Abstract
Uncontrolled endoplasmic reticulum (ER) stress responses are proposed to contribute to the pathology of chronic inflammatory diseases such as type 2 diabetes or atherosclerosis. However, the connection between ER stress and inflammation remains largely unexplored. Here, we show that ER stress causes activation of the NLRP3 inflammasome, with subsequent release of the pro-inflammatory cytokine interleukin-1β. This ER-triggered proinflammatory signal shares the same requirement for reactive oxygen species production and potassium efflux compared with other known NLRP3 inflammasome activators, but is independent of the classical unfolded protein response (UPR). We thus propose that the NLRP3 inflammasome senses and responds to ER stress downstream of a previously uncharacterized ER stress response signaling pathway distinct from the UPR, thus providing mechanistic insight to the link between ER stress and chronic inflammatory diseases.
Keywords: endoplasmic reticulum stress, NLRP3 inflammasome, innate immunity, macrophages
Inflammation is the first response of the immune system to infection or tissue injury, and is meant to protect the body from these insults. Prolonged or chronic inflammation, however, can exacerbate tissue damage and is implicated in the development of diseases, such as arthritis, neurodegenerative diseases and type 2 diabetes.1 The endoplasmic reticulum (ER) stress response is a potent, evolutionarily conserved response to misfolded proteins and cellular metabolic stress. The inflammatory response is frequently triggered as a consequence of ER stress, caused by metabolic problems or by the accumulation of misfolded proteins.2 Under such conditions, the ER initiates the unfolded protein response (UPR). This response is initially aimed at altering the cellular transcriptional and translational programs to resolve the protein-folding defect, but if the problem persists it initiates programed cell death. In addition to the intracellular responses, chronic ER stress can also, by mechanisms that remain poorly characterized, cause inflammation within affected tissues.3
Recent years have seen remarkable growth in our understanding of the cellular and molecular mechanisms that control the inflammatory response. In particular, proteins belonging to the NOD-like receptor (NLR) family have been identified as central players in innate immunity.4, 5 Some of these NLRs participate in multiprotein complexes termed inflammasomes, which mediate caspase-1-dependent maturation of the highly proinflammatory cytokine interleukin-1β (IL-1β). Of the thus far described inflammasomes, the NLRP3 inflammasome is most fully characterized.6 In addition to microbial and viral danger signals (PAMPs), the NLRP3 inflammasome is unique in that it can sense the presence of endogenous danger signals that are associated with cellular or tissue damage or stress (DAMPs), such as uric acid crystals.4 As the mechanism by which ER stress triggers inflammation remains poorly understood, we sought to investigate the role of the NLRP3 inflammasome as a potential sensor of ER stress.
Results
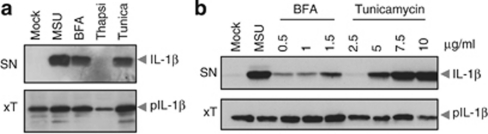
We first determined whether induction of ER stress triggers the maturation of IL-1β. Human macrophages were exposed to tunicamycin, an inhibitor of GlcNAc-1-phosphate transferase and thus of glycoprotein synthesis, in order to induce ER stress. The presence of processed IL-1β was detected in the supernatant of the human monocytic cell line THP-1 and primary human monocytes – macrophages following stimulation with tunicamycin (Figures 1a and b). Similarly, induction of ER stress using Brefeldin A (BFA), which reversibly blocks protein transport from the ER to the Golgi, also caused IL-1β maturation and secretion. (Figures 1a and b). Thapsigargin, which induces ER stress by blocking ER SERCA pumps and thereby depleting ER Ca2+ stores, also led to IL-1β secretion, although exclusively in primary cells (Figure 1a and data not shown). Taken together, these data suggest that induction of ER stress by three different compounds results in the release of mature IL-1β by human macrophages.
Figure 1.
ER stress triggers the release of mature interleukin-1β by macrophages. (a) PMA-differentiated THP-1 cells were stimulated with uric acid crystals (MSU, positive control), BFA, thapsigargin or tunicamycin for 6 h. Precipitated supernatant and whole-cell extracts were then analyzed by western blot. (b) Human primary macrophages were stimulated with MSU, BFA or tunicamycin for 6 h, and analyzed by western blot. IL-β, mature IL-1β; pIL-1β, proIL-1β; Casp1, mature caspase-1; pCasp1, procaspase-1; SN, supernatant; xT, whole-cell extract
The NLRP3 inflammasome is composed of NLRP3, Cardinal, the adaptor ASC and caspase-1, and mediates the production of active IL-1β in response to an ever-expanding list of stimuli.4 A second inflammasome is constituted of the NLR member NLRC4 and caspase-1, and senses the PAMP flagellin, while a third inflammasome senses DNA via AIM2. While all inflammasomes described thus far (the NLRP3, AIM2, NLRC4 and NLRP1 inflammasomes) sense various PAMPs, only the NLRP3 inflammasome has been shown to respond to a plethora of DAMPs as well. We therefore hypothesized that NLRP3 is the inflammasome sensor activated by ER stress.
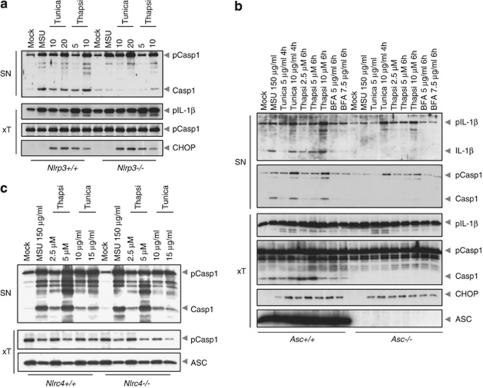
To confirm the specific involvement of the NLRP3 inflammasome, we induced ER stress in THP-1 cells in which NLRP3, ASC and caspase-1 were knocked-down using shRNA. We found that secretion of IL-1β was markedly reduced in cells deficient in NLRP3, ASC and caspase-1, but not NLRC4 (Supplementary Figure 1, and data not shown). The specific involvement of the NLRP3 inflammasome was further confirmed by performing similar experiments in murine macrophages isolated from Nlrp3−/−, Asc−/− or Nlrc4−/− mice (Figures 2a–c). Moreover, ER stress-induced inflammasome activation is independent of toll-like or RIG-I-like receptors, as IL-1β secretion was normal in MyD88-, Trif- and Mavs-deficient murine macrophages (Supplementary Figures 2A, B and C).
Figure 2.
ER stress specifically activates the NLRP3 inflammasome. (a–c) LPS-primed bone marrow-derived macrophages from Nlrp3+/+, Nlrp3−/−, Asc+/+, Asc−/−, Nlrc4+/+ and Nlrc4−/− mice, respectively, were stimulated with MSU, tunicamycin, thapsigargin or BFA as indicated for 6 h, and analyzed by western blot
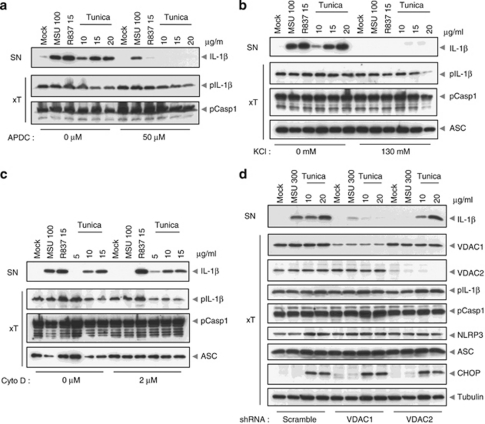
The mechanism by which the NLRP3 inflammasome is activated remains unclear,7, 8 though recent evidence suggests mitochondria sense and integrate various danger signals and relay the signal to the NLRP3 inflammasome.9 Nonetheless, both potassium (K+) efflux and an increase of reactive oxygen species (ROS) are required for NLRP3 inflammasome activation in response to all stimuli tested thus far. We therefore examined whether these factors are required for ER stress-induced IL-1β maturation. This was indeed the case as the addition of ROS scavengers (Figure 3a) or the inhibition of K+ efflux (Figure 3b) completely inhibited IL-1β secretion. Additionally, cytochalasin D treatment, which blocks actin polymerization, failed to block ER stress-induced IL-1β secretion, suggesting that phagocytosis is not required akin to other non-particulate NLRP3 agonists (Figure 3c).
Figure 3.
Mechanism of ER stress-induced inflammasome activation is similar to that of other known NLRP3 activators. (a, b and c) PMA-differentiated THP-1 cells were pre-incubated for 30 min with 50-μM ammonium pyrrolidinedithiocarbamate, 130-mM extracellular KCl or 2-μM cytochalasin D (Cyto D), respectively, stimulated with MSU, R837 or tunicamycin for a further 6 h, and analyzed by western blot. (d) PMA-differentiated THP-1 cells overexpressing an anti-VDAC1, anti-VDAC2 or scramble shRNA were stimulated with MSU or tunicamycin for 6 h, and analyzed by western blot
The finding that ER stress, like other NLRP3 activators, activates the NLRP3 inflammasome in a K+ efflux- and ROS-dependent manner suggests that it may also be sensed by mitochondria. Thus, it is conceivable that ER stress initiates a signal that is transmitted to mitochondria and then relayed to the NLRP3 inflammasome. This notion is consistent with the subcellular localization of NLRP3. In resting cells, NLRP3 is associated with ER membranes, and then upon activation translocates to membranes that are positive for markers of both ER and mitochondria, which likely correspond to mitochondria-associated membranes (MAMs).9
VDACs are the major channels for the exchange of metabolites and ions between the mitochondria and other cellular compartments, including the ER. They are therefore important for mitochondrial oxidative metabolism and the generation of ROS. We therefore tested whether dampening mitochondrial activity by knocking down the expression of VDACs has an impact on inflammasome activation induced by ER stress. Akin to MSU, inflammasome activation by tunicamycin was impaired in cells with downregulated VDAC1 (Figure 3d). Interestingly, VDAC2 knock-down did not affect IL-1β secretion by tunicamycin, while it substantially decreased inflammasome activity triggered by MSU or other NLRP3 activators, as previously reported (Figure 4a and data not shown).9 This intriguing observation suggests the existence of different inflammasome activation mechanisms downstream of ER stress compared with other known NLRP3 activators, possibly at the mitochondrial level.
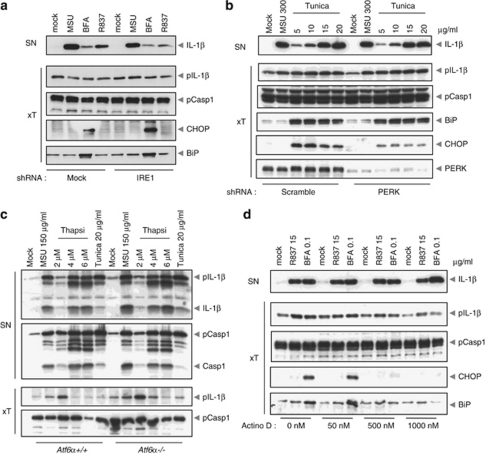
Figure 4.
ER stress activates the NLRP3 inflammasome independently of the UPR. (a) PMA-differentiated THP-1 cells overexpressing an shRNA against IRE1α or an empty vector were stimulated with MSU, BFA or R837 for 6 h, and analyzed by western blot. (b) PMA-differentiated THP-1 cells stably overexpressing a scramble or anti-PERK shRNA were stimulated with MSU or tunicamycin for 6 h, and analyzed by western blot. (c) LPS-primed BMDM isolated from Atf6α+/+ and Atf6α−/− mice were stimulated with MSU, thapsigargin or tunicamycin for 6 h, and analyzed by western blot. (d) PMA-differentiated THP-1 cells were pretreated with actinomycin D for 30 min, stimulated with R837 or BFA for 6 h, and analyzed by western blot
The cellular response to ER stress results in the activation of the UPR, which consists of the three main effector pathways that are initiated by ER-localized transmembrane proteins, namely IRE1α, PERK and ATF6α. We thus investigated the possible role of UPR effectors in inflammasome activation. THP-1 cells stably expressing shRNA against IRE1α or PERK displayed no alteration in the secretion of mature IL-1β in response to BFA or tunicamycin treatment (Figures 4a and b and Supplementary Figure 3). Additionally, macrophages derived from Atf6α−/− mice did not differ from WT littermates in their response to thapsigargin or tunicamycin (Figure 4c). Of note, strong NLRP3 activators like MSU or R837 did not induce CHOP upregulation (Figure 4a), suggesting that they do not induce ER stress and their ability to activate the inflammasome is thus also independent of an ER stress response.
The UPR results in widespread blockage of the translation of most proteins through phosphorylation of eIF2α. Simultaneously, it selectively induces the transcription and translation of proteins initially involved in pro-survival pathways to help the cell cope with and resolve the cause of ER stress, and subsequently to initiate programed cell death if the ER stress is excessive or prolonged. Overall, most downstream effectors of the UPR are thus transcription-dependent. While synthesis of the ER stress response effector CHOP was blocked by the transcriptional inhibitor actinomycin D, inflammasome activation was not affected (Figure 4d). This observation further suggests that activation of the NLRP3 inflammasome by ER stress is not dependent on the UPR.
As signaling pathways triggered by the UPR appeared to be dispensable, we next investigated the role of alternative signaling pathways that were shown to emanate from a stressed ER.2 ER stress is proposed to trigger NF-κB activation through the interaction between IRE1α and the IKK complex via the adaptor TRAF2.10, 11 In line with the observation that IRE1α is not required (see above), THP-1 cells expressing an shRNA against TRAF2 responded normally to NLRP3 agonists (Supplementary Figure 4a). We next investigated a possible role of the JNK signaling pathway. ER stress-induced JNK activation is thought to be triggered by a TRAF2- and ASK1- (a stress-activated MAP3K) dependent pathway. However, neither TRAF2 nor ASK1 were required for inflammasome activation in response to ER stress, as both THP-1 cells with knock-downed TRAF2 and macrophages from Ask1−/− mice responded normally (Supplementary Figures 4A and B).
Discussion
In this study, we demonstrated that three different compounds (BFA, tunicamycin and thapsigargin), that are known to induce ER stress, also activate the NLRP3 inflammasome in human and murine macrophages. The activation mechanism is similar to that of other known NLRP3 activators, requiring ROS generation and potassium efflux. A minor observed discrepancy lies in the VDAC1, but not VDAC2, requirement for activation of the inflammasome by ER stressors, possibly suggesting the existence of different signaling pathways triggered by the various NLRP3 activators.
Interestingly, we could not find a role for the well-known ER stress effectors PERK, IRE1α or ATF6α in NLRP3 inflammasome activation. Consequently, we thus rule out the involvement of the classical UPR in this signaling pathway, and suggest that an UPR-independent ‘fourth branch' of the ER stress response regulates NLRP3 inflammasome activation. As such, we do not offer definitive proof that ER stress per se is responsible for NLRP3 inflammasome activation. Indeed, it could be argued that BFA, tunicamycin and thapsigargin could each have specific, ER-independent, inflammasome-activating properties distinct from one another. However, in keeping with Occam's razor, we believe that the most straightforward explanation is that a previously uncharacterized response stemming from a stressed ER, that is distinct from the UPR, is responsible for the ability of these compounds to activate the inflammasome. The precise signaling pathway involved remains elusive, similar to other NLRP3 activators described to date.
Chronic inflammatory diseases such as diabetes and atherosclerosis are linked to chronic metabolic dysregulation and ER stress. ER membranes have long been known to co-sediment with those from the metabolic hub of the cell, namely mitochondria. These ER MAMs have a different composition than the rest of the ER, giving rise to the concept that they may have specialized functions. Interestingly, we have previously shown that activation of the NLRP3 inflammasome occurs at the MAMs.9 Mitochondria were recently shown to have a pivotal role in activation of the inflammasome, and here we show that ER stress also activates the inflammasome.
MAMs are known to regulate exchange of metabolites, lipids and Ca2+ between the ER and mitochondria. These factors, as well as tight regulation of mitochondrial fusion and fission, are critical for appropriate mitochondrial activity. We thus propose a model in which ER stress alters steady-state communication between the ER and mitochondria, leading to dysregulated mitochondrial activity, enhanced production of mitochondrial ROS and thus activation of the NLRP3 inflammasome. Our data suggest that this ER stress-induced inflammasome activation is independent of the UPR response. Apart from the UPR response, other signaling pathways have been described to radiate from the ER or mitochondria such as the activation of JNK and NF-κB, both of which were not necessary for inflammasome activation.
Chronic ER stress is observed in obesity, and in cellular systems ER stress leads to insulin resistance.12 The results presented here suggest that ER stress-induced NLRP3 inflammasome activation may contribute to ER stress-induced disease. This concept is supported by clinical trials demonstrating an important role of IL-1β (and thus the inflammasome) in the progression of T2D.13 Blocking IL-1β or IL-1R leads to an astonishing improvement of glucose and insulin levels. Another example of a clinical evidence for a connection between ER stress and the inflammasome in chronic inflammatory disease comes from patients with TNF-receptor-associated periodic syndrome (TRAPS).14 TRAPS is similar to cryopyrin-associated periodic syndromes (CAPS, caused by mutations in NLRP3), in that both diseases are characterized by recurrent fevers, abdominal and joint pain and myalgia, similar to symptoms found in CAPS patients. While CAPS is caused by mutations in NLRP3, TRAPS is caused by mutations in TNF-R1, which were originally thought to cause altered shedding of the extracellular portion of TNF-R1.15 More recent findings, however, suggest that the mutated TNF-R1 traffics abnormally and accumulates in the ER.14 Moreover, TRAPS-associated TNF-R1 mutants cause enhanced production of mitochondrial ROS,14 which is a requirement for NLRP3 inflammasome activation. It is therefore possible that accumulation of the TNF-R1 in the ER triggers ER stress and mitochondrial ROS production, thereby causing NLRP3 activation and subsequent IL-1β release. This would explain the surprising clinical finding that this classically TNF-driven disease can be treated with IL-1 inhibitors.16 Considering that the dynamics of ER–mitochondria interaction has a crucial role in NLRP3 inflammasome activation, targeting ER stress may, as previously suggested,3 represent an attractive therapeutic target to combat chronic inflammatory disease.
Materials and Methods
Reagents
Uric acid, LPS, ammonium pyrrolidinedithiocarbamate, nigericin and actinomycin D were purchased from (Sigma-Aldrich Chemie GmbH, Buchs, Switzerland). BFA, tunicamycin, thapsigargin, puromycin, phorbol 12-myristate 13-acetate (PMA) were from Alexis (Enzo life Sciences (ELS) AG, Lausen, Switzerland). Ultra-pure LPS was from Invivogen (LabForce AG, Nunningen, Switzerland), and potassium chloride from AppliChem (Axon Lab AG, Baden-Dättwil, Switzerland).
Antibodies
The primary antibodies used in this study were obtained from Sigma-Aldrich (tubulin), Apotech (Enzo Life Sciences (ELS) AG) (NLRP3), MBL (LabForce AG) (ASC), Cell Signaling (BioConcept, Allschwil, Switzerland) (human mature IL-1β, BiP, PERK and VDAC1), Santa Cruz Biotechnology, Inc. (Heidelberg, Germany) (human caspase-1 and IRE1α), Abcam plc (Cambridge Science Park, UK) (VDAC2) and Alexis (CHOP). The antibody against mouse caspase-1 (p20) is a generous gift from Dr Peter Vandenabeele (Ghent University). The antibody against human pro-IL-1β was produced in-house while that against mouse-IL-1β was a kind gift from Roberto Solari (GlaxoSmithKline AG, Münchenbuchsee, Switzerland). Primary antibodies were generally used at a 1 : 1000 dilution in blocking solution (PBS-0.05% Tween with 5% skim milk).
Generation of THP-1 cells stably expressing shRNA
THP-1 cells stably expressing shRNA were obtained by lentiviral infection as previously described.17Plasmids encoding for shRNA against NLRP3, ASC and caspase-1 were homemade and have been described.18 The other shRNA-encoding plasmids were from Sigma, and their respective sequences were as follows: IRE1α, 5′-CCGGGAGAAGATGATTGCGATGGATCTCGAGATCCATCGCAATCATCTTCTCTTTTT-3′ MyD88, 5′-CCGGGCAGAGCAAGGAATGTGACTTCTCGAGAAGTCACATTCCT TGCTCTGCTTTTT-3′ PERK, 5′-CCGGCCGTAGTAAGAAATGGATCATCTCGA GATGATCCATTTCTTACTACGGTTTTT-3′ TRAF2, 5′-CCGGCGAGACGGTAGA GGGTGAGAACTCGAGTTCTCACCCTCTACCGTCTCGTTTTT-3′ and 5′-CCGGCCCTTGCAGATTCCACGCCATCTCGAGATGGCGTGGAATCTGCAAGGGTTTTT-3′ VDAC1, 5′-CCGGGCAGTTGGCTACAAGACTGATCTCGAGATCAGTCTTGTAG CCAACTGCTTTTT-3′ VDAC2, 5′-CCGGGATCTCAACAAGAGCTGTATTCTCGA GAATACAGCTCTTGTTGAGATCTTTTTTG-3′.
Mice
Nlrp3−/−,19 Asc−/−,20 IPAF/Nlrc4−/−,20 Atf6α−/−21 and Ask1−/− 22 mice have been described. All mice were on C57BL/6 background and were housed at the University of Lausanne following the Swiss Federal Veterinary Office guidelines.
Cell preparation and stimulation
Human THP-1 cells were maintained in RPMI 1640 medium (Invitrogen, Life Technologies Europe BV, Zug, Switzerland), supplemented with 10% fetal calf serum (FCS, PAA Laboratories GmbH, Chemie Brunschwig AG, Basel, Switzerland) and 1% penicillin–streptomycin (Invitrogen) at 37°C and 5% CO2. For experiments, THP-1 cells were differentiated for 3 h with 100-nM PMA on the day before stimulation. Primary murine bone marrow-derived macrophages were isolated from tibial and femoral bone marrow progenitors as described, 23 and primed with 100 ng/ml ultra-pure LPS overnight the day before stimulation. Human primary monocytes were isolated from whole blood obtained from the Center for Blood Transfusion, Lausanne, Switzerland, using the OptiPrep TM protocol (Axis-Shield AG, Wädenswil, Switzerland) following the manufacturer's instructions. Cell extracts and precipitated supernatants were analyzed by western blot.
Acknowledgments
We thank Chantal Mattmann and Sylvie Hertig for excellent technical support, all the members of the Tschopp Lab for helpful discussions, and Christina Thomas, Kendle Maslowski and Fabio Martinon for critical reading of the manuscript. This work was supported by grants from the Swiss National Science Foundation (through an MD-PhD grant to PM), the CAS President's fund from the Chinese Academy of Sciences (to RZ), the Fundamental Research Funds for the Central Universities (to RZ), the start-up fund from the University of Science and Technology of China (to RZ), Apo-SYS and the Institute for Arthritis Research. This manuscript is dedicated to the memory of Professor Jürg Tschopp.
Glossary
- ER
endoplasmic reticulum
- AIM2
absent in melanoma 2
- APDC
ammonium pyrrolidinedithiocarbamate
- ASC
apoptosis-associated speck-like protein containing a CARD
- ASK1
apoptosis signal-regulating kinase 1
- ATF6
activating transcription factor 6
- BFA
brefeldin A
- BMDM
bone marrow-derived macrophages
- CAPS
cryopyrin-associated periodic syndrome
- CARD
caspase recruitment domain
- Caspase
cysteinyl aspartate-specific proteinase
- CHOP
C/EBP homologous protein
- DAMP
danger-associated molecular pattern
- eIF2α
eukaryotic initiation factor 2α
- ER
endoplasmic reticulum
- FCS
fetal calf serum
- IL-1β
interleukin-1β
- IL-1R
interleukin-1 receptor
- IPAF
interleukin-1β-converting enzyme protease-activating factor
- IRE1
inositol-requiring enzyme 1
- JNK
c-Jun N-terminal kinase
- LPS
lipopolysaccharide
- MAM
mitochondria-associated membranes
- MAVS
mitochondrial antiviral-signaling protein
- MSU
monosodium urate
- MyD88
myeloid differentiation protein 88
- NF-κB
nuclear factor kappaB
- NLR
NOD-like receptor
- NLRC4
NLR family CARD domain-containing protein 4
- NLRP
NACHT, LRR and PYD domains-containing protein
- NOD
nucleotide-binding oligomerization domain
- PAMP
pathogen-associated molecular pattern
- PMA
phorbol 12-myristate 13-acetate
- ROS
reactive oxygen species
- shRNA
small hairpin RNA
- SERCA
sarcoplasmic/endoplasmic reticulum calcium ATPase
- TNF-R1
tumor-necrosis factor receptor type 1
- TRAF
tumor necrosis factor receptor-associated factor
- TRAPS
tumor-necrosis factor receptor type 1-associated periodic syndrome
- TRIF
TIR domain-containing adapter inducing IFN-beta
- T2D
type 2 diabetes
- UPR
unfolded protein response
- VDAC
voltage-dependent anion-selective channel
- WT
wild-type
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Cell Death and Disease website (http://www.nature.com/cddis)
Edited by M Piacentini
Supplementary Material
References
- Dinarello CA. Blocking interleukin-1β in acute and chronic autoinflammatory diseases. J Intern Med. 2011;269:16–28. doi: 10.1111/j.1365-2796.2010.02313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol. 2008;8:923–934. doi: 10.1038/nri2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Ting JPY, Duncan JA, Lei Y. How the noninflammasome NLRs function in the innate immune system. Science. 2010;327:286–290. doi: 10.1126/science.1184004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostini L, Martinon F, Burns K, Mcdermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- Latz E. The inflammasomes: mechanisms of activation and function. Curr Opin Immunol. 2010;22:28–33. doi: 10.1016/j.coi.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp J, Schroder K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production. Nat Rev Immunol. 2010;10:210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol Cell Biol. 2006;26:3071–3084. doi: 10.1128/MCB.26.8.3071-3084.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Anti-inflammatory agents: present and future. Cell. 2010;140:935–950. doi: 10.1016/j.cell.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulua AC, Simon A, Maddipati R, Pelletier M, Park H, Kim K-Y, et al. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS) J Exp Med. 2011;208:519–533. doi: 10.1084/jem.20102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott MF, Aksentijevich I, Galon J, McDermott EM, Ogunkolade BW, Centola M, et al. Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell. 1999;97:133–144. doi: 10.1016/s0092-8674(00)80721-7. [DOI] [PubMed] [Google Scholar]
- Obici L, Meini A, Cattalini M, Chicca S, Galliani M, Donadei S, et al. Favourable and sustained response to anakinra in tumour necrosis factor receptor-associated periodic syndrome (TRAPS) with or without AA amyloidosis. Ann Rheum Dis. 2011;70:1511–1512. doi: 10.1136/ard.2010.143438. [DOI] [PubMed] [Google Scholar]
- Papin S, Cuenin S, Agostini L, Martinon F, Werner S, Beer H-D, et al. The SPRY domain of Pyrin, mutated in familial Mediterranean fever patients, interacts with inflammasome components and inhibits proIL-1beta processing. Cell Death Differ. 2007;14:1457–1466. doi: 10.1038/sj.cdd.4402142. [DOI] [PubMed] [Google Scholar]
- Pétrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout- uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- Mariathasan S, Weiss DS, Newton K, Mcbride J, O'rourke K, Roose-Girma M, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Sato T, Matsui T, Sato M, Okada T, Yoshida H, et al. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev Cell. 2007;13:365–376. doi: 10.1016/j.devcel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, et al. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2:222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didierlaurent A, Brissoni B, Velin D, Aebi N, Tardivel A, Käslin E, et al. Tollip regulates proinflammatory responses to interleukin-1 and lipopolysaccharide. Mol Cell Biol. 2006;26:735–742. doi: 10.1128/MCB.26.3.735-742.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.