Abstract
Background and Purpose
The interpretation of the results of previous anti-osteoporosis interventions after spinal cord injury (SCI) is undermined by incomplete information about the intervention dose or patient adherence to dose requirements. Rehabilitation research as a whole traditionally has struggled with these same issues. The purpose of this case report is to offer proof of the concepts that careful dose selection and surveillance of patient adherence should be integral components in rehabilitation interventions.
Case Description
A 21-year-old man with T4 complete paraplegia (7 weeks) enrolled in a unilateral soleus muscle electrical stimulation protocol. Compressive loads applied to the tibia approximated 1.4 times body weight. Over 4.8 years of home-based training, data logging software provided surveillance of adherence. Soleus muscle torque and fatigue index adaptations to training as well as bone mineral density (BMD) adaptations in the distal tibia were measured.
Outcomes
The patient performed nearly 8,000 soleus muscle contractions per month, with occasional fluctuations. Adherence tracking permitted intervention when adherence fell below acceptable values. The soleus muscle torque and fatigue index increased rapidly in response to training. The BMD of the untrained tibia declined approximately 14% per year. The BMD of the trained tibia declined only approximately 7% per year. The BMD was preferentially preserved in the posterior half of the tibia; this region experienced only a 2.6% annual decline.
Discussion
Early administration of a load intervention, careful estimation of the loading dose, and detailed surveillance of patient adherence aided in the interpretation of a patient’s adaptations to a mechanical load protocol. These concepts possess wider applicability to rehabilitation research and should be emphasized in future physical therapy investigations.
Bone demineralization after spinal cord injury (SCI) results from an increase in bone re-sorption without a compensatory increase in bone formation.1 This imbalance of bone anabolic and catabolic processes results from the disruption of several different homeostatic control mechanisms. Altered levels of hormones may be a causative or aggravating factor in bone loss after SCI.2 Bone has extensive connections with the sympathetic nervous system that, after SCI, lead to impaired blood flow and may contribute to demineralization.3 Additionally, several types of nerve fibers present in bone secrete neuropeptides that are known to affect bone homeostasis.4–6 Most intriguingly, new evidence suggests that the hypothalamus may directly regulate bone metabolism via descending spinal neural pathways.7–9 New awareness of these diverse bone homeostatic systems has raised questions about their relationship to mechanical load, long believed to be a fundamental determinant of bone health.
The importance of the loss of mechanical load after SCI has been emphasized10,11 or deemphasized1,12 by various theorists at various times. A wide range of studies has failed to demonstrate measurable efficacy of load interventions on bone mineral density (BMD) after SCI. Studies of passive standing,13,14 standing with low-level electrical stimulation,15,16 body weight-supported treadmill training,17 and electrically stimulated cycling18–20 have revealed no BMD benefit from these forms of mechanical loading. Three important considerations with these studies were that the magnitude of the compressive load was not estimated, long-term adherence to the intervention was not quantified, and/or the intervention was not implemented in a timely fashion after SCI. Because of these omissions, insight into whether mechanical loading was effective is lacking.
The apparent ineffectiveness of load interventions prompted one group to conclude, “Since treatment interventions based on physical activity … have no sufficient positive effects, pharmacologic therapy seems to be necessary.”1(p776) This conclusion is incongruent with the important therapeutic principle of stress adaptation,21 which forms the basis for many physical therapy treatments. Our experience with mechanical loading interventions after SCI has supported the notion that a mechanical load of a sufficient dose applied soon after SCI can lead to a significant preservation of BMD (31% higher than that in untrained limbs) in targeted limb segments for up to 2.5 years.22 We believe that the key to the success of this training approach was that the stress was timely, appropriately dosed, and closely monitored for adherence.
The purpose of this case report is to describe a single patient who continued with a prescribed protocol for more than 4.6 years. We describe how the dose of the mechanical load was estimated, how surveillance of the dose was achieved over a longitudinal training protocol, and how BMD outcomes supported the unique contribution of the mechanical load to BMD preservation, even in the context of the aforementioned hormonal, vascular, and neural disruptions. We offer this unique case as proof of the concepts that careful dose selection and surveillance of adherence should be integral components in longitudinal studies of antiosteoporosis interventions after SCI.
Case Description
Patient History
A 21-year-old man sustained sensory/ motor complete (American Spinal Injury Association [ASIA] classification A)23 T4 paraplegia consequent to a gunshot wound (hunting accident). He underwent standard emergency care at a tertiary medical facility, completed outpatient rehabilitation, and presented to our facility at 7 weeks after SCI to be screened for participation in a unilateral soleus muscle electrical stimulation protocol. The patient participated in no standing or walking protocols before enrollment and used a wheelchair full time. Although he occasionally experienced lower-extremity spasms, at no time during his enrollment did he take antispasmodic medication. The patient provided informed consent for the protocol, as approved by the University of Iowa Human Patients Institutional Review Board. Some data for this patient appeared in aggregate form in previous reports.22,24,25
Examination
During a screening session, we ruled out lower motor neuron involvement by eliciting strong soleus muscle contractile responses to transcutaneous electrical pulses. The patient denied any previous fracture to the lower extremities, medical conditions known to affect bone metabolism, deep-vein thrombosis, current systemic illness, or any pressure ulcers. The patient denied participation in any previous electrical muscle stimulation protocol since sustaining the SCI.
Upon being cleared of the above-described exclusion criteria, the patient elected to enroll in a 3-year treatment program of daily soleus muscle electrical stimulation training. Several characteristics identified him as a suitable candidate for participation in a longitudinal treatment program with a considerable degree of patient burden. First, the patient had a strong family support system that could provide transportation to and from laboratory sessions. Additionally, he had sustained no ancillary orthopedic injuries on the date of his SCI, and his course of outpa-tient rehabilitation had been uncomplicated. On the other hand, during an intake survey at the time of enrollment, he reported “moderately bothersome” back, leg, and shoulder pain and “moderately bothersome” feelings of anxiety and depression.26 Despite these challenges, the patient believed that he could commit to a regimen of soleus muscle training lasting approximately 30 minutes per day on 5 days per calendar week.
The patient began attending twice-weekly stimulation sessions in the laboratory in order to acclimate the right (randomly selected) soleus muscle to stimulation. The patient demonstrated no signs or symptoms of autonomic hyperreflexia at any time during the acclimatization period or thereafter during the training protocol. (Autonomic hyperreflexia is a risk factor during electrical stimulation protocols for people with SCI, particularly those with lesions at or above T4. Noxious cutaneous input to the spinal cord via intact peripheral afferent fibers yields activation of the sympathetic ganglia below the level of the lesion. This sympathetic outflow can yield an adverse autonomic response that includes increased heart rate and blood pressure, headache, nausea, and sweating. Although patients generally adapt to electrical stimulation protocols, care must be taken to monitor for autonomic hyperreflexia even after tolerance to stimulation has been achieved.)
Intervention
Time dependence of load intervention
The patient began the electrical stimulation training protocol at 7 weeks after SCI, as soon as was practicable after he completed outpatient rehabilitation. Although extensive muscle atrophy had likely already occurred,27 paralyzed muscle appears to respond rapidly to electrical stimulation protocols.28,29 Thus, although muscular loads delivered to the trained tibia were initially low, they had the potential to increase rapidly as the soleus muscle adapted to the demands of increased use. In the acute stage after SCI, however, tibia BMD is not appreciably different from that in the non-SCI state (BMD drops by approximately 2%– 4% per month after SCI30). Thus, if rapid adaptation of soleus muscle torque occurred, the tibia would remain capable of withstanding the muscular load. However, once osteoporosis after SCI has been established, it appears to be irreversible,31,32 limiting the suitability of electrical stimulation protocols in people with long-standing SCI. Muscle adaptations could rapidly out-strip the ability of bone to withstand muscular forces, leading to an increased risk for fracture. There is a window of opportunity shortly after SCI during which mechanical loading interventions can be most safely and effectively used. We aimed to capitalize on this factor by treating this patient as soon as was feasible after his SCI.
Physiologic level of load
The patient performed daily soleus muscle training for 3 years as described in a previous report.22 Throughout the treatment program, the patient subjectively reported that after stimulation bouts, his lower-extremity spasms were less frequent. Thus, at the end of the protocol, the patient expressed a desire to continue training; his request was granted after approval from the University of Iowa Human Subjects Institutional Review Board. At the time of this report, the patient had been involved in soleus muscle training for 4.6 years and is now 4.8 years post-SCI. He continues to perform home-based stimulation according to the requested training dose and comes to the laboratory for muscle physiology testing once per month. The patient continues to undergo bone density assessments at approximately 6-month intervals.
The patient used a portable stimulator and data logging system to deliver trains of 10 stimulus pulses (15 Hz) to one soleus muscle, leaving the other soleus muscle as an untrained within-patient control. The requested dose of stimulation was 4 bouts of 125 contractions, 5 days per week, for a total stimulation goal of 10,000 contractions per month. A limb fixation system ensured that the soleus muscle contractions did not elicit ankle plantar flexion. By constraining ankle motion, we hoped to maximize the forces that the patient developed in the soleus muscle (and therefore to maximize the mechanical load delivered to the tibia). On the basis of previous investigations,33–36 we designed the electrical stimulation protocol with the goals of triggering soleus muscle hypertrophy and taxing the soleus muscle excitation-contraction coupling system (involved in fatigue resistance). The desired outcome of the training protocol was a soleus muscle that delivered strong, sustained forces to the tibia for the duration of the daily 4-bout training sessions.
Because bone-mounted strain gauges are ethically problematic in human research, osteogenic strain thresholds in humans are poorly understood, and strains developed during load protocols are difficult to estimate. Therefore, we designed the soleus muscle protocol to elicit a “physiologic” level of load congruent with established exercise principles. Paralyzed muscle adapts most vigorously to overload conditions; that is, hypertrophy and fiber type adaptations are dependent on the load developed within the muscle.28 We elected to restrict joint motion and to use a supramaximal stimulation intensity (200 mA) to elicit a very strong isometric contraction. We determined through the use of a biomechanical model that soleus muscle contractions in this protocol delivered compressive loads approx-imating 1.4 times body weight to the tibia.22 (It is important to note, however, that the biomechanically modeled compressive load induced by this protocol was more likely to deliver a bending moment to the tibia because of the posterior orientation of the soleus muscle.) In contrast, previous protocols involving passive standing delivered loads of only about 40% of body weight to the limbs.13,14 We believe that previous protocols involving low-level electrical stimulation,15,16 body weight-supported treadmill training,17 and electrically stimulated cycling18–20 likely did not approach the magnitude of tibia compressive loads made possible by strong isometric soleus muscle contractions. The present protocol therefore represents the high end of a continuum of mechanical loading approaches, with non-isometric electrical stimulation protocols and passive standing each likely transmitting incrementally lower levels of mechanical loads to the limbs. (Vibration, another method for mechanically loading paralyzed limbs, engenders loads of the smallest magnitude. However, vibration possesses other characteristics that may cause it to be effective for preventing bone demineralization. For a review, see the article by Rubin and coauthors.37)
Because the patient retained metal fragments from his gunshot injury, we were unable to use magnetic resonance imaging (MRI) to directly measure soleus muscle hypertrophy in response to the training protocol. Instead, the patient underwent monthly assessments of soleus muscle peak torque and fatigability to determine muscle adaptations to training. The reliability and validity of these physiologic assessment techniques were previously reported.22,31,33,34 The fatigue index (FI) is the quotient of the smallest peak at the end of the training bout and the maximum peak torque for that bout. Starting at 1.4 years after SCI, the patient underwent periodic peripheral quantitative computed tomography assessments of BMD at the distal tibia. We previously determined that training effects must be larger than 4.6% to exceed the possible measurement error attributable to user-determined slice placement at this anatomic location.24 In addition to using routine peripheral quantitative computed tomography analysis algorithms, we developed an analysis technique to partition the tibia image into anterior and posterior regions.
Surveillance of dose
The patient’s portable stimulator included data logging software that provided surveillance of his adherence to the requested loading dose. The stimulator was programmable by laboratory personnel but had only a start or stop button at the patient interface. Once the stimulator was activated, the preprogrammed stimulus frequency, intensity, repetitions, and duration were delivered unless the patient aborted the bout. We tabulated the number of contractions performed each month and expressed adherence as a percentage of the monthly goal of 10,000 contractions. The patient returned to the laboratory at least once per month for measurement of soleus muscle torque and fatigue capacity. About once every 3 months he also underwent torque and fatigue testing of the untrained soleus muscle for comparison.
Outcomes
Adherence to Loading Dose
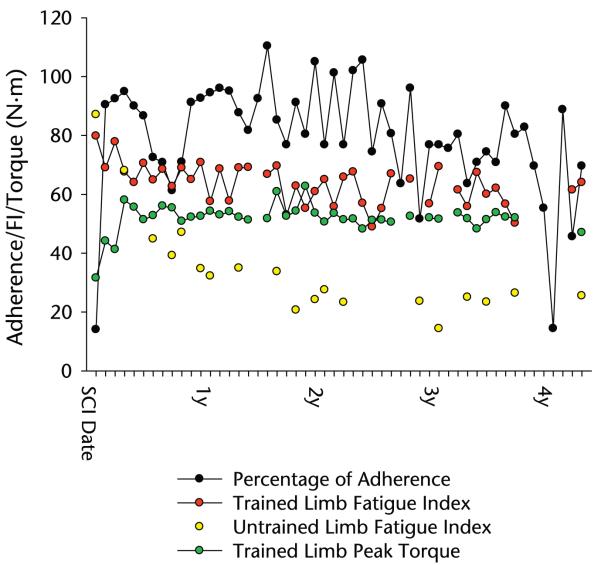
Mean percentage of adherence to the requested loading dose was 77.66% (~7,766 contractions per month) over the course of the patient’s training history (Fig. 1). By 4.6 years postinjury, the patient had completed almost 404,000 soleus muscle contractions administered on more than 800 days of training. Thus, over 4.6 years, the patient devoted more than 400 hours to the soleus muscle training protocol.
Figure 1.
Percentage of adherence, fatigue index (FI), and torque during the patient’s training history. SCI=spinal cord injury.
Training fluctuations were caused in various instances by illness, occupational responsibilities (the patient worked on his family’s farm), or vacations. The low percentage of adherence recorded in his fourth year post-SCI reflects a period when the patient’s stimulator malfunctioned and when he did not attend a routine laboratory session (Fig. 1).
Muscle Adaptation to Load
The patient’s trained limb peak torque nearly doubled by the third month of training (Fig. 1) and remained within 20% of this value for the remainder of the training history. The trained soleus muscle maintained an FI of approximately 60%, whereas the FI of the untrained limb dropped to approximately 30%. At the initial measurement, the untrained (left) limb FI was slightly higher than the trained (right) limb FI (Fig. 1). By the sixth month of training, the untrained limb FI dropped by 49%, whereas the trained limb FI dropped by only 19%. The slope of a first-order regression line through the trained limb data was −.238; that for the untrained limb data was −.845. Throughout the training history, the trained limb FI never dropped more than 39% below the baseline FI. In contrast, at its nadir, the untrained limb FI was 83.5% lower than the baseline value. Across all time points, the mean (SE) trained limb FI was 63.5 (1.0), within 20% of the initial measurement. At the final measurement point, nearly 5 years after SCI, the trained limb FI was 64.0, 60% higher than the untrained limb FI (25.5).
Bone Adaptation to Load
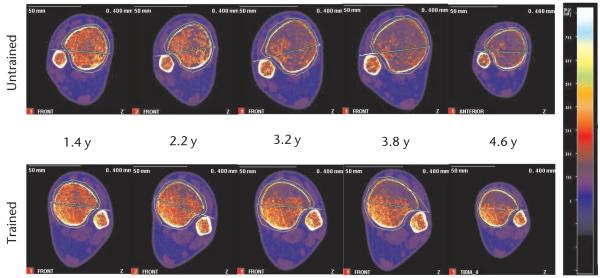
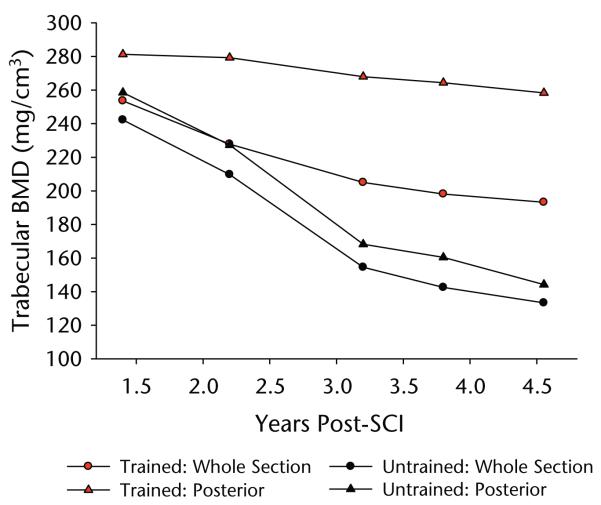
After 1.4 years of training, very little difference in trabecular architecture appeared to exist between the trained and untrained limbs (Fig. 2). Quantitative BMD measurements confirmed this observation, revealing only a 4.5% trabecular BMD difference between limbs at this time point (Fig. 3). Over the next 3 years of training, we noted a conspicuous deterioration of the trabecular lattice in the untrained limb (Fig. 2). The BMD of the untrained tibia fell by 44.96% between the first and last measurements, an average loss of 14.26% per year of surveillance (Fig. 3). The rate of this loss was most rapid between 1.4 and 3.2 years. The appearance of the destruction of the trabecular lattice was uniform throughout the untrained tibia cross section (Fig. 2), a finding corroborated by similar overall percent declines in BMD for the anterior and posterior regions (45.77% and 44.22%, respectively) (Fig. 3).
Figure 2.
Longitudinal peripheral quantitative computed tomography scans of the patient’s distal tibia at various times after spinal cord injury. The apparent size difference in the panels at the far right is an image processing artifact (the scan was collected with a larger image matrix; resolution did not differ). Scans are normalized to the density of fat (0 mg/cm3), depicted as bright blue; higher densities are represented by brighter colors (see color scale at right). Fully mineralized cortical bone appears white (~1,200 mg/cm3), and trabecular bone appears orange (~300 –500 mg/cm3). Over time, large areas of marrow fat (blue) replaced trabecular bone within the trabecular envelope. This destruction of the trabecular lattice was substantially minimized in the posterior region of the trained tibia.
Figure 3.
Bone mineral density (BMD) data corresponding to the scan images depicted in Figure 2. Data are shown for the whole tibia cross section and for the posterior subregions (delineated in Fig. 2 by a green line transecting the cross section). SCI=spinal cord injury.
In contrast, over time we observed destruction of the trabecular lattice only in the anterior portion of the trained tibia cross section (Fig. 2). The BMD of the trained limb whole cross section declined about half as rapidly as that of the BMD of the untrained limb whole cross section (only 7.55% per year of surveillance) (Fig. 3). Upon closer examination, we found that this divergence was attributable to the preservation of BMD in the posterior portion of the trained tibia. Although the anterior portion of the trained tibia experienced a 37.78% decline in BMD, similar to that in the untrained limb, the posterior portion BMD declined just 8.18% between the first and final measurements, a 2.59% annual decline. In fact, at the time of this report (4.8 years after SCI), the BMD of the posterior portion of the patient’s trained tibia (258 mg/cm3) continues to approximate non-SCI normative values for the distal tibia (~250 mg/cm3).24,38
Discussion
The objective of this case report was to demonstrate that a mechanical loading intervention administered soon after SCI and applied for nearly 5 years supports the theory that mechanical load is an important homeostatic input to bone after SCI. However, as described in this case report, rigorous control of the loading dose, protocol adherence, and timing of the intervention all are important considerations in the implementation of therapeutic stress to the limbs of people with SCI. We believe that the interpretation of many previous studies is hindered by incomplete control or reporting of these important factors, or both. The heuristic value of these studies is adversely affected because future researchers cannot interpret whether the loading dose was inadequate, not adhered to, or introduced at the wrong time or whether the mechanical loading itself was not effective. We maintain that mechanical loading has excellent potential as an antiosteoporosis therapeutic strategy after SCI; however, we emphasize that careful surveillance of the intervention is necessary to ensure that the requested dose is received by the client. We believe that this concept has far greater applicability than research on osteoporosis after SCI and may be useful for rehabilitation researchers participating in any intervention trials.
Timing of Intervention: Is Adaptation Still Possible?
For antiosteoporosis interventions after SCI to be effective, they must induce a net balance in osteoblast activity versus osteoclast activity. These cellular processes must occur on the surface of preexisting bone structural elements. The extensive destruction of the trabecular lattice in patients with long-term SCI thus limits the available surface area for bone remodeling, limiting the potential for adaptation to mechanical loading interventions. The plastic potential of bone tissue after SCI diminishes with time, suggesting that mechanical loading interventions have the greatest chance for success if they are administered soon after SCI. The inclusion of patients with long-standing SCI likely was a contributing factor in many studies that failed to demonstrate efficacy of mechanical loading.13–15,18,19 However, as was seen in 2 other studies, the inclusion of patients in the acute stage after SCI (<8 weeks) is no guarantee of success.17,20 Timely interventions designed to capitalize on available plasticity will be under-mined if the loading dose is insufficient to elicit desirable adaptations.
Interpretation of Adaptations to Mechanical Load
On the basis of the likely importance of vascular and neural factors in osteoporosis after SCI, one could argue that stimulation of the trained leg led to resolution of lacunar-canalicular venous stasis, enhanced local release of bone-active neuropeptides, or other nonmechanical differences from the untrained limb. We believe that these changes are in fact likely and are fertile ground for future research. Through the use of adherence-monitoring software, we determined that the trained limb received nearly 8,000 cycles of compressive load during each month of the treatment program. We initially conceptualized this load as compression distributed evenly throughout the tibia cross section. The compartmentalization of adaptations raises the possibility that the predominant load orientation attributable to soleus muscle contraction was a bending moment that placed the posterior tibia in compression and the anterior tibia in tension. This is an important area for future investigation; if other electrical stimulation protocols similarly reveal asymmetric effects of muscular contraction on BMD, then attention must be given to developing antiosteoporosis interventions that more symmetrically load the bone cross section through simultaneous co-contractions of synergistic muscles.
We do not present the absolute peak torque data for the untrained limb in our patient. A direct comparison of absolute peak muscle torque values between trained and untrained paralyzed limbs after chronic SCI is complicated because of known changes in passive elastic tissues in the non-exercised limb. (For a review of factors influencing untrained paralyzed muscle torque, see the article by Shields and Dudley-Javoroski.22)
Impact of Fluctuating Adherence
The demands of daily life caused our patient’s adherence to fluctuate over the course of his training history. However, we believe that his level of participation in the protocol was rather remarkable (~100 hours per year, not including transportation time); many people without SCI may not devote this level of time to physical training regimens. On the basis of our experience with other patients and similar protocols,22,31,39 it seems that patients with SCI can integrate longitudinal training protocols into their daily lives as long as reasonable accommodations are made to minimize patient burden. A key component of our approach was to use home-based, portable stimulators to obviate the need for daily laboratory visits. However, we believed that traditional methods for monitoring adherence (eg, logbooks and phone check-ins) would not be sufficiently reliable for tracking the loading dose. Our solution was to develop adherence-monitoring software that logged each contraction that our patient performed, along with date and time stamps. At laboratory visits, we measured soleus muscle torque and fatigue resistance, 2 other important factors contributing to the load experienced by the tibia. With these 2 surveillance methods, we obtained detailed information about patient adherence that allowed us to intervene if adherence showed a downward trend.
In response to the adherence fluctuations in our patient’s training history, the soleus muscle peak torque and FI were generally stable. Even after the substantial drop in adherence in his fourth year post-SCI, the torque and FI in successive months differed little from previous values. Between-day variations in the FI were greater than observed variations in peak force; this finding would be expected because of the method used for computing the FI (a quotient of 2 torque values, each of which has the potential to deviate from values on other days).
After we observed our patient’s drop in adherence, we engaged him in conversation to determine his desire to continue with the training protocol. He assured us that he did wish to continue, and we discussed the importance of maintaining an adequate loading dose. The patient renewed his training intensity and has subsequently maintained his adherence at a more typical level. We have had similar conversations with other patients who showed drops in adherence in longitudinal treatment programs. Most have responded to this feedback by intensifying training. In a few other cases, these conversations have helped smooth the exit process for patients who wished to cease training. Tracking detailed adherence data has also helped us to diplomatically inform chronically nonadherent patients that they will have to be discharged from treatment programs. Thus, adherence surveillance not only aids researchers in more confidently assessing data from longitudinal treatment programs but also helps researchers engage patients in honest dialogue about the level of patient burden of treatment programs. Understanding the extent to which clients adhere to prescribed physical therapy treatments is equally important as physical therapists strive to base clinical practice on evidence.
Use of Dose Information
Detailed adherence data can help characterize a dose-response curve for rehabilitation interventions. If a patient is chronically nonadherent yet still experiences the desired outcome, then the target dose of the intervention may require reevaluation. Without knowledge of a pa-tient’s adherence, no such refinement is possible. Dose refinements are highly desirable for interventions that have noteworthy financial or patient burden concerns.
Dose refinements are also made possible by adjusting the stimulus magnitude, assuming that this magnitude has been estimated. In our treatment program, we sought a specific level of mechanical load with theoretical potential, estimated it biomechanically, and administered it with appropriate adherence surveillance. If the soleus muscle stimulation protocol had not yielded bone adaptations, then we would have obtained a useful benchmark by which we could refine the magnitude of the load. In addition, future studies will be better able to replicate the load magnitude conditions that led to the desirable effects that we observed. In contrast, many previous mechanical loading studies reported only the number of minutes or repetitions spent in a loading activity, leaving many unanswered questions about load magnitude at the level of the skeletal system. For many types of rehabilitation research, there is a great need to estimate stimulus magnitude at the level of the target tissue, not just the individual.
Tissue-level estimation of stimulus magnitude also has important patient safety implications. The strong contractions possible with electrical muscle stimulation have the potential to deliver dangerous shear loads to the skeletal system. For example, a common electrical stimulation protocol is to elicit isometric quadriceps muscle contractions with the knee in 90 degrees of flexion.28 Shear forces across the distal femur have led to at least one reported fracture in a protocol of this type.40 However, quadriceps muscle testing can be safely performed when careful consideration is given to minimizing shear forces, either through limitation of quadriceps muscle torque10 or through biomechanical determination of a shear-minimizing posture.41 Thus, the issue of stimulus magnitude in rehabilitation research is certainly not a “more is better” proposition. Dose refinements must be based on adequate tissue-level inputs without violation of the clinical principles of patient safety.
Clinical Context
The present case report is intended to illustrate: (1) the possible specificity of the trabecular bone response to mechanical load orientation (compression versus tension) when hormonal, vascular, and neural factors are presumed to be uniform across the bone cross section and (2) proof of the concept that careful dose selection and surveillance of adherence should be integral components in longitudinal rehabilitation interventions. We strongly caution readers not to extrapolate a clinical course of action from this preliminary report. However, research into this promising intervention approach is ongoing. We are currently engaged in examining longitudinal treatment programs involving mechanical loading at other regions of the lower extremity. In the coming years, we hope to provide answers to the following questions:
Can bone density be preserved throughout the cross section of the bone?
Is the dose (magnitude, frequency, and duration) of mechanical loading similarly effective at other anatomical sites?
What is the dose-response relationship of bone density to mechanical loading?
Does the effectiveness of mechanical loading persist or wane over time?
Do mechanical loading protocols affect health quality after SCI?
Do the bone density adaptations observed affect outcomes (fracture risk) after SCI?
This last question is the most important of all.
These questions require more in-depth study before mechanical loading through electrical muscle stimulation can be recommended as a safe and effective clinical intervention. The safety considerations of electrical muscle stimulation after SCI are not trivial. Peak forces generated by even long-term untrained muscle can be considerable because of connective tissue adaptations from disuse.22
Additionally, the financial feasibility of long-term daily electrical stimulation training is an issue of concern. Administration of the majority of the training dose at home, rather than in a clinic, would greatly minimize the cost of such an intervention. However, as with any biologic system, bone in paralyzed extremities is likely to display positive adaptations only as long as the therapeutic stimulus is continuously administered, a requirement of any daily exercise regimen. Patients who receive a suboptimal loading dose or who experience gaps in training are unlikely to experience optimal outcomes. To be financially justifiable, long-term electrical muscle stimulation protocols must demonstrate broad benefits to the overall health and well-being of people with SCI. Although the efficacy of mechanical loading is rapidly gaining support through research studies, translation of this strategy to a standard set of clinical guidelines is still a long-term goal.
Conclusion
The careful approach to dose selection, adherence monitoring, and intervention timing described in this case report is somewhat reminiscent of pharmacologic studies. These practices helped us to interpret the outcomes of a long-term loading intervention with greater confidence. Rehabilitation research has traditionally struggled with issues of timeliness, dose, and adherence, but we believe that awareness of these factors is increasing. This case report illustrates one example of how careful intervention design and monitoring can aid the clear interpretation of treatment outcomes.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (R01 NR-010285-05) to Dr Shields. Ms Dudley-Javoroski received a scholarship from the Foundation for Physical Therapy.
Footnotes
Dr Shields provided concept/idea/project design, project management, fund procurement, facilities/equipment, institutional liaisons, and clerical support. Both authors provided writing, data collection and analysis, the patient, and consultation (including review of manuscript before submission). The authors thank Deanna Frei, RTR, CT, April Miller, RTR, and Daniel Schiferl for their technical expertise during peripheral quantitative computed tomography measurements.
Contributor Information
Shauna Dudley-Javoroski, Graduate Program in Physical Therapy and Rehabilitation Science, University of Iowa, Iowa City, Iowa..
Richard K Shields, Carver College of Medicine–Physical Therapy and Rehabilitation Science, 1–252 Medical Education Building, University of Iowa, Iowa City, IA 52242 (USA)..
References
- 1.Reiter AL, Volk A, Vollmar J, et al. Changes of basic bone turnover parameters in short-term and long-term patients with spinal cord injury. Eur Spine J. 2007;16:771–776. doi: 10.1007/s00586-006-0163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maimoun L, Lumbroso S, Paris F, et al. The role of androgens or growth factors in the bone resorption process in recent spinal cord injured patients: a cross-sectional study. Spinal Cord. 2006;44:791–797. doi: 10.1038/sj.sc.3101922. [DOI] [PubMed] [Google Scholar]
- 3.Chantraine A, van Ouwenaller C, Hachen HJ, Schinas P. Intra-medullary pressure and intra-osseous phlebography in paraplegia. Paraplegia. 1979;17:391–399. doi: 10.1038/sc.1979.75. [DOI] [PubMed] [Google Scholar]
- 4.Hohmann EL, Elde RP, Rysavy JA, et al. Innervation of periosteum and bone by sympathetic vasoactive intestinal peptide-containing nerve fibers. Science. 1986;232:868–871. doi: 10.1126/science.3518059. [DOI] [PubMed] [Google Scholar]
- 5.Imai S, Matsusue Y. Neuronal regulation of bone metabolism and anabolism: calcitonin gene-related peptide-, substance P-, and tyrosine hydroxylase-containing nerves and the bone. Microsc Res Tech. 2002;58:61–69. doi: 10.1002/jemt.10119. [DOI] [PubMed] [Google Scholar]
- 6.Serre CM, Farlay D, Delmas PD, Chenu C. Evidence for a dense and intimate innervation of the bone tissue, including glutamate-containing fibers. Bone. 1999;25:623–629. doi: 10.1016/s8756-3282(99)00215-x. [DOI] [PubMed] [Google Scholar]
- 7.Pogoda P, Egermann M, Schnell JC, et al. Leptin inhibits bone formation not only in rodents, but also in sheep. J Bone Miner Res. 2006;21:1591–1599. doi: 10.1359/jbmr.060709. [DOI] [PubMed] [Google Scholar]
- 8.Takeda S, Elefteriou F, Levasseur R, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 9.Patel MS, Elefteriou F. The new field of neuroskeletal biology. Calcif Tissue Int. 2007;80:337–347. doi: 10.1007/s00223-007-9015-3. [DOI] [PubMed] [Google Scholar]
- 10.Rittweger J, Gerrits K, Altenburg T, et al. Bone adaptation to altered loading after spinal cord injury: a study of bone and muscle strength. J Musculoskelet Neuronal Interact. 2006;6:269–276. [PubMed] [Google Scholar]
- 11.Shields RK, Dudley-Javoroski S, Law L Frey. Electrically-induced muscle contractions influence bone density decline after spinal cord injury. Spine. 2006;31:548–553. doi: 10.1097/01.brs.0000201303.49308.a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chantraine A. Actual concept of osteoporosis in paraplegia. Paraplegia. 1978;16:51–58. doi: 10.1038/sc.1978.8. [DOI] [PubMed] [Google Scholar]
- 13.Kunkel CF, Scremin AM, Eisenberg B, et al. Effect of “standing” on spasticity, contracture, and osteoporosis in paralyzed males. Arch Phys Med Rehabil. 1993;74:73–78. [PubMed] [Google Scholar]
- 14.Ben M, Harvey L, Denis S, et al. Does 12 weeks of regular standing prevent loss of ankle mobility and bone mineral density in people with recent spinal cord injuries? Aust J Physiother. 2005;51:251–256. doi: 10.1016/s0004-9514(05)70006-4. [DOI] [PubMed] [Google Scholar]
- 15.Needham-Shropshire BM, Broton JG, Klose KJ, et al. Evaluation of a training program for persons with SCI paraplegia using the Parastep 1 ambulation system, part 3: lack of effect on bone mineral density. Arch Phys Med Rehabil. 1997;78:799–803. doi: 10.1016/s0003-9993(97)90190-8. [DOI] [PubMed] [Google Scholar]
- 16.Thoumie P, Le Claire G, Beillot J, et al. Restoration of functional gait in paraplegic patients with the RGO-II hybrid orthosis. A multicenter controlled study. II: Physiological evaluation. Paraplegia. 1995;33:654–659. doi: 10.1038/sc.1995.137. [DOI] [PubMed] [Google Scholar]
- 17.Giangregorio LM, Hicks AL, Webber CE, et al. Body weight supported treadmill training in acute spinal cord injury: impact on muscle and bone. Spinal Cord. 2005;43:649–657. doi: 10.1038/sj.sc.3101774. [DOI] [PubMed] [Google Scholar]
- 18.Leeds EM, Klose KJ, Ganz W, et al. Bone mineral density after bicycle ergometry training. Arch Phys Med Rehabil. 1990;71:207–209. [PubMed] [Google Scholar]
- 19.BeDell KK, Scremin AM, Perell KL, Kunkel CF. Effects of functional electrical stimulation-induced lower extremity cycling on bone density of spinal cord-injured patients. Am J Phys Med Rehabil. 1996;75:29–34. doi: 10.1097/00002060-199601000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Eser P, de Bruin ED, Telley I, et al. Effect of electrical stimulation-induced cycling on bone mineral density in spinal cord-injured patients. Eur J Clin Invest. 2003;33:412–419. doi: 10.1046/j.1365-2362.2003.01156.x. [DOI] [PubMed] [Google Scholar]
- 21.Mueller MJ, Maluf KS. Tissue adaptation to physical stress: a proposed “physical stress theory” to guide physical therapist practice, education, and research. Phys Ther. 2002;82:383–403. [PubMed] [Google Scholar]
- 22.Shields RK, Dudley-Javoroski S. Musculo-skeletal plasticity after acute spinal cord injury: effects of long-term neuromuscular electrical stimulation training. J Neurophysiol. 2006;95:2380–2390. doi: 10.1152/jn.01181.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.International Standards for Neurological Classification of SCI. American Spinal Injury Association; Atlanta, Ga: 2002. [Google Scholar]
- 24.Shields RK, Dudley-Javoroski S, Boaldin KM, et al. Peripheral quantitative computed tomography: measurement sensitivity in persons with and without spinal cord injury. Arch Phys Med Rehabil. 2006;87:1376–1381. doi: 10.1016/j.apmr.2006.07.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shields RK, Dudley-Javoroski S, Littmann AE. Post-fatigue potentiation of paralyzed soleus muscle: evidence for adaptation with long-term electrical stimulation training. J Appl Physiol. 2006;101:556–565. doi: 10.1152/japplphysiol.00099.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dudley-Javoroski S, Shields RK. Assessing health-related quality of life and secondary complications after complete spinal cord injury. Disabil Rehabil. 2006;28:103–110. doi: 10.1080/09638280500163828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castro MJ, Apple DF, Jr, Staron RS, et al. Influence of complete spinal cord injury on skeletal muscle within 6 mo of injury. J Appl Physiol. 1999;86:350–358. doi: 10.1152/jappl.1999.86.1.350. [DOI] [PubMed] [Google Scholar]
- 28.Crameri RM, Cooper P, Sinclair PJ, et al. Effect of load during electrical stimulation training in spinal cord injury. Muscle Nerve. 2004;29:104–111. doi: 10.1002/mus.10522. [DOI] [PubMed] [Google Scholar]
- 29.Harridge SD, Andersen JL, Hartkopp A, et al. Training by low-frequency stimulation of tibialis anterior in spinal cord-injured men. Muscle Nerve. 2002;25:685–694. doi: 10.1002/mus.10021. [DOI] [PubMed] [Google Scholar]
- 30.Wilmet E, Ismail AA, Heilporn A, et al. Longitudinal study of the bone mineral content and of soft tissue composition after spinal cord section. Paraplegia. 1995;33:674–677. doi: 10.1038/sc.1995.141. [DOI] [PubMed] [Google Scholar]
- 31.Shields RK, Dudley-Javoroski S. Musculo-skeletal adaptation in chronic spinal cord injury: effects of long-term soleus electrical stimulation training. J Neurorehabil Neural Repair. 2006;21:169–179. doi: 10.1177/1545968306293447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parfitt AM. Trabecular bone architecture in the pathogenesis and prevention of fracture. Am J Med. 1987;82:68–72. doi: 10.1016/0002-9343(87)90274-9. [DOI] [PubMed] [Google Scholar]
- 33.Shields RK. Fatigability, relaxation properties, and electromyographic responses of the human paralyzed soleus muscle. J Neurophysiol. 1995;73:2195–2206. doi: 10.1152/jn.1995.73.6.2195. [DOI] [PubMed] [Google Scholar]
- 34.Shields RK, Chang Y-J. The effects of fatigue on the torque-frequency curve of the human paralysed soleus muscle. J Electromyogr Kinesiol. 1997;7:3–13. doi: 10.1016/s1050-6411(96)00015-6. [DOI] [PubMed] [Google Scholar]
- 35.Shields RK, Chang YJ, Ross M. Neuromuscular propagation after fatiguing contractions of the paralyzed soleus muscle in humans. Muscle Nerve. 1998;21:776–787. doi: 10.1002/(sici)1097-4598(199806)21:6<776::aid-mus10>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 36.Shields RK, Law LF, Reiling B, et al. Effects of electrically induced fatigue on the twitch and tetanus of paralyzed soleus muscle in humans. J Appl Physiol. 1997;82:1499–1507. doi: 10.1152/jappl.1997.82.5.1499. [DOI] [PubMed] [Google Scholar]
- 37.Rubin C, Judex S, Qin YX. Low-level mechanical signals and their potential as a non-pharmacological intervention for osteoporosis. Age Ageing. 2006;35(suppl 2):ii32–ii36. doi: 10.1093/ageing/afl082. [DOI] [PubMed] [Google Scholar]
- 38.Eser P, Frotzler A, Zehnder Y, et al. Relationship between the duration of paralysis and bone structure: a pQCT study of spinal cord injured individuals. Bone. 2004;34:869–880. doi: 10.1016/j.bone.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 39.Shields RK, Dudley-Javoroski S. Monitoring standing wheelchair use after spinal cord injury: a case report. Disabil Rehabil. 2005;27:142–146. doi: 10.1080/09638280400009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hartkopp A, Murphy RJ, Mohr T, et al. Bone fracture during electrical stimulation of the quadriceps in a spinal cord injured subject. Arch Phys Med Rehabil. 1998;79:1133–1136. doi: 10.1016/s0003-9993(98)90184-8. [DOI] [PubMed] [Google Scholar]
- 41.Law L Frey, Shields RK. Femoral loads during passive, active, and active-resistive stance after spinal cord injury: a mathematical model. Clin Biomech. 2004;19:313–321. doi: 10.1016/j.clinbiomech.2003.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]