Abstract
Background
Allograft tissues can undergo several freeze-thaw cycles between donor tissue recovery and final use by surgeons. However, there are currently no standards indicating the number of reasonable freeze-thaw cycles for allograft bone and it is unclear how much a graft may be degraded with multiple cycles.
Questions/purposes
We therefore asked whether (1) the mechanical properties of fibular allograft bone would remain unchanged with increasing numbers of freeze-thaw cycles and (2) histologic alterations from increased numbers of freeze-thaw cycles would correspond to any mechanical changes.
Methods
Fibular allograft segments were subjected to two, four, and eight freeze-thaw cycles and compared biomechanically and histologically with a control group (one freeze-thaw cycle). Two freeze-dried treatments, one after being subjected to one freeze-thaw cycle and the other after being subjected to three freeze-thaw cycles, also were compared with the control group.
Results
For all segments, the average ultimate stress was 174 MPa, average modulus was 289 MPa, average energy was 2.00 J, and the average stiffness was 1320 N/mm. The material properties of the freeze-thaw treatment groups were similar to those of the control group: ultimate stress and modulus were a maximum of 16% and 70% different, respectively. Both freeze-dried treatments showed increased stiffness (maximum 53% ± 71%) and energy to failure (maximum 117% ± 137%) but did not exhibit morphologic differences. There were no alterations in the histologic appearance of the bone sections in any group.
Conclusions
Fibular allograft segments can be refrozen safely up to eight times without affecting the biomechanical or morphologic properties. Freeze-dried treatments require further study to determine whether the detected differences are caused by the processing.
Clinical Relevance
Cryopreserved cortical allografts are thawed by surgeons in preparation for procedures and then occasionally discarded when not used. Refreezing allograft tissues can result in a cost savings because of a reduction in wasted graft material.
Introduction
Structural allograft bone has various applications in orthopaedic surgery, including nonunion reconstruction, bridging bone defects, periprosthetic fracture repair, tumor reconstruction, arthrodesis, treatment of avascular necrosis, and spine surgery [2–4, 7, 11, 15, 16]. Although such procedures generally are successful, complications relating to unanticipated allograft fracture have prompted investigation into possible sources of allograft failure, including storage and handling conditions [5]. Allograft bone typically is frozen at temperatures less than −40°C and can be stored up to 5 years according to American Association of Tissue Banks Standards [1], which specify how the grafts are stored and the temperature for long-term frozen storage. These grafts then are thawed before clinical use according to product specifications. However, there are no current regulations or guidelines that govern the number of times a graft can be thawed and refrozen. In reality, allografts might be subjected to multiple freeze-thaw cycles at the tissue bank and surgical center location.
In clinical settings, a surgeon might arrange for allograft bone to be available for a procedure, but then it might go unused and subsequently be discarded. As graft temperature is not closely monitored, it is possible grafts that are believed to be frozen but in fact have passed through the water phase transition temperature are placed back into the freezer for future use. Safely refreezing the graft without compromising strength, immunogenicity, or sterility would lower costs by minimizing wasted graft material. Regarding graft strength, it is postulated refreezing of water in the bone material could cause microdamage to the cortical structure via expansion, analogous to the damage that occurs to pavements in cold climates, thus resulting in a weaker graft.
Studies investigating the effects of freeze-thaw cycles on musculoskeletal tissue graft properties have reported mixed findings [6–10, 12–14, 17]. One study analyzing bone-patellar tendon-bone grafts found no differences in stress or strain between fresh grafts and those subjected to one or two freeze-thaw cycles [8]. Another found four freeze-thaw cycles decreased the ability of meniscal allografts to resist compressive forces [9]. A third, more-recent study reported that as many as eight freeze-thaw cycles do not affect the biomechanical properties of bone-patellar tendon-bone grafts [6]. However, it is difficult to ascertain the effect of freeze-thaw cycles on bone using data derived from soft tissue, and studies on allograft cortical bone are limited and differ in their findings. Two studies showed torsion and bending properties of freeze-dried allograft bone were weaker after processing and storage [12, 17]. In contrast, other studies found as many as five freeze-thaw cycles do not affect the biomechanical properties of cancellous bone [10, 14] and long-term freezing reduces immunogenicity of grafts but does not decrease the biomechanical properties [13]. Thus, additional research on how freezing cycles affect bone biomechanics and morphology is clearly warranted.
We therefore asked whether (1) the mechanical properties of fibular allograft bone would remain unchanged with increasing numbers of freeze-thaw cycles and (2) histologic alterations from increased numbers of freeze-thaw cycles would occur.
Materials and Methods
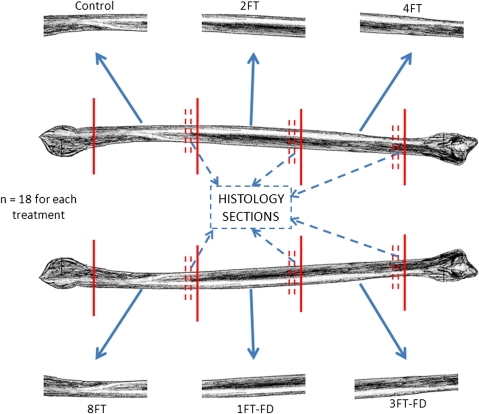
We recovered the left and right fibulae from 18 research-consented cadaveric donors (seven men, 11 women; 39–75 years old). Each fibula was thawed, debrided, cut into three 80-mm-long segments (Fig. 1), and subjected to the Allowash® (Lifenet Health; Virginia Beach, VA, USA) bioburden reduction process. One segment was randomly selected as the control and immediately tested, as a minimum of one freeze-thaw cycle is incurred between tissue recovery and processing. The remaining segments were randomly assigned to one of the five treatment groups: two, four, or eight total freeze-thaw cycles, one total freeze-thaw cycle plus freeze-drying, or three total freeze-thaw cycles plus freeze-drying. These specific treatments were chosen to span the range between the minimum and maximum cycles expected to be encountered by graft material between recovery and use. The maximum number of eight freeze-thaw cycles was chosen by consultation with American Association of Tissue Banks [1] and other published data [6]. Each treatment was represented once within a donor, thus each treatment had an n = 18. A freeze-thaw cycle is defined as a tissue being frozen (at −40°C or colder) and then allowed to warm until the residual water undergoes the solid to liquid phase change (~1°–2°C), as measured by calibrated thermistor. Freeze-drying was conducted according to validated protocols to result in a moisture content less than 6% by weight (Community Tissue Services; Dayton, OH, USA).
Fig. 1.
A schematic diagram shows the test segment preparation. Control = one total freeze-thaw cycle; 2FT, 4FT, 8FT = two, four, eight total freeze-thaw cycles; 1FT-FD = one total freeze-thaw cycle plus freeze-drying; 3FT-FD = three total freeze-thaw cycles plus freeze-drying. Each fibula (n = 18 pairs) was divided into six segments, one segment for each treatment so that all treatments were represented within a donor.
After the assigned number of freeze-thaw cycles was achieved, each specimen was thawed and then imaged in a CT scanner (Model XCT-2000; Stratec Medizintechnik GmbH, Pforzheim, Germany) to measure geometric parameters. CT slices were obtained at three locations in the middle portion of the segment (at the midpoint and ± 4 mm from it). The segment was placed on a radiotranslucent fixture with the same configuration as the bending fixture and in the same orientation as it would be for testing, ie, resting on two anvils with a span equivalent to the bending fixture. The measurements (ie, area moment of inertia, cortical thickness, and neutral axis dimension) used for the analysis were averaged over the three slices. The area moment of inertia is the resistance of an object to bending, and the neutral axis is the plane in the cross section where bending stresses are zero.
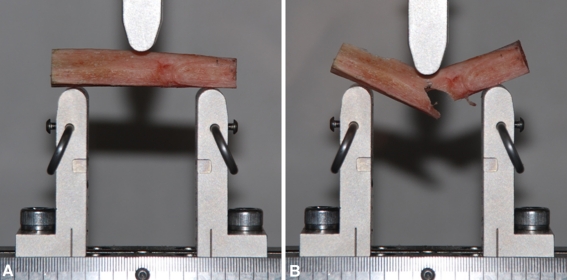
Immediately before mechanical testing, 5-mm sections were removed from the end of the segment (Fig. 1), placed in 10% buffered formalin, decalcified, dehydrated through a gradient of alcohols, infiltrated and embedded with paraffin, cut in cross section at 5-μm thickness, and stained with hematoxylin and eosin for histologic analysis. Remaining specimens were tested in three-point bending by loading to failure at a strain rate of 0.5 mm/second on a materials testing system (ElectroPuls™ E3000; Instron Corp, Norwood, MA, USA) (Fig. 2).
Fig. 2A–B.
The test setup is shown with a sample (A) before testing and (B) immediately after failure. The typical spiral failure pattern, as experienced in our study, is shown in this illustration.
Structural parameters (load, extension) were directly measured, from which material parameters (stress, strain) were derived using specimen dimensions obtained from the CT data. Stiffness and modulus were calculated as the slope of the force-displacement curve and stress-strain curve, respectively, between 20% and 80% of the peak force or stress. Stress (σ) and strain (ε) were calculated as:
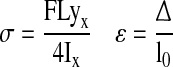 |
where F = measured load, L = span between anvils (50 mm), yx = maximum distance from cortical edge to horizontal axis, Ix = area moment of inertia about the horizontal plane, Δ = measured deflection, and l0 = cortical thickness. Energy was calculated by integrating the force-displacement curve to the point of failure. Ultimate stress, stiffness, energy, and modulus were normalized to the control for each donor as a percent error.
One author (GPB) evaluated each histologic section. One section from each treatment (n = 6 donors) was evaluated blind to the treatment. Sections were evaluated for cracking of the tissue, presence of osteocytes, alterations in the appearance of the Haversian canals, changes in the lamellae, and any alterations in the canaliculi. The sections were evaluated on a 0 to 3 scale with 0 = no significant alteration, 1 = mild change, 2 = moderate change, and 3 = severe change.
We used a two-way ANOVA (Minitab 16, State College, PA, USA) to test for differences between donors and/or treatments, and Tukey’s post hoc test for pairwise treatment comparisons. Pearson’s correlation coefficients were calculated for the response measures against donor age, gender, and segment location, and plotted in a correlation matrix. The Kruskal-Wallis nonparametric test was used to detect differences among the histologic measures.
Results
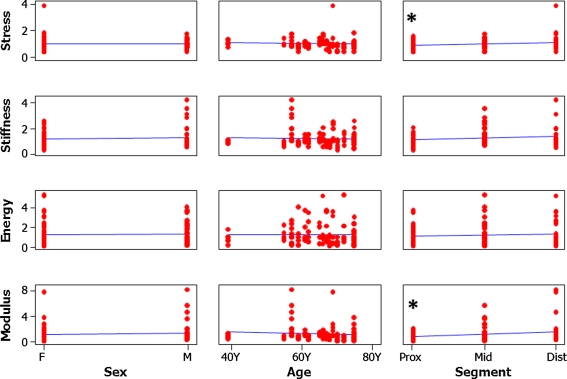
For all segments, the average ultimate stress was 174 ± 59 MPa, average modulus was 289 ± 217 MPa, average energy was 2.00 ± 1.83 J, and the average stiffness was 1320 ± 511 N/mm. ANOVA on the normalized data indicated differences between treatment groups and control for stiffness and energy. Post hoc analysis revealed an increase in stiffness between both freeze-dried treatments and the control and an increase in energy between two freeze-thaw cycles and the control (Table 1). All failures were characteristic of long-bone fractures resulting from bending, ie, a transverse or short oblique pattern originating at the application of load. We observed donor-to-donor variation for all biomechanical measures: stress p = 0.002, modulus p < 0.001, energy p = 0.036, and stiffness p < 0.001. None of the mechanical measures correlated with donor age or gender (Fig. 3). Segment, which indicates the longitudinal location of the sample, correlated with the stress and modulus, indicating the more distal portions had lower material properties values.
Table 1.
Mechanical results for all segments grouped by treatment
| Treatment | Ultimate stress | Modulus | Stiffness | Energy | ||||
|---|---|---|---|---|---|---|---|---|
| Average % ± SD % | P | Average % ± SD % | P | Average % ± SD % | P | Average % ± SD % | P | |
| Control | 0.00 | 0.00 | 0.00 | 0.00 | ||||
| 2FT | −0.49 ± 29.37 | 1.000 | 10.94 ± 103.67 | 0.999 | 2.22 ± 61.70 | 1.000 | 116.78* ± 136.88 | < 0.001 |
| 4FT | −7.47 ± 26.13 | 0.982 | −8.79 ± 45.61 | 1.000 | 2.84 ± 39.81 | 1.000 | 70.27 ± 124.36 | 0.123 |
| 8FT | 13.00 ± 80.29 | 0.892 | 40.23 ± 183.72 | 0.790 | 16.03 ± 64.34 | 0.878 | 77.97 ± 108.83 | 0.064 |
| 1FT-FD | −16.73 ± 23.72 | 0.737 | 50.44 ± 122.43 | 0.591 | 52.61* ± 71.16 | 0.006 | −64.46 ± 16.93 | 0.193 |
| 3FT-FD | 2.14 ± 37.46 | 1.000 | 70.71 ± 184.73 | 0.220 | 43.67* ± 93.33 | 0.038 | −49.73 ± 25.08 | 0.471 |
Values are expressed as average ± SD percent error from control; * treatments different (p < 0.05) from control; control = one total freeze-thaw cycle; 2FT, 4FT, 8FT = two, four, eight total freeze-thaw cycles; 1FT-FD = one total freeze-thaw cycle plus freeze-drying; 3FT-FD = three total freeze-thaw cycles plus freeze-drying.
Fig. 3.
The graph shows the measured parameters plotted versus each of the donor parameters. The asterisk (*) indicates parameters that are correlated (α = 0.05) to one another. F = female; M = male; prox = proximal; mid = middle; dist = distal. Segment (the longitudinal location of the sample) was correlated to Modulus (p = 0.0) and Stress (p = 0.0). Units for all measures are percent difference from the control segment.
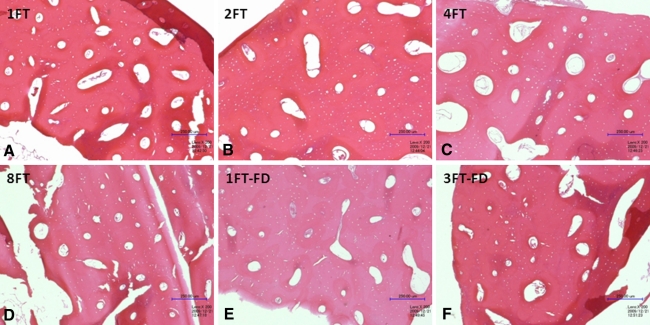
Structurally, the bones had no evidence of damage from the freeze-thaw process. Histologic changes included cellular debris in the Haversian canals in all but one section. Cells including osteocytes either were absent or were dead in the sections. There also was mild cracking in the lamellae of three sections. All three of these sections were over-decalcified, so this cracking likely is sectioning artifact. No differences were observed between treatment groups for any bone characteristics analyzed, including Haversian canals, lamellae, osteocytes, and canaliculi (Fig. 4). The size, shape, orientation, organization, physical damage, and staining characteristics of the Haversian canals, lamellae, and canaliculi were unaffected by the freeze-thaw processes. All parameters had scores of 0 indicating no change except for the three lamellae sections with scores of 1. The only consistent feature resulting from the freeze-thaw cycle was the death of the cells in the sections, which is an expected response in the tissue. We observed no differences in the appearances of the Haversian canals (p = 0.416), lamellae (p = 0.416), osteocytes (p = 0.416), or canaliculi (p = 1.000) between the treatments.
Fig. 4A–F.
Histologic samples of all treatments in a representative donor are shown (Stain, hematoxylin & eosin; original magnification, ×200): (A) 1FT = control, (B) 2FT = two freeze-thaw cycles, (C) 4FT = four freeze-thaw cycles, (D) 8FT = eight total freeze-thaw cycles; (E) 1FT-FD = one total freeze-thaw cycle plus freeze-drying; and (F) 3 FT-FD = three total freeze-thaw cycles plus freeze-drying. No morphologic changes were noted in the samples as a result of the treatments.
Discussion
Allograft bone is relied on to provide structural support in cases of substantial bone loss. Differences in graft handling by tissue banks and surgery centers before implantation could potentially introduce material property changes that may weaken the graft, which could lead to early failure and the need for additional surgery. All bone allografts endure one freeze-thaw cycle between donor tissue recovery and processing, and frozen allografts occasionally are subjected to an additional cycle between processing and distribution to surgical centers. Freeze-dried allografts are frozen once between recovery and processing and then frozen before the freeze-drying process. Subsequent freeze-thaw cycles can occur once the allograft has been distributed to a surgical center, but these grafts potentially are discarded if they have been thawed and not used. We therefore asked whether (1) the mechanical properties of fibular allograft bone would remain unchanged with increasing numbers of freeze-thaw cycles and (2) histologic alterations from increased numbers of freeze-thaw cycles would occur.
We acknowledge limitations to our study. First, the donor-to-donor variation was large. The analysis indicated age and gender were correlated to the moment of inertia, ie, the female donors in our sample population tended to be older than the male donors and thus had thinner cortical walls and a lower moment of inertia. In general, however, structural allografts are not typically created from cortical bone recovered from older, female donors. Further confounding the variability is the fact that the material parameters decreased along the length from proximal to distal indicating the heterogeneity of the samples added variability to the study design. Using more heterogeneous samples would likely reduce the variability. Second, we did not examine subfailure fatigue properties with cyclical loading that might better indicate mechanical differences between frozen and lyophilized grafts. Stiffness was greater for both. Third, in vitro testing does not reflect changes that occur in the allograft bone attributable to tissue remodeling and revascularization once the tissue is implanted. Although the structural properties do not change, incorporation and graft healing might be affected by increasing freeze-thaw cycles. Allograft bone serves as a scaffold with osteoconductive properties and is penetrated by vessels from the host bone for incorporation. One study found freezing bone grafts does not influence the structure for osteoconduction or change its ability to incorporate with the same biomechanical competence [12]. In vivo studies can now be performed to determine whether the osteoconductive and incorporative abilities remain with multiple freeze-thaw cycles. Fourth, only one author (GPB) reviewed the histologic morphometry and evaluated the changes using an unvalidated scoring system. However, this author is a trained veterinary pathologist and the scoring system used was an ordinal scale of increasing evidence of morphologic change.
The mechanical properties of fibular allograft bone remain unchanged with increasing numbers of freeze-thaw cycles. The material parameters, stress, and modulus were unaffected by increasing freeze-thaw cycles or even by freeze-drying. These results are in line with those of other authors regarding soft tissue [6, 8, 9] and hard tissue [10, 13, 14] as a result of freezing and thawing cycles. Two previous studies [12, 17] found that processing and storage caused freeze-dried allograft bone to be weaker, but we did not compare our samples with the same control group. However, the results for energy to failure and stiffness seem to indicate some subfailure differences might exist between freeze-dried and nonfreeze-dried bone segments when comparing the graft rather than the material, ie, fibula segment versus cortical bone.
Histologic alterations from increased numbers of freeze-thaw cycles do not occur. Our observations suggest there is no effect on the histologic appearance or failure properties of fibular bone segments when subjected to as many as eight freeze-thaw cycles and/or freeze-drying. Furthermore, there is no detectable trend of property change as the number of cycles increase.
Cryopreserved cortical allografts are thawed by surgeons in preparation for procedures and then occasionally discarded when not used. Refreezing allograft tissues can result in a cost savings because of a reduction in wasted graft material. Our observations suggest fibular allograft segments can be refrozen up to eight times without affecting the biomechanical or morphologic properties. Observations from the current study along with those of previous researchers [6, 8–10, 13, 14] indicate that soft tissue and hard tissue allografts may be safely refrozen for future use. Freeze-dried treatments require further study to determine whether the detected differences are caused by the processing or uncontrolled experimental variation.
Acknowledgments
We thank Dr. Michael Coffey for assistance in planning the study and sample preparation, Dr. Mark Stouffer for assistance in planning the study, and Scott Lucous for assistance in sample preparation and conducting the testing.
Footnotes
One or more of the authors have received funding from Community Tissue Services (JMS,SAH) and The Dayton Area Graduate Medical Education Council Grant Number 226700 (JCG).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Community Blood Center/Community Tissue Services™.
References
- 1.American Association of Tissue Banks. Standards for Tissue Banking. Ed 11. McLean, VA: American Association of Tissue Banks; 2007.
- 2.Buckley PD, Gearen PF, Petty RW. Structural bone-grafting for early atraumatic avascular necrosis of the femoral head. J Bone Joint Surg Am. 1991;73:1357–1364. [PubMed] [Google Scholar]
- 3.Buecker PJ, Gebhardt MC. Are fibula strut allografts a reliable alternative for periarticular reconstruction after curettage for bone tumors? Clin Orthop Relat Res. 2007;461:170–174. doi: 10.1097/blo.0b013e31804e8470. [DOI] [PubMed] [Google Scholar]
- 4.Crosby LA, Norris BL, Dao KD, McGuire MH. Humeral shaft nonunions treated with fibular allograft and compression plating. Am J Orthop (Belle Mead NJ) 2000;29:45–47. [PubMed] [Google Scholar]
- 5.Jones J, Yoo J, Hart R. Delayed fracture of fibular strut allograft following multilevel anterior cervical spine corpectomy and fusion. Spine (Phila PA 1976) 2006;31:E595–E599. doi: 10.1097/01.brs.0000229253.17108.03. [DOI] [PubMed] [Google Scholar]
- 6.Jung HJ, Vangipuram G, Fisher MB, Yang G, Hsu S, Bianchi J, Ronholdt C, Woo SL. The effects of multiple freeze-thaw cycles on the biomechanical properties of the human bone-patellar tendon-bone allograft. J Orthop Res. 2011;29:1193–1198. doi: 10.1002/jor.21373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar A, Chambers I, Maistrelli G, Wong P. Management of periprosthetic fracture above total knee arthroplasty using intramedullary fibular allograft and plate fixation. J Arthroplasty. 2008;23:554–558. doi: 10.1016/j.arth.2007.05.054. [DOI] [PubMed] [Google Scholar]
- 8.Lee GH, Kumar A, Berkson E, Verma N, Bach BR, Jr, Hallab N. A biomechanical analysis of bone-patellar tendon-bone grafts after repeat freeze-thaw cycles in a cyclic loading model. J Knee Surg. 2009;22:111–113. doi: 10.1055/s-0030-1247734. [DOI] [PubMed] [Google Scholar]
- 9.Lewis PB, Williams JM, Hallab N, Virdi A, Yanke A, Cole BJ. Multiple freeze-thaw cycled meniscal allograft tissue: a biomechanical, biochemical, and histologic analysis. J Orthop Res. 2008;26:49–55. doi: 10.1002/jor.20473. [DOI] [PubMed] [Google Scholar]
- 10.Linde F, Sørensen HC. The effect of different storage methods on the mechanical properties of trabecular bone. J Biomech. 1993;26:1249–1252. doi: 10.1016/0021-9290(93)90072-M. [DOI] [PubMed] [Google Scholar]
- 11.Neufeld SK, Uribe J, Myerson MS. Use of structural allograft to compensate for bone loss in arthrodesis of the foot and ankle. Foot Ankle Clin. 2002;7:1–17. doi: 10.1016/S1083-7515(01)00002-X. [DOI] [PubMed] [Google Scholar]
- 12.Pelker RR, Friedlaender GE, Markham TC. Biomechanical properties of bone allografts. Clin Orthop Relat Res. 1983;174:54–57. [PubMed] [Google Scholar]
- 13.Reikeras O, Sigurdsen UW, Shegarfi H. Impact of freezing on immunology and incorporation of bone allograft. J Orthop Res. 2010;28:1215–1219. doi: 10.1002/jor.21121. [DOI] [PubMed] [Google Scholar]
- 14.Santoni BG, Womack WJ, Wheeler DL, Puttlitz CM. A mechanical and computational investigation on the effects of conduit orientation on the strength of massive bone allografts. Bone. 2007;41:769–774. doi: 10.1016/j.bone.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 15.Schultz KD, Jr, McLaughlin MR, Haid RW, Jr, Comey CH, Rodts GE, Jr, Alexander J. Single-stage anterior-posterior decompression and stabilization for complex cervical spine disorders. J Neurosurg. 2000;93(2 suppl):214–221. doi: 10.3171/spi.2000.93.2.0214. [DOI] [PubMed] [Google Scholar]
- 16.Talbot M, Zdero R, Garneau D, Cole PA, Schemitsch EH. Fixation of long bone segmental defects: a biomechanical study. Injury. 2008;39:181–186. doi: 10.1016/j.injury.2007.08.026. [DOI] [PubMed] [Google Scholar]
- 17.Thalgott JS, Fogarty ME, Giuffre JM, Christenson SD, Epstein AK, Aprill C. A prospective, randomized, blinded, single-site study to evaluate the clinical and radiographic differences between frozen and freeze-dried allograft when used as part of a circumferential anterior lumbar interbody fusion procedure. Spine (Phila PA 1976) 2009;34:1251–1256. doi: 10.1097/BRS.0b013e3181a005d7. [DOI] [PubMed] [Google Scholar]