Introduction
One of the critical functions of intestinal motility is the propulsion of intestinal contents in the aborad direction. The small and large intestines have complex motor functions that produce the peristaltic response for the movement of chime (1), phase III of the interdigestive migrating motor complex to facilitate the movement of indigestible solids (2), and fluctuations in intestinal tone and capacitance that produce pressure gradients for the movement of gases (3). These phenomena can be grossly assessed by measurement of gastrointestinal transit, which is defined as the movement of intestinal contents or markers between two points in the gastrointestinal tract. Transit assessment of the small intestine and colon is relevant in the study of physiology, pathophysiology, pharmacodynamics, and there is increasing use of small bowel and colonic transit measurements in clinical practice as well. The purpose of this paper is to review the techniques available for the study of small bowel and colonic transit.
Evaluation of small bowel transit
Barium contrast studies
The earliest and most readily available method for assessing gastrointestinal transit is contrast radiography. Barium is an imperfect substitute for intestinal chyme because it is inert and, therefore, it does not initiate events induced by the biochemical components of food. Barium also has a greater density than water and it is hypo-osmolar compared to food. Nevertheless, barium contrast studies are immensely helpful in patients with suspected motility disorders to rule out mechanical obstruction, to evaluate whether there is local or diffuse dilatation, to identify the presence of intestinal diverticula. Careful radiographic observations can assess the integrity of segmental contractions and peristalsis in a qualitative way. In a structurally normal small intestine, the transit time of barium may also be measured from the moment the barium enters the duodenum until it reaches the cecum, although few studies have adhered to this definition.
There is a remarkable paucity of information regarding the normal transit time of barium in the small bowel, despite decades of application of this test. One textbook lists the normal range between 30 minutes and 6 hours (4). There is only one large study, performed in 1951, that has evaluated normal transit values in healthy subjects (5). In Lönnerblad's study (5), 111 students between the ages of 18 and 25 years underwent studies with the administration of a small volume (200 mL) barium meal. The transit time was measured from the time of when the subject had finished drinking the contrast meal to the time when the barium first entered the cecum (orocecal time). The mean orocecal time was 178 minutes with a standard deviation of 93 minutes, and 10% of the healthy subjects had a transit time of more than 5 hours. In a smaller study of 48 healthy volunteers (6), a much larger volume of barium (between 450 and 650 mL) was administered, and the transit time was measured from barium entry into the proximal jejunum to the time that it reached the cecum (jejuno-cecal time). The median transit time was 45 minutes. However, because of the different methodologies, these two studies are not comparable.
All other “normal” values for barium small bowel transit are derived from studies in symptomatic patients referred for small bowel radiography who had structurally negative exams. The volume of barium in these studies is also considerably greater than in the study of Lönnerblad. The largest such study (7) was comprised of 315 patients, given 16 ounces of barium (approximately 473 mL). The transit time was defined as the time from the completion of the upper gastrointestinal examination (usually 15 minutes) to the time the barium reached the cecum (small bowel transit time). This small bowel transit time was reported as a mean of 89.6 minutes with only 3 cases taking over 4 hours. In a different study of 26 patients who received 500 mL of barium, transit time was measured from the time of ingestion to the time to reach the terminal ileum (oro-terminal ileal time), with a mean time of 32.8 minutes (8). Finally, in another report of 21 patients who received 300 mL of barium (9), the orocecal transit time was 110 minutes (interquartile range 60-170 minutes).
Body position (right decubitus versus upright) (10) and use of pre-procedure laxatives (8) do not alter small bowel barium transit times. On the other hand, the use of effervescent agents (11), the addition of hyperosmolar contrast media to the barium suspension (12), and the use of prokinetics such as metoclopramide (13-15), ceruletide (9), prostigmin and cholecystokinin (4) have all been reported to accelerate transit.
In summary, small bowel transit time measured during barium radiography is inversely related to the volume of the barium administered, and what constitutes a normal barium small bowel transit time remains elusive because of lack of standardization of methods and definitions. In addition, contrast radiography studies provide substantial radiation exposure, thus precluding the use of this test for transit determination alone.
Breath hydrogen small bowel transit test
The first use of a breath test to measure orocecal transit was described by Bond and Levitt (16). The test is based on the observation that only bacteria are capable of producing hydrogen gas in the human body, and it is presumed that the hydrogen excreted in the breath is the product of colonic bacterial fermentation of unabsorbed carbohydrates ingested orally. Breath hydrogen tests have a number of appealing attributes including simplicity, safety, availability and low cost.
Lactulose Breath Test
The most widely used and, arguably, best studied carbohydrate substrate for transit determination is lactulose. The time between the ingestion of lactulose and the rise in breath hydrogen of at least 3, 5, or 10 parts per million (p.p.m.) above baseline, has been used to define the orocecal transit time (17,18). Validation studies have confirmed a high degree of correlation with simultaneous scintigraphy (19-22). Lactulose breath tests have been used to document pharmacological effects on gut motility (23-27) and to study disease states in adults and in children (28-33).
Early validation studies performed with doses of lactulose ranging from 5-40 g demonstrated that the transit times are shorter with increasing doses of lactulose (16,34). Comparison studies of lactulose breath hydrogen test and scintigraphy performed simultaneously show strong correlation (r = 0.945, P <0.01). However, when the studies performed with simultaneous scintigraphy and lactulose are compared to scintigraphy without lactulose in the same individuals, it appears that the lactulose markedly accelerates transit, with the median transit time of 56 minutes compared to a median transit time of 205 minutes when scintigraphy is used without co-administration of lactulose (35). This suggests that lactulose is non-physiologic since it accelerates small bowel transit, presumably due its osmotic activity, which is the basis for its use as a laxative.
Inulin Breath Test
An attempt to circumvent the problems with lactulose involves using inulin, which is a soluble fiber composed of fructose polymers (i.e., a fructan). Human enzymes cannot disrupt the β(1,2)-glycoside bonds of fructans; therefore, inulin is not broken down until it is acted upon by bacterial enzymes in the colon. Inulin is highly polymerized; therefore, fewer inulin molecules are needed, compared to lactulose, to produce an equivalent amount of hydrogen gas after full bacterial enzymatic hydrolysis. Thus, the inulin dose has lower osmotic activity compared to lactulose.
The inulin orocecal transit time is defined as the time from ingestion of the inulin containing meal until the rise in breath hydrogen content by 10 p.p.m. above baseline (36). Orocecal transit times were tested in 17 healthy subjects fed 5 g of inulin in a solid meal and, separately, with a lactose 13C-ureide marker (discussed in Breath stable-isotope small bowel transit test below). This study found no evidence that 5 g of inulin in a solid nutrient meal accelerates transit, since the transit times were 360 minutes (range 270-420 minutes) with inulin and 353 minutes (range 285-375 minutes) with lactose 13C-ureide (36). There were also no significant differences in orocecal transit time with 5 g and 10 g inulin. In a study of 29 subjects, Schneider et al. found a median orocecal transit time of 300 minutes (range 180-420 minutes) for a liquid nutrient test meal containing 5 g of inulin (37). Correlation between the inulin-based test and the lactose 13C-ureide test was moderate [r=0.85 (37) and r=0.72].
The pitfalls of the inulin based test include the finding that 7-10% of subjects were hydrogen non-producers in response to 5 g of inulin (36,37), and one of the studies showed that the median orocecal transit time was about 30 minutes shorter with inulin than with the lactose 13C-ureide test (37), suggesting the possibility that inulin may also accelerate orocecal transit time. At present, inulin based hydrogen breath tests have not demonstrated responsiveness to pharmacological manipulation of transit and have not been applied in disease states affecting motility.
Finally, for both breath test methods, the orocecal time can be confounded by the rate of gastric emptying, which may be delayed in patients with gastroparesis, thus rendering interpretation of small bowel transit suspect or impossible. In addition, the presence of small bowel bacterial overgrowth associated with intestinal diverticula or motility abnormalities can cause an early peak in breath hydrogen which may result in a spuriously low orocecal transit determination.
Breath stable-isotope small bowel transit test
A stable-isotope labeled sugar-urea (lactose 13C-ureide) transit test has been advocated as a favorable alternative to breath hydrogen transit tests. Such a stable isotope test requires a very small dose of the substrate (0.5 - 1.2 g) which is unlikely to have any significant accelerating osmotic effect (38,39). The enzyme to cleave the sugar-urea bond is not present at the human intestinal brush border, and very little of the molecule is absorbed undigested (40). After passing through the small intestine, the sugar-urea bond is cleaved by an allantoicase enzyme present among the colonic bacterial flora (and not present in Helicobacter pylori), thus liberating 13C-labeled ureide which subsequently undergoes hydrolysis with release of 13CO2 and excretion by the lungs. The ratio of breath 13CO2/12CO2 can be determined by isotope ratio mass spectrometry.
The time from ingestion of the lactose 13C-ureide containing meal to the first increase in breath 13CO2/CO2 ratio (measured at 2.5 standard deviations above the running average of previously measured 13CO2/CO2 values) indicates the orocecal transit time by this stable-isotope breath test (39). The lactose 13C-ureide test has been validated in a comparison with scintigraphy in 22 healthy volunteers (39). The orocecal transit time was 292 ± 58 minutes for the breath test and 283 ± 53 minutes for scintigraphy, and there was good correlation between the two tests (r=0.94). The lactose 13C-ureide test has been used to evaluate pharmacological perturbations of orocecal transit (41-43).
Since stable isotopes such as 13C are non-radioactive, the test has been used in children (44). Disadvantages include the relative expense of the lactose 13C-ureide substrate compared to lactulose and inulin, the expense of the mass spectrometry analytical equipment, the need for subjects to avoid physical activity to maintain constant CO2 production, and the potential of colonic type bacterial flora present in the small bowel to cause premature digestion of the lactose 13C-ureide. It has also been recommended that the colonic flora should be pre-conditioned or “spiked” with unlabeled lactose ureide the night before the test (39), although a subsequent study reported that this step may not be necessary (45).
Radiopaque marker small bowel transit test
A method utilizing radiopaque markers to evaluate small bowel transit has been described by Sadik et al. (46). The method consists of ingesting a 400 kcal meal with 20 radiopaque markers. The markers are 4 mm in size, and it has been shown previously that gastric emptying of indigestible solids in the range of 1.5 to 7 mm can occur independent of the phase III of the migrating motor complex (47). Fluoroscopy is then used to localize the markers every 30 minutes for 8 hours. The number of markers present in the small bowel is plotted against time. The small bowel residence time is defined as the area under the curve divided by the number of markers reaching the colon during the observation time (8 hours). The median small bowel residence times in 83 healthy subjects and in 16 patients with portal hypertension were 3.2 and 5.9 hours respectively. This method has also been used to determine differences in small bowel residence time between healthy men and women (48). Other methods utilizing radiopaque markers have been used in patients with small bowel transplant (49) and in patients who have had colectomy (50).
Although the use of radiopaque markers is fairly simple and inexpensive, there may be difficulty in attributing the position of the markers to a specific anatomical location within the gastrointestinal tract, and this can result in erroneous calculations. Additionally, there is substantial radiation exposure with this method and the need for multiple imaging every 30 minutes for up to 8 hours, which is inconvenient for the patient as well as the staff.
Small intestine scintigraphy
Small bowel scintigraphy is not commonly used outside of research, but an assessment of orocecal transit in the form of scintigraphic colonic filling at 6 hours is gaining increased use in conjunction with gastric emptying or whole gut transit tests (51).
The test involves ingestion of either a liquid [water (52,53)] or solid [resin beads or meal (54-56)] material labeled with 111indium or 99mtechnetium and obtaining sequential scans over several hours. Scintigraphic small bowel transit time can be calculated in several ways (57), but most commonly as the time for 10% or 50% of the activity to arrive at the terminal ileum or cecum, after correcting for gastric emptying by subtracting the time for the equivalent proportion to be emptied from the stomach (58). A valid surrogate for the 10% scintigraphic small bowel transit time is the percent of the meal filling the colon at 6 hours (54).
Normative data are limited (based on less than 30 subjects), with wide ranges (that are method-dependent) for scintigraphic small bowel transit times (55,58,59). The range of normal values for colonic filling at 6 hours is 11% go 70% with radiolabeled nondigestible particles (60,61) or 43% to 95% for radiolabeled digestible solids (62). Alternatively, rapid scintigraphic small bowel transit time has been defined as cecal arrival time of <90 minutes (54). Neither age nor gender appears to influence scintigraphic small bowel transit time (55,56). In a study of 95 participants (healthy volunteers and patients with irritable bowel syndrome), the mean 6 hour colonic filling was 51.4 ± 3%, and the estimated intersubject coefficient of variation was 56% (63). However, 6 hour colonic filling is a measure of orocecal transit time and could be significantly influenced by gastric emptying rate.
Responsiveness of scintigraphic small bowel transit time to treatment was shown in studies of the effect of cisapride in patients with gastroparesis and chronic intestinal dysmotility (64,65) and of tegaserod in patients with irritable bowel syndrome with constipation (66). Identification of delayed scintigraphic small bowel transit time has been shown to impact both initial diagnosis and clinical management (67), but data on clinical outcomes are limited. A confounder is that slow colonic transit delays small bowel transit; therefore scintigraphic small bowel transit time needs to be interpreted with caution in patients with delayed colonic transit or constipation (68). This criticism also applies to the measurement of small bowel transit by all methods.
A different scintigraphic method designed to measure duodeno-cecal (as opposed to orocecal) transit involves the use of 99mtechnetium-hepatobiliary iminodiacetic acid (99mTc-HIDA) intravenous tracer which is taken up by the liver and excreted in the bile directly into the duodenum; this avoids the influence of gastric emptying on scintigraphic measurement of small bowel transit (59). In 30 healthy subjects, the mean 99mTc-HIDA small bowel transit time was 77.9 + 31.1 minutes; in 14 of 17 patients who had dysmotility verified by manometry, the 99mTc-HIDA small bowel transit time was delayed. There is limited published information regarding this technique.
In summary, scintigraphy provides physiological and quantitative information. Of the methods reviewed thus far, scintigraphy has yielded the most reliable results (55). Disadvantages are lack of standardization in clinical practice, lack of general application, the wide range of normal values, and a potential pitfall in interpretation when gastric emptying and/or colonic transit are delayed. The costs of the imaging equipment, radiation exposure, and difficulty in delineating anatomy are other drawbacks.
Wireless motility capsule small bowel transit
Small bowel transit time can also be measured with wireless motility capsule [WMC (69)]. The WMC is 26 mm long by 13 mm wide and contains sensors that measure pH, pressure and temperature. It is approved for the measurement of regional (gastric, small bowel, and colonic) transit and whole gut transit time. In addition, the WMC can characterize pressure patterns and motility indices in the different regions of the gastrointestinal tract (70,71). The transit of a large indigestible solid like a WMC in the small intestine is presumed to depend upon phase II and phase III activity of the interdigestive motor complex (72), but the evidence is limited. It is unclear whether ileal prolonged propagated contractions ultimately expel the capsule from the small bowel to the colon (73).
Regional transit determination with WMC requires discontinuing motility altering drugs (as with all transit tests) and acid-suppressing medications for one week prior to the test. After an overnight fast, the patient consumes a standard meal (255 kcal nutrient bar) followed by swallowing the WMC with water. The patient then fasts for 6 hours. The gastric emptying time is determined by an abrupt rise in pH to >4 or a rise of 3 pH units over baseline, reflecting passage of the WMC across the pylorus into the duodenum. The WMC small bowel transit time is defined as the interval between this rise in pH to the time when the pH suddenly falls by more than 1 unit for at least 5 minutes as the WMC enters the cecum (74). The determination of colonic transit by WMC will be discussed separately.
In 9 healthy subjects (75) the median WMC small bowel transit time was 350 minutes (169–676 minutes), compared to the simultaneous scintigraphic transit median of 342 minutes (162–669 minutes). In another study involving 66 healthy subjects (76), the median normal WMC small bowel transit was 276 minutes (IQR 240-354 minutes). In the same study, 34 patients with gastroparesis had WMC small bowel transit time that was similar to that of healthy subjects with a median of 270 minutes (IQR 216-330 minutes). In a study of 157 constipated patients (77), median transit time was 234 minutes (IQR 201-293 minutes) and was very similar to transit by radiopaque markers. In 10 healthy subjects, dietary intervention with insoluble fiber reduced WMC whole gut transit time, but did not significantly change WMC small bowel transit time (78).
One of the chief advantages is that the WMC test is performed with the patient ambulatory and outside the clinic setting, and the information is sent by telemetry to a recording device that the patient wears. A practical disadvantage is the occasional difficulty in identifying the 1 unit pH drop signifying passage into the cecum, especially in patients with post-surgical changes (e.g. right hemicolectomy) or incompetent ileocecal valve resulting in bacterial colonization of the distal ileum. Another potential pitfall results from the lack of standardization of the second meal 6-hour fast after WMC ingestion. This may potentially affect the time for WMC transit through the distal small bowel. Finally, the nondigestible, 26 mm by 13 mm WMC may be retained longer than 6 days or become impacted in the gastrointestinal tract; hence, contra-indications include suspected mechanical obstruction, recent gastrointestinal surgery (within 3 months), and Crohn's disease. In three multicenter studies involving 495 subjects, 32 subjects retained the capsule longer than 6 days, but radiographic evidence showed all subjects had passed the capsule by day 26 (69,77,79).
Evaluation of colon transit
Radiopaque marker
Colonic transit studies are used to evaluate total and segmental colonic transit times. Radiopaque markers can be obtained commercially.
Several different methods to measure colonic transit with radiopaque markers have been described (80-82. The method which is perhaps the simplest and involves the least amount of radiation exposure is the method of Hinton et al. (80). This involves the ingestion of a gelatin capsule containing 24 radiopaque 4.5 mm rings, and performing a single plain abdominal x-ray 5 days later. In this method, normal transit is identified by 5 or less markers retained by day 5, and slow transit by 6 or more markers retained. These values were established in 25 healthy subjects (83). Localization of the retained markers in the rectosigmoid area suggests functional outlet obstruction, whereas diffuse distribution of the retained markers is suggestive of slow transit constipation. However, this is only suggestive and not diagnostic, since delayed rectosigmoid transit due to pelvic floor dyssynergia may inhibit proximal colonic transit and result in widespread distribution of markers.
The method described by Metcalf et al. (82) involves the ingestion of 24 radiopaque markers each day at 24 hour intervals for three days. A plain abdominal x-ray is taken on day 4 and then every third day until all the markers have passed. Calculations are based on adding the numbers of markers in the entire colon (or in segments of interest, if segmental transit is desired) on each of the x-rays (e.g., on days 4 and 7), and the sum of the number of markers on each x-ray equals the mean colonic transit time. Segmental transit times are measured in the right colon to the right of the vertebral spinous processes and above an imaginary line from the fifth lumbar vertebra to the pelvic outlet. The left colon is the area to the left of the vertebral spinous processes and the imaginary line above the fifth lumbar vertebra and the left anterior superior iliac crest. The rectosigmoid is the area under the imaginary line from the pelvic brim on the right to the superior iliac crest on the left. Normal values, based upon the study of 73 healthy subjects (82), are as follows: whole colon 35 hours (95th percentile: 68 hours), right colon 11.3 hours (95th percentile: 32 hours), left colon 11.4 hours (95th percentile: 39 hours), and rectosigmoid 12.4 hours (95th percentile: 36 hours).
The advantages of radiopaque marker colon transit tests are the well established normal values and standardization of methods; it is also readily available and reasonably inexpensive. Although the radiopaque markers do not provide sufficient information on rapid transit, a method has been described with shorter intervals of marker ingestion (8 instead of 24 hours) and dividing the sum of the number of markers by 3 (84). Similarly, Sadik et al. have quantitated rapid colonic transit using radiopaque markers (85).
Disadvantages include the radiation exposure and multiple visits for the 1 to 3 abdominal x-rays that may be required. It is also possible that the solid markers may move through the colon differently than stool. The localization of the markers relative to bone landmarks may not reflect their true position in the colon. These criticisms notwithstanding, the radiopaque markers are the reference standard for colon transit evaluation in clinical practice (86).
Colonic Scintigraphy
Colonic transit scintigraphy is a safe and noninvasive method that has been shown to correlate with radiopaque markers. Scintigraphy provides information on proximal (ascending colon emptying) and overall colon transit (87). In the most widely published method, subjects ingest a pH sensitive methacrylate-coated capsule containing 111indium-labeled activated charcoal particles after an overnight fast. The coated capsule dissolves upon reaching the alkaline terminal ileum, releasing the radioisotope into the lumen (61,88). The alternative method involves radiolabeling the liquid phase (water) of a standard solid-liquid meal with 111indium diethylene triamine pentaacetic acid (111In-DTPA). The transit of the radiolabeled water is then used to assess small bowel and colonic transit (52).
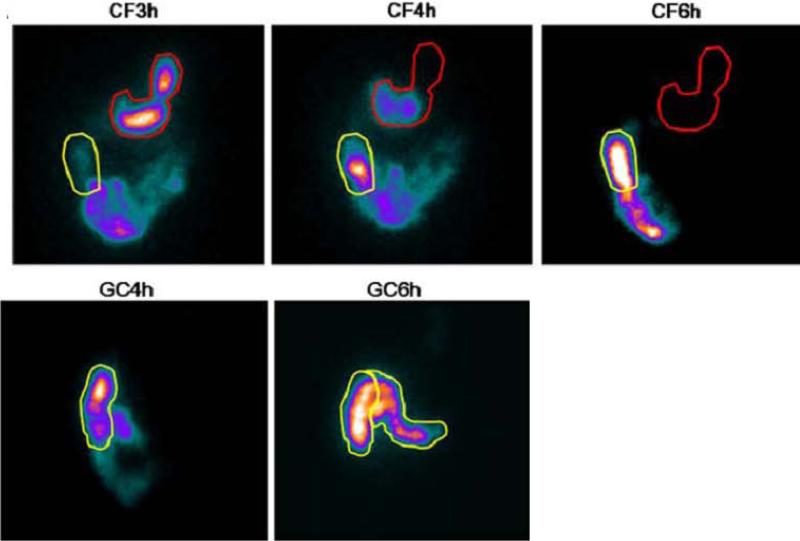
Regardless of how the scintigraphic material is delivered to the colon, the subject undergoes repeated scanning with a gamma camera to obtain anterior and posterior abdominal scans of 2 minutes duration each, at 4, 6, 8, 24, and 48 hours post-ingestion to appraise colonic transit [(89) Figure 1].
Figure 1.
Ileocolonic transit demonstrating the arrival of radiolabel in the right colon, and movement of isotope from the ascending to the transverse colon in response to meal ingestion at 4 hours. Reproduced from Deiteren A, Camilleri M, Burton D, et al: Effect of meal ingestion on ileocolonic and colonic transit in health and irritable bowel syndrome. Dig Dis Sci 2010;55:384-391.
A variable region of interest program is used to quantitate counts in each colonic segment: ascending colon (AC), transverse colon (TC), descending colon (DC) and rectosigmoid (RS), numbered as segments 1 to 4, respectively. Segment 5 refers to the expelled radioactivity in stool. In the 111In-DTPA labeled water method, the geometric center is based on 6 colonic regions (4 main regions plus hepatic and splenic flexures) and stool (52).
There are two primary endpoints to summarize colonic transit. Overall colonic transit is expressed as the numeric value of the geometric center, which is the weighted average of the isotope distribution within the colon and stool and is calculated by the sum of the products of the proportion of 111In counts in each segment and its weighting factor as follows (61): geometric center = [(%ascending colon × 1) + (%transverse colon × 2) + (%descending colon × 3) + (%rectosigmoid × 4) + (%stool × 5) ] / 100]. This formula would be expanded to include the 7 regions used with the 111In-DTPA labeled water method. The geometric center can be determined for every time point that a scan is obtained, and the times of greatest interest are at 24, 48 and 72 hours. The ascending colon emptying endpoint is summarized as the time for emptying half of the ascending colon (T1/2), which is calculated by linear interpolation of values on the ascending colon emptying curve (88). The delayed release capsule facilitates this measurement by delivering the radiolabeled charcoal to the ileocolonic region before capsule dissolution allows the particles to disperse. Since the emptying of the ileum into the colon occurs as bolus movements (90), there is relatively clear accumulation of 111In-charcoal in the ascending colon. At Temple University, the 111In-DTPA labeled water method is used with inclusion of a 72 hour image: isolated retention of isotope in the rectosigmoid colon is considered to be suggestive of functional rectal outlet obstruction among a group of 73 patients with constipation (52). Unpublished data from Mayo Clinic in 390 patients with documented evacuation disorders, and 61 with slow transit constipation without evacuation disorder confirm the findings at Temple University and show rectal evacuation disorder is associated with delayed overall colon transit at 48hours and ascending colon emptying t1/2 compared to health, and that distal colon and stool content differentiates evacuation disorder from slow transit constipation (Nullens, Nelsen, Camilleri et al , unpublished observation).
Normal values for scintigraphic colon transit have been established in 37 healthy volunteers with geometric center at four hours less than or equal to 1.4, geometric center at 24 hours between 1.7 and 4.0, and geometric center at 48 hours between 3.0 and 4.8 (87,91-93). Patients with geometric centers less than the above values are considered to have slow transit, and those with greater values are classified as accelerated transit.
Colonic transit measured by scintigraphy, especially proximal colon emptying, is significantly correlated with 24 hour stool weight (92), fecal consistency and bowel movement frequency (63).
Performance characteristics of scintigraphic colonic transit have also been assessed in healthy subjects and in patients with irritable bowel syndrome. In 21 healthy volunteers who underwent two separate scintigraphic colonic transit assessments 3 weeks apart, the interindividual coefficients of variation were 37% at 24 hours and 24% at 48 hours. The intraindividual coefficients of variation were predictably lower at 28% at 24 hours and 14% at 48 hours (94). In 86 patients with constipation-irritable bowel syndrome and 17 healthy volunteers, intraindividual coefficients of variation were 31% at 24 hours and 27% at 48 hours over a period of less than 3 weeks, and 38% at 24 hours and 30% at 48 hours over a median interval of two years (63).
The data suggest that the degree of variation is similar across different mean values of colonic transit, and the vast majority of individuals have replicate values within 1 geometric center unit of baseline measurement. The measured coefficient of variation reflects the physiological variation in colonic motor function that also manifests as natural variation in stool frequency and consistency.
Whole colonic scintigraphic transit and ascending colon emptying have been reported to be abnormal in several diseases of colonic motility including idiopathic constipation, functional diarrhea, carcinoid diarrhea (95), and different subtypes of irritable bowel syndrome, based on predominant bowel dysfunction [diarrhea, constipation, and mixed (96)]. It is, therefore, plausible that colonic transit measurement may serve as a biological marker of colonic function in disease and as a surrogate endpoint in the evaluation of drug therapy. For example, in the highly prevalent, multifactorial clinical condition of irritable bowel syndrome (96), 32% had abnormal colonic transit, with 16% of constipation-irritable bowel syndrome patients having slow transit and 46% with diarrhea-irritable bowel syndrome having fast transit. In addition, 14% of patients with mixed-irritable bowel syndrome had fast transit at 48 hours.
The measurement of scintigraphic colon transit in response to drugs in development has generally predicted the responses to treatment observed in phase IIB or III clinical trials (97). Thus, scintigraphic colonic transit has correctly predicted clinical efficacy with medications targeting different mechanisms including prokinetics such as 5-HT4 agonists, bisacodyl, neurotrophin-3 [(98) Figure 2]; medications retarding transit such as 5-HT3 antagonists, CCK1 antagonist; and secretagogues such as linaclotide, lubiprostone. Equally important, the colonic transit measurement has correctly predicted lack of efficacy of medications to alter bowel dysfunction in IBS in clinical trials when there were no significant effects of the drug on colonic transit. Examples include a CRF1-antagonist (99) and solabegron, a β3-adrenergic agonist (100).
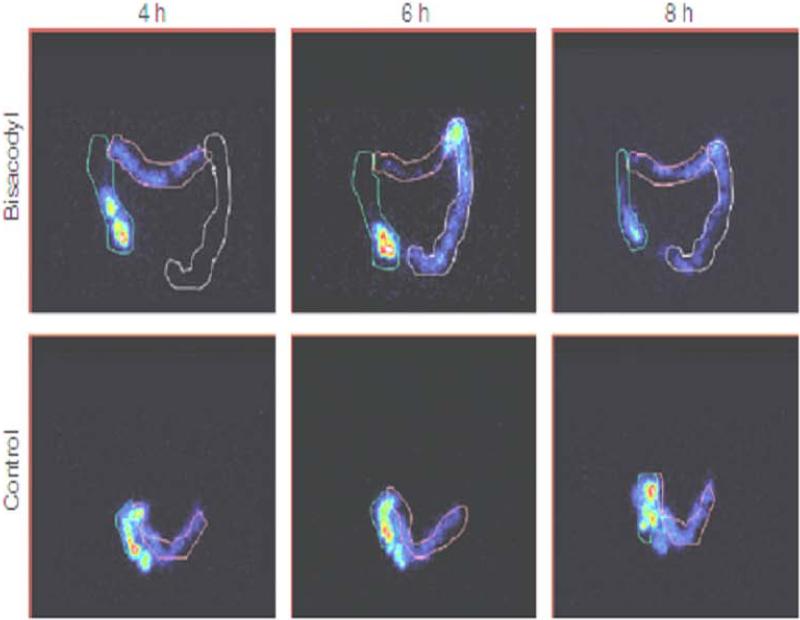
Figure 2.
Effect of the colonic stimulant laxative bisacodyl on colonic transit. Note the acceleration of transit of isotope through the colon with bisacodyl whereas, control treatment is associated with retention of isotope predominantly in the ascending and tortuous transverse colon. Reproduced from Manabe N, Cremonini F, Camilleri M, et al: Effects of bisacodyl on ascending colon emptying and overall colonic transit in healthy volunteers. Aliment Pharmacol Ther 2009;30:930-936.
In summary, scintigraphic assessment of colonic transit provides a noninvasive, safe, and relatively rapid (24 or 48 hours) assessment of whole colon and regional colonic transit. The technique is well validated and used in clinical practice and in numerous research studies. Disadvantages include radiation exposure, expense, and the limited availability of radiopharmaceuticals, gamma camera detection equipment, and operator expertise in tertiary medical centers.
Wireless motility capsule colonic transit
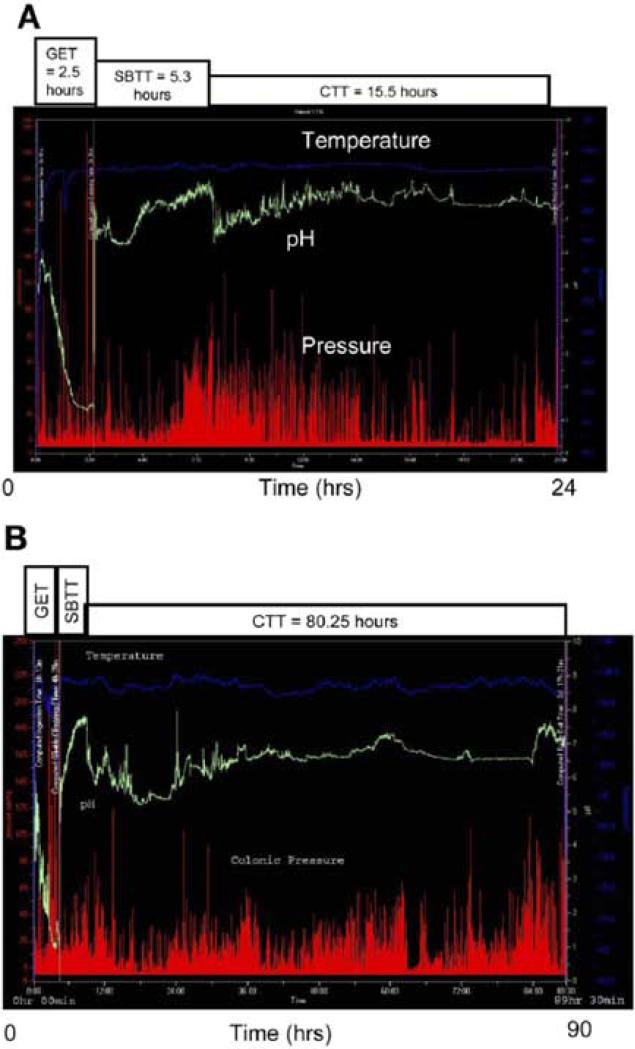
The basic attributes of the wireless motility capsule (WMC) and method of test administration were addressed previously (see Wireless motility capsule small bowel transit). The passage of the WMC into the cecum is determined by the sudden fall of pH by more than 1 unit which lasts for at least 5 minutes. The colonic transit time is the time from entry of the WMC into the cecum to the time the WMC passes out of the colon (Figure 3). The latter is determined by either the direct visualization (by the patient) of the device in the stool, a sudden drop in the temperature reading accompanied by loss of pressure recordings, the lack of WMC transmission to the receiver, or by noting the absence of the WMC on an abdominal x-ray.
Figure 3.
Wireless motility capsule recording transit in (A) healthy and (B) constipated individuals. Reproduced from Rao SS, Kuo B, McCallum RW, et al: Investigation of colonic and whole-gut transit with wireless motility capsule and radiopaque markers in constipation. Clin Gastroenterol Hepatol 2009;7:537–544
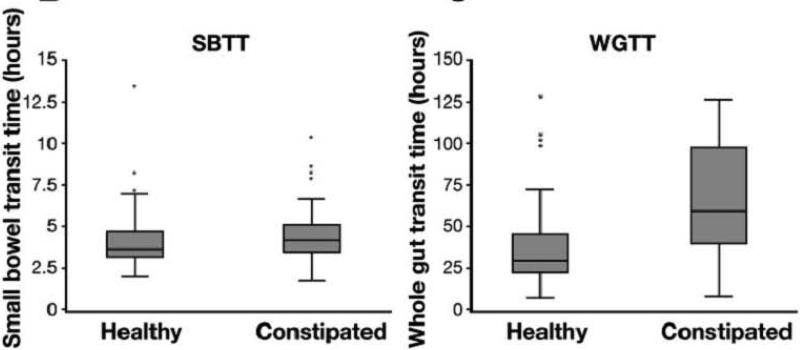
Colonic transit time assessed by WMC in 78 constipated and 87 healthy subjects (79) demonstrated a median value of 21.7 hours (IQR, 15.5-37.3 hours) in healthy subjects and 46.7 hours (IQR, 24.0-91.9 hours) in constipated patients, and small bowel and whole gut transit were delayed as well (Figure 4). The correlation of the WMC to percent of radiopaque markers retained on day 5 was r = 0.69 (p<0.001) in patients with constipation studied simultaneously with both methods. The upper limit of normal WMC colon transit time, based upon the 95th percentile of the normal subjects, was identified as 59 hours (79).
Figure 4.
Small bowel and whole gut transit times are prolonged in constipated participants compared to healthy controls. Reproduced from Rao SS, Kuo B, McCallum RW, et al: Investigation of colonic and whole-gut transit with wireless motility capsule and radiopaque markers in constipation. Clin Gastroenterol Hepatol 2009;7:537–544
In a subsequent large, multicenter study of 158 patients with constipation, the colonic transit assessment with WMC was compared with simultaneous radiopaque marker study, and there was overall agreement of 87% for classifying subjects as having slow or normal colonic transit (77). The WMC has also been used to characterize pressure activity in health and in constipated patients (101), although the clinical relevance of this has not been established.
The advantages of WMC assessment of colonic transit are the well validated method, standardization with automated analysis, and provision of a global assessment of gastrointestinal transit similar to scintigraphy. The WMC transit study is performed predominantly in the patients’ usual surroundings and does not require return visits for imaging. Also, there is no radiation exposure. Drawbacks include occasional difficulty with swallowing the 26 × 13 mm capsule, rare technical failure of device, difficulty with discernment of the 1 unit decrease in pH that marks the entry into the cecum, the potential for prolonged retention, and the relatively greater cost than the standard radiopaque marker study.
Acknowledgments
We thank Mrs. Cindy Stanislav for excellent secretarial support.
Support & Grants: Dr. Camilleri is supported in part by grants RO1-DK-1RC1-DK086182, R01-DK-079866 and RO1-DK-67071 from National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: No conflicts of interest exist.
References
- 1.Bayliss WM, Starling EH. The movements and innervation of the small intestine. J Physiol. 1899;24:99–143. doi: 10.1113/jphysiol.1899.sp000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kerlin P, Zinsmeister A, Phillips S. Relationship of motility to flow of contents in the human small intestine. Gastroenterology. 1982;82:701–706. [PubMed] [Google Scholar]
- 3.Tremolaterra F, Villoria A, Serra J, et al. Intestinal tone and gas motion. Neurogastroenterol Motil. 2006;18:905–910. doi: 10.1111/j.1365-2982.2006.00809.x. [DOI] [PubMed] [Google Scholar]
- 4.Plavsic BM, Robinson AE, Jeffrey RB. Gastrointestinal Radiology. McGraw-Hill; New York, NY: 1992. [Google Scholar]
- 5.Lönnerblad L. Transit time through small intestine: roentgenologic study on normal variability. Acta Radiologica - Supplementum. 1951;88:1–85. [PubMed] [Google Scholar]
- 6.Thompson WM, Halvorsen RA, Shaw M, et al. Evaluation of intramuscular ceruletide for shortening small bowel transit time. Gastrointest Radiol. 1982;7:141–147. doi: 10.1007/BF01887628. [DOI] [PubMed] [Google Scholar]
- 7.Kim SK. Small intestine transit time in the normal small bowel study. AJR. 1968;104:522–524. doi: 10.2214/ajr.104.3.522. [DOI] [PubMed] [Google Scholar]
- 8.Richards DG, Stevenson GW. Laxatives prior to small bowel follow-through: are they necessary for a rapid and good-quality examination? Gastrointest Radiol. 1990;15:66–68. doi: 10.1007/BF01888739. [DOI] [PubMed] [Google Scholar]
- 9.Summers DS, Roger MD, Allan PL, et al. Accelerating the transit time of barium sulphate suspensions in small bowel examinations. Eur J Radiol. 2007;62:122–125. doi: 10.1016/j.ejrad.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 10.Sampson MA, deLacey G, Twomey B, et al. The small bowel follow-through: time to sit up. Clin Radiol. 1994;49:478–487. doi: 10.1016/s0009-9260(05)81746-5. [DOI] [PubMed] [Google Scholar]
- 11.Brady AP. Use of effervescent agents in small bowel meal examination. Clin Radiol. 1994;49:434–435. doi: 10.1016/s0009-9260(05)81837-9. [DOI] [PubMed] [Google Scholar]
- 12.Fraser GM, Adam RD. Modifications to the gas-enhanced small bowel barium follow-through using Gastrografin and compression. Clin Radiol. 1988;39:537–554. doi: 10.1016/s0009-9260(88)80230-7. [DOI] [PubMed] [Google Scholar]
- 13.Paul N, Rawlinson J, Keir M. The use of metoclopramide for the small bowel meal examination: pre-procedural versus peri-procedural oral administration. Br J Radiol. 1996;69:1130–1133. doi: 10.1259/0007-1285-69-828-1130. [DOI] [PubMed] [Google Scholar]
- 14.James WB, Hume R. Action of metoclopramide on gastric emptying and small bowel transit time. Gut. 1968;9:203–205. doi: 10.1136/gut.9.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.James WB, Melrose AG. Metoclopramide in gastro-intestinal radiology. Clin Radiol. 1969;20:57–60. doi: 10.1016/s0009-9260(69)80059-0. [DOI] [PubMed] [Google Scholar]
- 16.Bond JH, Levitt MP. Investigation of small bowel transit time in man utilizing pulmonary hydrogen measurements. J Lab Clin Med. 1975;85:546–555. [PubMed] [Google Scholar]
- 17.Camboni G, Basilisco G, Bozzani A, et al. Repeatability of lactulose hydrogen breath test in subjects with normal or prolonged orocecal transit. Dig Dis Sci. 1988;33:1525–1527. doi: 10.1007/BF01535941. [DOI] [PubMed] [Google Scholar]
- 18.Hirakawa M, Iida M, Kohrogi N, et al. Hydrogen breath test assessment of orocecal transit time: comparison with barium meal study. Am J Gastroenterol. 1988;83:1361–1363. [PubMed] [Google Scholar]
- 19.Yu D, Cheeseman F, Vanner S. Combined oro-caecal scintigraphy and lactulose hydrogen breath testing demonstrate that breath testing detects oro-caecal transit, not small intestinal bacterial overgrowth in patients with IBS. Gut. 2011;60:334–340. doi: 10.1136/gut.2009.205476. [DOI] [PubMed] [Google Scholar]
- 20.Sciarretta G, Furno A, Mazzoni M, et al. Lactulose hydrogen breath test in orocecal transit assessment. Critical evaluation by means of scintigraphic method. Dig Dis Sci. 1994;39:1505–1510. doi: 10.1007/BF02088056. [DOI] [PubMed] [Google Scholar]
- 21.van Nieuwenhoven MA, Kovacs EM, Brummer RJ, et al. The effect of different dosages of guar gum on gastric emptying and small intestinal transit of a consumed semisolid meal. J Am Coll Nutr. 2001;20:87–91. doi: 10.1080/07315724.2001.10719019. [DOI] [PubMed] [Google Scholar]
- 22.Ternent CA, Thorson AG, Blatchford GJ, et al. Mouth to pouch transit after restorative proctocolectomy: hydrogen breath analysis correlates with scintigraphy. Am J Gastroenterol. 2001;96:1460–1463. doi: 10.1111/j.1572-0241.2001.03799.x. [DOI] [PubMed] [Google Scholar]
- 23.Staniforth DH. Effect of drugs on oro-caecal transit time assessed by the lactulose/breath hydrogen method. Eur J Clin Pharmacol. 1987;33:55–58. doi: 10.1007/BF00610380. [DOI] [PubMed] [Google Scholar]
- 24.Yuan CS, Foss JF, O'Connor M, et al. Gut motility and transit changes in patients receiving long-term methadone maintenance. J Clin Pharmacol. 1998;38:931–935. doi: 10.1002/j.1552-4604.1998.tb04389.x. [DOI] [PubMed] [Google Scholar]
- 25.Yuan CS, Foss JF, Osinski J, et al. The safety and efficacy of oral methylnaltrexone in preventing morphine-induced delay in oral-cecal transit time. Clin Pharmacol Ther. 1997;61:467–475. doi: 10.1016/S0009-9236(97)90197-1. [DOI] [PubMed] [Google Scholar]
- 26.Gorard DA, Libby GW, Farthing MJ. Effect of a tricyclic antidepressant on small intestinal motility in health and diarrhea-predominant irritable bowel syndrome. Dig Dis Sci. 1995;40:86–95. doi: 10.1007/BF02063948. [DOI] [PubMed] [Google Scholar]
- 27.Morali GA, Braverman DZ, Lissi J, et al. Effect of clonidine on gallbladder contraction and small bowel transit time in insulin-treated diabetics. Am J Gastroenterol. 1991;86:995–999. [PubMed] [Google Scholar]
- 28.Soares AC, Lederman HM, Fagundes-Neto U, et al. Breath hydrogen test after a bean meal demonstrates delayed oro-cecal transit time in children with chronic constipation. J Pediatr Gastroenterol Nutr. 2005;41:221–224. doi: 10.1097/01.mpg.0000167499.40074.d7. [DOI] [PubMed] [Google Scholar]
- 29.Ghoshal UC, Ghoshal U, Ayyagari A, et al. Tropical sprue is associated with contamination of small bowel with aerobic bacteria and reversible prolongation of orocecal transit time. J Gastroenterol Hepatol. 2003;18:540–547. doi: 10.1046/j.1440-1746.2003.03006.x. [DOI] [PubMed] [Google Scholar]
- 30.Lorena SL, de Souza Almeida JR, Mesquita MA. Orocecal transit time in patients with functional dyspepsia. J Clin Gastroenterol. 2002;35:21–24. doi: 10.1097/00004836-200207000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Iivonen MK, Ahola TO, Matikainen MJ. Bacterial overgrowth, intestinal transit, and nutrition after total gastrectomy. Comparison of a jejunal pouch with Roux-en-Y reconstruction in a prospective random study. Scand J Gastroenterol. 1998;33:63–70. doi: 10.1080/00365529850166220. [DOI] [PubMed] [Google Scholar]
- 32.Vajro P, Silano G, Longo D, et al. Orocoecal transit time in healthy and constipated children. Acta Paediatrica Scandinavica. 1988;77:583–586. doi: 10.1111/j.1651-2227.1988.tb10704.x. [DOI] [PubMed] [Google Scholar]
- 33.Cook GC. Delayed small-intestinal transit in tropical malabsorption. BMJ. 1978;2(6132):238–240. doi: 10.1136/bmj.2.6132.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Read NW, Miles CA, Fisher D, et al. Transit of a meal through the stomach, small intestine and colon in normal subjects and its role in the pathogenesis of diarrhea. Gastroenterology. 1980;79:1276–1282. [PubMed] [Google Scholar]
- 35.Miller MA, Parkman HP, Urbain JL, et al. Comparison of scintigraphy and lactulose breath hydrogen test for assessment of orocecal transit: lactulose accelerates small bowel transit. Dig Dis Sci. 1997;42:10–18. doi: 10.1023/a:1018864400566. [DOI] [PubMed] [Google Scholar]
- 36.Geboes KP, Luypaerts A, Rutgeerts P, et al. Inulin is an ideal substrate for a hydrogen breath test to measure the orocaecal transit time. Aliment Pharmacol Ther. 2003;18:721–729. doi: 10.1046/j.1365-2036.2003.01750.x. [DOI] [PubMed] [Google Scholar]
- 37.Schneider AR, Jepp K, Murczynski L, et al. The inulin hydrogen breath test accurately reflects orocaecal transit time. Eur J Clin Invest. 2007;37:802–807. doi: 10.1111/j.1365-2362.2007.01862.x. [DOI] [PubMed] [Google Scholar]
- 38.Heine WE, Berthold HK, Klein PD. A novel stable isotope breath test: 13C-labeled glycosyl ureides used as noninvasive markers of intestinal transit time. Am J Gastroenterol. 1995;90:93–98. [PubMed] [Google Scholar]
- 39.Geypens B, Bennink R, Peeters M, et al. Validation of the lactose-[13C]ureide breath test for determination of orocecal transit time by scintigraphy. J Nucl Med. 1999;40:1451–1455. [PubMed] [Google Scholar]
- 40.Ruemmele FM, Heine WE, Keller KM, et al. Metabolism of glycosyl ureides by human intestinal brush border enzymes. Biochimica et Biophysica Acta. 1997;1336:275–280. doi: 10.1016/s0304-4165(97)00037-8. [DOI] [PubMed] [Google Scholar]
- 41.Coremans G, Vos R, Margaritis V, et al. Small doses of the unabsorbable substance polyethylene glycol 3350 accelerate oro-caecal transit, but slow gastric emptying in healthy subjects. Dig Liver Dis. 2005;37:97–101. doi: 10.1016/j.dld.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 42.Priebe MG, Wachters-Hagedoorn RE, Landman K, et al. Influence of a subsequent meal on the oro-cecal transit time of a solid test meal. Eur J Clin Invest. 2006;36:123–126. doi: 10.1111/j.1365-2362.2006.01601.x. [DOI] [PubMed] [Google Scholar]
- 43.Cloetens L, De Preter V, Swennen K, et al. Dose-response effect of arabinoxylooligosaccharides on gastrointestinal motility and on colonic bacterial metabolism in healthy volunteers. J Am Coll Nutr. 2008;27:512–518. doi: 10.1080/07315724.2008.10719733. [DOI] [PubMed] [Google Scholar]
- 44.Van Den Driessche M, Van Malderen N, Geypens B, et al. Lactose-[13C]ureide breath test: a new, noninvasive technique to determine orocecal transit time in children. J Pediatr Gastroenterol Nutr. 2000;31:433–438. doi: 10.1097/00005176-200010000-00019. [DOI] [PubMed] [Google Scholar]
- 45.De Preter V, Verbeke K. Evaluation of the necessity of induction for lactose-[15N, 15N]-ureide to study the colonic ammonia metabolism. Scand J Gastroenterol. 2006;41:396–400. doi: 10.1080/00365520500279688. [DOI] [PubMed] [Google Scholar]
- 46.Sadik R, Abrahamsson H, Bjornsson E, et al. Etiology of portal hypertension may influence gastrointestinal transit. Scand J Gastroenterol. 2003;38:1039–1044. doi: 10.1080/00365520310004939. [DOI] [PubMed] [Google Scholar]
- 47.Stotzer PO, Abrahamsson H. Human postprandial gastric emptying of indigestible solids can occur unrelated to antral phase III. Neurogastroenterol Motil. 2000;12:415–419. doi: 10.1046/j.1365-2982.2000.00218.x. [DOI] [PubMed] [Google Scholar]
- 48.Sadik R, Abrahamsson H, Stotzer PO. Gender differences in gut transit shown with a newly developed radiological procedure. Scand J Gastroenterol. 2003;38:36–42. doi: 10.1080/00365520310000410. [DOI] [PubMed] [Google Scholar]
- 49.Pecchi A, De Santis M, Torricelli P, et al. Radiologic imaging of the transplanted bowel. Abdominal Imaging. 2005;30:548–563. doi: 10.1007/s00261-004-0288-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tomita R, Fujisaki S, Tanjoh K. Relationship between gastrointestinal transit time and daily stool frequency in patients after Ileal J pouch-anal anastomosis for ulcerative colitis. Am J Surg. 2004;187:76–82. doi: 10.1016/j.amjsurg.2002.12.002. [DOI] [PubMed] [Google Scholar]
- 51.Lin HC, Prather C, Fisher RS, et al. Measurement of gastrointestinal transit. Dig Dis Sci. 2005;50:989–1004. doi: 10.1007/s10620-005-2694-6. [DOI] [PubMed] [Google Scholar]
- 52.Bonapace ES, Maurer AH, Davidoff S, et al. Whole gut transit scintigraphy in the clinical evaluation of patients with upper and lower gastrointestinal symptoms. Am J Gastroenterol. 2000;95:2838–2847. doi: 10.1111/j.1572-0241.2000.03195.x. [DOI] [PubMed] [Google Scholar]
- 53.Krevsky B, Maurer AH, Niewiarowski T, et al. Effect of verapamil on human intestinal transit. Dig Dis Sci. 1992;37:919–924. doi: 10.1007/BF01300391. [DOI] [PubMed] [Google Scholar]
- 54.Camilleri M, Zinsmeister AR, Greydanus MP, et al. Towards a less costly but accurate test of gastric emptying and small bowel transit. Dig Dis Sci. 1991;36:609–615. doi: 10.1007/BF01297027. [DOI] [PubMed] [Google Scholar]
- 55.Argenyi EE, Soffer EE, Madsen MT, et al. Scintigraphic evaluation of small bowel transit in healthy subjects: inter- and intrasubject variability. Am J Gastroenterol. 1995;90:938–942. [PubMed] [Google Scholar]
- 56.Bennink R, Peeters M, Van den Maegdenbergh V, et al. Evaluation of small-bowel transit for solid and liquid test meal in healthy men and women. Eur J Nucl Med. 1999;26:1560–1566. doi: 10.1007/s002590050495. [DOI] [PubMed] [Google Scholar]
- 57.Camilleri M, Hasler WL, Parkman HP, et al. Measurement of gastrointestinal motility in the GI laboratory. Gastroenterology. 1998;115:747–762. doi: 10.1016/s0016-5085(98)70155-6. [DOI] [PubMed] [Google Scholar]
- 58.Maurer AH, Krevsky B. Whole-gut transit scintigraphy in the evaluation of small-bowel and colonic transit disorders. Semin Nucl Med. 1995;25:326–338. doi: 10.1016/s0001-2998(95)80006-9. [DOI] [PubMed] [Google Scholar]
- 59.Grybäck P, Jacobsson H, Blomquist L, et al. Scintigraphy of the small intestine: a simplified standard for study of transit with reference to normal values. Eur J Nucl Med Mol Imaging. 2002;29:39–44. doi: 10.1007/s00259-001-0687-z. [DOI] [PubMed] [Google Scholar]
- 60.Charles F, Camilleri M, Phillips SF, et al. Scintigraphy of the whole gut: clinical evaluation of transit disorders. Mayo Clin Proc. 1995;70:113–118. doi: 10.4065/70.2.113. [DOI] [PubMed] [Google Scholar]
- 61.Camilleri M, Zinsmeister AR. Towards a relatively inexpensive, noninvasive, accurate test for colonic motility disorders. Gastroenterology. 1992;103:36–42. doi: 10.1016/0016-5085(92)91092-i. [DOI] [PubMed] [Google Scholar]
- 62.Cremonini F, Mullan BP, Camilleri M, et al. Performance characteristics of scintigraphic transit measurements for studies of experimental therapies. Aliment Pharmacol Ther. 2002;16:1781–1790. doi: 10.1046/j.1365-2036.2002.01344.x. [DOI] [PubMed] [Google Scholar]
- 63.Deiteren A, Camilleri M, Bharucha AE, et al. Performance characteristics of scintigraphic colon transit measurement in health and irritable bowel syndrome and relationship to bowel functions. Neurogastroenterol Motil. 2010;22:415–423. e95. doi: 10.1111/j.1365-2982.2009.01441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Camilleri M, Brown ML, Malagelada JR. Impaired transit of chyme in chronic intestinal pseudoobstruction. Correction by cisapride. Gastroenterology. 1986;91:619–626. doi: 10.1016/0016-5085(86)90631-1. [DOI] [PubMed] [Google Scholar]
- 65.Camilleri M, Malagelada JR, Abell TL, et al. Effect of six weeks of treatment with cisapride in gastroparesis and intestinal pseudoobstruction. Gastroenterology. 1989;96:704–712. [PubMed] [Google Scholar]
- 66.Prather CM, Camilleri M, Zinsmeister AR, et al. Tegaserod accelerates orocecal transit in patients with constipation-predominant irritable bowel syndrome. Gastroenterology. 2000;118:463–468. doi: 10.1016/s0016-5085(00)70251-4. [DOI] [PubMed] [Google Scholar]
- 67.Balan K, Alwis L, Sonoda LI, et al. Utility of whole gut transit scintigraphy in patients with chronic gastrointestinal symptoms. Nucl Med Commun. 2010;31:328–333. doi: 10.1097/MNM.0b013e328335e5a9. [DOI] [PubMed] [Google Scholar]
- 68.Rao SS, Camilleri M, Hasler WL, et al. Evaluation of gastrointestinal transit in clinical practice: position paper of the American and European Neurogastroenterology and Motility Societies. Neurogastroenterol Motil. 2011;23:8–23. doi: 10.1111/j.1365-2982.2010.01612.x. [DOI] [PubMed] [Google Scholar]
- 69.Kuo B, McCallum RW, Koch KL, et al. Comparison of gastric emptying of a nondigestible capsule to a radio-labelled meal in healthy and gastroparetic subjects. Aliment Pharmacol Ther. 2008;27:186–196. doi: 10.1111/j.1365-2036.2007.03564.x. [DOI] [PubMed] [Google Scholar]
- 70.Cassilly D, Kantor S, Knight LC, et al. Gastric emptying of a non-digestible solid: assessment with simultaneous SmartPill pH and pressure capsule, antroduodenal manometry, gastric emptying scintigraphy. Neurogastroenterol Motil. 2008;20:311–319. doi: 10.1111/j.1365-2982.2007.01061.x. [DOI] [PubMed] [Google Scholar]
- 71.Kloetzer L, Chey WD, McCallum RW, et al. Motility of the antroduodenum in healthy and gastroparetics characterized by wireless motility capsule. Neurogastroenterol Motil. 2010;22:527–533. e117. doi: 10.1111/j.1365-2982.2010.01468.x. [DOI] [PubMed] [Google Scholar]
- 72.Sarr MG, Kelly KA. Patterns of movement of liquids and solids through canine jejunum. Am J Physiol. 1980;239:G497–G503. doi: 10.1152/ajpgi.1980.239.6.G497. [DOI] [PubMed] [Google Scholar]
- 73.Quigley EM, Borody TJ, Phillips SF, et al. Motility of the terminal ileum and ileocecal sphincter in healthy humans. Gastroenterology. 1984;87:857–866. [PubMed] [Google Scholar]
- 74.Parkman HP, McCallum RW, Rao SSC. GI Motility Testing. Slack Inc; Thorofare, NJ: 2011. [Google Scholar]
- 75.Zarate N, Mohammed SD, O'Shaughnessy E, et al. Accurate localization of a fall in pH within the ileocecal region: validation using a dual-scintigraphic technique. Am J Physiol. 2010;299:G1276–G1286. doi: 10.1152/ajpgi.00127.2010. [DOI] [PubMed] [Google Scholar]
- 76.Sarosiek I, Selover KH, Katz LA, et al. The assessment of regional gut transit times in healthy controls and patients with gastroparesis using wireless motility technology. Aliment Pharmacol Ther. 2010;31:313–322. doi: 10.1111/j.1365-2036.2009.04162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Camilleri M, Thorne NK, Ringel Y, et al. Wireless pH-motility capsule for colonic transit: prospective comparison with radiopaque markers in chronic constipation. Neurogastroenterol Motil. 2010;22:874–882. e233. doi: 10.1111/j.1365-2982.2010.01517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Timm D, Willis H, Thomas W, et al. The use of a wireless motility device (SmartPill®) for the measurement of gastrointestinal transit time after a dietary fibre intervention. Br J Nutr. 2011;105:1337–1342. doi: 10.1017/S0007114510004988. [DOI] [PubMed] [Google Scholar]
- 79.Rao SS, Kuo B, McCallum RW, et al. Investigation of colonic and whole-gut transit with wireless motility capsule and radiopaque markers in constipation. Clin Gastroenterol Hepatol. 2009;7:537–544. doi: 10.1016/j.cgh.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 80.Hinton JM, Lennard-Jones JE, Young AC. A new method for studying gut transit times using radioopaque markers. Gut. 1969;10:842–847. doi: 10.1136/gut.10.10.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Arhan P, Devroede G, Jehannin B, et al. Segmental colonic transit time. Dis Colon Rectum. 1981;24:625–629. doi: 10.1007/BF02605761. [DOI] [PubMed] [Google Scholar]
- 82.Metcalf AM, Phillips SF, Zinsmeister AR, et al. Simplified assessment of segmental colonic transit. Gastroenterology. 1987;92:40–47. doi: 10.1016/0016-5085(87)90837-7. [DOI] [PubMed] [Google Scholar]
- 83.Evans RC, Kamm MA, Hinton JM, et al. The normal range and a simple diagram for recording whole gut transit time. Intl J Colorectal Dis. 1992;7:15–17. doi: 10.1007/BF01647654. [DOI] [PubMed] [Google Scholar]
- 84.Anuras S, editor. Motility Disorders of the Gastrointestinal Tract: Principles and Practice. Raven Press; New York, NY: 1992. p. 129. [Google Scholar]
- 85.Sadik R, Stotzer PO, Simrén M, et al. Gastrointestinal transit abnormalities are frequently detected in patients with unexplained GI symptoms at a tertiary centre. Neurogastroenterol Motil. 2008;20:197–205. doi: 10.1111/j.1365-2982.2007.01025.x. [DOI] [PubMed] [Google Scholar]
- 86.Rao SSC. Constipation: evaluation and treatment of colonic and anorectal motility disorders. GE Clin NA. 2007;36:687–711. doi: 10.1016/j.gtc.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 87.Proano M, Camilleri M, Phillips SF, et al. Transit of solids through the human colon: regional quantification in the unprepared bowel. Am J Physiol. 1990;258:G856–G862. doi: 10.1152/ajpgi.1990.258.6.G856. [DOI] [PubMed] [Google Scholar]
- 88.Burton DD, Camilleri M, Mullan BP, et al. Colonic transit scintigraphy labeled activated charcoal compared with ion exchange pellets. J Nucl Med. 1997;38:1807–1810. [PubMed] [Google Scholar]
- 89.Deiteren A, Camilleri M, Burton D, et al. Effect of meal ingestion on ileocolonic and colonic transit in health and irritable bowel syndrome. Dig Dis Sci. 2010;55:384–391. doi: 10.1007/s10620-009-1041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Spiller RC, Brown ML, Phillips SF. Emptying of the terminal ileum in intact humans. Influence of meal residue and ileal motility. Gastroenterology. 1987;92:724–729. doi: 10.1016/0016-5085(87)90024-2. [DOI] [PubMed] [Google Scholar]
- 91.Stivland T, Camilleri M, Vassallo M, et al. Scintigraphic measurement of regional gut transit in idiopathic constipation. Gastroenterology. 1991;101:107–115. doi: 10.1016/0016-5085(91)90466-x. [DOI] [PubMed] [Google Scholar]
- 92.Vassallo M, Camilleri M, Phillips SF, et al. Transit through the proximal colon influences stool weight in the irritable bowel syndrome. Gastroenterology. 1992;102:102–108. doi: 10.1016/0016-5085(92)91789-7. [DOI] [PubMed] [Google Scholar]
- 93.Degen LP, Phillips SF. How well does stool form reflect colonic transit? Gut. 1996;39:109–113. doi: 10.1136/gut.39.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cremonini F, Mullan BP, Camilleri M, et al. Performance characteristics of scintigraphic transit measurements for studies of experimental therapies. Aliment Pharm Ther. 2002;16:1781–1790. doi: 10.1046/j.1365-2036.2002.01344.x. [DOI] [PubMed] [Google Scholar]
- 95.von der Ohe M, Camilleri M, Kvols LK, et al. Motor dysfunction of the small bowel and colon in patients with the carcinoid syndrome and diarrhea. N Engl J Med. 1993;329:1073–1078. doi: 10.1056/NEJM199310073291503. [DOI] [PubMed] [Google Scholar]
- 96.Camilleri M, McKinzie S, Busciglio I, et al. Prospective study of motor, sensory, psychologic, and autonomic functions in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2008;6:772–781. doi: 10.1016/j.cgh.2008.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Camilleri M. Scintigraphic biomarkers for colonic dysmotility. Clin Pharmacol Ther. 2010;87:748–753. doi: 10.1038/clpt.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Manabe N, Cremonini F, Camilleri M, et al. Effects of bisacodyl on ascending colon emptying and overall colonic transit in healthy volunteers. Aliment Pharmacol Ther. 2009;30:930–936. doi: 10.1111/j.1365-2036.2009.04118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sweetser S, Camilleri M, Linker Nord SJ, et al. Do corticotropin releasing factor-1 receptors influence colonic transit and bowel function in women with irritable bowel syndrome? Am J Physiol. 2009;296:G1299–G1306. doi: 10.1152/ajpgi.00011.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Grudell AB, Camilleri M, Jensen KL, et al. Dose-response effect of a beta3-adrenergic receptor agonist, solabegron, on gastrointestinal transit, bowel function, and somatostatin levels in health. Am J Physiol. 2008;294:G1114–G1119. doi: 10.1152/ajpgi.00051.2008. [DOI] [PubMed] [Google Scholar]
- 101.Hasler WL, Saad RJ, Rao SR, et al. Heightened colon motor activity measured by a wireless capsule in patients with constipation: relation to colon transit and IBS. Am J Physiol. 2009;297:G1107–G1114. doi: 10.1152/ajpgi.00136.2009. [DOI] [PubMed] [Google Scholar]