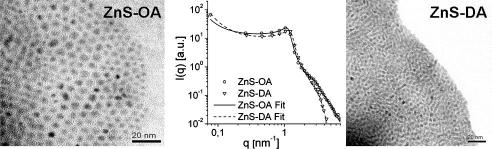
Graphical abstract
Highlights
► Combined X-ray and light scattering study was performed on ZnS nanoparticles. ► Ligands with different steric properties, dodecyl- and oleylamine, are compared. ► Nanoparticles exhibit sizes of 3–5 nm. ► Thickness of the ligand shell is about 1.9 nm.
Keywords: Zinc sulphide, SAXS, Electron microscopy, Nanoparticles, Ligand shell, Oleylamine
Abstract
Nanoparticles capped with amine ligands with different steric properties, dodecylamine and oleylamine, respectively, are investigated in the solid state as well as in solution. A combined X-ray diffraction, small angle X-ray scattering and electron microscopy investigation showed that the nanoparticles exhibit the sphalerite modification of ZnS as crystal phase with a diameter of 3–5 nm. A close packing of the monocrystalline nanoparticles in the solid state is observed. However, in the dodecylamine sample, besides spherical particles, a fraction of the nanoparticles is elongated. The nanoparticles are readily resoluble in apolar solvents like hexane. Dynamic light scattering (DLS) and SAXS investigations of the solutions reveal that the nanoparticles are dissolved as singular particles. In the case of oleylamine-capped ZnS, a defined core–shell structure with a ZnS core with a diameter of 4 nm and an organic shell with a thickness of approximately 2 nm have been found. Dodecylamine-capped nanoparticles slightly tend to form agglomerates with a diameter of approximately 40 nm.
1. Introduction
Zinc sulphide is one of the most important II–VI semiconductors with a band gap of about 3.6 eV [1]. ZnS-nanoparticles have a high potential in many different technological applications [2], e.g. as photonic crystals [3,4] or in optoelectronic devices [5,6]. There are numerous reports on the synthesis of nanocrystalline ZnS in the literature [7]. Without strong capping ligands, however, nanocrystalline ZnS is often obtained in micrometer-sized agglomerates [8]. Thus, for the preparation of soluble ZnS nanocrystals, usually bulky surfactants are used as stabilisers to prevent aggregation. Joo et al. [9] reported a general synthesis method towards metal sulphide nanocrystals using oleylamine (OA), trioctylphosphine oxide (TOPO) and elemental sulphur for the preparation of monodisperse sphalerite ZnS nanocrystals. Following this publication, the synthesis method became popular, was further developed and extended to ternary and quarternary sulphides and selenides [10–18].
The analytical foundation of most papers is based on X-ray diffraction (XRD)-analysis and transmission electron microscopy (TEM)-analysis. Although modern electron microscopy is inevitable in the structural characterisation, the selection of the images remains a quite subjective issue and sometimes does not really represent the sample in total. In this context, small angle X-ray scattering (SAXS) is a valuable complementary method analysing a larger amount of the sample. Although data analysis and interpretation are laborious and sometimes difficult, SAXS is a well suited method for investigating the size, shape and distribution for nanoparticles in the solid state [19] but also in solution [20]. This latter point is of special interest as many applications require stable and well dissolved nanoparticles. Here, a combination of SAXS and dynamic light scattering (DLS) delivers the desired information, e.g. if nanoparticles are separately dissolved or if agglomerates are formed. In contrast to the high number of articles dealing with the synthesis of ZnS nanoparticles, reports dealing with the structural characterisation of the prepared ZnS nanoparticles using SAXS [15,21–26] are less common.
The analysis of size and structural properties of nanoparticles is one of the fundamental tasks in colloid science, because material properties are directly influenced by these parameters. In this study, we discuss the structural details of amine-capped ZnS nanoparticles in the solid state as well as in solution by using complementary analysis methods. We chose two long-chained amines – oleylamine and dodecylamine – as solvent and capping agent in a simplified synthesis procedure for the preparation of ZnS nanoparticles [9]. We thereby investigate the influence of the organic moiety. Oleylamine exhibits a double bond in the middle of the chain; dodecylamine has only single bonds, whereas the chemical reactivity of the amine should not be affected, see Scheme 1. Thus, the only difference should stem from the length and size requirements of the organics.
Scheme 1.
Molecular structures of DA and OA.
The nanoparticles were structurally characterised by XRD, TEM and SAXS investigations in the solid state. The particles are still soluble in organic solvents, and the colloidal solution was characterised by DLS, SAXS and UV–Visible spectroscopy.
2. Experimental section
ZnCl2 (puriss. p.a. >98%, Sigma Aldrich), sulphur (powder, approx. 100 mesh, sublimated, Sigma Aldrich), oleylamine (technical grade, 70%, Sigma Aldrich), dodecylamine (98%, Sigma Aldrich), and methanol (Baker analysed reagent grade, J.T. Baker) were used without further purification. The reactions were carried out under nitrogen atmosphere.
2.1. Nanoparticle synthesis
2.1.1. ZnS-OA
ZnCl2 (272.6 mg, 2.00 mmol, 1.0 eq.) was dissolved in oleylamine (10 mL, 30.3 mmol, 15.1 eq.) by heating the mixture to 170 °C for 30 min. After the solution was cooled down, a solution of sulphur (192.4 mg, 6.00 mmol, 3 eq.) in oleylamine (3 mL, 9.08 mmol, 4.5 eq.) was added and the mixture was heated for 1 h at 210 °C. The solution was cooled down, and the particles were precipitated into methanol and separated by centrifugation. The particles were washed by suspending them in methanol and centrifugation. Then, they were dried under reduced pressure at ambient temperature.
2.1.2. ZnS-DA
ZnCl2 (272.6 mg, 2.00 mmol, 1.0 eq.) was dissolved in dodecylamine (7 mL, 30.2 mmol, 15.1 eq.) by heating the mixture to 170 °C for 30 min. After the reaction mixture was cooled down, a solution of sulphur (192.4 mg, 6.0 mmol, 3 eq.) in dodecylamine (3 mL, 13.0 mmol, 6.5 eq.) was added and the mixture was stirred for 1 h at 210 °C. The solution was cooled down, and the particles were precipitated into methanol and separated by centrifugation. The particles were washed by suspending them in methanol and centrifugation. Then, they were dried under reduced pressure at ambient temperature.
2.2. Characterisation techniques
X-ray diffraction (XRD) patterns were obtained on a Siemens D 501 diffractometer in Bragg–Brentano-geometry operated at 40 kV and 30 mA, using Cu Kα radiation (λ = 1.54178 Å) at a scan rate of 0.05° (50 s measurement time per step). The diameter of the primary crystallites of the ZnS-nanoparticles (DXRD) was determined according to the broadening of the diffraction peaks using the Scherrer relationship (Eq. (1)):
| (1) |
with Δ(2θ) is the full width at half-maximum (FWHM) of the peak in radians, θ is half of the scattering angle 2θ and K is the Scherrer form factor, which is 0.9 for spherical particles. In this context, it is very important to note that the Scherrer relationship is only a good approximation for spherical crystals. The size is inversely proportional to the integral width. Crystal size determination was performed using the 1 1 1 reflection at 2θ = 28.7°. The experimental line width was determined to be 0.12° at this 2θ-position by measuring a Si-reference standard (NIST 640c).
Transmission electron microscopy (TEM) investigations were carried out on a Philips CM20, 200 kV and LaB6 cathode, and the high resolution image was performed using a FEI TF20, 200 kV and field emission gun. Samples for TEM investigations were prepared by dispersing the powder particles on a holey polymer support film coated with carbon.
UV–Visible absorption spectra were recorded on Cary 50 Bio UV–Visible Spectrophotometer.
For dynamic light scattering (DLS) measurements, we used a laboratory built goniometer, which was equipped with single mode fibre optics and an ALV single photon detector. The light source was a Verdi V5 diode laser from Coherent with a wavelength of 532 nm and a maximum output power of 5 W (typically laser power used 0.5 W). The data acquisition was performed with an ALV 5000 multiple τ digital correlator. This allows a minimum time interval of 12.5 ns for the correlation function. The ALV-5000/E software package was used to record and store the correlation functions. All experiments were carried out at 25 °C and at a scattering angle of 90°. Each sample was measured 10 times for 60 s. The obtained correlation functions were averaged and used for the calculation of the size distributions. We used the ORT software [27] to determine the volume weighted size distribution functions.
2.2.1. Small angle X-ray scattering (SAXS)
The SAXS equipment was a SAXSess camera (Anton-Paar, Graz, Austria) using an X-ray generator (Philips, PW 1730/10) operated at 40 kV and 50 mA with a sealed-tube Cu anode. A Göbel mirror was used to convert the divergent polychromatic X-ray beam into a collimated line-shaped beam of Cu Kα radiation (λ = 0.154 nm). The 2D scattering pattern was recorded by an imaging-plate detector (model Fuji BAS1800 from Raytest, Straubenhardt, Germany) and integrated into the one-dimensional scattering function I(q) using SAXSQuant software (Anton-Paar).
Liquid samples were filled at room temperature into the sample holder (quartz capillary in a metal block, temperature controlled by a Peltier element, ±0.1 K) and measured at 25 °C.
Powder samples were pressed into a 1 mm slit within an Al-plate and mounted directly in the camera without using any additional window for the sample holder.
The evaluation of the SAXS data is presented in the Supporting information.
3. Results and discussion
Complementary methods were used to characterise the ZnS nanoparticles capped with oleylamine (OA) and dodecylamine (DA), respectively, in the solid state and in solution to gain information concerning size and structural properties.
3.1. Characterisation of the nanoparticles in the solid state
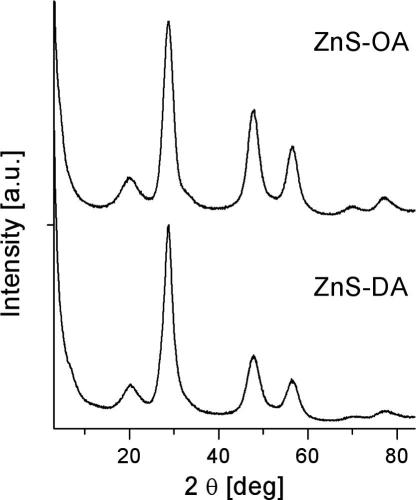
Phase analysis was performed by powder X-ray diffraction. Fig. 1 compares the diffraction patterns of the oleylamine-capped zinc sulphide nanoparticles (ZnS-OA) and the dodecylamine-capped ones (ZnS-DA). Intense peaks at 2θ = 28.7° (1 1 1), 47.6° (2 2 0), 56.3° (3 1 1) and smaller ones at 33.1° (2 0 0), 59.1° (2 2 2), 69.5° (4 0 0), 76.8° (3 3 1) and 79.1° (4 2 0) are observed, which are in good agreement with the Powder Diffraction File (PDF) 5-0566 of the International Centre for Diffraction Data for sphalerite-ZnS. An additional broad peak at 2θ = 20° is observed attributable to the organic capping ligand as this peak disappears upon annealing at temperatures above 250 °C. The width of the observed peaks indicates that the material is nanocrystalline. Using the Scherrer equation, a rough estimation of the crystallite size reveals 3.1 nm as value for the crystallite diameter of the sample ZnS-OA and 3.5 nm for ZnS-DA.
Fig. 1.
Powder X-ray diffraction of the prepared ZnS nanoparticles using oleylamine (ZnS-OA – upper trace) and using dodecylamine (ZnS-DA – lower trace) as capping ligand.
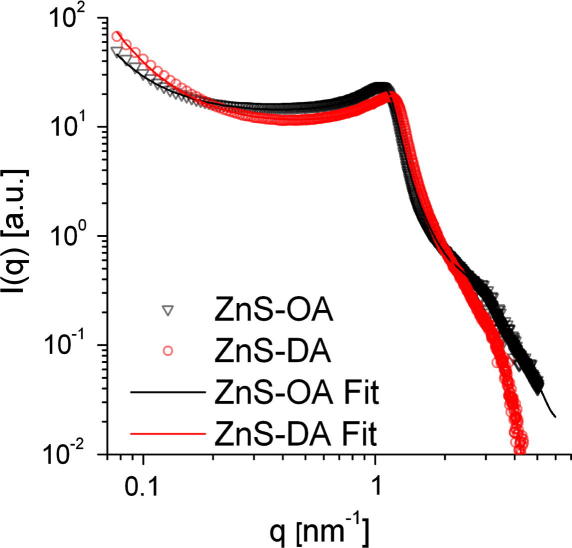
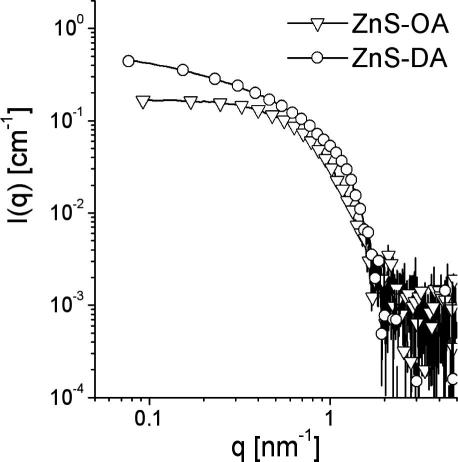
The XRD-patterns of both samples also show a high intensity at values of 2θ < 10°. To exploit this scattering range, SAXS measurements on powder samples have been carried out. Fig. 2 shows the SAXS data of both samples in the double logarithmic plot. Both samples show an interaction peak at a q value of approximately 1 nm−1. The upturn at low q-values indicates larger structures. The data were evaluated using the generalised indirect Fourier transformation (GIFT). The model for the structure factor used by GIFT was a modification of the one introduced by Hoekstra and co-workers for sticky hard spheres [28,29] and is described in more detail in the experimental section. The obtained model data are summarised in Table 1 and the approximation to the experimental data is shown in Fig. 2. For both samples, similar results were obtained. The scattering objects exhibit a radius of 2.9 (ZnS-OA) and 2.7 nm (ZnS-DA), with a volume fraction of approx. 0.46, which correlates well to a dense packing of spheres where the shell of the capping amine forms a continuous matrix in between. Taking these parameters and using core radii of 2.0 nm for ZnS-OA and 1.8 nm for ZnS-DA, one can estimate the volume fraction of the ZnS-cores within the solid to be 15% for the ZnS-OA and 14% for the ZnS-DA systems. The dense packing is also reflected in the high fractal dimension. The high polydispersity values might be due to imperfections of the structure factor model used, since they are not found in the model free description of the particle form factor.
Fig. 2.
SAXS data of the prepared ZnS powder samples using oleylamine (ZnS-OA) and dodecylamine (ZnS-DA) as capping ligand as well as the obtained fits obtained by GIFT.
Table 1.
Fit parameters and confident intervals obtained by the GIFT routine for both samples.
| ZnS-OA | ZnS-DA | |
|---|---|---|
| Volume fraction | 0.459 ± 0.001 | 0.466 ± 0.004 |
| Radius (nm) | 2.93 ± 0.01 | 2.71 ± 0.03 |
| Polydispersity | 0.372 ± 0.002 | 0.441 ± 0.024 |
| Fractal dimension | 2.80 ± 0.01 | 2.87 ± 0.02 |
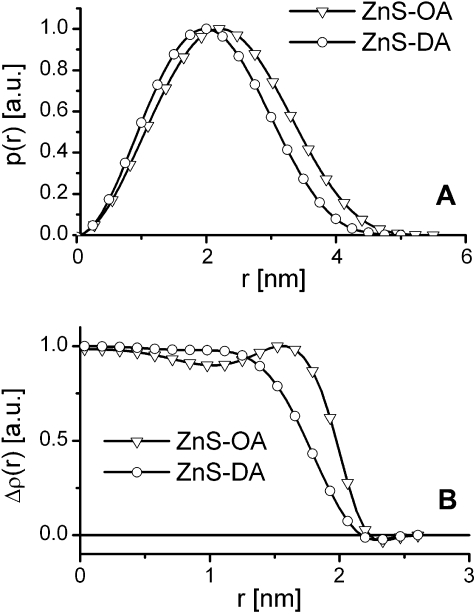
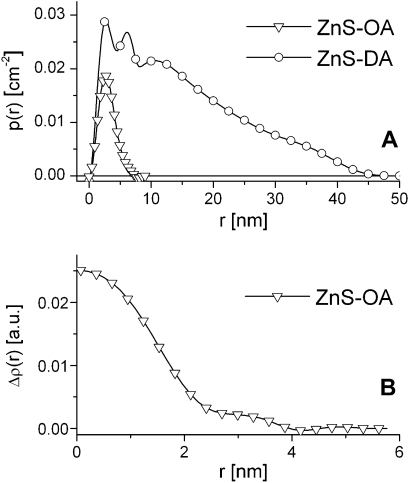
Using the GIFT routine, also the pair distance distribution function p(r) (PDDF) can be obtained. The PDDF is a real space analogue of the form factor of the scattering particles containing the same information, which is just easier to interpret than in the reciprocal space. The PDDF can be interpreted as a histogram of all distances between scattering centres present in the particle weighted by the contrast at the endpoint of the distances [30]. The PDDFs of both samples (Fig. 3A) are bell shaped, which is typically for globular particles. Deconvolution of the PDDF results in the radial contrast profile Δρ(r) (Fig. 3B). It shows high electron density up to a radius of 2.0 nm (ZnS-OA) and 1.8 nm (ZnS-DA), respectively. Due to the high contrast, these values correspond to the inorganic core of the particles. Comparison of these radii with the interaction radii obtained for the structure factor model, a core–shell model can be proposed, with an inorganic ZnS core with a diameter of 4 nm and 3.6 nm, respectively, and an organic capping shell of 0.9 nm. These values seem to be contrary to the XRD-results, which show smaller crystallites for ZnS-OA. It has to be noted that both methods are suitable to only a limited extent to give absolute numbers for such small nanoparticles. Differences between both analyses methods are often encountered [8]. However, the values for the inorganic core are in the same order as the estimations from the XRD-measurements, which indicate that nanoparticles are monocrystalline as the primary crystallite size and the scattering spheres are in the same range. The organic shell is lower as expected for isolated spheres but is in consistence with closely packed spheres – also indicated by the high volume fraction and the high fractal dimension, where the organic shells can interpenetrate to some extent. The deconvolution also results in an estimate of polydispersity, which was found to be 0.16 for both samples.
Fig. 3.
(A) Pair distance distribution functions p(r) of ZnS-OA and ZnS-DA obtained from the SAXS data using the GIFT routine (limits of uncertainty are lower than the symbol size). (B) Radial contrast profile Δρ(r) of both samples determined by deconvolution of the PDDFs (limits of uncertainty are lower than the symbol size).
In order to prove this model, TEM measurements have been undertaken and representative micrographs are shown in Fig. 4 for ZnS-OA and in Fig. 5 for ZnS-DA. The TEM-micrographs confirm the results of the SAXS measurements and XRD-analysis and show that the particle sizes are in the range between 2 and 5 nm. Having a closer look, the particles in the sample ZnS-OA (Fig. 4) have a mean diameter of approximately 3.5 nm and are well separated.
Fig. 4.
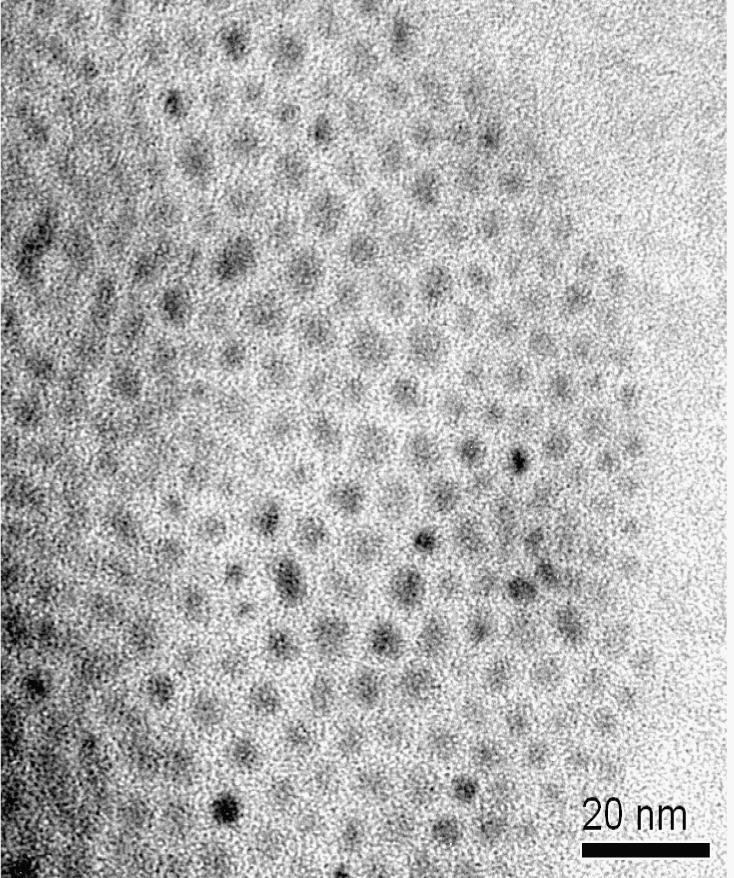
TEM image of sample ZnS-OA.
Fig. 5.
TEM image of sample ZnS-DA.
The particles in sample ZnS-DA (Fig. 5) are closer packed as in sample ZnS-OA, and two different types of particles can be observed, spherical particles with a diameter of approximately 2.6 nm and larger elongated particles with a length up to 6 nm. This could also give an explanation of the differences in particle size observed by XRD and SAXS, as ZnS-DA generally exhibits smaller nanoparticles but in addition also some larger nanostructures.
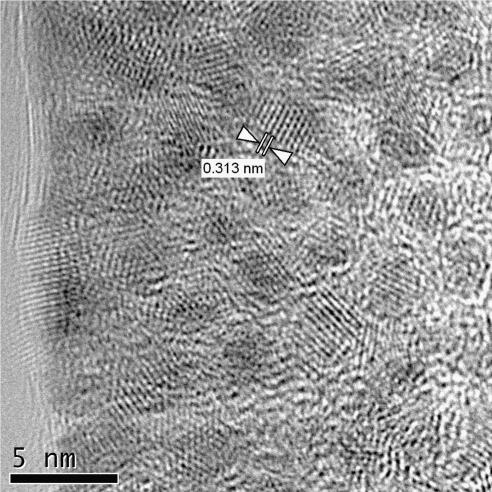
In Fig. 6, a high resolution TEM image of ZnS-DA nanoparticles is presented. The nanoparticles have sizes of 2–4 nm. The plane distance of 0.313 nm, which is found for a particle imaged down the [0 1 1] crystallographic zone axis, corresponds well to data base values (PDF 5-0566).
Fig. 6.
HRTEM image of ZnS-DA. The distance of 0.313 nm corresponds to the 1 1 1 netplanes of the cubic sphalerite crystal structure.
3.2. Characterisation of the nanoparticles in solution
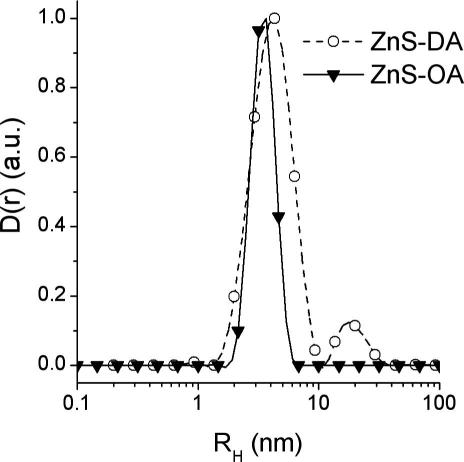
The nanoparticles can be redissolved in apolar solvents such as hexane and dichloromethane yielding stable solutions. This is an important issue for applications in solutions or the use of these nanoparticles in the preparation of nanocomposite materials. Dynamic light scattering (DLS) measurements as well as SAXS were used to investigate nanoparticle solutions in hexane. In Fig. 7, the volume weighted size distribution functions Dv(R) of both the samples, calculated via inverse Laplace transformation from the measured correlation functions, are shown. In both the samples, the main fraction has a hydrodynamic radius of about 3.5 nm for ZnS-OA and 4.3 nm for ZnS-DA. However, the dodecylamine-capped ZnS nanoparticles tend to agglomerate and form additional bigger sized aggregates with a radius of 20 nm. We assume that oleylamine is a better capping ligand because (1) of the longer carbon chain and (2) of the fact that the double bond leads to a more disordered shell, which facilitates dissolution.
Fig. 7.
Volume weighted size distribution functions Dv(R).
The SAXS scattering curves in double logarithmic representation (Fig. 8) show a horizontal regime at low q for ZnS-OA, indicating mostly small particles, while the slope found for ZnS-DA is due to the presence of the larger aggregates. Indirect Fourier transformation of the curves results in PDDFs (Fig. 9A). The curve obtained for ZnS-OA is asymmetrical with a tail at the right hand side. This can be well explained in terms of a core–shell particle, which has a high contrast in the core and a low contrast in the outer shell. The PDDF can be deconvoluted [31], resulting in an electron density profile (Fig. 9B). It shows the expected high contrast in the core and a low contrast in the shell. Approximating a two step model to the data results in a core radius of 2.1 nm and a total radius of 4.0 nm, which corresponds to a shell thickness of 1.9 nm, which matches well with a previous finding in literature [32]. As also reported by Keshari and Pandey [19], the core radius is again in good agreement with XRD data and the results from SAXS on the solid material, while the organic shell is now found to be considerably thicker. This is consistent with singular dissolved nanoparticles and a ligand shell with almost stretched molecules.
Fig. 8.
SAXS-curves of ZnS-solutions in hexane (every 10th point is shown).
Fig. 9.
(A) Pair distance distribution functions p(r) of ZnS-OA and ZnS-DA dissolved in hexane obtained from the SAXS data using IFT (limits of uncertainty are lower than the symbols) and (B) radial contrast profile Δρ(r) of ZnS-OA determined by deconvolution of the PDDFs (limits of uncertainty are lower than the symbols).
On the other hand, the PDDF of ZnS-DA shows a maximum at approximately the same position like the ZnS-OA curve but has further maxima at larger distances and decays slowly. This can be explained by the presence of particles that are of about the same size like in the case of ZnS-OA in combination with larger aggregates, where the maxima at larger distances are due to the local arrangement of the particles in the aggregates. The actual shape of these particles cannot be determined from the PDDF and it is possible that some of the features can be explained by elongation of the particles. The peaks at 7 nm and 11 nm are, however, a clear indication that partial aggregation of the particles occurred. This is also the reason, why a detailed analysis of the particle shape and ligand structure was not possible.
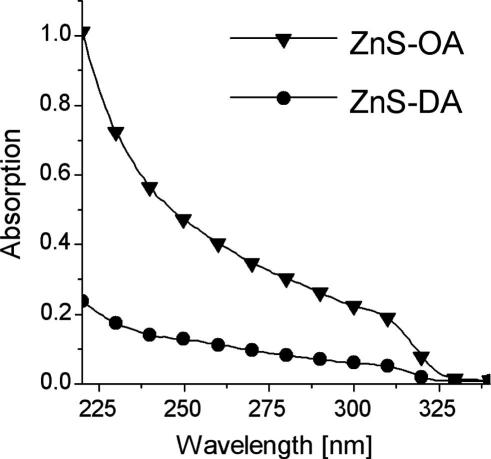
In Fig. 10, the UV-absorption spectra of both the samples in hexane solution are shown. Both samples have an absorption onset at approximately 325 nm, thus the absorption of light is shifted to lower wavelengths compared to bulk ZnS (bandgap: 3.66 eV, absorption onset: 338 nm) [33], as expected for nanoparticles.
Fig. 10.
UV-absorption spectra of ZnS-OA and ZnS-DA in hexane.
4. Conclusions
To gain more information on the structure of amine-capped metal sulphides, we studied ZnS nanoparticles capped with two different amines – oleylamine and dodecylamine – in the solid state as well as in solution with complementary characterisation methods (TEM, XRD, SAXS, DLS). The nanoparticles have been obtained by the versatile route originally introduced by Joo et al. [9].
In the solid state, determination of the particle size is often based on TEM and XRD. In this context, HR-TEM analysis is a very powerful method, as it can directly give information on the crystal size but also on crystal structure and defects. However, one should keep in mind that the sample cross-section of TEM is very low and is sometimes influenced by serendipity or the operator. Therefore, a comparison with the results from the XRD analysis using the Scherrer equation as well as with those from SAXS analysis, both using a larger fraction of the sample, is useful to obtain a more complete picture of the nanoparticles size and size distribution. The values obtained by TEM, SAXS and XRD are in rather good agreement as also seen by other groups [19,34]. The quite simple method using Scherrer formula to determine the particle size from XRD-peak broadening, especially, leads to rather accurate results. However, the primary crystallite size is smaller as the nanoparticle size itself, as an amorphous or imperfect surface is not included. If the polydispersity of the nanoparticles is not too large, SAXS analysis and Scherer equation usually are in good agreement [8].
The slight difference of the capping ligand regarding the space requirements yielded nanoparticles with different sizes and shapes but also influences the affinity to form agglomerates in solution. Both of the samples exhibit the sphalerite modification as crystal phase and have a diameter of approximately 3–5 nm. The nanoparticles in ZnS-OA are of spherical shape, whereas the sample ZnS-DA contains additionally a significant fraction of elongated particles, which can be only seen unambiguously by TEM. In the solid state, the nanoparticles are monocrystalline and well separated by the capping shell.
SAXS and DLS investigations on colloidal solutions of the nanoparticles reveal that the nanoparticles are dissolved as single particles in the case of ZnS-OA with a core–shell structure revealing a ZnS core with a diameter of 4 nm and an organic shell with a thickness of approximately 1.9 nm, which is reasonable compared to literature values [32] and the length of an oleylamine molecule. In the case of ZnS-DA, the main fraction is also dissolved as single particles. However, some agglomerates with a diameter of approximately 40 nm are formed. From the SAXS evaluation, there are some indications that elongated particles are present, but the formation of aggregates is not allowing a more detailed analysis.
Acknowledgments
Financial support by the Austrian Science Fund FWF (project N903-NAN “Hybrid Nanocomposites for Optoelectronics”) in the framework of the Austrian Nano-Initiative (research project cluster “Performance Optimization of Polymer Nanocomposites”), the Christian Doppler Research Association (CDG) and the Austrian Federal Ministry of Economy, Family and Youth (BMWFJ) is gratefully acknowledged.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.jcis.2011.12.032.
Contributor Information
Gabriele Kremser, Email: gabriele.kremser@yahoo.com.
Thomas Rath, Email: thomas.rath@tugraz.at.
Birgit Kunert, Email: birgit.kunert@tugraz.at.
Michael Edler, Email: edler@tugraz.at.
Gerhard Fritz-Popovski, Email: gerhard.popovski@unileoben.ac.at.
Roland Resel, Email: roland.resel@tugraz.at.
Ilse Letofsky-Papst, Email: ilse.papst@felmi-zfe.at.
Werner Grogger, Email: werner.grogger@felmi-zfe.at.
Gregor Trimmel, Email: gregor.trimmel@tugraz.at.
Appendix A. Supplementary material
References
- 1.Maity R., Maiti U.N., Mitra M.K., Chattopadhyay K.K. Physica E. 2006;33:104. [Google Scholar]
- 2.Murugan A.V., Heng O.Y., Ravi V., Viswanath A.K., Saaminathan V. J. Mater. Sci. 2006;41:1459. [Google Scholar]
- 3.Velikov K.P., van Blaaderen A. Langmuir. 2001;17:4779. [Google Scholar]
- 4.Liddell C.M., Summers C.J. J. Colloid Interface Sci. 2004;274:103. doi: 10.1016/j.jcis.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Dinsmore A.D., Hsu D.S., Gray H.F., Quadri S.B., Tian Y., Ratna B.R. Appl. Phys. Lett. 1999;75:802. [Google Scholar]
- 6.Mach R., Muller G.O. J. Crystal. Growth. 1988;86:866. [Google Scholar]
- 7.Fang X., Zhai T., Gautam U.K., Li L., Wu L., Bando Y., Golberg D. Prog. Mater. Sci. 2011;56:175. [Google Scholar]
- 8.Rath T., Kunert B., Resel R., Fritz-Popovski G., Saf R., Trimmel G. Inorg. Chem. 2008;47:3014. doi: 10.1021/ic7017715. [DOI] [PubMed] [Google Scholar]
- 9.Joo J., Na H.B., Yu T., Yu J.H., Kim Y.W., Wu F., Zhang J.Z., Hyeon T. J. Am. Chem. Soc. 2003;125:11100. doi: 10.1021/ja0357902. [DOI] [PubMed] [Google Scholar]
- 10.Choi S.-H., An K., Kim E.-G., Yu J.H., Kim J.H., Hyeon T. Adv. Funct. Mater. 2009;19:1645. [Google Scholar]
- 11.Kwon S.G., Hyeon T. Acc. Chem. Res. 2008;41:1696. doi: 10.1021/ar8000537. [DOI] [PubMed] [Google Scholar]
- 12.Wang D.-S., Zheng W., Hao C.-H., Peng Q., Li Y.-D. Chem. Eur. J. 2009;15:1870. doi: 10.1002/chem.200801815. [DOI] [PubMed] [Google Scholar]
- 13.Pein A., Baghbanzadeh M., Rath T., Haas W., Maier E., Amenitsch H., Hofer F., Kappe C.O., Trimmel G. Inorg. Chem. 2011;50:193. doi: 10.1021/ic101651p. [DOI] [PubMed] [Google Scholar]
- 14.Steinhagen C., Panthani M.G., Akhavan V., Goodfellow B., Koo B., Korgel B.A. J. Am. Chem. Soc. 2009;131:12554. doi: 10.1021/ja905922j. [DOI] [PubMed] [Google Scholar]
- 15.Riha S.C., Parkinson B.A., Prieto A.L. J. Am. Chem. Soc. 2009;131:12054. doi: 10.1021/ja9044168. [DOI] [PubMed] [Google Scholar]
- 16.Guo Q., Hillhouse H.W., Agrawal R. J. Am. Chem. Soc. 2009;131:11672. doi: 10.1021/ja904981r. [DOI] [PubMed] [Google Scholar]
- 17.Quan Z., Wang Z., Yang P., Lin J., Fang J. Inorg. Chem. 2007;46:1354. doi: 10.1021/ic061917n. [DOI] [PubMed] [Google Scholar]
- 18.Haas W., Rath T., Pein A., Rattenberger J., Trimmel G., Hofer F. Chem. Commun. 2011;47:2050. doi: 10.1039/c0cc04397d. [DOI] [PubMed] [Google Scholar]
- 19.Keshari A.K., Pandey A.C. J. Nanosci. Nanotechnol. 2008;8:1221. doi: 10.1166/jnn.2008.370. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H., Chen B., Banfield J.F. J. Phys. Chem. C. 2010;114:14876. [Google Scholar]
- 21.Belman N., Acharya S., Konovalov O., Vorobiev A., Israelachvili J., Efrima S., Golan Y. Nano Lett. 2008;8:3858. doi: 10.1021/nl802287h. [DOI] [PubMed] [Google Scholar]
- 22.Viswanatha R., Sapra S., Amenitsch H., Sartori B., Sarma D.D. J. Nanosci. Nanotechnol. 2007;7:1726. doi: 10.1166/jnn.2007.706. [DOI] [PubMed] [Google Scholar]
- 23.Mehta S.K., Kumar S., Chaudhary S., Bhasin K.K., Gradzielski M. Nanoscale Res. Lett. 2009;4:17. doi: 10.1007/s11671-008-9196-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodell C.M., Gilbert B., Weigand S.J., Banfield J.F. J. Phys. Chem. C. 2008;112:4791. [Google Scholar]
- 25.Narayanan J., Ramji R., Sahu H., Gautam P. IET Nanobiotechnol. 2010;4:29. doi: 10.1049/iet-nbt.2009.0010. [DOI] [PubMed] [Google Scholar]
- 26.Calandra P., Longo A., Liveri V.T. J. Phys. Chem. B. 2003;107:25. [Google Scholar]
- 27.Schnablegger H., Glatter O. Appl. Opt. 1991;30:4889. doi: 10.1364/AO.30.004889. [DOI] [PubMed] [Google Scholar]
- 28.Hoekstra H., Mewis J., Narayanan T., Vermant J. Langmuir. 2005;21:11017. doi: 10.1021/la051488q. [DOI] [PubMed] [Google Scholar]
- 29.Jensen H., Pedersen J.H., Jørgensen J.E., Pedersen J.S., Joensen K.D., Iversen S.B., Søgaard E.G. J. Exp. Nanosci. 2006;1:355. [Google Scholar]
- 30.Glatter O. J. Appl. Crystallogr. 1979;12:166. [Google Scholar]
- 31.Glatter O. J. Appl. Crystallogr. 1981;14:101. [Google Scholar]
- 32.Chen M., Feng Y.-G., Wang X., Li T.-C., Zhang J.-Y., Qian D.-J. Langmuir. 2007;23:5296. doi: 10.1021/la700553d. [DOI] [PubMed] [Google Scholar]
- 33.Öznülüer T., Erdogan I., Demir Ü. Langmuir. 2006;22:4415. doi: 10.1021/la052404g. [DOI] [PubMed] [Google Scholar]
- 34.Borchert H., Shevchenko E.V., Robert A., Mekis I., Kornowski A., Grübel G., Weller H. Langmuir. 2005;21:1931. doi: 10.1021/la0477183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.