Abstract
Although self-reported gambling urge intensities have clinical utility in the treatment of pathological gambling (PG), prior studies have not investigated their neural correlates. Functional magnetic resonance imaging (fMRI) was conducted while 10 men with PG and 11 control comparison (CON) men viewed videotaped scenarios of gambling, happy or sad content. Participants rated the intensity of their emotions and motivations and reported the qualities of their responses. Relative to the CON group, the PG group reported similar responses to sad and happy scenarios, but stronger emotional responses and gambling urges when viewing the gambling scenarios. Correlations between self-reported responses and brain activations were typically strongest during the period of reported onset of emotional/motivational response and more robust in PG than in CON subjects for all conditions. During this epoch, corresponding with conscious awareness of an emotional/motivational response, subjective ratings of gambling urges in the PG group were negatively correlated with medial prefrontal cortex activation and positively correlated with middle temporal gyrus and temporal pole activations. Sadness ratings in the PG group correlated positively with activation of the medial orbitofrontal cortex, middle temporal gyrus, and retrosplenial cortex, while self-reported happiness during the happy videos demonstrated largely inverse correlations with activations in the temporal poles. Brain areas identified in the PG subjects have been implicated in explicit, self-referential processing and episodic memory. The findings demonstrate different patterns of correlations between subjective measures of emotions and motivations in PG and CON subjects when viewing material of corresponding content, suggesting in PG alterations in the neural correlates underlying experiential aspects of affective processing.
Keywords: Emotion, fMRI, Gambling urges, Motivation, Temporal pole
Introduction
Pathological gambling (PG), categorized as an impulse control disorder, may be considered a disorder of misdirected motivation in which gambling-related stimuli have prominent saliency (Potenza et al. 2003b; Zack and Poulos 2004). Consistently, individuals with PG choose to gamble excessively despite adverse consequences. Emotional factors may contribute to engagement in gambling among individuals with PG. For example, PG frequently co-occurs with depression (Desai et al. 2007; Petry et al. 2005), gambling to escape dysphoria is represented in the inclusionary criteria for PG (APA 2004), and shared genetic contributions exist for PG and major depression (Potenza et al. 2005). Thus, an improved understanding of the biological processes underlying emotional and motivational states in PG is clinically important.
Gambling urges may contribute importantly to maintenance and relapse in PG (Frost et al. 2001; Ledgerwood and Petry 2006; Oei and Gordon 2008). Self-reported gambling urges have been related to treatment outcome. For example, PG patients, particularly those with moderate to high gambling urges at treatment onset, demonstrate significant symptom improvement with the opioid antagonist naltrexone, and gambling urge intensity has been positively associated with treatment outcome amongst individuals receiving the opioid antagonists naltrexone or nalmefene (Grant et al. 2008; Kim et al. 2001). Given the clinical significance of self-reported gambling urges, an improved understanding of how the neural underpinnings of gambling urges relate to self-reported intensities is clinically relevant. Few neuroimaging studies in PG have directly examined gambling urges (reviewed in Potenza 2008). A recent study by Goudriaan and colleagues (2010) found a correlation between subjective reports of gambling urges in a PG group with activity in the ventrolateral prefrontal cortex, left insula and caudate. However, studies examining gambling urges and cue reactivity in PG have yielded mixed results, potentially due to the use of different paradigms, as well as the inclusion of diverse groups with PG (van Holst et al. 2010). Gambling urges represent a persistent and complex phenomena that may include conscious as well as unconscious factors. There has been little investigation into stages of processing of gambling stimuli as they relate to gambling urges and further, there is little understanding how these may compare to the experience of other emotions.
A prior functional magnetic resonance imaging (fMRI) study from our group identified neural correlates of gambling urges and sad and happy emotional states in men with PG as compared to control (CON) subjects without PG (Potenza et al. 2003b). In this study, different temporal epochs of motivational and emotional processing were associated with between-group differences in regional brain activations. Three specific periods of tape-viewing were studied with respect to brain activation patterns: (1) the initial period of tape-viewing prior to the onset of subjective response, as compared to the pre-tape baseline; (2) the period following onset of emotional/motivational response as compared to the immediately preceding period of tape-viewing; and (3) the final period of tape-viewing as compared to post-tape baseline. The initial period of viewing of the gambling scenarios (a period involving the presentations of general “triggers” that have been linked to the initiation of gambling urges) identified the most robust between-group differences. Multiple cortical, basal ganglionic and thalamic brain regions showed relatively diminished activation in the PG group as compared to the CON group. This finding contrasted with those of cue provocation studies in obsessive compulsive disorder in which relatively increased activation of these regions has been reported (Breiter and Rauch 1996). Additionally, with respect to brain activations when viewing the gambling tapes, different patterns of brain activation differences were observed for the sad and happy tapes in which fewer between-group differences were observed in regional activation patterns. In the final period of viewing of the gambling scenarios (when the most robust gambling stimuli were presented), PG subjects showed relatively diminished activation of the ventromedial prefrontal cortex (vmPFC). This brain region had previously been implicated in risk/reward decision-making (Bechara 2003) and impulsive aggression (New et al. 2002; Siever et al. 1999) and has subsequently been implicated as showing relatively diminished activation in PG subjects during fMRI tasks involving cognitive control, simulated gambling, and decision-making (Potenza et al. 2003a; Reuter et al. 2005; Tanabe et al. 2007).
Here we extend our prior study by analyzing these data with respect to participants’ subjective responses. Given data on neural circuits involved in the retrieval of emotional memories (Buchanan 2007), we hypothesized that activation of brain regions involved in emotional memory retrieval (e.g., the temporal gyrus, particularly the temporal pole and parahippocampal areas) would correlate with subjective reports of motivational and emotional responses to the gambling, sad and happy videotapes, and that PG subjects in particular would show strong correlations between subjective responses and regional brain activations during the viewing of the gambling and sad scenarios. We further hypothesized that these associations would be particularly robust during the epoch of motivational and emotional processing linked to the subjective onset of motivational and emotional response, given the relevance of this epoch to subjective emotional and motivational state awareness.
Materials and Methods
Participants
Participants consisted of 10 men who met criteria for PG and 11 healthy control (CON) subjects. All were right-handed native English speakers between the ages of 18 and 65 years. Detailed demographic and assessment information on participants are described elsewhere (Potenza et al. 2003b). With the exception of nicotine dependence, participants in the PG group had no other current comorbid conditions. However, several individuals met criteria for past disorders (≥3 months) including two for marijuana abuse, one for alcohol and cocaine abuse, one for alcohol abuse and major depression, one for alcohol abuse and cocaine dependence and one for alcohol dependence and cocaine dependence (Potenza et al. 2003b). All control participants reported having gambled, and all PG participants reported current/past-year gambling.
Measures
South Oaks Gambling Screen (SOGS): the SOGS is a 16-item questionnaire used to assess to identify probable individuals with PG with good reliability and validity (Lesieur and Blume 1987; Stinchfield 2002). A Kolmogorov–Smirnov Test established that the distribution of self-report scores for each video type were normally distributed within each experimental group, with the exception of the CON group’s gambling urge responses to a gambling video to which participants provided responses of ‘0’. In order to gauge changes in gambling urge intensities before and after imaging, participants also completed a Gambling Urge Questionnaire (GUQ)—a modified version of the Alcohol Urge Questionnaire (AUQ)—prior to and following the imaging. This has been used in our prior studies (Potenza et al. 2003a, b). A similar version, the GUS, or Gambling Urge Scale (Raylu and Oei 2004), with nearly identical items based on the AUQ, has since been shown to have good predictive, concurrent and criterion-related validity. Although our version of the questionnaire was largely used as a precautionary measure, it is employed here in exploratory analyses with other subjective measures.
Experimental Task
The fMRI paradigm has been described previously (Potenza et al. 2003b; Wexler et al. 2001). Participants viewed two tapes each related to happy, sad and gambling scenarios in which young actors depicted each specific scene. The sad scenarios described a parental divorce and a loved one’s death, the happy scenarios depicted a wedding and a surprise visit from a loved one, while the gambling scenarios described scenes of casino gambling. Specifically, each gambling scenario began with a perceived stressor and expressed frustration followed by free time and the receipt of unexpected money. The actor then described going to a casino and the excitement and feeling of gambling. The sad and happy scenarios acted both as experimental and active control conditions for the gambling scenarios. Following the viewing of each tape type, participants described the quality of their emotional response (e.g., sadness, happiness) and rated them on a scale of 0 (no response) to 10 (intense emotional response). Similarly, participants rated their motivational response (i.e. urge to gamble) on a scale of 0 (no response) to 10 (intense motivational response). Participants rested for 2.5 min between videotapes. Participants viewed happy, sad or gambling scenarios in a counterbalanced order, with each scenario being approximately 4 min long.
Forty-five seconds of gray-screen exposure prior to and following each scenario provided imaging baselines. While viewing the scenarios, participants pushed a button to indicate the onset of emotional or motivational responses. This procedure allows for a more clear delineation of the epochs related to individual emotional experience. A schematic representation of the experimental timeline is depicted in Fig. 1.
Fig. 1.
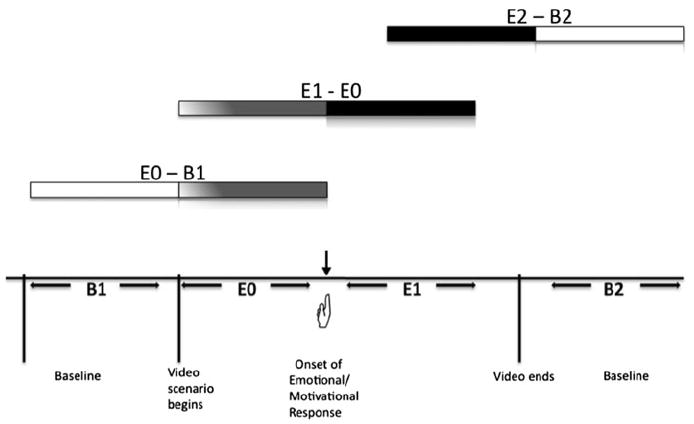
Schematic representation of time epoch comparisons for the study. B1 represents the first baseline; upon viewing of a the video, a button press by the individual indicates the onset of an emotional or motivational response (
 ); E0 represents the epoch prior to the button press; E1 represents the epoch immediately following the button press; E2 represents the final period of the video viewing. Horizontal bar lines represent comparisons between different epochs
); E0 represents the epoch prior to the button press; E1 represents the epoch immediately following the button press; E2 represents the final period of the video viewing. Horizontal bar lines represent comparisons between different epochs
Image Acquisition
Images were obtained using a 1.5-T MRI system equipped with an echoplanar imaging system (GE Signa; GE Medical Systems, Milwaukee, Wis), a standard quadrature head coil, and a T2*-sensitive gradient-recalled, single-shot, echoplanar pulse sequence (Potenza et al. 2003b; Wexler et al. 2001; Wexler 1998). Conventional T1-weighted spin-echo sagittal anatomic images (echo time, 11 ms; repetition time, 667 ms; field of view, 24; slice thickness, 5 mm; 256 × 128 × 1, number of excitations) were obtained first for slice localization. Next, 12 T1-weighted oblique-axial slices (echo time, 14 ms; repetition time, 500 ms; field of view, 20 × 20 cm; 256 × 192 × 1, number of excitations) were obtained parallel to the plane transecting the anterior and posterior commissures, covering the entire brain, to serve as underlays for functional images acquired at the same locations. Functional images were obtained using a single-shot, echo-planar-gradient echo sequence (repetition time, 1,500 ms; echo time, 60 ms; flip angle, 60°; matrix, 64 × 64; field of view, 20 × 20 cm; slice thickness, 8 mm; skip, 1 mm; number of images per slice, 240) at the same locations.
Data Analysis
Data were motion corrected for 3 translational directions and 3 possible rotations (Friston et al. 1995). Runs with motion in excess of 1.5 mm of displacement and 2 of rotation were rejected; as a result 2 runs of the Happy condition and 3 runs of the gambling condition were excluded in the control group and in the PG group 1 run of the Happy condition and 3 runs of the sad condition were excluded. Corrected images were spatially filtered using a Gaussian filter with a full-width half-maximum of 6.5 mm.
Changes in the echoplanar imaging signal were evaluated in 3 pairs of successive epochs (Potenza et al. 2003b; Wexler et al. 2001). The first comparison (E0–B1) represents differences from baseline (B1) to the period of scenario-viewing prior to the onset of an emotional response (E0) up to the first 45 s of viewing. The second comparison (E1–E0) contrasts the period following a subjective emotional response (E1) to the immediately preceding period of tape viewing prior to subjective emotional response (E0). The third comparison (E2–B2) is between the final 45 s of scenario viewing independent of emotional response (E2) to the final gray-screen baseline period after scenario viewing (B2). In this way, the E0–B1 comparison can be considered as representing neural correlates associated with exposure to gambling stimuli prior to the onset of an emotional or motivational response; the E1–E0 comparison corresponds with the brain activity associated with the onset of a conscious emotional or motivational response; lastly, the E2–B2 contrast represents the final period of emotional/motivational processing, during which there may be a culmination of emotional/motivational response.
Data were analyzed using MATLAB and the Yale fMRI Bioimage Suite analysis package (Potenza et al. 2003b; Skudlarski et al. 1999; Wexler et al. 2001). Correlations between brain activity and emotional motivational responses were computed at each voxel for the 10 slices in Talairach space from z = −12 to z = 50 (Shaywitz et al. 1999; Skudlarski et al. 1999; Wexler et al. 2001). Signal arising at the lower part of the brain was evaluated in all cases to ensure that it was greater than 75% of the signal from cortical regions in more uniform areas. Only voxels belonging to a contiguous set of 25 voxels, each meeting the specified significance threshold, were included in the maps.
Data were analyzed using MATLAB and the Yale fMRI analysis package (Skudlarski et al. 1999). For each subject, separate percent signal change maps of 3 pairs of successive epochs were calculated separately for gambling, sad and happy tapes. Images for each subject were transformed into common stereotactic space by piece-wise linear warping (Friston et al. 1995; Talairach and Tournoux 1988). The percent signal change maps from individual subjects were the derived measure of task-related activity used to conduct voxel-based (whole brain) correlations. These correlations were designed to examine associations between brain activation (as assessed by blood oxygen level dependent (BOLD) signal change) and ratings of subjective gambling urge or emotional responses. As participants viewed two of each videotape type (i.e. two gambling, two sad, and two happy videos), correlations were computed from the mean of each participants’ two subjective ratings with the mean percent signal change brain activation maps associated with each epoch.
The significance threshold for the voxel-based whole brain correlation analysis was set at r = 0.7 [uncorrected, extent threshold of 25 voxels, as has been done previously (Blumberg et al. 2003)]. The conjoint use of extent-based and voxel-based thresholds generates a more stringent significance threshold, as described elsewhere (Friston et al. 1995).
Results
Subjective Responses
As reported elsewhere (Potenza et al.2003b), there were no significant differences in the quality or magnitude of emotional responses to sad (P = .81) or happy (P = .56) scenarios between the PG and CON groups. Individuals with PG reported significantly greater intensities of emotional responses (P = .03) and gambling urges (P < .001) when viewing gambling scenarios than did those without PG (Potenza et al. 2003b). The quality of emotional responses appeared largely similar between experimental groups during the sad or happy scenarios. During the gambling scenarios, the PG group reported excitement, aggravation and desires to gamble, while the CON group frequently reported emotions of annoyance, pity and frustration.
Relationship Between Emotional/Motivational Responses and SOGS and GUQ Scores
In the PG group, Pearson correlation coefficients were calculated between SOGS scores and the video types, revealing a positive relationship between scores on this measure and gambling emotion reported following the gambling tape viewing (R = 0.64; P < 0.05). In the PG group, SOGS scores were not correlated with subjective responses to the sad or happy scenarios or the gambling urge ratings during gambling tape viewing (P > 0.05). In the PG group, mean scores of gambling urges on the GUQ prior to scanning were significantly greater than in the control group (P < 0.001). In the PG group, Pearson correlation coefficients were calculated between the GUQ and the video types, revealing a positive relationship between scores on this measure (prior to scanning) and gambling motivations reported following the tape viewing (R = 0.90; P < 0.001). There was also a relationship between the GUQ and gambling emotion ratings (R = 0.80; P < 0.01). There were no significant correlations between the GUQ and subjective responses to the sad or happy scenarios or with scores on the SOGS (P > 0.05).
E0–B1 Comparison
Sad Emotion
PG Group
During the initial period of viewing as compared to pre-tape baseline and prior to the reported onset of emotional/motivational response, subjective intensities of sadness in the PG group during viewing of the sad scenarios were positively correlated with activation of the left ventral prefrontal cortex, corresponding with Brodmann’s Area 4, and negatively correlated with activation of the right ventral medial prefrontal cortex and right cerebellum (Table 1). No correlations reached significance at r > 0.7 for PG subjects during this epoch for the happy and gambling scenarios, and none reached this threshold for the CON subjects for any of the tapes.
Table 1.
E0–B1 Subjective reports of motivational/emotional correlations with Talairach coordinates (x,y,z) of regional brain activity
| Group | r-Value | Brain region | x | y | z | BA | Size (mmˆ3) | Size (voxel) | Radius |
|---|---|---|---|---|---|---|---|---|---|
| A. Positive correlations | |||||||||
| Sad emotion | |||||||||
| PG | 0.76** | L Inferior frontal gyrus | −41 | 26 | −1 | 45 | 3,987 | 134 | 7.87 |
| HC | No correlations at 0.7 level | ||||||||
| B. Negative Correlations | |||||||||
| Sad Emotion | |||||||||
| PG | −0.75** | R Ventral medial prefrontal cortex | 16 | 37 | −5 | – | 3,005 | 101 | 9.45 |
| −0.75** | R Cerebellum | 17 | −49 | −16 | – | 1,339 | 45 | 5.08 | |
| −0.75** | L Cerebellum | −29 | −55 | −16 | – | 1,190 | 40 | 4.82 | |
| HC | No correlations at 0.7 level | ||||||||
BA Brodmann’s area
r < 0.7
P ≤ 0.01;
P ≤ 0.05
CON Group
No correlations reached significance at the r > 0.7 threshold for the CON subjects for any of the tapes.
E1–E0 Comparison
Gambling Urge
PG Group
Amongst PG subjects, subjective intensities of gambling urges correlated positively with activation in the temporal poles (bilateral), medial temporal gyrus and medial occipital gyrus and inversely with activation in left dorsal medial frontal cortex (Table 2; Fig. 2a).
Table 2.
E1–E0 subjective reports of motivational/emotional correlations with Talairach coordinates (x,y,z) of regional brain activity
| Group | r-Value | Brain region | x | y | z | BA | Size (mmˆ3) | Size (voxel) | Radius |
|---|---|---|---|---|---|---|---|---|---|
| A. Positive correlations | |||||||||
| Gambling motivation | |||||||||
| PG | 0.83** | L Middle temporal gyrus, medial occipital gyrus | −42 | −69 | 14 | 37, 39 | 1,398 | 47 | 5.43 |
| 0.75** | R Temporal pole | 38 | −1 | −16 | 38, 21 | 1,696 | 57 | 5.79 | |
| 0.73* | L Temporal pole | −40 | 4 | −16 | 21, 38 | 2,351 | 79 | 6.86 | |
| HC | 0.80** | R Radiatio optica | 30 | −68 | 14 | – | 744 | 25 | 3.84 |
| Gambling emotion | |||||||||
| PG | 0.76** | L Temporal pole | −39 | 8 | −18 | 21, 38 | 4,076 | 137 | 10.47 |
| 0.75** | R Temporal pole | 39 | 5 | −16 | 21, 38 | 1,517 | 51 | 5.23 | |
| HC | No correlations at 0.7 level | ||||||||
| Sad emotion | |||||||||
| PG | 0.87* | R Medial temporal gyrus | 49 | −63 | 5 | 37 | 3,302 | 111 | 8.02 |
| 0.86* | Medial temporal gyrus | −48 | −33 | −16 | 20,21 | 2,142 | 72 | 6.53 | |
| 0.82* | R Precentral gyrus | 56 | −15 | 38 | 4, 6 | 2,320 | 78 | 7.60 | |
| 0.82* | L Superior, medial frontal gyrus | −12 | 45 | 41 | 8 | 3,154 | 106 | 9.71 | |
| 0.81* | Medial precuneus | −1 | −68 | 23 | 31 | 1,190 | 40 | 4.96 | |
| 0.81* | R Inferior frontal gyrus | 51 | 29 | 14 | 46 | 893 | 30 | 5.90 | |
| 0.78 | Subthalamic nucleus | −48 | 12 | −11 | −5 | 893 | 30 | 4.15 | |
| 0.76 | Radiatio optica | −26 | −68 | 23 | – | 1,636 | 55 | 5.40 | |
| 0.72 | Hippocampus, parahippocampal gyrus | 8 | −46 | 0 | – | 18,893 | 635 | 24.26 | |
| 0.70 | Orbitofrontal cortex | 0 | 25 | 8 | – | 20,976 | 705 | 20.69 | |
| 0.70 | Precuneus | 13 | −42 | 50 | – | 2,529 | 85 | 8.31 | |
| HC | No correlations at 0.7 level | ||||||||
| Happy emotion | |||||||||
| PG | No positive correlations at 0.7 level | ||||||||
| HC | 0.77* | R Precuneus/cuneus | 0 | −63 | 14 | 31 | 922 | 31 | 4.33 |
| B. Negative correlations | |||||||||
| Gambling motivation | |||||||||
| PG | −0.80 | L Medial frontal gyrus | −5 | 32 | 41 | 8 | 1,488 | 50 | 5.12 |
| HC | No correlations at 0.7 level | ||||||||
| Happy emotion | |||||||||
| PG | −0.88** | Cingulate gyrus | −5 | 24 | 41 | 32, 8 | 833 | 28 | 3.81 |
| −0.85** | Fusiform gyrus | −32 | 39 | −16 | 37 | 744 | 25 | 3.68 | |
| −0.84** | L Medial frontal gyrus | −9 | 58 | −5 | 10 | 922 | 31 | 4.12 | |
| −0.82** | R Temporal pole | 44 | 0 | −5 | 22 | 833 | 28 | 3.94 | |
| −0.82** | L Middle temporal gyrus | −58 | −14 | −5 | 21 | 1,428 | 48 | 5.35 | |
| −0.80* | R Parahippocampal gyrus | 28 | −44 | −3 | – | 2,499 | 84 | 9.67 | |
| −0.78* | L Superior frontal gyrus | −8 | 57 | 14 | 10 | 2,351 | 79 | 8.9 | |
| HC | No negative correlations at 0.7 level | ||||||||
BA Brodmann’s area
r = 0.7
P ≤ 0.01;
P ≤ 0.05
Fig. 2.
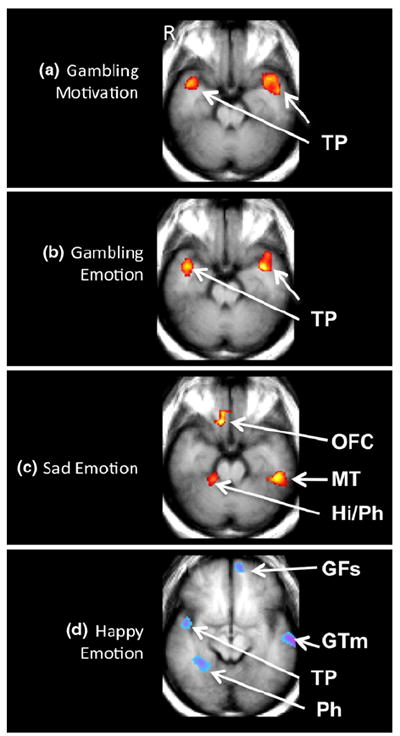
Functional magnetic resonance image correlational map of pathological gambling (PG) subjects (n = 10) after the reported onset of emotional or motivational response (E1–E0) during viewing of the Gambling (a, b), Sad (c), and Happy (d) scenarios. a Brain activation maps (z = −12) show positive correlations between the intensity of subjective reports of gambling motivations and bilateral temporal pole (TP) activity. b Brain activation maps (z = −12) show positive correlations between the intensity of subjective reports of gambling emotions and bilateral TP activity. c Brain activation map (z = −12) shows correlations between the intensity of subjective reports of sadness and brain activity in the orbitofrontal cortex (OFC), the right hippocampus (Hi) and parahippocampal gyrus (Ph) and the left medial temporal gyrus (MT) during viewing of the sad scenarios. d Brain activation map (z = −4) for the happy scenario show negative correlations between the intensity of subjective reports of happiness and brain activity in the left superior frontal gyrus (GFs), the left middle temporal gyrus (GTm), the right TP and the right Ph. Map displays correlations >0.7, with an extent threshold of 25 voxels. Blue/purple color indicates areas displaying inverse correlations and red/yellow color indicates areas displaying positive correlations. The left side of the brain is displayed on the right side of each image
CON Group
The CON group did not display any correlations at r > 0.7.
Gambling Emotion
PG Group
Intensities of gambling-related emotion, subjectively reported as excitement, aggravation and desire to gamble in the PG group, were positively correlated with bilateral temporal pole activation (Fig. 2b).
CON Group
CON subjects displayed no significant correlations at the 0.7 level.
Sad Emotion
PG Group
The largest number of brain regions showing significant correlations with subjective responses in the PG group was evidenced during the E1–E0 comparison. Subjective intensities of sadness in the PG group positively correlated with activation of the orbitofrontal cortex, medial temporal gyrus, precentral gyrus, precuneus, parahippocampal gyrus and the superior occipital gyrus (Fig. 2c).
CON Group
There were no significant correlations in CON subjects at the 0.7 level.
Happy Emotion
PG Group
In the PG group, subjective intensities of responses during viewing of the happy tape were negatively correlated with activation of the frontal, cingulate, parahippocampal, fusiform, and medial temporal gyri and the temporal pole (Fig. 2d).
CON Group
In the CON group, subjective intensities of responses during viewing of the happy tape positively correlated with bilateral cingulate activation.
E2–B2 Comparison
Happy Emotion
PG Group
During the final period of tape-viewing as compared to post-tape baseline, the PG group during the happy scenarios demonstrated negative correlations between subjective intensities of emotional responses and activation of the right superior and temporal gyrus and right medial frontal gyrus (Table 3). There were no correlations significant at r > 0.7 between the self-reported emotion and neural activations for the PG in the gambling and sad scenarios.
Table 3.
E2–B2 subjective reports of motivational/emotional correlations with Talairach coordinates (x,y,z) of regional brain activity
| Group | r-Value | Brain region | x | y | z | BA | Size (mmˆ3) | Size (voxel) | Radius |
|---|---|---|---|---|---|---|---|---|---|
| Negative correlations | |||||||||
| Happy emotion | |||||||||
| PG | −0.84** | R superior temporal gyrus | 59 | −26 | 14 | 42, 22 | 1,666 | 56 | 5.55 |
| −0.75** | R medial frontal gyrus | 7 | 54 | 5 | 10 | 1,577 | 53 | 5.56 | |
| HC | No correlations at 0.7 level | ||||||||
BA Brodmann’s area
r < 0.7
P ≤ 0.01;
P ≤ 0.05
CON Group
There were no correlations significant at r > 0.7 between the self-reported emotion and neural activations for the CON group for any scenario.
Discussion
This study is the first to directly examine the relationship between subjective intensities of gambling urges and emotional states and regional brain activations in PG as compared to non-PG subjects. Consistent with our hypotheses, activations within brain regions involved with emotional memory processing correlated with subjective reports of motivational and emotional responses. Furthermore, these responses to the videotapes showed one pattern of correlations in PG subjects and a different pattern in CON subjects. Also consistent with our hypotheses, subjective intensities correlated with activations in brain regions previously implicated in the retrieval and processing of emotional memories, particularly amongst the PG subjects with respect to the sad and gambling conditions. In addition, the most robust correlations were observed during the E1–E0 epoch, corresponding with the subjective onset of motivational and emotional responses. The biological and clinical implications of the findings are discussed in relation to the three experimental time epochs.
E0–B1
Neural Correlates of Sadness
The PG group demonstrated significant positive correlations between self-reported sadness and activations in the left ventral prefrontal cortex and inverse correlations with activations in the right ventromedial prefrontal cortex (vmPFC) and the right cerebellum. The vmPFC is implicated in coding for the affective significance of stimuli even when this is processed implicitly (Damasio 1994; Elliott et al. 2000). The role of the cerebellum in affective and executive processing is increasingly being noted as this area has significant projections through the thalamus to the cingulate and parahippocampal and prefrontal cortices (Bellebaum and Daum 2007; Lane et al. 1997; Malhi et al. 2007; Schmahmann 1996). Activation of these areas while viewing the sad scenarios prior to explicit report may signal differential sensitivity to negative cues. This observed pattern in PG but not CON subjects, seen prior to the conscious awareness of a negative emotion, suggests pathophysiological differences in affective processing that may reflect overlap between PG and other disorders (e.g., depression) involving affective dysregulation (Potenza et al. 2005).
E1–E0
Neural Correlates of Gambling Urges
The E1–E0 comparison contrasts the period of tape viewing immediately following subjective awareness of motivational or emotional response to the immediately preceding one, thereby representing the shift of attention from external to internal cues. Gambling urges in pathological gamblers recruited brain regions implicated in the retrieval and processing of emotion, including the temporal pole, medial prefrontal cortex and middle temporal gyrus; control participants did not show gambling-urge-related correlations at the same threshold (Gusnard et al. 2001; Kilts et al. 2001; Maguire and Mummery 1999).
Notably, gambling urge intensities were positively associated with temporal pole activations bilaterally (Fig. 2a), as well as with left middle temporal gyral activation. In addition to gambling urges, subjective reports of emotional responses in PG subjects were also associated with bilateral temporal pole activation (Fig. 2b). The similarities in neural correlation patterns between the motivational and emotional responses may reflect correlations between these measures as the most frequently reported emotional/motivational response in the PG group was ‘gambling’.
A role involving sensory-emotional linkage has been proposed for the temporal pole (Olson et al. 2007). The robust bilateral temporal pole association with gambling urges may relate to the affective saliency of addiction-related cues in the gambling film. Temporal pole activity is associated not only with episodic memory, but also more specifically with autobiographical memories (Maguire and Mummery 1999; Piefke et al. 2003). In particular, recent, positive, self-referential memories are linked with bilateral activation of the temporal poles, as well as the orbitofrontal cortex (OFC) and medial temporal lobe (Piefke et al. 2003). Therefore, although the videos viewed by participants were not autobiographical, temporal pole activation may reflect personally relevant information presented in the gambling videos or the retrieval of personally relevant emotional memories. Although the correlation of activation within this brain region with subjective reports of gambling urges is consistent with such a role, other possible explanations include the processing of acoustic stimuli as bilateral temporal pole activation has been observed during speech comprehension (Giraud et al. 2004).
The temporal pole has considerable and distinct reciprocal connections to the orbital and ventromedial prefrontal networks, as well as widespread projections to the amygdala (Kondo et al. 2005; Stefanacci et al. 1996). Connections to caudal OFC areas originate from the same temporal regions projecting to the amygdala, supporting the idea of this triadic network forming an anatomical substrate coding for emotionally significant events (Ghashghaei and Barbas 2002). Strong connections between the amygdala and temporal pole suggest an important role in long-term memory, whereby higher-order sensory signals are linked to an emotional context (Hoistad and Barbas 2008).
Gambling urges were also related to medial prefrontal cortex (MPFC) activity, specifically in an inverse fashion with left MPFC activity the PG group. The MPFC is implicated in performance monitoring, particularly in error-detection and response conflict (Ridderinkhof et al. 2004). Moderate doses of alcohol decrease activity in this area and produce deficits in error-monitoring (Ridderinkhof et al. 2002). In contrast, individuals with obsessive–compulsive disorder (OCD) demonstrate hyperactivity in this frontal-subcortical circuitry (Baxter et al. 1992; Ursu et al. 2003; Yucel et al. 2007), potentially producing a faulty increase in error-signaling that generates the need for corrective actions (Pitman 1987). In the current study, the inverse correlation between MPFC activity and subjective gambling urges may be related to a diminished ability to flexibly monitor and control behavior. Explicit representations of self-relevance, mindfulness, as well as the attribution of mental states to others also recruit MPFC activity (Castelli et al. 2000; Creswell et al. 2007; Gusnard et al. 2001). This brain area has been ascribed a role in the default mode of brain operation, in particular with the processing of embodied representations of the self (Raichle et al. 2001). The inverse correlation seen here between MPFC activation and self-reported gambling urges in PG is consistent with a role for the MPFC in monitoring personal affect and attention, and the notion that an accurate representation of internal state is necessary for adaptive decision-making (Damasio 1994). Although the data are consistent with such an explanation, it is also possible that negative correlations might reflect inattention or indifference in participants in response to the stimuli.
Gambling urges in PG subjects also positively correlated with activation in the left middle temporal gyrus, corresponding to Brodmann’s area 21. Kilts and colleagues (Kilts et al. 2001) reported inverse correlations between craving in cocaine-addicted individuals and left middle temporal gyral activation. Differences in the direction of the relationship (i.e. positive vs. negative) between our study and theirs may reflect differences in experimental paradigms or between the disorders, relating, for example, to cocaine influences on cortical structure and function (Beveridge et al. 2008). Nonetheless, the observation that function within this region relates to craving states in a “ behavioral” and drug addiction suggests that middle temporal gyral function may represent a target for treatment development across addictions.
Similar to Crockford et al. (2005), we found that gambling urges in the PG group correlated with left medial occipital gyrus activation. Visual cortex involvement may therefore be related to the sustained attention given by the PG group to gambling-related stimuli. Similar selective activation of the occipital cortex during drug-related cues has been observed in cocaine-dependent individuals (Grant et al. 1996).
While the current study identified brain areas implicated in emotional memory, correlations of gambling urges with activations in mesolimbic structures (ventral striatum, OFC, including ventromedial prefrontal cortex (vmPFC)) were largely not observed. Prior analysis of the data from the same cohort found that relatively diminished activation of the ventral striatum and OFC was observed in PG as compared to control subjects during viewing of the gambling scenarios, similar to activations when cocaine dependent subjects (as compared to control subjects) viewed cocaine scenarios (Potenza 2008). Although this brain area is frequently implicated in addiction studies, the relationship between OFC activation and craving is relatively inconsistently observed. For example, only one-third of studies report OFC signal changes with drug craving (Dom et al. 2005; Wilson et al. 2004). The current study examined conscious appraisal of motivational and emotional responses at specific points in time; it is possible that other brain areas (e.g., temporal cortices) may more closely link to cognizant aspects of these processes, such as emotionally or motivationally salient events (Wang et al. 1999). Mesolimbic regions may also be involved, albeit less directly or robustly; examination of the correlations between subjective gambling urges in the PG group during viewing of the gambling tapes and the fMRI responses at r > 0.6 revealed signal increases in the vmPFC, corresponding with Brodmann’s area 10. As such, and consistent with prior studies (Potenza et al. 2003a; Reuter et al. 2005; Tanabe et al. 2007), vmPFC appears relevant to responses to gambling cues, albeit not as robustly related to subjective responses as are some other brain regions.
There was also no signal change in the amygdala and medial temporal lobe during urge states. Given the instrumental role of the amygdaloid complex and medial temporal lobe structures in conditioned reinforcement, one might expect urge-related activations in these areas. Autobiographical memories, rather than nonautobiographical ones, may produce greater medial temporal lobe activity (Fink et al. 1996) and controlling for arousal and attention-grabbing effects may also preclude amygdala activity (Kilts et al. 2001). Alterations in striatal dopamine release in individuals with PG, may further influence striatal projection areas (Steeves et al. 2009). Therefore, the absence of a relationship between subjective urges and striatal activity may be due to the contrast methodology used in the current study which may not detect similar neural events occurring during both the E0 and the E1 epochs. That is, the methodology used in the current experiment may highlight most those brain changes associated with conscious urges. Additionally, the development of compulsive aspects of addiction away from voluntary control has been neurobiologically described as a shift from limbic to associative and sensorimotor corticostriatal circuits (Chambers et al. 2003; Everitt and Robbins 2005). Therefore it is perhaps not surprising that the relationship observed with gambling urges in the PG group were those involved in emotional memory and representations of self-relevance.
Neural Correlates of Sadness
During the E1–E0 comparison in the PG group subjective reports of sadness were related to middle temporal gyrus activation, suggesting an overlap in neural circuitry involved in sadness and in gambling urges. Other correlations in this group included the vmPFC (or more specifically the medial OFC), more dorsal regions of MPFC and the precuneus—areas that have previously been implicated in both positive and negative affect-induction (Habel et al. 2005; Malhi et al. 2007; Posner et al. 2009). In addition, sadness ratings correlated with left parahippocampal gyrus activity, an area connected to retrosplenial areas and often implicated in affect induction and memory (Fink et al. 1996; Lane et al. 1997; Vandekerckhove et al. 2005). Altogether, the correlations in the PG group during the E1–E0 epoch overlap considerably with bilateral activations reported in autobiographical memory processing (Steinvorth et al. 2006).
Happiness
The findings from happy epochs are also consistent with activation profiles associated with autobiographical, episodic memory. CON, but not PG, participant happiness ratings were positively correlated with right cuneus and precuneus activity. Similar correlations in the right posterior cingulate and precuneus are evidenced in opiate dependent subjects during happiness as well as during craving states (Sell et al. 2000). The activation patterns manifested in the CON participants may therefore pertain to explicit retrieval of happy memories (Shallice et al. 1994). In the PG group, however, happiness ratings were inversely associated with signal changes in the superior and medial frontal gyrus and temporal gyrus on the left side and the temporal pole on the right side. These results further support the idea of overlapping, but differentially activated, neural circuitry involved in the processing of gambling and happy scenarios. An inverse correlation with parahippocampal activation was also evident on the right side (Fig. 2d). These areas constitute a network of frontal-temporal-extended limbic areas implicated in affect induction and episodic memory (Jatzko et al. 2006; Lane et al. 1997; Vandekerckhove et al. 2005). The similarity in activations to those produced by autobiographical memories may represent a correspondence of these scenarios to previously experienced contexts (Vandekerckhove et al. 2005).
E2–B2
Happiness
During this last epoch (E2–B2), the PG group demonstrated inverse correlations between subjective responses and activations in the right superior temporal and medial frontal gyri during the happy scenario. This is consistent with findings from the previous epoch comparison (E1–E0), recruiting areas implicated in episodic memory.
Strengths, Limitations and Future Directions
The findings of the current study have multiple limitations including a small sample comprised exclusively of men, which limits generalizability to women who may show different patterns of brain activations associated with subjective reports of motivational and emotional states (McClernon et al. 2008). Other individual differences besides gender have also been shown to influence neural activations. For example, genetic characteristics have been associated with brain responses to emotional stimuli (Hariri et al. 2002), and future studies involving larger samples should investigate the possible influences of commonly occurring allelic variants. Methodologically, the fMRI environment presents another drawback given extraneous noises and the dark, confined space. While we were able to elicit gambling urges in the PG participants in this environment, it is unclear how this precisely would translate to real-life settings. In addition, it is possible that the PG and CON groups may differ in their baseline activations in the ROIs. However, the design of the current study, consisting of comparisons between the successive epochs, does not address this possibility. Future studies using other imaging techniques [e.g., arterial spin labelling (Rao et al. 2010)] could be used to investigate this possibility directly.
Although the current study employed a significance threshold of 0.7 for the correlational analyses and a conjoint cluster level threshold of 25 contiguous voxels (more stringent than used in some prior studies (Blumberg et al. 2003), Type I errors are possible due to the relatively lenient stringency threshold and number of comparisons in the current, preliminary study. Given the preliminary nature of the current study, we have tried to maintain a balance between both Type 1 and Type II errors. The initial data therefore attempt to highlight not only the most robust effects, but also more subtle neural responses that may warrant further investigation. Future studies with larger samples are necessary to examine further the relationships between subjective responses and neural activations in PG subjects, including how other features (e.g., co-occurring disorders) might influence these processes.
Single-item Likert-type rating of subjective urges to gamble presents another drawback. Urges represent a complex construct, and a single self-reported item may provide relatively little specificity or information relating domains involving cognitive, behavioral or psychophysiological processes (Blumberg et al. 2003). While the ratings of subjective urges to gamble correlated closely with the reports of gambling emotions in the PG group, we did not systematically examine further the self-reported psychological experience of the individual, nor how such measures may relate to actual behavior. Similarly, it is possible that the explicit questions about emotion and gambling urges may have brought greater attention to these feelings and potentially have influenced subjective ratings. Some convergent validity, however, is suggested by the correlation between subjective urges to gamble following viewing of the videos and responses on the GUQ (administered prior to scanning). Urges following exposure to gambling videos may therefore not only relate to immediate subjective state, but also to some stable propensities over time. Nonetheless, the single, subjective measure used to examine gambling urges following video presentation is limited in providing information on the accurate introspective abilities of the participant and on how this conscious experience relates to actual gambling intentions and behaviors. Future studies should examine directly the relationship between both subjective and neural responses to gambling and long-term outcomes.
It is also possible that specific affective states may play a role in contributing to the gambling urges in the PG group. This group consisted of men free of current comorbid conditions. However, past diagnoses of substance dependence or mood disorders may have influenced their susceptibility to gambling cues. This prospect can be addressed in future research with a larger and a more clinically diverse sample and including the use of substance dependent control populations. The use of structured measures of mood states, such as the Beck Depression Inventory, in future research could further examine the relationship between gambling urges and mood states. Indeed, future studies should incorporate clinical measures of depression or affective states, not only because of the high rates of comorbidity and shared genetic contributions to PG and major depression (Desai et al. 2007; Petry et al. 2005; Potenza et al. 2005), but also because of the close relationship observed between affective and gambling urge states in other PG studies (Romer Thomsen et al. 2009). Furthermore, emotion/motivation ratings could be collected immediately prior to and during viewing of the videotapes, in order to control for baseline differences and more clearly parcel out pre-existing versus experimentally-induced effects.
Another important factor to consider may be individual differences in access to gambling. Access to gambling out of the scanner may influence responses to cue exposure; although participants were informed as to the duration of the scanning procedure, gambling access was not directly assessed.
Conclusion
Prior experiments have not systematically examined the neural correlates of emotional and motivational states as related to self-reported responses in PG subjects. Given the relationship between self-reported gambling urges and clinical outcomes in treatment (Grant et al. 2008; Kim et al. 2001), these findings suggest neural targets for medication development in PG. Brain imaging techniques might help better define specific brain regions or circuits that are functioning differently in individuals with specific psychiatric disorders. This understanding could be used to investigate how therapies work, and whether different therapies (e.g., specific behavioral and pharmacological ones) might work in complementary or overlapping fashions (Brewer et al. 2008; Brody et al. 2004; Paulus et al. 2005). Additionally, individual differences in brain structure and function may exist such that individuals with similar subjective reports may display different patterns of brain activations. For example, PG and CON subjects may demonstrate similar subjective responses to sad scenarios but show differences in brain responses to sad stimuli. Such information could be relevant to developing improved treatment strategies for individuals with co-occurring PG and depression.
These results provide preliminary evidence that specific neural substrates of emotional and motivational responses are related to the magnitudes of subjective responses and provide further evidence of motivational and affective dysregulation in PG. Our results are consistent in implicating brain areas related to explicit as well as self-referential processing, similar to findings in drug addiction where the recall and imagery of autobiographical drug-related memories may in part account for the increased salience attribution and response disinhibition that characterize the disorder (Goldstein and Volkow 2002). Given the important role of urges in precipitating relapse in addictive disorders, future studies should further examine the interplay of these neural substrates and their role in the maintenance of gambling behaviors amongst individuals with PG.
Acknowledgments
The authors would like to thank Bruce Wexler and Todd Constable for their helpful feedback and suggestions. This research was supported by NIH grants R01 DA019039, RL1 AA017539, RL5 DA024858, the VA VISN1 MIRECC and REAP, UL1-DE19586 and the NIH Roadmap for Medical Research/Common Fund. This research was funded in part by a grant from the National Center for Responsible Gaming and its Institute for Research on Gambling Disorders.
Footnotes
Conflict of interest All authors reported no conflict of interest in the content of this paper. Dr. Potenza has received financial support or compensation for the following: Dr. Potenza consults for and is an advisor to Boehringer Ingelheim; has financial interests in Somaxon; has received research support from the National Institutes of Health, Veteran’s Administration, Mohegan Sun Casino, the National Center for Responsible Gaming and its affiliated Institute for Research on Gambling Disorders, and Forest Laboratories pharmaceuticals; has participated in surveys, mailings or telephone consultations related to drug addiction, impulse control disorders or other health topics; has consulted for law offices on issues related to addictions or impulse control disorders; has provided clinical care in the Connecticut Department of Mental Health and Addiction Services Problem Gambling Services Program; has performed grant reviews for the National Institutes of Health and other agencies; has guest-edited journal sections; has given academic lectures in grand rounds, CME events and other clinical or scientific venues; and has generated books or book chapters for publishers of mental health texts.
Contributor Information
Iris M. Balodis, Department of Psychiatry, Yale University School of Medicine, 1 Church Street, Rm 731, New Haven, CT, USA iris.balodis@yale.edu
Cheryl M. Lacadie, Diagnostic Radiology, Yale University School of Medicine, New Haven, CT, USA
Marc N. Potenza, Department of Psychiatry, Yale University School of Medicine, 1 Church Street, Rm 726, New Haven, CT, USA Child Study Center, Yale University School of Medicine, New Haven, CT, USA.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, D.C: American Psychiatric Press, Inc; 2004. Text Revision. [Google Scholar]
- Baxter LR, Jr, Schwartz JM, Bergman KS, Szuba MP, Guze BH, Mazziotta JC, et al. Caudate glucose metabolic rate changes with both drug and behavior therapy for obsessive-compulsive disorder. Archives of General Psychiatry. 1992;49(9):681–689. doi: 10.1001/archpsyc.1992.01820090009002. [DOI] [PubMed] [Google Scholar]
- Bechara A. Risky business: Emotion, decision-making, and addiction. Journal of Gambling Studies. 2003;19(1):23–51. doi: 10.1023/a:1021223113233. [DOI] [PubMed] [Google Scholar]
- Bellebaum C, Daum I. Cerebellar involvement in executive control. Cerebellum. 2007;6(3):184–192. doi: 10.1080/14734220601169707. [DOI] [PubMed] [Google Scholar]
- Beveridge TJ, Gill KE, Hanlon CA, Porrino LJ. Review. Parallel studies of cocaine-related neural and cognitive impairment in humans and monkeys. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2008;363(1507):3257–3266. doi: 10.1098/rstb.2008.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg HP, Leung HC, Skudlarski P, Lacadie CM, Fredericks CA, Harris BC, et al. A functional magnetic resonance imaging study of bipolar disorder: State- and trait-related dysfunction in ventral prefrontal cortices. Archives of General Psychiatry. 2003;60(6):601–609. doi: 10.1001/archpsyc.60.6.601. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Rauch SL. Functional MRI and the study of OCD: From symptom provocation to cognitive-behavioral probes of cortico-striatal systems and the amygdala. Neuroimage. 1996;4(3 Pt 3):S127–S138. doi: 10.1006/nimg.1996.0063. [DOI] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. Pretreatment brain activation during stroop task is associated with outcomes in cocaine-dependent patients. Biological Psychiatry. 2008;64(11):998–1004. doi: 10.1016/j.biopsych.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Lee G, Smith E, Sadeghi M, Saxena S, et al. Attenuation of cue-induced cigarette craving and anterior cingulate cortex activation in bupropion-treated smokers: A preliminary study. Psychiatry Research. 2004;130(3):269–281. doi: 10.1016/j.pscychresns.2003.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan TW. Retrieval of emotional memories. Psychological Bulletin. 2007;133(5):761–779. doi: 10.1037/0033-2909.133.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli F, Happe F, Frith U, Frith C. Movement and mind: A functional imaging study of perception and interpretation of complex intentional movement patterns. Neuroimage. 2000;12(3):314–325. doi: 10.1006/nimg.2000.0612. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: A critical period of addiction vulnerability. American Journal of Psychiatry. 2003;160(6):1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creswell JD, Way BM, Eisenberger NI, Lieberman MD. Neural correlates of dispositional mindfulness during affect labeling. Psychosomatic Medicine. 2007;69(6):560–565. doi: 10.1097/PSY.0b013e3180f6171f. [DOI] [PubMed] [Google Scholar]
- Crockford DN, Goodyear B, Edwards J, Quickfall J, el-Guebaly N. Cue-induced brain activity in pathological gamblers. Biological Psychiatry. 2005;58(10):787–795. doi: 10.1016/j.biopsych.2005.04.037. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Descartes’ error. New York, NY: Grosset/Putnam, G P Putnam’s Sons; 1994. [Google Scholar]
- de Ruiter MB, Veltman DJ, Goudriaan AE, Oosterlaan J, Sjoerds Z, van den Brink W. Response perseveration and ventral prefrontal sensitivity to reward and punishment in male problem gamblers and smokers. Neuropsychopharmacology. 2009;34(4):1027–1038. doi: 10.1038/npp.2008.175. [DOI] [PubMed] [Google Scholar]
- Desai RA, Desai MM, Potenza MN. Gambling, health and age: Data from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychology of Addictive Behaviors. 2007;21(4):431–440. doi: 10.1037/0893-164X.21.4.431. [DOI] [PubMed] [Google Scholar]
- Dom G, Sabbe B, Hulstijn W, van den Brink W. Substance use disorders and the orbitofrontal cortex: Systematic review of behavioural decision-making and neuroimaging studies. British Journal of Psychiatry. 2005;187:209–220. doi: 10.1192/bjp.187.3.209. [DOI] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ, Frith CD. Dissociable functions in the medial and lateral orbitofrontal cortex: Evidence from human neuroimaging studies. Cerebral Cortex. 2000;10(3):308–317. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nature Neuroscience. 2005;8(11):1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fink GR, Markowitsch HJ, Reinkemeier M, Bruckbauer T, Kessler J, Heiss WD. Cerebral representation of one’s own past: Neural networks involved in autobiographical memory. Journal of Neuroscience. 1996;16(13):4275–4282. doi: 10.1523/JNEUROSCI.16-13-04275.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline J-B, Heather JD, Frackowiak RSJ. Spatial registration and normalization of images. Human Brain Mapping. 1995;3:165–189. [Google Scholar]
- Frost RO, Meagher BM, Riskind JH. Obsessive-compulsive features in pathological lottery and scratch-ticket gamblers. Journal of Gambling Studies. 2001;17(1):5–19. doi: 10.1023/a:1016636214258. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H. Pathways for emotion: Interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115(4):1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Giraud AL, Kell C, Thierfelder C, Sterzer P, Russ MO, Preibisch C, et al. Contributions of sensory input, auditory search and verbal comprehension to cortical activity during speech processing. Cerebral Cortex. 2004;14(3):247–255. doi: 10.1093/cercor/bhg124. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. American Journal of Psychiatry. 2002;159(10):1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudriaan AE, de Ruiter MB, van den Brink W, Oosterlaan J, Veltman DJ. Brain activation patterns associated with cue reactivity and craving in abstinent problem gamblers, heavy smokers and healthy controls: an fMRI study. Addiction Biology. 2010;15:491–503. doi: 10.1111/j.1369-1600.2010.00242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JE, Kim SW, Hollander E, Potenza MN. Predicting response to opiate antagonists and placebo in the treatment of pathological gambling. Psychopharmacology (Berl) 2008;200(4):521–527. doi: 10.1007/s00213-008-1235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, et al. Activation of memory circuits during cue-elicited cocaine craving. Proceedings of the National Academy of Sciences of the Unites States of America. 1996;93(21):12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proceedings of the National Academy of Sciences of the Unites States of America. 2001;98(7):4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habel U, Klein M, Kellermann T, Shah NJ, Schneider F. Same or different? Neural correlates of happy and sad mood in healthy males. Neuroimage. 2005;26(1):206–214. doi: 10.1016/j.neuroimage.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297(5580):400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Hoistad M, Barbas H. Sequence of information processing for emotions through pathways linking temporal and insular cortices with the amygdala. Neuroimage. 2008;40(3):1016–1033. doi: 10.1016/j.neuroimage.2007.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jatzko A, Schmitt A, Demirakca T, Weimer E, Braus DF. Disturbance in the neural circuitry underlying positive emotional processing in post-traumatic stress disorder (PTSD). An fMRI study. European Archives of Psychiatry and Clinical Neuroscience. 2006;256(2):112–114. doi: 10.1007/s00406-005-0617-3. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, et al. Neural activity related to drug craving in cocaine addiction. Archives of General Psychiatry. 2001;58(4):334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Kim SW, Grant JE, Adson DE, Shin YC. Double-blind naltrexone and placebo comparison study in the treatment of pathological gambling. Biological Psychiatry. 2001;49(11):914–921. doi: 10.1016/s0006-3223(01)01079-4. [DOI] [PubMed] [Google Scholar]
- Kondo H, Saleem KS, Price JL. Differential connections of the perirhinal and parahippocampal cortex with the orbital and medial prefrontal networks in macaque monkeys. Journal of Comparative Neurology. 2005;493(4):479–509. doi: 10.1002/cne.20796. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Bradley MM, Lang PJ, Ahern GL, Davidson RJ, et al. Neuroanatomical correlates of pleasant and unpleasant emotion. Neuropsychologia. 1997;35(11):1437–1444. doi: 10.1016/s0028-3932(97)00070-5. [DOI] [PubMed] [Google Scholar]
- Ledgerwood DM, Petry NM. What do we know about relapse in pathological gambling? Clinical Psychology Review. 2006;26(2):216–228. doi: 10.1016/j.cpr.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Lesieur HR, Blume SB. The South Oaks Gambling Screen (SOGS): A new instrument for the identification of pathological gamblers. American Journal of Psychiatry. 1987;144(9):1184–1188. doi: 10.1176/ajp.144.9.1184. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Mummery CJ. Differential modulation of a common memory retrieval network revealed by positron emission tomography. Hippocampus. 1999;9(1):54–61. doi: 10.1002/(SICI)1098-1063(1999)9:1<54::AID-HIPO6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Malhi GS, Lagopoulos J, Owen AM, Ivanovski B, Shnier R, Sachdev P. Reduced activation to implicit affect induction in euthymic bipolar patients: An fMRI study. Journal of Affective Disorders. 2007;97(1–3):109–122. doi: 10.1016/j.jad.2006.06.005. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Kozink RV, Rose JE. Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsychopharmacology. 2008;33:2148–2157. doi: 10.1038/sj.npp.1301618. [DOI] [PubMed] [Google Scholar]
- New AS, Hazlett EA, Buchsbaum MS, Goodman M, Reynolds D, Mitropoulou V, et al. Blunted prefrontal cortical 18fluorodeoxyglucose positron emission tomography response to meta-chlorophenylpiperazine in impulsive aggression. Archives of General Psychiatry. 2002;59(7):621–629. doi: 10.1001/archpsyc.59.7.621. [DOI] [PubMed] [Google Scholar]
- Oei TP, Gordon LM. Psychosocial factors related to gambling abstinence and relapse in members of gamblers anonymous. Journal of Gambling Studies. 2008;24(1):91–105. doi: 10.1007/s10899-007-9071-7. [DOI] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y. The enigmatic temporal pole: A review of findings on social and emotional processing. Brain. 2007;130(Pt 7):1718–1731. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Archives of General Psychiatry. 2005;62(7):761–768. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- Petry NM, Stinson FS, Grant BF. Comorbidity of DSM-IV pathological gambling and other psychiatric disorders: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Clinical Psychiatry. 2005;66(5):564–574. doi: 10.4088/jcp.v66n0504. [DOI] [PubMed] [Google Scholar]
- Piefke M, Weiss PH, Zilles K, Markowitsch HJ, Fink GR. Differential remoteness and emotional tone modulate the neural correlates of autobiographical memory. Brain. 2003;126(Pt 3):650–668. doi: 10.1093/brain/awg064. [DOI] [PubMed] [Google Scholar]
- Pitman RK. A cybernetic model of obsessive-compulsive psychopathology. Comprehensive Psychiatry. 1987;28(4):334–343. doi: 10.1016/0010-440x(87)90070-8. [DOI] [PubMed] [Google Scholar]
- Posner J, Russell JA, Gerber A, Gorman D, Colibazzi T, Yu S, et al. The neurophysiological bases of emotion: An fMRI study of the affective circumplex using emotion-denoting words. Human Brain Mapping. 2009;30:883–895. doi: 10.1002/hbm.20553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN. The neurobiology of pathological gambling and drug addiction: An overview and new findings. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2008;363(1507):3181–3189. doi: 10.1098/rstb.2008.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN, Leung HC, Blumberg HP, Peterson BS, Fulbright RK, Lacadie CM, et al. An FMRI Stroop task study of ventromedial prefrontal cortical function in pathological gamblers. American Journal of Psychiatry. 2003a;160(11):1990–1994. doi: 10.1176/appi.ajp.160.11.1990. [DOI] [PubMed] [Google Scholar]
- Potenza MN, Steinberg MA, Skudlarski P, Fulbright RK, Lacadie CM, Wilber MK, et al. Gambling urges in pathological gambling: A functional magnetic resonance imaging study. Archives of General Psychiatry. 2003b;60(8):828–836. doi: 10.1001/archpsyc.60.8.828. [DOI] [PubMed] [Google Scholar]
- Potenza MN, Xian H, Shah K, Scherrer JF, Eisen SA. Shared genetic contributions to pathological gambling and major depression in men. Archives of General Psychiatry. 2005;62(9):1015–1021. doi: 10.1001/archpsyc.62.9.1015. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao H, Mamikonyan E, Detre JA, Siderowf AD, Stern MB, Potenza MN, et al. Decreased ventral striatal activity with impulse control disorders in Parkinson’s disease. Movement Disorders. 2010;25:1660–1669. doi: 10.1002/mds.23147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raylu N, Oei TP. The gambling urge scale: development, confirmatory factor validation, and psychometric properties. Psychology of Addictive Behaviors. 2004;18:100–105. doi: 10.1037/0893-164X.18.2.100. [DOI] [PubMed] [Google Scholar]
- Reuter J, Raedler T, Rose M, Hand I, Glascher J, Buchel C. Pathological gambling is linked to reduced activation of the mesolimbic reward system. Nature Neuroscience. 2005;8(2):147–148. doi: 10.1038/nn1378. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, de Vlugt Y, Bramlage A, Spaan M, Elton M, Snel J, et al. Alcohol consumption impairs detection of performance errors in mediofrontal cortex. Science. 2002;298(5601):2209–2211. doi: 10.1126/science.1076929. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306(5695):443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Romer Thomsen K, Callesen MB, Linnet J, Kringelbach ML, Moller A. Severity of gambling is associated with severity of depressive symptoms in pathological gamblers. Behavioural Pharmacology. 2009;20(5–6):527–536. doi: 10.1097/FBP.0b013e3283305e7a. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. From movement to thought: Anatomic substrates of the cerebellar contribution to cognitive processing. Human Brain Mapping. 1996;4:174–198. doi: 10.1002/(SICI)1097-0193(1996)4:3<174::AID-HBM3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Sell LA, Morris JS, Bearn J, Frackowiak RS, Friston KJ, Dolan RJ. Neural responses associated with cue evoked emotional states and heroin in opiate addicts. Drug and Alcohol Dependence. 2000;60(2):207–216. doi: 10.1016/s0376-8716(99)00158-1. [DOI] [PubMed] [Google Scholar]
- Shallice T, Fletcher P, Frith CD, Grasby P, Frackowiak RS, Dolan RJ. Brain regions associated with acquisition and retrieval of verbal episodic memory. Nature. 1994;368(6472):633–635. doi: 10.1038/368633a0. [DOI] [PubMed] [Google Scholar]
- Shaywitz S, Shaywitz BA, Pugh KR, Fulbright RK, Skudlarski P, Mencl WE, et al. Effect of estrogen on brain activation patterns in postmenopausal women during working memory tasks. JAMA. 1999;281:1197–1202. doi: 10.1001/jama.281.13.1197. [DOI] [PubMed] [Google Scholar]
- Siever LJ, Buchsbaum MS, New AS, Spiegel-Cohen J, Wei T, Hazlett EA, et al. d, l-fenfluramine response in impulsive personality disorder assessed with [18F]fluorodeoxyglucose positron emission tomography. Neuropsychopharmacology. 1999;20(5):413–423. doi: 10.1016/S0893-133X(98)00111-0. [DOI] [PubMed] [Google Scholar]
- Skudlarski P, Constable RT, Gore JC. ROC analysis of statistical methods used in functional MRI: Individual subjects. NeuroImage. 1999;9(3):311–329. doi: 10.1006/nimg.1999.0402. [DOI] [PubMed] [Google Scholar]
- Steeves TD, Miyasaki J, Zurowski M, Lang AE, Pellecchia G, Van Eimeren T, et al. Increased striatal dopamine release in Parkinsonian patients with pathological gambling: A [11C] raclopride PET study. Brain. 2009;132(Pt 5):1376–1385. doi: 10.1093/brain/awp054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanacci L, Suzuki WA, Amaral DG. Organization of connections between the amygdaloid complex and the perirhinal and parahippocampal cortices in macaque monkeys. Journal of Comparative Neurology. 1996;375(4):552–582. doi: 10.1002/(SICI)1096-9861(19961125)375:4<552::AID-CNE2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Steinvorth S, Corkin S, Halgren E. Ecphory of autobiographical memories: An fMRI study of recent and remote memory retrieval. Neuroimage. 2006;30(1):285–298. doi: 10.1016/j.neuroimage.2005.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchfield R. Youth gambling: How big a problem? Psychiatric Annals. 2002;32:197–202. [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme Medical; New York: 1988. [Google Scholar]
- Tanabe J, Thompson L, Claus E, Dalwani M, Hutchison K, Banich MT. Prefrontal cortex activity is reduced in gambling and nongambling substance users during decision-making. Human Brain Mapping. 2007;28(12):1276–1286. doi: 10.1002/hbm.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursu S, Stenger VA, Shear MK, Jones MR, Carter CS. Overactive action monitoring in obsessive-compulsive disorder: Evidence from functional magnetic resonance imaging. Psychological Science. 2003;14(4):347–353. doi: 10.1111/1467-9280.24411. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove MM, Markowitsch HJ, Mertens M, Woermann FG. Bi-hemispheric engagement in the retrieval of autobiographical episodes. Behavioural Neurology. 2005;16(4):203–210. doi: 10.1155/2005/460745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Holst RJ, van den Brink W, Veltman DJ, Goudriaan AE. Why gamblers fail to win: A review of cognitive and neuroimaging findings in pathological gambling. Neuroscience and Biobehavioral Reviews. 2010;34:87–107. doi: 10.1016/j.neubiorev.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Cervany P, Hitzemann RJ, Pappas NR, et al. Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Science. 1999;64(9):775–784. doi: 10.1016/s0024-3205(98)00619-5. [DOI] [PubMed] [Google Scholar]
- Wexler BE, Gottschalk CH, Fulbright RK, Prohovnik I, Lacadie CM, Rounsaville BJ, et al. Functional magnetic resonance imaging of cocaine craving. American Journal of Psychiatry. 2001;158(1):86–95. doi: 10.1176/appi.ajp.158.1.86. [DOI] [PubMed] [Google Scholar]
- Wexler BE, Stevens AA, Bowers AA, Sernyak MJ, Goldman-Rakic PS. Word and tone working memory deficits in schizophrenia. Archives of General Psychiatry. 1998;55(12):1093–1096. doi: 10.1001/archpsyc.55.12.1093. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: A neurocognitive analysis. Nature Neuroscience. 2004;7(3):211–214. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel M, Harrison BJ, Wood SJ, Fornito A, Wellard RM, Pujol J, et al. Functional and biochemical alterations of the medial frontal cortex in obsessive-compulsive disorder. Archives of General Psychiatry. 2007;64(8):946–955. doi: 10.1001/archpsyc.64.8.946. [DOI] [PubMed] [Google Scholar]
- Zack M, Poulos CX. Amphetamine primes motivation to gamble and gambling-related semantic networks in problem gamblers. Neuropsychopharmacology. 2004;29(1):195–207. doi: 10.1038/sj.npp.1300333. [DOI] [PubMed] [Google Scholar]


