, Singer S. Impact of intensity-modulated radiation therapy on local control in primary soft-tissue sarcoma of the extremity. J Clin Oncol. 2008;26:3340–3344. doi: 10.1200/JCO.2008.16.6249. [DOI] [PubMed] [Google Scholar]
- 22.Kaled MA, Hong L, Brennan M, Della-Bianca C, Singer S. Intensity modulated radiation therapy for primary soft tissue sarcoma of the extremity: preliminary results. Int J Radiat Oncol Biol Phys. 2007;68:458–464. doi: 10.1016/j.ijrobp.2006.12.054. [DOI] [PubMed] [Google Scholar]
- 23.Koizumi M, Inoue T, Yamazaki H, Teshima T, Tanaka E, Yoshida K, Imai A, Shiomi H, Kagawa K, Araki N, Kuratsu S, Uchida A, Inoue T. Perioperative fractionated high-dose rate brachytherapy for malignant bone and soft tissue tumors. Int J Radiat Oncol Biol Phys. 1999;43:989–993. doi: 10.1016/S0360-3016(98)00491-X. [DOI] [PubMed] [Google Scholar]
- 24.Kubo T, Sugita T, Shimose S, Matsuo T, Hirao K, Kimura H, Kenjo M, Ochi M. Nerve tolerance to high-dose-rate brachytherapy in patients with soft tissue sarcoma: a retrospective study. BMC Cancer. 2005;5:79. doi: 10.1186/1471-2407-5-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laskar S, Bahl G, Puri A, Agarwal MG, Muckaden M, Patil N, Jambhekar N, Gupta S, Deshpande DD, Shrivastava SK, Dinshaw KA. Perioperative interstitial brachytherapy for soft tissue sarcomas: prognostic factors for long term results of 155 patients. Ann Surg Oncol. 2007;14:560–567. doi: 10.1245/s10434-006-9137-2. [DOI] [PubMed] [Google Scholar]
- 26.Lee HY, Cordeiro PG, Mehrara BJ, Singer S, Alektiar KM, Hu QY, Disa JJ. Reconstruction after soft tissue sarcoma resection in the setting of brachytherapy: a 10 year experience. Ann Plast Surg. 2004;52:486–492. doi: 10.1097/01.sap.0000122649.64350.e3. [DOI] [PubMed] [Google Scholar]
- 27.Lewis JJ, Leung D, Espat J, Woodruff JM, Brennan MF. Effects of reresection in extremity soft tissue sarcoma. Ann Surg. 2000;231:655–663. doi: 10.1097/00000658-200005000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Livi L, Santoni R, Paiar F, Bastiani P, Beltrami G, Caldora P, Capanna R, Biase P, Detti B, Fondelli S, Maldolesi E, Pertici M, Polli C, Simontacchi G, Biti G. Late treatment-related complications in 214 patients with extremity soft-tissue sarcoma treated by surgery and postoperative radiation therapy. Am J Surg. 2006;191:230–234. doi: 10.1016/j.amjsurg.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 29.Nag S, Shasha D, Janjan N, Petersen I, Zaider M, The American Brachytherapy Society The American Brachytherapy Society recommendations for brachytherapy of soft tissue sarcoma. Int J Radiat Oncol Biol Phys. 2001;49:1033–1043. doi: 10.1016/S0360-3016(00)01534-0. [DOI] [PubMed] [Google Scholar]
- 30.O’Connor M, Pritchard DJ, Gunderson LL. Integration of limb-sparing surgery, brachytherapy, and external-beam irradiation in the treatment of soft-tissue sarcomas. Clin Orthop Relat Res. 1993;289:73–80. [PubMed] [Google Scholar]
- 31.Ormsby MV, Hilaris BS, Nori D, Brennan MF. Wound complications of adjuvant radiation therapy in patients with soft-tissue sarcomas. Ann Surg. 1989;210:93–99. doi: 10.1097/00000658-198907000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Sullivan B, Davis AM, Turcotte R, Bell R, Catton C, Chabot P, Wunder J, Kandel R, Goddard K, Sadura A, Pater J, Zee B. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359:2235–2241. doi: 10.1016/S0140-6736(02)09292-9. [DOI] [PubMed] [Google Scholar]
- 33.Petera J, Soumarova R, Růžičková J, Neumanová R, Dušek L, Sirák I, Mačingová Z, Paluska P, Kašaova L, Hodek M, Vošmik M. Perioperative hyperfractionated high-dose rate brachytherapy for the treatment of soft tissue sarcomas: multicentric experience. Ann Surg Oncol. 2010;17:206–210. doi: 10.1245/s10434-009-0684-1. [DOI] [PubMed] [Google Scholar]
- 34.Pisters PW, Harrison LB, Leung DH, Woodruff JM, Casper CS, Brennan MF. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol. 1996;14:859–868. doi: 10.1200/JCO.1996.14.3.859. [DOI] [PubMed] [Google Scholar]
- 35.Pisters PW, Harrison LB, Woodruff JM, Gaynor JJ, Brennan MF. A prospective randomized trial of adjuvant brachytherapy in the management of low-grade soft tissue sarcomas of the extremity and superficial trunk. J Clin Oncol. 1994;12:1150–1155. doi: 10.1200/JCO.1994.12.6.1150. [DOI] [PubMed] [Google Scholar]
- 36.Pollack A, Zagars GK, Goswitz MS, Pollock RA, Feig BW, Pisters PW. Preoperative vs. postoperative radiotherapy in the treatment of soft tissue sarcomas: a matter of presentation. Int J Radiat Oncol Biol Phys. 1998;42:517–523. doi: 10.1016/S0360-3016(98)00260-0. [DOI] [PubMed] [Google Scholar]
- 37.Stinson SF, Delaney TF, Greenberg J, Yang JC, Lampert MH, Hicks JE, Venzon D, White DE, Rosenberg SA, Glatstein EJ. Acute and long-term effects on limb function of combined modality limb sparing therapy for extremity soft tissue sarcoma. Int J Radiat Oncol Biol Phys. 1991;21:1493–1499. doi: 10.1016/0360-3016(91)90324-W. [DOI] [PubMed] [Google Scholar]
- 38.Strander H, Turesson I, Cavallin-Ståhl E. A systematic overview of radiation therapy effects in soft tissue sarcomas. Acta Oncol. 2003;42:516–531. doi: 10.1080/02841860310014732. [DOI] [PubMed] [Google Scholar]
- 39.Tepper JE, Suit HD. Radiation therapy of soft tissue sarcomas. Cancer. 1985;55(9 suppl):2273–2277. doi: 10.1002/1097-0142(19850501)55:9+<2273::AID-CNCR2820551434>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 40.Trojani M, Contesso G, Coindre JM, Rouesse J, Bui NB, Mascarel A, Goussot JF, David M, Bonichon F, Lagarde C. Soft-tissue sarcomas of adults; study of pathological prognostic variables and definition of a histopathological grading system. Int J Cancer. 1984;33:37–42. doi: 10.1002/ijc.2910330108. [DOI] [PubMed] [Google Scholar]
- 41.Willett CG, Czito BG, Tyler DS. Intraoperative radiation therapy. J Clin Oncol. 2007;25:970–977. doi: 10.1200/JCO.2006.10.0255. [DOI] [PubMed] [Google Scholar]
- 42.Yang JC, Chang AE, Baker AR, Sindelar WF, Danforth DN, Topalian SL, DeLaney T, Glatstein E, Steinberg SM, Merino MJ, Rosenberg SA. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998;16:197–203. doi: 10.1200/JCO.1998.16.1.197. [DOI] [PubMed] [Google Scholar]