Abstract
Background
Development of thoracic aortic aneurysms and aortic dissections (TAAD) is attributed to unbearable wall tension superimposed on defective aortic wall integrity and impaired aortic repair mechanisms. Central to this repair mechanisms are well-balanced and adequately functional cellular components of the aortic wall, including endothelial cells, smooth muscle cells (SMCs), inflammatory cells, and adventitial fibroblasts. Adventitial fibroblasts naturally produce aortic extracellular matrix (ECM), and, when aortic wall is injured, they can be transformed into SMCs, which in turn are involved in aortic remodeling. We postulated the hypothesis that adventitial fibroblasts in patients with TAAD may have defects in ECM production and SMC transformation.
Materials and Methods
Adventitial fibroblasts were procured from the adventitial layer of fresh aortic tissues of patients with TAAD (Group I) and of multi-organ donors (Group II), and 4-passage cell culture was performed prior to the experiment. To assess ECM production, cells were treated with TNF-α (50 pM) and the expression of MMP-2 / MMP-3 was analyzed using western blot technique. To assess SMC transformation capacity, cells were treated with TGF-β1 and expression of SM α-actin, SM-MHC, Ki-67 and SM calponin was evaluated using western blot technique. Fibroblasts were then treated with TGF-β1 (10 pM) for up to 10 days with TGF-β1 supplementation every 2 days, and the proportion of transformed SMC in the cell line was measured using immunofluorescence assay for fibroblast surface antigen every 2 days.
Results
MMP-3 expression was significantly lower in group I than in group II. TGF-β1-stimulated adventitial fibroblasts in group I expressed less SM α-actin, SM-MHC, and Ki-67 than in group II. SM-calponin expression was not different between the two groups. Presence of fibroblast was observed on immunofluorescence assay after more than 6 days of TGF-β1 treatment in group I, while most fibroblasts were transformed to SMC within 4 days in group II.
Conclusion
ECM production and SMC transformation are compromised in adventitial fibroblasts from patients with TAAD. This result suggests that functional restoration of adventitial fibroblasts could well be a novel approach for the prevention and treatment of TAAD.
Keywords: Aorta, Aneurysm, Aortic dissection, Fibroblast
INTRODUCTION
Although thoracic aortic aneurysm and aortic dissection (TAAD) is relatively uncommon compared to other vascular diseases, clinical implications of this disease entity are of utmost significance in that proper management in a timely fashion is the key to a successful outcome. Without treatment, spontaneous rupture occurs in 70% of the patients, and, once develops, would reportedly lead to 94% of mortality. Surgical intervention is known to be the most effective treatment modality, but most of the victims of TAAD are exposed to the risk of detrimental complications caused by disease progression. Delay in treatment may be caused either by delay in diagnosis or by bias towards improper conservative treatment based on the poor understanding of this condition. Genetic predisposition, regression, inflammation, artherosclerotic changes and aortic injury by toxic materials have been proposed to be the pathogenesis of the development of TAAD [1,2]. Although these etiologies would have individual molecular biologic mechanisms [3], progressive destruction of the aortic wall with inflammatory response and smooth muscle cell loss are the common features observed in the disease process [4]. Thus, it is prudent to state that the development of TAAD is attributed to unbearable wall tension superimposed on defective aortic wall integrity and impaired aortic repair mechanisms. Central to this repair mechanisms are well-balanced and adequately functional cellular components of the aortic wall, including endothelial cells, smooth muscle cells (SMCs), inflammatory cells, and adventitial fibroblasts. While the roles of former three cell types in the development of TAAD are relatively well known, information regarding adventitial fibroblast is sparse. Adventitial fibroblasts naturally produce aortic extracellular matrix (ECM), and, when aortic wall is injured, they migrate intro aortic media and can be transformed into SMCs, which in turn are involved in aortic remodeling. We postulated the hypothesis that adventitial fibroblasts in patients with TAAD may have defects in ECM production and SMC transformation. To test this hypothesis, we compared the functional characteristics of the fibrobasts from the patients with TAAD to those from normal subjects.
MATERIALS AND METHODS
1) Fibroblast cell culture
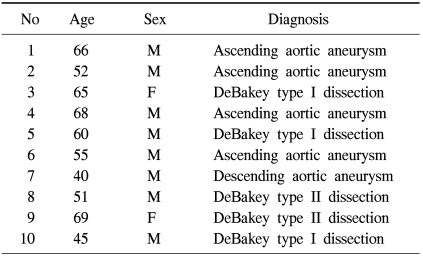
Fresh aortic tissue was procured from 10 patients with TAAD (group I) and 10 cardiac transplantation donors (group II). Age at operation in TAAD group was 57.1±10.1 years (40~69 years) (Table 1). Specimens were stored in culture media (DMEN, invitrogen, CA, USA) at 4℃ for less than 4 hours prior to the next step. Adventitial layer was then separated from the aortic wall, rinsed with the same culture media for three times, minced into small pieces, and stored in the culture media containing 5% of collagenase and elastinase at 37℃ for 30 minutes. Precipitates separated from culture media using centrifuge were then cultured in 10% fetal bovine serum containing antibiotics (100 U/mL of penicillin) in an incubator with 5% CO2 at 37℃. Fresh culture media were replaced every 48 hours, and, every 4 to 7 days, cultured cells were transferred to a new media for next-passage cell culture, to finally obtain 4-passage cell line.
Table 1.
Characteristics of the patients with thoracic aortic aneurysm and dissection
2) Western blot analysis
To assess the extracellular matrix production of adventitial fibroblast, cultured cells were treated with TNF-α (Tumor necrosis factor) (50 pM, ABCam, MA) for 2 days, rinsed with PBS for three times, and set to react with SDS sample buffer (Tris-Cl, SDS, b-mercaptoethanol, Glycerol, Bromophenol Blue) for 2~3 minutes. Degeneration of the proteins was induced by heating the sample for 5 to 10 minutes, and the sample was centrifuged and stored in the ice. Supernatant of the sample (15 µL) was then processed by 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE), treated with blocking buffer (5% skim milk) for 2 hours in the polyvinylidene fluoride (PVDF), and rinsed in the rinsing solution for 3 times. The reactor of this protein, matrix metalloproteinase 2, 3 (MMP-2, MMP-3, ABCam, MA) antibodies, was diluted to 1 : 1,000, and stored in the blocking buffer at 4℃ for 24 hours. Then, enhanced Chemiluminescence (ECL, Amersham Biosciences, NJ) was performed using the protein and the reactor. To assess the degree of SMC transformation of the fibroblasts, cultured cells were treated with TGFβ1 (10 pM, ABCam, MA) for 2 days, and the remaining process was the same as above except for that reactors were for smooth muscle (SM) α-actin, SM-MHC, Ki-67, and SM calponin (ABCam, MA).
3) Immunofluorescent staining
To conduct an in vivo test to assess SMC transformation of the fibroblasts, each fibroblast was placed in the round culture bottle, and TGFβ1 (10 pM, ABCam, MA) was supplemented every 2 days. Cell lines were observed at 0, 2, 4, 6, 8 and 10 days after the treatment of TGFβ1 to ascertain the proportion of transformed SMC from fibroblasts using immunofluorescence staining. For immunofluorescence staining, culture media was eliminated from the culture plate, and the precipitates were rinsed using PBS for 3 times. Fixation of the cells was performed using 3.7% formaldehyde, and the cells were then rinsed with PBS, methanol, 0.5% triton X-100T and 1% blocking solution. Antibodies for SM α-actin and fibroblast surface antigens were diluted (1 : 1,000) and stored with the cells at 4℃ in the dark room overnight. The cells were then rinsed with PBS to react with Texas red-labeled secondary antibodies (1 : 2,500) for 1 hour in the dark room, and subsequently to react with DAPI (5 mg/mL) for 5 seconds in the room temperature. After the cells were rinsed by PBS, SMCs transformed from adventitial fibroblasts were observed using immunofluorescent microscopy (Leica MPS 60, Germany).
4) Statistical analysis
Analyses were conducted using SPSS version 12.0 (SPSS inc. Chicago, IL). Data were presented as means with standard standard deviation. Comparison between the groups was performed using student's t-test. A p-value less than 0.05 was considered statistically significant.
RESULTS
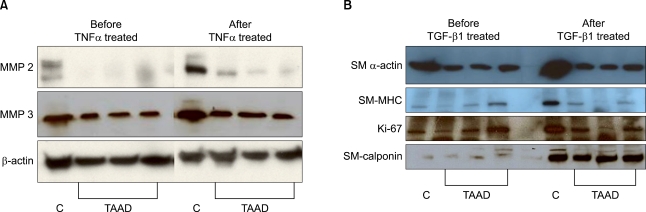
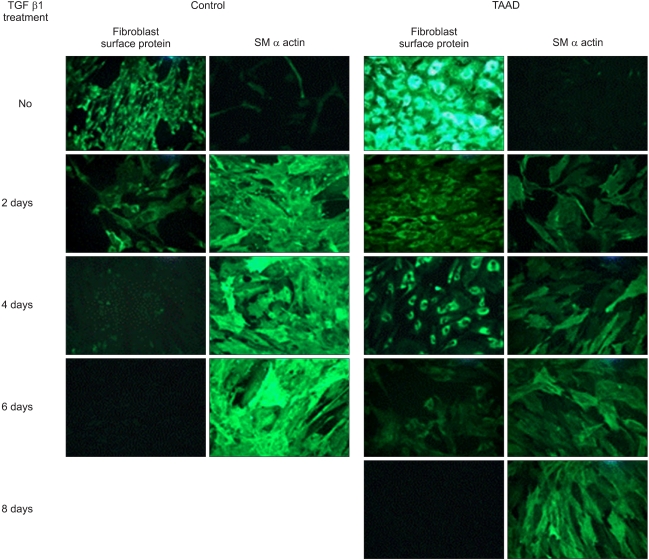
On western blot after the treatment of the fibroblasts with TNF-α (50 pM) for 2 days, MMP-3 expression in comparison to β actin was significantly lower in group I (83.9% to 97.5%) than in group II (99.7% to 140.9%, p<0.05). There was no inter-group difference in MMP-2 expression in comparison to β actin (98.6% to 108.9% versus 24.3% to 30.4%, p>0.05) (Fig. 1A). TGF-β1-stimulated adventitial fibroblasts in group I expressed less SM α-actin, SM-MHC, and Ki-67 than in group II (SM α-actin: 100.0% to 110.6% in group I, 94.8% to 141.6% in group II, P<0.01; SM-MHC: 66.2% to 68.5% in group I, 51.9% to 116.8% in group II, p<0.01; Ki-67: 77.1% to 72.3% in group I, 49.7% to 168.1% in group II, p<0.01). SM-calponin expression was not different between the two groups (60.9% to 143.4% in group I, 46.4% to 144.9% in group II, p>0.05) (Fig. 1B). Presence of fibroblast was observed on immunofluorescence assay after more than 6 days of TGF-β1 treatment (10 pM) in group I. To the contrary, SMC began to appear after 2 days of TGF-β1 treatment and most fibroblasts were transformed to SMC within 4 days in group II (Fig. 2).
Fig. 1.
(A) The extracellular matrix metabolism was reduced in adventitial fibroblasts from the patients with TAAD. The MMP-2 expression in the control group was 98.6% before TNFα (50 pM) treatment and 108.9% after treatment, and 24.3% before treatment and 30.4% after treatment in the TAAD group. The MMP-3 expression in the control group enhanced from 99.7% to 140.9% and from 83.9% to 97.5% in the TAAD group. Thus, TNFα treated adventitial fibroblasts from TAAD aortas expressed less MMP-3 than fibroblasts from healthy control group. (B) The smooth muscle cell transformation was reduced in adventitial fibroblasts from the patients with TAAD. The SM α-actin expression before TGFβ1 (10 pM) treatment in the control group was 94.8% and 141.6% after treatment, while 100.0% before treatment and 110.6% after treatment in the TAAD group. The SM-MHC expression in the control group was 51.9% before treatment and 116.8% after treatment while 66.2% before treatment and 68.5% after treatment in the TAAD group. The Ki-67 expression in the control group was 49.7% before treatment and 168.1% after treatment, while 77.1% before treatment and 72.3% after treatment in the TAAD group. The SM calponin expression in the control group was 46.4% and 144.9% after treatment, while 60.9% before treatment and 143.4% after treatment in the TAAD group. Thus, TGF-β1-stimulated adventitial fibroblasts from TAAD aortas expressed less SM α-actin and SM-MHC and Ki-67. Percent of controls were compared with the β actin expression. TNF=Tumor necrosis factor; MMP=Matrix metalloproteinase; C=Control group; TAAD=Thoracic aortic aneurysm and dissection; TGF=Transforming growth factor; SM=Smooth muscle; MHC=Major histocompatibility complex.
Fig. 2.
The immunohistochemical staining after fibroblasts were exposed to TGF-β1 (10 pM) for up to 10 days. TGF-β1 was supplemented every 2 days and the expressions of fibroblast specific marker (fibroblast surface protein) and smooth muscle cell specific marker (SM α actin) were examined every 2 days. At 4th day after treatment, the fibroblast surface protein was not expressed and SM α actin was fully expressed in the control group. In TAAD group, however, the fibroblast surface protein was still expressed at 6th day after treatment (×100). Control=Normal adventitial fibroblast; TGF=Transforming growth factor; SM=Smooth muscle; TAAD=Thoracic aortic aneurysm and dissection.
DISCUSSION
Thoracic aortic aneurysm is a life-threatening disease, and the pathogenesis of this condition is attributed to unbearable wall tension superimposed on defective aortic wall integrity and impaired repair mechanisms of the aortic wall. Cellular components, such as endothelial cells, smooth muscle cells (SMCs), inflammatory cells, adventitial fibroblasts, and extracellular matrix constitute the aortic wall. Remodeling of the aortic wall progresses toward the direction of maintaining adequate elastance to meet the hemodynamic requirements, and impairment of this remodeling mechanism leads to the formation of aortic aneurysm, aortic dissection and rupture. Aortic wall comprises three layers, and each layer plays a unique role in coping with the hydrauric stresses. The endothelial layer prevents the progression of wall tension initiated by the pulsatile blood flow to the outer layers. The media, the middle layer, is composed of SMCs and extracellular matrix (ECM). ECM consists of structural proteins, such as collagen and elastin, and glue proteins, such as laminin and fibronectin. SMCs and ECM determines the contractility and distensibility of the aortic wall. Thus, SMCs, collagen and elastin are the major determinants of aortic wall elastance. Decrease in ECM or unbalance of the ECM components leads to stiff aortic wall, which in turn results in the development of TAAD. Decrease in elastin is reported to stimulate the proliferation of the SMCs, and this disproportion of SMCs and ECM is observed in the diseases aorta, which signifies that balanced constitution of SMCs and ECM is of utmost importance in keeping the aortic wall integrity [5]. The third and outermost layer, adventitia, incurs little tensile stress from the blood stream in the physiologic setting because systolic pulse energy does not reach this layer [6]. In the pathologic condition of the aorta, however, adventitial layer is actively involved in the remodeling process by the production of ECM, internal migration of fibroblast with SMC transformation, and angiogenesis [7-12]. While disruption of the endothelial layer and inflammatory cell activation leads to the breakdown of the ECM, SMCs and fibroblast plays an important role in maintaining the optimal cell-matrix ratio.
To conduct a functional assessment of the adventitial fibroblast, we induced the degeneration of ECM by TNFα and measured MMP-2, which degradates collagen IV and regulates inflammatory process, and MMP-3, which degradates fibronectin, laminin, collagens III, IV, IX, X, and cartilage proteoglycan and regulates healing process and prevents artherosclerosis. On western blot, MMP-3 expression was less in patients with TAAD than in control group, which may signify overproduction of ECM may lead to aortic wall fibrosis.
In the remodeling process, adventitial fibroblasts have a potential to be transformed to SMCs or their analogues and to migrate to the media [11]. To assess the SMC transformation capacity of the fibroblasts, we stimulate the adventitial tissue with TGF-β1 and measured SM α-actin and actin, which are contractile proteins, SM-MHC, which regulates basic contraction, SM-calponin, which regulates smooth muscle contraction, and Ki-67, which is involved in SMC proliferation. On western blot, SM α-actin, SM-MHC and Ki-67 decreased in patients with TAAD compared to the control group, but there was no intergroup difference in SM-calponin level. It was also observed that SMC transformation was markedly delayed in the patients with TAAD on immunofluorescent staining. Thus, adventitial fibroblasts maintain aortic wall integrity as a central biologic regulator, and, once activated, conduct various missions such as cellular proliferation and ECM production [11,13-19], regulation of vascular tone [20,21], attenuation of oxidative stimulus and inflammation [22-25], cellular growth [26-28], and cellular migration and differentiation [29,30].
CONCLUSION
In patients with TAAD, adventitial fibroblasts are dysfunctional in terms of decreased ECM production and impaired SMC transformation. Therefore, restoration of fibroblast function may be the key to the prevention and treatment of TAAD. Further studies to elucidate the exact roles of adventitial fibroblast in the development of TAAD are mandatory.
References
- 1.Lee S, Solow-Cordero DE, Kessler E, Takahara K, Greenspan DS. Transforming growth factor-beta regulation of bone morphogenetic protein-1/procollagen C-proteinase and related proteins in fibrogenic cells and keratinocytes. J Biol Chem. 1997;272:19059–19066. doi: 10.1074/jbc.272.30.19059. [DOI] [PubMed] [Google Scholar]
- 2.Di Donato A, Ghiggeri GM, Di Duca M, et al. Lysyl oxidase expression and collagen cross-linking during chronic adriamycin nephropathy. Nephron. 1997;76:192–200. doi: 10.1159/000190168. [DOI] [PubMed] [Google Scholar]
- 3.Ross JJ, Tranquillo RT. ECM gene expression correlates with in vitro tissue growth and development in fibrin gel remodeled by neonatal smooth muscle cells. Matrix Biol. 2003;22:477–490. doi: 10.1016/s0945-053x(03)00078-7. [DOI] [PubMed] [Google Scholar]
- 4.Kenyon NJ, Ward RW, McGrew G, Last JA. TGF-beta1 causes airway fibrosis and increased collagen I and III mRNA in mice. Thorax. 2003;58:772–777. doi: 10.1136/thorax.58.9.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shanley CJ, Gharaee-Kermani M, Sarkar R, et al. Transforming growth factor-beta 1 increases lysyl oxidase enzyme activity and mRNA in rat aortic smooth muscle cells. J Vasc Surg. 1997;25:446–452. doi: 10.1016/s0741-5214(97)70254-4. [DOI] [PubMed] [Google Scholar]
- 6.Boak AM, Roy R, Berk J, et al. Regulation of lysyl oxidase expression in lung fibroblasts by transforming growth factor-beta 1 and prostaglandin E2. Am J Respir Cell Mol Biol. 1994;11:751–755. doi: 10.1165/ajrcmb.11.6.7946403. [DOI] [PubMed] [Google Scholar]
- 7.Kähäri VM, Chen YQ, Su MW, Ramirez F, Uitto J. Tumor necrosis factor-alpha and interferon-gamma suppress the activation of human type I collagen gene expression by transforming growth factor-beta 1. Evidence for two distinct mechanisms of inhibition at the transcriptional and posttranscriptional levels. J Clin Invest. 1990;86:1489–1495. doi: 10.1172/JCI114866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verrecchia F, Mauviel A. TGF-beta and TNF-alpha: antagonistic cytokines controlling type I collagen gene expression. Cell Signal. 2004;16:873–880. doi: 10.1016/j.cellsig.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Verrecchia F, Wagner EF, Mauviel A. Distinct involvement of the Jun-N-terminal kinase and NF-kappaB pathways in the repression of the human COL1A2 gene by TNF-alpha. EMBO Rep. 2002;3:1069–1074. doi: 10.1093/embo-reports/kvf219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iraburu MJ, Domínguez-Rosales JA, Fontana L, et al. Tumor necrosis factor alpha down-regulates expression of the alpha1(I) collagen gene in rat hepatic stellate cells through a p20C/EBPbeta- and C/EBPdelta-dependent mechanism. Hepatology. 2000;31:1086–1093. doi: 10.1053/he.2000.5981. [DOI] [PubMed] [Google Scholar]
- 11.Pischon N, Darbois LM, Palamakumbura AH, Kessler E, Trackman PC. Regulation of collagen deposition and lysyl oxidase by tumor necrosis factor-alpha in osteoblasts. J Biol Chem. 2004;279:30060–30065. doi: 10.1074/jbc.M404208200. [DOI] [PubMed] [Google Scholar]
- 12.Yamane K, Ihn H, Asano Y, Jinnin M, Tamaki K. Antagonistic effects of TNF-alpha on TGF-beta signaling through down-regulation of TGF-beta receptor type II in human dermal fibroblasts. J Immunol. 2003;171:3855–3862. doi: 10.4049/jimmunol.171.7.3855. [DOI] [PubMed] [Google Scholar]
- 13.Stetson SJ, Perez-Verdia A, Mazur W, et al. Cardiac hypertrophy after transplantation is associated with persistent expression of tumor necrosis factor-alpha. Circulation. 2001;104:676–681. doi: 10.1161/hc3101.093765. [DOI] [PubMed] [Google Scholar]
- 14.Siwik DA, Chang DL, Colucci WS. Interleukin-1beta and tumor necrosis factor-alpha decrease collagen synthesis and increase matrix metalloproteinase activity in cardiac fibroblasts in vitro. Circ Res. 2000;86:1259–1265. doi: 10.1161/01.res.86.12.1259. [DOI] [PubMed] [Google Scholar]
- 15.Hiraga S, Kaji T, Ueda Y, et al. Modulation of collagen synthesis by tumor necrosis factor alpha in cultured vascular smooth muscle cells. Life Sci. 2000;66:235–244. doi: 10.1016/s0024-3205(99)00586-x. [DOI] [PubMed] [Google Scholar]
- 16.Li YY, Feng YQ, Kadokami T, et al. Myocardial extracellular matrix remodeling in transgenic mice overexpressing tumor necrosis factor alpha can be modulated by anti-tumor necrosis factor alpha therapy. Proc Natl Acad Sci U S A. 2000;97:12746–12751. doi: 10.1073/pnas.97.23.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grande JP, Melder DC, Zinsmeister AR. Modulation of collagen gene expression by cytokines: stimulatory effect of transforming growth factor-beta1, with divergent effects of epidermal growth factor and tumor necrosis factor-alpha on collagen type I and collagen type IV. J Lab Clin Med. 1997;130:476–486. doi: 10.1016/s0022-2143(97)90124-4. [DOI] [PubMed] [Google Scholar]
- 18.Armendariz-Borunda J, Katayama K, Seyer JM. Transcriptional mechanisms of type I collagen gene expression are differentially regulated by interleukin-1 beta, tumor necrosis factor alpha, and transforming growth factor beta in Ito cells. J Biol Chem. 1992;267:14316–14321. [PubMed] [Google Scholar]
- 19.Zhang H, Facemire CS, Banes AJ, Faber JE. Different alpha-adrenoceptors mediate migration of vascular smooth muscle cells and adventitial fibroblasts in vitro. Am J Physiol Heart Circ Physiol. 2002;282:H2364–H2370. doi: 10.1152/ajpheart.00858.2001. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Lo C. Regulation of fibronectin expression by PDGF-BB and IGF-I in cultured rat thoracic aortic adventitial fibroblasts. Cell Biol Int. 1995;19:517–525. doi: 10.1006/cbir.1995.1096. [DOI] [PubMed] [Google Scholar]
- 21.Liu G, Eskin SG, Mikos AG. Integrin alpha(v)beta(3) is involved in stimulated migration of vascular adventitial fibroblasts by basic fibroblast growth factor but not platelet-derived growth factor. J Cell Biochem. 2001;83:129–135. doi: 10.1002/jcb.1208. [DOI] [PubMed] [Google Scholar]
- 22.Zhu DL, Herembert T, Marche P. Mitogenic events induced by vasopressin in aortic fibroblasts from spontaneously hypertensive rats. Clin Sci (Lond) 1993;85:57–61. doi: 10.1042/cs0850057. [DOI] [PubMed] [Google Scholar]
- 23.Zhu DL, Herembert T, Marche P. Increased proliferation of adventitial fibroblasts from spontaneously hypertensive rat aorta. J Hypertens. 1991;9:1161–1168. [PubMed] [Google Scholar]
- 24.Li G, Chen YF, Greene GL, Oparil S, Thompson JA. Estrogen inhibits vascular smooth muscle cell-dependent adventitial fibroblast migration in vitro. Circulation. 1999;100:1639–1645. doi: 10.1161/01.cir.100.15.1639. [DOI] [PubMed] [Google Scholar]
- 25.Zalewski A, Shi Y. Vascular myofibroblasts. Lessons from coronary repair and remodeling. Arterioscler Thromb Vasc Biol. 1997;17:417–422. doi: 10.1161/01.atv.17.3.417. [DOI] [PubMed] [Google Scholar]
- 26.Faggin E, Puato M, Zardo L, et al. Smooth muscle-specific SM22 protein is expressed in the adventitial cells of balloon-injured rabbit carotid artery. Arterioscler Thromb Vasc Biol. 1999;19:1393–1404. doi: 10.1161/01.atv.19.6.1393. [DOI] [PubMed] [Google Scholar]
- 27.Hu Y, Zhang Z, Torsney E, et al. Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J Clin Invest. 2004;113:1258–1265. doi: 10.1172/JCI19628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Darby I, Skalli O, Gabbiani G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest. 1990;63:21–29. [PubMed] [Google Scholar]
- 29.Smith JD, Bryant SR, Couper LL, et al. Soluble transforming growth factor-beta type II receptor inhibits negative remodeling, fibroblast transdifferentiation, and intimal lesion formation but not endothelial growth. Circ Res. 1999;84:1212–1222. doi: 10.1161/01.res.84.10.1212. [DOI] [PubMed] [Google Scholar]
- 30.Miano JM, Kitchen CM, Chen J, et al. Expression of human smooth muscle calponin in transgenic mice revealed with a bacterial artificial chromosome. Am J Physiol Heart Circ Physiol. 2002;282:H1793–H1803. doi: 10.1152/ajpheart.00875.2001. [DOI] [PubMed] [Google Scholar]