Abstract
In follicular lymphoma, nonmalignant immune cells are important. Follicular lymphoma depends on CD4+ cells, but CD8+ cells counteract it. We hypothesized that the presence of follicular lymphoma is associated with higher CD4+ than CD8+ cell numbers in the tumor microenvironment but not in the immune system. Using flow cytometry, pre-treatment and follow-up CD4/CD8 ratios were estimated in the bone marrow, blood and lymph nodes of untreated follicular lymphoma patients in two independent data sets (N1=121; N2=166). The ratios were analyzed for their relation with bone marrow lymphoma involvement. Bone marrows were also investigated with immunohistochemistry. In either data set, the bone marrow CD4/CD8 ratios were higher in bone marrows involved with lymphoma (P=0.043 and 0.0002, respectively). The mean CD4/CD8 ratio was 1.0 in uninvolved and 1.4 in involved bone marrows. Also higher in involved bone marrows were CD4/CD56 and CD3CD25/CD3 ratios. No blood or lymph node ratios differed between bone marrow-negative and -positive patients. Sequential samples showed increased bone marrow CD4/CD8 ratios in all cases of progression to bone marrow involvement. Immunohistochemistry showed CD4+, CD57+, programmed death-1+, forkhead box protein 3+ and CD21+ cells accumulated inside the lymphoma infiltrates, whereas CD8+, CD56+ and CD68+ cells were outside the infiltrates. This study provides evidence in vivo that the microenvironment changes upon follicular lymphoma involvement.
Keywords: follicular lymphoma, microenvironment, bone marrow, CD4/CD8 ratio
Introduction
Investigations of follicular lymphoma lymph nodes have shown that high levels of CD4+ cells,1, 2 low levels of CD8+ cells,2, 3, 4 high CD4/CD8 ratios,2 and more follicular than interfollicular CD4+ cells2, 5 correlate with inferior clinical outcome. Follicular lymphoma cells, as their normal counterparts, germinal center B cells, are supported and protected by signals from CD4+ follicular helper T cells but counteracted by CD8+ cytotoxic T cells, according to several in vitro studies.6, 7, 8, 9, 10, 11, 12, 13, 14
There thus seems to be a vital incentive for follicular lymphoma to recruit CD4+ cells and to reduce the number of CD8+ cells. We hypothesized that the presence of follicular lymphoma is associated with higher CD4+ than CD8+ cell numbers in the immune microenvironment but not in the immune system. Using flow cytometry, we analyzed pre-treatment and follow-up CD4/CD8 ratios in bone marrows, blood and lymph nodes with respect to bone marrow involvement in two independent data sets of untreated patients with follicular lymphoma. Bone marrow involvement, seen in 40–70% of cases, is also an important adverse factor for outcome,15 even in the current era of therapy with the anti-CD20 monoclonal antibody rituximab.16
Materials and methods
First data set
We first constructed a data set of patients treated in Stockholm between 1994 and 2010. Their bone marrows had been sampled at the time of their original diagnosis of follicular lymphoma at the Department of Pathology, Karolinska University Hospital, Huddinge, Sweden. Flow cytometry analysis was routinely performed on all unfixed material, as described previously.4 At least 10 000 cells were collected and analyzed. The antibody clones used for flow cytometry varied over these 16 years. The patients in the first data set were studied according to a protocol approved by the Ethics Committee in Stockholm. Clinical characteristics were obtained from the patient files.
Second data set
The independent second set consisted of other patients who participated in two similar Nordic Lymphoma Group trials where all patients received rituximab without chemotherapy (M39035(ref. 17) and ML16865).18 Bone marrows were sampled at trial inclusion, and flow cytometry was recommended in the trial protocols. The flow cytometry assays at the participating centers were conducted according to a central protocol, as reported previously.18 At least 10 000 cells were collected and analyzed. The CD4, CD8, CD25 and CD56 flow cytometry antibody clones were chosen according to local routines and, thus, the clones used for flow cytometry varied between different institutions. These patients were studied under protocols approved by the Ethics Committee at each participating center. Clinical characteristics were obtained from case report forms.
In both the first and the second set, every diagnosis of follicular lymphoma had been ascertained in central pathology reviews of diagnostic lymph nodes.4, 18 We selected patients in whom the first bone marrow sample had been obtained before any systemic therapy, because therapy might differentially affect different T-cell subsets.19, 20 A bone marrow sample consisted of an aspiration (for flow cytometry) and a biopsy (for morphological diagnosis of bone marrow involvement). Bone marrow involvement was established on the basis of local pathology reports of the bone marrow biopsies. All bone marrows (first set, N1=121; second set, N2=166; Ntotal=287) had been analyzed with flow cytometry enumerations of CD4+ and CD8+ cells and all results were reported as percentages of events within lymphocyte gate.
The bone marrow CD4/CD8 ratio
Bone marrow involvement in follicular lymphoma is the result of an invasion by malignant B cells into the tissue. Because the malignant B cells are counted in flow cytometry assays, the CD4+ and CD8+ cell percentages will be affected but not the CD4/CD8 ratio, which was therefore the primary variable. The CD4/CD56 and CD3CD25/CD3 ratios, when available (n=158 and 85, respectively), were also analyzed.
Blood and lymph nodes and sequential samples
Blood is seldom involved with follicular lymphoma, while the diagnostic lymph nodes by definition are. To investigate whether bone marrow involvement has an association with distant immune cells in uninvolved tissue (blood) or involved tissue (lymph nodes), we evaluated all contemporaneous flow cytometry assays of blood (n=108) and diagnostic lymph nodes (n=169). We also analyzed follow-up bone marrows (n=155), including nine cases with simultaneous blood flow cytometry.
Immunohistochemistry
In the first set, 47 bone marrow biopsies obtained at diagnosis had sufficient material for additional stainings. They were reviewed with additional immunohistochemistry stainings for CD79a (JCB117, Dako, Glostrup, Denmark), CD20 (L26, Dako), CD21 (2G9, Novocastra, Newcastle, UK), CD3 (565, Novocastra), CD4 (368, Novocastra), CD57 (NKI, Novocastra), programmed death-1 (PD-1; AB 52587, Abcam, Cambridge, UK), forkhead box protein 3 (FOXP3; AB 20034, Abcam), CD8 (C8/144B, Dako), CD56 (1B6, Novocastra) and CD68 (PG-M1, Dako). The stainings were analyzed without knowledge of the flow cytometry results.
Statistical analysis
To normalize the ratios, a logarithmic transformation was applied before statistical testing for association with bone marrow positivity. In univariate analysis, differences in ratios between patients with and without bone marrow involvement were estimated with Student's t-test (for sequential samples in the same patients the paired t-test was used). Multivariate analysis was performed with forward stepwise logistic regression. The multivariate models did not contain any interactions, and they passed post-estimation controls. Correlations between ratios were estimated with linear regression. Categoric data were compared using the χ2-test. All P-values are two-tailed and calculated using Stata 9.2 (StataCorp. LP, College Station, TX, USA).
Results
Bone marrow CD4/CD8 ratios are significantly higher in patients with bone marrow involvement
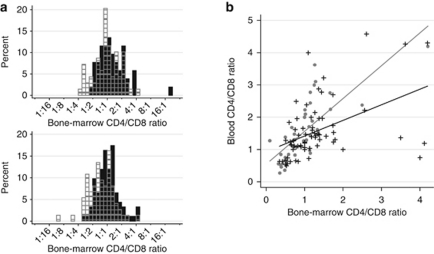
Lymphoma involvement of the bone marrow was seen in 52 out of 121 patients (43%) in the first data set and in 92 out of 166 (55%) in the second set, in total in 144 out of 287 (50%). In the first set, the bone marrow CD4/CD8 ratios were significantly higher in patients who had bone marrow involvement than in those who did not (P=0.043; Table 1). The finding was confirmed in the independent second set (P=0.0002). In the combined data set, the mean bone marrow CD4/CD8 ratio was 1.0 in patients without and 1.4 in patients with bone marrow involvement (overall P=0.0002; N=287). Graphically, the CD4/CD8 ratios showed a slight general shift to the right in the bone marrow-positive populations (Figure 1a). Some clinical factors correlated with bone marrow involvement in either data set (Table 2). The only clinical factor significant in both the first and the second set was more than four involved nodal areas. Multivariate analysis of the bone marrow CD4/CD8 ratios together with all clinical factors listed in Table 2 showed that the bone marrow CD4/CD8 ratios (first set, P=0.034; second set, P=0.001; overall, P=0.0003) and more than four involved nodal areas (first set, P<0.0001; second set, P<0.0001; overall, P<0.0001) were independently associated with bone marrow involvement. Bone marrow-positive samples also presented significantly higher bone marrow CD4/CD56 ratios (P=0.002; n=158). The mean bone marrow CD4/CD56 ratio was 1.8 in patients without and 2.8 in patients with bone marrow involvement. The CD3CD25/CD3 ratios were higher in involved (mean 0.13) than in uninvolved (mean 0.10) bone marrows but the difference was not significant (P=0.06; n=85).
Table 1. CD4/CD8 ratios in bone marrow, blood and lymph nodes.
| Ratio |
First data set (N1=121) |
Second data set (N2=166) |
Overall (N=287) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Bone marrow uninvolved |
Bone marrow involved |
P-value |
Bone marrow uninvolved |
Bone marrow involved |
P-value | P-value | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||
| Bone marrow CD4/CD8 | 1.2 | 0.7 | 1.7 | 2.8 | 0.043 | 0.9 | 0.6 | 1.2 | 0.8 | 0.0002 | 0.0002 |
| Blood CD4/CD8 | 1.9 | 1.2 | 2.2 | 1.2 | 0.52 | 1.5 | 0.9 | 1.4 | 0.8 | 0.79 | 0.54 |
| Lymph node CD4/CD8 | 4.0 | 2.1 | 4.7 | 3.6 | 0.36 | 4.5 | 3.1 | 4.7 | 2.5 | 0.48 | 0.25 |
Figure 1.
Bone marrow CD4/CD8 ratios and their correlations to blood CD4/CD8 ratios stratified by bone marrow lymphoma involvement. (a) Shows histograms of the bone marrow CD4/CD8 ratios in the first (above) and second (below) data sets. Black bars show the distribution of CD4/CD8 ratios in bone marrows involved with lymphoma and gray-striped transparent bars CD4/CD8 ratios in those uninvolved with lymphoma. (b) Shows the correlations between bone marrow and blood CD4/CD8 ratios. In patients without bone marrow lymphoma involvement (gray dots and gray fitted line), there was a general, systemic congruence between the two types of tissue. In patients with bone marrow involvement (black crosses and black fitted line), there was markedly less congruence.
Table 2. Clinical characteristics.
| Characteristic |
First data set (N1=121) |
Second data set (N2=166) |
Overall (N=287) | ||||
|---|---|---|---|---|---|---|---|
| Bone marrow uninvolved (N=69) | Bone marrow involved (N=52) | P-value | Bone marrow uninvolved (N=74) | Bone marrow involved (N=92) | P-value | P-value | |
| Histological subtype—% | 0.69 | 0.55 | 0.92 | ||||
| Grade 1 | 29 | 29 | 53 | 48 | |||
| Grade 2 | 55 | 50 | 43 | 48 | |||
| Grade 3A | 16 | 21 | 4 | 4 | |||
| Male sex—% | 49 | 56 | 0.48 | 54 | 49 | 0.51 | 0.95 |
| Age >60 years—% | 52 | 37 | 0.087 | 35 | 29 | 0.43 | 0.046 |
| LDH elevated—% | 33 | 31 | 0.77 | 20 | 36 | 0.028 | 0.17 |
| Hemoglobin <12 g/dl—% | 3 | 8 | 0.23 | 14 | 24 | 0.091 | 0.016 |
| >4 nodal areas involved—% | 13 | 56 | <0.0001 | 35 | 71 | <0.0001 | <0.0001 |
| B symptoms—% | 14 | 33 | 0.017 | 23 | 24 | 0.89 | 0.099 |
Abbreviation: LDH, lactate dehydrogenase.
Blood and lymph node CD4/CD8 ratios are similar in patients with and without bone marrow involvement
The CD4/CD8 ratios in blood (mean 1.6) and lymph nodes (mean 4.5) were similar in patients with and without bone marrow involvement in either data set as well as in the combined data set (Table 1). Also equal in bone marrow-positive and -negative patients were CD4/CD56 ratios in blood (mean 2.6; P=0.86; n=86) and lymph nodes (mean 51.4; P=0.70; n=97) as were CD3CD25/CD3 ratios in blood (mean 0.14; P=0.25; n=22) and lymph nodes (mean 0.23; P=0.92; n=62).
Bone marrow and blood CD4/CD8 ratios are closely related in bone marrow-negative patients but not in bone marrow-positive patients
In patients without bone marrow involvement, more than half of the variation of the bone marrow CD4/CD8 ratios could be explained by the blood CD4/CD8 ratios, and vice versa (R2=0.6; Figure 1b). In bone marrow-positive patients, the bone marrow and blood CD4/CD8 ratios showed a much weaker association (R2=0.2). The lymph node ratios were entirely unrelated to bone marrow or blood ratios (data not shown).
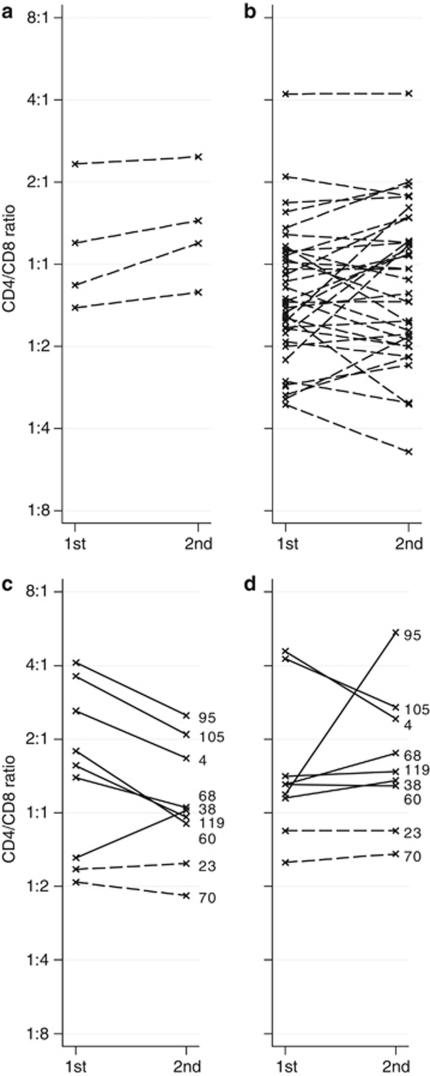
Sequential samples from bone marrow and blood suggest that the bone marrow CD4/CD8 ratio increases upon lymphoma involvement and decreases when the lymphoma disappears
Of the 155 patients with follow-up bone marrow samples, there were four initially bone marrow-negative patients whose follow-up bone marrow biopsies showed lymphoma involvement. This change to bone marrow involvement was accompanied by an increased bone marrow CD4/CD8 ratio in all four patients, and thus that more CD4+ than CD8+ cells were recruited (P=0.035; Figure 2a). One of these four patients remained untreated but the other three received rituximab between the first and the second biopsy. Rituximab could affect the T-cell subsets. To control for this, we investigated the 36 patients who showed no bone marrow involvement both before and after treatment with rituximab and found no difference between CD4/CD8 ratios in the first and second bone marrow biopsies (mean 1.0 at both time points; Figure 2b). Furthermore, in the total of 125 patients whose bone marrows were analyzed before and after rituximab therapy, there was no change in the bone marrow CD4/CD8 ratios (mean 1.2 at both time points). Bone marrow and blood were simultaneously analyzed in nine patients both before and after rituximab therapy; seven were initially bone marrow positive and two were negative. All nine patients were bone marrow negative at follow-up sampling. Six of the seven patients who changed from involvement to no involvement of the bone marrow had a marked decrease of the CD4/CD8 ratios in bone marrow (P=0.023; Figure 2c) but not in blood (P=0.83; Figure 2d). However, the decrease in bone marrow CD4/CD8 ratios was not significant when analyzing all 65 bone marrows that turned from positive to negative (P=0.22), although the mean changed from 1.5 to 1.1.
Figure 2.
CD4/CD8 changes in sequential samples. Dashed lines represent patients with initially no bone marrow lymphoma involvement and solid lines those with initial involvement. The y scales are logarithmic. (a) Shows how bone marrow CD4/CD8 ratios increased in all four bone marrow-negative patients who progressed to bone marrow involvement. Three of these four patients received rituximab in the meantime, but (b) demonstrates that rituximab therapy did not induce bone-marrow CD4/CD8 changes in patients who retained bone marrows free from lymphoma. (c, d) Show the nine patients with complete sequential samples of bone marrow and blood CD4/CD8 ratios. The numbers represent patient identity. All nine received rituximab between samples. Seven patients (solid lines) cleared their marrows from lymphoma, and six of these also showed decreased bone marrow CD4/CD8 ratios (c), but there was no similar tendency in the comparable blood CD4/CD8 ratios of which two decreased, two were stable and three increased (d).
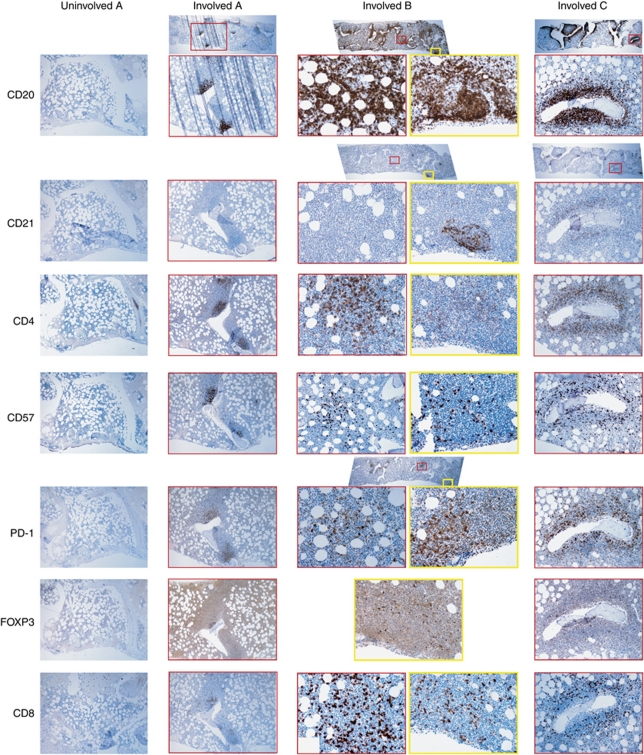
Immunohistochemistry analysis of involved bone marrows reveals higher frequencies and altered topographic distributions of T-cell subsets and follicular dendritic cells
In every investigated case, the archival report whether the bone marrow was involved with lymphoma (n=21) or not (n=26) was confirmed. The 26 bone marrow biopsies without lymphoma involvement showed invariably a sparse, diffuse distribution of cells positive for CD3, CD4, CD8, CD20, CD56, CD57, CD79a, FOXP3 and CD68; cells positive for CD21 were absent and those positive for PD-1 were extremely rare (Figure 3). In 16 out of the 21 biopsies positive for follicular lymphoma, CD21+ follicular dendritic cell networks surrounded the lymphoma cells, but a remarkable finding was that in five cases the follicular lympoma infiltrates lacked CD21+ networks. Furthermore, some biopsies contained a mix of CD21+ and CD21- lymphoma infiltrates (Figure 3). The CD4+ cells homed to the follicular lymphoma infiltrates in all cases, as did FOXP3+ and CD57+ cells. PD-1+ cells resided almost exclusively in the lymphoma infiltrates, usually in the central area of them. In the involved bone marrows, the CD8+ cells were few, found outside or in the periphery of the infiltrates, or sometimes sparsely throughout the biopsy. The CD68+ and CD56+ cells were diffusely distributed outside the lymphoma infiltrates but the areas with lymphoma infiltration contained very few CD68+ and CD56+ cells (Data not shown).
Figure 3.
Immunohistochemistry of bone marrows. All investigated bone marrows without follicular lymphoma involvement had the same appearance (left-most column, Uninvolved A), showing few and scattered cells positive for CD20, CD4, CD57, FOXP3 and CD8. There are no cells positive for CD21 or PD-1. This bone marrow is from the patient who later progressed to bone marrow involvement (Involved A), without any interceding therapy. The CD20 staining in the later biopsy shows a moderate infiltration of lymphoma cells. There are no accompanying CD21+ follicular dendritic cells. CD4+ and CD57+ T cells are mostly located in the infiltrates. PD-1+ T cells are exclusively found in the infiltrates and also FOXP3+ cells have homed there. There are some CD8+ cytotoxic T cells in the periphery of the infiltrates. Involved B is a bone marrow with heavy involvement of follicular lymphoma. Two involved areas (the red and yellow squares) have been examined in large magnification. CD21+ follicular dendritic cell networks are seen in one area, but not in the other, suggesting different stromal cells' support in the same specimen. The distributions of cells positive for CD4, CD57, PD-1 and CD8 seem to differ between the two areas. In both areas, FOXP3+ showed a perifollicular pattern. Involved C shows peritrabecular follicular lymphoma infiltrates. There is a CD21+ follicular dendritic cell network and an aggregation of CD4+, CD57+, PD-1+ and FOXP3+ T cells.
Increasing bone marrow involvement correlates with higher bone marrow CD4/CD8 ratios
At review, the percentage of lymphoma involvement in the involved bone marrow biopsies was estimated. There was a positive correlation between increasing areas of lymphoma in the biopsy and higher bone marrow CD4/CD8 (P=0.010) and CD4/CD56 (P=0.043) ratios in flow cytometry. The median percentage of bone marrow involvement was 15%. In bone marrows with <15% lymphoma infiltration, the mean bone marrow CD4/CD8 ratio was 1.2, while in those with ⩾15% lymphoma, the mean was 2.9. The corresponding figures for CD4/CD56 were 2.1 and 3.6.
Discussion
This report shows that follicular lymphoma cells in the bone marrow alter the local immune microenvironment. Using flow cytometry in two independent data sets of untreated patients, we found increased bone marrow CD4/CD8 ratios in bone marrows involved with follicular lymphoma. The blood and lymph node CD4/CD8 ratios did not change with bone marrow involvement, suggesting that the immunomodulating effect of follicular lymphoma is locally restricted. We also examined sequential samples of patients and found that initially bone marrow-negative patients who later progressed to bone marrow involvement showed accompanying increases in their bone marrow CD4/CD8 ratios. This did not appear to be an effect from interceding rituximab, because rituximab did not change the CD4/CD8 ratios in patients whose disease did not progress to bone marrow involvement.
Using flow cytometry, we investigated T-cell subsets positive for CD4, CD8 and CD25, as well as natural killer (NK) cells positive for CD56. We found that follicular lymphoma is connected with relatively more CD4+ T cells than CD8+ T cells and CD56+ NK cells. There was also a trend for larger proportions of T cells positive for CD25 in involved bone marrows. To further investigate these findings, we examined a fraction of the bone marrows using immunohistochemistry. CD4+ cells were abundant within follicular lymphoma infiltrates. The results were similar for FOXP3+ (regulatory T) and CD57+ T cells. PD-1+ cells were detected in the involved bone marrows, always inside the lymphoma infiltrates, but were virtually absent in lymphoma-negative marrows. CD21+ networks were found in 76% of the lymphoma-involved bone marrows but in none of those without lymphoma. CD8+ cells were scarce and located in the periphery of the lymphoma infiltrates or diffusely distributed outside them. CD56+ NK cells and CD68+ macrophages were located outside the infiltrates. These findings are similar to the microenvironment of follicular lymphoma lymph nodes. It appears that follicular lymphoma brings its entourage to the involved tissue.21
Multiple mechanisms, none of which are mutually exclusive, may be invoked to explain why follicular lymphoma needs its entourage. Follicular lymphoma cells, such as normal germinal center B cells, seem to be dependent on other cells in the microenvironment. Follicular helper T cells express CD40 ligand, which interacts with CD40 on malignant and nonmalignant B cells and protects them from apoptosis.6, 7, 8, 9 Follicular helper T cells also secrete interleukin (IL)-4 at very high levels, promoting survival and proliferation of follicular lymphoma cells.10, 11, 12 The IL-4 receptor gene is upregulated in follicular lymphoma cells.11, 22 IL-4 inhibits CD8+ cells.23 Likewise, both follicular lymphoma and germinal center B cells are supported by follicular dendritic cells.24, 25, 26 In this report, most, but not all, investigated lymphoma-infiltrated bone marrows contained CD21+ follicular dendritic cell networks. The CD21+ follicular dendritic cells are normally alien to the bone marrow. Migration of follicular dendritic cells from lymph tissue is unlikely. Rather, the follicular lymphoma cells induce local mesenchymal precursors to differentiate into CD21+ follicular dendritic cells.27, 28 Bone marrow mesenchymal cells have also been shown to nurse follicular lymphoma cells,13, 29, 30 and could possibly substitute for CD21+ follicular dendritic cell networks (Figure 3). The stromal cells' growth-promoting effects on follicular lymphoma cells are overruled by interferon-γ, which is mainly produced by CD8+ cytotoxic T cells and CD56+ NK cells within normal and malignant lymphoid organs.13 Interferon-γ also inhibits B-cell migration,31 plausibly preventing the spread of follicular lymphoma. Furthermore, interferon-γ modulates the development of naïve CD4+ T cells to helper1 T cells.32 The balance between helper1–cytotoxic T-cell cytokines on one hand and helper2–follicular helper T-cell cytokines on the other is probably skewed in follicular lymphoma. IL-4 is the only cytokine that is found in higher levels in follicular lymphoma than in reactive follicular hyperplastic lymph nodes.33
Follicular lymphoma cells, follicular helper T cells, follicular dendritic cells and bone marrow mesenchymal stem cells can secrete C–X–C motif chemokine 13 (CXCL13), which attracts C–X–C chemokine receptor type 5 (CXCR5)-positive follicular helper T and follicular lymphoma cells to the lymphoma area.34, 35, 36 B cells express membrane lymphotoxin α1β2 and tumor necrosis factor to stimulate CXCL13 production in mesenchymal cells.13 A subset of CXCR5+CD4+ follicular helper T cells is positive for PD-1, and we found PD-1+ cells exclusively in the lymphoma infiltrates. These CXCR5+CD4+ T cells will home to the follicular lymphoma infiltrate and contribute to an increased production of CXCL13 that will attract yet more CXCR5+ cells. Subsequently, this local aggregation of IL-4 secretors will further perturb the microenvironment, including a skewing of the differentiation of naïve CD4+ cells to the production of more B-cell helpers and an inhibition of the CD8+ cells. We also showed that regulatory T cells were prevalent in the lymphoma infiltrates, concurring with the results of an in vitro study, which reported that follicular lymphoma cells skew the microenvironment in favor of more regulatory T cells.37 It has experimentally been shown that regulatory T cells attenuate other T cells in the follicular lymphoma microenvironment.38, 39, 40 It has also been demonstrated that follicular lymphoma cells induce dysfunctions in neighboring normal T cells, by reducing their ability to form F-actin immune synapses.41 Our findings also agree with a recent study, which showed that there are more CXCR5+ (especially CXCR5+CD25+CD57+) T cells in follicular lymphoma nodes than in normal lymph nodes, but the authors also reported that the remaining helper1 T cells in the tumors should retain their intrinsic ability to elicit anti-tumor specific responses.42 A ‘vaccinal effect'43, 44 might explain why the prognostic impact of CD4 cells is reversed by rituximab.18
Normally, the approximate mean CD4/CD8 ratio in bone marrow is 1.0,45 in blood 1.5,46 and in cadaverous normal lymph nodes 3.3.47 In the present study, the mean CD4/CD8 ratio was normal (1.0) in bone marrows without lymphoma involvement but high (1.4) in bone marrows with lymphoma. The mean blood CD4/CD8 ratios in bone marrow-negative and -positive patients were similar, 1.53 and 1.57, respectively. The lymph node CD4/CD8 ratios were high, regardless of the distant bone marrow status (Table 1). This is consistent with the hypothesis that follicular lymphoma exerts a local rather than a systemic influence on immune cells. When we compared CD4/CD8 ratios in blood and negative bone marrows, we found that they were closely related (R2=0.6; Figure 1b) but markedly less so when the bone marrow was involved with lymphoma (R2=0.2). The residual squared correlation coefficient of 0.2 and the CD4/CD8 ratios' modest shift to the right (Figure 1a) in bone marrow-positive cases are explained by the parts of the bone marrows, which remained free from lymphoma. In these parts of the marrows there is no follicular lymphoma microenvironment and the systemic, host-specific immune-cell balances are intact (Figure 3). Indeed, the bone marrow CD4/CD8 ratios increased with the degree of follicular lymphoma involvement. Another explanation for the residual correlation between CD4/CD8 in blood and involved marrows is an inherent weakness of the bone marrow sampling method: aspiration will always dilute the marrow with peripheral blood. The CD4/CD8 ratios in normal bone marrows are lower if flow cytometry is conducted on biopsies instead of aspirates (mean 0.6 instead of 1.0).45 However, aspiration for flow cytometry is the sole method in clinical and research use and the dilution of the bone marrow cell suspension by peripheral blood would only slightly decrease the significant differences reported here, and would not lead to false-positive results.
Our study suggests that the microenvironment of the bone marrow changes in patients who progress to bone marrow involvement. This conclusion is, however, based on only four bone marrow-negative patients who later progressed to bone marrow involvement (Figure 2). The findings give some corroboration to the concept that follicular lymphoma changes its microenvironment, rather than the idea that host-specific factors in the bone marrow will make it more or less prone to being invaded. Other limitations to this study exist. This report focused on the CD4/CD8 ratio, an established assessment of the balance between the two major T-cell subsets, to show that follicular lymphoma alters the local microenvironment. Our data on the CD4/CD8 ratio provide only a small fraction of the full picture, because CD4+ and CD8+ cells are but two of many factors, which would be altered by the lymphoma, as suggested by the immunohistochemistry findings and by the CD4/CD56 and CD3CD25/CD3 ratios, and also by previous studies. However, a strength of this study is the large number of untreated patients in two independent data sets with sequential samples. Another strength is that the outcome variable (involved and uninvolved bone marrow) could be investigated exclusively in follicular lymphoma patients why we did not have to introduce bias by comparing tissues in patients and healthy controls. This allows us to conclude that follicular lymphoma is associated with, and probably causes, a local change of the microenvironment, as demonstrated with the CD4/CD8 and CD4/CD56 ratios. We present the first evidence in vivo that follicular lymphoma induces changes in its microenvironment, changes that are presumably vital for its persistence in the affected tissue. Identifying and blocking the mechanisms with which follicular lymphoma modulates the microenvironment could be of great clinical benefit.
Acknowledgments
We thank all trial investigators and flow cytometric labs participating in the Nordic Lymphoma Group trials. Financial support was provided through the regional agreement on medical training and clinical research (ALF) between the Stockholm County Council and Karolinska Institutet, and through the Swedish Research Council, the Swedish Cancer Society, the Nordic Cancer Union and the Cancer Research Funds of Radiumhemmet.
AUTHOR CONTRIBUTIONS
BEW designed research, performed research, analyzed data and wrote the paper. BS, BC, and EK designed research, performed research and wrote the paper. BØ, HH, PdB and CS performed research and wrote the paper.
The authors declare no conflict of interest.
References
- Byers RJ, Sakhinia E, Joseph P, Glennie C, Hoyland JA, Menasce LP, et al. Clinical quantitation of immune signature in follicular lymphoma by RT-PCR-based gene expression profiling. Blood. 2008;111:4764–4770. doi: 10.1182/blood-2007-10-115915. [DOI] [PubMed] [Google Scholar]
- Wahlin BE, Aggarwal M, Montes-Moreno S, Gonzalez LF, Roncador G, Sanchez-Verde L, et al. A unifying microenvironment model in follicular lymphoma: outcome is predicted by programmed death-1-positive, regulatory, cytotoxic, and helper T cells and macrophages. Clin Cancer Res. 2010;16:637–650. doi: 10.1158/1078-0432.CCR-09-2487. [DOI] [PubMed] [Google Scholar]
- Alvaro T, Lejeune M, Salvado MT, Lopez C, Jaen J, Bosch R, et al. Immunohistochemical patterns of reactive microenvironment are associated with clinicobiologic behavior in follicular lymphoma patients. J Clin Oncol. 2006;24:5350–5357. doi: 10.1200/JCO.2006.06.4766. [DOI] [PubMed] [Google Scholar]
- Wahlin BE, Sander B, Christensson B, Kimby E. CD8+ T-cell content in diagnostic lymph nodes measured by flow cytometry is a predictor of survival in follicular lymphoma. Clin Cancer Res. 2007;13:388–397. doi: 10.1158/1078-0432.CCR-06-1734. [DOI] [PubMed] [Google Scholar]
- Glas AM, Knoops L, Delahaye L, Kersten MJ, Kibbelaar RE, Wessels LA, et al. Gene-expression and immunohistochemical study of specific T-cell subsets and accessory cell types in the transformation and prognosis of follicular lymphoma. J Clin Oncol. 2007;25:390–398. doi: 10.1200/JCO.2006.06.1648. [DOI] [PubMed] [Google Scholar]
- Johnson PW, Watt SM, Betts DR, Davies D, Jordan S, Norton AJ, et al. Isolated follicular lymphoma cells are resistant to apoptosis and can be grown in vitro in the CD40/stromal cell system. Blood. 1993;82:1848–1857. [PubMed] [Google Scholar]
- Schmitter D, Koss M, Niederer E, Stahel RA, Pichert G. T-cell derived cytokines co-stimulate proliferation of CD40-activated germinal centre as well as follicular lymphoma cells. Hematol Oncol. 1997;15:197–207. doi: 10.1002/(sici)1099-1069(199711)15:4<197::aid-hon614>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Ghia P, Boussiotis VA, Schultze JL, Cardoso AA, Dorfman DM, Gribben JG, et al. Unbalanced expression of bcl-2 family proteins in follicular lymphoma: contribution of CD40 signaling in promoting survival. Blood. 1998;91:244–251. [PubMed] [Google Scholar]
- Travert M, Ame-Thomas P, Pangault C, Morizot A, Micheau O, Semana G, et al. CD40 ligand protects from TRAIL-induced apoptosis in follicular lymphomas through NF-kappaB activation and up-regulation of c-FLIP and Bcl-xL. J Immunol. 2008;181:1001–1011. doi: 10.4049/jimmunol.181.2.1001. [DOI] [PubMed] [Google Scholar]
- Umetsu DT, Esserman L, Donlon TA, DeKruyff RH, Levy R. Induction of proliferation of human follicular (B type) lymphoma cells by cognate interaction with CD4+ T cell clones. J Immunol. 1990;144:2550–2557. [PubMed] [Google Scholar]
- Pangault C, Ame-Thomas P, Ruminy P, Rossille D, Caron G, Baia M, et al. Follicular lymphoma cell niche: identification of a preeminent IL-4-dependent T(FH)-B cell axis. Leukemia. 2010;24:2080–2089. doi: 10.1038/leu.2010.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buske C, Twiling A, Gogowski G, Schreiber K, Feuring-Buske M, Wulf GG, et al. In vitro activation of low-grade non-Hodgkin′s lymphoma by murine fibroblasts, IL-4, anti-CD40 antibodies and the soluble CD40 ligand. Leukemia. 1997;11:1862–1867. doi: 10.1038/sj.leu.2400822. [DOI] [PubMed] [Google Scholar]
- Maby-El Hajjami H, Ame-Thomas P, Pangault C, Tribut O, DeVos J, Jean R, et al. Functional alteration of the lymphoma stromal cell niche by the cytokine context: role of indoleamine-2,3 dioxygenase. Cancer Res. 2009;69:3228–3237. doi: 10.1158/0008-5472.CAN-08-3000. [DOI] [PubMed] [Google Scholar]
- Epron G, Ame-Thomas P, Le Priol J, Pangault C, Dulong J, Lamy T, et al. Monocytes and T cells cooperate to favor normal and follicular lymphoma B-cell growth: role of IL-15 and CD40L signaling Leukemia 2011. e-pub ahead of print 26 July 2011; doi: 10.1038/leu.2011.179 [DOI] [PubMed]
- Harris NL, Swerdlow SH, Jaffe ES, Ott G, Nathwani BN, de Jong D, et al. Follicular lymphomaIn: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. (eds).WHO classification of tumours of haematopoietic and lymphoid tissues4 edn.IARC: Lyon; 2008220–226. [Google Scholar]
- Federico M, Bellei M, Marcheselli L, Luminari S, Lopez-Guillermo A, Vitolo U, et al. Follicular lymphoma international prognostic index 2: a new prognostic index for follicular lymphoma developed by the international follicular lymphoma prognostic factor project. J Clin Oncol. 2009;27:4555–4562. doi: 10.1200/JCO.2008.21.3991. [DOI] [PubMed] [Google Scholar]
- Kimby E, Jurlander J, Geisler C, Hagberg H, Holte H, Lehtinen T, et al. Long-term molecular remissions in patients with indolent lymphoma treated with rituximab as a single agent or in combination with interferon alpha-2a: a randomized phase II study from the Nordic Lymphoma Group. Leuk Lymphoma. 2008;49:102–112. doi: 10.1080/10428190701704647. [DOI] [PubMed] [Google Scholar]
- Wahlin BE, Sundstrom C, Holte H, Hagberg H, Erlanson M, Nilsson-Ehle H, et al. T cells in tumors and blood predict outcome in follicular lymphoma treated with rituximab. Clin Cancer Res. 2011;17:4136–4144. doi: 10.1158/1078-0432.CCR-11-0264. [DOI] [PubMed] [Google Scholar]
- Gamberale R, Galmarini CM, Fernandez-Calotti P, Jordheim L, Sanchez-Avalos J, Dumontet C, et al. In vitro susceptibility of CD4+ and CD8+ T cell subsets to fludarabine. Biochem Pharmacol. 2003;66:2185–2191. doi: 10.1016/j.bcp.2003.07.008. [DOI] [PubMed] [Google Scholar]
- Iwata S, Saito K, Tokunaga M, Yamaoka K, Nawata M, Yukawa S, et al. Phenotypic changes of lymphocytes in patients with systemic lupus erythematosus who are in longterm remission after B cell depletion therapy with rituximab. J Rheumatol. 2010;38:633–641. doi: 10.3899/jrheum.100729. [DOI] [PubMed] [Google Scholar]
- Bognar A, Csernus B, Bodor C, Reiniger L, Szepesi A, Toth E, et al. Clonal selection in the bone marrow involvement of follicular lymphoma. Leukemia. 2005;19:1656–1662. doi: 10.1038/sj.leu.2403844. [DOI] [PubMed] [Google Scholar]
- Husson H, Carideo EG, Neuberg D, Schultze J, Munoz O, Marks PW, et al. Gene expression profiling of follicular lymphoma and normal germinal center B cells using cDNA arrays. Blood. 2002;99:282–289. doi: 10.1182/blood.v99.1.282. [DOI] [PubMed] [Google Scholar]
- Kienzle N, Olver S, Buttigieg K, Groves P, Janas ML, Baz A, et al. Progressive differentiation and commitment of CD8+ T cells to a poorly cytolytic CD8low phenotype in the presence of IL-4. J Immunol. 2005;174:2021–2029. doi: 10.4049/jimmunol.174.4.2021. [DOI] [PubMed] [Google Scholar]
- Petrasch S, Kosco M, Perez-Alvarez C, Schmitz J, Brittinger G. Proliferation of non-Hodgkin-lymphoma lymphocytes in vitro is dependent upon follicular dendritic cell interactions. Br J Haematol. 1992;80:21–26. doi: 10.1111/j.1365-2141.1992.tb06395.x. [DOI] [PubMed] [Google Scholar]
- Lindhout E, Mevissen ML, Kwekkeboom J, Tager JM, de Groot C. Direct evidence that human follicular dendritic cells (FDC) rescue germinal centre B cells from death by apoptosis. Clin Exp Immunol. 1993;91:330–336. doi: 10.1111/j.1365-2249.1993.tb05904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Choi YS. Follicular dendritic cell-signaling molecules required for proliferation and differentiation of GC-B cells. Semin Immunol. 2002;14:259–266. doi: 10.1016/s1044-5323(02)00058-1. [DOI] [PubMed] [Google Scholar]
- Rademakers LH. Follicular dendritic cells in germinal centre development. Res Immunol. 1991;142:257–260. doi: 10.1016/0923-2494(91)90071-p. [DOI] [PubMed] [Google Scholar]
- Kapasi ZF, Qin D, Kerr WG, Kosco-Vilbois MH, Shultz LD, Tew JG, et al. Follicular dendritic cell (FDC) precursors in primary lymphoid tissues. J Immunol. 1998;160:1078–1084. [PubMed] [Google Scholar]
- Weekes CD, Pirruccello SJ, Vose JM, Kuszynski C, Sharp JG. Lymphoma cells associated with bone marrow stromal cells in culture exhibit altered growth and survival. Leuk Lymphoma. 1998;31:151–165. doi: 10.3109/10428199809057595. [DOI] [PubMed] [Google Scholar]
- Ame-Thomas P, Maby-El Hajjami H, Monvoisin C, Jean R, Monnier D, Caulet-Maugendre S, et al. Human mesenchymal stem cells isolated from bone marrow and lymphoid organs support tumor B-cell growth: role of stromal cells in follicular lymphoma pathogenesis. Blood. 2007;109:693–702. doi: 10.1182/blood-2006-05-020800. [DOI] [PubMed] [Google Scholar]
- Flaishon L, Lantner F, Hershkoviz R, Levo Y, Shachar I. Low levels of IFN-gamma down-regulate the integrin-dependent adhesion of B cells by activating a pathway that interferes with cytoskeleton rearrangement. J Biol Chem. 2001;276:46701–46706. doi: 10.1074/jbc.M103484200. [DOI] [PubMed] [Google Scholar]
- Zaretsky AG, Taylor JJ, King IL, Marshall FA, Mohrs M, Pearce EJ. T follicular helper cells differentiate from Th2 cells in response to helminth antigens. J Exp Med. 2009;206:991–999. doi: 10.1084/jem.20090303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo KR, Dabir B, Kovach A, Devor C, Bandle R, Bond A, et al. IL-4 protein expression and basal activation of Erk in vivo in follicular lymphoma. Blood. 2008;112:3818–3826. doi: 10.1182/blood-2008-02-138933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husson H, Freedman AS, Cardoso AA, Schultze J, Munoz O, Strola G, et al. CXCL13 (BCA-1) is produced by follicular lymphoma cells: role in the accumulation of malignant B cells. Br J Haematol. 2002;119:492–495. doi: 10.1046/j.1365-2141.2002.03832.x. [DOI] [PubMed] [Google Scholar]
- Kim CH, Lim HW, Kim JR, Rott L, Hillsamer P, Butcher EC. Unique gene expression program of human germinal center T helper cells. Blood. 2004;104:1952–1960. doi: 10.1182/blood-2004-03-1206. [DOI] [PubMed] [Google Scholar]
- Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192:1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZZ, Novak AJ, Ziesmer SC, Witzig TE, Ansell SM. Malignant B cells skew the balance of regulatory T cells and TH17 cells in B-cell non-Hodgkin′s lymphoma. Cancer Res. 2009;69:5522–5530. doi: 10.1158/0008-5472.CAN-09-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZZ, Novak AJ, Ziesmer SC, Witzig TE, Ansell SM. Attenuation of CD8(+) T-cell function by CD4(+)CD25(+) regulatory T cells in B-cell non-Hodgkin′s lymphoma. Cancer Res. 2006;66:10145–10152. doi: 10.1158/0008-5472.CAN-06-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZZ, Novak AJ, Stenson MJ, Witzig TE, Ansell SM. Intratumoral CD4+CD25+ regulatory T-cell-mediated suppression of infiltrating CD4+ T cells in B-cell non-Hodgkin lymphoma. Blood. 2006;107:3639–3646. doi: 10.1182/blood-2005-08-3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilchey SP, De A, Rimsza LM, Bankert RB, Bernstein SH. Follicular lymphoma intratumoral CD4+CD25+GITR+ regulatory T cells potently suppress CD3/CD28-costimulated autologous and allogeneic CD8+CD25- and CD4+CD25- T cells. J Immunol. 2007;178:4051–4061. doi: 10.4049/jimmunol.178.7.4051. [DOI] [PubMed] [Google Scholar]
- Ramsay AG, Clear AJ, Kelly G, Fatah R, Matthews J, Macdougall F, et al. Follicular lymphoma cells induce T-cell immunologic synapse dysfunction that can be repaired with lenalidomide: implications for the tumor microenvironment and immunotherapy. Blood. 2009;114:4713–4720. doi: 10.1182/blood-2009-04-217687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilchey SP, Rosenberg AF, Hyrien O, Secor-Socha S, Cochran MR, Brady MT, et al. Follicular lymphoma tumor infiltrating T-helper (TH) cells have the same polyfunctional potential as normal nodal TH cells despite skewed differentiation Blood 2011. E-pub ahead of print 5 August 2011; doi: 10.1182/blood-2011-03-340646 [DOI] [PMC free article] [PubMed]
- Cartron G, Watier H, Golay J, Solal-Celigny P. From the bench to the bedside: ways to improve rituximab efficacy. Blood. 2004;104:2635–2642. doi: 10.1182/blood-2004-03-1110. [DOI] [PubMed] [Google Scholar]
- Hilchey SP, Hyrien O, Mosmann TR, Livingstone AM, Friedberg JW, Young F, et al. Rituximab immunotherapy results in the induction of a lymphoma idiotype-specific T-cell response in patients with follicular lymphoma: support for a ″vaccinal effect″ of rituximab. Blood. 2009;113:3809–3812. doi: 10.1182/blood-2008-10-185280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark P, Normansell DE, Innes DJ, Hess CE. Lymphocyte subsets in normal bone marrow. Blood. 1986;67:1600–1606. [PubMed] [Google Scholar]
- Bofill M, Janossy G, Lee CA, MacDonald-Burns D, Phillips AN, Sabin C, et al. Laboratory control values for CD4 and CD8 T lymphocytes. Implications for HIV-1 diagnosis. Clin Exp Immunol. 1992;88:243–252. doi: 10.1111/j.1365-2249.1992.tb03068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan CF, Eastman PJ, Conner JB, Baier KA, Durham JB. Clinical utility of a lymph node normal range obtained by flow cytometry. Ann NY Acad Sci. 1993;677:404–406. doi: 10.1111/j.1749-6632.1993.tb38799.x. [DOI] [PubMed] [Google Scholar]