Abstract
How dendritic cells (DC) present antigen to cytotoxic T cells (CTL) without themselves being killed through contact mediated cytotoxicity (so called kiss of death) has proven to be controversial. Using mice deficient in Serine Protease Inhibitor 6 (Spi6) we show that Spi6 protects DC from the kiss of death by inhibiting granzyme B (GrB) delivered by CTL. Infection of Spi6 KO mice with Lymphocytic Choriomeningits virus (LCMV) revealed impaired survival of CD8α DC. The impaired survival of Spi6 KO CD8α DC resulted in impaired priming and expansion of both primary and memory LCMV-specific CTL, which could be corrected by GrB deficiency. The rescue in the clonal burst obtained by GrB elimination demonstrated that GrB was the physiological target through which Spi6 protected DC from CTL. We conclude that the negative regulation of DC priming of CD8 T lymphocyte immunity by CTL killing is mitigated by the physiological inhibition of GrB by Spi6.
Keywords: dendritic cells, granzyme B, serine protease inhibitor, cytotoxic T cell
Introduction
The killing of infected cells by CD8+ cytotoxic T cells (CTL) is critical for immunity to intracellular pathogens such as viruses (1). After the resolution of an acute viral infection, a sub-population of CTL differentiates into memory CD8 T cells (2). Memory cells exhibit long-lived protection from subsequent infections by the same virus through robust secondary responses involving immediate cytotoxic function from effector memory cells (Tem) (3) and rapid expansion of central memory (Tcm) into new CTL effectors (3, 4). Dendritic cells (DC) are the physiological antigen presenting cells that stimulate both naïve CD8 T cells and memory CD8 T cells to differentiate and proliferate into CTL (5, 6). In mice, the CD11c++ CD8α++ CD205+ DC population can best acquire a wide variety of cellular antigens (including viral proteins) from infected and apoptotic cells and present them on self class I MHC to cognate CD8 T cells in a process known as cross-presentation (7, 8). CD8αDC are also the subset specifically responsible for cross-presentation of LCMV antigens to CD8 T cells (7).
Contact-dependent, lymphocyte-mediated cytotoxicity proceeds through two pathways. The first pathway is triggered by members of the Tumor Necrosis Factor Receptor family of which Fas is the most important (1). The second involves the exocytosis of proteins present in CTL and natural killer cell granules (1). Exocytosis of perforin (Pfn) (1) facilitates the entry of serine proteases called granzymes, which trigger apoptosis in target cells (9). Granzymes A and B are the most abundant granzymes in mice and humans and are the best-characterized (1). Granzyme B (GrB) activates the caspase-dependent pathways of apoptosis and like caspases cleaves after aspartic-acid residues.
Given the effectiveness of the granule exocytosis pathway to deliver a kiss of death (1) it is not surprising that antigen-presenting DC are themselves killed by cognate CTL. Experiments with Pfn deficient CTL show that primary CTL eliminate DC that express cognate peptide-antigen/MHC (pMHC) as part of a negative-feedback mechanism that limits the expansion of the immune response to tumor (10) or virus (11). This is consistent with observations that the maximum primary clonal burst requires the continual presentation of antigen by DC to differentiated CTL over the course of several days in addition to the initial presentation for a few hours to cytotoxically inert naïve CD8 T cells (12, 13). A negative regulatory loop working through the killing of DC has also been observed in the memory CD8 T cell response (14) to allow the containment and efficient resolution of CTL expansion from cytotoxic Tem cells (15). However, the fact that DC are still highly effective at priming CTL expansion implies that they have mechanisms that protect them from the kiss of death.
The mouse serine protease inhibitor (serpin), Serine Protease Inhibitor 6 (Spi6), is a potent inhibitor of GrB (16). Spi6 lacks signal secretory sequences and so it has been suggested that this endogenous inhibitor protects cells from CTL-induced damage by inhibiting GrB in the cytoplasm (1). The up-regulation of Spi6 in DC upon maturation or through transgene expression results in the protection of DC from granule-mediated programmed cell death (PCD) in vitro (17). However, increased Spi6 expression in bone marrow-derived DC (BMDDC) is not sufficient to protect from direct killing by CTL in vivo (18). Therefore whether or not protection of DC from GrB-mediated killing is a physiological mechanism for the control of CTL immunity remains to be determined. We show that Spi6 when up-regulated in mature BMDDC is required to protect from GrB-mediated CTL killing. Importantly, we show a similar requirement for protection from GrB by Spi6 in the in vivo survival of CD8αDC after LCMV infection. The impaired survival of Spi6 KO CD8αDC resulted in defective expansion of primary CTL and secondary CTL from memory CD8 T cells. We conclude that the negative regulation of DC priming of CD8 T cells by CTL killing is mitigated by the physiological inhibition of GrB by Spi6.
Materials and Methods
Mice
Thy1.2+ and Thy1.1+ C57BL/6J wild-type and GrB KO mice (9) were purchased from Jackson Lab. C57BL/6J Spi6 KO mice and P14 mice were bred in house (19). GrB KO P14 Thy1.1 mice were generated by inter-crossing and screening by PCR for the GrB allele (9) and flow-cytometry for the P14 TCR (Vα2+) and CD90.1 in the blood (19). All animals were maintained in a pathogen-free environment at Imperial College London and all experiment were conducted in accordance to Home Office (UK) regulations.
Flow cytometry
The following fluorescently labelled monoclonal antibodies were purchased from eBiosciences: anti-CD86- (allophycocyanin [APC]-labeled), anti-I-Ab- (fluorescein isothiocynate [FITC]-labeled), anti-CD11c- (R-phycoerythrin [PE]-labeled), anti-CD8-APC, anti-CD90.1-AlexaFluor450. The following fluorescently labelled antibodies were purchased from BD Pharmingen: Ly-6C-PE-Cy7, CD4-FITC. The gp33 peptide [KAVYNFATM] /H-2Db-APC tetramer was purchased from Becton Dickinson (19). Lymph-node cells, splenocytes and BMDDCs were prepared and stained with tetramers and mAb as before (19). ICS with anti-EdU-AlexaFlour488 to detect EdU incorporation in dividing P14 CD8 cells was performed according the manufacturer’s protocol (Invitrogen). DC were subjected to ICS with rabbit anti-Spi6 antiserum (1/1000 dilution) or rabbit pre-immune serum (1/1000 dilution) (20) then goat anti-rabbit IgG-APC (1/100 dilution; Jacksons ImmunoResearch). Stained cells were acquired on a Cyan ADP machine (Beckman Coulter) and analyzed with FlowJo (TreeStar Inc.).
CTL assays
Bone marrow was flushed out of femoral bones taken from 6–8 weeks old mice and immature BMDDC generated by 8–10 of culture as described in Lutz et al. (21). BMDDC were matured by culture for 24h with LPS (1 μg/ml, Sigma). Spleen cells (106/ml) from wild-type or GrB KO P14 mice were cultured with LCMV gp33 peptide [KAVYNFATM] (10−6M) and IL-2 (10 U/ml) for 2 d to generate CTLs (19). To measure CTL-induced apoptosis, BMDDC targets were pulsed with gp33 (10−7M) for 1h then genomic DNA labelled with 3H-thymidine and then incubated with P14 CTL over a range of ratios in 12 replicates. The percentage apoptosis was determined after 4h as follows: % apoptosis = (3H-labelled DNA retained in target without CTL) – (3H-labelled DNA retained in target with CTL/ 3H-labelled DNA retained in target without CTL) × 100 (22). Apoptosis was induced by treatment with anti-mouse Fas mAb (clone Jo2) and cyclohexamide (BD Biosciences). To measure CTL-induced lysis, BMDDC targets were pulsed with gp33 (10−7M) for 1h and labelled with 51Cr- then incubated with P14 CTL over a range of ratios in quadruplicate. The percentage specific lysis was determined after 4 h as follows: % specific release = (specific release-spontaneous release)/(maximum release-spontaneous release) × 100. The percentage specific lysis of RMA cells in the absence of gp33 was <10% and the spontaneous release was <10% of the maximum release (19). In addition, gp33-pulsed BMDDC were also labelled with the cell-permanent non-fluorescent acetomethoxy derivate of calcein (Calcein AM) according to the manufacturer’s protocol (Invitrogen) then incubated with P14 CTL over a range of E/T ratios. After 4 or 8 h BMDDC lysis was measured by the release of fluorescent calcein into the supernatant as follows: % specific release = (specific release-spontaneous release)/(maximum release-spontaneous release) × 100. The percentage specific lysis of RMA cells in the absence of gp33 was <10% and the spontaneous release was <10% of the maximum release.
Adoptive transfer and LCMV infection
Naïve CD8+ cells were purified (>90%) from the spleens of wild-type or GrB KO P14 mice (Thy1.1+) by positively sorting with anti-CD8 magnetic beads (Miltenyi Biotec) and adoptively transferred (5×103) by i.v. injection into wild-type or Spi6 KO (Thy1.2+) and after 2 d infected with LCMV Armstrong (2×105 pfu i.p.). For memory cell experiments, LCMV Armstrong recipients were re-infected after 40d with clone 13 variant of LCMV Armstrong (106 pfu i.v.). A quantitative PCR method was used measure the level of LCMV Armstrong from infected mice exactly as described by McCauslan and Crotty (23). Briefly, total spleens were weighed then a known amount homogenized and total RNA recovered. First strand cDNA synthesis was performed then cDNA used as the template for real-time PCR using primers specific for the glycoprotein (GP forward and reverse primers) and nucleoprotein (NP2 forward and reverse primers) of LCMV. A standard curve with linearized plasmids encoding LCMV GP and NP genes to calculate the number of copies of LCMV genome per mg spleen.
Ex vivo DC analysis
Spleens were cut into small fragments and digested with Collagenase D grade II (1mg/ml) and DNaseI grade II (20μg/ml) (both from Roche) in RPMI 1640 medium + 10% FCS for 25 min at room temperature. EDTA (0.2mM) was successively added for another 5 min to disrupt DC-T cell interaction. Spleen fragments were filtered through mesh (70 μm) and centrifuged at 2, 000 × g for 5 min at room temperature (7). Cells were re-suspended in MACS buffer (2% BSA + 0.5M EDTA in PBS) and magnetically sorted with anti-CD11c-beads (according to Miltenyi Biotec protocol). The number of CD8α DC in each spleen was determined by determining the % of CD11c+ Ly6C− CD8α+ CD4− cells then multiplying by the number of enriched CD11c+ cells. The number of pDC in each spleen was determined by multiplying the % of CD11c+ Ly6C+ cells by the number of enriched CD11c+ cells. For ex vivo priming and expansion experiments, CD8α DC from the spleens of infected mice were FACS-sorted after antibody staining (MoFlo; DakoCytomation). Briefly, CD11c+ enriched cells (protocol as above) were stained for surface markers and CD11c++CD8+CD4−cells (8% CD11c+-enriched cells in wild-type, 3% in Spi6 KO) purified by FACS (>98% pure). FACS-purified CD8α DC (0–5 × 104 per 96 U-bottom well/0.2ml) were incubated with bead sorted CD8+ T cells from wild-type P14 mice (5 × 104 cells/well). Positive control cultures that were pulsed with gp33 peptide gave 55 % EdU+cells and negative control cultures (5 × 104 P14 CD8 T cells alone/well) gave 0.7 %. EdU+ cells. Cells were pulsed with EdU (5 μM) for 2.5 d and the percentage of EdU+ cells was then determined by ICS with anti-EdU-AlexaFlour488 and flow-cytometry according to manufacturer instructions (Click-iTR EdU Invitrogen).
Statistics
The significance of difference was measured using two-tailed Student’s t-tests.
Results
Spi6 is required to protect BMDDC from killing by GrB delivered by CTL
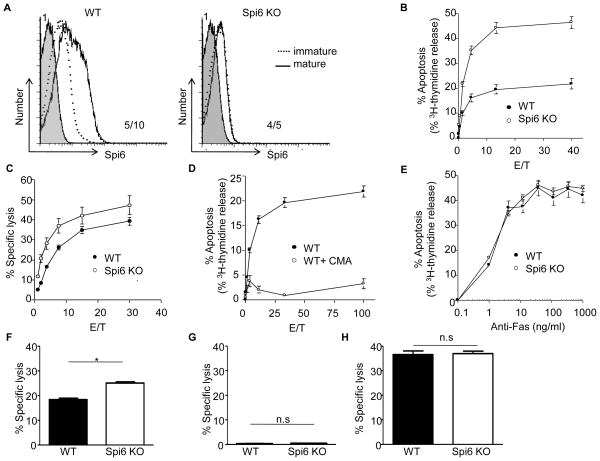
We used Spi6 KO mice to determine whether Spi6 was required to protect DC from CTL killing in vitro (19). BMDDC from C57BL/6 mice (H-2b) were generated then “matured” by activation with culture in LPS, as evidenced by the up-regulation of the CD86 and I-Ab markers (21) (Suppl. Fig. 1). Intracellular staining (ICS) with anti-Spi6 antiserum (20) revealed the intracellular expression of Spi6 after LPS activation of BMDDC from wild-type mice (Fig. 1A). Spi6 KO BMDDC showed an activated phenotype comparable to that of wild-type (Suppl. Fig. 1), but did not express Spi6 (Fig. 1A). We examined the sensitivity of matured BMDDC pulsed with the gp33 LCMV antigen peptide to killing by wild-type anti-LCMV CTL that express the P14 TCR specific for gp33/H-2Db (19). Spi6 KO BMDDC are more susceptible to PCD than wild-type BMDDC, as evidenced by DNA fragmentation measured after 4h (Fig. 1B). Furthermore, the specific lysis of Spi6 KO BMDDC was consistently greater than wild-type, as evidenced by Cr51 release from the cytoplasm (Fig. 1C). Our in vitro findings with BMDDC are in agreement with previous reports of increased expression of Spi6 upon maturation by LPS activation correlating with resistance to CTL killing (17, 18).
Figure 1. Spi6 up-regulation in mature BMDDC is required to protect from CTL killing.
(A) Histograms for staining with rabbit pre-immune serum (shaded histogram) and rabbit anti-Spi6 antiserum in immature (broken line) or LPS-matured BMDDC (line). MFI for pre-immune serum (top left) and anti-Spi6 serum (bottom right). (B) % Apoptosis based on 3H-thymidine release from LPS-matured and gp33-pulsed BMDDC after 4h culture with P14 CTL. (C) % Specific lysis based on 51Cr-release from BMDDC after 4h culture with P14 CTL. (D) % Apoptosis based on 3H-thymidine. (E) % Apoptosis based on 3H-thymidine after treatment with anti-Fas mAb. (F) % Specific lysis based on the release of calcein AM from BMDDC after 4h culture with WT P14 CTL assay at E/T = 20 after 4h. (G) % Specific lysis based on release of calcein AM BMDDC after 4h culture with GrB KO P14 CTL at E/T = 20. (H) % Specific lysis based on release of calcein AM with GrB KO P14 CTL assay at E/T = 20 after 8h. Values are median and ± SEM of triplicate measure and representative of 3 independent experiments.
Treatment of CTLs with concanamycin A (CMA) inhibits granule-mediated cytolysis, but does not affect Fas-mediated cytotoxicity (24). P14 CTLs treated with CMA did not induce the PCD of gp33-pulsed DCs, indicating that the increased lysis and DNA fragmentation of Spi6 KO BMDDCs was due to increased sensitivity to granule-mediated PCD and not Fas-killing (Fig. 1D). This conclusion is supported by Fig. 1E, in which we show that BMDDC from Spi6 KO mice are equally susceptible PCD induced by anti-Fas antibody. Finally, we used P14 CTL from GrB KO mice (9, 19) to address the specific role of GrB in the susceptibility of Spi6 KO BMDDC to CTL killing. GrB KO P14 CTLs did not induce the lysis of gp33-pulsed DCs after either 4h (Fig. 1G) or 8h (Fig.1H), indicating that the increased killing of Spi6 KO BMDCs was due to increased sensitivity to GrB. We conclude that Spi6 up-regulation is required to protect BMDDC from GrB-mediated killing by CTL.
Protection from GrB by Spi6 determines the survival of CD8α DC in vivo
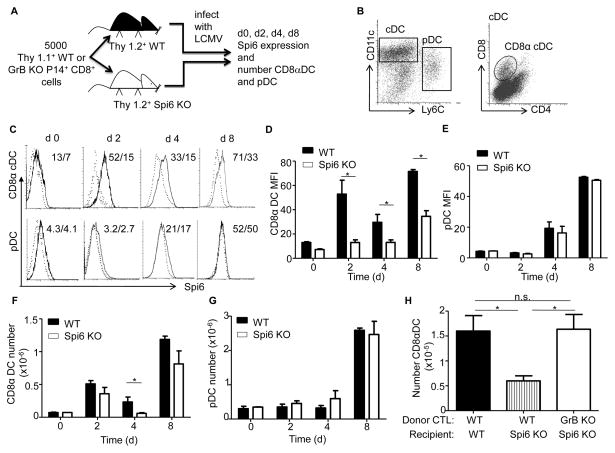
Expression correlation studies have given contradictory findings on the role of Spi6 in controlling the negative feedback loop of CTL expansion based on the killing of DC (17, 18). To address this issue we determined whether Spi6 was required for the protection of endogenous splenic DC from GrB delivered by CTL. Congenically marked (Thy1.1+) wild-type P14 CD8 T cells were adoptively transferred to either wild-type or Spi6 KO mice (both Thy1.2+), which were then infected with LCMV (Fig. 2A). After the infection of mice with LCMV, CD8α++ DC acquire LCMV proteins from infected cells and cross-presents them with class I MHC to cognate CD8 T cells (7). CD11c+ cells were purified with magnetic beads from the spleens of infected mice then CD8α+ DCs (CD11c++ CD8α+ CD4−) and plasmacytoid DCs (pDC) (CD11c+ Ly-6C+) populations were identified by antibody staining and flow-cytometry (Fig. 2B). We measured the expression of Spi6 in wild-type compared with Spi6 KO negative control DC by ICS with anti-Spi6 antibody (20). We observed the significant up-regulation of Spi6 in wild-type CD8α+ DC on d2 (MFI: wild-type 3.3-fold higher than Spi6 KO; P= 0.050), d4 (MFI: wild-type 2-fold higher than Spi6 KO; P= 0.047) and d8 (MFI: wild-type 2-fold higher than Spi6 KO; P= 0.017) after LCMV infection (Fig. 2 C&D). However, we did not observe any up-regulation of Spi6 in wild-type pDC over the level of Spi6 KO cells (Fig. 2E).
Figure 2. Spi6 up-regulation in CD8αDC is required for survival.
(A) Experimental plan. (B) Flow cytometry plots of staining for markers identifying DC subsets from CD11c+ magnetic bead sorted splenocytes. Left plot shows conventional DC (cDC) and plasmacytoid DC (pDC) and right plot the markers for CD8αDC of gated cDC cells. (C) Histograms for staining with anti-Spi6 antiserum on CD8αDC and pDC from the spleen after LCMV infection over time. Numbers: MFI WT/Spi6 KO. Mean MFI values for ICS anti-Spi6 serum in CD8α DC (C) and pDC (D). Mean absolute number of CD8α DCs (E) and pDC (F). (G) Mean absolute number of CD8α DC in WT mice or Spi6 KO after adoptive transfer of either WT or GrB KO P14 CD8 T cells. All means values are ± SEM (n=3–6) and are representative of 3 independent experiments, *p<0.05, not significant (n.s.) p>0.5.
We then measured the absolute number of CD8α+ DC and pDC in recipient mice after LCMV infection. On d4, we observed a 75% reduction (P=0.040) in the number of CD8α+ DC in Spi6 KO mice compared to wild-type recipients (Fig. 2E), but did not observe any difference in the number of pDC at any time point (Fig. 2F). The deficit in the level of CD8α+ DC in Spi6 KO mice is consistent with the expression of Spi6 from d2 onwards. Wild-type pDC do not express Spi6 and are present in wild-type levels in Spi6 KO mice, however the strong cross-reactivity with Spi6 antiserum (Fig. 2E) suggests that other serpins may play a role in pDC survival and function (1).
Staining for congenic markers revealed that >95% of the clonal burst of anti-gp33 CTL in Spi6 KO recipients was derived from adoptively transferred P14 CD8 T cells resulting in a 20-fold excess of the number of donor over endogenous primary CTL (Suppl. Fig. 2). Therefore the replacement of wild-type donor with GrB KO cells in Spi6 KO recipients was an effective means to reduce the potential for GrB-mediated killing of targets by CTL (Fig. 2A).
We next determined if the decrease in the level of viable CD8α+ DC in Spi6 KO mice was due to increased CTL killing by donor P14 CD8 T cells. The adoptive transfer of GrB KO P14 CD8 T cells resulted in the rescue of the number of Spi6 KO CD8α+ DC up to wild-type levels on d4 after LCMV infection (Fig. 2G). Therefore we conclude that Spi6 is required for the survival of CD8α+ DC through protection from GrB delivered by CTL. The specific up-regulation of Spi6 and corresponding requirement for survival in CD8α+ DC compared to pDC is consistent with the role of CD8 αDC in cross-presenting of LCMV to CD8 T cells (7).
Functionality of Spi6 KO CD8α DC
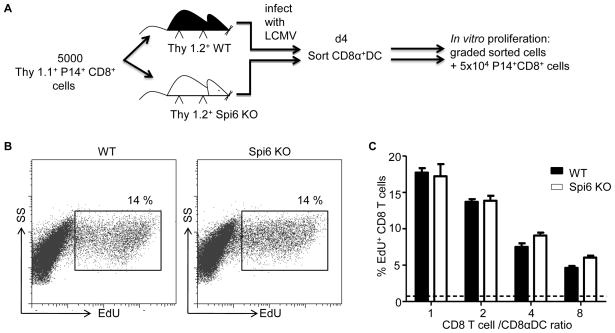
We next determined if Spi6 was required for CD8α DC function. Equal numbers of FACS-purified CD8α+ DC from recipient wild-type or Spi6 KO mice (Thy1.2+) on d4 after LCMV infection were co-cultured with naïve P14 CD8 responder T cells (Fig.3A). P14 CD8 T cell division was measured by EdU incorporation into genomic DNA (25)(Fig. 3B). We found that the number of CD8α+ DC from LCMV infected mice determined the extent of proliferation of wild-type P14 CD8 T cells, as evidenced by the titration of percentage EdU+ P14 CD8 T cell with the ratio of P14 CD8 T cell to CD8α+ DC (Fig. 3B). However, we observed no difference in the percentage of EdU+ P14 CD8 T cells after culture with Spi6 KO compared to wild-type CD8α+ DC (Fig. 3B & C). Therefore, ex vivo Spi6 KO DC are not qualitatively impaired in their ability to prime the proliferation of cognate CD8 T cells. We conclude that although Spi6 is required for the survival of CD8α DC it is not directly required for function.
Figure 3. Functionality of Spi6 KO CD8αDC.
A) Experimental plan. (B) Flow cytometry plots for ICS with anti-EdU mAb on P14 CTL after 60 h of culture with CD8α DC FACS-purified from LCMV infected mice. % EdU+ is indicated next to gates. (C) Mean % EdU+ P14 CD8 T cells. Broken line indicates the background level of proliferation. All means values are ± SEM (n=3–4) and are representative of 3 independent experiments, *p<0.05.
Spi6 is required for the priming of primary CTL responses in vivo
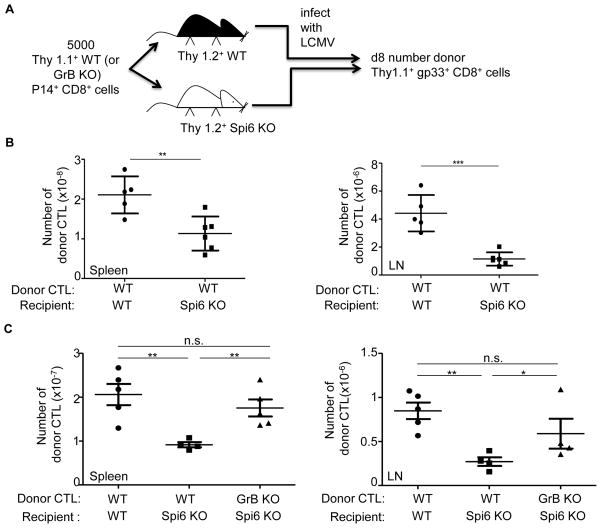
Given that the number of viable LCMV+ CD8α+ DC determines the proliferation of P14 CD8 T cells (Fig. 3B), we determined whether the impaired survival of Spi6 KO CD8α+ DC resulted in impaired expansion of primary CTL in vivo. Wild-type P14 CD8 T cells (Thy1.1+) were adoptively transferred to wild-type or Spi6 KO recipients (Thy1.2+) and the number of donor-derived gp33+ CD8+ cells determined in the clonal burst on d8 by flow-cytometry (Fig. 4A). We observed a 2-fold (P= 0.01) decrease in the level of donor-derived gp33+ CD8+ cells in the spleen and an 8-fold (P= 0.003) decrease in the lymph nodes of Spi6 KO compared to wild-type recipients (Fig. 4B). Therefore, the decrease in the number of Spi6 KO CD8α+ DC compared to wild-type resulted in impaired priming and expansion of anti-LCMV CTL. This defective expansion could be rescued when GrB KO P14 CD8 T cells were adoptively transferred demonstrating that the GrB-dependent deficit in Spi6 KO CD8α+ DC survival (Fig. 2G) also results in impaired CTL expansion (Fig. 4C). The defective expansion of wild-type anti-LCMV CTL in Spi6 KO compared to wild-type recipients lead to a corresponding 100-fold increase in the level of LCMV in the spleen on d6 after infection (Suppl Fig. 3). Therefore, Spi6 is required for the priming of functionally relevant CTL responses by DC in vivo.
Figure 4. Defective expansion of primary CTL in Spi6 KO mice.
(A) Experimental plan. (B) Mean absolute number of donor WT P14 CTL (gp33+Thy1.1+CD8+) at d8 after LCMV infection in the spleen and lymph-nodes (LN) of recipients. (C) Mean absolute number of donor P14 CTL (gp33+Thy1.1+CD8+) at d8 after LCMV infection. All means values (horizontal line) are ± SEM (vertical lines with bars) (n=3–6) and are representative of 3 independent experiments, *p<0.05, **=p<0.005, ***=p<0.0005, not significant (n.s.) p>0.5.
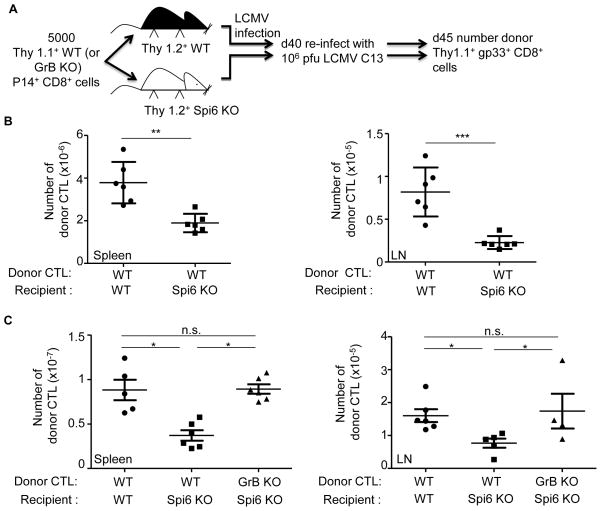
Spi6 is required for the priming of memory CTL responses in vivo
The elimination of DC by cytotoxic Tem cells also is through to provide a negative feedback mechanism for the control of secondary effectors (6, 14, 15, 26). Therefore, we determined whether Spi6 was required to protect CD8α+ DC from cytotoxic Tem cells. First, we established the level of memory CD8 T cells in wild-type and Spi6 KO mice. On d40 after primary LCMV infection, the level of donor memory gp33 CD8 T cells in the spleen of Spi6 KO mice was about 2-fold higher than wild-type, and in the lymph node the same as wild-type (Suppl. Fig. 4). This is consistent with pervious observations in the spleens of intact Spi6 KO mice that homeostatic mechanisms correct diminished clonal burst by increasing development in the memory phase (20).
We then examined the expansion of secondary CTL from anti-LCMV memory CD8 T cells Wild-type and Spi6 KO recipient mice were infected with LCMV then on d40 were reinfected with a high dose of the clone 13 variant of LCMV (106 PFU i.v.) and the re-expansion of gp33+ CD8+ cells measured after 5d (Fig. 5A) (4). We show in Fig. 5B that the number of donor secondary effectors was 2-fold lower in the spleen (P=0.0014) and 4-fold lower in the lymph-nodes (P=0.0006) of Spi6 KO compared to wild-type mice. This corresponds to defective expansion in number of anti-LCMV CTL in Spi6 KO mice upon reinfection over the level observed in d40 memory (Suppl. Fig 4). Such that the 4-fold expansion in the number of donor gp33+ CD8+ cells observed in the spleens of wild-type mice upon re-infection was completely abolished in Spi6 KO mice (Suppl. Fig. 4 Fig. 5B). The defect in memory CTL expansion in Spi6 KO recipients could be corrected by GrB deficiency of the donor P14 CD8 T cells (Fig. 5C). We conclude that Spi6 is required for the priming of secondary effectors from memory CD8 T cells and so dampens the negative feedback control of effector cell expansion exerted by DC elimination.
Figure 5. Defective expansion of secondary CTL in Spi6 KO mice.
(A) Experimental plan. (B) Mean absolute number of donor WT P14 CTL (gp33+Thy1.1+CD8+) at d5 after LCMV C13 re-infection in the spleen and lymph-nodes (LN) of recipients. (C) Mean absolute number of donor P14 CTL. All means values (horizontal line) are ± SEM (vertical lines with bars) (n=3–6) and are representative of 3 independent experiments, *p<0.05, **=p<0.005, ***=p<0.0005, not significant (n.s.) p>0.5.
Discussion
Understanding the mechanisms that initiate and terminate CTL responses are central to our understanding immunity to intracellular pathogens and tumor cells. A negative feedback mechanism in which priming DC expressing pMHC are killed by the CTL they prime, has been proposed for the control of the expansion of naïve and memory CD8 T cells levels (11, 15). This model predicts that factors that control the viability of DC in the face of CTL killing will exert control over the expansion of CTL. However, expression correlation (17, 18) give contradictory findings on the role of Spi6 in controlling this negative feedback loop at the level of DC viability. Collectively, our findings resolve the controversy on the role of Spi6 in protecting DC from CTL activity in vivo (17, 18). Using Spi6 KO mice, we demonstrate a non-redundant role for Spi6 in facilitating the priming and expansion of CTL through the protection of DC from the kiss of death.
Our in vitro finding that Spi6 KO BMDDC are susceptible to CTL killing is in agreement with previous reports of increased expression of Spi6 upon maturation by LPS activation correlating with resistance to CTL killing (17, 18). However, our in vivo findings differ from those of Andrew et al., 2008, who showed that LPS-treated BMDDC (Spi6hi) were no more resistant to killing by cognate CTL than non-LPS-treated BMDDC (Spi6 lo)(18). But an important point to consider is that the study of Andrew et al. correlated the expression of Spi6 mRNA to in vivo survival, whereas our study directly interrogated the role of Spi6 in DC by using Spi6-deficient cells. Furthermore, whether or not LPS-treated BMDDC continue to express Spi6 after adoptive transfer was not tested and it is well known that LPS induces PCD in DC (8, 21) and so this may have counteracted any cyto-protection afforded by initial Spi6 up-regulation.
A negative feedback loop in which priming DC expressing pMHC are killed by cognate CTL, has been proposed for the control of the expansion of primary CTL (11, 15). Our results indicate that Spi6 is a physiological factor that controls the viability of DC and so regulates the negative feedback of CTL expansion. Spi6 was up-regulated in CD8αDC but not pDC after LCMV infection and in Spi6 KO mice only the CD8αDC was diminished. CD8α+ DC are responsible for cross-presentation to anti-LCMV CD8 T cells, which them into direct contact with GrB+ cells, in contrast to pDC, which although they drive inflammation and support indirect CTL activation by secreting type I interferons (8) are as less-efficient than CD8α+ DC at cross-presentation to CTL (27). In addition our findings are consistent with reports that CD8αDC priming requires several days to generate the clonal burst of CTL in addition to the initial presentation for a few hours to non-cytolytic naïve CD8 T cells (12, 13).
The elimination of DC by cytotoxic Tem cells also is through to provide a negative feedback mechanism for the control of secondary effectors (6, 14, 15, 26). We observed that the expansion of wild-type donor derived secondary effectors was also impaired in Spi6 KO mice. Furthermore, the deficit could be corrected when GrB KO cells replaced wild-type donor CD8 T cells. We conclude that Spi6 is required for the priming of secondary effectors from memory CD8 T cells and so dampens the negative feedback control of effector cell expansion exerted by DC elimination by GrB.
The cyto-protective function we describe for Spi6 in DC can be placed in a wider context of protection from GrB by the intracellular serpin (1). Spi6 KO mice have revealed that Spi6 is also required to protect CTL (19) and invariant Natural Killer T cells (28) from their own GrB as well as mesencyhmal stem cells (MSC) from CTL-delivered GrB (29). Spi6 and the human homologue Proteinase Inhibitor 9 (PI9) (30, 31) also inhibit other proteases. For example Spi6 can inhibit neutrophil elastase (NE) (20) and PI9 inhibits caspase 1(32). However, the complete rescue of DC survival and expansion of anti-LCMV CTL by GrB deficiency argues that at least in this context GrB is the physiological target of Spi6.
GrB was first characterized as an effector molecule in the granule exocytosis pathway of killing (9). The discovery that CTL can be killed by their own GrB (19) or when this is delivered by a T regulatory cell (Treg) (33) has lead to the suggestion that perhaps GrB should also be viewed as a negative immuno-modulator (1). The survival of DC during the expansion phase is required for potent T cell responses and so our current findings would predict that preservation of DC by pharmacological inhibition of GrB would enhance not only priming of naïve CD8 T cells but also the boosting of vaccine responses by presentation to Tem cells.
Supplementary Material
Acknowledgments
The authors thank S. M. Byrne and S. Alyahya for help with mouse breeding and experiments, R. Sampson for help with FACS.
Abbreviations used in the paper
- DC
Dendritic cell
- CTL
cytotoxic T cell
- GrB
granzyme B
- Spi6
Serine Protease Inhibitor
- LCMV
Lymphocytic Choriomeningitis virus
- BMDDC
bone marrow derived dendritic cells
- LPS
Lipopolysaccharide
- CMA
concanamycin A
- Tem
cognate peptide-antigen/MHC pMHC, effector memory cells
- Tcm
central memory
- PCD
programmed cell death
- Pfn
perforin
- ICS
Intracellular staining
- pDC
plasmacytoid DC
- cDC
conventional DC
- FACS
Fluorescence activated cell sorting
Footnotes
This work was supported by the National Institutes of Health grant AI45108, The Wellcome Trust, and Cancer Research UK (to P.G.A-R.)
References
- 1.Ashton-Rickardt PG. Serine protease inhibitors and cytotoxic T lymphocytes. Immunol Rev. 2010;235:147–158. doi: 10.1111/j.0105-2896.2010.00892.x. [DOI] [PubMed] [Google Scholar]
- 2.Opferman JT, Ober BT, Ashton-Rickardt PG. Linear differentiation of cytotoxic effectors into memory T lymphocytes. Science. 1999;283:1745–1748. doi: 10.1126/science.283.5408.1745. [DOI] [PubMed] [Google Scholar]
- 3.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 4.Lau LL, Jamieson BD, Somasundaram T, Ahmed R. Cytotoxic T-cell memory without antigen. Nature. 1994;369:648–652. doi: 10.1038/369648a0. [DOI] [PubMed] [Google Scholar]
- 5.Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zammit DJ, Cauley LS, Pham QM, Lefrancois L. Dendritic cells maximize the memory CD8 T cell response to infection. Immunity. 2005;22:561–570. doi: 10.1016/j.immuni.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belz GT, Shortman K, Bevan MJ, Heath WR. CD8alpha+ dendritic cells selectively present MHC class I-restricted noncytolytic viral and intracellular bacterial antigens in vivo. J Immunol. 2005;175:196–200. doi: 10.4049/jimmunol.175.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heath WR, Belz GT, Behrens GM, Smith CM, Forehan SP, Parish IA, Davey GM, Wilson NS, Carbone FR, Villadangos JA. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev. 2004;199:9–26. doi: 10.1111/j.0105-2896.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 9.Heusel JW, Wesselschmidt RL, Shresta S, Russell JH, Ley TJ. Cytotoxic Lymphocytes Require Granzyme B for the Rapid Induction of DNA Fragmentation and Apoptosis in Allogeneic Target Cells. Cell. 1994;76:977–987. doi: 10.1016/0092-8674(94)90376-x. [DOI] [PubMed] [Google Scholar]
- 10.Hermans IF, Ritchie DS, Yang J, Roberts JM, Ronchese F. CD8+ T cell-dependent elimination of dendritic cells in vivo limits the induction of antitumor immunity. J Immunol. 2000;164:3095–3101. doi: 10.4049/jimmunol.164.6.3095. [DOI] [PubMed] [Google Scholar]
- 11.Yang J, Huck SP, McHugh RS, Hermans IF, Ronchese F. Perforin-dependent elimination of dendritic cells regulates the expansion of antigen-specific CD8+ T cells in vivo. Proc Natl Acad Sci U S A. 2006;103:147–152. doi: 10.1073/pnas.0509054103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curtsinger JM, Johnson CM, Mescher MF. CD8 T cell clonal expansion and development of effector function require prolonged exposure to antigen, costimulation, and signal 3 cytokine. J Immunol. 2003;171:5165–5171. doi: 10.4049/jimmunol.171.10.5165. [DOI] [PubMed] [Google Scholar]
- 13.Jusforgues-Saklani H, Uhl M, Blachere N, Lemaitre F, Lantz O, Bousso P, Braun D, Moon JJ, Albert ML. Antigen persistence is required for dendritic cell licensing and CD8+ T cell cross-priming. J Immunol. 2008;181:3067–3076. doi: 10.4049/jimmunol.181.5.3067. [DOI] [PubMed] [Google Scholar]
- 14.Wakim LM, Waithman J, van Rooijen N, Heath WR, Carbone FR. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science. 2008;319:198–202. doi: 10.1126/science.1151869. [DOI] [PubMed] [Google Scholar]
- 15.Wong P, Pamer EG. Feedback regulation of pathogen-specific T cell priming. Immunity. 2003;18:499–511. doi: 10.1016/s1074-7613(03)00081-5. [DOI] [PubMed] [Google Scholar]
- 16.Sun J, Ooms L, Bird CH, Sutton VR, Trapani JA, Bird PI. A New Family of 10 Murine Ovalbumin Serpins Includes Two Homologs of Proteinase Inhibitor 8 and Two Homologs of the Granzyme B Inhibitor (Proteinase Inhibitor 9) Journal of Biological Chemistry. 1997;272:15434–15441. doi: 10.1074/jbc.272.24.15434. [DOI] [PubMed] [Google Scholar]
- 17.Medema JP, Schuurhuis DH, Rea D, van Tongeren J, de Jong J, Bres SA, Laban S, Toes REM, Toebes M, Schumacher TNM, Bladergroen BA, Ossendorp F, Kummer JA, Melief CJM, Offringa R. Expression of the Serpin Serine Protease Inhibitor 6 Protects Dendritic Cells from Cytotoxic T Lymphocyte-induced Apoptosis: Differential Modulation by T Helper Type 1 and Type 2 Cells. Journal of Experimental Medicine. 2001;194:657–667. doi: 10.1084/jem.194.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrew KA, Simkins HM, Witzel S, Perret R, Hudson J, Hermans IF, Ritchie DS, Yang J, Ronchese F. Dendritic cells treated with lipopolysaccharide up-regulate serine protease inhibitor 6 and remain sensitive to killing by cytotoxic T lymphocytes in vivo. J Immunol. 2008;181:8356–8362. doi: 10.4049/jimmunol.181.12.8356. [DOI] [PubMed] [Google Scholar]
- 19.Zhang M, Park SM, Wang Y, Shah R, Liu N, Murmann AE, Wang CR, Peter ME, Ashton-Rickardt PG. Serine protease inhibitor 6 protects cytotoxic T cells from self-inflicted injury by ensuring the integrity of cytotoxic granules. Immunity. 2006;24:451–461. doi: 10.1016/j.immuni.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Zhang M, Byrne S, Liu N, Wang Y, Oxenius A, Ashton-Rickardt PG. Differential survival of cytotoxic T cells and memory cell precursors. J Immunol. 2007;178:3483–3491. doi: 10.4049/jimmunol.178.6.3483. [DOI] [PubMed] [Google Scholar]
- 21.Lutz MB, Kukutsch N, Ogilvie ALJ, Roβner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. Journal of Immunological Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 22.Matzinger P. The JAM test A simple assay for DNA fragmentation and cell death. Journal of Immunological Methods. 1991;145:185–192. doi: 10.1016/0022-1759(91)90325-a. [DOI] [PubMed] [Google Scholar]
- 23.McCausland MM, Crotty S. Quantitative PCR technique for detecting lymphocytic choriomeningitis virus in vivo. J Virol Methods. 2008;147:167–176. doi: 10.1016/j.jviromet.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kataoka T, Shinohara N, Takayama H, Takaku K, Kondo S, Yonehara S, Nagai K. Concanamycin A, a powerful tool for characterization and estimation of contribution of perforin- and Fas-based lytic pathways in cell-mediated cytotoxicity. J Immunol. 1996;156:3678–3686. [PubMed] [Google Scholar]
- 25.Yu Y, Arora A, Min W, Roifman CM, Grunebaum E. EdU incorporation is an alternative non-radioactive assay to [(3)H]thymidine uptake for in vitro measurement of mice T-cell proliferations. J Immunol Methods. 2009;350:29–35. doi: 10.1016/j.jim.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 26.Wolint P, Betts MR, Koup RA, Oxenius A. Immediate cytotoxicity but not degranulation distinguishes effector and memory subsets of CD8+ T cells. J Exp Med. 2004;199:925–936. doi: 10.1084/jem.20031799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Villadangos JA, Schnorrer P. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat Rev Immunol. 2007;7:543–555. doi: 10.1038/nri2103. [DOI] [PubMed] [Google Scholar]
- 28.Ansari AW, Temblay JN, Alyahya SH, Ashton-Rickardt PG. Serine protease inhibitor 6 protects iNKT cells from self-inflicted damage. J Immunol. 2010;185:877–883. doi: 10.4049/jimmunol.1000651. [DOI] [PubMed] [Google Scholar]
- 29.El Haddad N, Heathcote D, Moore R, Yang S, Azzi J, Mfarrej B, Atkinson M, Sayegh MH, Lee JS, Ashton-Rickardt PG, Abdi R. Mesenchymal stem cells express serine protease inhibitor to evade the host immune response. Blood. 2011;117:1176–1183. doi: 10.1182/blood-2010-06-287979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sprecher CA, Morgenstern KA, Mathewes S, Dahlen JR, Schrader SK, Foster DC, Kisiel W. Molecular Cloning, Expression, and Partial Characterization of Two Novel Members of the Ovalbumin Family of Serine Proteinase Inhibitors. Journal of Biological Chemistry. 1995;270:29854–29861. doi: 10.1074/jbc.270.50.29854. [DOI] [PubMed] [Google Scholar]
- 31.Sun J, Bird CH, Sutton V, McDonald L, Coughlin PB, De Jong TA, Trapani JA, Bird PI. A Cytosolic Granzyme B Inhibitor Related to the Viral Apoptotic Regulator Cytokine Response Modifier A Is Present in Cytotoxic Lymphocytes. Journal of Biological Chemistry. 1996;271:27802–27809. doi: 10.1074/jbc.271.44.27802. [DOI] [PubMed] [Google Scholar]
- 32.Annand RR, Dahlen JR, Sprecher CA, De Dreu P, Foster DC, Mankovich JA, Talanian RV, Kisiel W, Giegel DA. Caspase-1 (interleukin-1beta-converting enzyme) is inhibited by the human serpin analogue proteinase inhibitor 9. Biochem J. 1999;342(Pt 3):655–665. [PMC free article] [PubMed] [Google Scholar]
- 33.Cao X, Cai SF, Fehniger TA, Song J, Collins LI, Piwnica-Worms DR, Ley TJ. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27:635–646. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.