Abstract
Background
Laboratory tasks that measure various facets of impulsivity derived from self-report questionnaires are important for elucidating the behavioral consequences of impulsivity in humans and for back-translating these facets to non-human species. Negative urgency, or mood-based rash action, is a self-report facet of impulsivity linked to problem substance use; however, a valid behavioral task is lacking.
Method
The current studies were designed to bridge self-report questionnaire and behavioral measures of negative urgency in humans and to determine if this could be back-translated to rats.
Results
Humans scoring high in negative urgency showed greater behavioral responding and increased frustration following unexpected reward omission on a monetary-based task compared to subjects low in negative urgency. Rats also showed elevated responding for either sucrose pellets or intravenous amphetamine following unexpected reward omission.
Conclusion
These results suggest that impulsive behavior engendered by unexpected reward omission may represent a valid behavioral model of negative urgency linked to substance abuse.
Keywords: Impulsivity, Negative Urgency, Translational Research, Substance Abuse, Amphetamine, Self-Administration
1. Introduction
Impulsivity has been defined in many ways and measured by various self-report questionnaires (e.g., Eysenck and Eysenck, 1985; Patten et al., 1995; Tellegen, 1982). Whiteside and Lynam (2001) factor analyzed the scores on multiple impulsivity scales and identified four distinct facets: (1) negative urgency (acting rashly in response to distress); (2) lack of perseverance (inability to remain focused on a task); (3) lack of premeditation (acting without thinking); and (4) sensation seeking (seeking out novel experiences). These four facets are measured on a personality questionnaire referred to as the UPPS. While each of these facets may predict various risky behaviors, recent evidence suggests that negative urgency shows a prominent association with problem substance use (Cyders et al., 2009; Whiteside and Lynam, 2003; Zapolski et al., 2009).
Behavioral tasks related to these personality facets are needed to investigate how these facets are expressed to engender substance use and abuse. Unfortunately, while there are some exceptions (e.g., Kjome et al., 2010), performance on behavioral tasks purported to measure impulsivity often does not correspond to self-report scales (Reynolds et al., 2006). Behavioral tasks are also needed to investigate the neurobiological mechanisms involved in the relation between impulsivity and substance use with laboratory animals, which has become a highly active area of neurobehavioral research (Belin et al., 2008; Dalley et al., 2007; Economidou et al., 2010; Perry and Carroll, 2008; Winstanley et al., 2010). However, negative urgency, as defined by mood-based rash action, has not been targeted specifically in this preclinical research.
The goal of the current experiments was to develop a translational behavioral model of negative urgency in which a negative mood state is induced by omitting an expected reward. Human volunteers were trained on two alternating task components: (1) the presentation of a conditioned stimulus (CS) that predicted the presentation of a rewarding unconditioned stimulus (US); and (2) response-contingent reinforcement. In the second component, subjects acquired money contingent on button clicks. Following response stability, operant response rates were then measured following unexpected reward omission in which the CS was presented alone (no US). An increase in operant responding during reward omission trials (CS alone) compared to standard trials (CS+US) was taken as behavioral evidence for negative urgency. Subjects completed the UPPS prior to the behavioral task to determine individual differences in negative urgency and completed visual analog scales throughout the behavioral task to assess the effects of reward omission on mood. To model negative urgency in laboratory rats, a similar reward omission procedure was employed using a standard two-lever operant conditioning chamber, except that animals earned either palatable food pellets or intravenous amphetamine infusions in the second component.
2. Methods
EXPERIMENT 1
The goal of Experiment 1 was to determine if changes in operant responding following unexpected reward omission is associated with the onset of negative mood states and the personality construct of negative urgency in humans.
2.1 Subjects
Based on an anticipated moderate effect size, 38 nonsmoking males and females between the ages of 18 and 36 were recruited through flyers placed in local newspapers and in the community – mostly students at the University of Kentucky. Subjects were required to complete 2 sessions and could earn $9–$20 per session. Only data from subjects completing both sessions were analyzed.
2.2 Apparatus
The task was presented on a computer with an attached mouse located on a table in front of the subject. Subjects were permitted to adjust the location of the computer and mouse on the table.
2.3 Urgency Task
Subjects came into the laboratory and were instructed to abstain from drug and alcohol use prior to each session; absence of drug use was verified by urine (OnTrack TesTstik Bar, Varian) and breath (Alco-Sensor III, Intoximeters, Inc.) testing; no drug or alcohol use was detected during the study.
Session 1
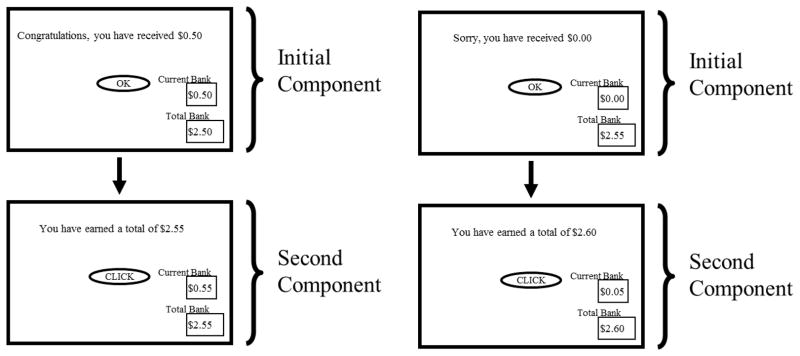
Subjects signed an informed consent form approved by the University of Kentucky Institutional Review Board, completed the UPPS, and were trained to complete the computer task. Subjects then completed the first session consisting of 20 trials, with each trial consisting of two components. In the initial component, cash register sounds signaled an increase of $0.50 on a ‘current trial’ counter and a ‘total session’ counter (Figure 1A). Subjects were required to click a button to acknowledge the initial monetary presentation and initiate the second component of the trial. During the terminal component, subjects could earn additional $0.05 increments on the ‘current trial’ and ‘total session’ counters by clicking a button on a mouse according to a fixed ratio 100 (FR-100) schedule of reinforcement. Time in the terminal component was variable, averaging 2 min. Subjects received text notification and a cash register sound each time they completed a ratio.
Figure 1.
Schematic of (A) reward and (B) omission trial in Experiment 1.
Session 2
The purpose of session 2 was to examine changes in response rate (clicks/sec) following unexpected reward omission. Session 2 consisted of 20 total trials; 18 were identical to those in session 1, while two randomly intermixed omission trials occurred in which the typical $0.50 and accompanying cash register sounds were not presented during the initial component (Figure 1B). The operant component, however, was identical to the reward trials both in session 1 and those randomly intermixed in session 2.
2.4 Statistics
Negative urgency performance on the UPPS was scored on a scale from 1.0–4.0, 1.0 being very low urgency, and 4.0 being very high urgency. All subjects were grouped into either low (1 –1.9; n = 9), medium (2.0 – 2.9; n = 20), or high (3.0 – 4.0; n = 6) urgency based on their UPPS scores to examine differences in demographic information (Table 1). Only behavioral data from session 2 were analyzed. Response rate (mouse clicks/sec) during the second component of trials was measured as a function of trial type and a reward omission effect (i.e., omission trial rate/total trial rate) was calculated. Hierarchical regression was used to determine the association between UPPS scores and the magnitude of the reward omission effect (for detailed description of the scale, see Whiteside and Lynam, 2001; 2003).
Table 1.
Demographic information from Experiment 1 grouped by urgency status.
| High (3–4) | Medium (2–2.9) | Low (1–1.9) | ||
|---|---|---|---|---|
| Male | 11 | 2 | 5 | 4 |
| Age (Mean ± SEM) | 21.46 ± 1.05 | 20.59 ± 0.95 | 22.58± 0.79 | |
| Ethnicity | ||||
| Caucasian | 11 | 2 | 5 | 4 |
| African American | 0 | 0 | 0 | 0 |
| Asian | 0 | 0 | 0 | 0 |
|
| ||||
| Female | 24 | 4 | 15 | 5 |
| Age (Mean ± SEM) | 22.95 ± 0.88 | 21.08 ±1.02 | 20.95 ±0.99 | |
| Ethnicity | ||||
| Caucasian | 16 | 2 | 11 | 3 |
| African American | 5 | 1 | 2 | 2 |
| Asian | 3 | 1 | 2 | 0 |
EXPERIMENT 2A
The goal of Experiment 2A was to determine if the reward omission effect could be modeled in laboratory rats using a variant of the human task with sucrose reward during both components, and to assess the impact of level of motivation on the magnitude of effect. Examination of the reward omission effect under different levels of motivation is important because surprising reward omission induces an aversive, emotional internal state (Papini and Dudley, 1997), and it is important to determine to what extent this state drives the reward omission effect to model negative urgency in rats. While a reward omission effect was demonstrated in rats more than 50 years ago using a runway procedure (Amsel and Roussel, 1952; Amsel and Ward, 1965), that specific procedure is not readily adaptable to humans.
2.5 Subjects
Twelve male rats (250–275 g at the beginning of experimentation) were obtained from Harlan Sprague-Dawley (Indianapolis, IN) and were acclimated to single housing in a colony room held at constant temperature prior to the experiment. Light and dark phases were on a 12:12 hour cycle, and all experimentation occurred in the light phase. Rats were restricted to 20g of rat chow given following their daily sessions, and had unlimited access to water in their home cage. Rats were cared for in accordance with the Institutional Animal Care and Use Committee at the University of Kentucky.
2.6 Apparatus
An operant conditioning chamber (ENV-001; MED Associates, St. Albans, VT) located inside a sound-attenuating chamber was used. Two walls of the operant chamber were made of aluminum, while the side walls were made of Plexiglas. A recessed food tray (5×4.2 cm) was located in the bottom-center of the front wall. A response lever was located on each side of the recessed food tray on the front wall. A 28-V white cue light was located 6 cm above each response lever. A white houselight was mounted in the center of the back wall of the chamber. All responses and scheduled consequences were controlled by a computer interface.
2.7 Procedure
Pretraining
Rats were given 10 sessions of a light-sucrose association. A white key light was illuminated on either the left or right side for 5 sec, followed by delivery of one sucrose pellet. The side of the key light was counterbalanced across rats. Following a 2-sec dark delay, the houselight was then illuminated for 10 sec (an intertrial interval; ITI). Rats were given 32 trials per session.
Operant Training
Following acquisition, rats were given sessions in which sucrose pellets were earned by completing a fixed ratio (FR) response requirement. Two levers were presented, one of which was inactive (no programmed consequence) and the other which was active (resulted in the delivery of one sucrose pellet). The response requirement increased every two sessions from an FR-1, to 3, to 5, to 10. Rats remained in this phase until responding stabilized (within 20 lever presses on the active lever over three sessions on FR-10 response requirement). Rats were given thirty two 2-min trials, separated by a 10-sec ITI.
Baseline Phase
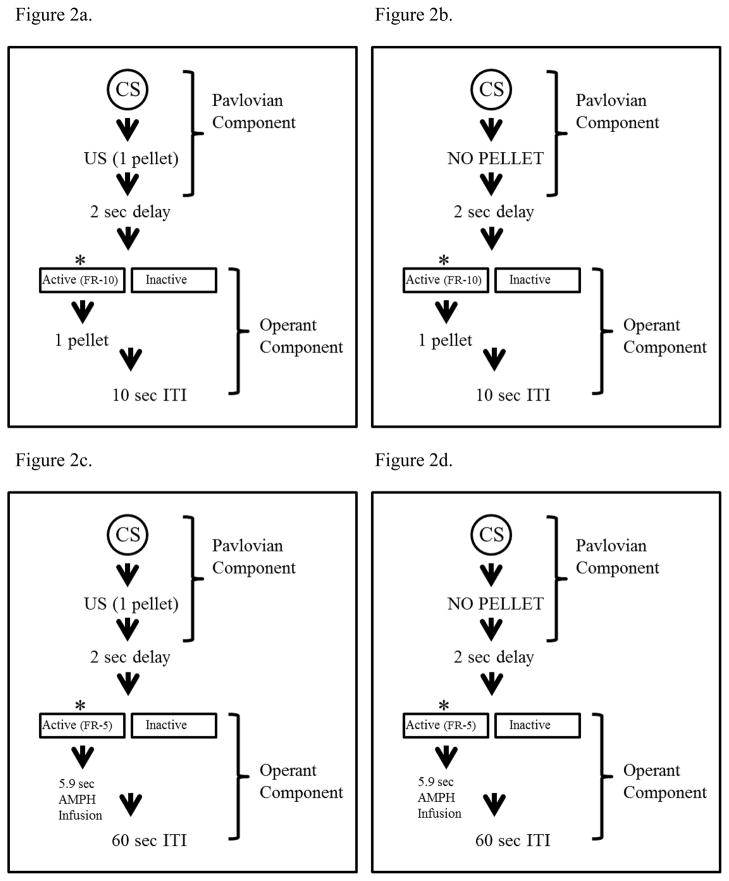
Following operant training, rats were moved to a baseline training phase. Trials began with a component in which a cue light was illuminated for 5 sec, followed by immediate delivery of one sucrose pellet. After the pellet was delivered, a 2-sec dark delay (no cue light) occurred to allow the rat to consume the pellet, and to separate the two components of the trial. Once the 2-sec delay ended, the operant component began. Two levers were presented, the active and inactive levers, for 2 min. Rats completed an FR-10 on the active lever to receive one pellet, and there was no time-out period following reinforcement delivery. Rats could continually complete the FR-10 schedule of reinforcement and receive an additional pellet for each requirement completed within the 2-min operant component. A 10-sec intertrial interval (ITI) then occurred, signaled by illumination of the houselight (Figure 2A). Rats received 32 trials per session. Once rats responded consistently, they were moved into the test phase.
Figure 2.
Schematic of (A) baseline and (B) test trial in Experiment 2A. Schematic of (C) baseline and (D) test trial in Experiment 2B.
Test Phase
Rats received an alternating schedule of training and test sessions, such that four training sessions separated each test session. Test sessions consisted of 24 reward trials and eight omission trials, randomly intermixed. Reward trials were identical to those presented in the baseline phase. Each omission trial was similar to the reward trial (Figure 2B), except no pellet was delivered in the initial Pavlovian component. As before, operant components were 2-min in duration. Rats were given one test session under food restriction conditions, and then were given free access to food in their home cage and allowed to re-stabilize responding in subsequent baseline sessions. Following response stability, rats were given an additional test session.
2.8 Statistics
Only data from the test sessions were analyzed using an ANOVA. Bonferroni-corrected t tests were conducted to further examine differences in response rates. Data from the two trial types were analyzed separately (reward and omission trials).
EXPERIMENT 2B
The goal of Experiment 2B was to determine if the reward omission effect also could be obtained using amphetamine as a reinforcer, rather than sucrose pellets, during the operant component. Two different unit doses were evaluated in separate groups of rats. 2.9
2.9 Subjects
Twenty six different male Sprague-Dawley rats were maintained as during Experiment 2A.
2.10 Apparatus
Similar operant chambers were used as those in Experiment 2A, except a photo beam was located within the food tray to record all head entries, and drug was delivered intravenously through a silastic tube from an infusion pump. All responses and scheduled consequences were recorded and controlled by a computer interface using Med-IV software.
2.11 Drug
d-Amphetamine sulfate was mixed in sterile 0.9% NaCl (saline) and infused intravenously in a volume of 0.1 ml over a 5.9 sec duration.
2.12 Surgery
Methods of implantation and maintenance of indwelling jugular catheters are described previously (Gipson and Bardo, 2009).
2.13 Procedure
Procedures were similar to those of Experiment 2A with the following modifications: (1) amphetamine rather than sucrose was delivered following completion of the response requirement in the operant component; (2) the FR requirement was decreased to 5; (3) the ITI was increased to 60 sec; and (4) the number of omission trials was reduced to two.
Pretraining
Rats were pretrained with a CS (white cue light) – US (sucrose pellet) association, and received 40 presentations of the 5-sec CS followed by delivery of three sucrose pellets. Each food presentation was followed by a 2-sec dark delay. Following this delay, a 60-sec ITI separated each CS. To determine if learning of the Pavlovian contingency occurred, a conditioned response (CR) was measured by recording photo beam breaks caused by head entry into the food tray; data were expressed as the ratio of beam breaks per second during the CS to beam breaks per second during the ITI. A stability criterion of 5:1 ratio of beam breaks/sec during the CS and ITI for three successive sessions was used. Following acquisition, catheters were implanted.
Operant Training
After recovery, rats were trained to lever press up to an FR-5 (1, to 3, to 5) for the delivery of amphetamine (0.03 or 0.1 mg/kg/infusion; n=10 or 8, respectively), cued by illumination of the houselight for 5.9 sec. The saline group (n=8) received training with the 0.03 mg/kg/infusion unit dose of amphetamine during this phase in order to ensure acquisition of lever pressing prior to saline substitution.
Baseline Phase
Rats were then moved into the baseline phase in which they received only reward trials (i.e., no omission trials). Reward trials began with a component in which a cue light was illuminated for 5 sec, followed by immediate delivery of three sucrose pellets and then a 2-sec dark delay (no cue light). Once the 2-sec delay ended, the operant component began. During the operant component, two levers were presented (an active and inactive lever, for two min). Rats completed an FR-5 on the active lever to receive one infusion (amphetamine or saline). Responses on the active lever during the infusion, as well as on the inactive lever, resulted in no programmed consequence. Once the 2-min operant component ended, a 60-sec dark ITI occurred immediately. Rats received 20 trials per session (Figure 2C).
Test Phase
Following response stability (≤ 20% variability over three sessions), rats were moved into the test phase. Rats received an alternating schedule of four training sessions and one test session, for a total of 5 test sessions. Training days consisted of 20 reward trials, whereas test sessions consisted of 15 reward trials (CS+US) and five omission trials (CS alone), randomly intermixed. Each omission trial was similar to a reward trial, except no sucrose pellets were delivered in the initial component (Figure 2D).
2.14 Statistics
Only data from the test sessions were analyzed using an ANOVA. Bonferroni-corrected t tests were conducted to further examine differences in response rates. Data from rats with faulty catheters were excluded from the analysis.
3. Results
3.1 Experiment 1
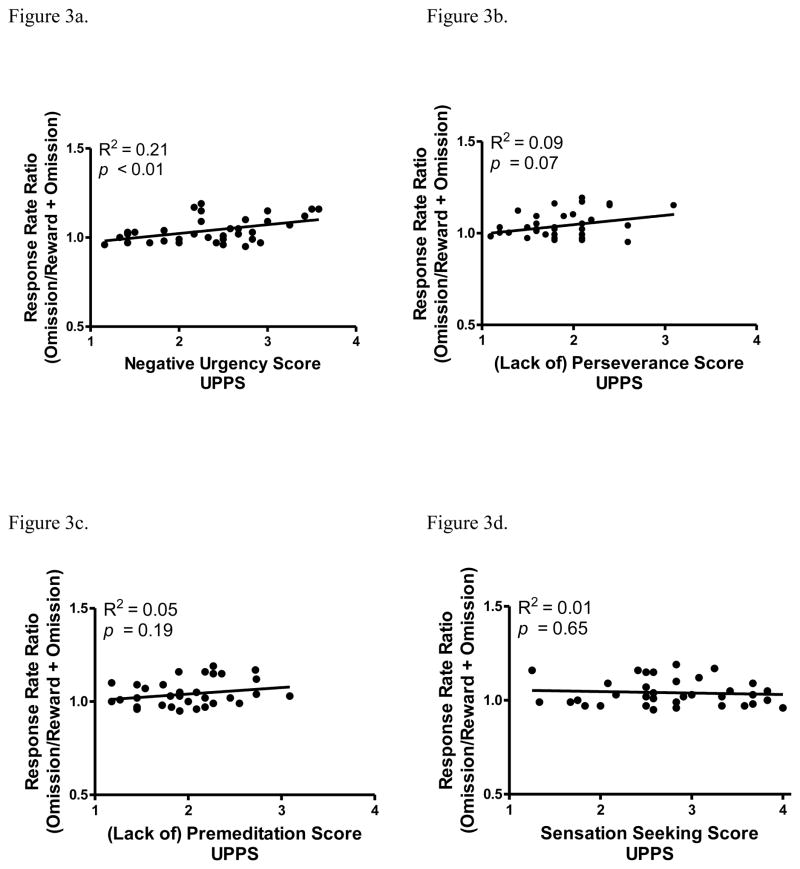
To examine differences in demographic information, humans were grouped according to negative urgency scale scores into high, medium, and low groups (Table 1; high: 3.0 – 4.0; medium: 2.0 – 2.9; low: 1 –1.9). No group differences in age, ethnicity, or gender were found. To examine the relationship between the reward omission effect and facets of impulsivity, linear regression analyses were conducted on each of the UPPS facets (Figures 3A–D). Only negative urgency scores were significantly correlated with the reward omission effect [R2 = 0.21, F(1,33) = 8.37, p < .01]. The (lack of) perseverance scores approached significance [R2 = 0.10, F(1,33) = 3.53, p = 0.07], but neither (lack of) premeditation or sensation seeking scores were related to the reward omission effect. The relation between impulsivity scores and the reward omission effect was further examined using hierarchical regression. When negative urgency was included as the first step, (lack of) perseverance added in the second step, and all four facets included the third predictor, correlations were significant at each step (p < .05).
Figure 3.
Linear regression between the response rate ratio and the UPPS facets (A) urgency, (B) (lack of) perseverance, (C) (lack of) premeditation, and (D) sensation seeking.
3.2 Experiment 2A
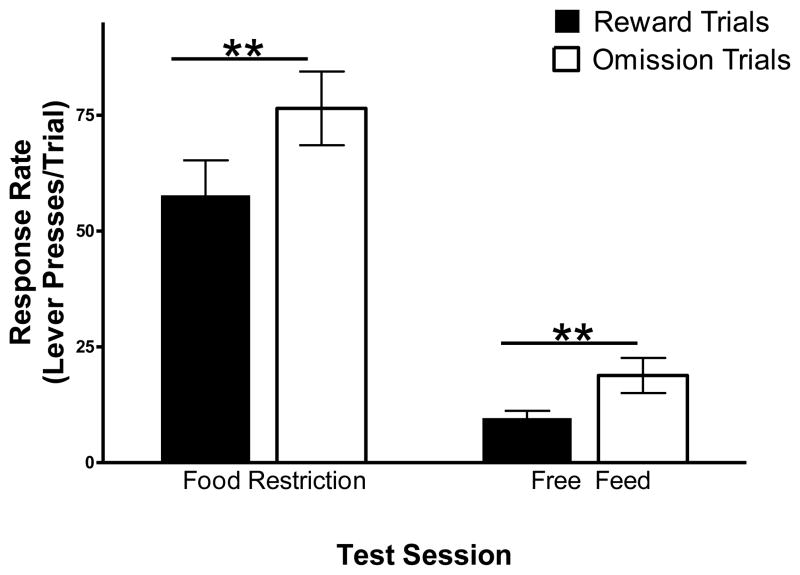
To examine differences in response rates on the two trial types under conditions of food restriction and free feed in rats, a 2 × 2 (condition × trial type) repeated-measures ANOVA was conducted. A significant main effect of condition [F(1,11) = 40.04, p < .0001] and trial type [F(1,11) = 30.43, p < .0001] was found. While response rate was decreased under free feed conditions, operant response rates were significantly increased following unexpected reward omission, regardless of feeding condition (Figure 4).
Figure 4.
Response rate (number of lever presses/trial) for sucrose pellets in Experiment 2A on test sessions in which both baseline and test trials were given under conditions of food restriction and free feed. (**p < .01).
3.3 Experiment 2B
Saline
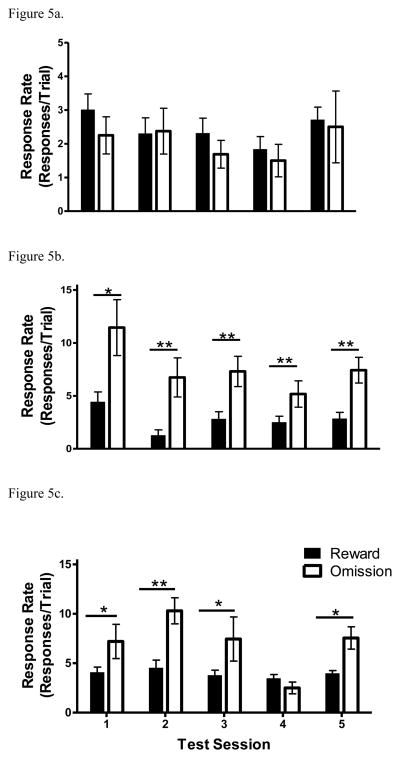
On the final three sessions of acquisition of the Pavlovian association, the rate of head entries observed during the CS was significantly higher than during the ITI [t(11) = 7.85, p < .0001; data not shown], indicating that acquisition was obtained. To examine the effects of unexpected sucrose reward omission on saline self-administration, a 2 × 5 (trial type × test session) repeated-measures ANOVA was conducted. No significant differences in the response rates for saline infusions during reward and omission trials were found; thus, there was no omission effect observed in saline controls (Figure 5A). Furthermore, no differences in saline intake were observed (results not shown).
Figure 5.
Response rate (responses/trial on reward and omission trials during each test session. (A) Results from the saline control group in Experiment 2B; (B) Results from the 0.03 mg/kg/infusion of amphetamine group in Experiment 2B; (C) Results from the 0.1 mg/kg/infusion of amphetamine group in Experiment 2B. (*p < .05; *p < .01).
Amphetamine (0.03 mg/kg/infusion)
Similar to the saline control group, the rate of head entries observed during the CS was significantly higher than during the ITI on the final three sessions of acquisition [t(11) = 7.85, p < .0001; data not shown]. Drug-maintained response rates [F(1,7) = 65.39, p < .0001; Figure 5B] and drug intake [F(1,7) = 136.11, p < .001; data not shown] during omission trials were significantly increased over rates during reward trials.
Amphetamine (0.1 mg/kg/infusion)
Rate of head entries during the CS was significantly higher than during the ITI on the final three sessions of acquisition [t(11) = 7.83, p < .01]. For both drug-maintained response rates (Figure 5C) and drug intake (data not shown), significant main effects of trial type ([F(1,8) = 14.93, p < .01], [F(1,8) = 36.08, p < .001]) and session ([F(4,32) = 3.71, p < .05], [F(1,8) = 5.74, p < .001]), as well as a session × trial interaction ([F(4,32) = 2.82, p < .05], [F(4,32) = 2.99, p < .05]), were observed. On the fourth test session, no difference was found, perhaps due to devaluation of the CS across trials; when rats were further food restricted prior to the fifth test session, there was a re-emergence of omission effect.
4. Discussion
The current study sought to develop a translational behavioral model of negative urgency. Although previous studies have indicated the relevance of performance on impulsivity tasks, such as the go-no go task, to urgency (Billieux et al., 2010), the current study is the first to develop a translational model specifically for individual differences in negative urgency. The model was validated in human volunteers by demonstrating that money-reinforced task performance varied as a function of individual differences in negative urgency, but not other facets of impulsivity measured by the UPPS. This behavioral model was then back-translated to rats using either food or amphetamine reinforcement to support future neurobehavioral mechanistic studies on mood-based rash action.
As a caveat to Experiment 1, it should be noted that the magnitude of the reward omission effect varying as a function of negative urgency in humans was obtained with a relatively small sample size. Thus, it will be important to study the task in a larger sample of adults to further validate the model. Further, more work is needed to establish the discriminative validity of the behavioral task used here by assessing its relation with personality scales beyond the UPPS. An additional limitation to the human and nonhuman tasks is the difference in dependent variable (clicks/second in the human task versus responses/trial in the nonhuman task). Although there are inherent complications in conducting cross-species studies (e.g., heterogeneity in procedural variables associated with the human and nonhuman tasks), it is important to establish both the cross-species generality and construct validity of preclinical animal models of impulsivity in order to effectively examine the role of impulsivity in drug abuse vulnerability under highly controlled conditions. A pre-clinical behavioral model of mood-based rash action may help elucidate the neurobehavioral causes of urgency, and reduce risk in a more effective way.
Although negative urgency is a relatively newly characterized facet of impulsivity, it is correlated strongly with various risky behaviors such as problem substance use, risky sex and eating disorders (Cyders et al., 2009; Doran et al., 2009; Fischer et al., 2004; 2007; Whiteside and Lynam, 2003; Zapolski et al., 2009). The current results from rats indicate that reward omission enhances the rate of amphetamine self-administration. This effect was not specific to amphetamine, however, as response rates for sucrose also increased following reward omission under both food restriction and free feed conditions. These findings extend earlier work using a food-motivated runway task (Amsel and Rossel, 1952; Amsel and Ward, 1965) to an operant task that is more amenable to translation between human and non-human animals. According to Papini and Dudley (1997), unexpected reward omission induces an aversive internal state, and emotional arousal is a critical component of the omission effect. Although the experiments in the current report focused on inducing behavioral invigoration as a result of unexpected reward omission (to model negative urgency), future experimentation should also address the possibility of behavioral invigoration following unexpected reward as a model of positive urgency (acting rashly in response to positive affect). However, Stout et al. (2003) found that the reward omission effect occurs as a result of two independent processes, with rats showing facilitated responding following unexpected reward omission, but suppressed responding following unexpected reward presentation. Thus, this procedure may not be a useful model of positive urgency. Although the current report focused only on negative urgency, findings indicate that reward omission tasks may produce a consistent pattern of results at both the preclinical and clinical levels of analysis, thus furthering our ability to examine the neurobehavioral risk factors involved in substance use and abuse.
In addition, the increase in response rates on omission trials compared to reward trials in both human and nonhuman animals may reflect behavioral contrast in which responding during the presentation of one stimulus is altered by the schedule of reinforcement associated with a different stimulus (Reynolds, 1961a; 1961d). In a multiple schedule, rate of responding increases in one component as a result of a change of schedule in the other component from reinforcement to extinction (Reynolds, 1961a). Frustration occurs following a comparison between expectation of reward value and actual reward value, when the actual value is less than the expected value (Crespi, 1942; Flaherty, 1984). Thus in the current studies, increased responding in the operant terminal component may be due to the decrease in reinforcement in the initial Pavlovian component, indicative of behavioral contrast.
Finally, impulsivity has been measured as both a state (transient behavior) and a trait (personality measure that is stable over time) variable (Odum and Baumann, 2010) and this has caused difficulty in interpretation. Indeed, previous preclinical research has focused on individual differences in impulsivity as a trait variable measured by performance on a delay discounting task (e.g., Perry et al., 2005). There are few behavioral tasks that reliably relate to or predict facets of trait impulsivity, although there are some exceptions (e.g., the Balloon Analogue Risk Task; Lejuez et al., 2002; 2010). Thus, developing behavioral paradigms that better correlate with trait measures of impulsivity may help in the development of effective prevention and treatment programs for drug abuse. In the current experiments, negative urgency was examined as both a state (response rate following reward omission in both humans and rats) and a trait (scores on the UPPS in humans) variable. Thus, a novel finding of the current report is that transient, state-based negative urgency increases drug use preclinically. Future studies are needed to determine if state-based negative urgency increases drug use in humans, as well as to determine if trait negative urgency also predicts drug-taking in rats. It may be especially valuable to study these questions using adolescent subjects, as this population is known to be at maximal risk for expressing risk-related impulsive behavior (Spear, 2000).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amsel A, Roussel J. Motivational properties of frustration: I. Effect on a running response of the addition of frustration to the motivational complex. J Exp Psychol. 1952;43:363–368. doi: 10.1037/h0059393. [DOI] [PubMed] [Google Scholar]
- Amsel A, Ward JS. Frustration and persistence: resistance to discrimination following prior experience with the discriminanda. Psychol Mon. 1965;79 doi: 10.1037/h0093879. [DOI] [PubMed] [Google Scholar]
- Billieux J, Gay P, Rochat L, Van der Linden M. The role of urgency and its underlying psychological mechanisms in problematic behaviours. Behav Res Ther. 2010;48:1085–1096. doi: 10.1016/j.brat.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespi LP. Quantitative variation of incentive and performance in the white rat. Am J Psychol. 1942;55:467–517. [Google Scholar]
- Cyders MA, Flory K, Rainer S, Smith GT. The role of personality dispositions to risky behavior in predicting first year college drinking. Addiction. 2009;104:93–202. doi: 10.1111/j.1360-0443.2008.02434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ESJ, Theobald DEH, Lääne K, Peňa Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran N, Cook J, McChargue D, Spring B. Impulsivity and cigarette craving: differences across subtypes. Psychopharmacology. 2009;207:365–373. doi: 10.1007/s00213-009-1661-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidou D, Pelloux Y, Robbins TW, Dalley JW, Everitt BJ. High impulsivity predicts relapse to cocaine-seeking after punishment-induced abstinence. Biol Psychiatry. 2009;65:851–856. doi: 10.1016/j.biopsych.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ, Eysenck SBG. Manual of the Eysenck Personality Scales (EPS Adult) Hodder and Stoughton; London: 1991. [Google Scholar]
- Flaherty CF. Incentive relativity. Cambridge University Press; New York: 1996. [Google Scholar]
- Fischer S, Anderson KG, Smith GT. Coping with distress by eating or drinking: the role of trait urgency and expectancies. Psychol Addict Behav. 2004;18:269–274. doi: 10.1037/0893-164X.18.3.269. [DOI] [PubMed] [Google Scholar]
- Fisher S, Smith GT, Annus A, Hendricks M. The relationship of neuroticism and urgency to negative consequences of alcohol use in women with bulimic symptoms. Pers Individ Dif. 2007;43:1199–1209. [Google Scholar]
- Gipson CD, Bardo MT. Extended access to amphetamine self-administration increases impulsive choice in a delay discounting task in rats. Psychopharmacology. 2009;207:391–400. doi: 10.1007/s00213-009-1667-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjome KL, Lane SD, Schmitz JM, Green C, Ma L, Prasla I, Swann AC, Moeller FG. Relationship between impulsivity and decision making in cocaine dependence. Psychiatry Res. 2010;178:299–304. doi: 10.1016/j.psychres.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejuez CW, Magidson JF, Mitchell SH, Sinha R, Stevens MC, de Wit H. Behavioral and biological indicators of impulsivity in the development of alcohol use, problems, and disorders. Alcohol Clin Exp Res. 2010;34:1334–1345. doi: 10.1111/j.1530-0277.2010.01217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, Brown R. Evaluation of a behavioral measure of risk taking: the balloon analogue risk task (BART) J Exp Psychol Appl. 2002;8:75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- Odum AL, Baumann AA. Delay discounting: state and trait variable. In: Madden GJ, Bickel WK, editors. Impulsivity: The Behavioral and Neurological Science of Discounting. American Psychological Association; Washington DC: 2010. [Google Scholar]
- Papini MR, Dudley RT. Consequences of surprising reward omissions. Rev Gen Psychol. 1997;1:175–197. [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology. 2008;200:1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology. 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- Reynolds GS. Behavioral contrast. J Exp Anal Behav. 1961a;4:57–71. doi: 10.1901/jeab.1961.4-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds GS. Contrast, generalization, and the process of discrimination. J Exp Anal Behav. 1961d;4:289–294. doi: 10.1901/jeab.1961.4-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B, Ortengren A, Richards JB, de Wit H. Dimensions of impulsive behavior: personality and behavioral measures. Pers Individ Dif. 2006;40:305–315. [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuro Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stout SC, Boughner RL, Papini MR. Reexamining the frustration effect in rats: of surprising reinforcement and nonreinforcement. Learn Motiv. 2003;34:437–456. [Google Scholar]
- Tellegen A. Multidimensional Personality Questionnaire manual. University of Minnesota Press; Minneapolis, MN: 1982. [Google Scholar]
- Whiteside SP, Lynam DR. The Five Factor Model and impulsivity: using a structural model of personality to understand impulsivity. Pers Individ Dif. 2001;30:669–689. [Google Scholar]
- Whiteside S, Lynam D. Understanding the role of impulsivity and externalizing psychopathology in alcohol abuse. Applications of the UPPS Impulsive Behavior Scale. Exp Clin Psychopharmacology. 2003;11:210–217. doi: 10.1037/1064-1297.11.3.210. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Olaussen P, Taylor JR, Jentsch JD. Insight into the relationship between impulsivity and substance abuse from studies using animal models. Alcohol Clin Exp Res. 2010;34:1–13. doi: 10.1111/j.1530-0277.2010.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapolski TCB, Cyders MA, Smith GT. Positive urgency predicts illegal drug use and risky sexual behavior. Psychol Addict Behav. 2009;23:348–354. doi: 10.1037/a0014684. [DOI] [PMC free article] [PubMed] [Google Scholar]