Abstract
Objective
The purpose of this study was to determine whether long-term electrical stimulation training of the paralyzed soleus could change this muscle’s physiological properties (torque, fatigue index, potentiation index, torque-time integral) and increase tibia bone mineral density.
Methods
Four men with chronic (>2 years) complete spinal cord injury (SCI; American Spinal Injury Association classification A) trained 1 soleus muscle using an isometric plantar flexion electrical stimulation protocol. The untrained limb served as a within-subject control. The protocol involved ~30 minutes of training each day, 5 days a week, for a period of 6 to 11 months. Mean compliance over 11 months of training was 91% for 3 subjects. A fourth subject achieved high compliance after only 5 months of training. Mean estimated compressive loads delivered to the tibia were ~110% of body weight. Over the 11 months of training, the muscle plantar flexion torque, fatigue index, potentiation index, and torque-time integral were evaluated periodically. Bone mineral density (dual-energy x-ray absorptiometry) was evaluated before and after the training program.
Results
The trained limb fatigue index, potentiation index, and torque-time integral showed rapid and robust training effects (P < .05). Soleus electrical stimulation training yielded no changes to the proximal tibia bone mineral density, as measured by dual-energy x-ray absorptiometry. The subject with low compliance experienced fatigue index and torque-time integral improvements only when his compliance surpassed 80%. In contrast, his potentiation index showed adaptations even when compliance was low.
Conclusions
These findings highlight the persistent adaptive capabilities of chronically paralyzed muscle but suggest that preventing musculoskeletal adaptations after SCI may be more effective than reversing changes in the chronic condition.
Keywords: Paralysis, Bone density, Muscle, Plasticity, Fatigue
The musculoskeletal system experiences extensive physiologic changes after spinal cord injury (SCI). Muscle paralyzed by an upper motor neuron lesion undergoes profound atrophy and gradual transformation to a fast-fatigable phenotype.1,2 In the chronically paralyzed state (>2 years), the soleus muscle demonstrates decreased oxidative enzymes (SDH, NADH),1 extensive fatigue,2,3 and decreased cross-sectional area.4 Deprived of its principal source of loading (voluntary muscle contraction5), bone rapidly demineralizes and osteoporosis develops in paralyzed extremities.6–9 The lifetime risk of extremity fracture for people with SCI is approximately double the risk for the able-bodied population.10
Despite the extensive changes that occur after SCI, evidence is accumulating that paralyzed tissues still adapt according to the overload principle and the specificity of training principle. Electrical stimulation training can improve the torque output11–13 and fatigue resistance13 of paralyzed muscle. As with neurologically intact muscle, repetitive muscular overload elicits the greatest improvements in muscle torque and endurance.11,12,14 We have recently demonstrated that the acutely paralyzed soleus muscle vigorously adapts to electrically elicited repetitive muscle contractions that generate muscular overload (~60% of the muscle’s maximal capacity).13,15,16 After 3 years, trained soleus muscles retained high torque-generating capacity and fatigue resistance, while the contralateral untrained muscles experienced typical post-SCI physiologic decline. The trained soleus muscles exhibited contractile speed characteristics that were typical of functionally slow muscle, similar to the nonparalyzed soleus.
Previous electrical stimulation training studies indicate that chronically paralyzed muscle (>2 years post-SCI) does indeed undergo hypertrophy12,17–20 and up-regulation of metabolic machinery12,18,21 in response to training. However, the influence of this training on the skeletal system after chronic SCI is not clear. Bone is believed to adapt according to the mechanostat principle,22 in which bone remodeling occurs when skeletal loads exceed a certain threshold. Several electrical muscle stimulation programs have only marginally influenced bone density,11,23,24 but the compressive dose of load delivered to the bone was not quantified. Our recent longitudinal soleus training program13 delivered compressive loads to the tibia that approximated 1 to 1.5 times body weight (BW).25 Early training in individuals with acute SCI attenuated the decline of tibia bone mineral density (BMD), yielding a 31% difference in trabecular bone density between trained and untrained limbs.13 Bone in chronically paralyzed extremities, on the other hand, has demonstrated less adaptive capability. Because of the extensive destruction of the trabecular lattice during post-SCI osteoporosis,26,27 it has been postulated that bone density loss after long-term paralysis may not be reversible.28
With the advent of a cure for SCI potentially on the horizon, it is imperative that we understand whether deleterious musculoskeletal adaptations after chronic SCI can be reversed. A thorough exploration of this issue has been hindered by the difficulties inherent to longitudinal research, a lack of quantifiable estimates of stress delivered to tissue, the use of protocols with excessive subject burden, and uncertainty that subjects received the prescribed dose of exercise (compliance). Based on our previous assessments of the soleus muscle,2,13,15,25,29 we designed an electrical stimulation training protocol to overload the muscle to increase torque production, to repetitively stress the muscle to increase its capacity for repetitive work, and to deliver loads to bone that approximated body weight (BW). Accordingly, the purpose of this study was to determine whether long-term electrical stimulation training of the chronically paralyzed soleus muscle could change the physiological properties of the soleus (peak torque [PT], fatigue index [FI], potentiation index [PI], and torque-time integral) and influence tibia BMD.
METHODS
Subjects
Four men with SCI participated in this longitudinal study (Table 1). The protocol was approved by the institution’s Human Subjects Institutional Review Board. All subjects provided written informed consent before participating. Inclusion criteria were a greater-than-2 year history of complete (American Spinal Injury Association classification A)30 spinal cord injury above T12 (as determined by neurological examination), passive ankle dorsiflexion to neutral, and passive knee flexion to at least 90° in a seated position. Exclusion criteria were pressure ulcers, lower motor neuron injury below T12 (which would prevent tibial nerve electrical activation), lower extremity trauma, or peripheral/systemic illness or infection.
Table 1.
Subject Demographics
| Subject | Spinal Cord Injury Level | Age | Years After Spinal Cord Injury | Trained Limb |
|---|---|---|---|---|
| 1 | T6 | 55 | 9.9 | Right |
| 2 | T1 | 37 | 11.8 | Right |
| 3 | T5 | 68 | 12.0 | Right |
| 4 | T7 | 49 | 2.0 | Left |
After an initial testing session (month 0), the subjects began a unilateral soleus electrical stimulation training protocol. Subjects were invited to train for a minimum of 6 months or a maximum of 11 months. At the end of 6 months, 3 subjects (subjects 1, 2, and 4) agreed to continue training through the remainder of the calendar year (month 11).
Test Apparatus and Protocol
Subjects remained in their wheelchairs during the test procedure. The ankle was stabilized in a system that measured isometric plantar flexion torque, as described previously.2,15,31 The knee was positioned at 90° of flexion, and the ankle was in neutral joint position. A nerve stimulation probe placed in the popliteal fossa delivered transcutaneous electrical impulses to the tibial nerve. The test position (flexed knee) minimized the contribution of the gastrocnemius to the plantar flexor torque.32 Stimulation was provided by a constant current electrical stimulator with a range of 0 to 200 mA at 400 V. It was triggered by digital pulses from a data-acquisition board (Metrabyte DAS 16F; Keithley Instruments Inc, Cleveland, Ohio) housed in a microcomputer under custom software control. The stimulation intensity was supramaximal (about 1.5 times the intensity required to produce a maximum compound muscle action potential). The stimulator delivered a 10-pulse train (15 Hz; 667-millisecond duration) every 2 seconds (Figure 1). Each stimulation bout included 125 trains.
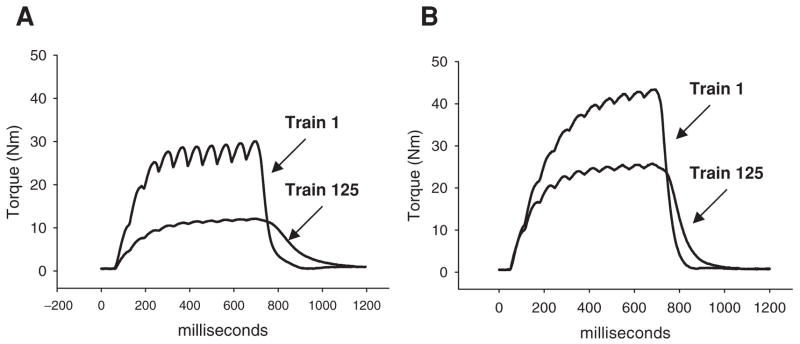
Figure 1.
Representative example of plantar flexion torque produced by 10-pulse stimulus trains during a 125-train fatigue bout (stimulation started with the muscle in a non-fatigued state). A) Subject 1, month 0; FI = 40%. B) Subject 1, month 11; FI = 58%. The area under the torque curve for train 125 is indicative of the minimum integral for each month.
Based on our previous work,15 we selected 15-Hz stimulation because it yields an unfused tetanic force that is approximately 60% of a completely fused tetanus. We reasoned that this level of muscle force would provide the necessary overload to induce muscle adaptations (as was observed in acute SCI subjects13). Likewise, based on our biomechanical model,25 this level of muscle contraction would yield a compressive load through the tibia of approximately 1 to 1.5 times BW, a stimulus believed to be sufficient to trigger bone remodeling.22 We used a Burke-like fatigue protocol (1-on:2-off work-rest cycle) to induce significant low-frequency fatigue without inducing neuromuscular transmission failure.29 On certain testing sessions, we delivered 4 fatigue bouts (each separated by 5 minutes of rest) to determine the magnitude of low-frequency fatigue and of subsequent postfatigue potentiation.16 These phenomena are believed to reflect the effectiveness of sarcoplasmic Ca2+ release33 and of myosin regulatory light chain phosphorylation,34–37 2 important components of the excitation-contraction process.
Training Protocol
Each subject trained the plantar flexor muscles of 1 limb. For purposes of training, all subjects activated the plantar flexors with reusable self-adhesive carbon electrodes. Two subjects (subjects 1 and 3) attended in-laboratory stimulation sessions 5 days per week for ~35 minutes per day. Each day, these subjects underwent 4 bouts of stimulation, each separated by a 5-minute rest period. Two other subjects (subjects 2 and 4) trained their limbs at home via a portable stimulator and a limb-constraining system identical to the one used in the laboratory. These 2 subjects returned to the laboratory for testing 2 to 4 times per month. Stimulation parameters were identical to parameters used in the laboratory sessions. The home-training subjects performed 4 stimulation bouts per day on 5 days each week, with 5 minutes of rest between each bout. The portable electrical stimulation system had a start-and-stop button at the subject interface. The stimulators were designed to operate when the subject’s skin impedance was recognized (based on a range previously established in the laboratory). The stimulators did not engage unless the electrodes were in place and the skin impedance matched that of the subject. Once activated, the preprogrammed stimulus frequency, intensity, repetitions, and duration were delivered unless the subject aborted the bout. A microprocessor and memory chip within the stimulator recorded the date, time, and number of stimulus pulses delivered to the subject. We were therefore able to precisely quantify each subject’s compliance with the home-based training protocol. The training protocol specified that 10000 electrically stimulated contractions be completed each month (4 bouts of 125 contractions per day × 5 days per week × 4 weeks = 10 000 contractions). Compliance was calculated as the percentage of the recommended number of contractions a subject completed in each month.
Data Processing
We present values from a single stimulation bout each month. The PT was defined as the highest torque produced during any of the 125 trains in a bout. The FI was computed according to the following formula: 100 × minimum torque in a bout/PT in a bout. Higher values indicate greater resistance to fatigue. For each stimulus train, we obtained the torque-time integral (Nm*s). The integral is influenced both by the PT and the temporal characteristics of the contraction (duration, degree of fusion, etc). We conceptualized the integral as an estimator of contractile work. We obtained the minimum integral (MI) for each bout of exercise to determine the amount of work being maintained after the development of fatigue.
During several testing sessions, we conducted 4 stimulation bouts, each separated by 5 minutes, to induce low-frequency fatigue. Upon resumption of stimulation, torque gradually increased, a process called postfatigue potentiation.16,38 We divided the PT of the fourth bout by the torque of the first contraction in the fourth bout. This yielded a PI, which served as an estimate of the degree of excitation-contraction coupling compromise present at the beginning of the fourth bout and the magnitude of torque recovery during potentiation.
To eliminate the offset in PT, FI, and MI among subjects, we normalized each of these variables to the pretraining (month 0) value. Thus, each of these variables is presented as a percentage of its pretraining value.
BMD Measurement
Subjects underwent dual x-ray absorptiometry (DEXA; Hologic QDR 2000 scanner; Hologic Inc, Waltham, Mass) scans of their proximal tibiae at the beginning and the end of the study. A radiology technician positioned each subject and collected each scan. Because the tibia is a nonstandard measurement site lacking routine clinical analysis algorithms, we used a custom protocol to measure tibia BMD.39 This method was shown to be highly reliable (intraclass correlation coefficients >0.90) and sensitive to tibia bone loss after paralysis.39 DEXA provided areal BMD in grams per centimeter squared.
Biomechanical Model: Calculation of Loading Dose
We used a biomechanical model25 to estimate the compressive loads delivered to the tibia via plantar flexor contraction. In brief, plantar flexion PT was divided by the moment arm of the Achilles tendon (6 cm during maximal voluntary contraction,40,41 yielding soleus contractile force). Because of the line of action of the Achilles tendon (parallel to the tibia), this force was transmitted as a compressive load to the tibia. Each subject’s compressive load was presented as a percentage of BW.
Calculation of Compliance
Compliance was calculated for each subject according to the following formula: (number of stimulations received/number of stimulations prescribed) × 100. To be included in the analysis, subjects needed to comply with at least 80% of the recommended dose. Subjects 1, 3, and 4 received 91.1% of the prescribed dose of 10000 contractions per month (~9110 contractions). Subject 2 had low compliance for the first 3 months, attaining only >80% compliance by month 5. Thus, subject 2 will be presented separately.
Statistical Analysis
A repeated-measures analysis of variance was used to test for significant effects across time for all dependent variables. Tukey post hoc analysis was used in the event that there was a significant main effect. The strength of the association between changes in FI and changes in the PI was established using a linear regression model and coefficient of determination (R2). Significance was set at P < .05 for all tests.
RESULTS
PT
A representative example of the training effect on torque appears in Figure 1. The mean (SE) normalized PT for subjects 1, 3, and 4 (the subjects who achieved high compliance) appears in Figure 2, while each subject’s PT is presented separately in Figure 3A. Trained-limb PT increased 19% and 31% by months 3 and 4, respectively (P < .05), after which there was no significant change. The 2 subjects who continued the protocol to 11 months showed no additional increase in PT between 4 and 11 months. Interestingly, mean (SE) untrained limb PT at month 6 also exceeded month 0 values (Figure 2). As depicted in Figure 3A, all 3 subjects with high compliance demonstrated enhancements in untrained limb PT during the course of the study.
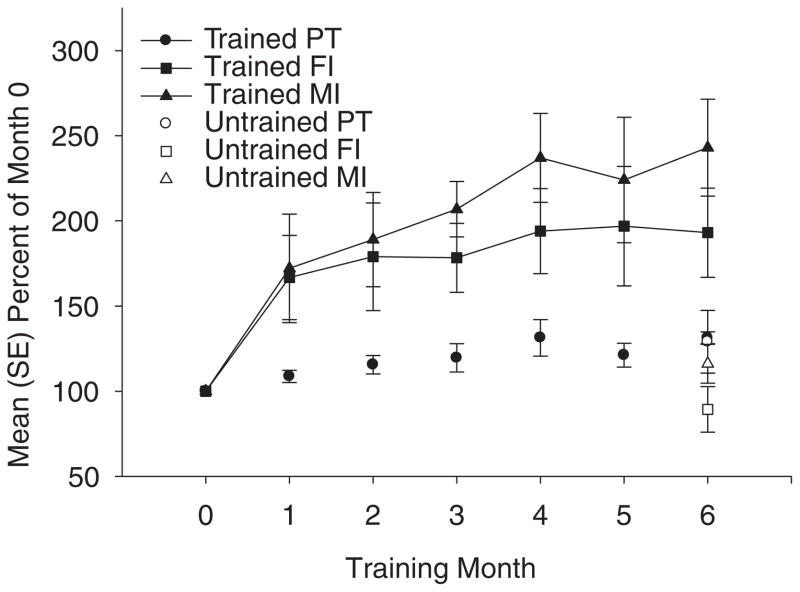
Figure 2.
Mean (SE) percentage of baseline (month 0) values for 3 subjects with high compliance (>80%). Two subjects continued training until month 11. Their month 11 data are presented individually (Figure 3). PT = peak torque; FI = fatigue index; MI = minimum integral.
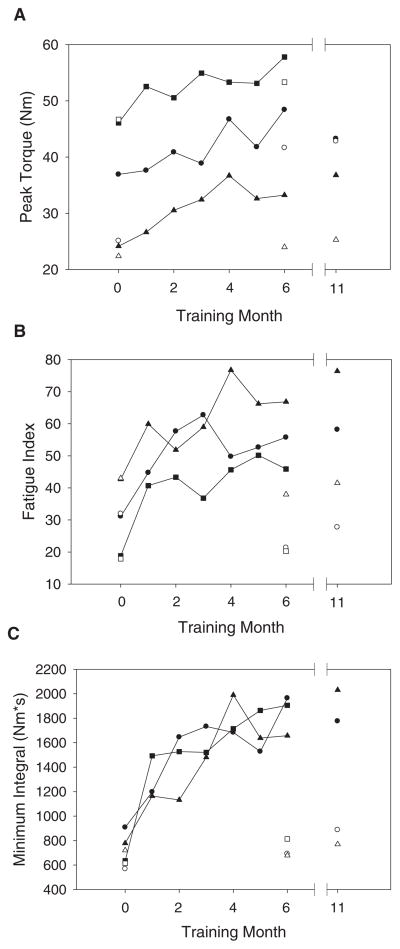
Figure 3.
A) Peak torque. B) Fatigue index. C) Minimum torque-time integral for 3 subjects with high compliance in training. Filled symbols represent the trained limbs, and unfilled symbols represent the untrained limbs. Circles, squares, and triangles represent subjects 1, 3, and 4, respectively (Table 1).
FI
A representative example of the training effect on FI appears in Figure 1. The mean (SE) normalized FI for subjects 1, 3, and 4 (the subjects who achieved high compliance) appears in Figure 2, while each subject’s individual FI is presented in Figure 3B. Group mean FI increased sharply during month 1, exceeding the baseline value by 66% (P < .05). A trend toward an increased FI continued until month 6, after which FI values stabilized. Examination of individual data reveals that the rapid increase in FI at month 1 occurred in all 3 subjects with high compliance (Figure 3B). By 11 months, the 2 subjects who continued to train showed consistent increases in FI when compared to the month 1 FI values.
The untrained limbs of the 3 subjects with high compliance showed no enhancements of FI at any point during the study (P = .32; Figure 2). Figure 3B illustrates the strong between-limb similarities in FI at the start of the study, followed by rapid differentiation between the trained and untrained limbs.
MI
A representative example of the training effect on minimum torque-time integral appears in Figure 1. The mean (SE) normalized MI for subjects 1, 3, and 4 (the subjects who achieved high compliance) appears in Figure 2, while each subject’s individual MI is presented in Figure 3C. Group mean MI increased sharply during month 1, exceeding the baseline value by 72% (P < .05). A trend toward increased MI continued over months 1 to 3, reaching significance by month 4. Thus, MI at months 4, 5, and 6 was significantly greater than MI at month 1 (P < .05). Examination of individual data reveals that the 2 subjects who continued to train beyond 6 months sustained the increased MI for 11 months (Figure 3C).
Similar to the FI data, MI differed widely between the trained and untrained limbs (P < .05; Figure 2). Again, Figure 3C illustrates the relative similarity in MI between limbs at the start of the study, followed by rapid differentiation between trained and untrained limbs.
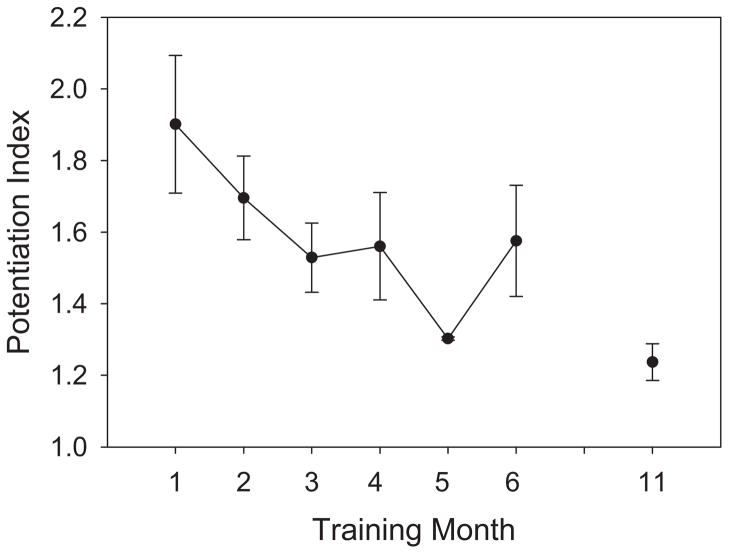
PI
A representative example of the training effect on postactivation potentiation appears in Figure 4. The 3 subjects with high compliance experienced a steady decline in PI over the first 3 months of training (Figure 5). PI was significantly lower than the month 1 value at months 3 to 6 and 11 (P < .05). Interestingly, 71% of the variance in the FI could be explained by the PI (R2 = 0.71).
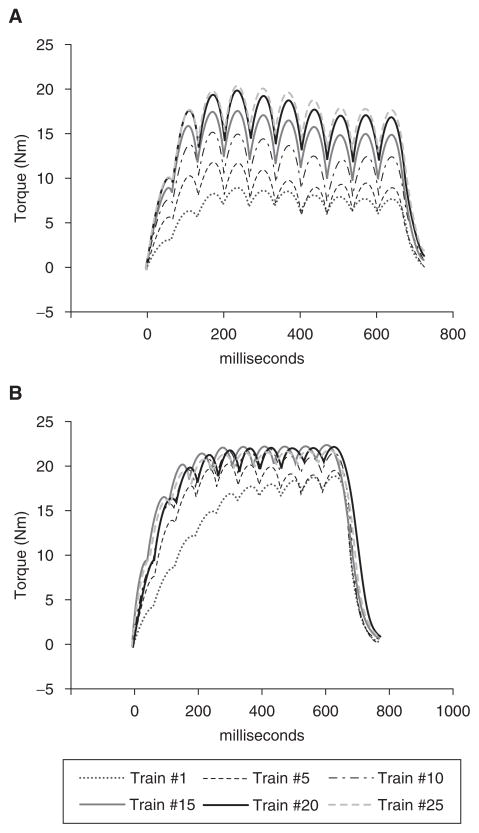
Figure 4.
Representative examples of plantar flexion torque produced by 10-pulse stimulus trains during the first 25 contractions of a 120-train fatigue bout performed 15 minutes after a fatigue protocol. A) Subject 1, month 0; potentiation index (PI) = 2.07. B) Subject 1, month 11; PI = 1.29.
Figure 5.
Mean (SE) potentiation index for 3 subjects with high compliance (>80%; months 1–6). Two subjects continued training until month 11.
Compliance Measures
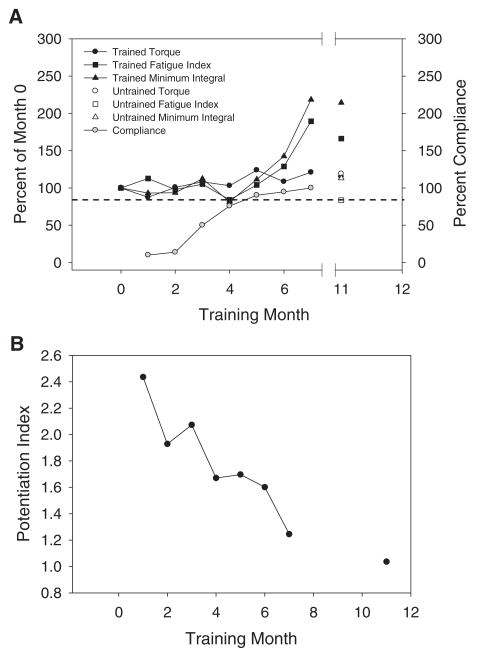
The 2 subjects that met the 80% compliance criterion had a mean compliance of 91.1% ± 3.6%. Subject 2 did not meet the threshold training compliance during the first 5 months of the study. Interestingly, this subject’s PT, FI, and MI remained unchanged until month 6, only after the 80% threshold level of compliance was met (Figure 6A). Similar to the subjects with high compliance, this subject experienced larger gains in FI and MI than in PT. Unexpectedly, this subject demonstrated reductions in PI with a minimal dose of training (Figure 6B).
Figure 6.
The single subject (subject 2 in Table 1) who demonstrated low compliance at the beginning of training. The dotted line represents 80% compliance. Data are given through month 7 to aid comparison between this subject’s magnitude of effect and that of the other 3 subjects. A) Torque, fatigue index, and minimum integral. B) Potentiation index.
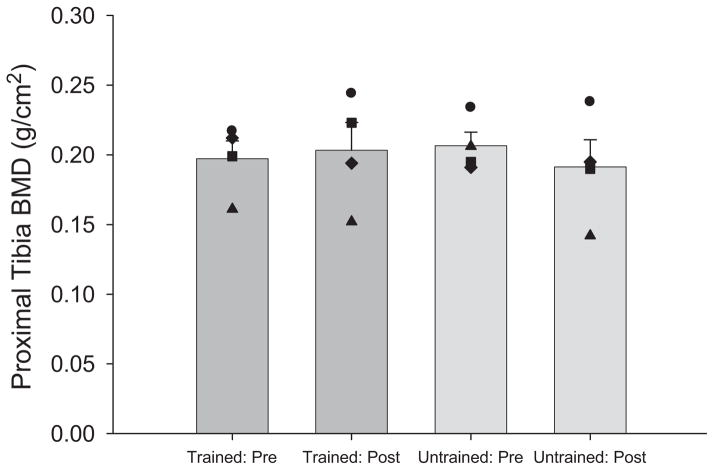
BMD
The mean estimated dose of compressive load to the tibia for the 3 subjects with high compliance increased from 89.2% ± 15.9% BW at the start of the study to 116.1% ± 17.9% BW by month 6 of training. Untrained limb BMD did not differ from trained limb BMD either before or after training (Figure 7). The BMD of the proximal tibia did not differ before and after training for either the trained or the untrained limb (P > .05; Figure 7). The trained limbs of 2 subjects demonstrated an approximately 0.02 g/cm2 gain in BMD. This degree of change in BMD would be unlikely to significantly alter the strength of the bone.
Figure 7.
Mean (SE) trained and untrained limb proximal tibia bone mineral density (BMD), before and after training. Black symbols represent each individual subject.
DISCUSSION
In 3 subjects with chronic spinal cord injury, 11 months of electrically stimulated soleus muscle training yielded a rapid and prolonged improvement in fatigue resistance. Similar improvements emerged for torque-time integral, an estimator of contractile work. Improvements in PT took longer to develop and were of lower magnitude than the other muscle physiology variables. The PI, an estimator of excitation-contraction compromise, declined steadily during the course of training. Proximal tibia BMD (as measured by DEXA) did not differ because of training.
FI
For subjects with high compliance, FI responded rapidly to the plantar flexor training protocol. Within the first month, FI exceeded the baseline value by 66%. Similar rapid increases have been observed in previous studies. Subjects with SCI who trained 1 tibialis anterior muscle (10 Hz) demonstrated FI values of ~39% before training and ~62% after 1 month of training.42 This magnitude of improvement (~75% over baseline) is in accord with the improvement observed by month 1 of the present study. Similarly, Gerrits and colleagues14 reported that after just 2 weeks of electrical stimulation training (15 Hz), quadriceps FI rose 43% above its baseline value.
Although up-regulation of mRNA for MHC-I and down-regulation of mRNA for MHC-IIX have been observed after 2 weeks of training,42 there is no evidence to suggest that these early changes correlate to muscle fatigability measures. Similarly, changes that would indicate enhanced oxidative metabolism, such as increased capillarization17 and citrate synthase levels,21 have not been reported within the first month of electrical stimulation training. Possible training-induced alterations to proteins involved in excitation-contraction coupling (sarco[endo]plasmic reticulum Ca2+-ATPase, ryanodine, dihydropyridine, myosin light chain kinase, etc) may be involved but have yet to be confirmed.
The improvement in FI observed in the 3 subjects with high compliance persisted in the 2 subjects who continued to train until month 11 of the study. This finding is in accord with numerous other studies that have examined the long-term effects of electrical stimulation cycling. Mohr et al showed that conversion of quadriceps myosin to MHC IIA was almost complete by 1 year,21 which would likely indicate higher FI. Moreover, they showed that citrate synthase levels remained high at 1 year, bolstering this supposition. Likewise, Andersen et al also showed a near-total conversion of quadriceps myosin to MHC IIA after 12 months of training.43 However, neither of these training protocols was designed to overload the muscle. Rather, these studies emphasized aerobic conditioning with low loads during electrical stimulation–induced cycling. We recently reported preservation of acutely paralyzed soleus FI for up to 3 years of electrical stimulation training that involved muscular overload.13 The findings of the present study support the observation that just as FI can be preserved in acutely paralyzed muscle, it can also be brought back in muscle with long-term paralysis.
Torque
For the 3 subjects with high compliance, group mean PT increased until month 4 and thereafter remained relatively steady at approximately 30% over the pretraining value. Thus, adaptations leading to increased torque occurred in a time frame consistent with volitional strength-training studies.44 For the 2 subjects who trained beyond 6 months, PT remained greater than pretraining values at month 11. Two studies of the tibialis anterior42,45 reported no increase in force after 9 and 30 weeks of training, respectively. Hartkopp et al46 reported only a modest increase in wrist extensor force after 12 weeks of training (in a subset of subjects who trained at 15 Hz). However, minimal load was placed on the muscles being trained. In contrast, quadriceps torque appears to respond readily to training. Gerrits et al14 reported that quadriceps force rose 20% over the initial value after 12 weeks of training (10 or 50 Hz). Crameri et al12 reported even larger gains in quadriceps force (51% to 122% over the initial force) in subjects who trained with 35 Hz for 10 weeks.
Our findings agree with previous studies that attribute large increases in torque to training. However, previous studies lacked a within-subject control limb for comparison. The increase in PT observed in the untrained limbs in the present study suggests that other noncontractile tissue adaptations47,48 or cross-educational effects49,50 minimized the difference in training despite visible external signs of hypertrophy. Since all of these subjects had clinically complete lesions, this may support a segmental contribution to cross-education with training.51,52
MI
The torque-time integral has important implications for the clinical use of electrical stimulation. The MI, which occurs at or near the end of a fatigue bout, provides a way to estimate the robustness of the training on the amount of work the muscle performs. Muscles with a high MI (like the trained limbs in the present study) are likely to be more useful for functional tasks than muscles whose integral is low during fatigue (like the untrained limbs). In the cohort of subjects with good compliance, group mean MI increased sharply during month 1, paralleling the rapid gain in FI. However, the influence of fluctuating PT can be seen in months 4 to 6, when the MI increases and decreases in tandem with PT.
Overall, the intervention increased the ability of chronically paralyzed muscle to perform repetitive work.
PI
Electrical stimulation training significantly decreased the magnitude of postfatigue potentiation observed over time in subjects with chronic SCI. The PI could diminish in 2 ways: 1) if the PT for bout 4 decreased or 2) if the contraction 1 torque for bout 4 increased. Further inspection of the data used to calculate the PI reveals that PT for bout 4 did not significantly differ among any of the training months (P > .05). In contrast, contraction 1 torque for months 3 to 6 and month 11 significantly exceeded month 1 torque (P < .05). In addition, month 11 starting torque significantly exceeded the starting torque for all other training months (P < .05). Thus, although bout 4 torque reached a similar maximum value at all time periods, the bout 4 starting torque increased with training. As such, the trained soleus muscles exhibited less fatigue after repetitive stimulation than before they underwent training. This is also supported by the large increase in bout 1 FI during the course of the study. Improved fatigue resistance was coupled with reduced postfatigue potentiation, suggesting that both may be influenced by excitation-contraction coupling mechanisms. Low-frequency fatigue, which is known to be attributable to excitation-contraction coupling compromise, may benefit most from this type of training protocol.
The PI demonstrated by the subjects in the present study is commensurate with PI values for a previous study of individuals with chronic SCI (~1.9).16 We have recently demonstrated that when started early after SCI (<6 months), this soleus training protocol prevents the emergence of postfatigue potentiation for as long as 2 years after SCI.16 These acutely trained subjects demonstrated minimal low-frequency fatigue, adding additional evidence for a possible effect of training on the excitation-contraction coupling mechanism.
Although there are exceptions,53 the preponderance of evidence from previous studies concurs that potentiation is more prominent in fast fibers than in slow fibers. In rat gastrocnemius, Moore and Stull34 found greater myosin light chain phosphorylation (the probable mechanism of potentiation) in fast fibers than in slow fibers. One human study found that vastus lateralis muscles demonstrating the most potentiation had more type 2 fibers and faster contraction time than did muscles that demonstrated less potentiation.54 Another study found that human gastrocnemius (with a mixed fiber type population) demonstrated greater twitch potentiation than the soleus did (which has mainly slow fibers).55 In ankle dorsiflexors, muscles with short twitch times (a characteristic of functionally fast muscle) also demonstrated the greatest posttetanic potentiation.56 In any event, our findings show that daily training has a robust effect on the mechanisms leading to postfatigue potentiation in chronically paralyzed muscle.
Bone Density
BMD is at least partially determined by the loads experienced by the skeletal system.22,57 The greatest contributor of loading is the activity of muscles,5 which introduce compressive and shear forces through the bones over which they cross. Extensive research has been undertaken to elucidate a method to improve post-SCI BMD via electrically stimulated contraction. We have recently demonstrated that the electrical stimulation protocol used in this study can partially preserve BMD at the proximal tibia25 and distal tibia13 if initiated in individuals with acute SCI. This finding, however, was in subjects who began the training protocol less than 4.5 months after SCI. The osteogenic potential of this protocol in subjects with chronic SCI appears to be limited; subjects demonstrated no improvement in BMD due to the training protocol. The subject’s age (37–68 years) did not appear to influence the responses in bone density to training in this study. According to the mechanostat theory, there is a certain threshold of bone strain that will trigger an osteogenic process under normal conditions. As individuals age or have reduced stress from SCI, the bone loses its density. Thus, the amount of stress required to strain the bone should become less as well and should make it easier to reach a threshold strain according to the mechanostat theory. However, the findings from this study suggest that when the bone deteriorates too extensively, it loses its capacity to adapt to strain, even though the threshold strain according to the mechanostat theory may be reached. Future studies may need to consider other methods, such as peripheral quantitative computed tomography, to assess only the highly metabolic trabecular lattice,58–60 in which bone remodeling may be most likely to occur. On the other hand, computed tomography images of the distal tibial epiphysis in people with chronic SCI often reveal a near-complete absence of the trabecular lattice,26,27 a development that is likely irreversible.28 Thus, a window of opportunity to influence bone density may exist in individuals with SCI within the first year, beyond which loading interventions have a smaller chance of a successful outcome.
Compliance
One subject in the present study (subject 2) demonstrated low compliance with stimulation dose recommendations during the first 3 months of home training. This development had the unexpected benefit of allowing us to explore the possible effect of stimulation dose on muscle physiology. This individual demonstrated no adaptive effects (PT, FI, or MI) until his percentage compliance exceeded approximately 80%. Afterward, although PT remained near baseline values, this subject demonstrated FI and MI improvements on par with the other 3 subjects. Values for PT, FI, and MI for this subject’s untrained limb resembled the subjects with high compliance. This finding further underscores the need for elucidation of dose-response relationships for this (and other) stimulation paradigms. It also highlights the critical importance of monitoring compliance with any long-term intervention study.
Interestingly, the subject with low compliance experienced improvements in PI that were of similar magnitude and timing as that of the group of subjects who had high compliance. This indicates that even before improvements in torque and fatigue resistance emerged, the soleus muscle in this individual was undergoing adaptations that minimized the effect of potentiation after repetitive stimulation. Low-dosage training completed by the subject in the first 3 months was sufficient to incite adaptations of the excitation-contraction coupling mechanism. Further work is needed to discern the doses of activity that are needed to yield adaptive effects on the various muscle parameters in individuals with chronic SCI.
The small sample size of this study clearly reflects the difficulties inherent in conducting longitudinal research of intensive training interventions in those with chronic paralysis. A daily training protocol requires significant subject commitment and an ability to attend frequent laboratory sessions. The home-based training protocol substantially reduced this subject burden but required careful monitoring of subject compliance. The precise understanding of compliance and stimulation dose obtained in this study helps to partially offset the normal limitations on conclusions that can be drawn from a study with a small sample. Despite the small sample size in this study, several muscle-dependent variables responded to training in a systematic and significant fashion and in a manner highly reminiscent of changes observed in acutely paralyzed muscle.13 We maintained more than 80% power in this study for all variables except bone density. Thus, we cannot state with certainty that the lack of an effect on bone was related to the small sample size.
CONCLUSIONS
Eleven months of soleus muscle electrical stimulation training yielded improvements in PT, fatigue resistance, torque-time integral, and postfatigue potentiation in 3 people with long-standing SCI. Because of the isometric muscle activation conditions, the tibia compressive loads were considerably higher than in previous training studies (~110% of BW), the training was of long duration (11 months), and the dose of exercise was high (~9110 contractions per month), yet proximal tibia BMD did not change significantly from the training. Future studies with 3-dimensional bone densitometry techniques and a larger sample size should more clearly elucidate the adaptive capacity of trabecular bone in chronic SCI with long-term stimulation. Similarly, future studies should explore the dose-response relationship between therapeutic loading strategies and muscle and bone parameters. To this end, careful monitoring of subject compliance should be a feature of future studies.
Acknowledgments
This work was supported by an award (R01-HD 39445) to R.K.S. from the National Center for Medical Rehabilitation Research (National Institutes of Health) and by the Christopher Reeve and Sam Schmidt Paralysis Foundations (to R.K.S.). S.D.-J. received a scholarship from the Foundation for Physical Therapy, Inc.
References
- 1.Grimby G, Broberg C, Krotkiewska I, et al. Muscle fiber composition in patients with traumatic cord lesion. Scand J Rehabil Med. 1976;8:37–42. [PubMed] [Google Scholar]
- 2.Shields RK. Fatigability, relaxation properties, and electromyographic responses of the human paralyzed soleus muscle. J Neurophysiol. 1995;73:2195–2206. doi: 10.1152/jn.1995.73.6.2195. [DOI] [PubMed] [Google Scholar]
- 3.Shields RK. Muscular, skeletal, and neural adaptations following spinal cord injury. J Orthop Sports Phys Ther. 2002;32:65–74. doi: 10.2519/jospt.2002.32.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castro MJ, Apple DF, Jr, Hillegass EA, et al. Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur J Appl Physiol. 1999;80:373–378. doi: 10.1007/s004210050606. [DOI] [PubMed] [Google Scholar]
- 5.Lu TW, Taylor SJ, O’Connor JJ, et al. Influence of muscle activity on the forces in the femur: an in vivo study. J Biomech. 1997;30:1101–1106. doi: 10.1016/s0021-9290(97)00090-0. [DOI] [PubMed] [Google Scholar]
- 6.Wilmet E, Ismail AA, Heilporn A, et al. Longitudinal study of the bone mineral content and of soft tissue composition after spinal cord section. Paraplegia. 1995;33:674–677. doi: 10.1038/sc.1995.141. [DOI] [PubMed] [Google Scholar]
- 7.Biering-Sorensen F, Bohr HH, Schaadt OP. Longitudinal study of bone mineral content in the lumbar spine, the forearm and the lower extremities after spinal cord injury. Eur J Clin Invest. 1990;20:330–335. doi: 10.1111/j.1365-2362.1990.tb01865.x. [DOI] [PubMed] [Google Scholar]
- 8.Garland DE, Adkins RH. Bone loss at the knee in spinal cord injury. Top Spinal Cord Inj Rehabil. 2001;6:37–46. [Google Scholar]
- 9.Garland DE, Stewart CA, Adkins RH, et al. Osteoporosis after spinal cord injury. J Orthop Res. 1992;10:371–378. doi: 10.1002/jor.1100100309. [DOI] [PubMed] [Google Scholar]
- 10.Vestergaard P, Krogh K, Rejnmark L, et al. Fracture rates and risk factors for fractures in patients with spinal cord injury. Spinal Cord. 1998;36:790–796. doi: 10.1038/sj.sc.3100648. [DOI] [PubMed] [Google Scholar]
- 11.Belanger M, Stein RB, Wheeler GD, et al. Electrical stimulation: can it increase muscle strength and reverse osteopenia in spinal cord injured individuals? Arch Phys Med Rehabil. 2000;81:1090–1098. doi: 10.1053/apmr.2000.7170. [DOI] [PubMed] [Google Scholar]
- 12.Crameri RM, Cooper P, Sinclair PJ, et al. Effect of load during electrical stimulation training in spinal cord injury. Muscle Nerve. 2004;29:104–111. doi: 10.1002/mus.10522. [DOI] [PubMed] [Google Scholar]
- 13.Shields RK, Dudley-Javoroski S. Musculoskeletal plasticity after acute spinal cord injury: effects of long-term neuromuscular electrical stimulation training. J Neurophysiol. 2006;95:2380–2390. doi: 10.1152/jn.01181.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerrits HL, Hopman MT, Sargeant AJ, et al. Effects of training on contractile properties of paralyzed quadriceps muscle. Muscle Nerve. 2002;25:559–567. doi: 10.1002/mus.10071. [DOI] [PubMed] [Google Scholar]
- 15.Shields RK, Chang Y-J. The effects of fatigue on the torque-frequency curve of the human paralysed soleus muscle. J Electromyogr Kinesiol. 1997;7:3–13. doi: 10.1016/s1050-6411(96)00015-6. [DOI] [PubMed] [Google Scholar]
- 16.Shields RK, Dudley-Javoroski S, Littmann AE. Postfatigue potentiation of paralyzed soleus muscle: evidence for adaptation with long-term electrical stimulation training. J Appl Physiol. 2006;101:556–565. doi: 10.1152/japplphysiol.00099.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chilibeck PD, Jeon J, Weiss C, et al. Histochemical changes in muscle of individuals with spinal cord injury following functional electrical stimulated exercise training. Spinal Cord. 1999;37:264–268. doi: 10.1038/sj.sc.3100785. [DOI] [PubMed] [Google Scholar]
- 18.Crameri RM, Weston A, Climstein M, et al. Effects of electrical stimulation-induced leg training on skeletal muscle adaptability in spinal cord injury. Scand J Med Sci Sports. 2002;12:316–322. doi: 10.1034/j.1600-0838.2002.20106.x. [DOI] [PubMed] [Google Scholar]
- 19.Hjeltnes N, Aksnes AK, Birkeland KI, et al. Improved body composition after 8 wk of electrically stimulated leg cycling in tetraplegic patients. Am J Physiol. 1997;273:R1072–R1079. doi: 10.1152/ajpregu.1997.273.3.R1072. [DOI] [PubMed] [Google Scholar]
- 20.Scremin AM, Kurta L, Gentili A, et al. Increasing muscle mass in spinal cord injured persons with a functional electrical stimulation exercise program. Arch Phys Med Rehabil. 1999;80:1531–1536. doi: 10.1016/s0003-9993(99)90326-x. [DOI] [PubMed] [Google Scholar]
- 21.Mohr T, Andersen JL, Biering-Sorensen F, et al. Long-term adaptation to electrically induced cycle training in severe spinal cord injured individuals [published correction appears in Spinal Cord. 1997;35:262] Spinal Cord. 1997;35:1–16. doi: 10.1038/sj.sc.3100343. [DOI] [PubMed] [Google Scholar]
- 22.Frost HM. Bone’s mechanostat: a 2003 update. Anat Rec. 2003;275A:1081–1101. doi: 10.1002/ar.a.10119. [DOI] [PubMed] [Google Scholar]
- 23.Bloomfield SA, Mysiw WJ, Jackson RD. Bone mass and endocrine adaptations to training in spinal cord injured individuals. Bone. 1996;19:61–68. doi: 10.1016/8756-3282(96)00109-3. [DOI] [PubMed] [Google Scholar]
- 24.Eser P, de Bruin ED, Telley I, et al. Effect of electrical stimulation-induced cycling on bone mineral density in spinal cord-injured patients. Eur J Clin Invest. 2003;33:412–419. doi: 10.1046/j.1365-2362.2003.01156.x. [DOI] [PubMed] [Google Scholar]
- 25.Shields RK, Dudley-Javoroski S, Frey Law L. Electrically-induced muscle contractions influence bone density decline after spinal cord injury. Spine. 2006;31:548–553. doi: 10.1097/01.brs.0000201303.49308.a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Modlesky CM, Majumdar S, Narasimhan A, et al. Trabecular bone microarchitecture is deteriorated in men with spinal cord injury. J Bone Miner Res. 2004;19:48–55. doi: 10.1359/JBMR.0301208. [DOI] [PubMed] [Google Scholar]
- 27.Slade JM, Bickel CS, Modlesky CM, et al. Trabecular bone is more deteriorated in spinal cord injured versus estrogen-free post-menopausal women. Osteoporos Int. 2005;16:263–272. doi: 10.1007/s00198-004-1665-7. [DOI] [PubMed] [Google Scholar]
- 28.Parfitt AM. Trabecular bone architecture in the pathogenesis and prevention of fracture. Am J Med. 1987;82:68–72. doi: 10.1016/0002-9343(87)90274-9. [DOI] [PubMed] [Google Scholar]
- 29.Shields RK, Chang YJ, Ross M. Neuromuscular propagation after fatiguing contractions of the paralyzed soleus muscle in humans. Muscle Nerve. 1998;21:776–787. doi: 10.1002/(sici)1097-4598(199806)21:6<776::aid-mus10>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 30.American Spinal Injury Association. International Standards for Neurological Classification of SCI. Atlanta, Ga: American Spinal Injury Association; 2002. [Google Scholar]
- 31.Shields RK, Law LF, Reiling B, et al. Effects of electrically induced fatigue on the twitch and tetanus of paralyzed soleus muscle in humans. J Appl Physiol. 1997;82:1499–1507. doi: 10.1152/jappl.1997.82.5.1499. [DOI] [PubMed] [Google Scholar]
- 32.Sale D, Quinlan J, Marsh E, et al. Influence of joint position on ankle plantarflexion in humans. J Appl Physiol. 1982;52:1636–1642. doi: 10.1152/jappl.1982.52.6.1636. [DOI] [PubMed] [Google Scholar]
- 33.Westerblad H, Duty S, Allen DG. Intracellular calcium concentration during low-frequency fatigue in isolated single fibers of mouse skeletal muscle. J Appl Physiol. 1993;75:382–388. doi: 10.1152/jappl.1993.75.1.382. [DOI] [PubMed] [Google Scholar]
- 34.Moore RL, Stull JT. Myosin light chain phosphorylation in fast and slow skeletal muscles in situ. Am J Physiol. 1984;247:C462–C471. doi: 10.1152/ajpcell.1984.247.5.C462. [DOI] [PubMed] [Google Scholar]
- 35.Tubman LA, MacIntosh BR, Maki WA. Myosin light chain phosphorylation and posttetanic potentiation in fatigued skeletal muscle. Pflugers Arch. 1996;431:882–887. doi: 10.1007/s004240050081. [DOI] [PubMed] [Google Scholar]
- 36.Tubman LA, Rassier DE, MacIntosh BR. Attenuation of myosin light chain phosphorylation and posttetanic potentiation in atrophied skeletal muscle. Pflugers Arch. 1997;434:848–851. doi: 10.1007/s004240050474. [DOI] [PubMed] [Google Scholar]
- 37.Vandenboom R, Houston ME. Phosphorylation of myosin and twitch potentiation in fatigued skeletal muscle. Can J Physiol Pharmacol. 1996;74:1315–1321. [PubMed] [Google Scholar]
- 38.Rankin LL, Enoka RM, Volz KA, et al. Coexistence of twitch potentiation and tetanic force decline in rat hindlimb muscle. J Appl Physiol. 1988;65:2687–2695. doi: 10.1152/jappl.1988.65.6.2687. [DOI] [PubMed] [Google Scholar]
- 39.Shields RK, Schlechte J, Dudley-Javoroski S, et al. Bone mineral density after spinal cord injury: a reliable method for knee measurement. Arch Phys Med Rehabil. 2005;86:1969–1973. doi: 10.1016/j.apmr.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maganaris CN, Baltzopoulos V, Sargeant AJ. Changes in Achilles tendon moment arm from rest to maximum isometric plantarflexion: in vivo observations in man. J Physiol (Lond) 1998;510:977–985. doi: 10.1111/j.1469-7793.1998.977bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maganaris CN, Baltzopoulos V, Sargeant AJ. In vivo measurement-based estimations of the human Achilles tendon moment arm. Eur J Appl Physiol. 2000;83:363–369. doi: 10.1007/s004210000247. [DOI] [PubMed] [Google Scholar]
- 42.Harridge SD, Andersen JL, Hartkopp A, et al. Training by low-frequency stimulation of tibialis anterior in spinal cord-injured men. Muscle Nerve. 2002;25:685–694. doi: 10.1002/mus.10021. [DOI] [PubMed] [Google Scholar]
- 43.Andersen JL, Mohr T, Biering-Sorensen F, et al. Myosin heavy chain isoform transformation in single fibres from m. vastus lateralis in spinal cord injured individuals: effects of long-term functional electrical stimulation (FES) Pflugers Arch. 1996;431:513–518. doi: 10.1007/BF02191897. [DOI] [PubMed] [Google Scholar]
- 44.Moritani T, deVries HA. Potential for gross muscle hypertrophy in older men. J Gerontol. 1980;35:672–682. doi: 10.1093/geronj/35.5.672. [DOI] [PubMed] [Google Scholar]
- 45.Stein RB, Gordon T, Jefferson J, et al. Optimal stimulation of paralyzed muscle after human spinal cord injury. J Appl Physiol. 1992;72:1393–1400. doi: 10.1152/jappl.1992.72.4.1393. [DOI] [PubMed] [Google Scholar]
- 46.Hartkopp A, Harridge SD, Mizuno M, et al. Effect of training on contractile and metabolic properties of wrist extensors in spinal cord-injured individuals. Muscle Nerve. 2003;27:72–80. doi: 10.1002/mus.10290. [DOI] [PubMed] [Google Scholar]
- 47.Maganaris CN, Reeves ND, Rittweger J, et al. Adaptive response of human tendon to paralysis. Muscle Nerve. 2006;33:85–92. doi: 10.1002/mus.20441. [DOI] [PubMed] [Google Scholar]
- 48.McDonald MF, Garrison MK, Schmit BD. Length-tension properties of ankle muscles in chronic human spinal cord injury. J Biomech. 2005;38:2344–2353. doi: 10.1016/j.jbiomech.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 49.Moritani T, deVries HA. Neural factors versus hypertrophy in the time course of muscle strength gain. Am J Phys Med. 1979;58:115–130. [PubMed] [Google Scholar]
- 50.Hortobagyi T, Scott K, Lambert J, et al. Cross-education of muscle strength is greater with stimulated than voluntary contractions. Motor Control. 1999;3:205–219. doi: 10.1123/mcj.3.2.205. [DOI] [PubMed] [Google Scholar]
- 51.Aagaard P, Simonsen EB, Andersen JL, et al. Neural adaptation to resistance training: changes in evoked V-wave and H-reflex responses. J Appl Physiol. 2002;92:2309–2318. doi: 10.1152/japplphysiol.01185.2001. [DOI] [PubMed] [Google Scholar]
- 52.Enoka RM. Neural adaptations with chronic physical activity. J Biomech. 1997;30:447–455. doi: 10.1016/s0021-9290(96)00170-4. [DOI] [PubMed] [Google Scholar]
- 53.Stuart DS, Lingley MD, Grange RW, et al. Myosin light chain phosphorylation and contractile performance of human skeletal muscle. Can J Physiol Pharmacol. 1988;66:49–54. doi: 10.1139/y88-009. [DOI] [PubMed] [Google Scholar]
- 54.Hamada T, Sale DG, MacDougall JD, et al. Postactivation potentiation, fiber type, and twitch contraction time in human knee extensor muscles. J Appl Physiol. 2000;88:2131–2137. doi: 10.1152/jappl.2000.88.6.2131. [DOI] [PubMed] [Google Scholar]
- 55.Vandervoort AA, McComas AJ. A comparison of the contractile properties of the human gastrocnemius and soleus muscles. Eur J Appl Physiol. 1983;51:435–440. doi: 10.1007/BF00429079. [DOI] [PubMed] [Google Scholar]
- 56.O’Leary DD, Hope K, Sale DG. Posttetanic potentiation of human dorsiflexors. J Appl Physiol. 1997;83:2131–2138. doi: 10.1152/jappl.1997.83.6.2131. [DOI] [PubMed] [Google Scholar]
- 57.Frost HM. Bone “mass” and the “mechanostat”: a proposal. Anat Rec. 1987;219:1–9. doi: 10.1002/ar.1092190104. [DOI] [PubMed] [Google Scholar]
- 58.Jarvinen TL, Kannus P, Sievanen H. Have the DXA-based exercise studies seriously underestimated the effects of mechanical loading on bone? J Bone Miner Res. 1999;14:1634–1635. doi: 10.1359/jbmr.1999.14.9.1634. [DOI] [PubMed] [Google Scholar]
- 59.Rubin C, Turner AS, Mallinckrodt C, et al. Mechanical strain, induced noninvasively in the high-frequency domain, is anabolic to cancellous bone, but not cortical bone. Bone. 2002;30:445–452. doi: 10.1016/s8756-3282(01)00689-5. [DOI] [PubMed] [Google Scholar]
- 60.Snyder WS, editor. Report of the Task Group on Reference Man. Oxford, UK: Pergamon; 1975. [Google Scholar]