Abstract
Background
The relationship between the degree of expression of matrix metalloproteinases or tissue inhibitor of metalloproteinases and venous reflux remains to be investigated.
Materials and Methods
Primary varicose vein tissues were obtained from 23 patients, 18 females and 5 males, aged from 19 to 73. Cephalic or basilic veins were obtained for the control group from 10 patients who underwent vascular access for maintenance hemodialysis. Two operative techniques (high ligation with stripping or endovenous laser coagulation) were used. The expression of matrix metalloproteinase-2 and 13 and tissue inhibitor of metalloproteinase-4 in the varicose vein group and control group was assessed semi-quantitatively by immunohistochemical slides stained with primary antibodies.
Results
Twenty (87%) of the varicose vein group patients had greater or lesser saphenous vein diseases with reflux. The focal weak (+) stain for matrix metalloproteinases-2, and 13, and tissue inhibitor of matrix metalloproteinase-4 was dominant in the varicose vein group; the focal or diffuse strong stain (++ or +++) was prevalent in the control group. The differences were statistically significant (p<0.01). The degree of reflux and the duration of symptoms were not significantly related to the expression of MMP-13 (p=0.317 and p=0.654, respectively).
Conclusion
Further study should be performed to investigate the relationship between the clinical characteristics related to venous hypertension or reflux and expression of MMPs and TIMP in varicose veins.
Keywords: Veins, Varicose veins, Extracellular matrix
INTRODUCTION
Reflux, or pathologic retrograde flow, caused by an incompetent venous valve has been reported as one of the most important etiologic factors of varicose veins (VV) in the lower extremities. Furthermore, venous hypertension followed by reflux can cause changes in the physical properties of the venous walls by increased expression of matrix metalloproteinases (MMPs), which will lead to a dilated and serpentine morphology of VV [1]. However, the relationship between venous hypertension or reflux and the expression of various subtypes of MMPs, or role of each subtype of MMPs, in creating morphologic changes is still controversial [2]. This study is focused on comparing the level of expression of MMP-2, MMP-13, and tissue inhibitor of metalloproteinase-4 (TIMP-4) between VV and non-varicose veins in order to investigate the relationship between the expression of MMPs and the clinical characteristics related to reflux in VV.
MATERIALS AND METHODS
Twenty-three patients who underwent surgery for primary varicose veins from October 2009 to February 2010 were included. Informed consent was obtained from all the patients, and this study was approved by Institutional Review Board of Jeju National University Hospital. The specimens used from the control group of patients (n=10) who underwent surgery for vascular access formation for maintenance hemodialysis, were cephalic or basilica veins. None of the control group patients had varicose veins in their lower extremities.
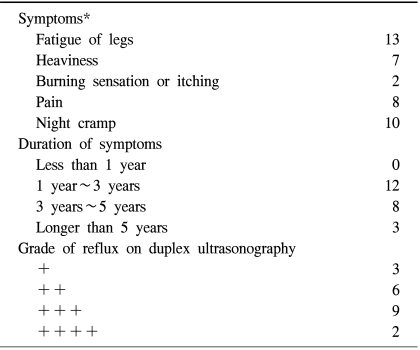
The mean age of the patients with VV was 52.5 (range 19~73) and 78.3% (n=18) were female. The patients' characteristics based on the classification of chronic venous disease in the "Reporting standards in venous disease" [3] are presented presented in Table 1.
Table 1.
Patient characteristics based on the classification of chronic venous disease (n=23)
Symptoms, cosmetic needs, or concerns about complications were treated by surgical resection. A careful medical history, physical examination, and routine preoperative laboratory studies were performed for each patient, and duplex ultrasonography of deep and superficial veins of the legs was performed in order to rule out secondary varicose veins and detect venous reflux. Two operative techniques for VV were used: high ligation with stripping and endovenous laser coagulation with an 810 nm diode laser (Dinona Inc., Daejeon, South Korea) with or without additional individual ligation and excision. In cases of severely tortuous and dilated VV, high ligation and stripping was preferred because the insertion and progression of the laser catheter can be difficult and incomplete obliteration or recurrence should be prevented. In the cases in which no clear differences between the two techniques were predicted, one of the techniques was selected based on the patient's preference.
Immunohistochemical analysis for the expression of the MMPs and TIMP-4 was performed as follows. Ten percent formalin-fixed, paraffin-embedded tissue was cut at 4 µm slices, deparaffinized in xylene, and rehydrated with graded ethanol. A standard immunohistochemical technique was performed using a Ventana BenchMark XT immunostainer (Ventana Medical Systems Inc., AZ, USA). Heat epitope retrieval provided by the immunostainer was performed for 60 minutes. The enzymatic reactivity was visualized with 3,3'-diaminobenzidine. The primary antibodies used were anti-MMP-2 (Diagnostic BioSystems, California, USA; clone A-Gel VC2; dilution 1 : 25), anti-MMP-13 (American Diagnostica inc., Connecticut, USA; polyclonal; 1 : 50), and anti- TIMP-4 (Diagnostic BioSystems, California, USA; polyclonal; dilution 1 : 50). They were diluted with PBS buffer- based dilution solution and incubated for 32 minutes at 37℃. For the negative controls, the slides were stained by omitting the primary antibody from the protocol and substituting it with commercially available nonimmune mouse immunoglobulin G serum (DAKO, Carpinteria, CA, USA).
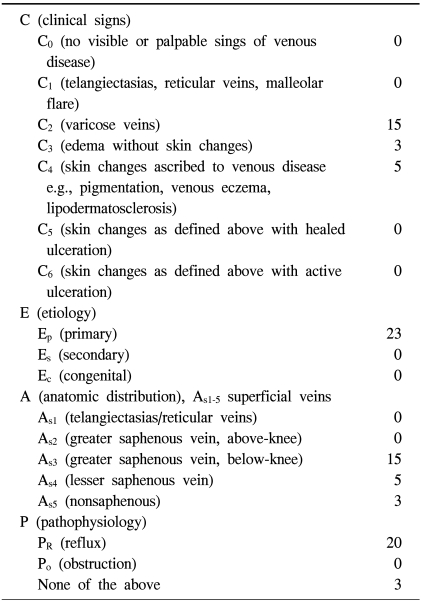
The immunohistochemical slides were evaluated and interpreted by a pathologist. The slides were scored semi-quantitatively for staining intensity and quantity as follows: - no signal detectable, + focal weak staining, ++ focal (<50%) strong or diffuse (≥50%) weak staining, and +++ diffuse strong staining (Fig. 1).
Fig. 1.
Photomicrographs show immunohistochemical findings of MMP2 (A: +, B: +++), MMP13 (C: +, D: +++), and TIMP4 (E: +, F: +++) in the vascular wall (×40). MMP=Matrix metalloproteinase; TIMP=Tissue inhibitor of metalloproteinase.
A Pearson chi squared test (SPSS, version 12.1 for Windows, Chicago, IL, USA) was used for determining the differences in expression between MMPs and TIMP-4; a p-value <0.05 was considered statistically significant.
RESULTS
The patients' clinical characteristics including their symptoms, the duration of symptoms, and grade of reflux on duplex ultrasonography are presented in Table 2. Twenty (87%) patients had greater or lesser saphenous vein disease with reflux. Ligation of the sapheno-femoral or sapheno-popliteal junction with stripping was performed for 17 patients and endovenous laser coagulation with additional individual excisions was performed for 6 patients.
Table 2.
Preoperative clinical characteristics (n=23)
*Each patient presented more than one symptom.
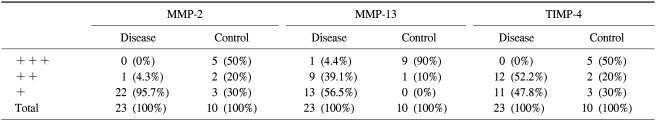
Immunohistochemical analysis showed that focal or diffuse strong staining for MMP-2 and MMP-13 tended to be prominent in the control group, whereas focal weak staining was prevalent in the disease group (Table 3). No specimen showed diffuse strong staining for MMP-2 in the varicose vein group. Expression of TIMP-4 also showed a similar pattern to that of MMPs. Half (n=5) of the control group showed diffuse strong staining for TIMP-4, but strong staining for TIMP-4 in the varicose vein group was not indentified. When focal or diffuse strong staining (++ or +++) is considered to identify a 'significantly stained group' and focal weak stain (+) is considered to identify a 'group that is not significantly stained', the differences in the expression of MMP-2, MMP-13, and TIMP-4 between the disease and the control groups were all statistically significant (p<0.001).
Table 3.
Differences in expression of MMPs and TIMP between the disease group and the control group
MMP=Matrix metalloproteinase; TIMP=Tissue inhibitor of metalloproteinase.
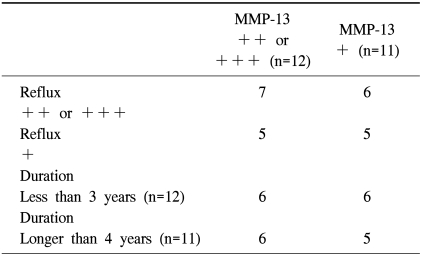
The relationship between the degree of reflux observed on the duplex ultrasonography or the duration of symptoms and expression of MMP-13 is presented in Table 4, and there is no significant increase in expression of MMP-13 with a rise in the degree of reflux or a longer duration of symptoms (p=0.317 and p=0.654, respectively).
Table 4.
Expression of MMP-13 according to the duration of symptoms or reflux
MMP=Matrix metalloproteinases.
DISCUSSION
The etiologic factors causing structural changes observed in VV remain to be clarified. Although venous hypertension followed followed by valvular incompetence was believed to be the primary cause, the biochemical or molecular mechanisms connecting venous hypertension and structural changes are still unclear. Vascular wall remodeling is reported to be related to the activity of macrophages, smooth muscle cells, endothelial cells, fibroblasts [4], MMPs, the enzymes secreted by macrophages, collagen, fibronectin, laminin, and other components of the extracellular matrix.
It was initially hypothesized that this study would reveal the increased expression of MMP-2 and -13 and TIMP-4 in VV because most of the studies that have compared the expressions of MMPs and TIMPs between human VV and non-varicose vein specimens showed up-regulation of the various subtypes of MMPs [5]. Those results agree with the existing hypothesis that morphologic characteristics of VV are caused by degradation of the extracellular matrix. Furthermore, Raffetto and colleagues [6] reported that the magnitude and the duration of the venous wall tension in vitro are related to over-expression of MMP-2. This supported our hypothesis about over-expression of MMPs and TIMP in VV because the duration of the symptoms or the awareness of protruded veins was present for at least one year in all of the patients who were included. It was speculated that this study would show a significant relationship between the over-expression of MMPs or TIMP and clinical characteristics such as duration of symptoms or grade of reflux on duplex ultrasonography. However, the results of this study did not show any relationships between the degree of expression of MMPs and clinical characteristics. The expression patterns of MMPs and TIMP between the two groups were quite contrary to the initial hypothesis (The fact that the control group consisted of upper extremity veins may not have had an effect on the results because they had not been exposed to the effects of gravity or venous hypertension.). Reasons for such results could be assumed to be as follows. First, observing the degree of reflux on the duplex ultrasonography was not the proper method for measuring the stimulatory effect that causes an increase in expression of MMPs. Ishikawa et al. [7] reported that levels of expression of MMP-2 and TIMPs in varicose veins were not different from those in the corresponding normal vessels and shear stress or increased intravascular pressure may lead to vascular wall remodeling. The degree of reflux can be subjective and may not properly represent hemodynamic stress. Second, the effect of prolonged exposure to venous hypertension in vivo on changes in the venous wall remains to be clarified. Raffetto and colleagues [6] suggested that increased venous wall tension causes over-expression of MMPs and consequently promotes degradation of the extracellular matrix, but they also mentioned that in vitro tension time was 24 hours in their experiment, and whether or not time-dependent acute venodilatory effects of MMPs have prolonged effect in vivo should be investigated.
The decrease, lack of change, or regional variation of MMP-2 or MMP-13 in varicose veins has also been previously noted [8,9]. MMPs are zinc-dependent endopeptidases that degrade all kinds of extracellular matrix and process bioactive molecules. Grouping or subtyping MMPs is based on the substrate specificity and cellular localization, but it is difficult to clearly classify the subtypes because the substrates or locations of MMPs overlap. Likewise, the precise role of each MMP subtype during the pathogenesis of VV remains to be clarified.
CONCLUSION
MMP-2, 13, and TIMP-4 did not show significantly increased expression in VV when compared to the control group. Clinical characteristics such as degree of reflux on duplex ultrasonography or duration of symptoms were not significantly related to the increased expression of MMP-13. Further studies should be performed to investigate the relationship between the clinical characteristics related to venous hypertension or reflux and the expression of MMPs and TIMP in VV.
Footnotes
This study is supported by a clinical research grant of Jeju National University Hospital.
References
- 1.Somers P, Knaapen M. The histopathology of varicose vein disease. Angiology. 2006;57:546–555. doi: 10.1177/0003319706293115. [DOI] [PubMed] [Google Scholar]
- 2.Lim CS, Davies AH. Pathogenesis of primary varicose veins. Br J Surg. 2009;96:1231–1242. doi: 10.1002/bjs.6798. [DOI] [PubMed] [Google Scholar]
- 3.Eklöf B, Rutherford RB, Bergan JJ, et al. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg. 2004;40:1248–1252. doi: 10.1016/j.jvs.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 4.Woodside KJ, Hu M, Burke A, et al. Morphologic characteristics of varicose veins: possible role of metalloproteinases. J Vasc Surg. 2003;38:162–169. doi: 10.1016/s0741-5214(03)00134-4. [DOI] [PubMed] [Google Scholar]
- 5.Sansilvestri-Morel P, Rupin A, Jullien ND, et al. Decreased production of collagen Type III in cultured smooth muscle cells from varicose vein patients is due to a degradation by MMPs: possible implication of MMP-3. J Vasc Res. 2005;42:388–398. doi: 10.1159/000087314. [DOI] [PubMed] [Google Scholar]
- 6.Raffetto JD, Qiao X, Koledova VV, Khalil RA. Prolonged increases in vein wall tension increase matrix metalloproteinases and decrease constriction in rat vena cava: potential implications in varicose veins. J Vasc Surg. 2008;48:447–456. doi: 10.1016/j.jvs.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishikawa Y, Asuwa N, Ishii T, et al. Collagen alteration in vascular remodeling by hemodynamic factors. Virchows Arch. 2000;437:138–148. doi: 10.1007/s004280000200. [DOI] [PubMed] [Google Scholar]
- 8.Gillespie DL, Patel A, Fileta B, et al. Varicose veins possess greater quantities of MMP-1 than normal veins and demonstrate regional variation in MMP-1 and MMP-13. J Surg Res. 2002;106:233–238. doi: 10.1006/jsre.2002.6455. [DOI] [PubMed] [Google Scholar]
- 9.Badier-Commander C, Verbeuren T, Lebard C, Michel JB, Jacob MP. Increased TIMP/MMP ratio in varicose veins: a possible explanation for extracellular matrix accumulation. J Pathol. 2000;192:105–112. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH670>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]