Abstract
Purpose
Two physical function questions on the widely used SF-12 Health Related Quality of Life questionnaire appear less than optimal for people with complete spinal cord injury (SCI). Physical function questions typically receive the lowest score unless the individual is ambulatory. Additionally, the influence of secondary complications of SCI on quality of life is largely unknown. The purposes of this report are: (1) to determine whether two SF-12 physical function follow-up questions provide increased information in subjects with complete SCI; and (2) to describe the secondary complications of SCI in this group.
Method
Ten subjects with complete SCI completed two survey instruments (the SF-12 and a Secondary Complications survey) every 3 months. The SF-12 included two physical function follow-up questions designed to improve the sensitivity and appropriateness of the SF-12 for a population with complete SCI.
Results
The SF-12 follow-up questions revealed differences in physical function that did not appear with the original SF-12 items. With the new questions, subject scores approximated population normative values. The most common secondary complications (back pain, shoulder pain, leg spasms, leg joint stiffness, and difficulty coughing) were the most likely to be rated as moderately or greatly bothersome.
Conclusion
We advocate the use of follow-up questions for the SF-12 in complete SCI populations that are not ambulating to better discriminate changes in physical function. Secondly, we advocate further investigations to better understand the incidence and the severity of secondary complications after SCI.
Keywords: Quality of life, paralysis, SF-12, health status, disability
Introduction
People with spinal cord injury (SCI) face significant emotional, social, and economic challenges in the years after injury. They have high rates of depression [1,2], and higher rates of suicide [3], divorce [4], and unemployment [5] than the general population. For these reasons, quality of life (QOL) after spinal cord injury has become a topic of great interest [6]. QOL can generally be defined as ‘the value assigned to duration of life as modified by the impairments, functional states, perceptions, and social opportunities that are influenced by disease, injury, treatment, and policy’ [7].
Certain demographic factors such as being employed [8,9], being married [8,10,11], being young at age of injury [8], and being young at age of test administration [9] tend to correlate with higher QOL scores. Time since injury exerts an uncertain effect on QOL, with some studies showing a positive correlation [8,12] and others finding no specific relationship [13]. Although one study showed that people with quadriplegia report lower QOL than people with paraplegia [14], unexpectedly, many studies have shown that level of lesion does not correlate with QOL [8,9,5]. Previous studies have found that certain secondary complications of SCI, such as neurogenic pain, spasticity, and neurogenic bladder and bowel problems exert a powerful negative influence on QOL [8]. It remains unknown, however, whether the total incidence of complications present in an individual can also negatively affect QOL. Likewise, few studies [16] have investigated the relative severity of complications, opting instead for a present/absent reporting scheme. No comprehensive method yet exists to quantify both the incidence and severity of secondary complications after SCI, and the relationship between such a ‘global’ measure and QOL is unknown.
An international panel of SCI experts recently recommended that the Medical Outcomes Study 36-Item Short Form (SF-36) [17] be used to track QOL in SCI patients after discharge from acute care [18]. The SF-36 assesses eight dimensions of health-related quality of life: bodily pain, physical function, physical and emotional role function, mental health, social function, vitality, and overall health. It has good internal consistency and discriminate and convergent validity for populations with chronic illness [19,20]. Evidence is beginning to accumulate to support the reliability and validity of the SF-36 for the SCI population [21,22].
A shorter version of the SF-36, the 12-item SF-12, is used to study populations where respondent burden is a concern [23]. For example, the SF-12 may be more acceptable than the SF-36 to people with high tetraplegia, who may have difficulty manipulating writing instruments. The SF-12 has repeatedly been shown to have a high correlation with the SF-36 [22,23]. A drawback of the SF-12 is that two questions regarding physical function appear less than optimal for respondents with complete SCI (Table I). These questions ask whether respondents can climb stairs or perform other activities that require ambulation, and have been rated to be inappropriate and offensive by individuals with SCI [22,24]. People with complete thoracic or cervical SCI generally are not ambulatory and may therefore earn the lowest possible score on the physical function subscale of the SF-12. However, they may have full functional mobility in the community, in their vocation, and even in vigorous wheelchair sports. From a rehabilitation specialist perspective, the original SF-12 physical function questions yield little important information about the functional mobility status of people with complete SCI [9]. Recently, investigators have proposed modifications to the SF-12, to enhance its usability in this specialized population [22,25].
Table I.
Complete SCI follow-up questions in the Physical Function domain of the SF-12.
| The following questions are about activities you might do during a typical day. Does your health now limit you in these activities? If so, how much? | |
| Original SF-12: |
Moderate activities, such as moving a table, pushing a vacuum cleaner, bowling, or playing golf. |
| Complete SCI follow-up: |
Moderate activities, such as using your wheelchair around your home. |
|
Original SF-12: Complete SCI-follow-up: |
Climbing several flights of stairs Going rapidly in your wheelchair for several blocks. |
We have adapted the standard SF-12 questionnaire to include two follow-up items that gauge the respondent’s ability to perform wheelchair-specific functional tasks (Table I). In addition, we have developed a questionnaire that investigates the incidence and severity of secondary complications of SCI. The purposes of this study were: (1) to determine whether the SF-12 physical function follow-up questions provide enhanced information about the physical function of people with complete SCI; and (2) to describe the secondary complications associated with SCI in this group.
Methods
Subjects
Subjects completed an SF-12 Health-Related Quality of Life Survey [26] (US version) every 3 months during a 2.5-year study period. Pilot data indicated that QOL scores could fluctuate considerably in response to life events in individuals with SCI. (Examples include attending rehabilitation, becoming ill, and weathering relationship difficulties). We used repeated measures to help account for these unavoidable events, in order to obtain a more stable, longitudinal view of the QOL status of these subjects. Participation in the study ranged from 1 to 10 survey periods (that is, the two subjects who participated longest completed 10 surveys; see Table II). A total of 10 subjects (one female) participated in the study.
Table II.
Subject demographics. Average time between repeated surveys was 3 months.
| No. | SCI Level |
Sex | Age at SCI |
Age at 1st survey |
Years post-SCI at 1st survey |
No. of surveys |
|---|---|---|---|---|---|---|
| 1 | T7 | M | 47 | 52.15 | 4.69 | 6 |
| 2 | T11 | M | 48 | 54.30 | 6.28 | 10 |
| 3 | T10 | M | 29 | 31.03 | 1.77 | 8 |
| 4 | T10 | M | 22 | 23.88 | 1.62 | 7 |
| 5 | C6 | F | 54 | 55.94 | 1.68 | 6 |
| 6 | T4 | M | 17 | 17.79 | 0.45 | 4 |
| 7 | T4 | M | 37 | 37.45 | 0.43 | 7 |
| 8 | T4 | M | 22 | 22.96 | 0.56 | 10 |
| 9 | T4 | M | 21 | 21.52 | 0.19 | 6 |
| 10 | C6 | M2 | 9 | 35.21 | 5.47 | 1 |
Subjects elected to participate in this study for varying lengths of time, up to 2.5 years.
Subject demographic data appear in Table II. All subjects had complete SCI (ASIA-A; no motor or sensory function below level of lesion [27]). Subjects were recruited from among people with SCI enrolled in a separate study within our laboratory. All subjects provided written informed consent, according to institutional guidelines. After receiving surveys during visits to the laboratory, subjects had the option to complete the survey on-site or to complete it at home. One subject with quadriplegia dictated item responses to her in-home nurses’ aide.
SF-12 follow-up questions
The QOL questionnaire included follow-up items for SF-12 questions #2 and #3 (Table I). Based on our clinical experience and observations, we devised survey items that would be functionally relevant to wheelchair users and that would approximate the ‘Moderate’ and ‘Vigorous’ activity categories delineated by the original survey wording. Several of the original SF-12 questions probe the effect of a subject’s ‘health’ on various dimensions of QOL. Subjects in this study frequently asked whether they should consider their SCI to be a component of their ‘health’, or whether that term should be interpreted as ‘presence or absence of illness’, omitting the influence of SCI. As suggested by Tate et al., we offered no clarification of this term, and encouraged subjects to decide for themselves how to interpret these questions [25]. This was done to preserve test validity and to facilitate the comparison of these results with previous SF-12 studies and with normative data. SF-12 scores are expressed as a percentile rank, with a higher score indicating a better perception of that dimension of quality of life.
Secondary complications questionnaire
On the same date as completing the SF-12, subjects also completed a Secondary Complications survey (Table III). The questionnaire included a section listing 17 common secondary complications of SCI, along with a scale to rate the incidence and severity of each complication. The 17 questionnaire items were compiled from two sources: previous reports describing secondary complications [16,28 – 30] and our own clinical observations of this subject population. The five severity ratings in the survey instrument were assigned numerical values from 1 to 5, with 1 indicating absence of that complication and 5 indicting a ‘greatly bothersome’ complication (Table III). Each subject’s mean severity of complications reported for each time point was computed in the following fashion: (Total severity score of all complications rated greater than or equal to 2)/(number of complications rated greater than or equal to 2). Because complications scored as 1 (‘I do not have this problem’) were omitted, mean severity only refers to the severity of complications that were reported to be present.
Table III.
Incidence and relative severity of secondary complications for 10 subjects.
| ‘I don’t have this problem’. |
‘Problem is not at all bothersome’. |
‘Problem is slightly bothersome’. |
‘Problem is moderately bothersome’. |
‘Problem is greatly bothersome’. |
|
|---|---|---|---|---|---|
| Leg swelling | 73.02 | 9.35 | 10.12 | 7.50 | 0.00 |
| Leg spasms | 1.25 | 21.56 | 55.05 | 16.79 | 5.36 |
| Leg joint stiffness | 34.86 | 14.77 | 33.82 | 13.87 | 2.68 |
| Shortness of breath | 70.88 | 19.26 | 11.11 | 0.00 | 0.00 |
| Difficulty coughing | 49.38 | 14.00 | 22.24 | 8.79 | 3.93 |
| Bowel constipation | 75.52 | 9.44 | 10.93 | 4.11 | 0.00 |
| Diarrhea | 92.64 | 2.00 | 2.86 | 0.00 | 0.00 |
| Indigestion | 77.19 | 2.00 | 17.38 | 3.43 | 0.00 |
| Urinary tract infection | 54.20 | 19.80 | 20.82 | 1.43 | 0.00 |
| Urinary incontinence | 50.46 | 4.69 | 39.86 | 1.25 | 0.00 |
| Problem w/ bladder program | 68.94 | 10.93 | 16.38 | 2.50 | 0.00 |
| Headaches | 74.30 | 4.00 | 21.70 | 0.00 | 0.00 |
| Back pain | 20.04 | 11.29 | 40.64 | 18.33 | 9.70 |
| Shoulder pain | 40.25 | 5.36 | 34.15 | 16.31 | 2.68 |
| Pain below level of injury | 69.67 | 5.00 | 21.23 | 2.86 | 1.25 |
| Feeling blue or sad | 58.74 | 8.61 | 25.81 | 5.60 | 0.00 |
| Feeling isolated/lonely | 67.90 | 8.43 | 22.42 | 1.25 | 0.00 |
Values are mean percent of total subjects (subjects were surveyed on up to 10 occasions, spaced 3 months apart). Categories that do not sum to 100% reflect rounding error. Grey shading denotes complications which were present greater than 50% of the time. Note that these complications were the most frequent ones to be rated ‘moderately’ or ‘greatly’ bothersome.
To explore potential associations between QOL scores and level of SCI, we assigned numerical values to the SCI levels of the subjects. Two strategies appeared possible: assigning a numerical value to each individual level, or grouping SCI levels into large coded ‘bins’. (For example, ‘Cervical’ = 1, ‘Thoracic’ = 2, and ‘Lumbar’ = 3). We chose to assign each level its own value because particularly for cervical injuries, one functioning segment distal or proximal can have important functional consequences.
Data and statistical analysis
The mean, standard error, and range of the physical function scores for the revised questions were contrasted with the zero score of the standard physical function questions. A one-way repeated measures ANOVA was used to compare the incidence and severity of complications. Univariate analysis and an associated R-square value were calculated to determine the strength of the relationships among the dependent variables.
Results
Quality of life
Mean quality of life scores for these subjects exceeded population norms in all SF-12 subscales except Physical Function (Figure 1). With the original SF-12 questions, all subjects scored 0% on the physical function subscale (that is, both tasks were reported to be ‘limited a lot’). If the specialized SCI follow-up questions are used, physical function percentile scores ranged between 50 and 100%. The mean score for the group exceeded the US SF-36 population norm (84.15%; SF-12 population norms are unavailable) (Figure 1). These amended physical function scores did not correlate with either subject age at test administration (r 2 = 0.00560) or with time post-SCI (r 2 = 0.0004528). Amended physical function scores did not correlate with numerically coded SCI lesion level (r 2 = 0.02813).
Figure 1.
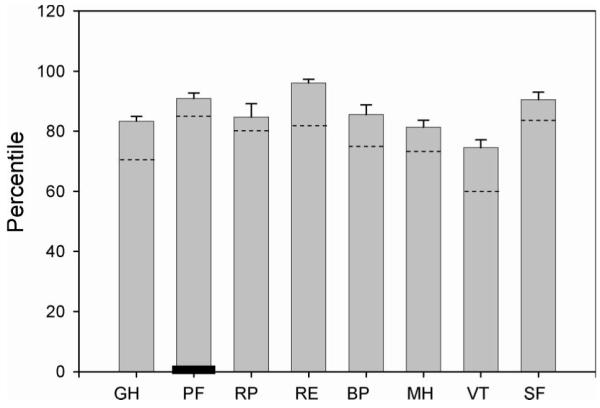
Mean SF-12 subscale scores for 10 subjects. Each subject contributed between 1 and 10 survey samples to the population mean values (see Table II). Dotted lines indicate US population norms. The black bar in the second column indicates the Physical Function score when computed with the standard SF-12 questions, rather than the SCI-specific version. GH, general health; PF, physical function; RP, role physical; RE, role emotional; BP, bodily pain; MH, mental health; VT, vitality; ?SF, social function.
Four male subjects began the study during the acute phase of SCI (before 6 months post-SCI). Further inspection revealed that at the first survey period (approximately 0.41 years post-SCI), this cohort’s scores were lower than male population normative values for six SF-12 subscales (Figure 2). By the third survey period (approximately 0.91 years post-SCI), this cohort exceeded male normative values in seven of eight subscales. The bodily pain subscale demonstrated the largest relative improvement during the first year after SCI.
Figure 2.
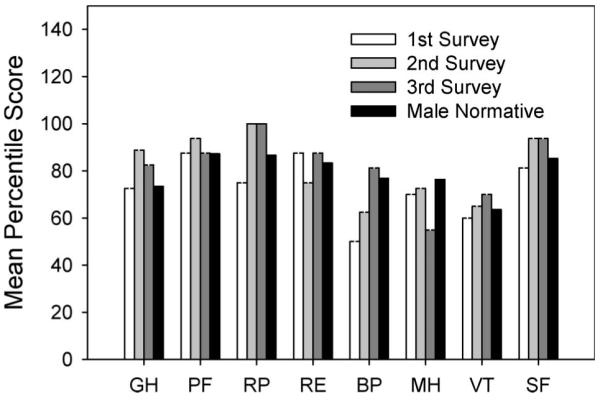
Mean SF-12 subscale scores for four subjects who entered the study during the acute phase of SCI. Results are presented for the first year after SCI. Surveys 1, 2, and 3 occurred at the following mean times post-SCI: 0.4123, 0.6582, and 0.9103 years following injury. Population normative data for males appear as the black bar. PF scores were obtained with the SCI-specific follow-up questions. GH, general health; PF, physical function; RP, role physical; RE, role emotional; BP, bodily pain; MH, mental health; VT, vitality; SF, social function.
Secondary complications
All 17 secondary complications in the survey instrument were reported to be present during the study period. The most common complication, leg spasms, was reported to be present 98.75% of the time (Table III). The least common complication, diarrhea, was absent 92.64% of the time. Five complications were reported to be present greater than 50% of the time: leg spasms, leg joint stiffness, difficulty coughing, back pain, and shoulder pain. These five complications were also the ones most commonly rated as ‘moderately’ or ‘greatly’ bothersome. Of these, back pain was most often reported to be ‘greatly bothersome’. Further inspection of individual results revealed that all 10 subjects reported back pain at some time, and four subjects had experienced ‘greatly bothersome’ back pain.
Overall mean severity of secondary complications for all subjects at all times was 2.9825 (on the 1 – 5 severity scale). Overall mean number of complications reported to be present by all subjects at all times was 6.8594. Mean severity of complications did not correlate with age (r 2 = 0.0017) or time since SCI (r 2 = 0.0970). A weak positive correlation existed between the number of reported complications and age (r 2 = 0.2745). Similarly, a weak positive correlation existed between the number of reported complications and time since SCI (r 2 = 0.2073). No correlations existed between numerically coded SCI level and mean severity of complications (r 2 = 0.0232). Similarly, no correlations existed between SCI level and number of reported complications (r 2 = 0.0161).
Discussion
Quality of life
The first purpose of this study was to determine whether a set of follow-up questions to the Physical Function domain of the SF-12 survey yielded enhanced information regarding the functional status of people with complete SCI. With the exception of the physical function subscale, SF-12 quality of life scores for people with complete SCI exceeded population norms. This is a novel finding that has not been reported in previous SF-12 or SF-36 studies. Three previous studies (in Sweden, Canada, and the US) have found that people with SCI score lower on all eight SF-36 subscales than population normative values [8,9,21]. In another study, people with SCI scored lower than population norms for all subscales except Role Emotional and Social Function [31]. One possible reason that our subjects scored higher than subjects in previous studies is that our proportion of people with quadriplegia was low (20%). In the four studies mentioned above, 33 – 47% of the subjects had quadriplegia. Although the effect of lesion level on QOL remains uncertain, some previous investigations have reported lower quality of life scores in people with quadriplegia than in people with paraplegia [14]. Although level of lesion in the present study did not correlate with physical function scores (r 2 = 0.0281), our small sample size likely did not have an adequate range of lesion levels to show such a correlation.
In the previously mentioned SF-12 and SF-36 studies of people with SCI [8,9,22], physical function scores of people with SCI deviated more from population norms than any other domain. However, physical function scores in those studies did not demonstrate the pronounced floor effect apparent in the present study. A possible reason for this difference is that at least two of the previous studies included people with incomplete injuries [8,21]. The remaining two studies do not specifically exclude people with incomplete injuries, so it is probable that such individuals were included [9,22]. In groups of subjects with similar levels of injury, people with incomplete injuries have been shown to report higher physical function scores than people with complete injuries [8]. A sample population that includes people with incomplete SCI may be more likely to have a mid-range mean physical function value that avoids a floor effect.
Although the demographic makeup of our group may explain why they reported different QOL scores than previous SCI groups, it does not explain why our subjects’ scores exceeded population normative values. These 10 subjects were concurrently enrolled in a separate study that required frequent laboratory visits, which in turn required good general health, a desire to be involved in activities outside of the home, and a method of transportation. Seven of the 10 subjects held jobs outside the home. From a social and vocational standpoint, these subjects differed little from the able-bodied population. Vocational status has previously been shown to influence QOL [5], and may help explain why in general, these subjects reported high QOL scores. Another possible explanation for the high QOL values reported by our SCI subjects may be scaling differences between the SF-12 and the SF-36. Because SF-12 population normative values are unavailable, we compared the SF-12 scores in this study to SF-36 normative data. Although the SF-12 demonstrates excellent correlation with the SF-36, an offset in the absolute value of percentile scores may exist between the two surveys.
In the present study, the original SF-12 physical function scores did not offer detailed information about the functional status of subjects with complete SCI. All subjects reported that the physical function items were ‘limited a lot’. However, clinical observation of our subjects revealed that levels of function ranged from substantial dependence on aides for all activities of daily living (subject 5) to participation in collegiate wheelchair basketball (subject 4). The SF-12 physical function questions lacked sufficient sensitivity to delineate levels of function in this group of subjects with complete SCI. The two follow-up questions, however, revealed differences in perceived physical function among these subjects (scores ranged from 50 to 100%). A more heterogeneous group, including more individuals with low functional independence, may display an even wider range of scores.
We believe that a great need exists for a sensitive yet succinct measure of subjective physical function in subjects with complete SCI, because complete SCI is a useful research model. For example, studies that wish to avoid the confounding effects of descending spinal input in incomplete injury often focus exclusively on people with complete injuries. In such studies, the ability to detect a change in physical function due to a therapeutic intervention is critical. While a given intervention may not enable a person with complete SCI to go bowling or play golf, it may still yield a notable functional change. For example, a therapeutic intervention might enable a deconditioned person with complete SCI to wheel community distances. A functional change of this magnitude would be undetected by the original SF-12 physical function questions.
The follow-up questions used in this investigation yielded a range of responses that provided enhanced information about the physical function of these subjects. One previous study used alternative wording for physical function items [25], and found that physical function scores obtained with the altered questions correlated strongly with Functional Independence Measure scores. These authors attempted to preserve the objective of the original SF-36 questions (locomotion) without using language that necessitated ambulation. However, they retained the SF-36 question regarding stair-climbing, and used the caveat that assistive devices were permissible. We argue that because people with complete SCI rarely, if ever, use stairs, our question regarding rapid wheeling for several blocks may be more applicable to the routine functional demands encountered by this population.
Using physical function follow-up questions for subjects with complete SCI can improve the quality and heuristic value of the information obtained. Although it is important to retain the original SF-12 questions, for reasons of generalizability and comparison, we advocate using follow-up questions in order to obtain information that more accurately reflects functional status in this group.
Four subjects in this study enrolled during the acute phase of SCI, and demonstrated notable improvements during the first year post-injury. Only the Mental Health subscale never exceeded male population norms. This is in accord with other studies that highlight the particular mental health challenges experienced by people with SCI [1,2]. Longitudinal QOL data for acute SCI are scarce. One previous study reported that SF-36 scores remained stable between 1 and 6 months post-SCI, with the exception of Role Emotional scores, which declined [32]. Similar results were reported in a study that used a Quality of Life and Needs Assessment Questionnaire [33]. Because dramatic functional gains routinely occur during rehabilitation in the first year post-SCI, QOL scores may change more rapidly at this time than at other times post-SCI. Future studies should track QOL during this critical time period.
Secondary complications
The second purpose of the study was to describe the incidence and severity of secondary complications in this population of people with complete SCI. Five complications were reported to be present greater than 50% of the time (leg spasms, leg joint stiffness, difficulty coughing, back pain, and shoulder pain). These were also the complications most frequently reported to be moderately or greatly bothersome. Back pain was reported to be at least moderately bothersome 22% of the time (Table III), and 40% of our subjects reported experiencing severe back pain at some time. During the tenure of this study, two subjects (numbers 3 and 4) sought surgical intervention for back pain. Subjects in another study reported that back pain was present 10% of the time, and that back pain was more bothersome than pain in other regions (neck, arm/shoulder) [16]. Numerous studies have shown that pain in general is a very frequent (and often very severe) complication of SCI [34 – 36].
Weak positive correlations existed between subject age and number of complications. Similarly, weak positive correlations existed between time since SCI and number of complications. Klotz et al. [28] reported significant positive correlations between age and incidence of pain and between time since SCI and pain. (These authors did not ask their subjects to specify the location of their pain). Their findings are in accordance with numerous other studies [34 – 37]. One study concluded that the best predictor of presence or absence of pain after SCI was subject age [37].
Other secondary complications have a less certain relationship with age and time post injury. Noreau et al. [30] found that the incidence of some complications increased with subject age (urinary tract infection, hypotension, and shoulder overuse), while the incidence of others decreased with age (spasticity, autonomic dysreflexia, pressure sores). Only shoulder overuse symptoms were reported significantly more frequently in older than in younger subjects. Other studies have noted correlations between secondary complications and time since SCI. Johnson et al. [16] reported that back pain complaints were more prevalent in subjects with longer history of SCI. In contrast, Noureau found that several complications (including urinary tract infection, spasticity, and shoulder pain) were reported most frequently in people with a short duration of injury (2 – 7 years), and less frequently in people with longer time post-SCI [30]. Since no subjects in our study had an SCI longer than 7 years, we are unable to compare our results to this finding.
No previous studies have employed the approach used in the present study to conceptualize prevalence of secondary complications (mean incidence and mean severity across a number of possible complications). While many studies have investigated the relationship between the incidence (number) of complications and time (age or time post-injury), we are aware of only one previous study that discusses the relationship between severity of complications and time [16]. While it was tempting to explore correlations between quality of life scores and secondary complications, our small sample size prevented us from doing so. This limitation of the study clearly bears on certain findings, such as the group mean QOL scores and the correlations between incidence/severity of complications and time or lesion level. It does not, however undermine the utility of the SF-12 follow-up questions, nor does it contravene the potential usefulness of this method to globally describe both the incidence and severity of secondary complications.
Conclusion
Two physical function follow-up questions to the SF-12 yielded enhanced information about the functional status of people with complete SCI. Although the original questions appear adequately suited for mixed subject populations (incomplete and complete injuries), we suggest that researchers consider adding follow-up questions to increase the sensitivity with which functional status can be assessed in people with complete SCI. Secondly, we advocate further investigation of global methods to assess secondary complications after SCI. Some information is known about how specific complications affect quality of life. However, it is unknown how the overall incidence and severity of complications, when taken as a whole, affect the quality of life of people with complete SCI.
Acknowledgements
This work was supported by an award to Richard K. Shields (R01-HD 39445) from the National Center for Medical Rehabilitation Research (NIH) and by an award from the Christopher Reeve Paralysis Foundation. Shauna Dudley-Javoroski received a Doctoral Scholarship from the Foundation for Physical Therapy.
References
- 1.Fuhrer MJ, Rintala DH, Hart KA, Clearman R, Young ME. Depressive symptomatology in persons with spinal cord injury who reside in the community. Archives of Physical Medicine & Rehabilitation. 1993;74:255–260. [PubMed] [Google Scholar]
- 2.Hancock KM, Craig AR, Dickson HG, Chang E, Martin J. Anxiety and depression over the first year of spinal cord injury: a longitudinal study. Paraplegia. 1993;31:349–357. doi: 10.1038/sc.1993.59. [DOI] [PubMed] [Google Scholar]
- 3.Charlifue SW, Gerhart KA. Behavioral and demographic predictors of suicide after traumatic spinal cord injury. Archives of Physical Medicine & Rehabilitation. 1991;72:488–492. [PubMed] [Google Scholar]
- 4.DeVivo MJ, Hawkins LN, Richards JS, Go BK. Outcomes of post-spinal cord injury marriages [erratum appears in Arch Phys Med Rehabil 1995 Apr;76(4):397] Archives of Physical Medicine & Rehabilitation. 1995;76:130–138. doi: 10.1016/s0003-9993(95)80022-0. [DOI] [PubMed] [Google Scholar]
- 5.Conroy L, McKenna K. Vocational outcome following spinal cord injury. Spinal Cord. 1999;37:624–633. doi: 10.1038/sj.sc.3100904. [DOI] [PubMed] [Google Scholar]
- 6.Whiteneck GG. Evaluating outcome after spinal cord injury: what determines success? 1996 Donald Munro Lecture. Journal of Spinal Cord Medicine. 1997;20:179–185. [PubMed] [Google Scholar]
- 7.Patrick DL, Erickson P. Health status and health policy. Oxford University Press; New York: 1993. [Google Scholar]
- 8.Westgren N, Levi R. Quality of life and traumatic spinal cord injury. Archives of Physical Medicine & Rehabilitation. 1998;79:1433–1439. doi: 10.1016/s0003-9993(98)90240-4. [DOI] [PubMed] [Google Scholar]
- 9.Leduc BE, Lepage Y. Health-related quality of life after spinal cord injury. Disability & Rehabilitation. 2002;24:196–202. doi: 10.1080/09638280110067603. [DOI] [PubMed] [Google Scholar]
- 10.Post MW, de Witte LP, van Asbeck FW, van Dijk AJ, Schrijvers AJ. Predictors of health status and life satisfaction in spinal cord injury. Archives of Physical Medicine & Rehabilitation. 1998;79:395–401. doi: 10.1016/s0003-9993(98)90139-3. [DOI] [PubMed] [Google Scholar]
- 11.Holicky R, Charlifue S. Ageing with spinal cord injury: the impact of spousal support. Disability & Rehabilitation. 1999;21:250–257. doi: 10.1080/096382899297675. [DOI] [PubMed] [Google Scholar]
- 12.Richards JS, Bombardier CH, Tate D, et al. Access to the environment and life satisfaction after spinal cord injury. Archives of Physical Medicine & Rehabilitation. 1999;80:1501–1506. doi: 10.1016/s0003-9993(99)90264-2. [DOI] [PubMed] [Google Scholar]
- 13.Post MW, Van Dijk AJ, Van Asbeck FW, Schrijvers AJ. Life satisfaction of persons with spinal cord injury compared to a population group. Scandinavian Journal of Rehabilitation Medicine. 1998;30:23–30. doi: 10.1080/003655098444282. [DOI] [PubMed] [Google Scholar]
- 14.Kannisto M, Merikanto J, Alaranta H, Hokkanen H, Sintonen H. Comparison of health-related quality of life in three subgroups of spinal cord injury patients. Spinal Cord. 1998;36:193–199. doi: 10.1038/sj.sc.3100543. [DOI] [PubMed] [Google Scholar]
- 15.Putzke JD, Richards JS, Hicken BL, DeVivo MJ. Predictors of life satisfaction: a spinal cord injury cohort study. Archives of Physical Medicine & Rehabilitation. 2002;83:555–561. doi: 10.1053/apmr.2002.31173. [DOI] [PubMed] [Google Scholar]
- 16.Johnson RL, Gerhart KA, McCray J, Menconi JC, Whiteneck GG. Secondary conditions following spinal cord injury in a population-based sample. Spinal Cord. 1998;36:45–50. doi: 10.1038/sj.sc.3100494. [DOI] [PubMed] [Google Scholar]
- 17.Ware JE, Snow KK, Kosinski M. SF-36 health survey: Manual and interpretation guide. New England Medical Center, the Health Institute; Boston: 1993. [Google Scholar]
- 18.Wood-Dauphinee S, Exner G, Bostanci B, et al. Quality of life in patients with spinal cord injury – basic issues, assessment, and recommendations. Restorative Neurology & Neuroscience. 2002;20:135–149. [PubMed] [Google Scholar]
- 19.VanderZee KI, Sanderman R, Heyink J. A comparison of two multidimensional measures of health status: the Nottingham Health Profile and the RAND 36-Item Health Survey 1.0. Quality of Life Research. 1996;5:165–174. doi: 10.1007/BF00435982. [DOI] [PubMed] [Google Scholar]
- 20.McHorney CA, Ware JE, Jr, Rogers W, Raczek AE, Lu JF. The validity and relative precision of MOS short- and long-form health status scales and Dartmouth COOP charts. Results from the Medical Outcomes Study. Medical Care. 1992;30:MS253–265. doi: 10.1097/00005650-199205001-00025. [DOI] [PubMed] [Google Scholar]
- 21.Forchheimer M, McAweeney M, Tate DG. Use of the SF-36 among persons with spinal cord injury. American Journal of Physical Medicine & Rehabilitation. 2004;83:390–395. doi: 10.1097/01.phm.0000124441.78275.c9. [DOI] [PubMed] [Google Scholar]
- 22.Andresen EM, Fouts BS, Romeis JC, Brownson CA. Performance of health-related quality-of-life instruments in a spinal cord injured population. Archives of Physical Medicine & Rehabilitation. 1999;80:877–884. doi: 10.1016/s0003-9993(99)90077-1. [DOI] [PubMed] [Google Scholar]
- 23.Jenkinson C, Layte R, Jenkinson D, et al. A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies? Journal of Public Health Medicine. 1997;19:179–186. doi: 10.1093/oxfordjournals.pubmed.a024606. [DOI] [PubMed] [Google Scholar]
- 24.Mattson-Prince J. A rational approach to long-term care: comparing the independent living model with agency-based care for persons with high spinal cord injuries. Spinal Cord. 1997;35:326–331. doi: 10.1038/sj.sc.3100453. [DOI] [PubMed] [Google Scholar]
- 25.Tate DG, Kalpakjian CZ, Forchheimer MB. Quality of life issues in individuals with spinal cord injury. Archives of Physical Medicine & Rehabilitation. 2002;83:S18–25. doi: 10.1053/apmr.2002.36835. [DOI] [PubMed] [Google Scholar]
- 26.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 27.International Standards for Neurological Classification of SCI . American Spinal Injury Association; Atlanta, GA: 2002. Revised 2002. [Google Scholar]
- 28.Klotz R, Joseph PA, Ravaud JF, Wiart L, Barat M, Tetrafigap G. The Tetrafigap Survey on the long-term outcome of tetraplegic spinal cord injured persons: Part III. Medical complications and associated factors. Spinal Cord. 2002;40:457–467. doi: 10.1038/sj.sc.3101317. [DOI] [PubMed] [Google Scholar]
- 29.McKinley WO, Jackson AB, Cardenas DD, DeVivo MJ. Long-term medical complications after traumatic spinal cord injury: a regional model systems analysis. Archives of Physical Medicine & Rehabilitation. 1999;80:1402–1410. doi: 10.1016/s0003-9993(99)90251-4. [DOI] [PubMed] [Google Scholar]
- 30.Noreau L, Proulx P, Gagnon L, Drolet M, Laramee MT. Secondary impairments after spinal cord injury: a population-based study. American Journal of Physical Medicine & Rehabilitation. 2000;79:526–535. doi: 10.1097/00002060-200011000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Andresen EM, Vahle VJ, Lollar D. Proxy reliability: health-related quality of life (HRQoL) measures for people with disability. Quality of Life Research. 2001;10:609–619. doi: 10.1023/a:1013187903591. [DOI] [PubMed] [Google Scholar]
- 32.Lucke KT, Coccia H, Goode JS, Lucke JF. Quality of life in spinal cord injured individuals and their caregivers during the initial 6 months following rehabilitation. Quality of Life Research. 2004;13:97–110. doi: 10.1023/B:QURE.0000015284.95515.17. [DOI] [PubMed] [Google Scholar]
- 33.Kennedy P, Rogers B. Reported quality of life of people with spinal cord injuries: a longitudinal analysis of the first 6 months post-discharge. Spinal Cord. 2000;38:498–503. doi: 10.1038/sj.sc.3101021. [DOI] [PubMed] [Google Scholar]
- 34.Stormer S, Gerner HJ, Gruninger W, et al. Chronic pain/ dysaesthesiae in spinal cord injury patients: results of a multicentre study. Spinal Cord. 1997;35:446–455. doi: 10.1038/sj.sc.3100411. [DOI] [PubMed] [Google Scholar]
- 35.Demirel T, Braun W, Reimers CD. Results of spinal cord stimulation in patients suffering from chronic pain after a two year observation period. Neurochirurgia. 1984;27:47–50. doi: 10.1055/s-2008-1053725. [DOI] [PubMed] [Google Scholar]
- 36.Anke AG, Stenehjem AE, Stanghelle JK. Pain and life quality within 2 years of spinal cord injury. Paraplegia. 1995;33:555–559. doi: 10.1038/sc.1995.120. [DOI] [PubMed] [Google Scholar]
- 37.Richards JS. Chronic pain and spinal cord injury: review and comment. Clinical Journal of Pain. 1992;8:119–122. doi: 10.1097/00002508-199206000-00009. [DOI] [PubMed] [Google Scholar]


