Abstract
The Ets transcription factor, Fli-1 is activated in murine erythroleukemia and overexpressed in various human malignancies including Ewing's sarcoma, induced by the oncogenic fusion protein EWS/Fli-1. Recent studies by our group and others have demonstrated that Fli-1 plays a key role in tumorigenesis, and disrupting its oncogenic function may serve as a potential treatment option for malignancies associated with its overexpression. Herein, we describe the discovery of 30 anti-Fli-1 compounds, characterized into six functional groups. Treatment of murine and human leukemic cell lines with select compounds inhibits Fli-1 protein or mRNA expression, resulting in proliferation arrest and apoptosis. This anti-cancer effect was mediated, at least in part through direct inhibition of Fli-1 function, as anti-Fli-1 drug treatment inhibited Fli-1 DNA binding to target genes, such as SHIP-1 and gata-1, governing hematopoietic differentiation and proliferation. Furthermore, treatment with select Fli-1 inhibitors revealed a positive relationship between the loss of DNA-binding activity and Fli-1 phosphorylation. Accordingly, anti-Fli-1 drug treatment significantly inhibited leukemogenesis in a murine erythroleukemia model overexpressing Fli-1. This study demonstrates the ability of this drug-screening strategy to isolate effective anti-Fli-1 inhibitors and highlights their potential use for the treatment of malignancies overexpressing this oncogene.
Keywords: erythroleukemia, Fli-1, drug inhibition
Introduction
The progression of cancer is a multistage process arising from the additive or cooperative activities of multiple pathways, resulting from the activation of proto-oncogenes and inactivation of tumor suppressor genes. Delineation of the genetic processes involved in the development of cancer will provide a better understanding of how disruption of the homeostatic balance between proliferation, differentiation and apoptosis leads to malignancy, as well as to provide indications of potential therapeutic targets.
A well-studied example of multistage malignancy in mice is erythroleukemia induced by different strains of Friend leukemia virus. Studies using this cancer model have led to the identification of fli-1, a member of the Ets family of transcription factors, activated through retroviral insertional mutagenesis in 90% of Friend Murine Leukemia virus (F-MuLV)-induced erythroleukemias.1, 2 The constitutive activation of fli-1 in erythroblasts leads to a dramatic shift in the Epo/Epo-R signal transduction pathway, blocks erythroid differentiation, and activates the Ras pathway, ultimately resulting in massive Epo-independent proliferation of erythroblasts.3, 4
In addition to Friend erythroleukemia, proviral integration at the fli-1 locus also occurs in leukemias induced by the Cas-Br-E virus5 and Fli-1 aberrant expression is associated with chromosomal abnormalities in humans. In Ewing's sarcoma, a chromosomal translocation generates a fusion of the 5′ transactivation domain of EWS with the 3′ Ets domain of Fli-1. The resulting fusion oncoprotein, EWS/Fli-1, acts as an aberrant transcriptional activator with strong transforming capabilities.6 The importance of Fli-1 in the development of human leukemia, such as acute myelogenous leukemia, has been demonstrated in studies regarding the Tel transcription factor that interacts with Fli-1 through protein–protein interactions.7 Fli-1 overexpression has also been detected in various types of human sarcomas and hematological malignancies.8
Although Fli-1 overexpression has been detected in a wide range of malignancies, the specific role of Fli-1 in tumorigenesis has remained unclear. Our group has recently demonstrated that RNAi-mediated downregulation of Fli-1 in both human and murine erythroleukemias results in growth inhibition and rapid cell death in vitro.9 This observation is also supported by another report in which downregulation of Fli-1 in murine erythroleukemia results in massive cell death associated with downregulation of several previously identified and novel Fli-1 target genes.10 Together, this data strongly suggests that Fli-1 overexpression is vital in maintaining the malignant phenotype and Fli-1 may serve as a potential therapeutic target in a number of human malignancies. In this study, using a sensitive screening assay, we have identified 30 small molecules/compounds capable of inhibiting Fli-1 expression and function. Herein, we describe the capacity of select drugs to inhibit the expression and/or transactivational activity of Fli-1 in several murine and human malignancies resulting in inhibition of cell growth and apoptosis in vitro and inhibition of leukemic progression in an F-MuLV-induced erythroleukemia mouse model in vivo.
Materials and methods
Cell lines
Murine Friend virus-induced erythroleukemic cell lines DP17-17, CB3, human erythroleukemic cell line HEL, and 293T cells were maintained in α-Modified Eagle Medium supplemented with 5% fetal bovine serum (Gibco, Grand Island, NY, USA).
Drug screening
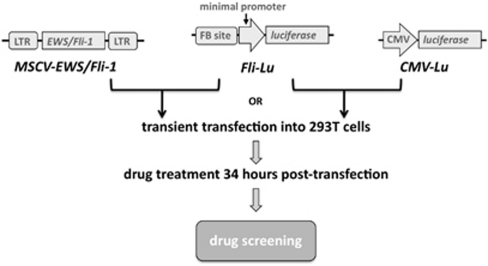
A schematic representation for Fli-1 drug screening is shown in Figure 1. A DNA fragment selected by CAST containing two Fli-1 binding sites was cloned into the pGL4.28 luciferase reporter (Promega, Madison, WI, USA) containing a minimal promoter, designated Fli-Lu. Construction of the expression vector, MSCV-EWS/Fli-1, was described previously.9 Fli-Lu was transiently transfected with the MSCV-EWS/Fli-1, MSCV empty vector or CMV-Lu into 293T cells using Lipofectamine 2000 (Invitrogen, Burlington, Canada). Cells were treated with compounds from various libraries 34 h post-transfection and screened for efficient downregulation of luciferase activity. Lead anti-Fli-1 compounds were chosen for their ability to reduce luciferase activity by at least 50%.
Figure 1.
Schematic representation of the Fli-1 drug-screening strategy. Fli-Lu: Multiple copies of the Fli-1 consensus-binding site (FB site) cloned upstream a minimal promoter immediately upstream the luciferase gene. MSCV-EWS/Fli-1: EWS/Fli-1 gene cloned into the MSCV retroviral vector. CMV-Lu: Luciferase gene driven by the CMV promoter. Fli-1–Lu was co-transfected with either MSCV-EWS/Fli-1 or CMV-Lu into 293T cells. Cells were treated with various drugs 34 h post transfection and screened for efficient downregulation of luciferase activity.
Immunoblotting and antibodies
Cells were lysed with radio immunoprecipitation assay buffer (0.5% Nonidet P-40, 50 m Tris HCl (pH 8.0), 120 m NaCl, 50 m NaF, plus 1 m Na3VO4, 10 g/ml aprotinin, 100 g/ml leupeptin and 10 m phenylmethylsulfonyl fluoride). 40 μg lysates were fractioned by SDS-Polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane (Immobilon-P, Millipore, Billerica, MA, USA). The following antibodies were used: Gata-1, SHIP-1 and Fli-1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA); β-actin (Sigma-Aldrich, Oakville, Canada); Bcl-2 (Cell signaling, Beverly, MA, USA) and goat-anti-mouse and goat anti-rabbit HRP-conjugated secondary antibodies (Promega). CB3 cell lysates treated with calcimycin (0.5 μ) and cantharidin acid (Can A) (1.0 μ) for 24 h were immunoprecipitated overnight at 4 °C using the Fli-1 antibody, and washed three times. Fli-1 protein was resolved on SDS-Polyacrylamide gel electrophoresis, transferred to a polyvinylidene fluoride membrane (Immobilon-P, Millipore), and immunoblotted using the phospho-threonine (42H4) mouse antibody (Cell Signalling, Danvers, MA, USA).
RNA extraction and northern blotting
Total RNA preparation and northern blotting was performed as previously described.2
Cell proliferation assays
Cancer cell lines CB3, HEL, A-673 and DP17-17 infected with MigR1-Fli-1 retrovirus11 (1 × 104) were plated in triplicate and treated with the indicated concentration of anti-Fli-1 drugs prepared from a 10 m stock solution dissolved in DMSO. The Trypan-blue exclusion assay was performed at 24-hour intervals for the Trypan-blue exclusion assay. Data is representative of three independent experiments.
Electrophoretic mobility shift assay (EMSA)
Nuclear extracts were isolated from HEL cells using the method described previously.11 Single-stranded oligonucleotides containing a Fli-1 binding site located on the SHIP-1 promoter, 5′-CCTGAAACAGGAAGTCAGTCAG-3′, were radioactively-labeled (γ-32P)ATP with T4 polynucleotide kinase (New England Biolabs, Pickering, Canada), purified using NUCTrap probe purification columns (Agilent Technologies, Santa Clara, CA, USA), annealed by boiling for 2 min and cooled at room temperature for 1 h. For competition assays, 100-fold excess cold single-stranded oligonucleotides were added. Fli-1 or c-Myc antibodies (2 μl; Santa Cruz Biotechnology) were used for the supershift assay. Samples were electrophoresed on a 5% acrylamide gel in 0.5 × TBE buffer.
Chromatin immunoprecipitation (ChIP) and quantitative PCR
HEL cells (1 × 108) were washed twice with PBS (Gibco) and crosslinked with 1% formaldehyde at 37 °C for 15 min, followed by addition of 125 m glycine for 5 min at room temperature. Fixed cells were washed in PBS and incubated on ice for 50 min in swelling buffer (20 m 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) pH 7.9, 10 m KCl, 1 m EDTA, 10% glycerol, 1 m DTT, 0.5 m phenylmethylsulfonyl fluoride (PMSF), 0.1 m Na3VO4). Cells were homogenized on ice using a Dounce homogenizer, nuclei were pelleted by centrifugation, resuspended in lysis buffer (10 m Tris-HCl pH 8.0, 140 m NaCl, 0.025% NaAzide, 1% TritonX-100, 0.1% SDS, 1 m DTT, 0.5 m PMSF, 0.1 m Na3VO4, 1% deoxycholate) and sonicated using the Branson250 Sonifier, followed by centrifugation. Fragmented chromatin was pre-cleared by incubation on ice with ProteinA sepharose beads for 1 h. A chromatin aliquot was removed for input control. Immunoprecipitations were performed overnight at 4 °C with 2 μg of Fli-1 (Santa Cruz Biotechnology) or nonspecific normal rabbit immunoglobulin G (IgG; Promega) antibody. ProteinA sepharose beads were added and incubation continued for 1 h. Precipitates were washed once in 0.1% SDS, 1% TritonX-100, 2 m EDTA, 150 m NaCl, 20 m Tris-HCl; four times in 0.1% SDS, 1% TritonX-100, 2 m EDTA, 500 m NaCl, 20 m Tris-HCl; once in 250 m LiCl, 1% NP-40, 1% deoxycholate, 1 m EDTA, 10 m Tris-HCl; three times in TE buffer, and extracted by adding 200 μl each of 1% SDS and 100 m NaHCO3. NaCl was added to a final concentration of 300 m and incubated overnight at 65 °C to reverse crosslinking. DNA was incubated with proteinase K at 50 °C for 2 h, purified with phenol chloroform and resuspended in TE buffer. PCR was performed to amplify a SHIP-1 promoter fragment containing the Fli-1 binding site, as previously described.11 Primer forward: 5′-CATGCCTTTGGCCTATTCAC-3′, Reverse: 5′-TGAGTGCCTGAAACAGGAAGT-3′. ChIP was quantified using the real-time PCR Qiagen QuantiFast SYBR green PCR kit (Qiagen, Mississauga, Canada). The level of SHIP-1 enrichment is expressed as the percentage of input chromatin precipitated by Fli-1 or control IgG antibodies.
Tumor induction and in vivo drug studies
Viral supernatants from NIH-3T3 cells containing F-MuLV clone 571 were harvested and frozen at –80 °C. Newborn mice were inoculated by intraperitoneal F-MuLV (clone 57) injections with 100 μl, 3000 focus-forming units, within 48 h of birth. Six weeks post infection, leukemic mice were treated every other day for a total of six injections with peruvoside, camptothecin (CPT), calcimycin, Can A (1 mg/kg) or DMSO, and monitored for signs of disease. Hematocrit values were measured by tail blood collection in 200 μl heparinized capillary tubes (Drummond Scientific, Broomall, PA, USA), centrifugation at 100 × g for 10 min, and evaluated using a hematocrit gauge. All animal studies followed the institutional guidelines.
Survival and statistical analysis
Mice survival rates were computed and plotted according to the nonparametric Kaplan–Meier analysis. Statistical analysis was performed using the two-tailed student's t-test with significance considered at P<0.05 by analysis of variance using Origin 3.5 software (Microcal Software, Northampton, MA, USA).
Results
Isolation of EWS/Fli-1 inhibitors from known small molecule/compound libraries
To identify small molecules/compounds capable of inhibiting the transactivation ability of Fli-1, luciferase assays were designed using a vector with the Fli-1 Ets DNA binding site9 cloned in front of a minimal promoter, immediately upstream of the luciferase reporter gene, designated Fli-1–Lu (Figure 1). The oncoprotein, EWS/Fli-1, functions as a strong transcriptional activator compared with Fli-1 alone, and possesses a strong transforming ability mediated through the Fli-1 3′ Ets domain.12 EWS/Fli-1 exogenous expression results in more robust luciferase activity compared with Fli-1 alone.9 Accordingly, the EWS/Fli-1 expression vector, MSCV-EWS/Fli-1, was used for the screening assay in lieu of Fli-1. The Fli-1–Lu construct was co-transfected with the EWS/Fli-1 vector into 293T cells (Figure 1). 293T cells co-transfected with Fli-1–Lu and MSCV empty vector or with the CMV-Lu luciferase vector driven by the CMV constitutively active promoter, served as negative controls compulsory for the exclusion of drugs that inhibit luciferase activity through an indirect pathway independent of Fli-1 activity.
Screening experiments were conducted in high-throughput formats using criteria to define positive hits as described previously.13, 14 To this end, we employed biologically and pharmacologically active compounds, natural products and marketed drugs distributed in the LOPAC collection (1280 samples, SIGMA), Prestwick Chemical Library (1200 samples, Prestwick Chemical) and Spectrum collection (2000 samples, MicroSource). Screening the above libraries led to the identification of 30 compounds that efficiently downregulated EWS/Fli-1-driven luciferase activity by at least 50%, in three independent experiments. Lead compounds were clustered into six distinct functional groups (full list in Table 1). The original screening was performed at a concentration of 5 μ, repeating a similar screening at lower concentrations revealed that the majority of the lead compounds have an IC(50) ranging between 0.165 and 5.62 μ (Table 1). The six functional drug groups identified in our study are classified as follows:
Table 1. Classification of lead anti-Fli-1 compounds.
| Drug classes | Number of drugs | Drug name | Effective dose (μ) IC(50)a |
|---|---|---|---|
| Cardenolides | 10 | Peruvoside, digitoxin, dihydroouabain, quabain, strophanthidin, proscillaridin A, cymarin, convalatoxin, gitioxin, lanatoside C | 0.165–0.33 |
| Calcium ionophores | 5 | Calcimycin, alexidine dihydrochloride, gossypol-acetic acid complex, oligomycin A, thapsigargin | 0.66–1.33 |
| Topoisomerase I inhibitors | 1 | Camptothecin | 0.33 |
| Protein synthesis inhibitors | 2 | Emetine, anisomycin | 0.165–0.66 |
| Chemotherapeutic drugs | 4 | Etoposide, dactinomycin, cycloheximide, puromucine dihydrochloride, | 0.66–2.66 |
| Others | 8 | Cedrelone, 5-azacytidine, anthothecol, cephaline, dihydrochloride heptahydrate, helveticoside, deacetoxy-7-oxogedunin, pyrvinion pamoate, cantharidin acid | 0.66–5.62 |
Compound library screening resulted in the identification of six drug classes with the indicated number in each class.
IC(50) was calculated as the lowest drug concentration resulting in a 50% reduction of EWS/Fli-1 luciferase activity. Column 3 displays one example of each inhibitor drug class. Column 4 displays the calculated range of effective dose response for each class.
Group I - Cardenolides
Cardenolides also called cardiotonic steroids, are potent inhibitors of Na+/K+-ATPase and have known anti-cancer properties.15 We identified all ten known Cardenolides as inhibitors of EWS/Fli-1 transactivation ability. Peruvoside was chosen from this group for its use in the treatment for congestive heart failure and has the lowest IC(50).
Group II - Calcium ionophores
These are used to increase intracellular Ca2+ levels in intact cells. The best example is calcimycin (A23187), which induces differentiation of erythroleukemic cells through unknown pathways.16 Out of the five calcium ionophores identified, calcimycin displayed the greatest Fli-1 inhibitory effect.
Group III - Topoisomerase I inhibitors
It has been previously shown that CPT, a topoisomerase I inhibitor, inhibits Friend virus-induced erythroleukemia progression.17 CPT indeed acts as a potent inhibitor of EWS/Fli-1 transcriptional activity at very low concentrations.
Group IV - Protein synthesis inhibitors
Two inhibitors of protein synthesis inhibitors were identified to block EWS/Fli-1 activity at very low concentrations.
Group V - Chemotherapeutic drugs
Four chemotherapeutic drugs were identified in our screening. Etoposide and Dactinomycin, known potent topoisomerase inhibitors, inhibit EWS/Fli-1 activity at nanomolar concentrations. These drugs are in current use for the treatment of Ewing's sarcoma.18
Group VI - Other inhibitors
This group consists of eight drugs that exert anti-Fli-1 activity at various concentrations, not fitting the above classifications.
To examine the ability of the lead anti-Fli-1 compounds to inhibit Fli-1 in cancer cells overexpressing Fli-1, representative drugs with different biological activities from group I, II and III, namely peruvoside, calcimycin and CPT, were chosen for further anti-Fli-1 drug analysis. Comparison of specificity, potency and mechanisms of action for each member would facilitate the selection of lead anti-Fli-1 compounds for potential use as single drugs or, if appropriate, combined formulations with potentially enhanced efficacy. This study did not include the investigation of compounds from group IV (protein synthesis inhibitors), as in addition to anti-Fli-1 activity these drugs may obtain the ability of broad, nonspecific protein inhibition of other factors having a role in Fli-1–associated malignancies, and group V (chemotherapeutic drugs), as the clinical efficacy of these drugs has already been demonstrated, as well as group VI, as inhibition is detected at high dosages, where toxicity may be of concern.
Similarly, additional luciferase assays were performed to confirm the level of EWS/Fli-1 inhibition through treatment with peruvoside, CPT and Calcimycin. Drugs from groups I–III also inhibited EWS/Fli-1 activity in an Ewing's sarcoma cell line leading to a block in cellular proliferation and induction of apoptosis (data not shown, to be published in an independent study).
Anti-EWS/Fli-1 drugs inhibit proliferation and reduce survival of Fli-1 overexpressing erythroleukemic cells
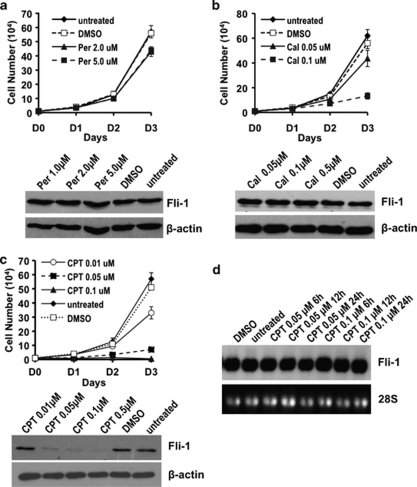
To investigate whether the identified anti-EWS/Fli-1 compounds also inhibit endogenous Fli-1 expression or activity, and as a result exert the toxic effects previously observed through RNAi-mediated Fli-1 downregulation,9, 10 Fli-1 overexpressing murine erythroleukemic CB3 cells2 were treated with representative drugs from groups I–III. Although treatment with peruvoside did not significantly affect the growth of CB3 cells in culture, CPT and calcimycin drastically inhibited the proliferation of these cells in a dose-dependent manner (Figure 2). Interestingly, only treatment with CPT reduced Fli-1 expression in a dose-dependent manner (Figure 2c); however, it did not affect transcription levels (Figure 2d), suggesting post-transcriptional regulation.
Figure 2.
Effect of anti-Fli-1 drugs on the proliferation of the erythroleukemia cell line CB3. CB3 cells (1 × 104) were plated in triplicate and treated with the indicated concentrations of (a) peruvoside (Per), (b) calcimycin (Cal), (c) CPT or vehicle DMSO control for 3 days (D1-3). Viable cells were counted on the indicated days using trypan blue exclusion assay. Fli-1 expression was analyzed 24 h post drug treatment. (d) CB3 cells were treated with the indicated concentrations of CPT for 24 h and subjected to northern blot analysis using a fli-1 cDNA probe.
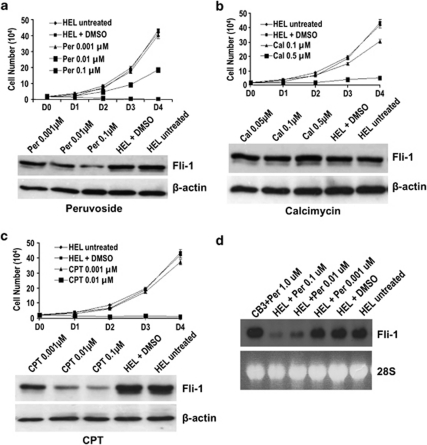
Growth inhibition by treatment with groups I–III drugs also occurs in the Fli-1 overexpressing human erythroleukemia cell line termed HEL (Figures 3a–c). However, although treatment with peruvoside in CB3 cells had only a minor inhibitory effect on proliferation leaving Fli-1 protein expression unaltered (Figure 2a), treatment of HEL cells significantly inhibited proliferation and significantly reduced Fli-1 protein levels at a concentration of 0.1 μ (Figure 3a). Similarly, treatment of CB3 cells with other lead compounds from group I, digitoxin, quabain and strophanthidin did not affect growth; however, treatment of HEL cells again resulted in a marked growth inhibition (data not shown). These results are consistent with the previous observation demonstrating a weak Na+/K+-ATPase activity and reduced drug penetration in mice compared with human cells.19 Treatment of HEL cells with peruvoside also resulted in downregulation of Fli-1 mRNA levels (Figure 3d), suggesting inhibition of Fli-1 transcription.
Figure 3.
Effect of anti-Fli-1 drugs on the proliferation of human erythroleukemia cell line HEL. HEL cells (1 × 104) were plated in triplicate and treated with the indicated concentrations of (a) peruvoside (Per), (b) calcimycin (Cal), (c) CPT or the vehicle control DMSO for 4 days (D1-4). The viable cells were counted on the indicated days using trypan blue exclusion assay. At 24 h post drug treatment the leukemic cells were subjected to western blot analysis using an anti-Fli-1 antibody. (d) HEL cells were treated with the indicated concentrations of peruvoside for 24 h and subjected to northern blot analysis using a fli-1 cDNA probe.
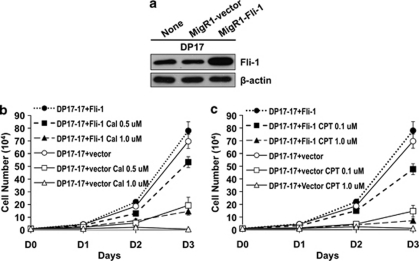
To determine whether the anti-proliferative effect of lead anti-Fli-1 drug treatment was directly mediated through inhibition of Fli-1, we investigated the effects of drug treatment in DP17-17 erythroleukemic cells with endogenous Fli-1 and ectopic Fli-1 overexpression. This cell line, derived from murine erythroleukemia induced by Friend virus, overexpresses another ETS transcription factor20 but expresses endogenous Fli-1 (Figure 4a). DP17-17 cells were transduced with Fli-1-expressing retrovirus resulting in ectopic Fli-1 overexpression compared with the parental line (Figure 4a). Treatment of Fli-1 overexpressing DP17-17 cells with calcimycin and CPT led to a marked growth inhibition only at higher dosages (Figures 4b and c). Treatment with lower doses of calcimycin (0.5 μ) and CPT (0.1 μ) somewhat reduced the survival of these cells, albeit at a lower level compared with vector alone treated cells displaying marked inhibition with the same drug concentration (Figures 4b and c). The ectopic overexpression of Fli-1, or increase in functional protein concentration, reduced the effectiveness observed with treatment of lower dosages. This data further supports the role of Fli-1 in leukemic proliferation and demonstrates that the representative anti-Fli-1 drugs exert their toxic effects at least in part through inhibition of Fli-1.
Figure 4.
Ectopic Fli-1 overexpression in erythroleukemic cells partially overcome growth inhibition induced by anti-Fli-1 drug treatment. (a) Increased levels of Fli-1 protein expression in the murine erythroleukemic cell line DP17-17, infected with retrovirus expressing Fli-1 (b–c) DP17-17 cell transduced with the vector control or Fli-1 expressing virus were treated with the indicated concentrations of (b) calcimycin (Cal), (c) CPT or the vehicle control DMSO for 3 days, and viable cells were counted on the indicated days using trypan blue exclusion assay.
Selective anti-Fli-1 drugs induce Fli-1 downregulation and/or block its DNA binding activity
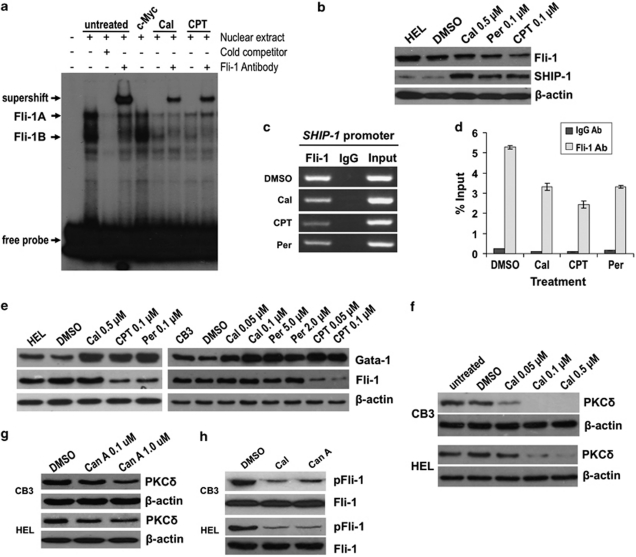
As described above, although CPT and peruvoside downregulate the expression of Fli-1, calcimycin blocks proliferation of Fli-1 overexpressing leukemic cells without effecting the protein levels of this oncogene (Figures 2 and 3). We hypothesized that calcimycin-dependent growth inhibition may result from its ability to inhibit the binding of Fli-1 to its target gene DNA. To test this hypothesis, EMSA were performed using nuclear extracts from HEL cells and labeled oligonucleotides containing two tandem known Fli-1 Ets DNA-binding sites.11 Two dominant bands (Fli-1A and Fli-1B) were detected and supershifted by the addition of Fli-1 antibody, although the addition of c-Myc antibody did not result in supershift (Figure 5a). The Fli-1A band was supershifted more efficiently than the Fli-1B band although both bands disappeared when nuclear extracts were incubated with cold competitor. To examine the effect of anti-Fli-1 drugs on DNA binding, nuclear extracts from HEL cells treated with CPT (0.1 μ) and calcimycin (0.5 μ) for 24 h were subjected to EMSA. As CPT reduces the levels of Fli-1 in HEL cells (Figure 3), reduced binding to Fli-1A and Fli-1B bands and consequently a weaker supershift was detected (Figure 5a). Similarly, cells treated with calcimycin displayed a lower intensity of Fli-1A and Fli-1B bands as well as the Fli-1 supershifted band (Figure 5a).
Figure 5.
Binding of Fli-1 to target gene DNA is inhibited by anti-Fli-1 drug treatment. (a) Nuclear extracts from drug treated (calcimycin 0.5 μ, CPT 0.1 μ) and untreated HEL cells were incubated with γ-32P-labelled oligonucleotides containing a Fli-1-binding site, in the presence or absence of Fli-1 antibody and subjected to EMSA. Addition of the c-Myc antibody was used as a negative control (lane 5). Competition assays were performed in the presence of 100–fold excess unlabeled oligonucleotides (cold competitor). Arrows indicate the position of Fli-1-specific bands, Fli-1A and Fli-1B. (b) Inhibition of Fli-1 through anti-Fli-1 drug treatment results in protein upregulation of the Fli-1 target gene SHIP-111 in HEL cells, as indicated by western blot. (c) Reduced binding of Fli-1 to the SHIP-1 promoter (lane 1) in HEL cells treated with anti-Fli-1 drugs (calcimycin 0.5 μ, CPT 0.1 μ, peruvoside 0.1 μ), as determined by ChIP using 2 μg Fli-1 or rabbit IgG antibody. (d) ChIP quantitative-PCR using Fli-1 and rabbit IgG antibodies illustrating reduced Fli-1 chromatin occupancy on the SHIP-1 promoter in HEL cells treated with the indicated anti-Fli-1 drugs. The level of SHIP-1 enrichment is expressed as the percentage of input chromatin precipitated by Fli-1 or control IgG antibodies. (e) Similar to SHIP-1, inhibition of Fli-1 resulted in upregulation of Gata-1 protein expression in HEL and CB3 cells treated with the indicated dosages of anti-Fli-1 drugs or DMSO control. Treatment of CB3 and HEL cells with the indicated concentration of (f) calcimycin and (g) Can A inhibits the protein expression of PKCδ in a dose-dependent manner. (h) CB3 and HEL cells treated with calcimycin (0.5 μ) and Can A (1.0 μ) display reduced levels of phosphorylated Fli-1 protein, as detected by western blot analysis using an anti-threonine antibody. Anti-Fli-1 antibody was used to demonstrate total protein levels.
The EMSA results demonstrate the ability of calcimycin to significantly lower the DNA-binding activity of Fli-1. Subsequently, we examined the effect of anti-Fli-1 drug treatment on the expression of known Fli-1 target genes, such as SHIP-1.11 SHIP-1 has been identified in our laboratory as a direct target gene, negatively regulated by Fli-1.11 Reduced expression of SHIP-1 correlates with significantly accelerated erythroleukemia progression in vitro and in vivo.11 Accordingly, treatment of the human erythroleukemia cell line HEL with CPT, peruvoside and calcimycin at dosages previously shown to inhibit cell growth (Figure 3), resulted in elevated levels of SHIP-1 (Figure 5b). Inhibition of Fli-1 binding to the SHIP-1 promoter by CPT, peruvoside and calcimycin was also confirmed by ChIP assay, using a Fli-1 antibody and analyzed by PCR amplifying a Fli-1-binding region within the SHIP-1 promoter.11 Results in Figure 5c reveal that anti-Fli-1 drug-treated HEL cells display a reduction in the binding of Fli-1 to its binding site on the SHIP-1 promoter, compared with control DMSO-treated cells. ChIP quantitative PCR (Figure 5d) also illustrated reduced Fli-1 chromatin occupancy on the SHIP-1 promoter. We also examined the expression of Gata-1, a gene known to have a key role in erythroid differentiation and proliferation, whose expression is negatively regulated by Fli-1.7 Accordingly, treatment of both HEL and CB3 cells with each of the three anti-Fli-1 drugs upregulated the expression of Gata-1 (Figure 5e).
Selective anti-Fli-1 drugs block phosphorylation of Fli-1 resulting in inhibition of DNA binding
Calcimycin inhibits Fli-1 DNA binding without affecting its expression levels (Figure 2b). Both isoforms of Fli-1, p51 and p48, are phosphorylated, on serine residues, in part by protein phosphatase 2A (PP2A) in the Jurkat human T-cell leukemia cell line.21 Fli-1 is also phosphorylated at threonine 312 by protein kinase C delta (PKCδ), which promotes its interaction with p300/CREB-binding protein-associated factor and subsequent acetylation in response to transforming growth factor beta.22 Calcimycin may alter Fli-1 phosphorylation status at specific sites, thereby affecting its DNA-binding activity. To test this hypothesis, expression levels of PKCδ were detected in both CB3 and HEL cells treated with calcimycin. Treatment with calcimycin downregulated PKCδ protein expression in a dose-dependent manner (Figure 5f). Interestingly, Can A one of the class VI Fli-1 inhibitors identified in our drug screen (Table 1) previously shown to inhibit PP2A,21 also inhibits PKCδ expression (Figure 5g). Indeed, we have shown that both calcimycin and Can A inhibit phosphorylation of Fli-1 in erythroleukemic cells using a pan-specific-anti-threonine antibody (Figure 5h). Taken together, this data suggests an important role for phosphorylation in the regulation of Fli-1 activity.
Inhibition of leukemogenesis by in vivo anti-Fli-1 drug treatment
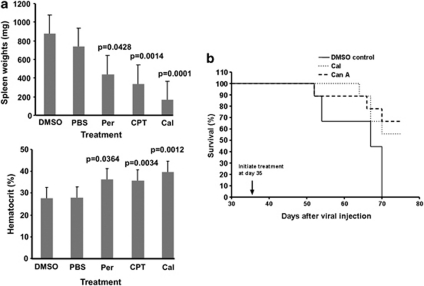
To demonstrate the effect of groups I–III drugs on in vivo cancer progression, we utilized the Friend leukemia mouse model in which Fli-1 overexpression is critical for the induction of this disease.2 Normal Balb/c mice display tolerance to treatment with groups I–III drugs at dosages of 1 mg/kg and 3 mg/kg, without signs of major cytotoxicity when administered for a period of 2 weeks (data not shown). Previous studies demonstrated that erythroleukemia development, associated with fli-1 activation, can be detected as early as 4 weeks post-viral infection.23 Treatment of six-week old leukemic mice (n=9/group) with groups I–III drugs (1 mg/kg) revealed a significant reduction in tumor burden by CPT and calcimycin, as supported by increased hematocrit values and decreased spleen weights, compared with PBS or DMSO-treated control mice (Figure 6a). Consistent with the weak growth inhibitory effect observed with the treatment of peruvoside in murine erythroleukemic cells (Figure 2a), we also detected an inferior and less significant inhibition effect by this drug in our leukemia mouse model. As both calcimycin and Can A were shown to inhibit Fi-1 phosphorylation (Figure 5h), we have also shown that treatment of F-MuLV-infected mice with these drugs significantly extended the survival of these mice (Figure 6b).
Figure 6.
Inhibition of leukemogenesis by anti-Fli-1 drugs. (a) 6-week old mice, infected at birth with F-MuLV, were treated every other day with the indicated drugs (1 mg/kg), for a period of 2 weeks. At the end of drug treatment, mice were sacrificed, and characteristic indicators of leukemia development, spleen weights and hematocrit levels were measured. Treatment with representative drugs from groups I-III significantly delayed leukemia development, associated with increased hematocrit values and reduced spleen size. Statistical significance (P value) is shown for each group. (b) F-MuLV-infected mice treated at day 35 post-infection with calcimycin (Cal; 1 mg/kg), Can A (1 mg/kg) or DMSO and observed for the development of leukemia. All DMSO control mice died 70 days post viral infection; 5 days later (75 dpi) anti-Fli-1 drug-treated mice were killed for purposes of comparison.
Discussion
Recent studies from our group and others have demonstrated that RNAi-mediated Fli-1 downregulation in both murine and human erythroleukemia blocks proliferation and induces apoptosis.9, 10 These results, for the first time, demonstrate the importance of Fli-1 in the induction and progression of both mouse and human erythroleukemia. Aberrant regulation of Fli-1 is also observed in a wide variety of human and murine disorders, suggesting that targeting Fli-1 for inhibition may be a potent treatment strategy for cancers overexpressing this oncogene. In this study, we have identified 30 compounds, classified into six functional groups that block Fli-1 transactivation ability, and associated with growth inhibition in leukemic cell lines overexpressing this oncogene. More importantly, treatment with representative anti-Fli-1 compounds from drug classes I–III also inhibited Friend virus-induced erythroleukemia development.
Among 30 anti-Fli-1 drugs identified in our study, 10 can be characterized as glycosides, a drug class known for their potent ability to inhibit Na+, K+-ATPase, contributing to their anti-cancer properties. Some members of this group like digoxin and ouabain have strong differentiation effects on both murine and human erythroleukemic cells.24, 25 Within this group, the activity and function of peruvoside in cancer is undetermined, as this drug is currently used for the treatment of heart failure. We have shown that treatment with peruvoside downregulates Fli-1 protein expression in humans (Figure 3a) at the transcriptional level (Figure 3d), not in murine erythroleukemic cells (Figure 2a). This result is consistent with weaker Na+/K+-ATPase channel activity reported in murine cells.19 Although treatment of F-MuLV-induced erythroleukemic mice with peruvoside has minimal effects on the induction and progression of disease, this drug may be more efficient for the treatment of human cancers overexpressing Fli-1.
Although peruvoside inhibits Fli-1 expression at the transcriptional level, CPT inhibits expression at the post-transcriptional level (Figures 2c and 3c). CPT has been characterized as a potent topoisomerase I inhibitor.26 A previous study has shown that the degree to which glycosides inhibit cancer cell growth correlates with the ability to inhibit Topoisomerase II-activity.27 It has been previously shown that glycosides also obtain the ability to inhibit Topoisomerase I.28 This suggests that inhibition of Fli-1 by peruvoside and CPT are likely mediated through the inhibition of topoisomerases. In accordance with weak inhibition observed in murine cells treated with glycosides, our results suggest that CPT and Peruvoside utilize different pathways to alter topoisomerase activity.
Etoposide, another potent topoisomerase inhibitor, can be classified within the chemotherapeutic group of drugs, named group V. Including Etoposide, 12 out of 30 anti-Fli-1 drugs identified in our screening strategy are potent inhibitors of topoisomerases. Thus, cancers in which Fli-1 overexpression has a key role in survival and proliferation, topoisomerase inhibitors may exert anti-cancer activity, in part, through inhibition of this transcription factor. This observation suggests that our drug-screening strategy serves as a strong assay for the identification of additional and more potent members of anti-topoisomerase drugs for the treatment of cancer.
Five calcium ionophores with potent anti-Fli-1 activity were identified in our drug screening, suggesting the importance of intracellular Ca+ levels on the regulation of Fli-1 transactivation ability. Although calcimycin is known to induce differentiation and promote erythroleukemic cell death,16, 29 the mechanism of its anti-cancer activity is yet to be identified. As downregulation of Fli-1 in erythroleukemic cells induces differentiation and cell death,9 calcimycin likely induces these events by inhibiting Fli-1 transactivation ability. Indeed, calcimycin inhibited Fli-1 and EWS/Fli-1 transactivation ability without affecting their expression. These results were supported by EMSA and ChIP assays, which revealed reduced DNA binding of Fli-1 in erythroleukemic cells treated with calcimycin. As expected, these drugs also upregulated the expression of the anti-proliferative Fli-1 target gene gata-1 and SHIP-1. Thus, calcimycin and likely other anti-Fli-1 drugs inhibit Fli-1 transactivation ability through a mechanism that does not involve downregulation of this oncoprotein.
It has been shown that Fli-1 is phosphorylated at specific sites, likely altering its transactivational activity.21, 22 In this study, we have shown that reduced Fli-1 DNA binding by calcimycin is associated with a dramatic downregulation of PKCδ expression, a kinase known to phosphorylate Fli-1 at threonine 312.22 Another striking observation in our study was that cantharidin, a known PP2A phosphatase inhibitor,21 also inhibits PKCδ expression in erythroleukemic cells. Indeed, both calcimycin and Can A block phosphorylation on the threonine residue of erythroleukemic cells in vitro and, accordingly, administration of these drugs in mice inhibited erythroleukemia development. These results demonstrate that inhibition of Fli-1 phosphorylation, mediated by PKCδ downregulation, blocks Fli-1 transactivation ability through restriction of its DNA-binding capacity. The above observations also suggest that this drug-screening strategy is a potent method to uncover transcription factor function, as we have demonstrated in the case of Fli-1. Moreover, our results also raise the possibility that several uncharacterized anti-Fli-1 drugs may alter other aspects of Fli-1 activity that could further provide insight into the role of this transcription factor in oncogenesis and development.
In summary, our mechanistic drug-screening strategy aimed at targeting EWS/Fli-1 has led to the identification of 30 compounds capable of inhibiting the function of this fusion oncoprotein. Selected compounds from three distinct drug classes blocked the expression and/or the activity of Fli-1 in leukemic cells resulting in cell growth arrest and apoptosis. In a mouse model of erythroleukemia overexpressing Fli-1, these drugs have also demonstrated strong anti-leukemic activity. Taken together, these results revealed the potential and importance of targeting Fli-1 activity for the improvement of cancer therapy, and have identified a simple and sensitive strategy to identify potent anti Fli-1 compounds for the treatment of various cancers overexpressing Fli-1.
Acknowledgments
This work was supported by a grant from the Ontario Institute for Cancer Research (OICR) and Canadian Institute of Health Research (CIHR) to YBD (MOP-110952). We would like to thank Ms Melanie Suttar for her excellent secretarial assistance.
The authors declare no conflict of interest.
References
- Ben-David Y, Giddens EB, Bernstein A. Identification and mapping of a common proviral integration site Fli-1 in erythroleukemia cells induced by Friend murine leukemia virus. Proc Natl Acad Sci USA. 1990;87:1332–1336. doi: 10.1073/pnas.87.4.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-David Y, Giddens EG, Letwin K, Bernstein A. Erythroleukemia induction by Friend murine leukemia virus: insertional activation of a new member of the ets gene family, Fli-1, closely linked to c-ets-1. Genes Dev. 1991;5:908–918. doi: 10.1101/gad.5.6.908. [DOI] [PubMed] [Google Scholar]
- Tamir A, Howard J, Higgins RR, Li YJ, Berger L, Zacksenhaus E, et al. Fli-1, an Ets-related transcription factor, regulates erythropoietin-induced erythroid proliferation and differentiation: evidence for direct transcriptional repression of the Rb gene during differentiation. Mol Cell Biol. 1999;19:4452–4464. doi: 10.1128/mcb.19.6.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zochodne B, Truong AH, Stetler K, Higgins RR, Howard J, Dumont D, et al. Epo regulates erythroid proliferation and differentiation through distinct signaling pathways: implication for erythropoiesis and Friend virus-induced erythroleukemia. Oncogene. 2000;19:2296–2304. doi: 10.1038/sj.onc.1203590. [DOI] [PubMed] [Google Scholar]
- Bergeron D, Poliquin L, Kozak CA, Rassart E. Identification of a common viral integration region in Cas-Br-E murine leukemia virus-induced non-T, non-B cell lymphomas. J Virol. 1991;65:7–15. doi: 10.1128/jvi.65.1.7-15.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delattre O, Zucman J, Plougastel B, Desmaze C, Melot T, Peter M, et al. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature. 1992;359:162–165. doi: 10.1038/359162a0. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski BA, Bastian LS, Bauer TR, Tsai S, Zielinska-Kwiatkowska AG, Hickstein DD. The ets family member Tel binds to the Fli-1 oncoprotein and inhibits its transcriptional activity. J Biol Chem. 1998;273:17525–17530. doi: 10.1074/jbc.273.28.17525. [DOI] [PubMed] [Google Scholar]
- Mhawech-Fauceglia P, Herrmann F, Bshara W, Odunsi K, Terracciano L, Sauter G, et al. FLI-1 Expression in 4323 malignant and benign tumours: a multiple tumour tissue microarray analysis using polyclonal antibody. J Clin Pathol. 2006;60:694–700. doi: 10.1136/jcp.2006.039230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui JW, Vecchiarelli-Federico LM, Li YJ, Wang GJ, Ben-David Y. Continuous Fli-1 expression plays an essential role in the proliferation and survival of F-MuLV-induced erythroleukemia and human erythroleukemia. Leukemia. 2009;23:1311–1319. doi: 10.1038/leu.2009.20. [DOI] [PubMed] [Google Scholar]
- Juban G, Giraud G, Guyot B, Belin S, Diaz JJ, Starck J, et al. Spi-1 and Fli-1 directly activate common target genes involved in ribosome biogenesis in Friend erythroleukemic cells. Mol Cell Biol. 2009;29:2852–2864. doi: 10.1128/MCB.01435-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhanpal GK, Vecchiarelli-Federico LM, Li YJ, Cui JW, Bailey ML, Spaner DE, et al. The inositol phosphatase SHIP-1 is negatively regulated by Fli-1 and its loss accelerates leukemogenesis. Blood. 2010;116:428–436. doi: 10.1182/blood-2009-10-250217. [DOI] [PubMed] [Google Scholar]
- May WA, Gishizky ML, Lessnick SL, Lunsford LB, Lewis BC, Delattre O, et al. Ewing sarcoma 11;22 translocation produces a chimeric transcription factor that requires the DNA-binding domain encoded by FLI1 for transformation. Proc Natl Acad Sci USA. 1993;90:5752–5756. doi: 10.1073/pnas.90.12.5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JH, Chung TD, Oldenburg KR. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- Brideau C, Gunter B, Pikounis B, Liaw A. Improved statistical methods for hit selection in high-throughput screening. J Biomol Screen. 2003;8:634–647. doi: 10.1177/1087057103258285. [DOI] [PubMed] [Google Scholar]
- Prassas I, Paliouras M, Datti A, Diamandis EP. High-throughput screening identifies cardiac glycosides as potent inhibitors of human tissue kallikrein expression: implications for cancer therapies. Clin Cancer Res. 2008;14:5778–5784. doi: 10.1158/1078-0432.CCR-08-0706. [DOI] [PubMed] [Google Scholar]
- Sparatore B, Pessino A, Patrone M, Passalacqua M, Melloni E, Pontremoli S. Changes in calcium influx affect the differentiation of murine erythroleukaemia cells. Biochem J. 1995;305 (Pt 1:285–290. doi: 10.1042/bj3050285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priel E, Aflalo E, Chechelnitsky G, Benharroch D, Aboud M, Segal S. Inhibition of retrovirus-induced disease in mice by camptothecin. J Virol. 1993;67:3624–3629. doi: 10.1128/jvi.67.6.3624-3629.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia S, Krailo MD, Chen Z, Burden L, Askin FB, Dickman PS, et al. Therapy-related myelodysplasia and acute myeloid leukemia after Ewing sarcoma and primitive neuroectodermal tumor of bone: a report from the Children's Oncology Group. Blood. 2007;109:46–51. doi: 10.1182/blood-2006-01-023101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perne A, Muellner MK, Steinrueck M, Craig-Mueller N, Mayerhofer J, Schwarzinger I, et al. Cardiac glycosides induce cell death in human cells by inhibiting general protein synthesis. PLoS ONE. 2009;4:e8292. doi: 10.1371/journal.pone.0008292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau-Gachelin F, Tavitian A, Tambourin P. Spi-1 is a putative oncogene in virally induced murine erythroleukaemias. Nature. 1988;331:277–280. doi: 10.1038/331277a0. [DOI] [PubMed] [Google Scholar]
- Zhang XK, Watson DK. The FLI-1 transcription factor is a short-lived phosphoprotein in T cells. J Biochem. 2005;137:297–302. doi: 10.1093/jb/mvi032. [DOI] [PubMed] [Google Scholar]
- Asano Y, Trojanowska M. Phosphorylation of Fli1 at threonine 312 by protein kinase C delta promotes its interaction with p300/CREB-binding protein-associated factor and subsequent acetylation in response to transforming growth factor beta. Mol Cell Biol. 2009;29:1882–1894. doi: 10.1128/MCB.01320-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard JC, Ung Y, Adachi D, Ben-David Y. p53-independent tumor growth and in vitro cell survival for F-MuLV-induced erythroleukemias. Cell Growth Differ. 1996;7:1651–1660. [PubMed] [Google Scholar]
- Mager D, Bernstein A. The program of Friend cell erythroid differentiation: early changes in Na+/K+ ATPase function. J Supramol Struct. 1978;8:431–438. doi: 10.1002/jss.400080405. [DOI] [PubMed] [Google Scholar]
- Bernstein A, Hunt DM, Crichley V, Mak TW. Induction by ouabain of hemoglobin synthesis in cultured Friend erythroleukemic cells. Cell. 1976;9:375–381. doi: 10.1016/0092-8674(76)90082-9. [DOI] [PubMed] [Google Scholar]
- Rasheed ZA, Rubin EH. Mechanisms of resistance to topoisomerase I-targeting drugs. Oncogene. 2003;22:7296–7304. doi: 10.1038/sj.onc.1206935. [DOI] [PubMed] [Google Scholar]
- Winnicka K, Bielawski K, Bielawska A. Cardiac glycosides in cancer research and cancer therapy. Acta Pol Pharm. 2006;63:109–115. [PubMed] [Google Scholar]
- Lawrence TS. Reduction of doxorubicin cytotoxicity by ouabain: correlation with topoisomerase-induced DNA strand breakage in human and hamster cells. Cancer Res. 1988;48:725–730. [PubMed] [Google Scholar]
- Levenson R, Housman D, Cantley L. Amiloride inhibits murine erythroleukemia cell differentiation: evidence for a Ca2+ requirement for commitment. Proc Natl Acad Sci USA. 1980;77:5948–5952. doi: 10.1073/pnas.77.10.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]